Graphical abstract

Keywords: Fish cakes, Soybean oil, Thermal-oxidation, 1H NMR, Oxidation products
Highlights
-
•
The modified DPPH method elucidated the content of TPC and the antioxidant capacity in the frying system.
-
•
The increase of TPC was more relevant to the oxidation of frying medium than the effect of fish cakes.
-
•
Oxidation and hydrolysis products during frying were simultaneously determined by 1H NMR.
Abstract
The oxidation process of soybean oil (SBO) during frying fish cakes was investigated. The TOTOX value of before frying (BF) and after frying (AF) was significantly higher than control (CK). However, the total polar compound (TPC) content of AF reached 27.67% in frying oil continuously frying at 180℃ for 18 h, and 26.17% for CK. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) loss in isooctane and methanol significantly decreased with the extension of frying time and then tended to be stable. The decrease of DPPH loss was related to the increase of TPC content. Antioxidant and prooxidant balance (APB) value below 0.5 was obtained after 12 h for heated oil. (E)-2-alkenals, (E, E)-2,4-alkadienals, and n-alkanals were dominant ingredients among the secondary oxidation products. Traces of monoglycerides (MAG) and diglycerides (DAG) were also detected. These results may improve our understanding of the oxidation deterioration in SBO during frying.
1. Introduction
Fish cakes are surimi products with a certain elasticity. They are popular with consumers due to their high nutritional value and low price, which could provide high quality of protein and unsaturated fatty acids for human (Li et al., 2022). There are many cooking methods for surimi products, such as steaming, boiling, roasting, and frying. Frying is one of traditional cooking methods for surimi products. It involves soaking food in edible oil and transferring heat and mass, which endows fried food with the desired taste, quality, and golden color (Yamsaengsung & Moreira, 2002). Owing to the high-temperature environment, triglycerides simultaneously undergo oxidation, polymerization, and hydrolysis reactions, producing compounds with higher polarity than typical triglycerides. The insoluble and non-volatile substances produced by these reactions make negative effects on the oil (Xu et al., 2019). These compounds are referred to as polar compounds. TPC includes triglyceride oligomer (TGO), triglyceride dimer (TGD), diglyceride (DAG), monoglyceride (MAG), free fatty acid (FFA), and oxidized triglyceride monomer (oxTGM), which could be divided into oxidative polymerization products and hydrolysis products according to their respective formation pathways (Li et al., 2019). The formation of TPC in frying oil not only affects the quality and frying performance of the oil, but also causes food safety problems in fried foods. Intakes of foods containing high content of TPC may cause growth arrest, liver dysfunction, and an increased risk of developing cancer (Saguy & Dana, 2003). The TPC content in frying oil is used in many countries to determine whether the oil exceeds safety thresholds (Ahmad Tarmizi et al., 2019, Li et al., 2020, Zhou et al., 2019). In China, frying oil should be discarded when the TPC content exceeds 27%.
It was reported that 1H nuclear magnetic resonance (NMR) spectroscopy could be used to analyze the oxidation and hydrolysis products in edible oils (Guillén & Ruiz, 2003a). This method does not require complex pre-processing of samples, and allows the rapid collection of abundant information. 1H NMR technology can provide the ratio of different acyl groups in the oil, which is proportional to the number of hydrogen atoms that produced the signal. It could provide information about primary oxidation, including hydroperoxides with cis, trans- and trans, trans- conjugated double bonds, and secondary oxidation products, including mostly aldehydes such as (E)-2-alkenals, (E, E)-2,4-alkadienals, n-alkanals, 4-hydroxy-(E)-2-alkenals, 4-hydroperoxy-(E)-2-alkenals, 4,5-epoxy-(E)-2-alkenals, and 4-oxoalkanals (Goicoechea & Guillén, 2010). 4-hydroxy-(E)-2-alkenals and 4,5-epoxy-(E)-2-alkenals are genotoxic and cytotoxic, and were identified as the cause of diseases such as cancer, Alzheimer and Parkinson, among others (Martínez-Yusta & Guillén, 2015). The information provided by 1H NMR spectroscopy could be used to analyze the evolution of oil during frying cycles.
Soybean oil (SBO) was a representative and most consumed vegetable oil containing a high content of polyunsaturated fatty acids (Hwang, Ball, Doll, Anderson, & Vermillion, 2020). It’s well known that cooking oil with a high level of unsaturated fatty acids could promote heart benefits (Hwang, Winkler‐Moser, Doll, Gadgil, & Liu, 2019). In the process of industrial production, frying oil is often reused. Many studies have been reported the oxidative degradation of frying oil on a commercial scale (Adjonu et al., 2019, Ahmad Tarmizi et al., 2019, Li et al., 2017). There are few studies focusing on the influence of foodstuff on the oxidative degradation of frying oil, particularly the surimi product. In this study, the oxidation process of SBO was monitored during deep-frying fish cakes in order to investigate the effect of foodstuff on the oil oxidation. The DPPH loss and TPC content were used to confirm the discarding point of SBO during frying. 1H NMR technology was used to simultaneously quantify the oxidation products and hydrolysis compounds of SBO. Combining several conventional physicochemical indexes, a more comprehensive understanding of oxidation deterioration was further explored.
2. Materials and methods
2.1. Materials and chemicals
Frozen surimi (AAA grade) produced from silver carp was kindly provided by Jingli Co. ltd. (Honghu, Hubei, China). SBO was purchased from Zhongliang Co., ltd. (China), and salt (Hubei Salt Industry Group Co., ltd, Wuhan, Hubei, China) was purchased from the Huazhong Agricultural University market (Wuhan, Hubei, China).
Chloroform, methanol, NH4SCN, FeSO4·7H2O, BaCl2·2H2O, glacial acetic acid, isooctane, 4-methoxybenzeneamine, potassium hydroxide, sodium bisulfate, 2,2-diphenyl-1-picrylhydrazyl were purchased from Sinopharm Chemical Reagent Co., ltd. (Shanghai, China). Isooctane (Chromatography grade) and methanol (Chromatography grade) were acquired from Fisher Scientific Co. (Fair Lawn, NJ, USA). Deuterated chloroform (CDCl3) was supplied by Cambridge Isotope Laboratories, Inc. (Andover, MA, USA).
2.2. Sample preparation
The frozen surimi was thawed at 4℃. Subsequently, the moisture content of surimi was measured and adjusted to 78 g/100 g. The surimi was chopped at 1200 rpm for 2 min using a conditioning machine (K600, Frankfurt, Germany). Subsequently, the salt was added to the surimi in the proportion of 2 g/100 g, and the frozen surimi was then chopped at 1500 rpm for 2 min. Thereafter, surimi was added to casings (35 mm diameter) to produce surimi sausages. The surimi sausages were heated at 40℃ for 1 h to form surimi gel and then cut into 1.5 cm thick slices to produce the fish cakes. The ready-to-fry fish cakes were stored in a refrigerator at 4℃ until the requirement.
For the frying procedure, 5.7 L of SBO was added to a deep-fryer (Model DF-6L, Guangdong, China), and heated to 180 ± 5℃ in 10 min. The solid-to-liquid ratio of 1:30 (kg:L) was set. Subsequently, the prepared fish cakes were fried for 4 min every 3 h as a frying cycle. 150 mL of fried oil was sampled before (BF) and after (AF) each frying cycle respectively. In each frying cycle, BF means the oil sampled before the fish cakes added to the fryer, and AF means the oil sampled after the fish cakes fried for 4 min. The oil heated at 180 ± 5℃ without frying any fish cakes was as control (CK), and 300 mL of oil was sampled for every 3 h. The oil samples were stored at −80℃ until further analysis.
2.3. Determination of color
The measurement of the color of frying oil was carried out in a Lovibond colorimeter (Model F, Tintometer, Britain) followed the the official AOCS method Cc 13e-92 (AOCS, 2017). The yellow and red units was measured using a cuvette (Optical path 25.4 mm, 1 in.).
2.4. Determination of total oxidation (TOTOX) value
Following a comprehensive determination of primary oxidation products by peroxide value (PV) and the secondary oxidation products via p-anisidine value (p-AV) (Chew et al., 2017), the TOTOX value of the oil samples could be calculated using the following equation:
| (1) |
The PV was determined according to the method described by Shantha and Decker (Shantha & Decker, 1994). To prepare ferrous chloride solution, 0.4 g BaCl2·2H2O was dissolved in 50 mL water and mixed with ferrous sulfate solution (0.5 g FeSO4·7H2O dissolved in 50 mL water). Then, 2 mL of 10 N concentrated hydrochloric acid was added to the mixture, which was filtered to remove the barium sulfate precipitate and obtain the clarified ferrous chloride solution. To determine the PV, 0.1–0.3 g oil samples were weighed into a 15 mL centrifuge tube. Then, the oil sample was mixed with 9.8 mL chloroform–methanol (7:3, v:v) by vortex for 30 s. Fifty microliters of ammonium thiocyanate solution (3 g ammonium thiocyanate dissolved in 10 mL water) were added to the 15 mL centrifuge tube. Next, 50 µL of ferrous chloride solution was added to the tube and vortexed for 2–4 s. After reacting at room temperature for 5 min, the absorbance of the solution was determined at 500 nm using a UV–visible spectrophotometer. The measurement process was completed within 10 min.
The PV was calculated using the following equation:
| (2) |
where As is the absorbance of the sample, Ab is the absorbance of the blank, and m0 is the sample weight.
The determination of p-AV was performed according to the AOCS Official Method Cd 18–90 (AOCS, 2013). Samples (0.5–4.0 g) were weighed into 25 mL volumetric flasks. The oil sample was dissolved and diluted to volume with isooctane as a test solution. The absorbance of the test solution was measured at 350 nm, denoted as Ab, using the solvent as a blank. Five milliliters of the test solution and 5 mL isooctane were added to separate test tubes, and 1 mL p-anisidine reagent (0.25 g p-anisidine dissolved in 100 mL glacial acetic acid) was added to both tubes. The absorbance of the test solution (As) with 1 mL p-anisidine reagent was measured at 350 nm after 10 min, and the solvent with 1 mL p- anisidine reagent was used as a blank.
The p-AV of the sample was calculated using the following equation:
| (3) |
where As is the absorbance of the test solution that reacts with the p-anisidine reagent, Ab is the absorbance of the test solution, and m is the weight of the oil sample.
2.5. Determination of TPC
TPC was measured using a Testo™270 cooking oil tester (Testo, Lenzkirch, Germany) according to the manufacturer’s instructions. The results of the rapid instrument method were shown to be highly correlated with those of the column chromatography method (Chen et al., 2013, Song et al., 2017). The probe of the instrument was immersed in the deep fryer until the data was stable. The measurement was carried out in hot oil (150–190°C).
2.6. A modified DPPH method
DPPH was dissolved in methanol and isooctane at a concentration of 0.1 mM. DPPH solution (0.1 mM, 0.75 mL) was mixed with 0.25 mL of methanol and isooctane containing 40,000 ppm oil (w/v). To prepare the oil sample in methanol, the sample was dissolved in methanol and then vortexed for 1 min. The mixture was centrifuged at 3000 rpm for 3 min. Then, 0.25 mL of the supernatant was reacted with 0.75 mL of the 0.1 mM DPPH solution. To prepare the oil sample in isooctane, the sample was dissolved in isooctane and vortexed for 1 min. An aliquot (0.25 mL) of the solution was mixed with 0.75 mL DPPH solution. After 30 min of incubation in the dark, the absorbance of the mixtures was measured using a microplate reader (Multiskan FC, Thermo Fisher Scientific Inc., Waltham, MA, USA). The DPPH absorbances in methanol and isooctane were determined to be 517 and 509 nm, respectively. The DPPH loss was calculated using the following equation:
| (4) |
where A0 is the absorbance of blank sample, A1 is the absorbance of sample, k is the slope of conversion of DPPH absorbance into DPPH concentration (L⁄µmol DPPH), V is the volume of sample solution (L), m is the weight of the oil sample (g).
The antioxidant-prooxidant balance (APB) was calculated using the following equation:
| (5) |
where Dm is the DPPH loss in methanol, DI is the DPPH loss in isooctane.
2.7. Determination of fatty acid composition
To convert the frying oil to fatty acid methyl esters, 60 mg of oil was weighed into a test tube and dissolved in 4 mL isooctane (HPLC grade). KOH-methanol solution (0.2 mL of the solution comprising 13.2 g KOH dissolved in 80 mL chromatography grade methanol) was added to the test tube and vortexed for 1 min. Then, 1 g of NaHSO4 was added to the test tube and vortexed for 30 s to neutralize the excess NaOH. After centrifuging at 4000 rpm for 5 min, the supernatant was filtered through a 0.45 µm filter membrane and then transferred to the sample bottle for detection.
The fatty acid composition was determined by measuring the fatty acid methyl ester content using an Agilent gas chromatograph (Agilent Technologies, Beijing, China) equipped with a HP-INNOWAX (30 m × 0.32 mm × 0.25 µm) capillary column. For GC analysis, nitrogen was used as the carrier gas at a flow rate of 1.5 mL/min. The inlet temperature was set at 260℃. The temperature program was as follows: initial temperature, 210℃ for 9 min; 20℃/min until 250℃; and held for 10 min. The split ratio was 80:1. The content of each fatty acid was expressed as a percentage of the total identified fatty acid peaks.
2.8. 1H NMR spectroscopy
The 1H NMR spectra of the frying oil were collected according to the method described by Jia et al. (2020). Briefly, 50–100 mg of the oil sample was dissolved in 0.6–0.7 mL of CDCl3 and vortexed for 30 s. Then, the mixture was transferred to an NMR tube for further analysis using Bruker Advance III 600 spectrometer. The measurement conditions were as follows: 12,335.5 Hz spectral width, 16 scans, 2.656 s acquisition time, and 25℃. Using the tetramethylsilane as a basis at δ = 0 ppm, the chemical shifts were recorded with MestReNova 11.0 software.
The concentrations of primary and secondary oxidation products, MAG, and DAG were estimated and reported as millimoles per mole of triglyceride (mmol/mol TG). The 1H NMR spectra of the SBO are shown in Fig. 1A. The signals represented the number of hydrogen atoms in the triacylglycerols (Guillén & Ruiz, 2003a). Saturated acid, oleic acid, linoleic acid, and linolenic acid acyl groups of methyl hydrogen atoms corresponded to the area of each signal in the spectrum. The proportions of oleic (O), linoleic (L), linolenic (Ln), and saturated (S) acyl groups were determined using the following equation (Guillén & Ruiz, 2003b):
| (6) |
| (7) |
| (8) |
| (9) |
where S1 is the area of signal 1, S2 is the area of signal 2, S3 is the area of signal 5, S4 is the area of signal 6, and S5 is the area of signal 7.
Fig. 1.
Enlargement of the 1H NMR spectral regions, (A) 1H NMR spectral signal of soybean oil, (B) 1H NMR spectral signals of Z, E- and E, E- conjugated hydroperoxides, (C) 1H NMR spectral signals of secondary oxidation products, a: (E)-2-alkenals, b: (E, E)-2,4-alkadienals, c: n-alkanals, d: 4-hydroperoxy-(E)-2-alkenals, e: 4-hydroxy-(E)-2-alkenals, f: 4,5-epoxy-(E)-2-alkenals, g: 4-oxoalkanals, (D, E) 1H NMR spectral signals of hydrolysis products.
2.9. Statistical analysis
Data were expressed as the mean ± standard deviation, using Origin 8.0 software for mapping, SAS 9.2 statistical software for variance analysis, and significance analysis (p < 0.05).
3. Results and discussion
3.1. Variations of color with frying time
The color of the oil measured by Lovibond colorimeter was shown in Fig. 2A and B. The red and yellow value of BF, AF and CK significantly increased with frying time. From 0 h of BF to 24 h of AF, the red value of SBO increased by 5.72 times and the yellow value increased by 10.87 times. The polymer produced by the thermal oxidation of the oil caused the frying oil to darken in color (Yi-Chang et al., 1996). The red values of BF and AF at 24 h were higher than CK, which possibly due to the Maillard reaction in fish cakes (Udomkun et al., 2018). The pigment produced by Maillard reaction entered into the fryer, making the oil used to fried fish cakes with a higher red value.
Fig. 2.
Variations of (A) red value, (B) yellow value, (C) PV, (D) p-AV, (E) TOTOX, (F) total polar compound, (G) DPPH· loss and (H) APB value with the frying time. Different lowercase letters indicated significant differences among samples with frying time, where different capital letters indicated significant differences among BF, AF and CK. BF – before frying, AF – after frying, CK – control.
3.2. Variations of PV, p-AV, and TOTOX with frying time
PV was used to calculate the hydroperoxides in the oil. As shown in Fig. 2C, the PV of frying oil initially increased. Then the PV decreased and tended to be stable. The reason was that hydroperoxides was unstable and tended to break down under high frying temperature (Ahmad Tarmizi et al., 2019). Thus, it’s not accurate to use only PV for the description of oil oxidation.
p-AV was used to measure the secondary oxidation products in the oil. As shown in Fig. 2D, the p-AV of frying oil significantly increased with the extension of frying time. The nonvolatile aldehydes formed by the decomposition of hydroperoxides accumulated in the frying oil. And the aldehydes developed during the secondary oxidation process were more stable than hydroperoxides (Xu et al., 2020), which resulted in the constant increase of p-AV.
The TOTOX value was a combined measurement of both PV and p-AV, which was used to assess the overall degree of oxidation in the industry (Matthäus et al., 2009). As shown in Fig. 2E, the TOTOX value of SBO increased significantly with the extension of frying time (p < 0.05). There was no significant difference among BF, AF, and CK in the early stage (0–9 h) of the frying process (p > 0.05), and the TOTOX value of the BF and AF group was significantly higher than the CK group after 12 h. The results showed that the introduction of fish cakes promoted oxidation of frying oil. Lazarick et al. (2014) evaluated components of food coatings on the stability of frying oil, they found that protein-based food material caused the greatest oil deterioration. And particles formed in the frying process may fall into the oil, resulting in oil deterioration.
3.3. Variations of TPC content with frying time
The change in TPC content was shown in Fig. 2F. The TPC content of BF, AF, and CK groups significantly increased throughout frying (p < 0.05). The TPC contents of the oil used to fry fish cakes and the control almost exceeded the safety threshold after 18 h. The initial TPC content of oil used to fry fish cakes was 10.83%, reaching 33.17% after frying fish cakes for 24 h. This was consistent with the results of other studies, in which the TPC content significantly increased with the increment of frying cycles (Houhoula et al., 2003, Li et al., 2017). However, there was no significant difference between the oil used to fry fish cakes and the control. The percentage of the weight of polar compounds might be affected by many factors. One of them could be the migration of water from the fish cakes into the frying medium diluting the polar compounds (Martínez-Yusta & Guillén, 2015). Abidi et al. (1999) reported that the addition of 1% and 2% water into the oil inhibited the formation of TGP under simulated frying conditions. However, the addition of water increased the content of DAG. Hence it could be inferred that the introduction of fish cakes change the composition of polar compounds.
3.4. Antioxidant and prooxidant balance (APB) from DPPH loss
The modified DPPH method simultaneously provided information about the oxidation product content and antioxidants. The DPPH loss in isooctane gave information about oxidation products and/or free radical scavenging antioxidants, whereas DPPH loss in methanol supplied information on the antioxidant content (Song et al., 2016). Some studies indicated that DPPH loss in isooctane is highly correlated with TPC, especially in the middle and late stages of the frying cycle (Song et al., 2016). As shown in Fig. 2G, the DPPH loss in isooctane decreased from the beginning of the frying cycle, which indicated that the antioxidant substances decomposed during the frying cycle. The reduced antioxidant capacity of the frying oil resulted in easier oxidation during frying. After 15 h of frying, the DPPH loss gradually tapered off. This was consistent with the change in TPC content. The decrease in the antioxidant capacity and accumulation of oxidative products meant that the frying oil needed to be discarded. The application of fish cakes had no significant effect on the antioxidant capacity of frying oil. This was similar to the change in TPC content. It could be inferred that the oxidation reaction that occurred in the frying medium was the main reason for the increase of the oxidation products in the frying system.
The APB value reflected the balance between the content of antioxidant and pro-oxidation products (Choi et al., 2018). The antioxidant substances represent a much lower proportion than polar compounds in the frying oil while the APB value was lower than 0.5. It meant that the frying oil was already in a highly oxidized state. Song et al. (Song et al., 2017) reported that antioxidant content decreased and oxidation products increased after multiple frying cycles, which led to a decrease in the APB value. The APB value decreased at the beginning of the frying process. After heating for 12 h, the APB value of the frying oil was lower than 0.5 (Fig. 2H). This means that the frying oil was in the late stage of oxidation.
3.5. Comparison of fatty acid composition by GC and 1H NMR
Both the GC and 1H NMR methods could be used to obtain accurate information on the change in fatty acid composition. The 1H NMR results (Table 1) demonstrated that linoleic and linolenic acids in SBO decreased by 9.66% and 2.08% respectively after being heated for 24 h. These two fatty acids contain unsaturated double bonds (Multari et al., 2019), which are prone to oxidation. The increment magnitudes of oleic and saturated fatty acids were 3.68% and 8.06%. The decrease of polyunsaturated fatty acids and the increase of monounsaturated fatty acids or saturated fatty acids might have resulted from the subsequent degradation of hydroperoxide into secondary oxidation products and thermal polymerization between fatty acids (Adjonu et al., 2019).
Table 1.
Fatty acid composition of soybean oil during frying process by 1H NMR (%).
| Time/h | Oleic acid | Linoleic acid | Linolenic acid | Saturated fatty acid | |
|---|---|---|---|---|---|
| BF | 0 | 26.15 ± 0.75Aa | 49.71 ± 0.03Ba | 6.59 ± 0.64Aa | 17.55 ± 0.07Ad |
| 6 | 26.50 ± 0.89Aa | 48.34 ± 2.18Aa | 7.07 ± 0.05Aa | 18.09 ± 1.34Bd | |
| 12 | 27.02 ± 0.47Aa | 44.81 ± 1.01Bb | 6.39 ± 0.37Aa | 21.79 ± 1.12Ac | |
| 18 | 26.30 ± 2.20Aa | 43.63 ± 0.67ABb | 4.66 ± 0.36Ab | 25.41 ± 1.16Ab | |
| 24 | 27.17 ± 1.33Ba | 38.19 ± 1.25Cc | 4.19 ± 0.11Bb | 30.45 ± 0.03Aa | |
| AF | 0 | 25.49 ± 0.25Acd | 50.52 ± 0.55Aa | 7.22 ± 0.21Aa | 17.32 ± 1.15Ad |
| 6 | 27.18 ± 2.45Abc | 46.89 ± 1.25Ab | 6.56 ± 0.32Ba | 19.36 ± 1.52Bcd | |
| 12 | 24.31 ± 0.91Bd | 48.89 ± 1.40Aab | 5.21 ± 0.49Bbc | 21.59 ± 1.82Abc | |
| 18 | 28.76 ± 0.10Aab | 42.35 ± 1.49Bc | 5.33 ± 0.70Ab | 23.56 ± 0.70Aab | |
| 24 | 29.83 ± 0.59Aa | 40.05 ± 0.20Bd | 4.51 ± 0.24ABc | 25.61 ± 0.56Ba | |
| CK | 0 | 26.15 ± 0.75Aab | 49.71 ± 0.03Ba | 6.59 ± 0.64Aa | 17.55 ± 0.07Ac |
| 6 | 25.76 ± 1.53Aab | 45.6 ± 1.10Ab | 6.21 ± 0.17Ba | 22.43 ± 0.27Ab | |
| 12 | 28.36 ± 2.01Aa | 44.33 ± 0.30Bbc | 4.99 ± 0.22Bb | 22.33 ± 1.48Ab | |
| 18 | 26.23 ± 1.87Aab | 45.50 ± 1.49Ab | 4.60 ± 0.36Ab | 23.66 ± 3.72Ab | |
| 24 | 24.15 ± 1.31Cb | 43.24 ± 0.18Ac | 4.86 ± 0.35Ab | 27.75 ± 1.85Ba | |
Note: Different lowercase letters indicated significant differences among samples with frying time, where different capital letters indicated significant differences among BF, AF and CK. BF – before frying, AF – after frying, CK – control.
The changes in fatty acid composition detected by GC were shown in Table 2. The SBO was found to contain linoleic acid (52.83%) and oleic acid (27.13%) initially, which were the two main fatty acids in SBO. The linoleic acid content decreased from 52.83% to 49.22% after 24 h of heating. The linolenic acid content decreased from 5.74% to 4.44%. Owing to the different principles of the two detection methods, the results are not the same, but the variation tendency was consistent. 1H NMR was relatively more convenient and acceptable for the qualitative analysis of fatty acid composition (Guillén and Ruiz, 2003a, Wu et al., 2021).
Table 2.
Fatty acid composition of soybean oil during frying process by GC (%).
| Time/h | 14:0 | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | 20:0 | |
|---|---|---|---|---|---|---|---|---|
| BF | 0 | 0.06 ± 0.00Bf | 10.68 ± 0.03Ai | 3.56 ± 0.01Ai | 27.13 ± 0.00Bi | 52.83 ± 0.05Aa | 5.74 ± 0.00Ba | ND |
| 3 | 0.07 ± 0.01Ade | 10.82 ± 0.00Bh | 3.61 ± 0.01Bh | 27.51 ± 0.02Ah | 52.23 ± 0.07Ab | 5.48 ± 0.11Bb | 0.27 ± 0.00Bg | |
| 6 | 0.07 ± 0.00Be | 11.01 ± 0.01Cg | 3.69 ± 0.00Bg | 27.72 ± 0.00Bg | 51.79 ± 0.01Ac | 5.44 ± 0.00ABb | 0.28 ± 0.00Bf | |
| 9 | 0.08 ± 0.01Abcd | 11.20 ± 0.01Bf | 3.75 ± 0.00Bf | 27.91 ± 0.09Bf | 51.48 ± 0.09Ad | 5.29 ± 0.01Ac | 0.28 ± 0.00Be | |
| 12 | 0.08 ± 0.00Bcde | 11.33 ± 0.00Ce | 3.83 ± 0.02Be | 28.38 ± 0.01Ae | 51.01 ± 0.01Ae | 5.08 ± 0.01Bd | 0.29 ± 0.00Bd | |
| 15 | 0.08 ± 0.00Bbcd | 11.59 ± 0.00Bd | 3.91 ± 0.00Cd | 28.80 ± 0.00Ad | 50.41 ± 0.01Af | 4.90 ± 0.01Be | 0.30 ± 0.00Cc | |
| 18 | 0.09 ± 0.00Ab | 11.79 ± 0.00Bc | 3.99 ± 0.00Bc | 29.13 ± 0.01Ac | 50.00 ± 0.01Ag | 4.70 ± 0.01Bf | 0.30 ± 0.00Bb | |
| 21 | 0.08 ± 0.00BCc | 12.00 ± 0.01Bb | 4.07 ± 0.00Ab | 29.50 ± 0.00Ab | 49.52 ± 0.02Bh | 4.52 ± 0.01Cg | 0.30 ± 0.00Cb | |
| 24 | 0.10 ± 0.01Aa | 12.26 ± 0.03Aa | 4.22 ± 0.02Aa | 29.95 ± 0.04Aa | 48.86 ± 0.09Bi | 4.30 ± 0.01Ch | 0.31 ± 0.00Ba | |
| AF | 0 | 0.06 ± 0.00Ai | 10.68 ± 0.00Ai | 3.53 ± 0.01Bi | 27.15 ± 0.01Ai | 52.52 ± 0.01Ba | 5.78 ± 0.01Aa | 0.27 ± 0.00Ag |
| 3 | 0.07 ± 0.00Ah | 10.83 ± 0.01Bh | 3.61 ± 0.00Bh | 27.44 ± 0.01Ah | 52.16 ± 0.02Ab | 5.62 ± 0.01Ab | 0.27 ± 0.00Bf | |
| 6 | 0.07 ± 0.00Bg | 11.03 ± 0.00Bg | 3.69 ± 0.00Bg | 27.73 ± 0.00Ag | 51.77 ± 0.01Bc | 5.43 ± 0.02Bc | 0.28 ± 0.00Be | |
| 9 | 0.08 ± 0.00Af | 11.18 ± 0.01Cf | 3.76 ± 0.01Bf | 28.02 ± 0.00Af | 51.41 ± 0.01Ad | 5.27 ± 0.01Bd | 0.28 ± 0.00Be | |
| 12 | 0.08 ± 0.00Be | 11.35 ± 0.00Be | 3.84 ± 0.00Be | 28.39 ± 0.00Ae | 50.96 ± 0.01Be | 5.09 ± 0.01Be | 0.29 ± 0.00Bd | |
| 15 | 0.08 ± 0.00Ad | 11.63 ± 0.02Ad | 3.93 ± 0.00Bd | 28.78 ± 0.01Bd | 50.41 ± 0.01Af | 4.88 ± 0.00Cf | 0.30 ± 0.00Bc | |
| 18 | 0.09 ± 0.00Bc | 11.82 ± 0.01Ac | 4.00 ± 0.00Bc | 29.13 ± 0.00Ac | 49.98 ± 0.01Bg | 4.69 ± 0.01Bg | 0.30 ± 0.00Cc | |
| 21 | 0.09 ± 0.00Ab | 12.03 ± 0.00Ab | 4.08 ± 0.02Ab | 29.51 ± 0.02Ab | 49.46 ± 0.00Ch | 4.53 ± 0.01Bh | 0.31 ± 0.00Bb | |
| 24 | 0.09 ± 0.00ABa | 12.25 ± 0.00Aa | 4.19 ± 0.00ABa | 29.89 ± 0.01Ba | 48.93 ± 0.00Bi | 4.33 ± 0.01Bi | 0.32 ± 0.00Aa | |
| CK | 0 | 0.06 ± 0.00Bi | 10.68 ± 0.03Ai | 3.56 ± 0.01Ag | 27.13 ± 0.00Bi | 52.83 ± 0.05Aa | 5.74 ± 0.00Ba | ND |
| 3 | 0.07 ± 0.00Ah | 10.93 ± 0.02Ah | 3.80 ± 0.08Af | 27.30 ± 0.09Bh | 51.93 ± 0.06Bb | 5.67 ± 0.04Ab | 0.29 ± 0.01Ac | |
| 6 | 0.07 ± 0.00Ag | 11.12 ± 0.01Ag | 3.82 ± 0.01Aef | 27.70 ± 0.01Cg | 51.54 ± 0.00Cc | 5.45 ± 0.00Ac | 0.30 ± 0.00Ac | |
| 9 | 0.08 ± 0.00Af | 11.25 ± 0.00Af | 3.85 ± 0.00Ae | 28.04 ± 0.01Af | 51.22 ± 0.01Bd | 5.28 ± 0.00ABd | 0.29 ± 0.00Ac | |
| 12 | 0.08 ± 0.00Ae | 11.40 ± 0.00Ae | 3.91 ± 0.01Ad | 28.36 ± 0.01Be | 50.84 ± 0.00Ce | 5.11 ± 0.00Ae | 0.30 ± 0.00Ac | |
| 15 | 0.08 ± 0.00Bd | 11.58 ± 0.01Bd | 3.98 ± 0.00Ac | 28.73 ± 0.01Cd | 50.40 ± 0.01Af | 4.93 ± 0.01Af | 0.31 ± 0.00Ab | |
| 18 | 0.08 ± 0.00Cc | 11.71 ± 0.01Cc | 4.01 ± 0.01Ac | 29.07 ± 0.01Bc | 49.99 ± 0.01Ag | 4.81 ± 0.00Ag | 0.31 ± 0.00Aab | |
| 21 | 0.09 ± 0.00Bb | 11.90 ± 0.01Cb | 4.08 ± 0.00Ab | 29.31 ± 0.01Bb | 49.68 ± 0.01Ah | 4.63 ± 0.00Ah | 0.31 ± 0.00Aab | |
| 24 | 0.09 ± 0.00Ba | 12.08 ± 0.00Ba | 4.17 ± 0.02Ba | 29.69 ± 0.00Ca | 49.22 ± 0.02Ai | 4.44 ± 0.00Ai | 0.32 ± 0.00Aa | |
Note: Different lowercase letters indicated significant differences among samples with frying time, where different capital letters indicated significant differences among BF, AF and CK. BF – before frying, AF – after frying, CK – control.
3.6. Oxidation and hydrolysis products by 1H NMR
The changes in the oxidation products of SBO during frying were given in Table 3. The primary oxidation products were mostly Z, E- and E, E- conjugated hydroperoxides. The Z, E- conjugated hydroperoxides were between 6.5 and 6.6 ppm, and E, E- conjugated hydroperoxides were between 6.2 and 6.3 ppm (Fig. 1B) (Goicoechea & Guillén, 2010). The secondary oxidation products were between 9.5 and 9.8 ppm. Fig. 1C showed the 1H NMR spectra of six types of secondary oxidation products, mostly aldehydes such as (E)-2-alkenals, (E, E)-2,4-alkadienals, n-alkanals, 4-hydroxy-(E)-2-alkenals, 4-hydroperoxy-(E)-2-alkenals, and 4,5-epoxy-(E)-2-alkenals (Goicoechea & Guillén, 2010). The concentrations of hydroperoxides were calculated based on the area of these peaks. Z, E- and E, E- conjugated hydroperoxides increased in concentration during frying. The hydroperoxide decomposes into low molecular weight aldehydes and ketones, increasing secondary oxidation. The main aldehydes found were (E)-2-alkenals, (E, E)-2,4-alkadienals, and n-alkanals. These aldehydes were not observed in fresh SBO. As frying proceeded, the concentrations of these aldehydes began to increase due to the oxidation of SBO under high-temperature treatments. The secondary oxidation of SBO resulted in a large quantity of non-volatile aldehydes (Jiang et al., 2018).
Table 3.
Oxidation products of soybean oil during frying process by 1H NMR (mmol/mol oil).
| Primary oxidation products |
Secondary oxidation products |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time/h | E, E conjugated | Z, E conjugated | (E)-2-alkenals | (E, E)-2,4-alkadienals | n-alkanals | 4-hydroxy-(E)-2-alkenals + 4-hydroperoxy-(E)-2-alkenals | 4,5-epoxy-(E)-2-alkenals | 4-oxoalkanals | |
| BF | 0 | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 6.38 ± 0.20Bd | ND | 1.78 ± 0.01Cd | 3.10 ± 0.07Bc | 1.96 ± 0.04Bd | 0.75 ± 0.09Bc | 0.27 ± 0.14Bc | ND | |
| 12 | 11.07 ± 0.19Ac | ND | 3.44 ± 0.12Bc | 4.80 ± 0.29ABb | 3.78 ± 0.15Bc | 0.87 ± 0.03Bbc | 0.47 ± 0.12Abc | 0.24 ± 0.02Ab | |
| 18 | 13.15 ± 0.97Ab | 2.34 ± 0.02Ab | 5.15 ± 0.49Ab | 6.41 ± 0.81A a | 5.66 ± 0.64Bb | 1.07 ± 0.16Bab | 0.58 ± 0.13Ab | 0.40 ± 0.05Ab | |
| 24 | 14.36 ± 0.43Aa | 2.71 ± 0.20Aa | 6.78 ± 0.58Aa | 6.26 ± 0.32Aa | 8.32 ± 0.00Aa | 1.12 ± 0.11Ba | 0.82 ± 0.07Aa | 0.83 ± 0.27Aa | |
| AF | 0 | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 6.54 ± 0.53Bc | ND | 1.99 ± 0.04Bd | 3.23 ± 0.15Bb | 1.98 ± 0.05Bd | 0.59 ± 0.05Cb | 0.52 ± 0.06Aa | ND | |
| 12 | 10.79 ± 0.97Ab | ND | 4.23 ± 0.45Ac | 4.04 ± 0.96Bb | 3.62 ± 0.13Bc | 1.22 ± 0.28Aa | 0.55 ± 0.12Aa | 0.21 ± 0.00Ac | |
| 18 | 11.23 ± 1.29Bb | 1.11 ± 0.00Bb | 5.41 ± 0.43Ab | 6.01 ± 0.68Aa | 6.46 ± 0.03Ab | 1.06 ± 0.11Ba | 0.53 ± 0.02Aa | 0.30 ± 0.03Bb | |
| 24 | 15.66 ± 2.16Aa | 1.97 ± 0.05ABa | 7.12 ± 0.78Aa | 6.09 ± 0.43Aa | 8.18 ± 0.00Aa | 1.09 ± 0.13Ba | 0.61 ± 0.07Ba | 0.56 ± 0.03Aa | |
| CK | 0 | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 7.67 ± 0.04Ac | ND | 2.11 ± 0.02Ad | 3.71 ± 0.19Ac | 2.24 ± 0.20Ad | 0.93 ± 0.06Ac | 0.18 ± 0.10Bb | ND | |
| 12 | 9.83 ± 0.77Ab | 0.55 ± 0.13Ab | 3.06 ± 0.30Bc | 5.80 ± 0.08Ab | 4.84 ± 0.04Ac | 1.47 ± 0.11Ab | 0.22 ± 0.00Bb | 0.11 ± 0.04Bc | |
| 18 | 14.52 ± 0.24Aa | 2.24 ± 0.36Aa | 5.25 ± 0.13Ab | 6.17 ± 0.28Aab | 6.37 ± 0.14ABb | 1.80 ± 0.04Aa | 0.26 ± 0.01Bb | 0.33 ± 0.04ABb | |
| 24 | 14.91 ± 0.34Aa | 1.55 ± 0.78Bab | 6.71 ± 0.04Aa | 6.50 ± 0.55Aa | 7.21 ± 0.49Ba | 1.94 ± 0.16Aa | 0.36 ± 0.04Ca | 0.67 ± 0.03Aa | |
Note: Different lowercase letters indicated significant differences among samples with frying time, where different capital letters indicated significant differences among BF, AF and CK. BF – before frying, AF – after frying, CK – control.
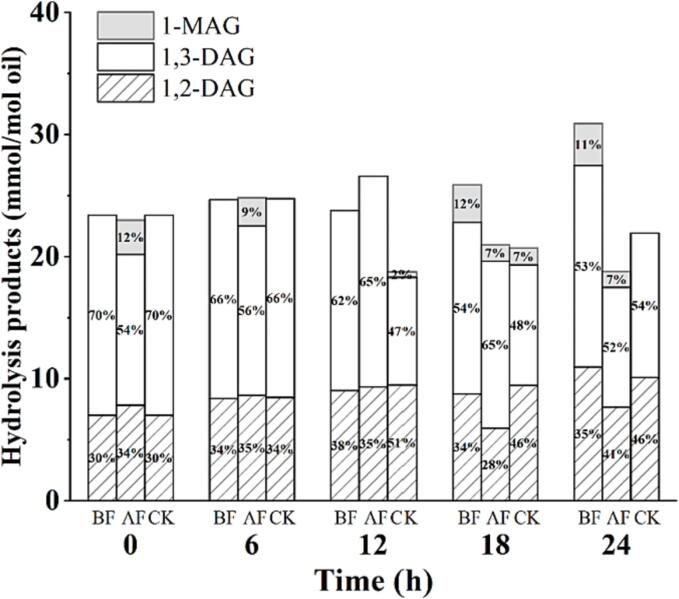
The hydrolysis of oil mainly led to the formation of DAG, MAG, and free fatty acids (Dobarganes et al., 2000). The hydrolysis products of SBO of 1,2-DAG, 1,3-DAG, and 1-MAG were also simultaneously detected through 1H NMR (Fig. 3). There was no significant difference in the generation of hydrolytic products among BF, AF and CK, and 1-MAG hardly formed during the frying process. Some other studies also found that hydrolysis products were minor products during the frying process (Arroyo et al., 1995, Marmesat et al., 2008, Sebedio et al., 1990). This may be due to the incomplete hydrolysis of SBO under frying conditions. Additionally, oxidation was faster than hydrolysis in the oil system. The further oxidation of the hydrolysis products resulted in the formation of polymers with higher molecular weight (Kmiecik et al., 2018, Rodriguez et al., 2021).
Fig. 3.
Hydrolysis products of soybean oil during the frying process. BF – before frying, AF – after frying, CK – control.
4. Conclusions
In the present study, the oxidation process of SBO during deep-frying of fish cakes at 180℃ was investigated. According to the result of the modified DPPH method, the low APB value (<0.5) indicated that the oil was highly oxidized after being heated for 12 h. And, TPC content was 27.67% after continuous frying for 18 h, reached the maximum limitation of 27%. The significant decrease of linolenic and linoleic acids, together with the increase of TOTOX value, suggested the accumulation of oxidation products during the frying process. The application of fish cakes promoted the oxidation of frying oil, but it had no significant effect on the change of TPC content. (E)-2-alkenals, (E, E)-2,4-alkadienals, and n-alkanals were the most retained aldehyde, and were related to the heating time of frying oil. The degradation of hydroperoxides prompted the accumulation of nonvolatile aldehydes. Further studies are required to comprehensively elaborate on the effect of different substrate foods on the oxidation process of frying oil.
Role of funding sources
This work was supported by the National Key R&D Program of China, Grant/Award Number: 2018YFD0901005 and 2021YFD1600502.
CRediT authorship contribution statement
Benlun Hu: Conceptualization, Methodology, Writing – original draft. Runlin Wu: Validation, Data curation. Jingwen Sun: Investigation, Software. Haonan Shi: Investigation, Validation. Caihua Jia: Supervision, Resources, Writing – review & editing. Ru Liu: Validation. Jianhua Rong: Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Caihua Jia, Email: chjia@mail.hzau.edu.cn.
Ru Liu, Email: liuru@mail.hzau.edu.cn.
Data availability
Data will be made available on request.
References
- Abidi S.L., Kim I.H., Rennick K.A. Determination of nonvolatile components of heated soybean oils separated with high-efficiency mixed-bed polystyrene/divinylbenzene columns. Journal of the American Oil Chemists Society. 1999;76(8):939–944. [Google Scholar]
- Adjonu R., Zhou Z., Prenzler P.D., Ayton J., Blanchard C.L. Different processing practices and the frying life of refined canola oil. Foods. 2019;8(11):527. doi: 10.3390/foods8110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Tarmizi A.H., Abd Razak R.A., Abdul Hammid A.N., Kuntom A. Effect of anti-clouding agent on the fate of 3-monochloropropane-1,2-diol esters and glycidyl esters in palm olein during repeated frying. Molecules. 2019;24(12):2332. doi: 10.3390/molecules24122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOCS. (2013). Official methods and recommended practices (6 ed.). Urbana, IL: American Oil Chemists' Society (Method Cd 18-90).
- AOCS. (2017). Official methods and recommended practices (7 ed.). Urbana, IL: American Oil Chemists' Society (Method Cd 13e-92).
- Arroyo R., Cuesta C., Sanchezmontero J.M., Sanchezmuniz F.J. High-performance size-exclusion chromatography of palm olein used for frying. Fett-Lipid. 1995;97(7–8):292–296. [Google Scholar]
- Chen W.-A., Chiu C., Cheng W.-C., Hsu C.-K., Kuo M.-I. Total polar compounds and acid values of repeatedly used frying oils measured by standard and rapid methods. Journal of Food and Drug Analysis. 2013;21:58–65. [Google Scholar]
- Chew S.C., Tan C.P., Nyam K.L. Optimization of bleaching parameters in refining process of kenaf seed oil with a central composite design model. Journal of Food Science. 2017;82(7):1622–1630. doi: 10.1111/1750-3841.13758. [DOI] [PubMed] [Google Scholar]
- Choi H., Kim M.-J., Lee J. Effect of polar and non-polar compounds from oxidized oils on oxidative stability in corn oil. European Journal of Lipid Science and Technology. 2018;120(3):1700312. [Google Scholar]
- Dobarganes C., Márquez-Ruiz G., Velasco J. Interactions between fat and food during deep-frying. European Journal of Lipid Science and Technology. 2000;102(8–9):521–528. [Google Scholar]
- Goicoechea E., Guillén M.D. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100 degrees C. Journal of Agricultural and Food Chemistry. 2010;58(10):6234–6245. doi: 10.1021/jf1005337. [DOI] [PubMed] [Google Scholar]
- Guillén M.D., Ruiz A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. European Journal of Lipid Science and Technology. 2003;105(9):502–507. [Google Scholar]
- Guillén M.D., Ruiz A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. European Journal of Lipid Science and Technology. 2003;105(11):688–696. [Google Scholar]
- Houhoula D.P., Oreopoulou V., Tzia C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. Journal of the Science of Food and Agriculture. 2003;83(4):314–319. [Google Scholar]
- Hwang H.S., Ball J.C., Doll K.M., Anderson J.E., Vermillion K. Investigation of polymers and alcohols produced in oxidized soybean oil at frying temperatures. Food Chemistry. 2020;317 doi: 10.1016/j.foodchem.2020.126379. [DOI] [PubMed] [Google Scholar]
- Hwang H.S., Winkler-Moser J.K., Doll K.M., Gadgil M., Liu S.X. Factors affecting antioxidant activity of amino acids in soybean oil at frying temperatures. European Journal of Lipid Science and Technology. 2019;121(7) [Google Scholar]
- Jia C., Zhang M., Ma W., Li J., Zhao S., Xiong S.…Li X. Evaluation of antioxidant properties of the different tissues of vine tea (Ampelopsis grossedentata) in stripped canola oil and sunflower oil. Journal of Food Science. 2020;85(4):1082–1089. doi: 10.1111/1750-3841.15092. [DOI] [PubMed] [Google Scholar]
- Jiang X., Huang R., Wu S., Wang Q., Zhang Z. Correlations between 1H NMR and conventional methods for evaluating soybean oil deterioration during deep frying. Journal of Food Measurement and Characterization. 2018;12(2):1420–1426. [Google Scholar]
- Kmiecik D., Kobus-Cisowska J., Kulczyński B. Thermal decomposition of partially hydrogenated rapeseed oil during repeated frying traditional and fast fries. Journal of the American Oil Chemists' Society. 2018;95(4):473–483. [Google Scholar]
- Lazarick K., Aladedunye F., Przybylski R. Effect of breading and battering ingredients on performance of frying oils. European Journal of Lipid Science and Technology. 2014;116(6):763–770. [Google Scholar]
- Li T., Niu L., Li X., Wang F., Huang Y., Liu Y. Formation of advanced glycation end-products in silver carp (Hypophthalmichthys molitrix) surimi products during heat treatment as affected by freezing-thawing cycles. Food Chemistry. 2022;395 doi: 10.1016/j.foodchem.2022.133612. [DOI] [PubMed] [Google Scholar]
- Li X., Li J., Wang Y., Cao P., Liu Y. Effects of frying oils' fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chemistry. 2017;237:98–105. doi: 10.1016/j.foodchem.2017.05.100. [DOI] [PubMed] [Google Scholar]
- Li X., Wu G., Wu Y., Karrar E., Huang J., Jin Q.…Wang X. Effectiveness of the rapid test of polar compounds in frying oils as a function of environmental and compositional variables under restaurant conditions. Food Chemistry. 2020;312 doi: 10.1016/j.foodchem.2019.126041. [DOI] [PubMed] [Google Scholar]
- Li X., Wu G., Yang F., Meng L., Huang J., Zhang H.…Wang X. Influence of fried food and oil type on the distribution of polar compounds in discarded oil during restaurant deep frying. Food Chemistry. 2019;272:12–17. doi: 10.1016/j.foodchem.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Marmesat S., Velasco L., Ruiz-Mendez M.V., Fernandez-Martinez J.M., Dobarganes C. Thermostability of genetically modified sunflower oils differing in fatty acid and tocopherol compositions. European Journal of Lipid Science and Technology. 2008;110(8):776–782. [Google Scholar]
- Martínez-Yusta A., Guillén M.D. Monitoring compositional changes in sunflower oil-derived deep-frying media by 1H Nuclear Magnetic Resonance. European Journal of Lipid Science and Technology. 2015;118(7):984–996. [Google Scholar]
- Matthäus B., Haase N.U., Unbehend G. Chemical and sensory characteristics of products fried in high-oleic, low-linolenic rapeseed oil. Journal of the American Oil Chemists' Society. 2009;86(8):799–808. [Google Scholar]
- Multari S., Marsol-Vall A., Heponiemi P., Suomela J.P., Yang B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Research International. 2019;122:318–329. doi: 10.1016/j.foodres.2019.04.026. [DOI] [PubMed] [Google Scholar]
- Rodriguez G., Squeo G., Estivi L., Quezada Berru S., Buleje D., Caponio F.…Hidalgo A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sacha inchi (Plukenetia volubilis) oil during French fries deep-frying. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.127942. [DOI] [PubMed] [Google Scholar]
- Saguy I.S., Dana D. Integrated approach to deep fat frying: Engineering, nutrition, health and consumer aspects. Journal of Food Engineering. 2003;56(2):143–152. [Google Scholar]
- Sebedio J.L., Bonpunt A., Grandgirard A., Prevost J. Deep fat frying of frozen prefried French fries: Influence of the amount of linolenic acid in the frying medium. Journal of Agricultural and Food Chemistry. 1990;38(9):1862–1867. [Google Scholar]
- Shantha N.C., Decker E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. Journal of Aoac International. 1994;77(2):421–424. [PubMed] [Google Scholar]
- Song J., Jang E.Y., Kim M.-J., Kim Y.-J., Lee J. Development of a spectroscopic method to determine the content of free radical scavenging compounds and oxidation products in thermally oxidised oils. International Journal of Food Science and Technology. 2016;51(11):2424–2432. [Google Scholar]
- Song J., Kim M.J., Kim Y.J., Lee J. Monitoring changes in acid value, total polar material, and antioxidant capacity of oils used for frying chicken. Food Chemistry. 2017;220:306–312. doi: 10.1016/j.foodchem.2016.09.174. [DOI] [PubMed] [Google Scholar]
- Udomkun P., Innawong B., Siasakul C., Okafor C. Utilization of mixed adsorbents to extend frying oil life cycle in poultry processing. Food Chemistry. 2018;248:225–229. doi: 10.1016/j.foodchem.2017.12.070. [DOI] [PubMed] [Google Scholar]
- Wu G.C., Han S.Y., Li X., Karrar E., Xu L.R., Jin Q.Z.…Wang X.G. Effect of the phenolic extract of Camellia oleifera seed cake on the oxidation process of soybean oil by 1H nuclear magnetic resonance during frying. LWT - Food Science and Technology. 2021;150 [Google Scholar]
- Xu L., Yang F., Li X., Zhao C., Jin Q., Huang J., Wang X. Kinetics of forming polar compounds in frying oils under frying practice of fast food restaurants. LWT - Food Science and Technology. 2019;115 [Google Scholar]
- Xu L., Zhang Y., Gong M., Huang J., Jin Q., Wang X., Wang X. Change of fatty acid esters of MCPD and glycidol during restaurant deep frying of fish nuggets and their correlations with total polar compounds. International Journal of Food Science & Technology. 2020;55(7) [Google Scholar]
- Yamsaengsung R., Moreira R.G. Modeling the transport phenomena and structural changes during deep fat frying: Part II: Model solution & validation. Journal of Food Engineering. 2002;53(1):11–25. [Google Scholar]
- Yi-Chang T., Moreira R., Sun X. Total frying-use time effects on soybean-oil deterioration and on tortilla chip quality. International Journal of Food Science & Technology. 1996;31(3):287–294. [Google Scholar]
- Zhou X., Chen Y., Yang Q., Liu Y., Wu Y., Lu R., Ni Z. Optimization of total polar compounds quantification in frying oils by low-field nuclear magnetic resonance. Analytical Sciences. 2019;35(12):1381–1384. doi: 10.2116/analsci.19P268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.