Abstract
During de novo plant organ regeneration, auxin induction mediates the formation of a pluripotent cell mass called callus, which regenerates shoots upon cytokinin induction. However, molecular mechanisms underlying transdifferentiation remain unknown. Here, we showed that the loss of HDA19, a histone deacetylase (HDAC) family gene, suppresses shoot regeneration. Treatment with an HDAC inhibitor revealed that the activity of this gene is essential for shoot regeneration. Further, we identified target genes whose expression was regulated through HDA19-mediated histone deacetylation during shoot induction and found that ENHANCER OF SHOOT REGENERATION 1 and CUP-SHAPED COTYLEDON 2 play important roles in shoot apical meristem formation. Histones at the loci of these genes were hyperacetylated and markedly upregulated in hda19. Transient ESR1 or CUC2 overexpression impaired shoot regeneration, as observed in hda19. Therefore, HDA19 mediates direct histone deacetylation of CUC2 and ESR1 loci to prevent their overexpression at the early stages of shoot regeneration.
Keywords: shoot regeneration, histone deacetylation, epigenetics, transdifferentiation
Significance Statement.
We found that the de novo shoot regeneration from a pluripotent cell mass called callus is regulated by a histone deacetylase family gene, HDA19. The two key genes responsible for shoot apical meristem formation are directly deacetylated by HDA19 at the early stages of shoot regeneration.
Introduction
Plants possess high pluripotent competency (1, 2), enabling de novo shoot organogenesis from explants using in vitro tissue culture techniques, which have been extensively used in various studies including those on transgenic breeding and propagation of economically important traits. The knowledge of molecular mechanisms underlying de novo organogenesis would not only enhance our understanding of plant differentiation but also help improve tissue culture techniques for agricultural and horticultural applications. During de novo shoot regeneration, first, a mass of undifferentiated pluripotent cells called callus is formed from an explant following incubation on an auxin-rich callus-inducing medium (CIM) (3, 4). Second, shoots are regenerated from the callus following incubation on a cytokinin-rich shoot-inducing medium (SIM) (3). During callus formation, plants are reprogrammed to acquire pluripotent competency in the middle layer of callus, which is required for shoot regeneration (3, 5, 6). Following incubation on SIM, shoot apical meristems (SAMs) are formed from the callus (7, 8).
The molecular pathway of de novo shoot regeneration has been gradually revealed (1, 9, 10). During shoot induction, the transcription factors PLETHORA (PLT) 3, PLT5, and PLT7 upregulate CUP-SHAPED COTYLEDON (CUC) 2 and the transcription factor ENHANCER OF SHOOT REGENERATION 1/DORNRÖSCHEN (ESR1/DRN) upregulates CUC1 (11, 12). At the early stages of shoot induction, spatial expression of CUC1 and CUC2 (13, 14) determines the location of SAM on the callus (13, 14). At subsequent stages, WUSCHEL (WUS) functions as a core transcription factor regulating the development of SAMs from shoot progenitors on the callus (14, 15). WUS expression is activated by cytokinin signaling transcription factors, including type-B ARABIDOPSIS RESPONSE REGULATOR 1 (ARR1), ARR2, ARR10, and ARR12 (16, 17).
Moreover, the expression of genes involved in shoot regeneration is epigenetically regulated through chromatin modification and transcriptional activity (18). For instance, at the early stages of shoot regeneration, WUS expression is repressed through DNA methylation via DNA methyltransferases (19–21). Furthermore, during shoot regeneration, histone modifiers, such as KRYPTONITE, JUMONJI14, and HISTONE ACETYLTRANSFERASE OF CBP FAMILY 1, and histone methyltransferases of the polycomb repressive complex 2 have been implicated in dynamic WUS expression through the regulation of histone methylation and acetylation status at the WUS locus (16, 19). The ARABIDOPSIS TRITHORAX-RELATED 2–ARR1 complex activates the type-A ARR genes ARR5 and ARR7 through H3K36me3 methylation, thereby repressing cytokinin signaling and inhibiting WUS expression at an inappropriate timing (22). Additionally, some histone modifiers play crucial roles in the acquisition of shoot regenerative competency during callus formation. Upon incubation on CIM, GENERAL CONTROL NONREPRESSED PROTEIN 5—a histone acetyltransferase (HAT) of the GNAT/MYST superfamily 1—activates the expression of WUSCHEL RELATED HOMEOBOX 5 (WOX5), WOX14, SCARECROW, PLT1, and PLT2, which establish callus pluripotency (23). Further, LYSINE-SPECIFIC DEMETHYLASE 1-LIKE3-mediated H3K4me2 demethylation of shoot regeneration-related genes, such as CBL-INTERACTING PROTEIN KINASE 23, NADH DEPENDENT GLUTAMATE SYNTHASE 1, and UBIQUITIN-PROTEIN LIGASE 4, during callus formation is required for their activation in response to shoot induction (24). Thus, epigenetic regulation plays a pivotal role throughout shoot regeneration, although the precise mechanism remains to be fully elucidated.
Here, we focus on a counterpart of HAT, histone deacetylase (HDAC), which represses gene expression by catalyzing histone H3 and H4 deacetylation (25, 26). Among HDACs in Arabidopsis thaliana, we showed that the function of HISTONE DEACETYLASE 19 (HDA19) is essential for shoot regeneration upon incubation on SIM. Further, we found that HDA19 directly deacetylates histones at CUC2 and ESR1 loci, preventing their overexpression upon shoot induction. In addition, transgenic plants transiently overexpressing CUC2 or ESR1 during incubation on SIM phenocopied hda19 in terms of shoot regeneration efficiency. Based on these results, we propose that although CUC2 and ESR1 induction is essential for SAM formation at the early stages of shoot regeneration, their expression level must be stringently regulated, and HDA19-mediated histone deacetylation plays a crucial role in this process.
Results
HDA19 is required for de novo shoot regeneration from callus
To investigate the roles of HDACs in de novo shoot regeneration, we performed shoot regeneration assays using root explants excised from regions at 0 to 1 cm from the root tip (0 to 1 cm root explants) of wildtype (WT) and hdac mutant plants (Fig. S1). We examined hdac mutants, including hda6, hda9, hda10, hda14, hda17, hda18, hda19, and histone deacetylase 2b (hd2b), and found that shoot regeneration was suppressed only in hda19, indicating that HDA19 is specifically involved in shoot regeneration from calli (Fig. S1). On day 10 of CIM incubation, there were no differences in callus formation between WT and hda19 (hda19-3: T-DNA insertion allele; hda19-5: insertion allele using CRISPR/Cas9) (Fig. 1B and C). In contrast, on day 21 of SIM incubation, the shoot regeneration efficiency of root explants from WT and hda19 calli was 100% and 20%, respectively (Fig. 1C and D). Similarly, shoot regeneration from different tissues, such as the hypocotyl, first leaf, and petal of hda19 explants was suppressed (Fig. S2). Moreover, in hda19 calli from which shoot regeneration was confirmed, the number of regenerated shoots was extremely low (one or two) and their morphology was abnormally thin and long leaf-like (Fig. 1C). This reduction in the shoot regeneration rate of hda19-3 (hereafter referred to as hda19) was recovered by HDA19-GFP expression under the control of its own promoter (HDA19p::HDA19g-sGFP) (Fig. 1C and D). Taken together, these results demonstrate that HDA19 is required for de novo shoot regeneration from calli.
Fig. 1.
Shoot regeneration phenotypes in hda19 and WT under suppressed HDAC activity. (A) Schematic of de novo shoot regeneration. Root tip (0 to 1 cm root explants) were excited from seedlings at 7 days after germination and incubated on CIM for 10 days, followed by SIM for 21 days. Phenotypes were observed at 10 days on CIM, 7 days on SIM, and 21 days on SIM. (B) Gene structure of HDA19 and the sites of T-DNA insertion and T-residue insertion using CRISPR/CAS9. Boxes: exons; bars: introns. (C) Phenotypes of WT, hda19-3, hda19-5, and a complementation line (complement, HDA19p-HDA19g-sGFP in hda19-3) at 10 days on CIM, 7 days on SIM, and 21 days on SIM. Scale bars = 2 mm. Arrow heads: regenerated shoots. (D) Shoot regeneration rate (the ratio of shoot-forming explants to the total explants) in WT, hda19-3, and complement explants at 21 days on SIM. Results are presented as means ± SE of at least three independent experiments (***P < 0.001, Student’s t-test). (E) Phenotypes of WT explants at 7 and 21 days on SIM with 1 μM Ky-2 on CIM and/or SIM. Ky-2 treatment is indicated by magenta letters. Scale bars = 2 mm. Yellow arrowheads indicate regenerated shoots.
Next, to verify that the observed phenotype of hda19 explants was due to defective HDA19 activity, we examined whether treatment with Ky-2 (27, 28), an inhibitor of HDAC family RPD3-like class I [e.g., HDA6, HDA7, HDA9, and HDA19 (29)], produced a phenotype similar to hda19. WT root explants were incubated on CIM and SIM with or without Ky-2, and the shoot regeneration phenotype was evaluated. During both CIM and SIM incubation, Ky-2 treatment suppressed shoot regeneration (Fig. 1E). Furthermore, shoot regeneration was observed in the presence of Ky-2 only during CIM incubation, whereas it was suppressed in the presence of Ky-2 only during SIM incubation (Fig. 1E). These results suggest that the HDAC activity of HDA19 during SIM incubation plays a pivotal role in shoot regeneration.
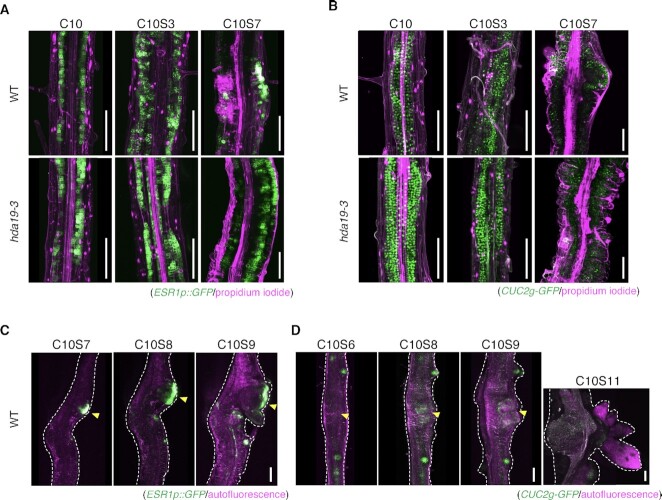
Finally, we examined the expression pattern of HDA19 during shoot regeneration in root explants using a transgenic line expressing HDA19p::HDA19g-sGFP (HDA19-GFP). Imaging analysis showed that under normal growth conditions,HDA19-GFP was mainly localized in the nuclei and was expressed in all tissues at the root tip and at two distinct stages of lateral root primordia (LRP) (Fig. 2A and B). Just before shoot induction (10 days on CIM: C10), HDA19-GFP expression was observed in all callus tissues, except epidermal cells. This expression pattern of HDA19-GFP was also seen in calli at 3 and 7 days after shoot induction following the incubation on CIM for 10 days (C10S3 and C10S7). At C10S14, HDA19-GFP was expressed in and around the SAM; however, it was weakly expressed in callus (Fig. 2C).
Fig. 2.
Expression pattern of HDA19 in roots and callus during shoot induction. (A to C) Expression pattern of the HDA19 translational reporter (HDA19p::HDA19g-sGFP, shown in green) in (A) root apical meristem at 7 days after germination (7 DAG), (B) LRP at 11 DAG, and (C) calli at 10 days on CIM (C10) and 3, 7, and 14 days on SIM (C10S3, C10S7, and C10S14, respectively). Cell outlines were visualized using propidium iodide (PI) staining (shown in magenta). Images in (A) and (B) represent a single optical section. Images in (C) represent z-projections. Scale bars = 45 μm. A yellow arrowhead indicates a developing SAM, and red arrowheads indicate leaf primordia.
HDA19 affects gene expression by regulating histone acetylation status during shoot induction
To clarify the role of HDA19-mediated histone deacetylation during shoot regeneration, we performed integrated analysis with RNA-seq and chromatin immunoprecipitation, followed by sequencing (ChIP-seq), on callus incubated on SIM for 7 days after 10 days of incubation on CIM (Data S1 and S2). HDA19 affects the acetylation status of histones H4 and H3 (29, 30). ChIP-seq of acetylated histone H4 (H4ac) showed that the histone acetylation level of 450 genes was significantly elevated (q < 0.01, FC > 1.5) in hda19 compared with that in WT (Fig. 3A, Data S2). Positional profiles of H4ac in the genic region (gene body plus 2 kb sequences up- and downstream) of 450 hyperacetylated genes revealed higher H4ac levels throughout the gene-coding region in hda19 than in WT (Fig. 3B). Meanwhile, histone H3 accumulation and distribution profiles in the genic region of hyperacetylated genes were comparable between WT and hda19 (Fig. 3A and B). We next analyzed whether the histone acetylation and gene expression levels were positively correlated in hda19. We classified genes into six groups (i to vi, low to high) based on the histone acetylation level in hda19 relative to that in WT and compared their expression between WT and hda19 (Fig. 3C). Histone acetylation level was correlated with gene expression level in hda19 (Fig. 3C). We identified 202 candidate target genes of HDA19, whose expression (q < 0.01, FC > 1.5) and histone acetylation levels were increased in hda19 compared with those in WT (Fig. 3D, Data S1).
Fig. 3.
Histone acetylation and gene expression levels in hda19 during shoot induction. (A) Histone H3 and acetylated histone H4 levels in hda19 compared with WT at 7 days on SIM. Each black dot represents the square root of the count of mapped reads of all genes. Magenta dots indicate 450 genes with histone H4 hyperacetylation in hda19 compared with WT (q < 0.01, FC > 1.5). (B) Positional profiles of histone H3 and acetylated histone H4 in WT and hda19 on genic region (gene plus 2 kb sequence up- and downstream) of 450 genes with histone H4 hyperacetylation in hda19 on SIM shown in (A). (C) Association between histone acetylation and gene expression level in hda19. Based on differences in histone H4 acetylation level between WT and hda19 [log2(RPM _hda19/RPM_WT)] at 7 days on SIM shown in (A), genes were divided into six groups (x-axis). Gene expression fold-changes between WT and hda19 [log2(RPM_hda19/RPM_WT)] at 7 days on SIM were calculated and plotted for group. (D) Venn diagram of genes with histone H4 hyperacetylation in hda19 on SIM shown in (A) and upregulated genes on SIM in hda19 compared with WT (q < 0.01, FC > 1.5).
HDA19 regulates shoot regeneration through ESR1 and CUC2 repression
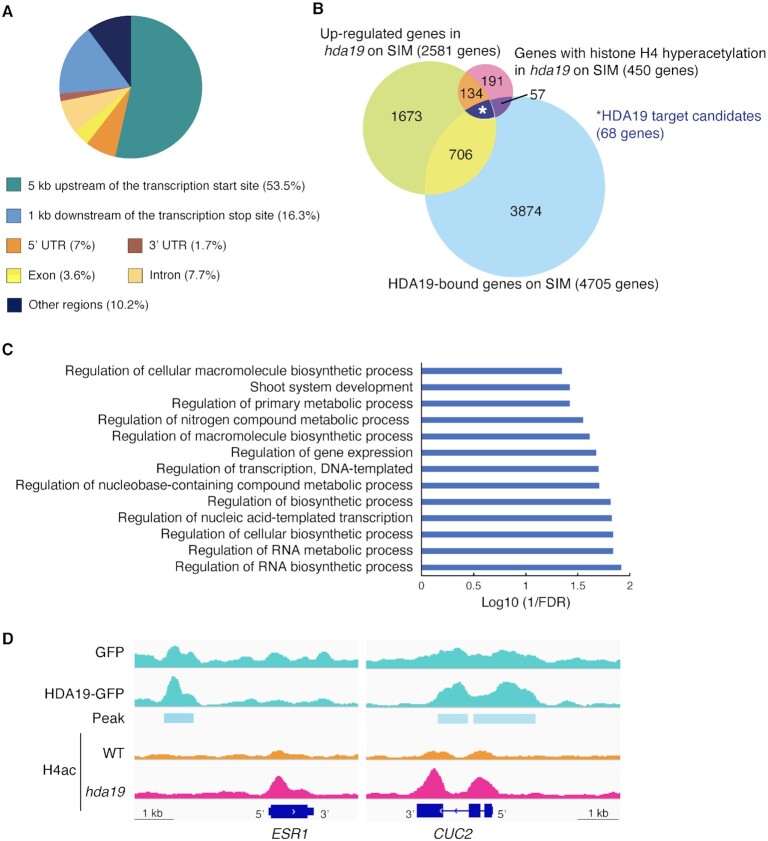
Next, we investigated the binding of HDA19 at 7 days on SIM using ChIP-seq in an hda19 line harboring HDA19-GFP and an anti-GFP antibody. We identified 8,823 HDA19-binding sites in the genome and found that HDA19 was bound to the upstream regions of majority of the genes (Fig. 4A, Data S4). From these, we identified 68 genes whose expression and histone acetylation levels were increased in hda19 compared with those in WT and to which HDA19 bound (Fig. 4B, Data S1). These were considered candidate genes that may be directly regulated by HDA19 and implicated in conferring the shoot regeneration phenotype of hda19. In Gene Ontology analysis of the candidates, genes involved in shoot system development were significantly enriched (FDR < 0.05) (Fig. 4C).
Fig. 4.
Identification of HDA19 target genes involved in shoot regeneration. (A) Pie chart showing the percentage of gene regions to which HDA19 could bind. HDA19 binding sites were determined using MACS2 peak calling (q < 0.001). A total of 8,823 peaks were analyzed. (B) Venn diagram of genes with histone H4 hyperacetylation in hda19 on SIM (q < 0.01, FC > 1.5), upregulated in hda19 on SIM (q < 0.01, FC > 1.5), bound to HDA19 on SIM [q < 0.001, −log10(P-value) > 20]. Genes that met all conditions were considered HDA19 target candidates. (C) Gene Ontology analysis of HDA19 target candidates (FDR < 0.05). (D) Distribution of acetylated histone H4 and HDA19 binding sites around the ESR1 and CUC2 loci at 7 days on SIM. Blue boxes indicate exons and blue lines indicate introns.
Of the 68 genes, we focused on two transcription factors responsible for shoot formation: ESR1 and CUC2 (31, 32). HDA19-binding sites were detected in the upstream regions of CUC2 and ESR1 and the gene-coding region of CUC2 (Fig. 4D). Therefore, HDA19 may directly reduce the histone acetylation level in both gene-coding regions by binding at the periphery of the genic regions of CUC2 and ESR1.
Next, to evaluate whether the hda19 phenotype during shoot regeneration could be attributed to the increased expression of ESR1 and CUC2, we conditionally overexpressed ESR1 or CUC2 and observed the resulting phenotypes (Fig. 5). ESR1 was transiently induced using pER8-ESR1, which induced ESR1 through 17β-estradiol application (31). CUC2 was transiently induced using CUC2g-m4-GR, which induced CUC2 through dexamethasone (DEX) application. CUC2g-m4-GR carries a mutation in the miR164 target sequence, which prevents CUC2 mRNA degradation to induce its overexpression (33). Following the incubation of pER8-ESR1 or CUC2g-m4-GR root explants on CIM for 10 days, shoot regeneration was analyzed at 7 and 21 days on SIM with/without the inducer. Expectedly, ESR1 or CUC2 overexpression through the application of the respective inducers on both CIM and SIM or SIM alone resulted in defects in shoot regeneration, similar to that in hda19 (Fig. 5A and B). Meanwhile, ESR1 or CUC2 overexpression in CIM alone did not affect shoot regeneration. Furthermore, we noted a pin-like leaf morphology of regenerated shoots in all conditions under ESR1 overexpression (Fig. 5A).
Fig. 5.

Effects of ESR1 and CUC2 overexpression on the shoot regeneration phenotype. (A) Shoot regeneration phenotypes of ESR1 conditional overexpression line pER8-ESR1 explants at 7 and 21 days on SIM with 5 μM 17β-estradiol on CIM and/or SIM. 17β-Estradiol treatment is indicated by magenta letters. (B) Shoot regeneration phenotypes of CUC2 conditional overexpression line CUC2g-m4-GR explants at 7 and 21 days on SIM with 1 μM DEX on CIM and/or SIM. DEX treatment is indicated by magenta letters. Shoot rate is the ratio of normal shoot-forming explants to the total explants. Pin rate is the ratio of shoot-forming explants with a pin-like leaf to the total shoot-forming explants. Shoot average is the number of shoots per explant tested. Scale bars = 2 mm. Red and orange arrowheads indicate regenerated shoots and a pin-like leaf, respectively.
Therefore, HDA19 may be involved in shoot regeneration during SIM incubation by directly regulating the histone acetylation levels and, ultimately, the expression levels of ESR1 and CUC2. The comparable expression levels of ESR1 and CUC2 in a complement line of hda19 and in WT at C10S7 support this hypothesis (Fig. S3).
Lack of localized expression of ESR1 and CUC2 may inhibit shoot regeneration in hda19
To investigate why ESR1 or CUC2 overexpression inhibited shoot regeneration, we introduced the respective reporters ESR1p::GFP and CUC2g-GFP into hda19 through crossing and observed fluorescent signals from callus formation to shoot regeneration. Both transgenes were considered to be regulated in the same manner as endogenous genes because ESR1p::GFP and CUC2g-GFP have upstream regions of approximately 4.8 and 3.1 kb, respectively, which overlap with the upstream HDA19-binding regions of approximately 2.8 and 1 kb, respectively (Data S3). In WT, ESR1p::GFP fluorescence signals were detected throughout the root explant at the time of callus formation (C10) and early stages of shoot regeneration (C10S3) (Fig. 6A). Subsequently, ESR1p::GFP fluorescence signals were concentrated at several locations of WT root explants at 7 days of incubation on SIM (Fig. 6A). Meanwhile, in hda19, ESR1p::GFP fluorescence signals were observed throughout the root explants, similar to that in WT, after 3 days of incubation on SIM, although the signals were stronger and expressed in more cells of root explants in the mutant (Fig. 6A). Even on day 7 of incubation on SIM, ESR1p::GFP fluorescence signals were observed throughout hda19 root explants, although no localized expression of ESR1p::GFP fluorescence was observed in calli (Fig. 6A).
Fig. 6.
Dynamics of ESR1 and CUC2 expression during shoot induction in hda19. Expression patterns of (A) ESR1p::GFP and (B) CUC2g-GFP during shoot induction in WT and hda19. Time-lapse images of ESR1p::GFP (C) and CUC2g-GFP (D) expression. The same explants were observed at each time point. GFP fluorescence is shown in green, and the outline of PI-stained cells or autofluorescence is shown in magenta. The images represent z-projections. Scale bar = 100 µm. Arrowheads indicate developing SAMs.
Next, in WT, the fluorescence signals of CUC2g-GFP were observed throughout the root explants after 3 days of incubation on SIM and accumulation of fluorescence was noted after 7 days of incubation on SIM (Fig. 6B). In hda19, the fluorescence signals of CUC2g-GFP were detected throughout the root explants, similar to that in WT after 3 days of incubation on SIM, although accumulation of fluorescence was not observed after 7 days of incubation on SIM (Fig. 6B). To examine the link between the localized expression of ESR1 and CUC2 and regeneration of SAM, we performed time-lapse observations of ESR1p::GFP and CUC2g-GFP fluorescence in WT (Fig. 6C and D). SAM was regenerated from the regions of fluorescence accumulation in root explants (Fig. 6C and D). Therefore, the inhibition of shoot regeneration in hda19 may be caused by the lack of localized expression of ESR1 and CUC2 during SAM regeneration. This hypothesis is further supported by the data using Ky-2. The inhibition of HDAC activity only during incubation on SIM increased the expression of ESR1 and CUC2 transcripts (Fig. S3) and was responsible for the lack of specific localization of ESR1p::GFP and CUC2g-GFP fluorescence at C10S7 as observed in hda19 (Fig. S4).
Discussion
In the present study, we showed that supplementing SIM with an HDAC inhibitor during incubation remarkably repressed shoot regeneration (Fig. 1E). In addition, at the early stage of shoot induction, hda19 exhibited an increased level of histone acetylation in 202 genes, which also showed elevated expression (Fig. 3D). These results strongly suggest that the HDAC activity of HDA19 during incubation on SIM is essential for the repression of specific genes to establish proper shoot regeneration. However, the regulation of histone acetylation status by HDA19 does not appear to be involved in the acquisition of regenerative competency during callus development.
Similar to many HDACs (30, 34–40), HDA19 binds to the upstream regions of genes and mediates histone deacetylation primarily near the transcription start site (Fig. 4A). This property of HDA19 is consistent with previous reports that HDA19 regulates differentiation and stress responses by binding to the upstream and coding regions of genes (30, 35, 38, 41–43). In the case of shoot induction, the expression level of WUS, a core factor regulating SAM development in the later stages of shoot induction (14, 15), was equivalent in WT and hda19 at C10S7 (Fig. S3), suggesting that HDA19 regulates genes that function before the stage of WUS activity. Intriguingly, we found that two essential genes for the early stage of shoot regeneration, namely ESR1 and CUC2 (31, 44, 45), were included in the 68 genes whose histone acetylation and expression were repressed via HDA19 binding (Fig. 4B and D). The expression of both these genes is elevated during the early stages of shoot regeneration, followed by decline (13, 31). In addition, optimal level of ESR1 expression during shoot induction is essential; in explants constitutively overexpressing ESR1, shoot regeneration is inhibited and abnormal leaf morphology is observed (31). Accordingly, the inability to suppress the expression of these genes to optimal levels in the appropriate cells at the appropriate time of shoot regeneration is a possible cause of the hda19 phenotype. In fact, constitutive ESR1 or CUC2 overexpression, at least during incubation on SIM, inhibited shoot regeneration (Fig. 5). Since the expression of 46 of the candidate targets of HDA19 was upregulated upon shoot induction, even in the WT (Fig. S5, Data S1: C10 vs. C10S7), HDA19 may be also responsible for regulating the expression of these genes to the correct level in response to shoot induction.
CUC2 was expressed throughout the callus during its development, and upon transfer to SIM, its expression was progressively restricted to the zone of SAM emergence (14). Consistent with observations reported by Matsuo et al. (46), our image analysis results confirmed that the dynamics of ESR1 expression in WT calli was similar to those of CUC2 during shoot regeneration. However, such restricted expression patterns of both CUC2 and ESR1 were not detected in hda19 (Fig. 6). Therefore, HDA19 likely represses ESR1 and CUC2 expression in cells other than the SAM-generating cells, thus contributing to their localized expression in callus upon shoot induction. Moreover, ESR1 upregulates the paralog of CUC2, CUC1, whose expression is also restricted to SAM-generating cells upon shoot induction (13, 14, 46). We found that CUC1 expression was significantly enhanced in hda19 incubated on SIM (Data S1), implying that HDA19 is indirectly implicated in regulating CUC1 dynamics in calli. Since HDA19 was expressed throughout the callus, including SAM cells, even after transfer to SIM, we speculate that there are functional differences in HDA19 between SAM-generating and other cells, which may be shaped by other factors, such as HDA19 interacting proteins. Indeed, HDA19 interacts with an array of partners during various developmental processes and stress responses (30, 35, 38, 41–43).
Furthermore, the 68 HDA19 target genes included other regulators that may be involved in various shoot regenerative processes, such as cell proliferation, auxin biosynthesis, photosynthesis, and pluripotency-related gene regulation (Fig. S5, Data S1). One of them is the transcription factor WOX11, which is known to activate LATERAL ORGAN BOUNDARIES DOMAIN16(LBD16) and WOX5 and is involved in the acquisition of pluripotency in callus cells (6, 23, 47–49). In WT, the expression of WOX11 in calli was repressed upon transfer to SIM (23, 49) (Data S1). Therefore, the direct repression of WOX11 expression by HDA19 may contribute to the differentiation of pluripotent callus cells into SAM-generating cells. However, expression levels of WOX5 in hda19 were less than that in WT in response to shoot induction (Data S1). In addition, the expression pattern of WOX5p::GFPer during shoot induction was similar between WT and hda19 (Fig. S6). These observations suggest that defects in the repression of other unknown WOX11 downstream may be a possible cause of the hda19 phenotype.
In conclusion, we showed that conditional fine-tuning of the expression of multiple key developmental genes through HDA19-mediated epigenetic regulation is important for determining the cell fate and loci of SAM formation during early shoot regeneration. Shoot progenitor formation in callus occurs sparsely, from which the developmental program proceeds sequentially to regenerate the shoot. Therefore, clarifying the spatial and temporal control mechanisms by determining which cells use which differentiation program at what time in callus tissue will lead to the development of methods to improve regeneration efficiency. The discovery and analysis of interacting factors will help us understand the mechanisms by which HDA19 regulates the spatiotemporal patterns and expression levels of genes important for shoot regeneration.
Materials and methods
Plant materials and growth conditions
The following mutant alleles were used in the present experiment: hda19-3 (SALK_139445), hda19-5 (a genome-edited allele in Col-0) (29), hda6 (axe1-4), hda18 (SAIL_1289_F05), hda9 (SALK_007123), hda10 (SM_3_28667), hda14 (SALK_144995), hda17 (SALK_090088), and hd2b-2 (a genome-edited allele in Col-0); all mutants are on a Col-0 background. Col-0 plants harboring pER8-ESR1 (31) were used for conditional ESR1 overexpression. Col-0 plants harboring ESR1p::GFP (50) and WOX5p::GFPer (51) were used for imaging analysis. Col-0 plants harboring 35Sp::GFP (24) were used for ChIP-seq. The generation of marker lines, a CUC2-overexpressing line, and an hd2b mutant line is described below. Combinations of the hda19-3 mutation and markers were generated by crossing. Plants homozygous for the mutation and markers were selected for genotyping and antibiotic treatment. Plants were grown in soil or MGRL medium (52) under a long-day (16 h light/8 h dark) photoperiod.
Regeneration assays
Root explants (0 to 1 cm from the root tip) were excised from seedlings 7 days after germination and cultured on CIM containing Gamborg’s B-5 medium (Wako, Osaka, Japan) with 20 g L−1 glucose (Wako), 0.5 g L−1 MES (Wako), 1 × Gamborg’s vitamin solution (Sigma–Aldrich, St. Louis, MO, USA), 500 µg L−1 2,4-D (Sigma–Aldrich), 50 µg L−1 kinetin (Sigma–Aldrich), and 0.8% gellan gum (Wako); the pH was adjusted to 5.7 using 1.0 M KOH. Continuous light was used for callus induction.
After culturing for 10 days on CIM, the explants were transferred to SIM containing Gamborg’s B-5 medium, 10 g L−1 sucrose, 0.5 g L−1 MES, 1 × Gamborg’s vitamin solution, 2 µg mL−1 trans-zeatin, 0.4 µg mL−1 indole-3-butyric acid, 1 µg mL−1 d-biotin, and 0.8% gellan gum; the pH was adjusted to 5.7 using 1.0 M KOH. Continuous light was used for shoot induction. For the chemical treatment, Ky-2, 17β-estradiol, or DEX were added to CIM or SIM.
After incubation on SIM for 21 days, the number of explants that regenerated shoots on all explants was evaluated. All phenotypic assays and microscopic observations were performed at least twice.
Microscopic imaging
To observe GFP fluorescent marker lines, 0.1 mg mL−1 propidium iodide (PI) (Sigma–Aldrich) was applied to the samples prior to imaging for counterstaining the cell outlines.
Root explants at the late stages of callus induction and at all stages of shoot induction (10 days on CIM and 3 to 14 days on SIM) and the root tips and LRP of seedlings (7 and 11 days after germination, respectively) were observed using an Olympus FVMPE-RS multiphoton microscope with an XLPLN 25 × WMP2 (N.A. = 1.05, WD = 2.00 mm) water immersion objective lens (Olympus, Shinjuku, Tokyo, Japan). To detect GFP, PI, and autofluorescence signals, the laser setting was tuned to 920 nm (for GFP) with a fixed 1,040 nm wavelength (for PI and autofluorescence). All lights were reflected by an FV30-SDM-M mirror. Signals were collected using an FV30-FGR filter mounted in front of GaAsP-PMTs. The z-stacks were reconstructed into a projection view using ImageJ. More than 10 samples were imaged for each marker line to confirm that the observed patterns were representative of the respective markers.
Generation of transgenic plants
The hd2b-2 allele was generated by genome editing as described previously (53, 54). To express a single guide RNA (sgRNA) and the CRISPR/Cas9 protein, pZH_OsU3gYSA_FFCas9 and pUC_AtU6oligo vectors were used for targeted mutagenesis in HD2B. The primer pair (5′-ATTGGTGGTTTTGAGTGTGACTGT-3′/5′-AAACACAGTCACACTCAAAACCAC-3′) was used for sgRNA to target HD2B. HD2B mutagenesis in Col-0 plants resulted in the generation of the hd2b-2 allele by inserting a thymine at 140 nt downstream from the HD2B translation initiation codon, inducing a nonsense mutation at 151 nt from the translational initiation codon.
To generate HDA19p::HDA19g-sGFP, a genomic DNA fragment (1.5 kb upstream of ATG to the stop codon) from HDA19 was amplified using 5′-CACCGGTAAAGCTTAAGATGGAAGCATGTGC-3′ and 5′- CGGAGCAGGCGTTTCCTCCTAAAACA-3′ primers and cloned into pENTR/D-TOPO using the Gateway system (Invitrogen, Carlsbad, CA, USA). The HDA19 genomic sequence was recombined upstream of sGFP in the pGWB504 (55) binary vector using the Gateway LR Clonase II Enzyme Mix (Invitrogen) to generate HDA19p::HDA19g-sGFP, which was used for plant transformation (hda19-3 for reporter generation complementation experiments). The constructed plasmid was transformed into Agrobacterium tumefaciens (Rhizobium radiobacter) strain GV3101::pMP90 and then into hda19-3 using the floral dip method (56). Homozygotes were selected using antibiotics and analyzed.
For CUC2g-m4-GR, the stop codon of CUC2g-m4 (33) was replaced with the coding sequence of rat GR receptor domain (57) with the linker sequences AAGCTTATCGATACCGTCGACCTCGAC and TGACTCGAG at the 5′ and 3′ ends, respectively. The entire fragment was cloned into the binary vector pBIN50 (58). For CUC2g-GFP, a genomic fragment of CUC2 spanning from the 3.1 kb promoter to the end of the coding sequence was amplified using 5′-GGGGACAACTTTGTATAGAAAAGTTGACTAGAGGAAGAGTTAAGAGATG-3′ and 5′-GGGGACTGCTTTTTTGTACAAACTTGCGTAGTTCCAAATACAGTCAAG-3′ primers and cloned into pDONR P4-P1R (Invitrogen) using the Gateway system. The CUC2 terminator was amplified using 5′-GGGGACAGCTTTCTTGT ACAAAGTGGCATCACAAAAGAGGTGACTTATA-3′ and 5′-GGGGACAACTTTGTATAATAAAGTTGAAATCATCTAACCGAAGATTCG-3′ primers and cloned into pDONR P2R-P3 (Invitrogen). The inserts in these two plasmids, together with the GFP coding sequence in pDONR207, were transferred to the pGWBmultisite vector (59) to generate CUC2p::CUC2-GFP::CUC2ter (CUC2g-GFP). The constructed plasmids were transformed into Agrobacterium tumefaciens strain GV3101::pMP90 and then into Col-0 using the floral dip method. Homozygotes were crossed with hda19-3.
RNA-seq
Root explants derived from WT (Col-0) and hda19-3 seedlings were collected on day 10 of CIM (C10) and day 7 of SIM (C10S7) incubation. Total RNA was isolated from the explants using the PureLink Plant RNA Reagent (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of purified RNA was assessed using the 2100 Bioanalyzer (Agilent, Hachioji, Tokyo, Japan). The extracted RNA (1,000 ng) was used to construct a transcriptome library with TruSeq RNA Sample Preparation v.2 (Illumina, San Diego, CA, USA). Libraries were pooled and 36 to 86 bp single-read sequences were obtained using the NextSeq 500 sequencer (Illumina). Three independent biological replicates were analyzed for each genotype.
RNA-seq data analysis
Quality-filtered reads were mapped onto the cDNA sequences of annotated genes and other transcripts of TAIR10 using Bowtie (60) with-all-best-strata settings (Data S4). Differentially expressed genes (DEGs) were identified using the edgeR package in R version 3.16.5 (61), treating biological triplicates as paired samples. Genes with adjusted q < 0.01 and fold change (FC) > 1.5 or < 0.66 in each comparison were identified as DEGs (Data S1). To calculate relative gene expression (hda19/WT), half of the minimum RPM (except for 0) value was added to all RPM values.
Gene expression analysis
Total RNA was extracted from explants on day 7 of SIM (C10S7) incubation using the Monarch Total RNA Miniprep Kit (New England Biolabs, Sumida, Tokyo, Japan) following the manufacturer’s protocol. Approximately 1000 ng of total RNA was reverse transcribed using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) following the manufacturer’s protocol. The resultant cDNA was used as a template for real-time PCR with 1 or 5 times dilution. Quantitative real-time RT-PCR was performed using Luna Universal qPCR Master Mix (New England Biolabs) on a Thermal Cycler Dice Real Time System II (TAKARA Bio, Kusatsu, Shiga, Japan). Expression level of PP2A was used for the normalization of expression levels of genes of interest. The primer sets used for the analysis were as follows: 5′-TAGCACCAACACAACCGTCACA-3′ (F) and 5′-AGTTAACGTCTAAGCCCAAGGC-3′ (R) for CUC2 (62); 5′-ACAGCTGTCATTATGCCTGAACCA-3′ (F) and 5′-GGTAGAGGAATCTAACGGTAGAGA-3′ (R) for ESR1 (63); 5′-CTTCCAGATGGCACCACTAC-3′ (F) and 5′-GCGATGCTTATCTGGAACAT-3′ (R) for WUS (64); 5′-GACCAAGTGAACCAGGTTATTGG-3′ (F) and 5′-TACTCTCCAGTGCCTGTCTTCA-3′ (R) for PP2A (63).
ChIP-seq
Root explants derived from WT (Col-0) and hda19-3 seedlings were collected on day 10 of CIM (C10) and day 7 of SIM (C10S7) incubation; 0.1 g of explants was frozen in liquid nitrogen, ground into a fine powder, cross-linked, and nuclear-extracted in the nucleus isolation buffer (1% formaldehyde, 0.6% Triton X-100, and 14.4 mM 2-mercaptoethanol) with 1 mM Pefabloc SC (Sigma–Aldrich) and complete protease inhibitor cocktail (Sigma–Aldrich). The samples were sonicated using S2 or M220 focused ultrasonicators (Covaris, Woburn, MA, USA) and milliTUBE 1 ml AFA fiber (Covaris). The sonicated samples were incubated with anti-acetyl-histone H4 (Merck Millipore, Burlington, MA, USA) and antihistone H3 (ab1791; Abcam, Cambridge, UK) antibodies at 4°C overnight. Protein G Magnetic Dynabeads (Thermo Fisher Scientific) were used for immunoprecipitation. The beads were washed two times each with PBS buffer, low-salt RIPA buffer [50 mM Tris–HCl (pH 7.8), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate, and 1% complete protease inhibitor (Roche Basel, Switzerland)], high-salt RIPA buffer [50 mM Tris–HCl (pH 7.8), 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate, and 1% complete protease inhibitor (Roche)], LNDET buffer [250 mM LiCl, 1% IGEPAL, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris–HCl (pH 7.8)], and with TE buffer. After adding the elution buffer [10 mM Tris–HCl (pH 7.8), 0.3 M NaCl, 5 mM EDTA, and 0.5% SDS], all beads were incubated overnight at 65°C. Lysates were treated with 200 ng mL−1 RNaseA at 37°C for 30 min and then treated with 800 ng mL−1 proteinase K and 400 ng mL−1 glycogen at 37°C for 2 h. After phenol–chloroform extraction and ethanol precipitation, the pellet was suspended in buffer EB (Qiagen, Venlo, Netherlands). The collected DNA was quantified using the Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific), and 1 ng DNA was used to construct a sequencing library with the KAPA Hyper Prep Kit for Illumina (Kapa Biosystems, Wilmington, USA). Dual-size selection was performed using Agencourt AMPure XP (Beckman Coulter, Brea, CA, USA) to enrich 300 to 500 bp fragments. Libraries were pooled, and 75 bp single-read sequences were obtained using the NextSeq 500 sequencer (Illumina).
Genome-wide localization patterns of the HDA19 protein were analyzed using HDA19p::HDA19g-sGFP transgenic plants on an hda19-3 background and control transgenic plants expressing 35Sp:GFP on a Col-0 background. Calli at 7 days of SIM (C10S7) incubation derived from the HDA19p::HDA19g-sGFP and 35Sp::GFP plants were subjected to ChIP-seq analysis using an anti-GFP antibody (ab290; Abcam), as described above. Two independent biological replicates were analyzed for each genotype.
ChIP-seq data analysis
Quality-filtered reads were aligned to the Arabidopsis reference genome TAIR10 using Bowtie (60) with -m 1 -S parameters to report only uniquely mapped reads (Data S4). The resulting SAM files were converted to sorted BAM files using SAMtools (65) and then converted to BED files using BEDTools (66). The “slop” function of BEDTools was used to extend the 5′-end of ChIP-seq reads toward the 3′-direction to fit the average insertion size (250 bp) of the sequenced libraries. Then, the “coverage” function of BEDTools was used to calculate the number of reads that overlapped with each annotation unit (Data S2). HDA19-binding sites were detected by model-based analysis of ChIP-seq data (MACS2) (67) using reads from the anti-GFP (35Sp::GFP) sample as controls (q < 0.001) (Data S3). For visualization, TDF files were created using igvtools (extension factor: 200) from BAM files and visualized using the Integrative Genome Viewer (68). The ngs.plot.r program (69) was used to determine the acetylation profiles and HDA19-binding regions of gene bodies. Scatter and NGS plots of ChIP-seq results are shown only for one of the biological replicates, because the two replicates showed very high reproducibility (Fig. S7). To calculate the acetylation level ratio (hda19/WT), half of the minimum RPM value was added to all RPM values.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr H. Banno at Chubu University, Japan, and Dr W. Werr at Cologne University, Germany, for providing the plants harboring pER8-ESR1 and ESR1p::GFP, respectively, and Dr M. Endo and Dr S. Toki at the National Agriculture and Food Research Organization, Japan, for providing the pZH_OsU3gYSA_FFCas9 and pUC_AtU6 oligo vectors for the CRISPR/Cas9 endonuclease constructs, and Dr M. Yoshida at RIKEN Center for Sustainable Resource Science, Japan, for providing Ky-2 reagent.
Notes
Competing Interest: The authors declare no competing interest.
Contributor Information
Haruka Temman, Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan.
Takuya Sakamoto, Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan.
Minoru Ueda, Plant Genomic Network Research Team, RIKEN Center for Sustainable Resource Science, 1-7-22 Suehiro, Tsurumi, Yokohama, Kanagawa 230-0045, Japan; Plant Epigenome Regulation Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan.
Kaoru Sugimoto, Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan.
Masako Migihashi, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan.
Kazunari Yamamoto, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan.
Yayoi Tsujimoto-Inui, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan.
Hikaru Sato, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan.
Mio K Shibuta, Academic Assembly (Faculty of Science), Yamagata University, Kojirakawa, Yamagata 990-8560, Japan.
Norikazu Nishino, Graduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, 2-4 Hibikino, Wakamatsu-ku, Kitakyushu-shi, Fukuoka 808-0196, Japan.
Tomoe Nakamura, Plant Genomic Network Research Team, RIKEN Center for Sustainable Resource Science, 1-7-22 Suehiro, Tsurumi, Yokohama, Kanagawa 230-0045, Japan; Department of Biological Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan.
Hiroaki Shimada, Department of Biological Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan.
Yukimi Y Taniguchi, School of Science and Technology, Kwansei Gakuin University, 2-1 Gakuen, Sanda, Hyogo 669–1337, Japan.
Seiji Takeda, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Shimogamo Hangi-cho, Sakyo-ku, Kyoto 60-8522, Japan; Biotechnology Research Department, Kyoto Prefectural Agriculture Forestry and Fisheries Technology Centre, 74 Kitaina Yazuma Oji, Seika, Kyoto 619-0244, Japan.
Mitsuhiro Aida, International Research Organization for Advanced Science and Technology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan; International Research Center for Agricultural and Environmental Biology, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-855, Japan.
Takamasa Suzuki, College of Bioscience and Biotechnology, Chubu University, 1200 Matsumoto-cho, Kasugai, Aichi 487-8501, Japan.
Motoaki Seki, Plant Genomic Network Research Team, RIKEN Center for Sustainable Resource Science, 1-7-22 Suehiro, Tsurumi, Yokohama, Kanagawa 230-0045, Japan; Plant Epigenome Regulation Laboratory, RIKEN Cluster for Pioneering Research, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan.
Sachihiro Matsunaga, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan.
Funding
This research was supported by grants from MXT/JSPS KAKENHI (15H05962, 19H03259, 20H05911, and 22H00415) to S.M. and MXT/JSPS KAKENHI (20H05425) to T.Sa.
Authors' Contributions
H.T.: conception and design, data analysis and interpretation, article drafting and revision, project initiation, and data acquisition; T.Sa.: article drafting and revision, data analysis and interpretation, and data acquisition; K.S.: conception and design, data analysis and interpretation; M.M., H.Sa., Y.K., Y.I. and M.K.S.: data acquisition; M.U., M.S., N.N., T.N., H.Sh., Y.Y.T. and S.T.: material generation; M.A.: conception and design; T.Su.: sequencing; S.M.: conception and design, data interpretation, and article revision. The manuscript was written based on inputs from all authors.
Data Availability
Data generated or analyzed during this study are included in this published article (and its supplementary data files). Data S1 contains source data corresponding to the Fig. 3C and D. Data S2 contains source data corresponding to Figs. 3A to C and 4A. Data S3 contains source data corresponding to Fig. 4C and Fig. S3. Data S4 contains the summary of RNA-seq and ChIP-seq experiments corresponding to Fig. 3. RNA-seq and ChIP-seq data that support the findings of this study have been deposited in the DNA Data Bank of Japan (Accession No. DRA13879 and DRA13880, respectively).
References
- 1. Ikeuchi M, et al. 2019. Molecular mechanisms of plant regeneration. Annu Rev Plant Biol. 70:377–406. [DOI] [PubMed] [Google Scholar]
- 2. Gaillochet C, Lohmann JU. 2015. The never-ending story: from pluripotency to plant developmental plasticity. Development. 142:2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skoog F, Miller CO. 1957. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 11:118–130. [PubMed] [Google Scholar]
- 4. Valvekens D, Montagu MV, Lijsebettens MV. 1988. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci. 85:5536–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christianson ML, Warnick DA. 1983. Competence and determination in the process of in vitro shoot organogenesis. Dev Biol. 95:288–293. [DOI] [PubMed] [Google Scholar]
- 6. Zhai N, Xu L. 2021. Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat. Plants. 7:1453–1460. [DOI] [PubMed] [Google Scholar]
- 7. Motte H, Vereecke D, Geelen D, Werbrouck S. 2014. The molecular path to in vitro shoot regeneration. Biotechnol Adv. 32:107–121. [DOI] [PubMed] [Google Scholar]
- 8. Radhakrishnan D, et al. 2018. Shoot regeneration: a journey from acquisition of competence to completion. Curr Opin Plant Biol. 41:23–31. [DOI] [PubMed] [Google Scholar]
- 9. Shin J, Bae S, Seo PJ. 2020. De novo shoot organogenesis during plant regeneration. J Exp Bot. 71:63–72. [DOI] [PubMed] [Google Scholar]
- 10. Sugimoto K, Temman H, Kadokura S, Matsunaga S. 2019. To regenerate or not to regenerate: factors that drive plant regeneration. Curr Opin Plant Biol. 47:138–150. [DOI] [PubMed] [Google Scholar]
- 11. Kareem A, et al. 2015. PLETHORA genes control regeneration by a two-step mechanism. Curr Biol. 25:1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuo N, Mase H, Makino M, Takahashi H, Banno H. 2009. Identification of ENHANCER OF SHOOT REGENERATION 1-upregulated genes during in vitro shoot regeneration. Plant Biotechnol. 26:385–393. [Google Scholar]
- 13. Cary AJ, Che P, Howell SH. 2002. Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana. Plant J. 32:867–877. [DOI] [PubMed] [Google Scholar]
- 14. Gordon SP, et al. 2007. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 134:3539–3548. [DOI] [PubMed] [Google Scholar]
- 15. Lardon R, Wijnker E, Keurentjes J, Geelen D. 2020. The genetic framework of shoot regeneration in Arabidopsis comprises master regulators and conditional fine-tuning factors. Commun Biol. 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang T-Q, et al. 2017. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell. 29:1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meng WJ, et al. 2017. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 29:1357–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu L-Y, et al. 2022. Dynamic chromatin state profiling reveals regulatory roles of auxin and cytokinin in shoot regeneration. Dev Cell. 57:526–542.e7. [DOI] [PubMed] [Google Scholar]
- 19. Li W, et al. 2011. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 7:e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shemer O, Landau U, Candela H, Zemach A, Eshed Williams L. 2015. Competency for shoot regeneration from Arabidopsis root explants is regulated by DNA methylation. Plant Sci. 238:251–261. [DOI] [PubMed] [Google Scholar]
- 21. Liu H, Zhang H, Dong YX, Hao YJ, Zhang XS. 2018. DNA METHYLTRANSFERASE1-mediated shoot regeneration is regulated by cytokinin-induced cell cycle in Arabidopsis. New Phytol. 217:219–232. [DOI] [PubMed] [Google Scholar]
- 22. Lee K, et al. 2021. Arabidopsis ATXR2 represses de novo shoot organogenesis in the transition from callus to shoot formation. Cell Rep. 37:109980. [DOI] [PubMed] [Google Scholar]
- 23. Kim J, et al. 2018. Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis. EMBO J. 37:e98726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishihara H, et al. 2019. Primed histone demethylation regulates shoot regenerative competency. Nat Commun. 10:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dutta A, Abmayr SM, Workman JL. 2016. Diverse activities of histone acylations connect metabolism to chromatin function. Mol Cell. 63:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Y, Lu Y, Zhao Y, Zhou D-X. 2019. Histone acetylation dynamics integrates metabolic activity to regulate plant response to stress. Front Plant Sci. 10:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sako K, et al. 2016. Ky-2, a histone deacetylase inhibitor, enhances high-salinity stress tolerance in Arabidopsis thaliana. Plant Cell Physiol. 57:776–783. [DOI] [PubMed] [Google Scholar]
- 28. Kurita K, et al. 2017. Live imaging of H3K9 acetylation in plant cells. Sci Rep. 7:45894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueda M, et al. 2017. The distinct roles of class I and II RPD3-like histone deacetylases in salinity stress response. Plant Physiol. 175:1760–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, et al. 2013. HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 25:134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banno H, Ikeda Y, Niu Q-W, Chua N-H. 2001. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 13:2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aida M, Vernoux T, Furutani M, Traas J, Tasaka M. 2002. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 129:3965–3974. [DOI] [PubMed] [Google Scholar]
- 33. Nikovics K, et al. 2006. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell. 18:2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu K, Malik K, Tian L, Brown D, Miki B. 2000. Functional analysis of a RPD3 histone deacetylase homologue in Arabidopsis thaliana. Plant Mol Biol. 44:167–176. [DOI] [PubMed] [Google Scholar]
- 35. Krogan NT, Hogan K, Long JA. 2012. APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Dev Camb Engl. 139:4180–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo M, et al. 2012. Histone deacetylase HDA6 is functionally associated with AS1 in repression of KNOX genes in Arabidopsis. PLoS Genet. 8:e1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo M, et al. 2015. Regulation of flowering time by the histone deacetylase HDA5 in Arabidopsis. Plant J. 82:925–936. [DOI] [PubMed] [Google Scholar]
- 38. Mehdi S, et al. 2016. The WD40 domain protein MSI1 functions in a histone deacetylase complex to fine-tune abscisic acid signaling. Plant Cell. 28:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gorham SR, Weiner AI, Yamadi M, Krogan NT. 2018. HISTONE DEACETYLASE 19 and the flowering time gene FD maintain reproductive meristem identity in an age-dependent manner. J Exp Bot. 69:4757–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hung F-Y, et al. 2018. The Arabidopsis LDL1/2-HDA6 histone modification complex is functionally associated with CCA1/LHY in regulation of circadian clock genes. Nucleic Acids Res. 46:10669–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song C-P, et al. 2005. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 17:2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu X, et al. 2014. Transcriptional repression by histone deacetylases in plants. Mol. Plant. 7:764–772. [DOI] [PubMed] [Google Scholar]
- 43. Pi L, et al. 2015. Organizer-derived WOX5 signal maintains root columella stem cells through chromatin-mediated repression of CDF4 expression. Dev Cell. 33:576–588. [DOI] [PubMed] [Google Scholar]
- 44. Matsuo N, Banno H. 2008. The Arabidopsis transcription factor ESR1 induces in vitro shoot regeneration through transcriptional activation. Plant Physiol Biochem. 46:1045–1050. [DOI] [PubMed] [Google Scholar]
- 45. Daimon Y, Takabe K, Tasaka M. 2003. The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on Calli. Plant Cell Physiol. 44:113–121. [DOI] [PubMed] [Google Scholar]
- 46. Matsuo N, Makino M, Banno H. 2011. Arabidopsis ENHANCER OF SHOOT REGENERATION (ESR)1 and ESR2 regulate in vitro shoot regeneration and their expressions are differentially regulated. Plant Sci. 181:39–46. [DOI] [PubMed] [Google Scholar]
- 47. Liu J, et al. 2014. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 26:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu X, Xu L. 2016. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 172:2363–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J, et al. 2018. The WOX11–LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture. Plant Cell Physiol. 59:739–748. [DOI] [PubMed] [Google Scholar]
- 50. Cole M, et al. 2009. DORNR Ö SCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development. 136:1643–1651. [DOI] [PubMed] [Google Scholar]
- 51. Blilou I, et al. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 433:39–44. [DOI] [PubMed] [Google Scholar]
- 52. Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S. 1992. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schiml S, Fauser F, Puchta H. 2014. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 80:1139–1150. [DOI] [PubMed] [Google Scholar]
- 54. Fauser F, Schiml S, Puchta H. 2014. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J. 79:348–359. [DOI] [PubMed] [Google Scholar]
- 55. Nakagawa T, et al. 2007. Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem. 71:2095–2100. [DOI] [PubMed] [Google Scholar]
- 56. Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735–743. [DOI] [PubMed] [Google Scholar]
- 57. Aoyama T, Chua N-H. 1997. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11:605–612. [DOI] [PubMed] [Google Scholar]
- 58. Takano S, Niihama M, Smith HMS, Tasaka M, Aida M. 2010. gorgon, a novel missense mutation in the SHOOT MERISTEMLESS gene, impairs shoot meristem homeostasis in Arabidopsis. Plant Cell Physiol. 51:621–634. [DOI] [PubMed] [Google Scholar]
- 59. Yi K, Menand B, Bell E, Dolan L. 2010. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet. 42:264–267. [DOI] [PubMed] [Google Scholar]
- 60. Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spinelli SV, Martin AP, Viola IL, Gonzalez DH, Palatnik JF. 2011. A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol. 156:1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iwase A, et al. 2017. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell. 29:54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Uchida N, Shimada M, Tasaka M. 2013. ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant Cell Physiol. 54:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li H, et al. 2009. The sequence alignment/map format and samtools. Bioinformatics. 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, et al. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robinson JT, et al. 2011. Integrative genomics viewer. Nat Biotechnol. 29:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shen L, Shao N, Liu X, Nestler E. 2014. ngs.plot: quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics. 15:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are included in this published article (and its supplementary data files). Data S1 contains source data corresponding to the Fig. 3C and D. Data S2 contains source data corresponding to Figs. 3A to C and 4A. Data S3 contains source data corresponding to Fig. 4C and Fig. S3. Data S4 contains the summary of RNA-seq and ChIP-seq experiments corresponding to Fig. 3. RNA-seq and ChIP-seq data that support the findings of this study have been deposited in the DNA Data Bank of Japan (Accession No. DRA13879 and DRA13880, respectively).