Highlights
-
•
Only Cys containing peptides showed good DPPH· scavenging activity.
-
•
Only Tyr containing peptides showed significant ABTS·+ scavenging activity.
-
•
DPPH/ABTS scavenging correlated to reducing power/oxidation inhibition respectively.
-
•
Peptides containing both Cys and Tyr had strong in vitro and cellular antioxidation.
-
•
Antioxidation evaluation should use indexes with different action mechanisms.
Keywords: Abalone viscera, Antioxidant peptide, DPPH radical scavenging activity, ABTS radical scavenging activity, Cell damage, Structure-activity relationship
Abstract
The in vitro antioxidation and cytoprotection of abalone visceral peptides against oxidative damage were investigated. Results show that the DPPH· scavenging activities of the 16 chemically synthesized peptides were significantly and positively correlated with their reducing power. Their scavenging activities against ABTS·+ were positively correlated with their ability to inhibit linoleic acid oxidation. Only Cys containing peptides exhibited good DPPH· scavenging activity, while only Tyr containing peptides showed significant ABTS·+ scavenging activity. In the cytoprotection assay, all four representative peptides significantly increased the viability of H2O2-damaged LO2 cells and the activities of GSH-Px, CAT, and SOD, and all decreased MDA levels and LDH leakage, in which the Cys-containing peptides were more effective at increasing the activities of antioxidant enzymes, while the Tyr-containing peptides were more effective at decreasing MDA levels and LDH leakage. Abalone visceral peptides containing both Cys and Tyr exhibit strong in vitro and cellular antioxidation.
1. Introduction
Bioactive peptides are composed of two to dozens of amino acids, having a relative molecular weight of less than 6 kDa (Dominguez-Perez, Beltran-Barrientos, Gonzalez-Cordova, Hernandez-Mendoza, & Vallejo-Cordoba, 2020). Bioactive peptides have many biological activities such as antioxidant (Wen, Zhang, Feng, Duan, Ma, & Zhang, 2020), antihypertensive (Sitanggang, Lesmana, & Budijanto, 2020), and anti-cancer (Cakir & Tunali-Akbay, 2021) properties. Bioactive peptides play important roles in the regulation of human metabolism and other vital activities and have great prospects for development in the fields of food and medicine (Gorguc et al., 2020, Xiong et al., 2022). Food-derived antioxidative peptides are considered to be safe and economical and contain healthy compounds with stable structures and easy absorption (Yang et al., 2021). The antioxidant effect of amino acids was first reported by Marcuse in 1960 (Marcuse, 1960), and since then, the antioxidant activities of amino acids and peptides have been widely studied. Many studies have demonstrated the potential of in vitro enzymatic digestion of marine biological protein resources as a way to produce antioxidant peptides (Majura et al., 2022).
China’s abalone (genus Haliotis, family Haliotidae, order Archaeogastropoda) aquaculture and consumption are the largest in the world, with an annual production of 20.3 × 104 t in 2020 (The People's Republic of China Ministry of Agriculture, 2021). Abalone viscera accounts for about 15–25 % of the total body weight of abalone (Zhou et al., 2012) and is often discarded during processing. Abalone viscera are rich in protein which can be used as precursors of bioactive peptides (Zhou et al., 2016). Previous studies have shown that abalone viscera protein hydrolysate have antioxidant (Hu, Yang, He, Wei, Wu, Xiong, et al., 2022), anti-inflammatory (Suleria, Addepalli, Masci, Gobe, & Osborne, 2017), and antitumor (Chen, Wei, Ye, Chen, & Weng, 2018) effects, and some iron-chelating peptides have been identified from the abalone viscera hydrolysates, which might be used as ingredients in function foods (Wu, Jia, Wen, Yu, Zhao, & Hu, 2021). However, these studies only involved the activities of the hydrolysates and small peptide mixtures isolated from visceral protein, which were not deep enough. In order to develop new methods for the utilization of these abalone viscera protein resources, we isolated and identified 16 peptides with potential antioxidant activity from abalone viscera protein hydrolysates in our previous study (Hu, et al., 2022). In this paper, the 16 abalone viscera peptides previously discovered were chemically synthesized. Their free radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,20-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), reducing power, and ability to inhibit linoleic acid oxidation were used as evaluation indexes to compare the in vitro antioxidant ability of each peptide. The peptides with strong in vitro antioxidant activity were selected for further analysis, specifically with regard to their ability to inhibit H2O2-induced oxidative damage of human LO2 hepatocytes. On this basis, the correlation among the evaluation indexes of in vitro antioxidant activity and the influence of amino acid composition on the antioxidant activity of the peptides were investigated, providing a reference for the industrial development of abalone viscera peptides.
2. Materials and methods
2.1. Materials
Sixteen peptides were previously identified from abalone viscera protein hydrolysate. These peptides were fractionated by a combination of Sephadex G-15 and Toyopearl HW-40F chromatography and were further purified by reversed-phase high-performance liquid chromatography (Hu, et al., 2022). Their amino acid sequences were determined to be: METY, YHGF, QCVR, QSCARF, AAPAVSGR, NRFGVSR, PVPPYKA, AAQYSRN, VHAEPTK, GCYVPKC, NSHVVR, AANNSTR, TIDCDR, CIGYDR, DDITRD, and DVAFMR. They were chemically synthesized by Shanghai Ease-Bio Technology Co., Ltd. (Shanghai, China). The purity of the synthesized peptides was over 90 %. LO2 cells were purchased from China Pharmaceutical University.
Chemical reagents: DPPH, linoleic acid, and ammonium thiocyanate were purchased from Sigma Chemical Co. (St. Louis, USA). Other chemicals used in the experiments were of analytical grade.
2.2. Determination of scavenging activity against DPPH free radicals
The DPPH free radicals (DPPH·) scavenging activities were determined according to the previously reported method (Hu, et al., 2022). 2 mL peptide solutions (1 mg/mL) were mixed with 2 mL DPPH solution (50 mg/L in absolute ethanol). The mixtures were incubated for 30 min at room temperature. Then, the absorbance of the mixture was measured at 517 nm. For the blank group, 2 mL peptide solutions were mixed with 2 mL of absolute ethanol. For the control group, 2 mL of DPPH solution was mixed with 2 mL of absolute ethanol. The scavenging activity was calculated as follows: scavenging activity (%) = 1 - [(Asample - Ablank) / Acontrol] × 100 %, where Acontrol means the absorbance of the control reaction, Ablank means the absorbance of the blank reaction, and Asample means the absorbance of the peptide sample reaction.
The IC50 value defined as the concentration of peptide required to scavenge 50 % of DPPH· was determined from the peptide scavenge activity curve equation by measuring the DPPH· scavenge activity at different concentrations of a peptide.
2.3. Determination of scavenging activity against ABTS free radicals
ABTS free radicals (ABTS·+) scavenging activity was measured using a Total Antioxidant Capacity Test Kit (T-AOC) (Nanjing jiancheng bioengineering institute, Nanjing, China) according to the kit instructions, at a sample concentration of 1 mg/mL. The determination of IC50 values of the peptides for scavenging ABTS·+ was similar to those for DPPH·.
2.4. Reducing power determination
The reducing powers of the abalone viscera peptides were measured according to Deng’s method (Deng et al., 2021), modified as follows. 1 mL peptide solutions were mixed with 1 mL 0.2 mol/L phosphate buffer solution (pH 6.6) and 1 mL 1 % (quality fraction) potassium ferricyanide, and the mixture reacted at 50 °C for 20 min. After incubation, 1 mL 10 % (v/v) trichloroacetic acid was added. The solution was then centrifuged. 2 mL supernatant was mixed with 0.4 mL 0.1 % (w/v) ferric chloride and 2 mL distilled water, and the mixture was incubated at room temperature for 10 min. Finally, the absorbance of the mixture was measured at 700 nm.
2.5. Determination of inhibition activity against linoleic acid oxidation
The inhibition of linoleic acid oxidation was measured using the method described by Ben Yakoub et al. (Ben Yakoub et al., 2020) with slight modification. The synthetic peptides were dissolved in 0.05 mol/L sodium phosphate buffer (pH 7.0) to prepare 0.5 mg/mL peptide solutions. 1 mL peptide solution was added to 1 mL 1.5 % (v/v) linoleic acid solution (in 95 % ethanol), and the mixture was diluted with distilled water to a final volume of 2.5 mL. For the control assay, 1 mL sodium phosphate buffer was added to the linoleic acid solution. The mixture was kept at 50 ± 1 °C in the dark for 144 h. 0.1 mL assay solution was mixed with 4.7 mL 75 % ethanol, 0.1 mL 30 % (w/v) ammonium thiocyanate and 0.1 mL 0.02 mol/L ferrous chloride that was dissolved in HCl. The absorbance was measured at 500 nm after 3 min incubation. The inhibition of linoleic acid oxidation was calculated as follows: Inhibition of linoleic acid oxidation (%) = [(A1-A2) / (A3-A4)] × 100 %, where A1 is the absorbance of the control reaction at 144 h, A2 is the absorbance of the peptide sample reaction at 144 h, A3 is the absorbance of the control reaction at 0 h, and A4 is the absorbance of the peptide sample reaction at 0 h.
2.6. Effect of abalone viscera peptides on LO2 cell viability
LO2 cells (1 × 105 /mL) in the logarithmic phase were seeded and incubated in 96 well plates at 37 °C for 24 h in a 5 % CO2 incubator. The supernatant (culture medium) was then removed. Synthetic peptide sample solutions at different concentrations were added as the experimental samples. The concentrations of peptide solutions were 0.001, 0.01, 0.1, 0.25, 0.5, 1, and 2 mg/mL, respectively. The medium was added instead of the peptide solution to create the control samples, and the blank samples consisted of medium only. After 24 h of incubation, the supernatant was discarded and 20 µL of 3-(4,5-dimethyl-2-thiazolyl)-2,5diphenyl-2-H-tetrazolium bromide (MTT) was added. After 4 h of incubation, 150 µL of dimethyl sulfoxide was added, followed by shaking for 5 min, and finally the absorbance of the mixture was measured at 490 nm. Cell viability was calculated as follows: Cell viability (%) = (ODsample – ODblank) / (ODcontrol – ODblank) × 100, where ODsample, ODcontrol, and ODblank represent the absorbance values of the peptide sample, control, and blank groups, respectively.
2.7. Cytoprotective effect of the abalone viscera peptides against oxidative damage induced by H2O2
LO2 cells were cultured according to the procedure in section 2.6. 100 µL of the solution containing synthetic peptide at different concentrations (0.1 mg/mL, 0.25 mg/mL, 0.5 mg/mL, 1 mg/mL) was added to LO2 cells as the experimental samples. For both the damage model and the controls, 100 µL medium was added. After 24 h of incubation, the supernatant was discarded. 100 μL 4 mmol/L H2O2 solution was added to both the experimental group and the damage model group, and 100 μL medium was added to the blank group. The cell viability was measured by the MTT method after 2 h of incubation. The calculation formula is the same as that used in section 2.6, above.
2.8. Determination of cellular antioxidant activity
LO2 cells (5 × 105 /mL) in the logarithmic phase were seeded and incubated in 6 well plates at 37℃ for 24 h in a 5 % CO2 incubator. Thereafter, the supernatant (culture medium) was discarded. The synthetic peptide solution was added to the cell precipitate for the experimental samples, vitamin C (Vc) for the positive control samples, and the medium for the negative control samples and H2O2 damage model group. After 24 h of incubation, the supernatant (culture medium) was removed. 1.5 mL 4 mmol/L H2O2 solution was added to the damage model group, experimental samples and positive control samples. 1.5 mL medium was added to the negative control samples. The mixture was incubated for 2 h. The culture solution was then collected and used for measuring the lactate dehydrogenase (LDH) content. The cells were centrifuged (600 r/min, 10 min, 4℃) to collect cell precipitate after 0.25 % trypsin digestion, and then 1 mL phosphate buffered saline (PBS) was added for cell ultrasonic disruption in an ice bath. After centrifugation (10000 r/min, 10 min, 4℃), the supernatant was taken to determine the contents of glutathione peroxidase (GSH-Px), catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA). Data calculation for each index was expressed as cellular protein content. All of the indexes were measured by assay kits (Nanjing jiancheng bioengineering institute, Nanjing, China) according to kit instructions.
2.9. Statistical analysis
The experimental data were statistically analyzed using SPSS 17.0 software. The Duncan method was used for group comparison. The correlations were analyzed by Pearson coefficients.
3. Results
3.1. In vitro antioxidant activity of abalone viscera peptides and its relationship to amino acid composition
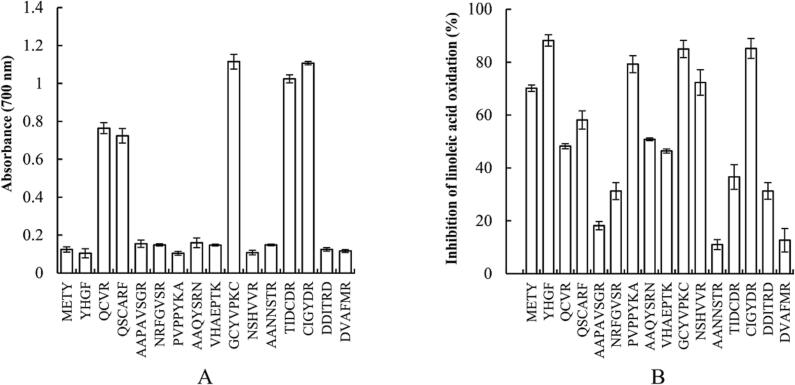
The scavenging activity of the peptides against DPPH· and ABTS·+ is shown in Fig. 1, with a peptide concentration of 1 mg/mL. All the Cys containing peptides (QCVR, QSCARF, GCYVPKC, TIDCDR, and CIGYDR) showed strong DPPH· scavenging activity, with IC50 values of 0.392 mg/mL, 0.416 mg/mL, 0.405 mg/mL, 0.267 mg/mL, and 0.207 mg/mL, respectively. The scavenging activity of peptides without Cys was not obvious, at less than 20 %. All the Tyr containing peptides (METY, YHGF, PVPPYKA, AAQYSRN, GCYVPKC and CIGYDR) showed strong ABTS·+ scavenging ability, with IC50 values of 1.200 mg/mL, 0.268 mg/mL, 0.860 mg/mL, 0.804 mg/mL, 0.389 mg/mL, and 0.144 mg/mL, respectively. The ABTS·+ scavenging ability of QCVR, QSCARF and TIDCDR, which contained Cys but not Tyr, ranged from 20 % to 40 %, and was clearly lower than that of the Tyr containing peptides. Meanwhile, the scavenging activity of the peptides without Cys or Tyr was not obvious, at less than 20 %. The peptides GCYVPKC and CIGYDR, which contain both Cys and Tyr, had strong scavenging activities against both DPPH· and ABTS·+.
Fig. 1.
Free radical scavenging activity of abalone viscera peptides.
The reducing power of the abalone viscera peptides and their ability to inhibit linoleic acid oxidation are illustrated in Fig. 2. The Cys containing peptides showed strong reducing power, while the others showed less. The reducing power of each peptide was consistent with its DPPH· scavenging activity. Most of the peptides exhibited the ability to inhibit linoleic acid oxidation. Tyr containing peptides showed strong inhibition, while peptides containing only Cys had relatively less inhibition, consistent with the ABTS·+ scavenging activity of each peptide. However, peptides NRFGVSR, VHAEPTK, NSHVVR, and DDITRD, which showed no significant activity in scavenging DPPH· and ABTS·+ or reducing power, exhibited some ability to inhibit linoleic acid oxidation.
Fig. 2.
The reducing power (A) and ability to inhibit linoleic acid oxidation (B) of abalone viscera peptides.
3.2. Correlation among the evaluation indexes of the in vitro antioxidant activities of abalone visceral peptides
The results of the correlation analysis of the evaluation indexes of in vitro antioxidant activity of abalone viscera peptides are shown in Table 1. The DPPH· scavenging activity of the peptides displayed a significant positive correlation with their reducing power. Scavenging activity against ABTS·+ was positively correlated with the inhibition of linoleic acid oxidation. By contrast, there was no correlation between DPPH· scavenging activity and ABTS·+ scavenging activity.
Table 1.
Correlation analysis among different evaluation indexes of in vitro antioxidation.
| Inhibition of linoleic acid autoxidation | ABTS·+ scavenging activity | DPPH· scavenging activity |
Reducing power | |
|---|---|---|---|---|
| Inhibition of linoleic acid autoxidation | 1 | |||
| ABTS·+ scavenging activity | 0.777** | 1 | ||
| DPPH·scavenging activity | 0.279 | 0.396 | 1 | |
| Reducing power | 0.274 | 0.439 | 0.973** | 1 |
** indicates significant correlation (P < 0.01).
3.3. Effect of abalone viscera peptides on the viability of LO2 cells damaged by H2O2
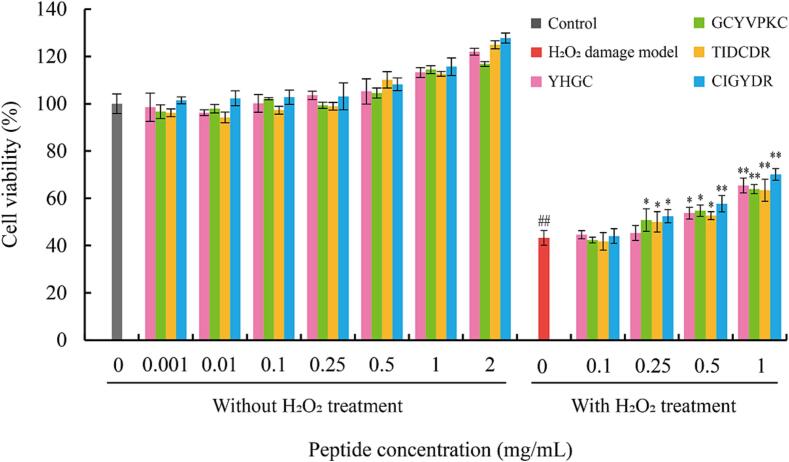
Among the 16 peptides, GCYVPKC and CIGYDR have both strong ABTS·+ and DPPH· scavenging activities, YHGF has strong ABTS·+ scavenging activity and TIDCDR has strong DPPH· scavenging activity. Therefore, these four peptides were selected to analyze their effects on the viability of LO2 cells without and with H2O2 injury. As seen in Fig. 3, all four peptides showed a tendency to promote the growth of LO2 cells, indicating that none of the four synthetic peptides was cytotoxic. The cell viability was greater than 90 % at the peptide concentration range of 0.10–1.00 mg/mL, with a maximum of 115.66 %.
Fig. 3.
Effect of abalone viscera peptides on the viability of LO2 cells and cytoprotective effect against LO2 cell damage induced by H2O2. ## indicates a significant difference (P < 0.01) between the H2O2 damage model group and the control group; * and ** indicate significant differences between the peptide treatment group and H2O2 damage model group at P < 0.05 and P < 0.01, respectively.
After H2O2 treatment, the cell viability of the damage model group decreased significantly compared to the control group. Compared to the H2O2 damage model group, the cell viability of each peptide treatment group did not change significantly when the peptides were added at a concentration of 0.10 mg/mL. However, when the peptide concentration was 0.25 mg/mL, the cell viability increased significantly in all the peptide treatment groups except for the YHGF treatment group. When the peptide concentration reached 1 mg/mL, the cell viability of all the peptide treatment groups increased significantly. Therefore, peptide concentrations of 0.25 mg/mL and 1 mg/mL were selected for subsequent experiments.
3.4. Cytoprotective effect of the abalone viscera peptides against cell damage
The effects of abalone viscera peptides on extracellular LDH leakage are shown in Fig. 4 (A). Compared to the control group, LDH leakage increased significantly in the damage model group. However, LDH leakage was significantly reduced in all peptide treatment groups compared with the damage model group. Peptide CIGYDR had the best effect, which was not significantly different from that of the Vc treatment group.
Fig. 4.
The effects of abalone viscera peptides on LDH leakage (A), MDA level (B), GSH-Px activity (C), CAT activity (D), and SOD activity (E) in LO2 cells. Different lowercase letters indicate significant differences among groups (P < 0.05).
The effects of abalone viscera peptides on cellular MDA levels are shown in Fig. 4 (B). Compared to the control group, the MDA level in the damage model group increased significantly, while the MDA levels in the peptide treatment groups decreased significantly compared to the damage model group, except for the 0.25 mg/mL TIDCDR treatment group. CIGYDR had the strongest effect, while TIDCDR, which does not contain Tyr, had the weakest effect, significantly weaker than those of the other peptide groups.
As shown in Fig. 4 (C), (D), and (E), the intracellular GSH-Px, CAT, and SOD activities in the damage model group decreased significantly compared to the control group, while all the peptide treatment groups showed, to different degrees, increased activity of these enzymes. Among them, the activities of intracellular GSH-Px, CAT, and SOD increased significantly in the Cys containing peptides GCYVPKC, TIDCDR, and CIGYDR treatment group compared to the damage model group. CIGYDR had the strongest effect, which was not significantly different from that of the Vc treatment group. The effect of YHGF, which does not contain Cys, was significantly less than those of the other peptide treatment groups.
4. Discussion
4.1. Selection of evaluation indexes for in vitro antioxidant activity
Indexes such as DPPH· scavenging activity, ABTS·+ scavenging activity, reducing power, and inhibition of linoleic acid oxidation are commonly used for the evaluation of in vitro antioxidant activity (Czelej, Garbacz, Czernecki, Wawrzykowski, & Wasko, 2022). The selection of the evaluation index is of vital importance when screening antioxidant peptides. In this study, abalone visceral peptide QCVR had a strong DPPH· scavenging activity but a weak ABTS·+ scavenging activity, while peptide YHGF showed the opposite behavior. Although NSHVVR had very weak scavenging activities against DPPH· and ABTS·+, it had good activity at inhibition of linoleic acid oxidation. It is generally considered that both DPPH· scavenging activity and reducing power assays are based on single electron transfer reaction mechanisms (Apak, Gorinstein, Bohm, Schaich, Ozyurek, & Guclu, 2013), while both ABTS·+ scavenging activity assays and inhibition of linoleic acid oxidation are based on hydrogen atom transfer reaction mechanisms (Ilyasov et al., 2020, Nwachukwu, Sarteshnizi, Udenigwe, & Aluko, 2021). In this study, the DPPH· scavenging activity of abalone visceral peptides had a high correlation with their reducing power (correlation coefficient 0.973), and their ABTS·+ scavenging activity was positively correlated with their ability to inhibit linoleic acid oxidation (correlation coefficient 0.777). These results are similar to that of Ohashi et al. (Ohashi et al., 2015) which showed that antioxidant activity of peptides against peroxidation of linoleic acid positively correlated with the ABTS assay. However, the study only focused on the chemically synthesized tripeptides containing Tyr and His, while our study involved peptides containing more various amino acid residues. The results of evaluation indexes with similar action mechanisms showed clear consistency. Therefore, a good evaluation index for antioxidant peptide activity should be composed of evaluation indexes with different action mechanisms, so as to achieve comprehensiveness, high efficiency, and economy of evaluation.
4.2. Effect of amino acid residues Cys and Tyr on the antioxidant activities of abalone visceral peptides
The structure of an antioxidant peptide determines its activity. Antioxidant peptides are mostly small molecules (Carrasco-Castilla et al., 2012, Hu et al., 2022, Wen et al., 2020) and thus do not have the same complex tertiary structure as biological macromolecules. Instead, the antioxidant activity of a peptide is closely related to the presence and position of specific amino acid residues in the peptide chain. The literature reports that peptides containing Cys exhibit significant DPPH· and ABTS·+ scavenging activity (Lu, Zhang, Sun, Song, & Huang, 2019), and peptides containing Tyr show significant ABTS·+ scavenging activity and increase the viability of oxidative damaged cells (Saito et al., 2003, Wong et al., 2020). The higher the amount of Cys, the stronger the antioxidant activity (Huang et al., 2012). The presence or absence of Cys and Tyr is critical to determining whether a peptide has ABTS·+ scavenging activity (Saito et al., 2003, Zheng et al., 2016). The peptides Tyr-X have been shown to have a higher ABTS·+ scavenging activity than peptides X-Tyr (Zheng, 2015). The abalone visceral peptides contain 18 amino acid residues (i.e. all except Leu and Trp) and each residue is present in at least two of the peptides under study. Using these peptides to explore the effect of amino acid residues on antioxidant activity is thus very representative of the behavior of peptides in general. The results in this study showed that only Cys containing peptides had obvious scavenging activity against DPPH·. Cys has a highly reducing -SH side chain group that can react with very stable DPPH· radicals, while other amino acid residues cannot reduce them to DPPHH (Zheng, Lin, Su, Zhao, & Zhao, 2015). In cellular assays, Cys containing peptides also exhibited a significant increase in cellular antioxidant enzyme activity. The keap1-Nrf2-ARE signaling pathway is the most important regulator of the cytoprotective response of cells to oxidative stress (Mirdamadi, Mirzaei, Soleymanzadeh, Safavi, Bakhtiari, & Zandi, 2021). Antioxidant peptides exerted cellular protective effects via the keap1-Nrf2-ARE signaling pathway. Antioxidant peptides led to the dissociation of Nrf2-keap1 complex, inhibited ubiquitination, activated Nrf2 pathway, and promoted the transcriptional activation of antioxidant enzymes (e.g. CAT, SOD, GSH-Px) to avoid oxidative damage in cells (Huo et al., 2022). Mirdamadi et al. indicated that the Cys in peptides could form hydrogen bonds with key amino acid residues in the binding site for Nrf2 peptide in the keap1 kelch domain, disrupted the keap1-Nrf2 interaction and promote antioxidant enzyme expression (Mirdamadi, Mirzaei, Soleymanzadeh, Safavi, Bakhtiari, & Zandi, 2021). However, in this study, the peptide GCYVPKC, which contains two Cys residues, showed lower in vitro free radical scavenging activity and cellular antioxidant activity than CIGYDR, which contains only one Cys. There was, therefore, no simple relationship between Cys quantity and peptide effect. This might be due to the position of Cys in the peptide and the type of adjacent amino acid residues. Cys at position C2 in GCYVPKC may form -S-S- after participating in scavenging free radicals, which increases the volume of the side chain at the N-terminus of Tyr and produces a steric clash with the phenolic hydroxyl group of Tyr, leading to a decrease in activity (Elias, Kellerby, & Decker, 2008).
The results of this study also showed that only peptides containing Tyr or Cys had obvious ABTS·+ scavenging activity, although the peptides containing only Cys had less activity than the Tyr containing peptides. All the peptides METY, YHGF, PVPPYKA, and AAQYSRN contain only one Tyr, but YHGF had a significantly higher ABTS·+ scavenging activity than the other peptides. This may be due to the fact that the Tyr is situated at the N-terminus of the peptide. If Tyr is at the C-terminus, the C-terminal carboxyl group can form a hydrogen bond with the side chain phenolic hydroxyl group of Tyr, leading to an inhibition of its hydrogen supply capacity (Zheng, 2015). Peptides containing both Cys and Tyr showed good DPPH· and ABTS·+ scavenging activity and cellular antioxidant capacity, and CIGYDR had especially strong activity. The carboxyl group of Asp adjacent to Tyr in CIGYDR has an electron-absorbing effect, which weakens the oxygen electron cloud on the phenolic hydroxyl group of Tyr and enhances its hydrogen supply capacity (Liu et al., 2022).
4.3. Effect of other amino acid residues on the activity of abalone visceral peptides
The literature has also reported that peptides containing Trp (Zheng, Dong, Su, Zhao, & Zhao, 2016), Arg (Carrasco-Castilla, et al., 2012), His (Saito, et al., 2003), and Asp (Wen, Zhang, Feng, Duan, Ma, & Zhang, 2020) exhibit clear antioxidant activity. However, in this study, the abalone visceral peptides containing Arg alone (AAPAVSGR, NRFGVSR, AANNSTR, DDTTRD, and DVAFMR) did not show in vitro antioxidant activity; this result is inconsistent with the literature (Carrasco-Castilla et al., 2012, Liang et al., 2018). Although the peptides containing His alone (VHAEPTK and NSHVVR) did not exhibit DPPH· and ABTS·+ scavenging activity and had very little reducing power, they showed significant inhibition of lipid oxidation. Not only can the imidazole group of His effectively quench free radicals, but the oxidized product, imidazolinone, also has a strong chelating effect on metal ions, thereby inhibiting lipid oxidation (Kohen, Yamamoto, Cundy, & Ames, 1988).
5. Conclusion
The DPPH· scavenging activity of abalone visceral peptides was significantly correlated with reducing power. The ABTS·+ scavenging activity of abalone visceral peptides was positively correlated with their ability to inhibit linoleic acid oxidation. There was no correlation between the DPPH· scavenging activity and the ABTS·+ scavenging activity of the peptides. The 16 synthetic abalone visceral peptides contained all amino acid residues except Leu and Trp. Only the peptides containing Cys showed good DPPH· scavenging activity. Only the peptides containing Tyr or Cys showed obvious ABTS·+ scavenging activity, and the peptides containing only Cys had less activity than the peptides containing Tyr. The peptides containing both Cys and Tyr had strong scavenging activity toward both DPPH· and ABTS·+. Peptides containing only His had little in vitro scavenging activity against DPPH· and ABTS·+, nor did they have strong reducing power, but they had a clear ability to inhibit linoleic acid oxidation. The peptides TIDCDR, YHGF, GCYVPKC, and CIGYDR all showed good cellular antioxidant activity, in which the peptides containing Tyr were more effective in reducing intracellular MDA levels and LDH leakage than those without Tyr, while the peptides containing Cys were significantly more effective at increasing the activities of CAT, SOD, and GSH-Px than the Cys-free peptides. Among those four peptides, CIGYDR showed the strongest protective effect against cellular oxidative damage, with no significant difference from the effect of Vc treatment. The results of this study lay a foundation for further research and industrial development of abalone visceral antioxidant peptides.
CRediT authorship contribution statement
Jing Liu: Formal analysis, Writing – original draft. Guohong Wu: Methodology, Writing – review & editing. Jiahong Yang: Data curation, Investigation. Chuanbo He: Methodology, Writing – review & editing. Hejian Xiong: Project administration, Supervision, Writing – review & editing. Ying Ma: Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Science and Technology Foundation of Fujian Province (grant no.:2019N0014) and Xiamen Science and Technology Planning Project (grant no.: 2022CXY0307), China. We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Contributor Information
Hejian Xiong, Email: hjxiong@jmu.edu.cn.
Ying Ma, Email: maying@jmu.edu.cn.
Data availability
Data will be made available on request.
References
- Apak R., Gorinstein S., Bohm V., Schaich K.M., Ozyurek M., Guclu K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure and Applied Chemistry. 2013;85(5):957–998. doi: 10.1351/Pac-Rep-12-07-15. [DOI] [Google Scholar]
- Ben Yakoub A.R., Abdehedi O., Jridi M., Elfalleh W., Bkhairia I., Nasri M., Ferchichi A. Bioactive polysaccharides and their soluble fraction from Tossa jute (Corchorus olitorius L.) leaves. Food Bioscience. 2020;37 doi: 10.1016/j.fbio.2020.100741. [DOI] [Google Scholar]
- Cakir B., Tunali-Akbay T. Potential anticarcinogenic effect of goat milk-derived bioactive peptides on HCT-116 human colorectal carcinoma cell line. Analytical Biochemistry. 2021;622 doi: 10.1016/j.ab.2021.114166. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Jacinto-Hernández C., Alaiz M., Girón-Calle J.…Dávila-Ortiz G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chemistry. 2012;135(3):1789–1795. doi: 10.1016/j.foodchem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chen S.R., Wei P.X., Ye Y.J., Chen J., Weng W.Y. Preparation of peptides derived from enzymatic hydrolysate of abalone viscera and their inhibitory effect on MDA-MB-231 human breast cancer cells. Food Science. 2018;39(23):120–125. [Google Scholar]
- Czelej M., Garbacz K., Czernecki T., Wawrzykowski J., Wasko A. Protein hydrolysates derived from animals and plants-A review of production methods and antioxidant activity. Foods. 2022;11(13) doi: 10.3390/foods11131953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Mai R.Y., Zhang C.Y., Liu J.Q., Ren Y.C., Li G., Chen J.J. Synthesis and pharmacological evaluation of a novel synthetic peptide CWHTH based on the Styela clava-derived natural peptide LWHTH with improved antioxidant, hepatoprotective and angiotensin converting enzyme inhibitory activities. International Journal of Pharmaceutics. 2021;605 doi: 10.1016/j.ijpharm.2021.120852. [DOI] [PubMed] [Google Scholar]
- Dominguez-Perez L.A., Beltran-Barrientos L.M., Gonzalez-Cordova A.F., Hernandez-Mendoza A., Vallejo-Cordoba B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. Journal of Functional Foods. 2020;73 doi: 10.1016/j.jff.2020.104134. [DOI] [Google Scholar]
- Elias R.J., Kellerby S.S., Decker E.A. Antioxidant activity of proteins and peptides. Critical Reviews In Food Science and Nutrition. 2008;48(5):430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Gorguc A., Gencdag E., Yilmaz F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments – A review. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109504. [DOI] [PubMed] [Google Scholar]
- Hu Y.Q., Yang J.H., He C.B., Wei H.C., Wu G.H., Xiong H.J., Ma Y. Fractionation and purification of antioxidant peptides from abalone viscera by a combination of Sephadex G-15 and Toyopearl HW-40F chromatography. International Journal of Food Science and Technology. 2022;57(2):1218–1225. doi: 10.1111/ijfs.15504. [DOI] [Google Scholar]
- Huang G.J., Deng J.S., Chen H.J., Huang S.S., Liao J.C., Hou W.C., Lin Y.H. Defensin protein from sweet potato (Ipomoea batatas [L.] Lam 'Tainong 57') storage roots exhibits antioxidant activities in vitro and ex vivo. Food Chemistry. 2012;135(3):861–867. doi: 10.1016/j.foodchem.2012.05.082. [DOI] [PubMed] [Google Scholar]
- Huo J., Ming Y., Li H., Li A., Zhao J., Huang M.…Zhang J. The protective effects of peptides from Chinese baijiu on AAPH-induced oxidative stress in HepG2 cells via Nrf2-mediated signaling pathway. Food Science and Human Wellness. 2022;11(6):1527–1538. doi: 10.1016/j.fshw.2022.06.010. [DOI] [Google Scholar]
- Ilyasov I.R., Beloborodov V.L., Selivanova I.A., Terekhov R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. International Journal of Molecular Sciences. 2020;21(3) doi: 10.3390/ijms21031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R., Yamamoto Y., Cundy K.C., Ames B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. PNAS. 1988;85(9):3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Wang Z., Li H., Cai L., Pan J., He H.…Yang L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food and Chemical Toxicology. 2018;115:315–328. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Liu H.F., Liang J.X., Xiao G.S., Vargas-De-La-Cruz C., Simal-Gandara J., Xiao J.B., Wang Q. Active sites of peptides Asp-Asp-Asp-Tyr and Asp-Tyr-Asp-Asp protect against cellular oxidative stress. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130626. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang L.X., Sun Q., Song G.H., Huang J.N. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Research International. 2019;116:707–716. doi: 10.1016/j.foodres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Majura J.J., Cao W.H., Chen Z.Q., Htwe K.K., Li W., Du R.…Gao J.L. The current research status and strategies employed to modify food-derived bioactive peptides. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.950823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuse R. Antioxidative effect of amino-acids. Nature. 1960;186:886–887. doi: 10.1038/186886a0. [DOI] [PubMed] [Google Scholar]
- Mirdamadi S., Mirzaei M., Soleymanzadeh N., Safavi M., Bakhtiari N., Zandi M. Antioxidant and cytoprotective effects of synthetic peptides identified from Kluyveromyces marxianus protein hydrolysate: Insight into the molecular mechanism. LWT. 2021;148 doi: 10.1016/j.lwt.2021.111792. [DOI] [Google Scholar]
- Nwachukwu I.D., Sarteshnizi R.A., Udenigwe C.C., Aluko R.E. A concise review of current in vitro chemical and cell-based antioxidant assay methods. Molecules. 2021;26(16) doi: 10.3390/molecules26164865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y., Onuma R., Naganuma T., Ogawa T., Naude R., Nokihara K., Muramoto K. Antioxidant properties of tripeptides revealed by a comparison of six different assays. Food Science and Technology Research. 2015;21(5):695–704. doi: 10.3136/fstr.21.695. [DOI] [Google Scholar]
- Saito K., Jin D.H., Ogawa T., Muramoto K., Hatakeyama E., Yasuhara T., Nokihara K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. Journal of Agricultural and Food Chemistry. 2003;51(12):3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Sitanggang A.B., Lesmana M., Budijanto S. Membrane-based preparative methods and bioactivities mapping of tempe-based peptides. Food Chemistry. 2020;329 doi: 10.1016/j.foodchem.2020.127193. [DOI] [PubMed] [Google Scholar]
- Suleria H.A.R., Addepalli R., Masci P., Gobe G., Osborne S.A. In vitro anti-inflammatory activities of blacklip abalone (Haliotis rubra) in RAW 264.7 macrophages. Food and Agricultural Immunology. 2017;28(4):711–724. doi: 10.1080/09540105.2017.1310186. [DOI] [Google Scholar]
- The People's Republic of China Ministry of Agriculture, Fisheries Bureau. (2021). China fishery statistical yearbook. 2021. Production (Chapter 2).
- Wen C., Zhang J.X., Feng Y.Q., Duan Y.Q., Ma H.L., Zhang H.H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chemistry. 2020;327 doi: 10.1016/j.foodchem.2020.127059. [DOI] [PubMed] [Google Scholar]
- Wong F.C., Xiao J.B., Wang S.Y., Ee K.Y., Chai T.T. Advances on the antioxidant peptides from edible plant sources. Trends in Food Science and Technology. 2020;99:44–57. doi: 10.1016/j.tifs.2020.02.012. [DOI] [Google Scholar]
- Wu W.F., Jia J., Wen C.R., Yu C.P., Zhao Q., Hu J.N. Optimization of ultrasound assisted extraction of abalone viscera protein and its effect on the iron-chelating activity. Ultrasonics Sonochemistry. 2021;77 doi: 10.1016/j.ultsonch.2021.105670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Luo T., Wang L., Weng Z., Song H., Wang F., Shen X. Potential of food protein-derived peptides for the improvement of osteoarthritis. Trends in Food Science and Technology. 2022;129:544–557. doi: 10.1016/j.tifs.2022.11.004. [DOI] [Google Scholar]
- Yang F.J., Chen X., Huang M.C., Yang Q., Cai X.X., Chen X.…Wang S.Y. Molecular characteristics and structure-activity relationships of food-derived bioactive peptides. Journal of Integrative Agriculture. 2021;20(9):2313–2332. doi: 10.1016/S2095-3119(20)63463-3. [DOI] [Google Scholar]
- Zheng, L. (2015). Structure-activity relationship and directional preparation of antioxidant peptide. Doctor, South China University of Technology.
- Zheng L., Dong H., Su G., Zhao Q., Zhao M. Radical scavenging activities of Tyr-, Trp-, Cys- and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chemistry. 2016;197(Pt A):807–813. doi: 10.1016/j.foodchem.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Zheng L., Lin L., Su G., Zhao Q., Zhao M. Pitfalls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its susceptibility to interference and low reactivity towards peptides. Food Research International. 2015;76(Pt 3):359–365. doi: 10.1016/j.foodres.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhao Y.J., Dong H.Z., Su G.W., Zhao M.M. Structure-activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. Journal of Functional Foods. 2016;21:485–496. doi: 10.1016/j.jff.2015.12.003. [DOI] [Google Scholar]
- Zhou D.Y., Ma D.D., Zhao J., Wan X.L., Tong L., Song S.…Zhu B.W. Simultaneous recovery of protein and polysaccharide from abalone (Haliotis discus hannaiIno) gonad using enzymatic hydrolysis method. Journal of Food Processing and Preservation. 2016;40(2):119–130. doi: 10.1111/jfpp.12589. [DOI] [Google Scholar]
- Zhou D.Y., Zhu B.W., Qiao L., Wu H.T., Li D.M., Yang J.F., Murata Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food and Bioproducts Processing. 2012;90(2):148–154. doi: 10.1016/j.fbp.2011.02.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.