Abstract
Cystic fibrosis airway disease is characterised by chronic Pseudomonas aeruginosa infection. Successful eradication strategies have been hampered by a poor understanding of the mechanisms underlying conversion to chronicity. The cystic fibrosis transmembrane conductance receptor (CFTR)-knockout (KO) rat harbours a progressive defect in mucociliary transport and viscosity. KO rats were infected before and after the appearance of the mucus defect, using a clinical mucoid-isolate of P. aeruginosa embedded in agarose beads. Young KO rats that were exposed to bacteria before the development of mucociliary transport defects resolved the infection and subsequent tissue damage. However, older KO rats that were infected in the presence of hyperviscous and static mucus were unable to eradicate bacteria, but instead had bacterial persistence through 28 days post-infection that was accompanied by airway mucus occlusion and lingering inflammation. Normal rats responded to infection with increased mucociliary transport to supernormal rates, which reduced the severity of a second bacterial exposure. We conclude that the aberrant mucus present in the CF airway permits persistence of P. aeruginosa in the lung.
@ERSpublications
This study identifies the airway mucus response to Pseudomonas aeruginosa infection, which protects the normal airway against reinfection. The failure of this response in the cystic fibrosis lung leads to chronic infection and mucus occlusion. https://bit.ly/3nDiZCj
Introduction
Cystic fibrosis (CF) is characterised by recurrent and chronic lung infections with pathogens such as Pseudomonas aeruginosa [1], a Gram-negative organism which operates as an opportunistic pathogen [2]. By the age of 20 years, ~60% of people with CF are intermittently or chronically infected with P. aeruginosa [3], despite aggressive antibiotic regimens. These patients have poorer long-term outcomes, including a more rapid rate of decline [4, 5]. Despite the clinical importance, mechanisms that permit P. aeruginosa infections to convert to a chronic phenotype in the CF lung are poorly understood [6], hampered by the lack of an appropriate animal model. Normal rodents that are infected with P. aeruginosa clear the bacteria quickly or develop acute, disseminated infections that are rapidly fatal [7, 8]. Even when models allow bacteria to persist beyond 3 days, the kinetics of these infections show a natural decline in CFUs over time, which is counter to the human experience. However, current models lack hyperconcentrated and static mucus similar to that present in the human CF airway [9, 10]. Because mucus contributes to clearance of bacteria from the airway, a process that is impaired in the CF lung [11], this abnormality may be responsible for enabling P. aeruginosa to persist.
To address this question, our laboratory has developed a novel cystic fibrosis transmembrane conductance receptor (CFTR)-knockout (KO) rat model that exhibits normal mucus at 2 months of age, but has severely hyperviscous and static mucus by 6 months [12]. Here we used this model to study infection with mucoid P. aeruginosa, embedded in agarose beads. We hypothesised that the abnormal mucus present in the older KO rat would enable the development of chronic infection with P. aeruginosa; that exposure to P. aeruginosa in the younger KO rat would prematurely worsen the mucus defect, causing delayed mucociliary transport before the natural development of the defect and initiating the feed-forward mechanism that perpetuates CF decline [13]; and we questioned whether increased mucin secretion in response to infection would be responsible for the conversion of acute P. aeruginosa to a chronic colonisation phenotype.
Materials and methods
CF rat model
All animal experiments at the University of Alabama at Birmingham (UAB; Birmingham, AL, USA) were conducted in accordance with UAB institutional animal care and use committee (IACUC)-approved protocols. All experiments used sd-CFTRtm1sage rats (Horizon Discovery, St Louis, MO, USA), either the CFTR−/− (termed KO) or their littermate controls, CFTR+/+ (termed wild type (WT)). This rat strain was bred and genotyped as described previously [14]. Animals were housed in standard cages maintained on a 12 h light/dark cycle with ad libitum access to food and water, in temperatures ranging from 71°F to 75°F. Infected animals were housed in standard cages in a separate designated room. WT and KO rats were maintained on a standard rodent diet with supplemental DietGel 76A (Clear H20, Westbrook, ME, USA) and 50% Go-LYTELY (Braintree Laboratories, Braintree, MA, USA) added to the water from weaning, as a means to reduce mortality from gastrointestinal obstruction. Groups were split evenly between males and females.
Pseudomonas aeruginosa
Rats were infected with the mucoid P. aeruginosa strain PAM57–15, a clinical isolate used previously [15, 16], and a kind gift of Tracy Bonfield (Case Western Reserve University, Cleveland, OH, USA). Bacteria were grown in tryptic soy broth (TSB) to absorbance at 600 nm of 1.0–1.5, collected, centrifuged and washed in PBS, pH 7.4. Bacteria were embedded in agarose using previously established methods, and adjusted to a concentration of 1×106 CFU 100 μL−1 [8, 15–17]. Agarose bead preparations were deemed acceptable if the CFU mL−1, the bead volume per millilitre and the bead size were all consistent, in order to maintain reproducibility. Sham infections were performed with sterile agarose beads.
Infection
P. aeruginosa-laden agarose beads were administered at a concentration of 1×106 CFU 100μL−1, via orotracheal administration. Rats were deeply anaesthetised using isoflurane and suspended on an intubation stand (Braintree Scientific) by their incisors. The tongue was pulled out and aside using blunt forceps, and a syringe attached to a blunt 18-gauge Luer stub (Instech Laboratories, Plymouth Meeting, PA, USA) inserted past the tongue and into the trachea to ensure delivery to the lung. 300 μL of the beads were delivered via syringe, for an inoculum of 3×106 CFU, followed by 50 μL of air. Rats remained upright on the intubation stand for ⩾5 s to complete inhalation of the inoculum, and were then allowed to recover. Rats were weighed before infection (day 0) and daily until sacrifice. Rats were sacrificed at days 3, 7, 14 and 28 following infection. In some experiments, rats were reinfected at day 28 using the same method and inoculum, and sacrificed 3 days later (day 31). Infections were repeated at least twice per time point.
Bronchoalveolar lavage
Rats were euthanised via intraperitoneal injection of 500 μL pentobarbital sodium (390 mg mL−1). Rats were exsanguinated, the thoracic cavity exposed, and intubated via the trachea. 5 mL of sterile cold PBS was pushed into the lungs and recollected into a separate sterile syringe using a two-way stopcock. Bronchoalveolar lavage fluid (BALF) was centrifuged at 270×g for 7 min, and the supernatant transferred for cytokine and mucin analysis. The cell pellet was resuspended, counted and adjusted to 1×104 cells mL−1 for cytocentrifuge. After centrifugation of the slide in the cytocentrifuge at 500×g for 5 min, slides were allowed to dry and stained with the Diff-Quick kit (Siemens Medical Solutions, Malvern, PA, USA). Total cells, neutrophils and macrophages were counted.
CFUs
Lungs were mechanically homogenised in 5 mLof F-12 media (Gibco, ThermoFisher Scientific, Grand Island, NY, USA) and aliquots of 200μL serially diluted in TSB for manual counting on Pseudomonas Isolation Agar (Millipore Sigma, St Louis, MO, USA).
Histology
Tracheae and lungs were immersion fixed in 10% phosphate-buffered formalin and embedded in paraffin blocks for sectioning. Sections were stained with haematoxylin and eosin or Alcian Blue–Periodic Acid–Schiff (ABPAS). Slides were imaged; induced bronchus-associated lymph tissue (iBALT) area, iBALT number, goblet cell area and intra-airway mucus area were measured morphometrically, as described previously [14]. Number of iBALT detected and iBALT area were normalised to lung section. Goblet cell area and intra-airway mucus area were normalised to length of the basal lamina. All slides were evaluated by trained personnel blinded to genotype and exposure group.
Cytokine and mucin analysis
Supernatant from bronchoalveolar lavage was used to analyse cytokine concentrations by ELISA. Undiluted samples were tested for interleukin (IL)-1β, tumour necrosis factor (TNF)-α and neutrophil elastase using sandwich ELISA kits (Abcam, Cambridge, MA, USA). Each cytokine was compared to a standard curve using known concentrations of recombinant rat cytokine. Muc5b and Muc5ac were detected on nitrocellulose membranes using modified dot-blot methods [18] and antibodies selective for each mucin. Mucin antibodies were validated against purified rat recombinant mucin proteins (LSBio, Seattle, WA, USA) and nonspecific binding ruled out with use against Fc blocker (supplementary figure S4). Antibodies were selected that were generated in goat host and that bind to highly conserved internal motifs (Muc5b: epitope QHTYTHIDECN, Abcam; Muc5ac: M1-b epitope [19]; Abcam). Signal was detected with horseradish peroxidase secondary antibodies and the SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific). Blots were detected for chemiluminescence and analysed by densitometry using ImageJ software. Samples were normalised to uninfected age-matched WT controls.
Micro-optical coherence tomography image acquisition and analysis
Functional microanatomic measurements of ex vivo tissue were performed using micro-optical coherence tomography [12, 14, 20, 21], as described previously. Trachea were excised and images were acquired at five controlled points along the ventral surface with the optical beam scanned along the longitudinal direction, using the cranial end as a reference. Mucociliary transport (MCT) rate was determined using time elapsed and distance travelled of native particulates in the mucus over multiple frames, using the pixel to micron conversion factor determined with image calibration, as done previously [12].
Statistics
Statistical analysis was performed in GraphPad Prism (LaJolla, CA, USA) version 7.0 or higher. Inferential statistics (mean, standard deviation and standard error of the mean) were computed using ANOVA or two-tailed paired t-test, as appropriate. For multiple comparisons, post hoc testing was applied only if ANOVA was significant. p-values <0.05 were considered significant. Statistics are presented as mean±sem. For all analyses, mean values per animal are reported.
Study approvals
Procedures involving animals were approved by the IACUC at UAB (IACUC-09479 and IACUC 20532).
Results
Pseudomonas aeruginosa becomes chronic only in the presence of abnormal mucus
Based on previous studies in the CFTR-KO rat that determined the progressive development of abnormal mucociliary transport and viscosity [12], we identified the ages that would replicate both early lung disease and more established lung disease in the CF patient population. Young, 2-month-old KO rats, which do not have any decrements to mucociliary transport or viscosity, and older, 6-month-old KO rats, which are characterised by a severe mucus defect including mucus hyperviscosity and delayed transport were exposed to a mucoid clinical isolate of P. aeruginosa (PAM57–15) embedded in agarose beads. There was no difference in mortality between genotypes at either age and mortality was only observed in the infected animals (supplementary figure S1a and b). In the young rats, the number of CFUs recovered from lung homogenates in both genotypes was close to the inoculum (figure 1a) at 3 days post-infection (dpi), but decreased to below the limit of detection by 7 dpi. There was no difference in weight loss between the genotypes following infection (figure 1c; sham-infected, supplementary figure S1c).
FIGURE 1.
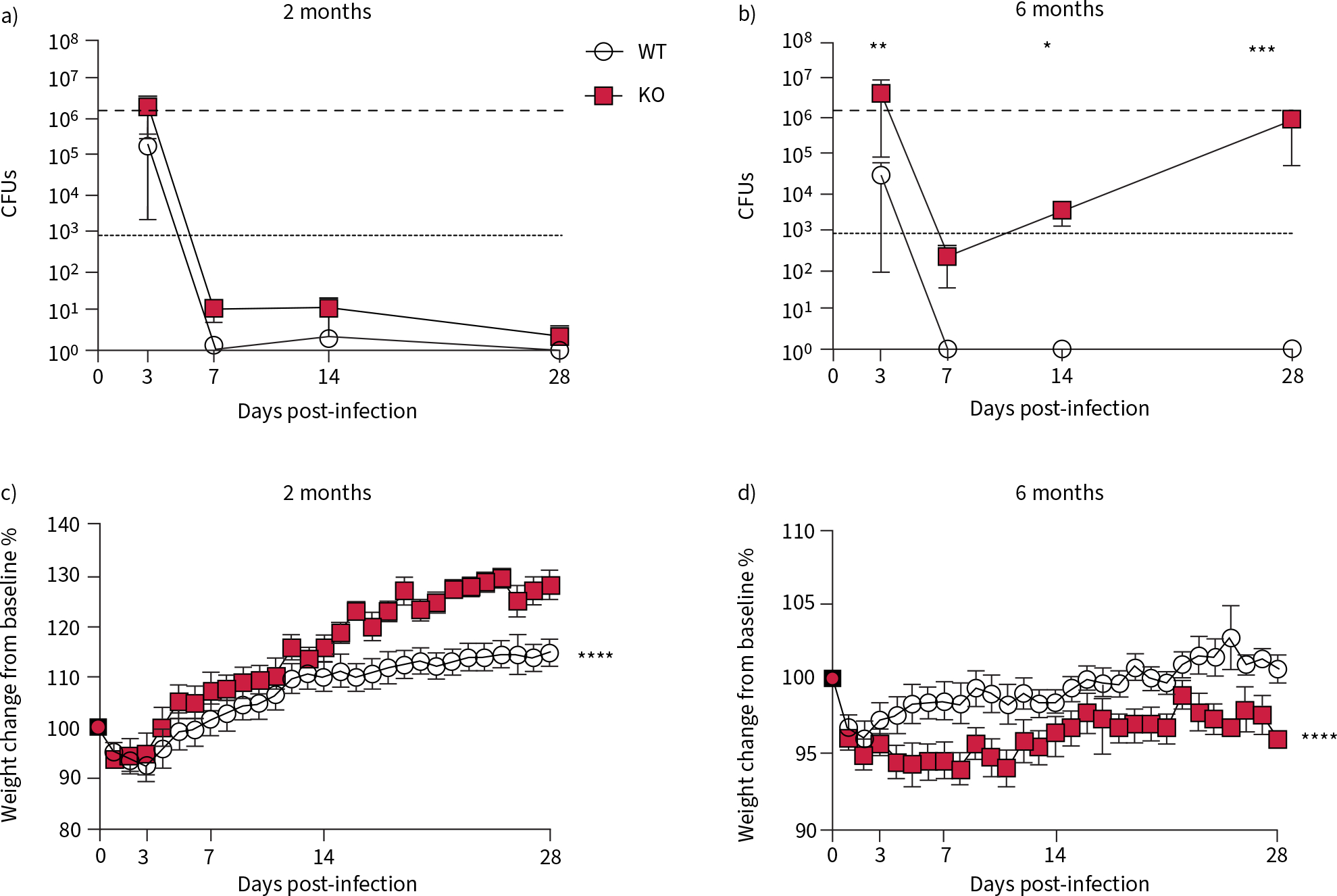
Pseudomonas aeruginosa converts to a chronic infection in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats. Wild type (WT) and KO rats were infected with P. aeruginosa at both 2 and 6 months of age. CFUs recovered from lung homogenates at indicated time points after infection in a) 2-month-old and b) 6-month-old rats. Percentage weight change from day 0 in c) 2-month-old and d) 6-month-old rats following infection. Data are presented as mean±sem and analysed via two-way ANOVA with Sidak post-test. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001. n=6–8 per group.
The bacteria recovered from the lungs of the 6-month-old rats showed more dynamic kinetics. Bacteria were recovered from the lungs of WT rats at 3 dpi only. However, the bacteria recovered from the lungs in the KO rat decreased at 7 dpi and then rebounded throughout the rest of the study (figure 1b). These kinetics indicate that the older KO rat cannot eradicate the initial acute infection, but rather promote regrowth of the remaining bacteria after the acute phase. The WT rats were able to recover from an initial weight loss by 16 dpi, while the KO rats never recovered their baseline weights (figure 1d; sham-infected, supplementary figure S1d).
Older CFTR-KO rats have an aberrant inflammatory response
Percentage of neutrophils and macrophages in BALF was determined. At 2 months of age, there was no difference between the total cell numbers in the BALF (figure 2a), the percentage of cells that were neutrophils (figure 2c) or the percentage of macrophages (figure 2e). In the 6-month-old rat, there were no differences in total cell count in the BALF between WT and KO, at any time point (figure 2b), but there was a higher percentage of neutrophils in the airway at 7 dpi in the KO compared to the WT specimens (figure 2d). This was accompanied by a higher percentage of macrophages in the BALF at 28 dpi (figure 2f). Percentages of monocytes and lymphocytes present in the BALF are reported (supplementary figure S2a–d).
FIGURE 2.
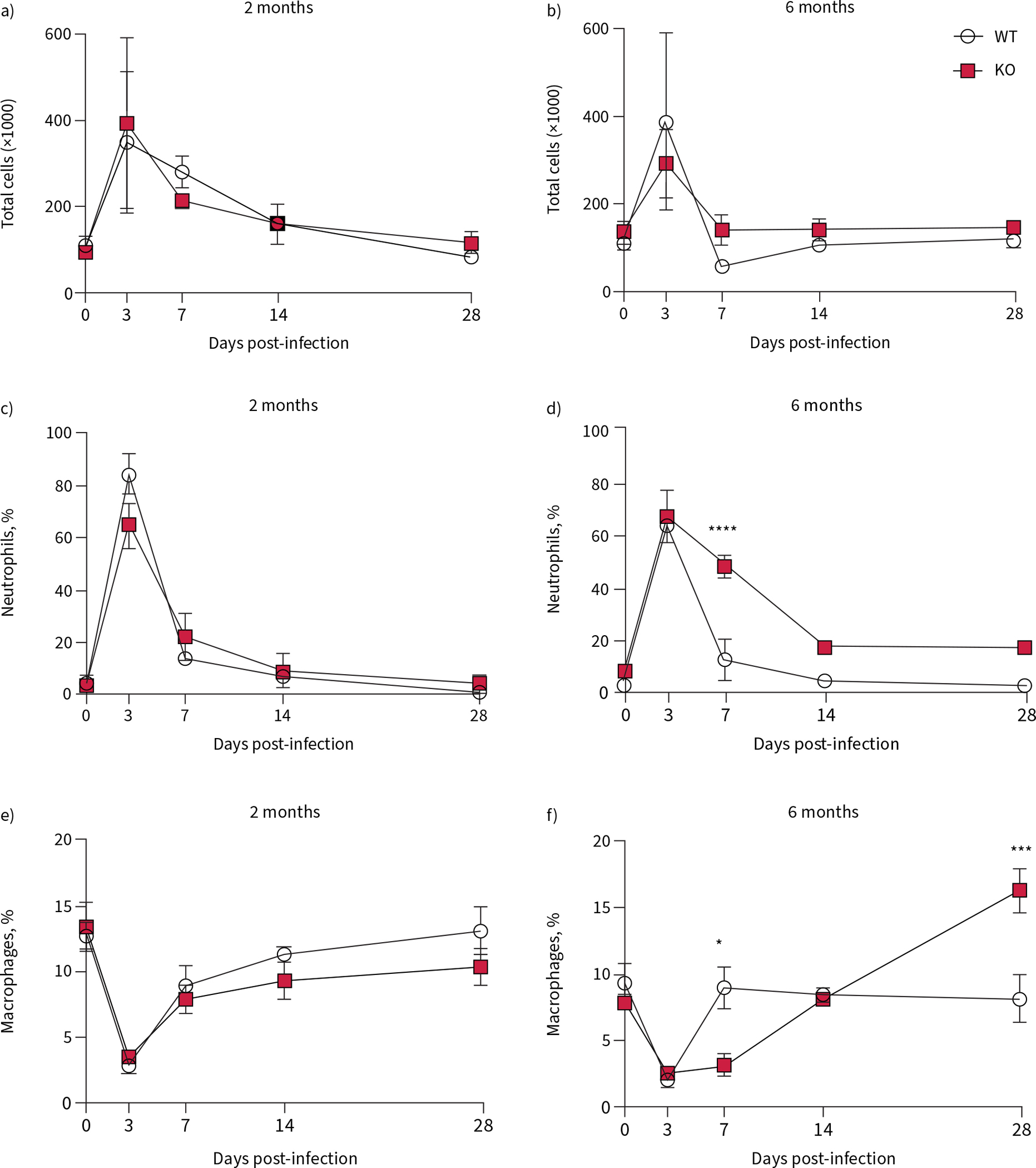
Aberrant inflammatory response in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats infected with Pseudomonas aeruginosa. Cells were collected from the airways of wild-type (WT) and KO rats via bronchoalveolar lavage and counted with differentiation. Total cell counts from a) 2-month-old and b) 6-month-old rats yielded percentage of neutrophils from c) 2-month-old and d) 6-month-old rats; and percentage of macrophages from e) 2-month-old and f) 6-month-old rats. Data are presented as mean±sem and analysed via Sidak post-test. *: p<0.05, ***: p<0.001, ****: p<0.0001. n=6–8 per group.
Representative histological images of WT and KO rats, from each time point throughout the course of infection are shown from both the 2-month-old (figure 3a) and the 6-month-old (figure 3b) studies. Histology images were scored, by a blinded reader, to quantitate the number (supplementary figure S2e and f ) and size of iBALT at each time point. At 2 months of age, there was no difference between genotypes (figure 3c). In the 6-month-old rat, the area of the iBALT increased throughout the experiment, with significantly larger iBALT in the KO lung at 28 dpi (figure 3d). This confirms pathogenesis previously observed in CF [22].
FIGURE 3.
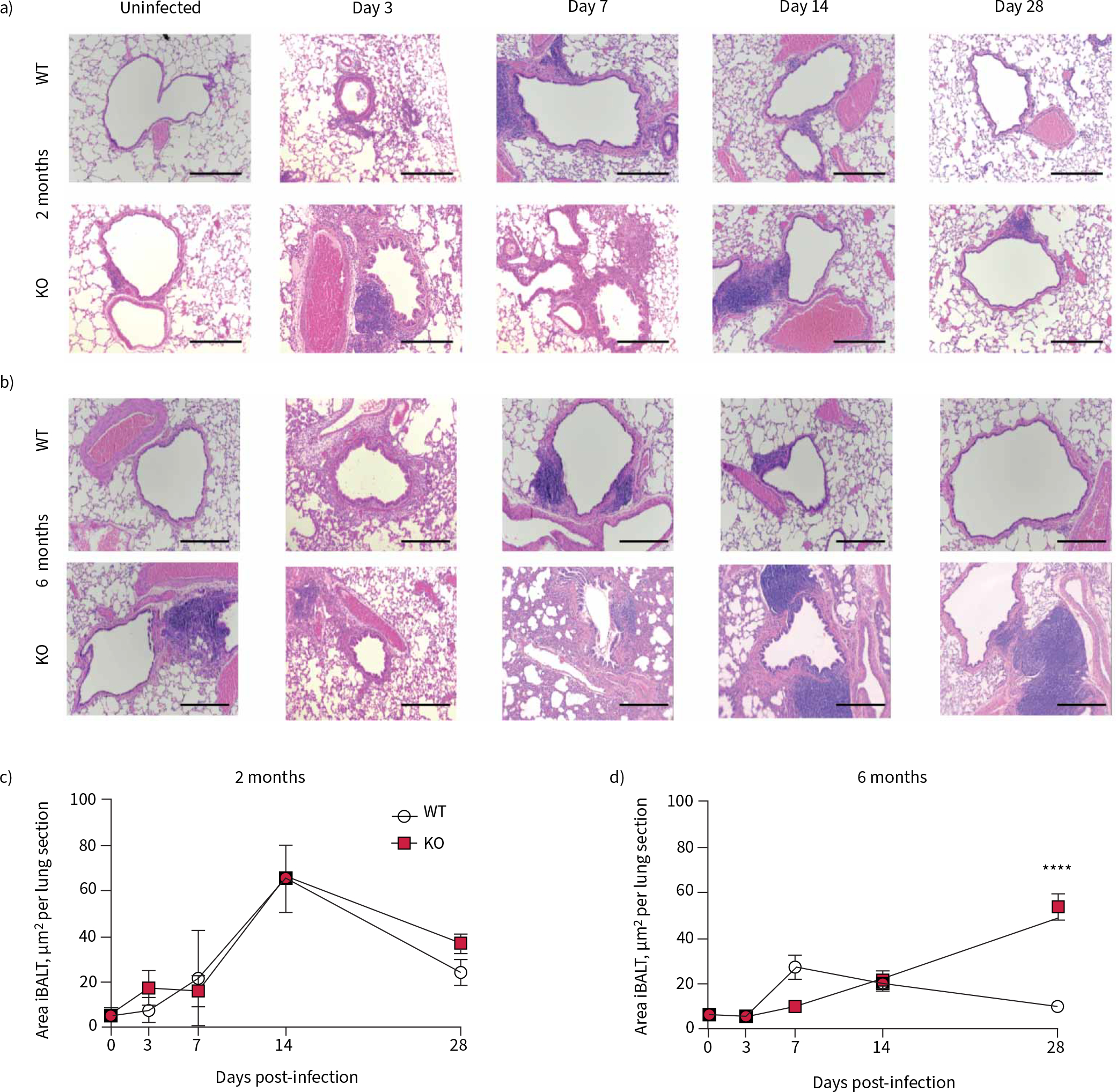
Resolution of inflammatory response to Pseudomonas aeruginosa infection is impaired in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats. Representative haematoxylin and eosin images are shown from 0, 3, 7, 14 and 28 days post-infection in a) 2-month-old wild-type (WT) and KO rats and b) 6-month-old WT and KO rats. Morphometric analysis of the slides yielded area of each individual induced bronchus-associated lymph tissue (iBALT) per lung section in c) 2-month-old WT and KO rats and d) 6-month-old WT and KO rats. Data are presented as mean±sem and analysed via two-way ANOVA with Sidak post-test. ****: p<0.0001. n=6–8 per group. Scale bars=200 μm.
Cytokine concentrations of common inflammatory mediators were measured in BALF collected from these animals. The 2-month-old rats had higher concentrations than the WT littermates of neutrophil elastase at 3 dpi (figure 4a), and TNF-α at 3 and 7 dpi (figure 4c) which both returned to baseline by 28 dpi. At this age there was no difference in IL-1β concentration between the two genotypes (figure 4e).
FIGURE 4.
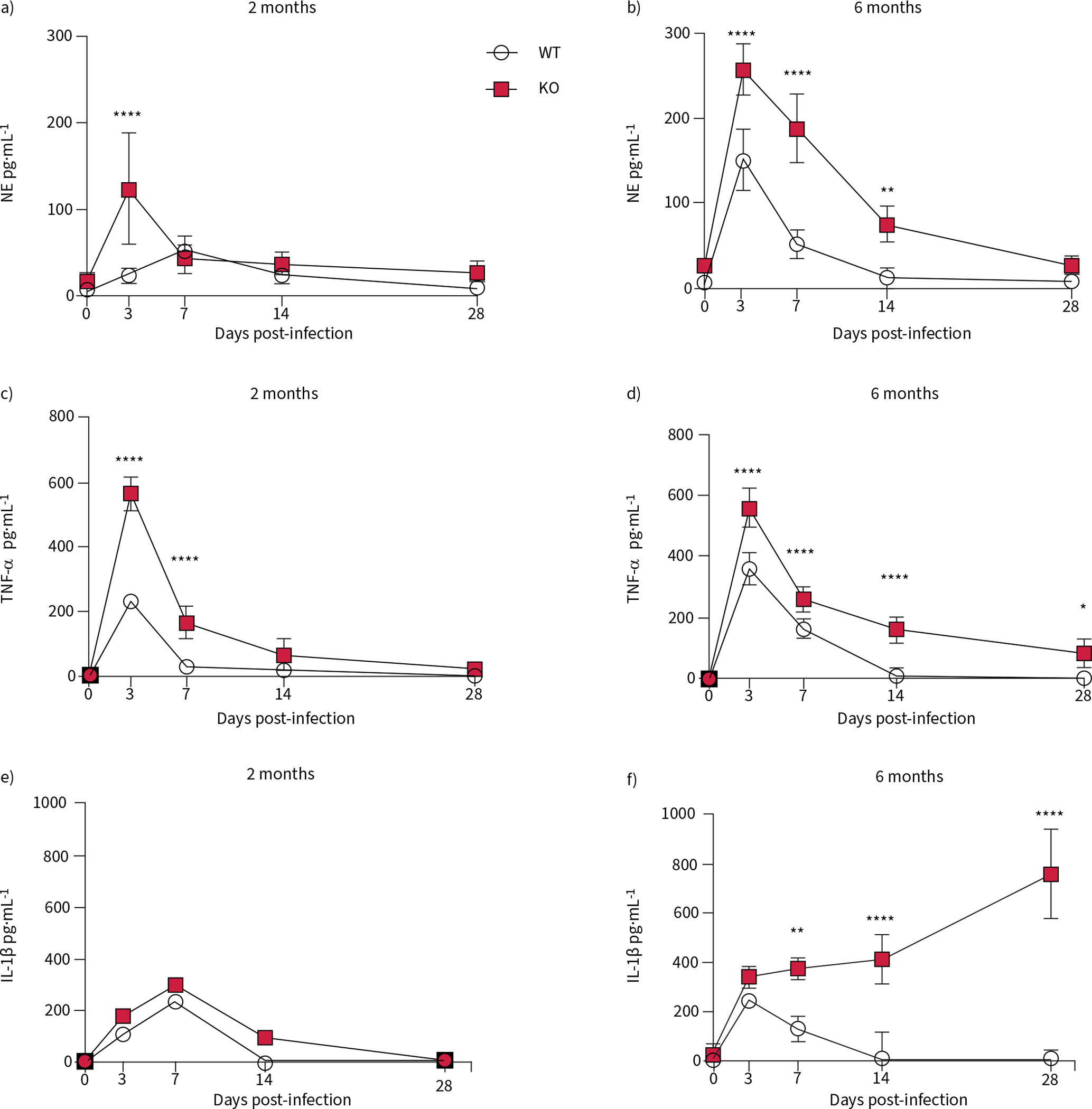
The cytokine response to Pseudomonas aeruginosa infection is heightened in cystic fibrosis transmembrane conductance receptor knockout (KO) rats at both 2 and 6 months of age. Bronchoalveolar lavage fluid collected from wild-type (WT) and KO rats at 0, 3, 7, 14 and 28 days post-infection were analysed by ELISA for neutrophil elastase (NE) concentration in a) 2-month-old and b) 6-month-old rats; tumour necrosis factor (TNF)-α concentration in c) 2-month-old and d) 6-month-old rats; and interleukin (IL)-1β concentration in e) 2-month-old and f) 6-month-old rats. Data are presented as mean±sem and analysed via two-way ANOVA with Sidak post-test. *: p<0.05, **: p<0.01, ****: p<0.0001. n=6–8 per group.
In contrast, the 6-month-old KO rats expressed higher neutrophil elastase concentration in the BALF at 3, 7 and 14 dpi (figure 4b). TNF-α concentration was elevated in the older KO rats at all four time points following infection (figure 4d). IL-1β concentrations in the 6-month-old KO rat were higher than WT at 7, 14 and 28 dpi. While neutrophil elastase and TNF-α are pan-inflammatory markers, indicating the neutrophilia and inflammatory response typical of a bacterial infection [23], IL-1β is also of interest, as it has been directly linked to increased mucus production in CF and other lung diseases [24]. In total, these data show a more severe inflammatory response in the older KO rats compared to all the other groups.
Mucus overproduction is not resolved in the older CFTR-KO rat following infection
We hypothesised that the P. aeruginosa infection would worsen the mucus expression in the KO rat at both 2 and 6 months of age. Representative histology images, stained with ABPAS, are shown at each time point, in both the 2-month-old (figure 5a) and the 6-month-old (figure 5b) rats. The 2-month-old KO rats had similar histology to their WT littermates at day 0, and expressed more mucus in the small airways at 3 dpi; however, by day 28, the mucus expression had returned to baseline levels. The morphometric analyses of the images show that in rats infected at 2 months of age, there was no statistically significant difference between the two genotypes in either the goblet cell area (supplementary figure S2g) or the mucus in the airways (figure 5c) at any time point post-infection.
FIGURE 5.
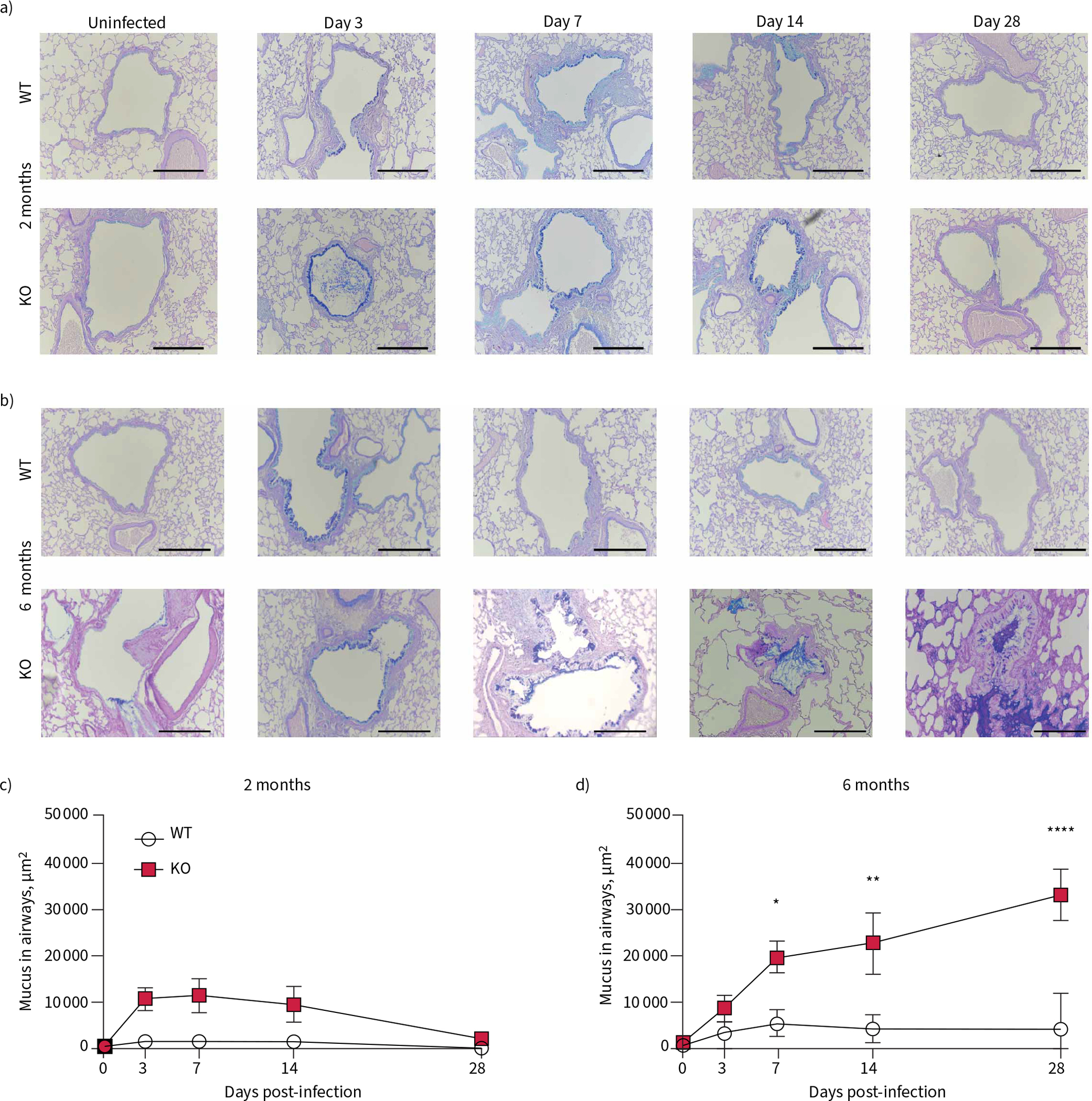
Mucus obstruction develops following Pseudomonas aeruginosa infection in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats. Representative Alcian Blue–Periodic Acid–Schiff images are shown from 0, 3, 7, 14 and 28 days post-infection in a) 2-month-old wild-type (WT) and KO rats and b) 6-month-old WT and KO rats. Morphometric analysis of the slides yielded area of mucus accumulation in the airways, in μm2, was calculated in c) 2-month-old and d) 6-month-old rats. Data are presented as mean±sem and analysed via two-way ANOVA with Sidak post-test. *: p<0.05, **: p<0.01, ****: p<0.0001. n=6–8 per group; 15–20 small airways per animal were measured. Scale bars=200 μm.
In contrast, the 6-month-old KO rat continued to accumulate mucus throughout infection. The uninfected 6-month-old KO rat had more mucus in the airways at day 0 (figure 5b), consistent with our initial studies [12], and showed increased mucus throughout the 4 weeks following infection, resulting in small-airway plugs by 28 dpi (figure 5d). This is accompanied by increased goblet cell staining in the airway, with worsening in the KO at each time point following exposure (supplementary figure S2h). This indicated that the older 6-month-old KO rat is the only group unable to resolve excessive mucus accumulations in the airways following exposure to P. aeruginosa.
We measured the relative amount of the secreted mucins Muc5b and Muc5ac collected from the lung via BALF following infection, compared to the WT uninfected control. In the KO rats infected at 2 months of age, Muc5b peaked at 7 dpi, significantly higher compared to the same time in the WT rats, but by 14 dpi there was no difference between the two genotypes (figure 6a). At this same age, Muc5ac increased at 3 dpi compared to the baseline, but there was no difference between genotypes, and in both groups, Muc5ac had returned to baseline by day 28 (figure 6c). As with the 2-month-old WT rats, Muc5b in the BALF did not change after infection in the 6-month-old WT rats. At this age, KO rats had an increase in Muc5b at 3 dpi, but had also returned to baseline by 28 dpi (figure 6b). In contrast, in older KO rats Muc5ac in the BALF remained significantly higher at day 28 (figure 6d). To understand how the exposure to mucoid P. aeruginosa alters mucins present in the airway, the ratios of Muc5ac to Muc5b were compared at day 0 and day 28. In the 2-month-old rats, the ratio of detected Muc5ac:Muc5b was approximately 1.0, indicating equal amounts of each, at day 0 in both genotypes (figure 6e). The ratio of Muc5ac:Muc5b decreased slightly at 28 dpi, but was not statistically different between the genotypes (figure 6e). In the 6-month-old group, the ratio of Muc5ac:Muc5b was again equal in both genotypes at day 0 (figure 6f). However, at 28 dpi, the ratio of Muc5ac:Muc5b dropped to <1 in the WT, indicating more Muc5b present, but was increased to an average of 3 in the KO, indicating much more Muc5ac in this group (figure 6f). These data indicate that there are significant increases of mucins following infection in 6-month-old KO rats, with a switch to predominantly Muc5ac, probably contributing to the observed mucus plugs and worsening airway obstruction seen during chronic infection.
FIGURE 6.
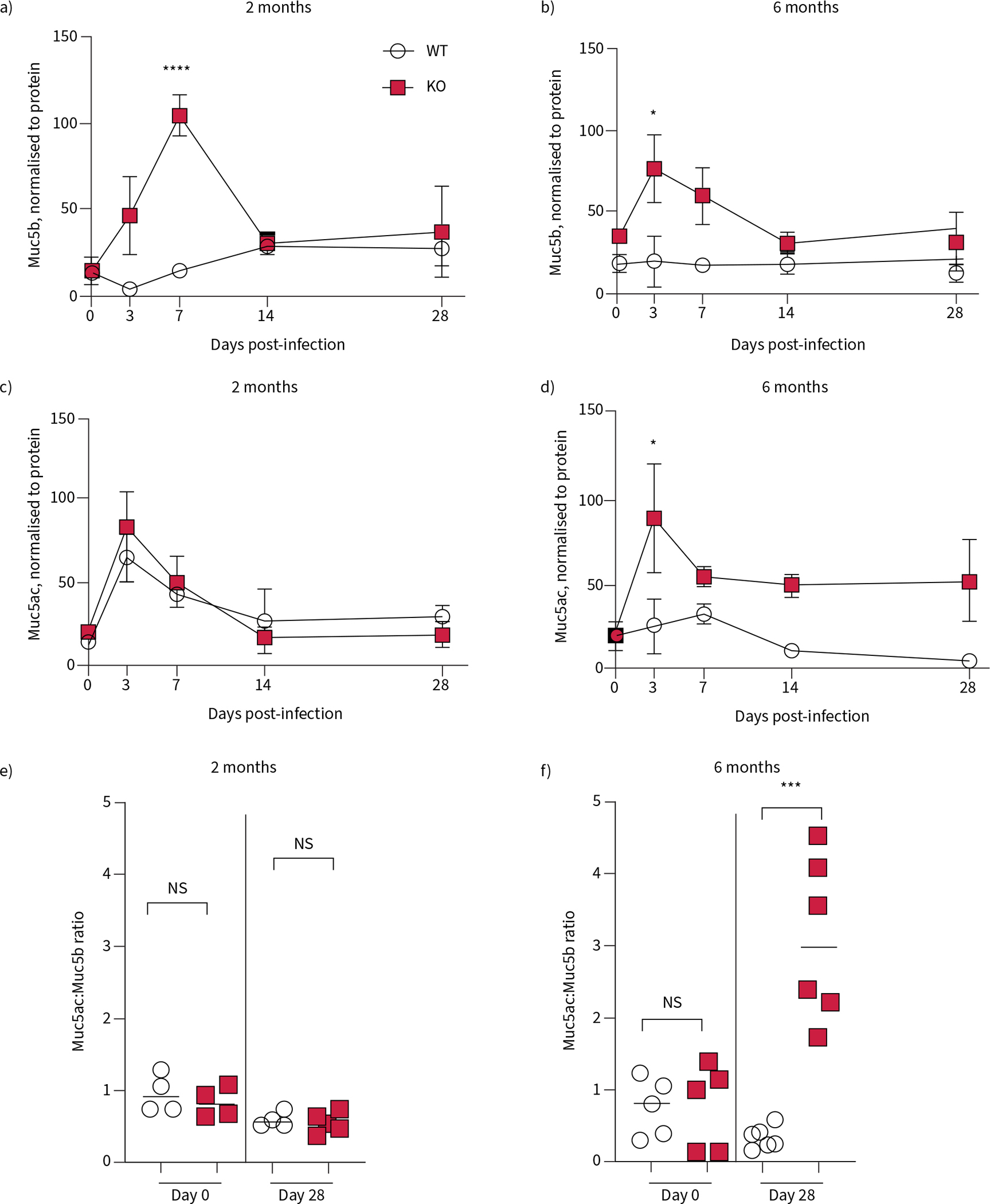
Mucin secretion is increased in cystic fibrosis transmembrane conductance receptor knockout (KO) rats following infection with Pseudomonas aeruginosa. Bronchoalveolar lavage fluid (BALF) samples collected from wild-type (WT) and KO rats at 0, 3, 7, 14 and 28 dpi were analysed for mucin content. Muc5b was measured in a) 2-month-old and b) 6-month-old rats. Muc5ac was measured in c) 2-month-old and d) 6-month-old rats. All data are normalised to protein concentrations in the BALF and analysed by two-way ANOVA. Mucin ratios from each age group at day 0 and day 28 are presented in e and f ), and data from each genotype analysed by two-tailed t-test. Data are presented as mean±sem. ns: nonsignificant. *: p<0.05, ***: p<0.001, ****: p<0.0001. n=4–6 per group.
Defective mucociliary clearance enables bacterial persistence
One major physiological function of mucus is to clear pathogens and other foreign particles from the airways. Therefore, we measured the airway surface liquid (ASL) depth and MCT rates in trachea collected from 2- and 6-month-old rats following infection. The ASL is the thin layer of fluid and mucus covering the surface of the epithelium that is reduced in the CF airway. Changes in the depth of the ASL can cause changes in MCT rates, shown previously by our laboratory [21].
Following infection, we found that ASL depth increased in both the WT and KO rats infected at 2 months of age, but returned to baseline by 28 dpi (figure 7a). At 2 months of age, both genotypes infected had reduced MCT rates at 3 dpi, but had returned to baseline by 28 dpi (figure 7c). At 6 months of age, ASL depth decreased in the WT rat at 3 dpi, but then increased until the resultant ASL depth at 28 dpi was higher than baseline (figure 7b), while the KO had a slight but steady increase in ASL depth throughout the course of the infection. WT rats at both 2 and 6 months of age experienced a reduction in MCT rates at 3 dpi that normalised following infection. However, the 6-month-old WT rat showed a dramatic increase in MCT rates at 14 dpi and 28 dpi (figure 7d). These data represent a difference in response to infection both compared to the younger WT and to the 6-month-old KO rat, and suggest that the older WT respond to infection with heightened secretion and clearance of mucus from the airway, while which the KO rats at this age are unable to do.
FIGURE 7.
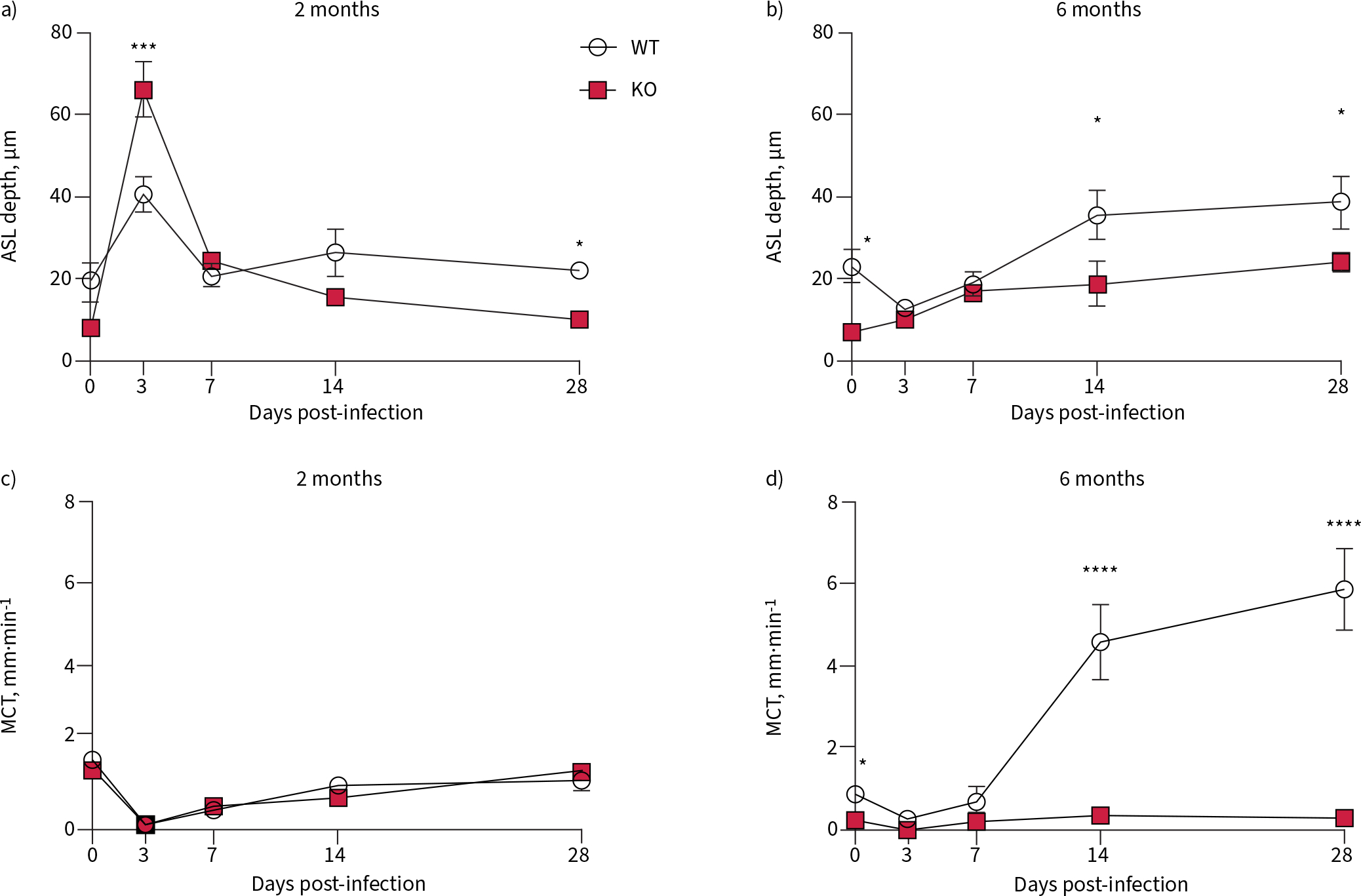
Infection with Pseudomonas aeruginosa alters airway functional parameters that remain unresolved in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats. Tracheae from wild-type (WT) and KO rats infected with P. aeruginosa were collected at 0, 3, 7, 14 and 28 days post-infection and imaged using micro-optical coherence tomography. Quantitation of the resultant images yielded airway surface liquid (ASL) at a) 2 months and b) 6 months; and mucociliary transport (MCT) at c) 2 months and d) 6 months of age. Data are presented as mean±sem. All data were analysed via two-way ANOVA with Sidak post-test. *: p<0.05, ***: p<0.001, ****: p<0.0001. n=6–8 per group.
We suspected that the abnormally high MCT rates seen in the 6-month-old WT rat would be protective against repeat insult, an advantage that would be missing in the KO rats of the same age. Therefore, 6-month-old WT and KO rats received a second infection, at 28 dpi. This group of WT rats had lower CFUs at 3 dpi compared to the first exposure (figure 8a), while the KO rats had higher CFUs at that time point. WT rats regained their baseline weight more quickly after the second exposure, compared to both the 6-month-old KO and the first infection (figure 8b). While KO rats had a higher percentage of neutrophils in their BALF at day 3 following the second exposure compared to the first, WT rats had no change (figure 8c). At 3 dpi following the repeat infection, both WT and KO rats had the same ASL depth compared to the same time point following the first infection (supplementary figure S3a). However, WT rats had baseline rates of MCT following the repeat infection (supplementary figure S3b), while MCT rates were not different between KO rats that had received one versus two infections. These data suggest that the supernormal mucociliary transport present in the 6-month-old WT at the time of repeat exposure resulted in a milder infection, while the KO rats had a worsened response to a repeat exposure.
FIGURE 8.
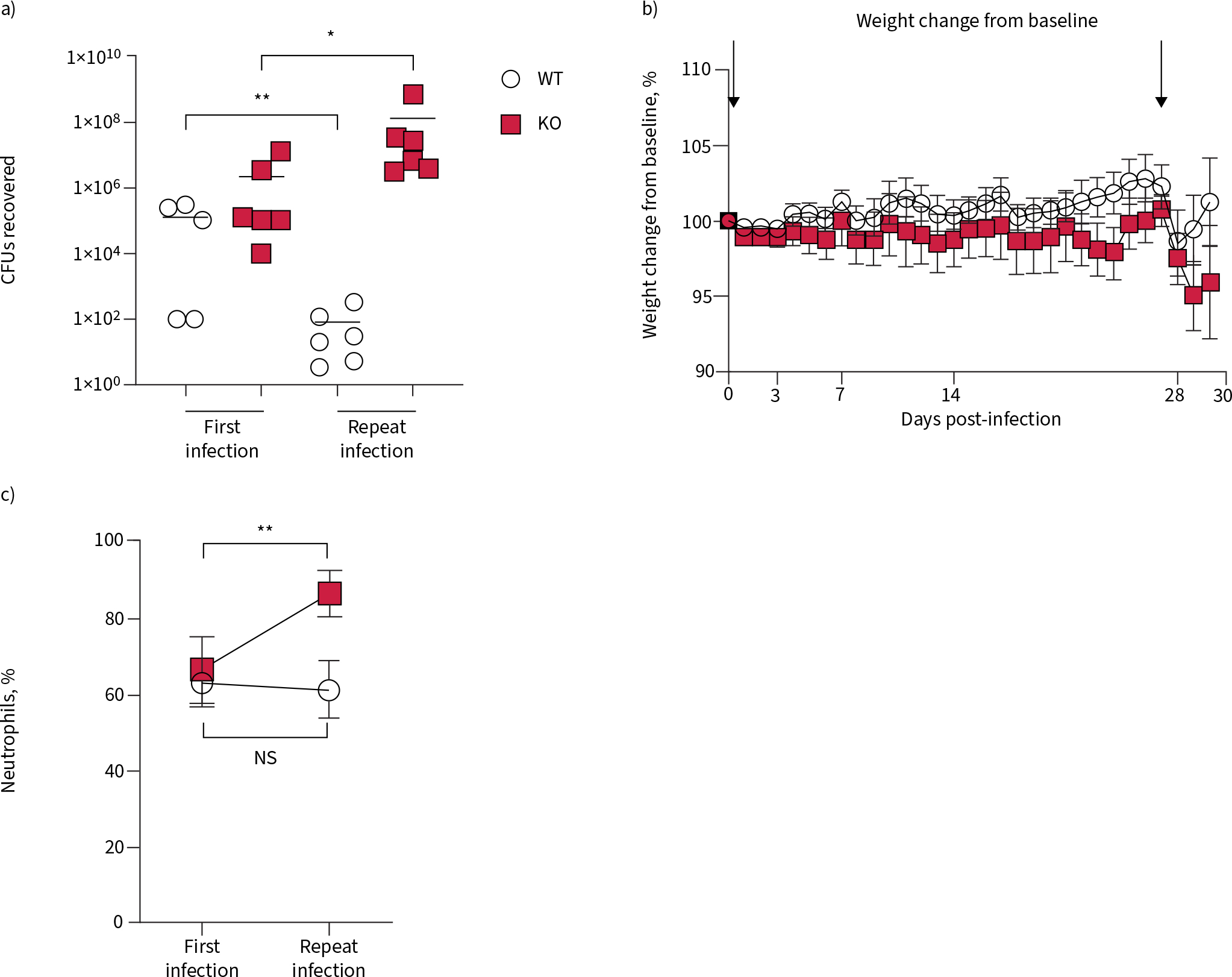
A second exposure following infection with Pseudomonas aeruginosa is poorly tolerated in 6-month-old cystic fibrosis transmembrane conductance receptor knockout (KO) rats. 6-month-old wild-type (WT) and KO rats infected with P. aeruginosa at day 0 were re-inoculated with the same strain at day 28. a) Lung homogenates were plated for CFU counts; data were analysed via one-way ANOVA with Tukey’s post-test. b) Weights were collected in both groups, and percentage change from baseline calculated; data were analysed via two-way ANOVA. c) Percentage of neutrophils in the bronchoalveolar lavage fluid were calculated; data between each genotype were analysed by a two-tailed t-test. ns: nonsignificant. *: p<0.05, **: p<0.01. n=5–6 per group.
Discussion
Chronic infection with P. aeruginosa is a hallmark of CF lung disease, but has been difficult to model in the laboratory setting [1, 6]. The abnormal mucus present in the older KO rat allows bacterial regrowth kinetics, with increasing CFUs collected from the lungs between days 7 and 28 after infection, that have not been shown in any other experimental model and may be more replicative of the dynamics of P. aeruginosa in the human CF airway [25].
Many distinct aspects of CF airway disease have been connected to increased susceptibility of chronic P. aeruginosa infection in people with CF. Dehydrated airway epithelium has been suggested to be the physical link between the CF airway and the development of P. aeruginosa biofilms [26]. Decreased airway surface pH is suggested to reduce the efficiency of bacterial killing in the CF airway [27]. Mucociliary clearance has been shown to be important in clearing bacteria from the normal airway [28]. A strength of the CFTR-KO rat model is that these pathologies are differentially expressed as the rat ages [12]. The KO rat at both ages tested have a dehydrated airway epithelium and hyperacidic pH [12]. Overall, the 2-month-old KO rat had only a minimally different response to infection compared to the age-matched WT group, with only temporarily worsened inflammation and mucin secretion that were each completely resolved by 4 weeks following infection. This differed sharply in the older 6-month-old KO rat, which had persistent bacterial infection and corresponding inflammation and mucus accumulation. The major difference between the 2 and 6-month-old KO rat is the presence of hyperviscous and static mucus at 6 months of age [12]. These older rats were unable to eradicate the bacteria. Instead, the presence of abnormal mucus in the airways appears to have allowed the development of chronic P. aeruginosa infection. Therefore, we conclude that the mucus transportability is the overriding factor permitting infection persistence. This conclusion was corroborated by the changes to the airway surface. While the 2-month-old WT and KO rats showed a drop in MCT rates during the acute phase of the infection, both genotypes were able to recover to baseline. This finding is key, and may explain why the 2-month-old rats are able to completely eradicate bacteria while the older KO rats are not.
Recent data from Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) programme captured mucus flakes in the airways of young, healthy patients, prior to bacterial infection [29]. These were associated with higher mucins in BALF analysis compared to non-CF samples, with proportionally more MUC5B than MUC5AC. The function of these different isoforms of mucin is unclear, but suggests that the separate mucins may contribute in a specific and differential way to the maintenance of the airway. One objective of the study presented here was to determine how expression of each isoform changes influences the switch to chronicity. In the younger rats, which eradicated infection quickly, Muc5b and Muc5ac were unchanged in the KO at days 0 and 28, compared to the age-matched WT. While the 6-month-old KO rats had more Muc5b than the WT before infection, it was Muc5ac that remained higher in the KO in the chronic phase at day 28. The increase of Muc5ac in the chronic phase of this model, possibly a result of accumulation and mucus stasis, replicates a finding of increased MUC5AC in people with CF following exacerbation [9, 30]. In our study, altered mucin ratios occurred simultaneously with reduced MCT rates in the 6-month-old KO rats and in the absence of any change to ciliary function, and therefore may be an aspect of pathogenesis not previously recognised. These data suggest two different possibilities underlying the switch from acute to chronic infection. Either the pre-exposure increase in Muc5b allows P. aeruginosa to gain a foothold in the lung, or the lack of resolution of Muc5ac in the airway allows the bacteria to remain and regrow. Future studies will test these hypotheses to determine if one is predominant, or if the pathophysiology is a combination of both.
As mentioned earlier, a major function of the MCT apparatus is to clear infection from the airways. In this study, the 6-month-old WT rat has a significant increase in MCT rates following infection; becoming faster than baseline by 14 dpi. This response was surprising, given the rapid eradication of the infection, and was postulated to be a protective measure. WT rats re-infected at 28 dpi, when MCT rates are elevated, but all other parameters have returned to baseline, have a mild response, with lower CFUs than the first exposure. While the adaptive immune system may be a factor in the reduced bacterial burden, the inflammatory sequelae of the first infection had resolved prior to the second exposure. Therefore, we feel that the higher MCT rates are responsible for the improved outcomes after the second infection in the WT rats. Conversely, KO rats infected at 28 dpi, when their lungs remain burdened with bacteria, excess mucus and increased inflammation, have the expected worsened response; higher CFUs, higher neutrophil influx and more significant weight loss. Although the second bacterial infection did not result in additive CFUs in the KO rats, probably due to the presence of neutrophils in the airway from the first infection, the KO rats still failed to completely eradicate bacteria. These data suggest that promoting mucus clearance following infection may help to eradicate bacteria and may even prevent the switch from acute to chronic in the CF lung.
This study has several limitations. The use of the agarose beads, while ensuring that the bacteria remain in the lung long enough to establish a chronic infection, still represent an experimental manipulation that is not fully representative of the natural course of infection in patients. Additionally, the experiments were performed with a clinical isolate that is adapted to infection in the lung and expresses a mucoid phenotype. Future studies will examine the mechanisms by which P. aeruginosa adapts to remain in the lung. Nevertheless, the data offered here present a groundbreaking model that recapitulates chronic P. aeruginosa infection in the CF airway, and can be used to continue mechanistic studies into this phenomenon. The divergence between eradication and conversion to chronicity appears to hinge on the presence of occlusive mucus, explaining why younger, more stable people with CF are more readily able to recover from infection [31], while infection in older patients leads to worsened outcomes [32], and why lung function declines with each additional exacerbation [13].
Supplementary Material
Acknowledgements:
The authors acknowledge the assistance of Dezhi Wang (Department of Anatomic Physiology), and the Histomorphometry and Molecular Analysis Core (Center for Metabolic Bone Disease, University of Alabama at Birmingham, Birmingham, AL, USA).
Support statement: This work was supported by the National Heart, Lung, and Blood Institute (1K08HL131867, S.E. Birket), the National Institute of Diabetes, Digestive and Kidney Diseases (DK072482, S.M. Rowe) and the Cystic Fibrosis Foundation (R464-CF, ROWE19R0, BIRKET20A0-KB). Funding information for this article has been deposited with the Crossref Funder Registry.
Footnotes
Conflict of interest: A.G. Henderson has nothing to disclose. J.M. Davis has nothing to disclose. J.D. Keith has nothing to disclose. M.E. Green has nothing to disclose. A.M. Oden has nothing to disclose. S.M. Rowe has a patent “Use of μOCT as a tool for diagnosis and drug discovery” issued. S.E. Birket reports grants from NIH NHLBI and Cystic Fibrosis Foundation, during the conduct of the study.
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168: 918–951. [DOI] [PubMed] [Google Scholar]
- 2.Mulcahy LR, Isabella VM, Lewis K. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 2014; 68: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2014 Annual Data Report. 2015. Available from: www.cff.org/medical-professionals/patient-registry
- 4.Nixon GM, Armstrong DS, Carzino R, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001; 138: 699–704. [DOI] [PubMed] [Google Scholar]
- 5.Milczewska J, Wołkowicz T, Zacharczuk K, et al. Clinical outcomes for cystic fibrosis patients with Pseudomonas aeruginosa cross-infections. Pediatr Pulmonol 2020; 55: 161–168. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann N, Rasmussen TB, Jensen PØ, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun 2005; 73: 2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeckeren A, Walenga R, Konstan MW, et al. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997; 100: 2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 2002; 36: 291–312. [DOI] [PubMed] [Google Scholar]
- 9.Henderson AG, Ehre C, Button B, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 2014; 124: 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352: 1992–2001. [DOI] [PubMed] [Google Scholar]
- 11.Ermund A, Meiss LN, Rodriguez-Pineiro AM, et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun 2017; 492: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birket SE, Davis JM, Fernandez CM, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018; 3: e97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waters V, Stanojevic S, Atenafu EG, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 2012; 40: 61–66. [DOI] [PubMed] [Google Scholar]
- 14.Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One 2014; 9: e91253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feola DJ, Garvy BA, Cory TJ, et al. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob Agents Chemother 2010; 54: 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cory TJ, Birket SE, Murphy BS, et al. Azithromycin increases in vitro fibronectin production through interactions between macrophages and fibroblasts stimulated with Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68: 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nacucchio MC, Cerquetti MC, Meiss RP, et al. Short communication. Role of agar beads in the pathogenicity of Pseudomonas aeruginosa in the rat respiratory tract. Pediatr Res 1984; 18: 295–296. [DOI] [PubMed] [Google Scholar]
- 18.Thornton DJ, Carlstedt I, Sheehan JK. Identification of glycoproteins on nitrocellulose membranes and gels. Mol Biotechnol 1996; 5: 171–176. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Yuan X, Zou L, et al. Effects of 1,25-dihydroxyvitamin D3 on the prevention of chronic obstructive pulmonary disease (COPD) in rats exposed to air pollutant particles less than 2.5 micrometers in diameter (PM2.5). Med Sci Monit 2018; 24: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One 2013; 8: e54473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birket SE, Chu KK, Liu L, et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 2014; 190: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frija-Masson J, Martin C, Regard L, et al. Bacteria-driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis. Eur Respir J 2017; 49: 1601873. [DOI] [PubMed] [Google Scholar]
- 23.Cantin AM, Hartl D, Konstan MW, et al. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 2015; 14: 419–430. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Sun L, Kato T, et al. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest 2019; 129: 4433–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra S, Hayes D Jr, Wozniak DJ. Cystic fibrosis and Pseudomonas aeruginosa: the host–microbe interface. Clin Microbiol Rev 2019; 32: e00138–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui H, Wagner VE, Hill DB, et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA 2006; 103: 18131–18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012; 487: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ermund A, Meiss LN, Dolan B, et al. The mucin bundles responsible for airway cleaning are retained in cystic fibrosis and by cholinergic stimulation. Eur Respir J 2018; 52: 1800457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esther CR Jr, Muhlebach MS, Ehre C, et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med 2019; 11: eaav3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgel PR, Montani D, Danel C, et al. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 2007; 62: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders DB, Hoffman LR, Emerson J, et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol 2010; 45: 127–134. [DOI] [PubMed] [Google Scholar]
- 32.de Boer K, Vandemheen KL, Tullis E, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011; 66: 680–685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


