Abstract
Adult mosquito females, through their bites, are responsible for the transmission of different zoonotic pathogens. Although adult control represents a pillar for the prevention of disease spread, larval control is also crucial. Herein we characterized the effectiveness of a suitable tool, named “MosChito raft”, for the aquatic delivery of a Bacillus thuringiensis var. israelensis (Bti) formulate, a bioinsecticide active by ingestion against mosquito larvae. MosChito raft is a floating tool composed by chitosan cross-linked with genipin in which a Bti-based formulate and an attractant have been included. MosChito rafts (i) resulted attractive for the larvae of the Asian tiger mosquito Aedes albopictus, (ii) induced larval mortality within a few hours of exposure and, more importantly, (iii) protected the Bti-based formulate, whose insecticidal activity was maintained for more than one month in comparison to the few days residual activity of the commercial product. The delivery method was effective in both laboratory and semi-field conditions, demonstrating that MosChito rafts may represent an original, eco-based and user-friendly solution for larval control in domestic and peri-domestic aquatic habitats such as saucers and artificial containers in residential or urban environments.
Subject terms: Entomology, Microbiology
Introduction
Mosquitoes (Diptera: Culicidae) are a major threat in public health since adult females are able to transmit parasites and pathogens to humans and animals during the blood meal1–3. In addition, globalization and climate change have loosened biogeographic barriers and species with high invasive potential have spread worldwide creating concerns about exotic vector-borne zoonoses outbreaks2,4–6. In this scenario, the Asian tiger mosquito Aedes albopictus (Skuse, 1894) (Diptera: Culicidae) represents a case point because records of appearance in novel habitats have increased exponentially in the last decades7,8. Indeed, indigenous to South-East Asia, islands of the Western Pacific and Indian Ocean, Ae. albopictus is now present worldwide7,8. Ae. albopictus females are aggressive biters throughout the day, and they are competent vector for at least 22 arboviruses2,7–9. Since its first appearance in Europe in 1979, this species has been implicated in dengue and chikungunya outbreaks and reasonable concern is rising about Zika emergence in Europe in the near future2,4,10,11. Thus, mosquito control definitely relieves the biting pressure by aggressive species but, most importantly, it represents the pillar of disease prevention. Therefore research efforts to develop novel effective and sustainable control strategies are strongly encouraged4,7,12–14.
To face the growing and global challenges in the control of vector-borne diseases, mosquito control must be tackled by Integrated Vector Management (IVM) that is a rational decision-making process consisting of a multi-level approach to optimize the use of different tools and strategies to make it efficient, cost effective, and sustainable12,15,16. Based on the constant engagement and mobilization of the communities, IVM includes vector surveillance and larval control that can significantly complement adulticiding in the mitigation of disease spread15,17–20. In particular, public education and community-based interventions for larval control are crucial in the case of highly anthropophilic and container-inhabiting species (e.g., Ae. albopictus) for which larval habitats are ephemeral, unpredictable and ubiquitous within domestic and peridomestic environments11,12,21,22.
When feasible, the primary intervention for larval source management is the reduction of the availability of larval habitats, e.g., avoiding stagnation of water by everyday observation and elimination of small water containers4,11,12,19,21,22. In addition, in Europe, several larvicides are available (a complete list can be found on the European CHemicals Agency website at https://echa.europa.eu/it/information-on-chemicals/biocidal-products) and their adoption is regulated by the legislative act EU 528/2012 on biocide registration and use, that aims to encourage the exploitation of products with low impact on human and animal health and on the environment11,12. Essentially, two product categories are available for mosquito larvae control in EU, namely insect growth regulators (IGRs, i.e., chitin synthase inhibitors and hormonal disruptors) and microbial bioinsecticides [i.e., formulates based on Bacillus thuringiensis var. israelensis (Bti) or on the combination Bti-Lysinibacillus sphaericus (Ls)]11,12,21. Although ascribed as chemicals, IGRs specifically target insect development and thus are relatively safe for non-target organisms with minor effects on aquatic insect fauna11,23. IGR-based formulations are important components in IVM since they are effective and long-lasting, especially diflubenzuron-based products. Nevertheless, resistance records have been described and the incidence of resistance should be taken into consideration when using these products extensively, for example planning the rotation of products with different active ingredients4,12,24.
On the other hand, microbial larvicides based on Bti and Ls are considered safe for the environment and very specific25,26; in particular, they are active by ingestion since the bacteria produce proteinaceous toxins that target the midgut epithelium of mosquito larvae26–28. Products based singly on Bti or Ls present advantages and drawbacks with respect to each other and to other insecticides. Bti (i) is scarcely persistent, especially in polluted and organically enriched water, and requires multiple applications11,29 however (ii) produces a blend of toxins (several Cry and Cyt toxins) and resistance outbreaks have never been registered, although a mild decrease in susceptibility and resistance to single toxins have been described28,29. Conversely, Ls (i) persists longer and recycle in the environment through infected larvae but (ii) insects are more prone to develop resistance to the binary toxin (Bin) responsible for its acute toxicity26,30. To avoid resistance spread no formulates with Ls alone are available on the market, whereas bioinsecticides based on both Bti and Ls have been developed to synergize the toxic effects of both and to partially compensate the lack of persistence of Bti. Nevertheless, combining Bti and Ls or Bti and IGRs (e.g., as in VectoPrime®) imposes unnecessarily a selection pressure by Ls and IGRs with a real chance of resistance alleles spread in mosquito populations. The use of these combinations would be avoided in the case of persistent Bti-based products, by making larval control more targeted and sustainable.
The present work aims to protect the benefits of the use of Bti-based bioinsecticides that suffer from lack of persistence but are highly recommended for their environmental sustainability and for their mode of action that prevent resistance development. We have recently developed a suitable delivery method for the oral administration of microorganisms or molecules to mosquito larvae using a chitosan-based hydrogel31. Herein the potential of this tool (i.e., “MosChito rafts”) for the targeted and long-lasting delivery of a Bti-based formulate to Ae. albopictus larvae was investigated.
Results
MosChito rafts attractiveness for Ae. albopictus larvae
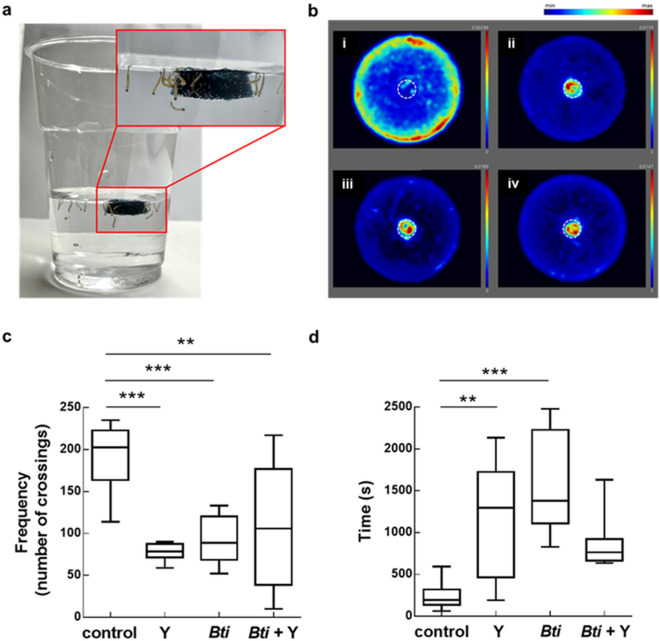
In the present study we intended to validate floating hydrogel rafts consisting of chitosan crosslinked with genipin that have been recently developed31, for the delivery of a Bti-based formulate to control mosquito larvae with bioassays on Ae. albopictus larvae (Fig. 1a).
Figure 1.
Exposure of Ae. albopictus larvae to MosChito rafts (a) and results of attractiveness assays (b–d). (b) Heatmaps of larval movement during the attraction experiment with control (i), Y (ii), Bti (iii), and Bti + Y (iv) rafts (see Methods for colour interpretation). In (c,d), crossing frequency of larvae in the zone at the border of the Petri dish and the cumulative duration of larvae permanence in the center zone are respectively represented. Data are reported as mean ± standard errors (**P < 0.01, ***P < 0.001).
Larvae movement was measured and represented by cumulative heatmaps (Fig. 1b). In the case of control rafts (Fig. 1b, i), light blue-white halos were present in the whole test area except for the borders where yellow and red signals were intense, demonstrating that the larvae were inclined to move intensely and rest for long time at the borders, likely because control rafts were not attractive to them. In contrast, larvae exposed to Y, Bti, and Bti + Y (Fig. 1b, ii, iii, and iv respectively) rafts showed a tendency of staying around the raft itself, where a red-yellow halo is present, while the rest of the test area remained dark-blue coloured due to fewer movements and/or shorter permanence. In summary, Y, Bti and Bti + Y rafts attracted the larvae that perceived the presence of yeast, Bti or both in the rafts, and tended to stay close to them. These results were confirmed by a higher mean number of crossings of the larvae close to the border of the Petri dish in the case of control rafts compared to the other rafts (Fig. 1c) (F(3, 35) = 9.548, P < 0.001, with P < 0.001 for control vs Y; P < 0.001 for control vs Bti, and P < 0.01 for control vs Bti + Y) and higher larvae permanence in the center zone in the case of Y and Bti compared to control (Fig. 1d) (F(3, 29) = 8.495, P < 0.001 with P < 0.01 for control vs Y, P < 0.001 for control vs Bti). In the case of Bti + Y rafts no significant difference compared to controls was observed, although a tendency was present (P = 0.106) (Fig. 1d). Overall, contrary to previous reports, yeast did not act as a lure32–35 and attractiveness assays have shown that MosChito rafts attracted larvae per se, thus without the addition of yeast in the hydrogel.
Insecticidal activity of MosChito rafts against Ae. albopictus larvae
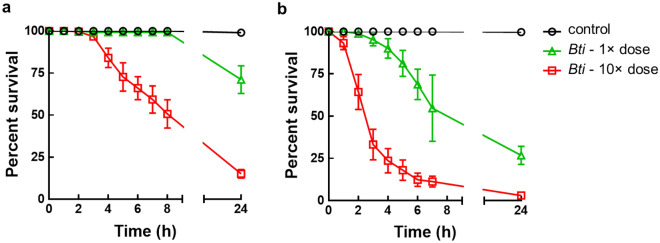
Bioassays clearly showed that the insecticidal effect of MosChito rafts is (i) dose-dependent, and (ii) slower and lower on 3rd instar larvae compared to 4th instar larvae (Fig. 2). Indeed after 7 h, 3rd instar larvae exposed to the 1× dose were almost all alive, whereas about 50% of 4th instar larvae were dead (Fig. 2a,b). On the contrary, MosChito rafts with the higher dose were highly effective on both larval instars. Survival of 3rd and 4th instar larvae decreased significantly compared to the controls after 4 or 2 h of exposure to the higher dose respectively (for 3rd instar larvae F(2, 21) = 11.49, P < 0.001; for 4th instar larvae F(2, 33) = 10.13, P < 0.001) (Fig. 2a,b). After 24 h, MosChito rafts with the 10× dose killed more than 80% of 3rd and almost all 4th instar larvae (for 3rd instar larvae F(2, 27) = 89.68, P < 0.001; for 4th instar larvae F(2, 33) = 387.00, P < 0.001).
Figure 2.
Bioassays with 3rd (a) or 4th (b) instar Ae. albopictus larvae exposed to control and Bti rafts. Two different doses of Bti were included in MosChito rafts (see Methods for the details) and survival of larvae was recorded at different times during 24 h. The values reported are the mean ± standard errors. Statistical significance of survival decrease in Bti exposed larvae compared to controls is reported along with the description of the results obtained.
To unequivocally demonstrate that MosChito rafts are active by ingestion of the hydrogel, a bioassay was performed exposing larvae to the water in which the rafts were left 24 h and then removed (Fig. 3). The data clearly showed that there was no toxicity in the water itself after removing MosChito rafts and thus the toxicity reported in the insecticidal activity test was almost entirely due to the consumption and direct ingestion of the hydrogel with Bti by Ae. albopictus larvae (Fig. 3).
Figure 3.
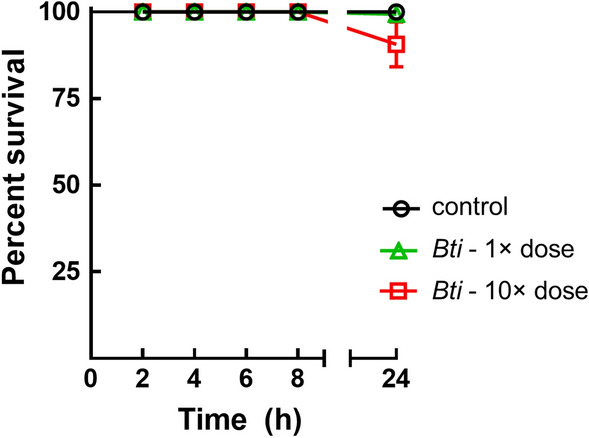
Bioassays with a mix of 3rd and 4th instar Ae. albopictus larvae to check whether Bti is released by MosChito rafts. Briefly, the rafts were left in 100 ml of tap water for 24 h and then the water was used to perform a 24 h time-course assay of survival with the larvae. The values reported are the mean ± standard error. The only statistically significant difference was observed at 24 h where 10× dose Bti induced a statistically significant, albeit small, survival decrease compared to other rafts (F(2, 15) = 10.76, P = 0.0013, P < 0.01).
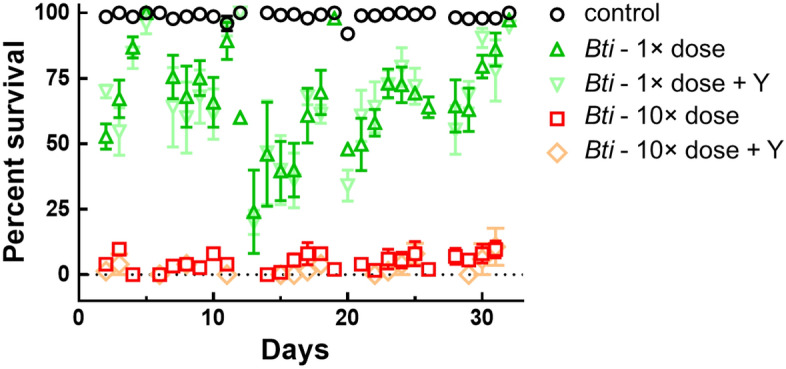
At the same time, data obtained during a month period demonstrated that MosChito rafts maintained unaltered toxicity against Ae. albopictus larvae over time for both tested doses of Bti (P < 0.001) (Fig. 4). It is worth mentioning that the slightly reduced toxicity in 3rd instar larvae (Figs. 2a, 4a) compared to 4th instar larvae (Figs. 2b, 4b) was likely due to the ingestion of lower quantities of hydrogel containing the Bti during 24 h of exposure.
Figure 4.
Bioassays with 3rd (a) or 4th (b) instar Ae. albopictus larvae exposed to control and MosChito rafts during long periods. Two different doses of Bti were included in the rafts (see Methods for the details) and 24 h survival of the larvae was recorded over a period of at least 30 days with the same rafts. The values reported are the mean ± standard errors. MosChito rafts toxicity was maintained during the test period (P < 0.001).
Although MosChito rafts attractiveness assays (Fig. 1) did not show a significant difference in the movement of the larvae between Bti and Bti + Y rafts, a bioassay was performed to check whether the presence of yeast may have any effect on MosChito rafts toxicity, for instance by phagostimulating the larvae and thus boosting toxicity. This hypothesis was not supported by the results (Fig. 5), indeed the presence of yeast in the rafts with 1× dose Bti did not show increased toxicity expected if the presence of yeast would have induced a higher consumption of the rafts (P = 0.156). As expected, Bti and Bti + Y rafts with the higher dose caused similar mortality, closed to 100% (P = 0.156).
Figure 5.
Bioassays with 4th instar Ae. albopictus larvae exposed to control, Bti, Bti + Y rafts (at two different doses, 1× and 10×). 24 h survival of larvae was recorded over a period of at least 30 days. The values reported are the mean ± standard errors. No statistically significant difference due to the presence of yeast between Bti and Bti + Y rafts was observed (P = 0.156).
Semi-field bioassays
Efficacy of MosChito rafts in the natural context was then tasted in semi-field bioassays: both strains were highly susceptible to Bti-containing rafts since after 1 day of exposure less than 50% survival was observed and after 5 days the mortality increased to almost 100%, compared to the controls (Fig. 6). The results showed that Ae. albopictus larvae with a genetic background presumably similar to that of the wild populations were also highly susceptible to Bti and did not show any behavioural characteristic that may cause control failure (e.g., lack of attractiveness or erosion activity and ingestion of MosChito rafts in a natural environment), thus validating MosChito rafts as an effective control tool.
Figure 6.
Bioassays in semi-field conditions with laboratory strains of Ae. albopictus established about 20 years ago (Rimini strain) or established less than 1 year prior to the experiments (Levate strain). Larvae of Rimini (a) or Levate (b) strain were exposed to control, Bti or Bti + Y rafts and survival was recorded every 24 h. The results are represented as mean ± standard error: curves that significantly differ from controls are indicated with an asterisk (P < 0.0001).
Discussion
The control of Ae. albopictus mosquitoes is tricky because they can breed in almost any type of water-filled containers and dry-resistant eggs can survive over several months12,36. Nevertheless, their attitude to vector arboviruses compels the development of novel and sustainable control strategies. MosChito rafts represent an original, eco-based and user-friendly solution for larval control in aquatic habitats such as saucers and artificial containers in residential or urban environments to prevent the development of the immature stages. Control of floodwater mosquitoes is often performed by predictable, extensive and inundative treatments of wetlands or water bodies by professionals (e.g., by backpack sprayer or even helicopter). On the contrary, larval control of container breeding mosquitoes as Ae. albopictus or Ae. aegypti requires a localized and targeted treatment of breeding sites which can be better accomplished by hand-application of larvicides to specific containers in private or public urban contexts11,12,22,36.
MosChito rafts have been conceived with highly tested biomaterials. Chitosan, the major component of the hydrogel, is a renewable resource since it is produced by deacetylation of chitin, the second most widely occurring biopolymer in nature after cellulose37,38. Besides availability, chitosan is characterized by non-toxicity, biodegradability, and biocompatibility. It is widely used in food packaging, water and wastewater treatments, cosmetics, and agriculture to improve crops growth39–41. Chitosan also represents a valuable raw material for innovative biomedical applications, as carriers for a variety of drugs, bandages and wound dressing, tissue engineering, and in nerve reparation38,41. In addition, genipin was adopted in the hydrogel as chitosan cross-linker, since biobased cross-linkers of plant origin are safe, environmentally sustainable, and renewable42–44.
The insecticidal activity of MosChito rafts relies on the inclusion of a Bti-based formulation to the initial hydrogel. Bti is a safe and effective bioinsecticide targeting mosquito larvae and is implemented in current control programs all over the world, including Europe11,26,36. The major concern about Bti use is the low persistence in the environment, mainly due to UV light exposure and microbic degradation, and thus multiple applications are required26,28,45. Available products stuffed with Bti as Mosquito Dunks® or Culinex® tabs are designed to be more or less rapidly dissolved in water and thus Bti is immediately exposed to water pH and UV light after its release. In addition, the amount of released Bti is not adjusted according to larval instar or density but is instead released in very high amounts to guarantee vector control. MosChito rafts’ mechanism of delivery is completely different. First, MosChito rafts containing a Bti formulate (i.e., VectoBac® 12AS) to be attractive to Ae. albopictus larvae even in the absence of a lure, to be highly effective during the 1-month testing period (while the commercial liquid formulate that was used to make MosChito rafts persists fully active only for a few days, as reported in VectoBac® 12AS data sheet), and that toxicity is mediated by the erosion of the soft hydrogel by the larvae mouthparts followed by ingestion. This characteristic is extremely important since it avoids dispersion of the bioinsecticide that is protected by the hydrogel from environmental abiotic and biotic stressors. Importantly, MosChito rafts allow to overcome the need for the addition of other insecticides (as Ls or IGRs) in Bti formulates to prolong the insecticidal effectiveness, a practice that dangerously imposes a selective pressure on larvae which may evolve in resistance to Ls and IGRs.
The European directive No. 528/2012 on biocidal products regulation made Bti one of the few larvicides authorized for mosquito control. Notwithstanding the individuation of new bioinsecticides remains a core effort for the improvement of mosquito larvae control strategies, the protection of the benefits of Bti use and the optimization of its performances by developing new delivery methods and/or by combining this bioinsecticide with other control strategies are also key issues. Recent works have demonstrated that the effectiveness of a B. thuringiensis strain active on lepidopteran pests (B. thuringiensis var. aizawaii, Bta) is enhanced when target insects are immune impaired by RNAi-mediated silencing of genes involved in cellular immune responses46,47. In addition, this approach can be exploited in the field by co-administration of the Bta-based formulate with transformed bacteria or plants as delivery vectors for immune silencing dsRNAs48,49. Similarly, MosChito rafts could be used as vectors for Bti in association with dsRNA nanocarriers50–52 or dsRNA-expressing microorganisms, as mosquito larvae have proved to be susceptible to environmental RNAi vectored by microorganisms, including S. cerevisiae34,53–56. S. cerevisiae in MosChito rafts can therefore be exploited as expression and delivery system for interfering RNAs or for other molecules able to complement or synergize Bti formulate activity. Research efforts in this direction are ongoing in our laboratory.
Likewise, the potential of this device has yet to be assessed for other mosquito species. Indeed, the possibility to control mosquito species that often share breeding containers will expand its potential. For instance, in Italy, the overlapping ecological niche and seasonal activities of the populations of Ae. albopictus and Culex pipiens57,58, could play to our benefit for a targeted control of both species with a single product. Furthermore, the application of MosChito rafts could be fruitful and promising against the larvae of the species Cx. pipiens which normally develop in containers characterized by a larger volume (Ae. albopictus: < 5 L; Cx. pipiens (s.l.): > 5 L)59.
In conclusion the present work represents a significant proof of concept that sets the stage for the development of diverse and effective control strategies for mosquito larvae. Indeed, in principle, any bioinsecticide active by ingestion (e.g., formulates that combine both Bti and Ls) can be included and delivered, and suitably transformed S. cerevisiae cells can boost the bioinsecticide activity.
Methods
Mosquitoes, microorganisms and reagents
Bioassays were performed using larvae of the Asian tiger mosquito Ae. albopictus. The Rimini strain was established in 2004 from mosquitoes collected in Rimini, Italy60 and some egg clusters were transferred to the insectary of the Department of Biosciences (University of Milan). The Levate strain was recently established (September 2020) from larvae collected in Levate (Bergamo, Italy). Unless differently indicated, the Rimini strain was used for the experiments. The colonies were maintained in the insectary under standard rearing conditions (27 ± 1 °C, 65%–80% relative humidity, 12:12 h light/dark photoperiod). Both strains were fed with fish food (Tetra-fish, Melle) for all larval instars and with sucrose solution (10% w/v in distilled water) at the adult stage. Females were fed with animal blood to allow egg development. The eggs were stored dry in the insectary and used, by rehydration, no later than 2 months after the laying. For hatching, tap water and different hatching media (broths referred to as HM from now on) were tested, including the medium suggested in literature that include beef meat extracts (0.029 g Lab-Lemco powder, 0.14 g peptone, 0.14 g yeast extract, 0.14 g NaCl in 1 L of distilled water, HM3)61–63 and 2 media developed in our laboratory (0.14 g Bacto™ tryptone, 0.14 g yeast extract, 0.14 g NaCl in 1 L of distilled water, HM1; 0.14 g Primatone® peptone, 0.14 g yeast extract, 0.14 g NaCl in 1 L of distilled water, HM2). The simplest and cheap medium HM1 was preferred as hatching solution, since no statistical differences were observed in the percentage of hatched larvae after 24 h compared to more complex media (i.e., 70% of egg hatching after 24 h, see Supplementary Fig. S1 online). This method allowed to optimally synchronize the larvae development which is important to perform bioassays with several conditions and replicates at the same time.
S. cerevisiae cells, strain SY2080, included into hydrogels (see “MosChito raft production”) were grown in generic yeast extract peptone dextrose (YPD) medium enriched with 2% w/v glucose as nutrient source and with chloramphenicol (1 µg/ml) added as antibiotic. Thirty ml of yeast culture were placed into a 50 ml tube and pelleted by centrifugation for 10 min at 3500×g at room temperature, and the supernatant was discarded. For heat inactivation the pellet was placed in a 70 °C water bath for 2 h (protocol modified from Mysore et al., 2017)34. A suspension of 107 heat killed cells/ml in water was used for rafts production. The commercial Bti-based product used in our experiment is VectoBac® 12AS (Sumitomo Chemicals Italia SRL, Valent Biosciences).
Unless differently indicated, all reagents were provided by Sigma-Aldrich, Italy.
MosChito raft production
A detailed description of the formulation of floating hydrogel baits (rafts) and their properties was reported in Piazzoni et al., 202231. Briefly, “control” rafts were prepared by mixing 1 ml of chitosan solution (10 mg chitosan dissolved in 1 ml of a 1% v/v acetic acid solution and added with 10 µl of 20 mM sodium dodecyl sulphate in water) with 100 µl of 44 mM genipin solution in 10% ethyl alcohol. The other rafts used in the experiments were prepared by adding to the control rafts (i) SY2080 strain S. cerevisiae cells (50 µl of an aqueous suspension with 107 cells/ml) (i.e., “Y” rafts), or (ii) the Bti-based insecticide VectoBac® 12AS (5 µl or 50 µl of the liquid formulate for the rafts used in laboratory tests and 100 µl for semi-field tests) (i.e., “Bti” rafts), or (iii) both S. cerevisiae cells and VectoBac® 12AS (50 µl with 107 yeast cells/ml and 5 µl or 50 µl of the Bti formulate) (i.e., “Bti + Y” rafts). Then, air bubbles were injected using a syringe pump (KD Scientific, Thermo Fisher Scientific) to allow raft flotation in water; finally, they were placed in aluminum moulds (1.260 ml volume per well) to obtained rafts of discoidal shape of 1.6 cm (diameter) × 0.5 cm (thickness) (i.e., 1.2 ml of volume). The final concentration of VectoBac® 12AS in “MosChito rafts” was thus 4.2 µl/ml (indicated as “1× dose”), 42 µl/ml (indicated as “10× dose”), or 420 µl/ml (in semi-field tests). Moulds were then incubated overnight in a ventilated oven at 37 °C. For mosquito attractiveness assays, the rafts were cut to obtain smaller ones.
MosChito rafts attractiveness assays
To assess whether mosquito larvae were attracted or repelled by control, Y, Bti, or Bti + Y rafts, attractiveness assays were performed. The dose of VectoBac® 12AS used in these Bti, and Bti + Y rafts was the 10× dose (i.e., 42 µl/ml). For each assay one raft (0.5 × 0.5 cm) was fixed with a needle in the center of a Petri dish (90 × 15 mm) containing 5 ml of tap water. To record larvae movement, Petri dishes were then placed in the DanioVision™ observation chamber (Noldus Inc., Wageningen, The Netherlands) with a plateholder filled with water to maintain the temperature at 27 °C. One single Ae. albopictus 3rd instar larva was tested for each recording. At least 10 larvae for each raft type (control, Y, Bti or Bti + Y) were tested. During each acquisition, lasting one hour, larvae movements to 3 pre-identified concentric areas in the Petri dish were recorded by automated video tracking (EthoVision XT® software, Noldus Inc.). Starting from the dish center these areas are referred as “central zone”, 0.5 cm to 2 cm from the center of the dish, “border zone”, corresponding to the most external zone of the dish, near the border, with a 0.7 cm width.
The data acquired were used to generate heatmaps describing larval movements in different zones (the images offer an intuitive and unique view of the data, where the colour represents the relative time spent in a certain area (blue, low; red, high), averaged over all larvae of each experiment) and to establish the frequency of crossings or the duration of the permanence of the larva in a particular zone. Data were then processed with the GraphPad Prism (GraphPad Software Inc. version 8, San Diego, CA, USA).
Laboratory bioassays
Third and 4th instar larvae were exposed separately to the rafts according to the guidelines for laboratory and field testing of mosquito larvicides64. Briefly, batches of 25 larvae were transferred by means of plastic Pasteur pipettes to disposable plastic cups containing 100 ml of tap water. Rafts (controls and, depending on the experiment, 1× dose or 10× dose Bti, 1× dose Bti + Y, or 10× dose Bti + Y) were thus gently introduced in the water and experimental cups were put in the insectary (27 ± 1 °C, 65–80% relative humidity, 12:12 h light/dark photoperiod). In the case of the time course analysis of MosChito rafts toxicity (Fig. 2), survival was recorded at different time points within the 24 h period of exposure. In order to check whether the insecticidal activity was exclusively due to the ingestion of the hydrogel containing Bti and/or the Bti that was released into the water in the cups, the MosChito rafts were left in 100 ml of tap water for 24 h and then the water alone was used to perform a time-course assay of survival with a mix of 3rd and 4th instar larvae (cumulative survival of 3rd and 4th instar larvae was recorded after 2, 4, 6, 8 and 24 h). The experiment was performed in duplicate with 3 cups for each condition (control, 1× dose Bti and 10× dose Bti) and with 25 larvae for each cup. To measure the insecticidal activity of the rafts during time, after each 24 h bioassay (i.e., larval survival was measured 24 h after the exposure to MosChito rafts), rafts were moved to a new plastic cup, with fresh tap water and 25 larvae, to start a new 24 h bioassay. The insecticidal activity of MosChito rafts was recorded across a 30 days-period. Three batches of 25 larvae were used to measure survival for each experimental condition and experiments were repeated with rafts obtained with at least 2 independent preparations.
Semi-field bioassays
Ae. albopictus larvae were tested in bioassays under semi-field conditions to evaluate the larvicidal efficacy of control, Y, Bti and Bti + Y rafts over time. These experiments were performed in the backyard of the Department of Biosciences of the University of Milan, from June to September 2021. Fifty Ae. albopictus larvae (Rimini strain) at different developmental stage were added to plastic containers with 200 ml of rainwater plus pebbles, leaves and sand to mimic the peridomestic environment where these mosquitoes normally breed and larvae develop. Survival was recorded every 24 h, until all larvae died or until all control larvae were pupated. Each bioassay was performed in triplicate and repeated 3 times. The same bioassays were performed following the same protocol using Levate strain of Ae. albopictus, a strain that has been established in the laboratory less than one year before the bioassays.
Statistical analysis
Data obtained in attractiveness assays were checked for normality using GraphPad Prism (GraphPad Software Inc. version 8) and statistical significance of differences was assessed with One-way ANOVA tests followed by Tukey’s multiple comparison post-hoc test. Insect survival in laboratory tests was analysed by one-way ANOVA followed by Tukey’s post-hoc test (Figs. 2 and 3) or by General Linear Model (GLM, performed with RStudio v2022.2.3.492, RStudio Team 2020)65 (Figs. 4 and 5). Data from semi-field bioassays were analysed by Log-rank (Mantel-Cox) test and the comparison between groups was adjusted with FDR (false discovery rate). If not differently stated, statistical analysis was performed using GraphPad Prism.
Supplementary Information
Acknowledgements
We thank Prof. Cristina Lenardi and Prof. Federico Lazzaro (University of Milan) for their valuable suggestions during the development of the research work. We thank Prof. Morena Casartelli for her comments on the manuscript.
Author contributions
C.B., S.C., S.E. and S.U. conceived and designed research. S.C., S.L., A.N., G.P., M.P. and S.P. performed experiments. R.Q. performed the experiments with yeasts. S.C., S.E., P.G., V.M. and D.P. analyzed data. S.C. wrote the manuscript. S.E. revised the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the Italian Ministry of Education, University and Research, PRIN 2017 Prot. 2017J8JR57 (to CB, SC and SU) and by Fondazione Cariplo (grant no. 2017-0798) to PG. This research was partially funded by the European Union—NextGenerationEU, Italian National Recovery and Resilience Plan, Mission 4, Component 2, Investment 1.5 “Innovation Ecosystems”, project MUSA (to SE and PG).
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information. In addition, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Simone Pitton and Agata Negri.
Contributor Information
Sara Epis, Email: sara.epis@unimi.it.
Silvia Caccia, Email: silvia.caccia@unimi.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-29501-3.
References
- 1.Ferguson NM. Challenges and opportunities in controlling mosquito-borne infections. Nature. 2018;559(7715):490–497. doi: 10.1038/s41586-018-0318-5. [DOI] [PubMed] [Google Scholar]
- 2.Jánová E. Emerging and threatening vector-borne zoonoses in the world and in Europe: A brief update. Pathog. Glob. Health. 2019;113(2):49–57. doi: 10.1080/20477724.2019.1598127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Vector-Borne Diseases. World Health Organization; 2020. p. 2020. [Google Scholar]
- 4.Dahmana H, Mediannikov O. Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. Pathogens. 2020;9:310. doi: 10.3390/pathogens9040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negri A, et al. Evidence for the spread of the alien species Aedes koreicus in the Lombardy region, Italy. Parasit. Vectors. 2021;14(1):534. doi: 10.1186/s13071-021-05031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnoldi I, et al. Assessing the distribution of invasive Asian mosquitoes in Northern Italy and modelling the potential spread of Aedes koreicus in Europe. Acta Trop. 2022;232:106536. doi: 10.1016/j.actatropica.2022.106536. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer MUG, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lwande OW, et al. Globe-trottingAedes aegyptiandAedes albopictus: Risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 2020;20(2):71–81. doi: 10.1089/vbz.2019.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratz N. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control (ECDC) and European Food Safety Authority (EFSA). Aedes albopictus—Current Known Distribution: March 2021. https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (European Center Disease Prevention Control, 2021).
- 11.Baldacchino F, et al. Control methods against invasiveAedesmosquitoes in Europe: A review. Pest Manag. Sci. 2015;71(11):1471–1485. doi: 10.1002/ps.4044. [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control (ECDC). Vector Control with a Focus on Aedes aegypti and Aedes albopictus Mosquitoes: Literature Review and Analysis of Information. https://www.ecdc.europa.eu/en/publications-data/vector-control-focus-aedes-aegypti-and-aedes-albopictus-mosquitoes-literature (European Center Disease Prevention Control, 2017).
- 13.World Health Organization (WHO). Global Vector Control Response 2017–2030. https://scholar.google.com/scholar_lookup?title=Global+Vector+Control+Response+2017%E2%80%932030&author=WHO&publication_year=2017 (World Health Organization, 2017).
- 14.Epis S, et al. Chimeric symbionts expressing a Wolbachia protein stimulate mosquito immunity and inhibit filarial parasite development. Commun. Biol. 2020;3(1):105. doi: 10.1038/s42003-020-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO). Handbook for Integrated Vector Management. https://apps.who.int/iris/handle/10665/44768 (World Health Organization, 2012).
- 16.U.S. Environmental Protection Agency (EPA). Success in Mosquito Control: an Integrated Approach. https://www.epa.gov/mosquitocontrol/success-mosquito-control-integrated-approach (U.S. Environment Protect Agency, 2021).
- 17.World Health Organization (WHO). Larval Source Management: A Supplementary Malaria Vector Control Measure: An Operational Manual. https://apps.who.int/iris/handle/10665/85379. (World Health Organization, 2013).
- 18.Ingabire CM, et al. Community-based biological control of malaria mosquitoes usingBacillus thuringiensisvar.israelensis(Bti) in Rwanda: Community awareness, acceptance and participation. Malar. J. 2017;16(1):399. doi: 10.1186/s12936-017-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonio-Nkondjio C, Sandjo NN, Awono-Ambene P, Wondji CS. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: Key parameters for success. Parasit. Vectors. 2018;11(1):57. doi: 10.1186/s13071-018-2627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartumeus F, Oltra A, Palmer JRB. Citizen science: A gateway for innovation in disease-carrying mosquito management? Trends Parasitol. 2018;34(9):727–729. doi: 10.1016/j.pt.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Baldacchino F, et al. An integrated pest control strategy against the Asian tiger mosquito in northern Italy: A case study. Pest Manag. Sci. 2017;73(1):87–93. doi: 10.1002/ps.4417. [DOI] [PubMed] [Google Scholar]
- 22.Faraji A, Unlu I. The eye of the tiger, the thrill of the fight: Effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), North America. J. Med. Entomol. 2016;53(5):1029–1047. doi: 10.1093/jme/tjw096. [DOI] [PubMed] [Google Scholar]
- 23.Lawler SP. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicol. Environ. Saf. 2017;139:335–343. doi: 10.1016/j.ecoenv.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Namias A, Jobe NB, Paaijmans KP, Huijben S. The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. Elife. 2021;10:e65655. doi: 10.7554/eLife.65655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brühl CA, et al. Environmental and socioeconomic effects of mosquito control in Europe using the biocideBacillus thuringiensissubsp.Israelensis(Bti) Sci. Total Environ. 2020;724:137800. doi: 10.1016/j.scitotenv.2020.137800. [DOI] [PubMed] [Google Scholar]
- 26.Silva-Filha M, et al. Bacterial toxins active against mosquitoes: Mode of action and resistance. Toxins. 2021;13:523. doi: 10.3390/toxins13080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Hua G, Adang MJ. Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. 2017;24(5):714–729. doi: 10.1111/1744-7917.12401. [DOI] [PubMed] [Google Scholar]
- 28.Valtierra de Luis D, Villanueva M, Berry C, Caballero P. Potential for Bacillus thuringiensis and other bacterial toxins as biological control agents to combat dipteran pests of medical and agronomic importance. Toxins. 2020;12:773. doi: 10.3390/toxins12120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho KDS, et al. Long-term exposure of Aedes aegypti to Bacillus thuringiensis var. israelensis did not involve altered susceptibility to this microbial larvicide or to other control agents. Parasit. Vectors. 2018;11:673. doi: 10.1186/s13071-018-3246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos EMDM, et al. Frequency of resistance alleles toLysinibacillus sphaericusin a Culex quinquefasciatuspopulation treated with aL. sphaericus /Bti biolarvicide. Biol. Control. 2019;132:95–101. doi: 10.1016/j.biocontrol.2019.02.006. [DOI] [Google Scholar]
- 31.Piazzoni M, et al. Biodegradable floating hydrogel baits as larvicide delivery systems against mosquitoes. Soft Matter. 2022;18(34):6443–6452. doi: 10.1039/D2SM00889K. [DOI] [PubMed] [Google Scholar]
- 32.Souza RS, Diaz-Albiter HM, Dillon VM, Dillon RJ, Genta FA. Digestion of yeasts and beta-1,3-glucanases in mosquito larvae: Physiological and biochemical considerations. PLoS ONE. 2016;11(3):e0151403. doi: 10.1371/journal.pone.0151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahouédo GA, et al. Effect of three larval diets on larval development and male sexual performance of Anopheles gambiae ss. Acta Trop. 2014;132:S96–S101. doi: 10.1016/j.actatropica.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Mysore K, et al. Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malar. J. 2017;16(1):1–2. doi: 10.1186/s12936-017-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joy TK, Arik AJ, Corby-Harris V, Johnson AA, Riehle MA. The impact of larval and adult dietary restriction on lifespan, reproduction and growth in the mosquito Aedes aegypti. Exp. Gerontol. 2010;45:685–690. doi: 10.1016/j.exger.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takken, W. & van den Berg, H. Manual on Prevention of Establishment and Control of Mosquitoes of Public Health Importance in the WHO European Region (with Special Reference to Invasive Mosquitoes). https://apps.who.int/iris/handle/10665/343056 (WHO/Europe, 2019).
- 37.Kou S, Peters L, Mucalo M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022;282:119132. doi: 10.1016/j.carbpol.2022.119132. [DOI] [PubMed] [Google Scholar]
- 38.Maliki S, et al. Chitosan as a tool for sustainable development: A mini review. Polymers. 2022;14:1475. doi: 10.3390/polym14071475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, et al. Fabrication, characterization and functional attributes of zein-egg white derived peptides (EWDP)-chitosan ternary nanoparticles for encapsulation of curcumin: role of EWDP. Food Chem. 2022;372:131266. doi: 10.1016/j.foodchem.2021.131266. [DOI] [PubMed] [Google Scholar]
- 40.Stasińska-Jakubas M, Hawrylak-Nowak B. Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules. 2022;27(9):2801. doi: 10.3390/molecules27092801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, et al. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022;367:130727. doi: 10.1016/j.foodchem.2021.130727. [DOI] [PubMed] [Google Scholar]
- 42.Indurkar A, Pandit A, Jain R, Dandekar P. Plant based cross-linkers for tissue engineering applications. J. Biomater. Appl. 2021;36(1):76–94. doi: 10.1177/0885328220979273. [DOI] [PubMed] [Google Scholar]
- 43.Yu Y, Xu S, Li S, Pan H. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2021;9(5):1583–1597. doi: 10.1039/D0BM01403F. [DOI] [PubMed] [Google Scholar]
- 44.Alavarse AC, et al. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022;202:558–596. doi: 10.1016/j.ijbiomac.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Duchet C, et al. Persistence and recycling of bioinsecticidalBacillus thuringiensis subsp.israelensisspores in contrasting environments: Evidence from field monitoring and laboratory experiments. Microb. Ecol. 2014;67(3):576–586. doi: 10.1007/s00248-013-0360-7. [DOI] [PubMed] [Google Scholar]
- 46.Caccia S, et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA. 2016;113(34):9486–9491. doi: 10.1073/pnas.1521741113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Lelio I, et al. Evolution of an insect immune barrier through horizontal gene transfer mediated by a parasitic wasp. PloS Genet. 2019;15(3):e1007998. doi: 10.1371/journal.pgen.1007998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caccia S, et al. Enhancement of Bacillus thuringiensis toxicity by feeding Spodoptera littoralis larvae with bacteria expressing immune suppressive dsRNA. J. Pest Sci. 2020;93(1):303–314. doi: 10.1007/s10340-019-01140-6. [DOI] [Google Scholar]
- 49.Di Lelio I, et al. Transgenic plants expressing immunosuppressive dsRNA improve entomopathogen efficacy against Spodoptera littoralis larvae. J. Pest. Sci. 2022;95(3):1413–1428. doi: 10.1007/s10340-021-01467-z. [DOI] [Google Scholar]
- 50.Whitten MM. Novel RNAi delivery systems in the control of medical and veterinary pests. Curr. Opin. Insect Sci. 2019;34:1–6. doi: 10.1016/j.cois.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan S, Ren BY, Shen J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021;28(1):21–34. doi: 10.1111/1744-7917.12822. [DOI] [PubMed] [Google Scholar]
- 52.Mysore K, Flannery EM, Tomchaney M, Severson DW, Duman-Scheel M. Disruption of Aedes aegypti olfactory system development through chitosan/siRNA nanoparticle targeting of semaphorin-1a. PLOS Negl. Trop. Dis. 2013;7(5):e2215. doi: 10.1371/journal.pntd.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Ekert, E., Powell, C. A., Shatters Jr, R. G. & Borovsky, D. Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J. Insect Physiol.70, 143–150 (2014). [DOI] [PubMed]
- 54.Mysore K, et al. Characterization of a broad-based mosquito yeast interfering RNA larvicide with a conserved target site in mosquito semaphorin-1a genes. Parasit. Vectors. 2019;12:256. doi: 10.1186/s13071-019-3504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mysore K, et al. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol. Biol. 2019;1858:213–231. doi: 10.1007/978-1-4939-8775-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez SBG, et al. RNAi-based bioinsecticide for Aedes mosquito control. Sci. Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-39666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003;32(6):1313–1321. doi: 10.1603/0046-225X-32.6.1313. [DOI] [Google Scholar]
- 58.Marini G, et al. The effect of interspecific competition on the temporal dynamics of Aedes albopictus and Culex pipiens. Parasit. Vectors. 2017;10(1):102. doi: 10.1186/s13071-017-2041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Müller R, et al. Larval superiority ofCulex pipienstoAedes albopictus in a replacement series experiment: Prospects for coexistence in Germany. Parasit. Vectors. 2018;11:80. doi: 10.1186/s13071-018-2665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellini, R. et al. Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial. in Area-Wide Control of Insect Pests (Vreysen, M.J.B., Robinson, A.S., Hendrichs, J. eds.). 505–515 (Springer, 2007).
- 61.Oliva CF, Damiens D, Vreysen MJB, Lemperière G, Gilles J. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE. 2013;8(11):e78884. doi: 10.1371/journal.pone.0078884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng M-L, Zhang D, David D, Lees R, Gilles J. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae)—II—Egg storage and hatching. Parasit. Vectors. 2015;8:42. doi: 10.1186/s13071-014-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Food and Agriculture Organization of the United Nations (FAO) and International Atomic Energy Agency (IAEA). Guidelines for routine colony maintenance of Aedes mosquito species. Version 1.0. in Insect Pest Control Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. (Maïga, H., Yamada, H., Severin, B.S.N., Carvalho, D.O., Mamai, W., Herrero, R.A., Bourtzis, K. & Bouyer, J. eds.). https://www.iaea.org/sites/default/files/21/06/nafa-ipc-manual-guidelines-for-routine-colony-maintenance-of-aedes-mosquito-species-v1.0.pdf (2017).
- 64.World Health Organization (WHO). Guidelines for Laboratory and Field Testing of Mosquito Larvicides. https://apps.who.int/iris/handle/10665/69101 (World Health Organization, 2005).
- 65.RStudio Team 2020. RStudio: Integrated Development for R. (RStudio, PBC, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. In addition, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.