Highlights
-
•
The quality of salted tilapia fillets is dependent on salting conditions.
-
•
The yields decrease at high ionic strength due to the salting-out effects.
-
•
Released proteins increase as NaCl concentration and salting time increase.
-
•
Myofibers swell after shrinking, contributing to the improved quality traits.
Keywords: Microstructure, Physicochemical properties, Mass transfer, Fish fillets, Salting
Abstract
The effects and mechanisms of salting on quality properties of tilapia fillets were investigated in the present study. Salting under high NaCl concentrations (12 % and 15 %) resulted in low water content and decreased yields, due to the salting-out effects and low pH. Water in fillets increased in the later stage of salting in 3 % and 6 % NaCl solutions (p < 0.05). The released proteins accumulated with increasing time (p < 0.05). The TBARS value increased from 0.01 to 0.20 mg/kg after 10 h in 15 % NaCl solution (p < 0.05). The quality changes were mainly correlated to the shrinking or swelling of myofibers, extracellular spaces, and existential state of muscle proteins. In consideration of fish quality and increasing call for low sodium intake, it was recommended to prepare fillets below 9 % NaCl with short times. The finding provided instructions to obtain target quality properties from tilapia by controlling salting conditions.
1. Introduction
Tilapia is an omnivorous fish with a worldwide production at 4514.6 thousand tons in 2020, and nearly 50 % of the global tilapia is cultured in China (FAO, 2022). Tilapia, known as “the fish of the 21st century”, is delicious and has high nutritional value, being expected to play a critical role in eradicating poverty and malnutrition worldwide (Wong et al., 2020). Therefore, tilapia has a great potential for utilization because of its high nutritional value and low price.
Fish is, however, highly perishable during distribution and marketing after slaughter due to the high moisture content and fragile muscle tissue. Great efforts have been made to develop effective technologies for quality analysis and preservation of fish and fish products, such as spectroscopy, histology, modified atmosphere packaging, and application of essential oils (Hassoun and Karoui, 2016, Hassoun and Çoban, 2017). Salting is a traditional technique used in the aquatic processing industries to prevent quality deterioration for some fish species (Jiang et al., 2019a, Jiang et al., 2023). Since excessive sodium intake causes hypertension, cardiovascular disease, and other health problems (He, Tan, Ma, & MacGregor, 2020), NaCl is primarily added at a relatively low level to improve the quality attributes (such as water-holding capacity, and springiness), and to modify the functionalities of muscle proteins with the general application of cryogenic technology to guarantee the shelf-life of aquatic products (Inguglia et al., 2017, Jiang et al., 2019b, Jiang et al., 2022, Shen et al., 2020).
Mass transfer, mainly involving salt uptake and water loss or absorption occurs in fish flesh during brine salting. The varying rates of mass transfer are dependent on many factors, such as NaCl concentration, salting time, fish species, and postmortem states. (Dimakopoulou-Papazoglou & Katsanidis, 2020). There are numerous studies on the kinetics of mass transfer during fish salting, which provide instructions to obtain fish products with a specified salt content or at equilibrium (Laub-Ekgreen et al., 2019, Van Nguyen et al., 2010). Osmotic pressures and concentration differences rise between fish flesh and salting media when NaCl concentration increases, which are the driving forces for the movement of salt, water, and water-soluble components (Dimakopoulou-Papazoglou & Katsanidis, 2020). Tissue microstructure can also affect the changing rate because the extracellular spaces can be channels for salt ions, water, and water-soluble components (Jiang et al., 2019a, Jiang et al., 2019b, Jiang et al., 2019).
Textural properties and color characteristics are determinants of consumer desire to purchase, which is also affected by salting (Inguglia et al., 2017). However, the relationship between the mass transfer and the quality changes during fish salting has not been well documented. NaCl concentration and salting time affect protein structure, spatial arrangement, and protein–protein interactions in fish flesh, modifying the composition and states of the intracellular and extracellular matrices, water content, and yield during salting (Hughes, Oiseth, Purslow, & Warner, 2014). It has been found that myofibrillar proteins are degraded and myosin heavy chains decompose into fragments during salting (Thorarinsdottir, Arason, Geirsdottir, Bogason, & Kristbergsson, 2002). Under optimal salting conditions, the improvements in water-holding capacity and texture properties of fish flesh are related to the extraction/solubilization of myofibrillar proteins and the swelling of myofibrillar lattice (Jiang et al., 2019a). In contrast, high salt concentrations and prolonged salting time cause protein aggregation and denaturation, resulting in high drip loss and texture deterioration (Jiang et al., 2019c). However, detailed information on the effects of salting conditions on the quality properties of tilapia fillets and the underly mechanisms is limited. Therefore, the present study aimed to reveal the effects of salting conditions on the quality of tilapia fillets and to delve into the underlying mechanisms during salting under various conditions.
2. Materials and methods
2.1. Preparation of samples
Totally fifty tilapia (Oreochromis niloticus) with a weight of 586.1 ± 33.1 g and a total length of 29.9 ± 0.8 cm were bought and transported to the treatment room of our university with oxygen supply to keep them alive. The fish were de-headed after a heavy blow to the head using a rubber hammer, and they were eviscerated and washed clearly with water within 30 min. The fish used in the present study were handled humanely according to the aquatic animal health code (World Organization for Animal Health, 2012). Fish fillets (10.0 cm × 2.0 cm × 1.0 cm) were obtained from the dorsal muscle, and two pieces were obtained from each fish. The fillets were brined in NaCl solutions with different concentrations (3 %, 6 %, 9 %, 12 %, and 15 %) at a fish to salt solution ratio of 1:3 (4 °C), with the fresh fillets as a control group. It should be stated that the unsalted group was marked as 0 h to compare with their salted counterparts. The fillets were removed from the solutions at a specified salting time (1, 2, 4, 6, 8, and 10 h), and packed separately after wiping the surface moister. The samples were then minced and stored at − 80 °C before determining physicochemical properties.
2.2. Determination of yield
The weight of the fillets before salting (W0) and after salting (W1) was recorded, and the yield (Y) was calculated according to equation (1):
| (1) |
2.3. Determination of changes in NaCl weight and water weight
The salt content was determined using a PAL-ES1 salinometer (Atago Co., ltd., Japan) following Jiang et al. (2019a). Distilled water (10 mL) was mixed with 1.00 g of samples (W2) and homogenized. After filtration, the NaCl content (X0) was measured using the salinometer, and the NaCl content of fish flesh was calculated using equations (2):
| (2) |
The water content of fish flesh was determined following the AOAC method (AOAC, 1990). The changes in the NaCl weight () and water weight () were calculated using equation (3), (4), respectively:
| (3) |
| (4) |
where was the water content at salting time of t; was the NaCl content at salting time of t; and were the water content and the NaCl content of unsalted fish, respectively; was the yield at salting time of t.
2.4. Determination of pH
Fish samples (1.00 g) were homogenized with cold distilled water (10 mL) and centrifuged. After filtration, the pH of the filtrate was measured using a pH meter (FE20, Mettler Toledo Co., China).
2.5. Measurement of texture properties
The fillets (2.0 cm × 2.0 cm × 1.0 cm) were compressed twice perpendicularly to the myofiber direction using a texture analyzer (TA.XT plus, Stable Micro System, UK). The cylindrical probe of P50 was used, and the trigger force was 5 g. The test rate was 1.0 mm/s, and the highest compression ratio was 40 %. Hardness was the maximum force at the first compression, and springiness was the height recovery after the first compression. Cohesiveness was a measure of the internal bonds and the resistance to chewing foods, and resilience represented the springback capacity at the first compaction.
2.6. Measurement of color characteristics
The color characteristics of tilapia fillets were measured using a colorimeter (CR-40, Konica Minolta Sensing Inc., Japan), and the changes in lightness (L*), redness (a*), and yellowness (b*) were analyzed.
2.7. Measurement of thiobarbituric acid reactive substances (TBARS) value
The TBARS value was measured following Vyncke (1975) with slight modifications. Fish samples (1.00 g) were added into 10 mL of 7.5 % trichloroacetic acid solution, containing 0.1 % EDTA, and homogenized. After filtration, the mixtures of thiobarbituric acid solution (0.02 mol/L) and the filtrate were incubated in boiling water for 40 min, and then cooled to room temperature. By referencing a standard curve using 1,1,3,3-tetraethoxypropane, the absorbance at 532 nm was used to calculate the TBARS value in fish flesh.
2.8. Determination of content of released proteins during salting
The content of released proteins during salting was analyzed following Jiang et al. (2019a). The biuret method was used to was determine the protein concentration using bovine serum albumin as the standard substance (Gornall, Bardawill, & David, 1949). The content of released proteins was calculated using equation (5):
| (5) |
where C was the protein concentration (g/mL); V was the volume of the remaining NaCl solutions (mL); W was the weight of the fish fillets before salting (g).
2.9. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The SDS-PAGE was performed following Shi et al. (2015) with slight modifications to analyze the composition of released proteins in the remaining solutions. The solutions were (0.5 mL) mixed with 0.5 mL of 20 mmol/L Tris-HCl buffer (8 mol/L urea, 2 % SDS, 2 % β-mercaptoethanol, pH 8.8). After the proteins were dissolved sufficiently at 98 °C (10 min), the solutions were cooled. Each protein sample (6 μg) was uploaded onto the precast polyacrylamide gels (5 %−20 %, Sangon Biotech Co., ltd., China). Coomassie brilliant blue staining solution and methanol and acetic acid solution were used to stain and destain the gels until the protein bands were clear.
2.10. Observation of tissue microstructure
The effects of salting conditions on the microstructure of tilapia fillets were evaluated using scanning electron microscopy (SEM) following Jiang et al. (2019b). Sample fixation was conducted using 2.5 % glutaraldehyde overnight (4 °C). The specimens were post-fixed, dehydrated, freeze-dried, and coated with gold after washing with 0.1 mol/L phosphate buffer (pH 7.3) three times. The SEM images were obtained using an SU5000 scanning electron microscope (Hitachi High-Technologies (Shanghai) Co., ltd., China).
2.11. Statistical analyses
The two-way analysis of variance and plotting were performed using Origin Pro8.5. When the effect of salt concentration or salting time was significant, the means were compared using Duncan’s method at p < 0.05.
3. Results and discussion
3.1. Changes in yield, salt weight, water weight, and pH in tilapia fillets during salting
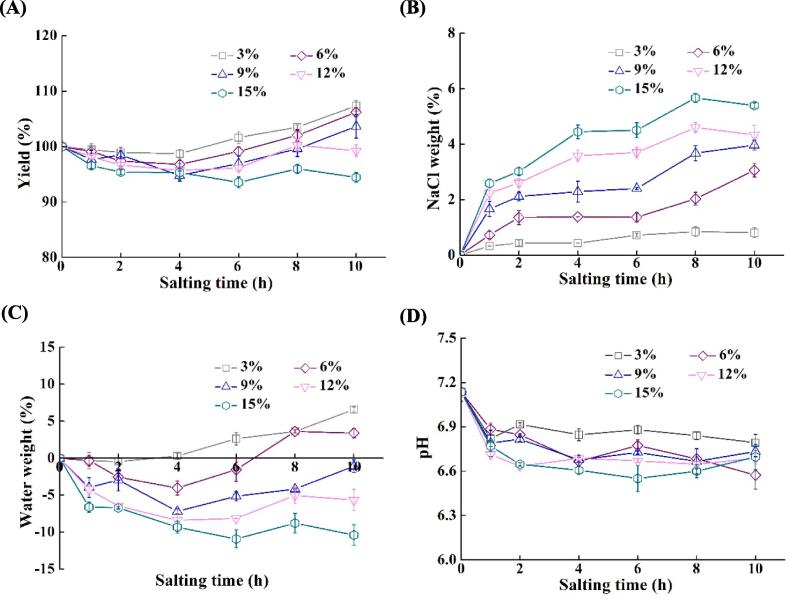
The effects of NaCl concentration and salting time on yield, salt weight, water weight, and pH in tilapia fillets were studied, and the results are shown in Fig. 1. The variation in yield of fish fillets is a result of the weight changes in salt, water, water-soluble proteins, small peptides, and other nitrogenous compounds during salting (Laub-Ekgreen et al., 2018, Thorarinsdottir et al., 2002, Thorarinsdottir et al., 2011). Because the weight did not change in the control group (0 h), the yield of these fillets was presented as 100 % to compare with their salted counterparts. As shown in Fig. 1A, the yield of tilapia fillets tended to decrease with increasing NaCl concentration at the same salting time. The yield of the control group decreased after the first hour of salting irrespective of NaCl concentration, which was be attributable to the shrinkage of myofibers and the loss of water and water-soluble substances from fish fillets (Jiang et al., 2019a, Szymczak and Kołakowski, 2012). After 10 h of salting, the yield of the fillets salted in 15 % NaCl solution was 94.4 %, which was 13.0 %, 11.8 %, 9.2 %, and 4.8 % lower than the fillets salted in 3 %, 6 %, 9 %, and 12 % NaCl solutions, respectively (p < 0.05). With the increase of salting time, the yield raised at various degrees after a fall in the initial stage of salting. The time to reach the lowest yields and the decreased degree of yields of fish fillets were concentration-dependent during salting. The yields exceeded 100 % after salting in 3 %, 6 %, and 9 % NaCl solutions for 6 h, 8 h, and 10 h, respectively, while it was no higher than 100 % for the fillets salted in 12 % and 15 % NaCl solutions within 10 h. Though salting in solutions with high NaCl concentrations (12 % and 15 %) was expected to accelerate the diffusion of salt into fillets, it might facilitate the formation of hydrophobic interactions between protein molecules, namely the salting-out effect on proteins, resulting in lower yields after salting (Shen et al., 2020). The changes in the yield were not only related to the uptake of salt but also to the increased water-holding capacity of tissues due to the binding of salt ions (Hamm, 1960, Jiang et al., 2019). Therefore, the dynamics of salt and water weight in fish flesh should be considered for the quality properties of salted fish products.
Fig. 1.
Changes in yield, NaCl weight, and water weight in tilapia fillets during salting. (A) Yield; (B) NaCl weight; (C) Water weight; (D) pH. The symbols of 3%, 6%, 9%,12%, and 15% indicate NaCl concentration for salting.
During salting, sodium and chloride ions diffuse into fish fillets, and water permeates out because of the differences in salt concentration and osmotic pressure (Van Nguyen et al., 2010). As shown in Fig. 1B, the NaCl weight increased more rapidly in the solutions with high NaCl concentrations, which was attributable to the large concentration differences between the solutions and the flesh. It could form large osmotic pressure differences and accelerate the uptake of NaCl (Thorarinsdottir et al., 2011b). With the extension of salting, differences in NaCl concentration and osmotic pressure between the two systems decreased, which resulted in a reduced rate of NaCl diffusion and a slow increase in NaCl weight. Salting under high salt concentrations might cause muscle fibers to shrink to a greater extent, and form large extracellular spaces, which would facilitate the uptake of salt ions and the loss of tissue water (Jiang et al., 2019, Shen et al., 2020). The NaCl weight in tilapia fillets increased along with the prolongation of salting time as expected.
During salting, a positive value of water weight represents a net increase in water, and a negative value indicates a net loss of water from fish tissues. As illustrated in Fig. 1C, the water weight was consistently negative in the samples salted in 9 %, 12 %, and 15 % NaCl solutions for up to 10 h. In comparison, it became positive after salting in 3 % and 6 % NaCl solutions for 4 h and 6.5 h, respectively. With the increase of salting time, the water weight decreased initially and increased in the salt solutions, apart from the 15 % NaCl solution. The variations in the water weight were closely related to the salting time and NaCl concentration. The water was lost from fish fillets at the beginning of salting due to high osmotic pressures (Jiang et al., 2019a, Jiang et al., 2019). The higher the NaCl concentration, the more water loss from the fish fillets. With the prolongation of salting, the salt content of fish muscle remained below the concentration for salting-out effects, and the myofibrils would swell and absorb water from the salt solutions due to the binding of salt ions, namely the salting-in effect on proteins (Corzo and Bracho, 2004, Dimakopoulou-Papazoglou and Katsanidis, 2020). At a high NaCl concentration (15 %), the proteins would form stronger protein–protein interactions, leading to severer tissue shrinkage and dehydration (Thorarinsdottir et al., 2002). No turning point was observed in the fillets salted in 15 % NaCl solution in terms of water weight, which could be attributable to the aggregation/denaturation of proteins and the resultant decrease in water-holding capacity of tissues (Van Nguyen et al., 2010). The decreased pH (see Fig. 1D) might also be responsible for the loss of water from the fillets salted under high NaCl concentrations (Herrero et al., 2005, Hughes et al., 2014, Szymczak et al., 2012).
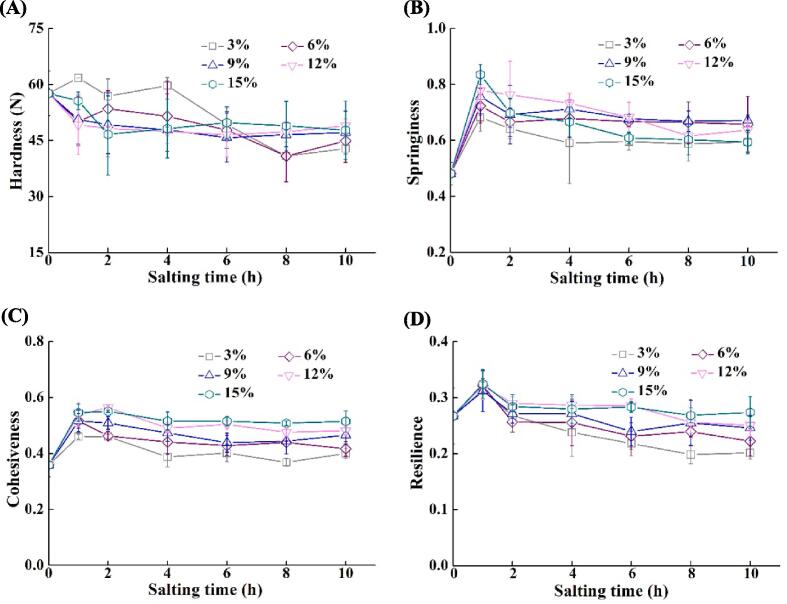
3.2. Changes in texture of tilapia fillets during salting
Texture properties are the most important quality traits of muscle foods, which are usually expressed by hardness, springiness, cohesiveness, and resilience (Schreuders, Schlangen, Kyriakopoulou, Boom, & van der Goot, 2021), and texture softening is undesirable for fish quality (Herrero et al., 2005). As shown in Fig. 2A, the hardness of tilapia fillets decreased significantly after 8 h and 10 h in 3 % NaCl solution (p < 0.05). In contrast, salting under other conditions reduced the problem of texture softening in tilapia fillets. The springiness of tilapia fillets increased after salting for 1 h significantly (p < 0.05), irrespective of NaCl concentrations (see Fig. 2B). The springiness of the fillets salted in 3 %, 6 %, and 9 % NaCl solutions did not change significantly after salting for 1–10 h (p > 0.05). The springiness decreased from 8 h in 12 % NaCl solution and 4 h in 15 % NaCl solution after reaching the maximum values (p < 0.05). Salt might cause salt-soluble proteins to dissolve, which possibly formed an elastic protein network and increased the springiness of fish fillets (Jiang et al., 2019b, Li et al., 2018). The protein network was characterized by an ordered spatial structure, which would contribute to the envelope of a large amount of water (Jiang et al., 2019a, Jiang et al., 2023). Bahuaud, Gaarder, Veiseth-Kent, and Thomassen (2010) reported that the decrease in springiness was related to cathepsin. During salting, the proteins remained in the flesh would be denatured due to high ionic strength, resulting in a decrease in springiness.
Fig. 2.
Changes in texture properties of tilapia fillets during salting. (A) Hardness; (B) Springiness; (C) Cohesiveness; (D) Resilience. The symbols of 3%, 6%, 9%,12%, and 15% indicate NaCl concentration for salting.
The cohesiveness of tilapia fillets increased after salting for 1 h significantly (p < 0.05), irrespective of NaCl concentrations (see Fig. 2C). Thereafter the cohesiveness showed a gradual decreasing trend as the salting time increased, except for the samples salted in 15 % NaCl solution. It was attributable to the continuous increase in water weight, resulting in a dilution effect on protein concentration and molecular interactions (Jiang et al., 2019a, Laub-Ekgreen et al., 2018). Degradation of myofibrillar proteins was probably related to the decrease in cohesiveness. Compared with the unsalted fillets, the resilience increased after 1 h and decreased at various degrees thereafter (see Fig. 2D). The fillets salted in 3 % NaCl solution had the lowest resilience after 8 h and 10 h (p < 0.05), probably due to the low solubility of muscle proteins (Jiang et al., 2019c).
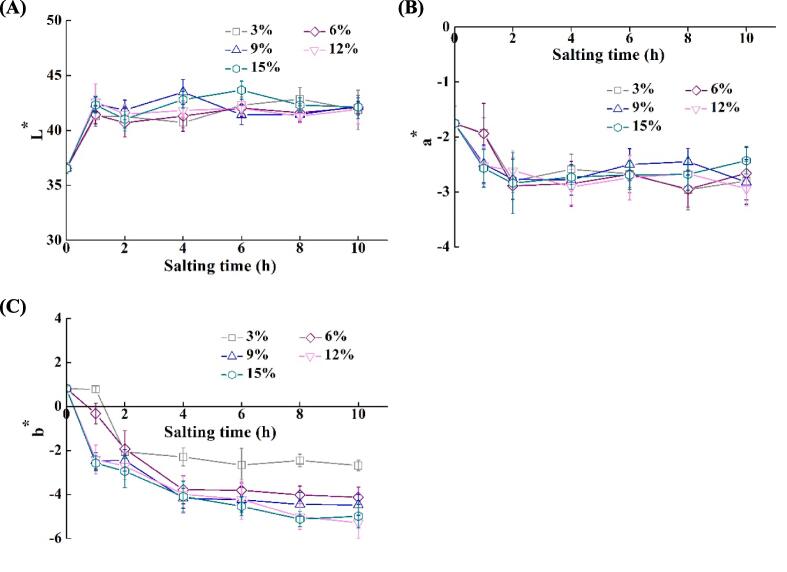
3.3. Changes in color of tilapia fillets during salting
Color is the first quality parameter evaluated by consumers, which is essential for the acceptance of foods (Hughes et al., 2014). As seen from Fig. 3A, the L* value of tilapia fillets increased considerably after salting (p < 0.05). NaCl concentration had no notable effect on the L* value for the fillets after 2 h and 10 h (p > 0.05). The L* value of the fillets reached a maximum value of 42.86 after 8 h in 3 % NaCl solution, and it reached a maximum value of 43.66 after 6 h in 15 % NaCl solution. The increase in the L* values might be because the water in the extracellular spaces of tissue could reflect more light into eyes, while the decrease was related to the swelling of muscle fibers and the increase in water-holding capacity, absorbing more light (Jiang et al., 2019b). The a* values of tilapia fillets were all negative irrespective of salting time and NaCl concentration (see Fig. 3B). The a* values of the fillets reached a minimum value after 4 h in 3 % and 6 % NaCl solutions, while it reached a minimum value after 2 h for other groups. NaCl concentration had no considerable effects on the a* values when the salting time was 2–6 h (p > 0.05). The decreased a* values were attributed to the loss of water-soluble hemoglobin and the increase in water weight (Jiang et al., 2019a). As shown in Fig. 3C, the b* values of tilapia fillets decreased significantly with the increasing NaCl concentration and salting time (p < 0.05). The changes in the a* and b* values were related to the loss of pigment and the oxidation degree of myoglobin and lipids (Jiang et al., 2019a, Mariutti and Bragagnolo, 2017).
Fig. 3.
Changes in color characteristics of tilapia fillets during salting. (A) L*; (B) a*; (C) b*. The symbols of 3%, 6%, 9%,12%, and 15% indicate NaCl concentration for salting.
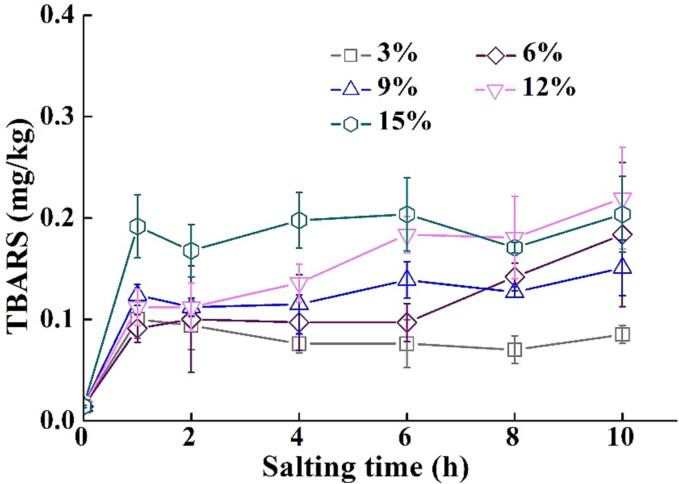
3.4. Changes in lipid oxidation in tilapia fillets during salting
The TBARS value is a measure of malondialdehyde content in muscle, which is widely used as an indicator of lipid oxidation. As illustrated in Fig. 4, the TBARS value of tilapia fillets increased after salting in all NaCl solutions (p < 0.05), while salting time had no considerable effects on the TBARS value except for the 12 % NaCl salted samples. No significant differences were observed in the TBARS value after salting for 8 h and 10 h in 6 %−15 % NaCl solutions (p > 0.05). It is widely accepted that NaCl has a promotional effect on lipid oxidation (Mariutti & Bragagnolo, 2017). NaCl might disrupt cellular integrity, replace or activate iron atoms in myoglobin molecules to provide a catalyst for lipid oxidation, and might also inhibit the activity of the antioxidant enzymes and accelerate lipid oxidation (Mariutti & Bragagnolo, 2017). The products of lipid oxidation and the intertwining between lipid oxidation and protein oxidation would influence the color characteristics of fish fillets (Jiang et al., 2022).
Fig. 4.
Changes in lipid oxidation in tilapia fillets during salting. The symbols of 3%, 6%, 9%,12%, and 15% indicate NaCl concentration for salting.
3.5. Changes in protein properties in tilapia fillets during salting
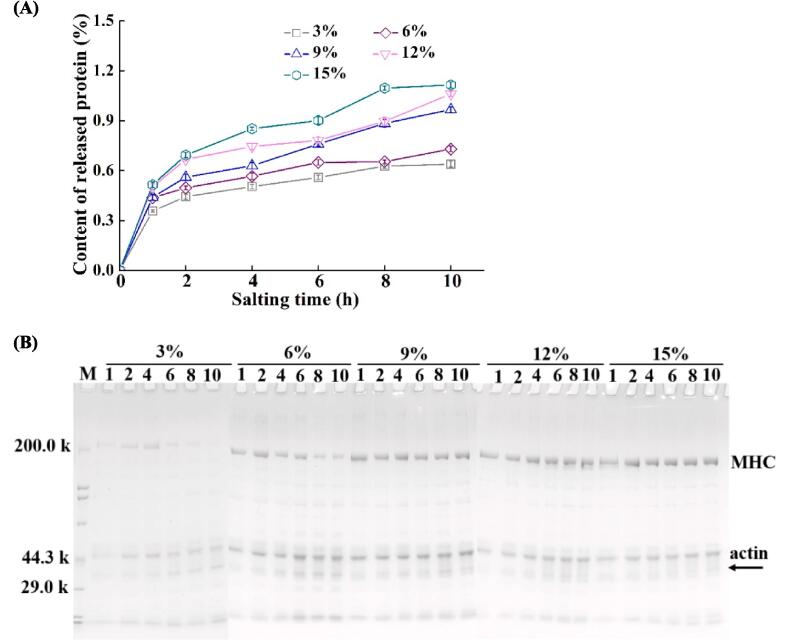
As the main component of fish muscle, the content, composition, and existential state of muscle proteins influence quality attributes of fish substantially (Jiang et al., 2019a, Jiang et al., 2022). The content of the released proteins was measured during salting, and the result is presented in Fig. 5A. The content of the released proteins from tilapia fillets increased markedly as NaCl concentration and salting time increased (p < 0.05). It might be because the solubilization of sarcoplasmic and myofibrillar proteins increased with the raised ionic strength in tissue (Chaijan, 2011).
Fig. 5.
Properties of released proteins from tilapia fillets during salting. (A) Content; (B) SDS-PAGE profile. MHC-myosin heavy chain; M-marker; 3%, 6%, 9%,12%, and 15%-NaCl concentration for salting; 1, 2, 4, 6, 8, and 10-salting time.
As shown in the SDS-PAGE profile of the released proteins (see Fig. 5B), the myofibrillar proteins were less soluble in the solutions with low NaCl concentration (3 %), and the intensities of myosin heavy chain and actin were obviously increased in other groups. New protein bands (marked by arrow, 30–40 kDa) were gradually clear with increasing salting time in salt solutions except for 3 % NaCl solution, which was supposed to be myosin light chains or subunits of tropomyosin (Cai, Nian, Cao, Zhang, & Li, 2020). The decrease in myosin heavy chains might be due to the degradation of proteins during salting, which was consistent with the increased protein bands with low molecular weight (Cai et al., 2020, Martinez-Alvarez and Gomez-Guillen, 2006). Proteolysis was probably accelerated by the increased enzyme activity during salting (Thorarinsdottir et al., 2011a). The changes in the content and composition of the released proteins indicated alterations in tissue ultrastructure during salting, which might affect quality characteristics of fish flesh. The loss of water-soluble proteins including enzymes might affect storage stability for fish and related products (Jiang et al., 2019b, Jiang et al., 2022).
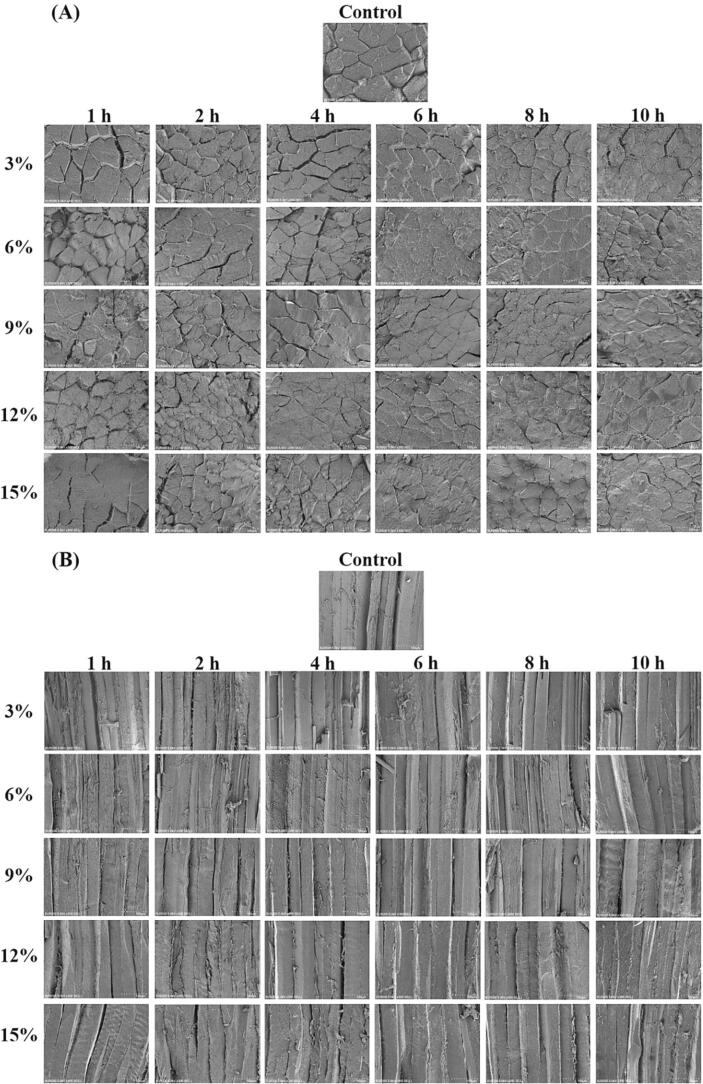
3.6. Changes in microstructure of tilapia fillets during salting
Fish quality is closely related to tissue microstructure, which can also reflect the association between protein and water molecules (Herrero et al., 2005, Thorarinsdottir et al., 2011). The effects of salting conditions on the microstructure of tilapia fillets were investigated using SEM transversely and longitudinally, and the results are presented in Fig. 6. The myofibers in the control fillets were intact with clear boundaries, and no obvious extracellular spaces were observed because fresh tilapia fillets were used and well handled. The myofibers shrank when the fillets were soaked in NaCl solutions due to high osmotic pressures. The following changes in tissue microstructure were NaCl concentration-dependent. With the increase of NaCl concentration and salting time, the extracellular spaces became small gradually, which was supposedly related to the solubilization/extraction of myofibrillar proteins and the swelling of myofilament lattice (Jiang et al., 2019c). It was consistent with the changes in the water weight and texture properties of fish fillets after salting (see Fig. 1 and Fig. 2). After 6–10 h in 6 % and 9 % NaCl solutions, the myofibers were smooth and plump, and they stayed close to each other. It was likely to be caused by the solubilization of myofibrillar proteins and disintegration of pericellular connective tissue. The texture properties of fish fillets were affected by the modified properties of muscle proteins and the interstices between myofibers (Herrero et al., 2005, Jiang et al., 2019). The fish fillets shrank to a high degree with a simultaneous loss of water and water-soluble substances into the salt solutions when salted in 12 % and 15 % NaCl solutions (see Fig. 5). A tight arrangement of myofibers and a reduction of extracellular spaces were also observed with prolonged salting time under these conditions, but these surfaces were not smooth. It might be related to protein denaturation/aggregation due to the salting-out effects, resulting in the loss of water and the decrease in cohesiveness (Thorarinsdottir et al., 2011b).
Fig. 6.
Changes in tissue microstructure of tilapia fillets during salting. (A) Transverse section; (B) Longitudinal section.
4. Conclusions
The changes in quality attributes of tilapia fillets were found to be dependent on NaCl concentration and salting time in the present study. The yields decreased initially and then increased under low NaCl concentrations (<12 %) with prolonged salting time. The yields were not only affected by mass transfer but also by water-holding capacity of tissues as indicated by the water absorption with prolonged salting time. Water in fillets increased in the later stage of salting in 3 % and 6 % NaCl solutions (p < 0.05). Muscle proteins were released into salt solutions gradually as the salting time increased. The fillets lost 1.12 % proteins after 10 h in 15 % NaCl solution. Solubilization and degradation of myofibrillar proteins were much severer when the NaCl concentration was higher than 3 %. The changes in microstructural properties were consistent with the quality variations, mainly reflected by the shrinking or swelling of myofibers, extracellular spaces, and disintegration of connective tissue. The finding provided instructions to obtain target quality properties from fish fillets by salting under proper conditions. The changes in nutritional and organoleptic properties and the mechanisms need be systematically studied in the future to control the quality of related fish products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2022YFD2100902) and Shanghai “Chenguang” Program (No. 20CG58).
Data availability
Data will be made available on request.
References
- AOAC . Association of Official Analytical Chemists Inc; Washington: 1990. Official methods of analysis. [Google Scholar]
- Bahuaud D., Gaarder M., Veiseth-Kent E., Thomassen M. Fillet texture and protease activities in different families of farmed Atlantic salmon (Salmo salar L.) Aquaculture. 2010;310(1–2):213–320. [Google Scholar]
- Cai L.Y., Nian L.Y., Cao A.L., Zhang Y.H., Li X.X. Effect of carboxymethyl chitosan magnetic nanoparticles plus herring antifreeze protein on conformation and oxidation of myofibrillar protein from Red Sea Bream (Pagrosomus major) after freeze-thaw treatment. Food and Bioprocess Technology. 2020;13(2):355–366. [Google Scholar]
- Chaijan M. Physicochemical changes of tilapia (Oreochromis niloticus) muscle during salting. Food Chemistry. 2011;129(3):1201–1210. doi: 10.1016/j.foodchem.2011.05.110. [DOI] [PubMed] [Google Scholar]
- Corzo O., Bracho N. Effects of brine concentration and temperature on equilibrium distribution coefficients during osmotic dehydration of sardine sheets. LWT-Food Science and Technology. 2004;37:475–479. [Google Scholar]
- Dimakopoulou-Papazoglou D., Katsanidis E. Osmotic processing of meat: Mathematical modeling and quality parameters. Food Engineering Reviews. 2020;12(1):32–47. [Google Scholar]
- Fao . Food and Agriculture Organization; Rome: 2022. The state of world fisheries and aquaculture 2022: Towards blue transformation. [Google Scholar]
- Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. The Journal of biological chemistry. 1949;177:751–766. [PubMed] [Google Scholar]
- Hamm R. Biochemistry of meat hydration. Advances in Food Research. 1960;10:355–463. doi: 10.1016/s0065-2628(08)60141-x. [DOI] [PubMed] [Google Scholar]
- Hassoun A., Karoui R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chemistry. 2016;200:343–353. doi: 10.1016/j.foodchem.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Hassoun A., Çoban Ö.E. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends in Food Science & Technology. 2017;68:26–36. [Google Scholar]
- He F.J., Tan M., Ma Y., MacGregor G.A. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. Journal of the American College of Cardiology. 2020;75(6):632–647. doi: 10.1016/j.jacc.2019.11.055. [DOI] [PubMed] [Google Scholar]
- Herrero A.A., Carmona P., Garcia M.L., Solas M.T., Careche M. Ultrastructural changes and structure and mobility of myowater in frozen-stored hake (Merluccius merluccius L.) muscle: Relationship with functionality and texture. Journal of Agricultural and Food Chemistry. 2005;53(7):2558–2566. doi: 10.1021/jf0490706. [DOI] [PubMed] [Google Scholar]
- Hughes J.M., Oiseth S.K., Purslow P.P., Warner R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Science. 2014;98(3):520–532. doi: 10.1016/j.meatsci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Inguglia E.S., Zhang Z.H., Tiwari B.K., Kerry J.P., Burgess C.M. Salt reduction strategies in processed meat products - A review. Trends in Food Science & Technology. 2017;59:70–78. [Google Scholar]
- Jiang Q.Q., Nakazawa N., Hu Y.Q., Osako K., Okazaki E. Microstructural modification and its effect on the quality attributes of frozen-thawed bigeye tuna (Thunnus obesus) meat during salting. LWT-Food Science and Technology. 2019;100:213–219. [Google Scholar]
- Jiang Q.Q., Nakazawa N., Hu Y.Q., Osako K., Okazaki E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chemistry. 2019;293:178–186. doi: 10.1016/j.foodchem.2019.04.091. [DOI] [PubMed] [Google Scholar]
- Jiang Q.Q., Jia R., Nakazawa N., Hu Y.Q., Osako K., Okazaki E. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. Food Chemistry. 2019;271:550–560. doi: 10.1016/j.foodchem.2018.07.219. [DOI] [PubMed] [Google Scholar]
- Jiang Q.Q., Du Y.F., Nakazawa N., Hu Y.Q., Shi W.Z., Wang X.C.…Okazaki E. Effects of frozen storage temperature on the quality and oxidative stability of bigeye tuna flesh after light salting. International Journal of Food Science and Technology. 2022;57(5):3069–3077. [Google Scholar]
- Jiang Q.Q., Du Y.F., Huang S.Y., Gu J.H., Shi W.Z., Wang X.C., Wang Z.H. Physicochemical and microstructural mechanisms for quality changes of lightly salted tilapia (Oreochromis Niloticus) fillets during frozen storage. Journal of the Science of Food and Agriculture. 2023;103:308–316. doi: 10.1002/jsfa.12142. [DOI] [PubMed] [Google Scholar]
- Laub-Ekgreen M.H., Jessen F., Martinez-Lopez B. Mechanistic modelling of the coupled salt and water transport in herring during brining and curing. Journal of Food Engineering. 2019;250:18–25. [Google Scholar]
- Laub-Ekgreen M.H., Martinez-Lopez B., Frosch S., Jessen F. The influence of processing conditions on the weight change of single herring (Clupea herengus) fillets during marinating. Food Research International. 2018;108:331–338. doi: 10.1016/j.foodres.2018.03.055. [DOI] [PubMed] [Google Scholar]
- Li D.Y., Huang Y., Wang K.X., Dong X.P., Yu D., Ge L.H.…Yu C.X. Microstructural characteristics of turbot (Scophthalmus maximus) muscle: Effect of salting and processing. International Journal of Food Properties. 2018;21(1):1291–1302. [Google Scholar]
- Mariutti L.R.B., Bragagnolo N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Research International. 2017;94:90–100. doi: 10.1016/j.foodres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez O., Gomez-Guillen M.C. Effect of brine salting at different pHs on the functional properties of cod muscle proteins after subsequent dry salting. Food Chemistry. 2006;94(1):123–129. [Google Scholar]
- Schreuders F.K.G., Schlangen M., Kyriakopoulou K., Boom R.M., van der Goot A.J. Texture methods for evaluating meat and meat analogue structures: A review. Food Control. 2021;127 [Google Scholar]
- Shen S.K., Chen Y.W., Dong X.P., Liu F.J., Cai W.Q., Wei J.L.…Lin M.M. The effect of different salt concentration and time combinations in physicochemical properties and microstructure of Russian sturgeon (Acipenser gueldenstaedtii) fillets under vacuum impregnation. Journal of Food Processing and Preservation. 2020;44(12):e14967. [Google Scholar]
- Shi Y., Li R.Y., Tu Z.C., Ma D., Wang H., Huang X.Q., He N. Effect of gamma-irradiation on the physicochemical properties and structure of fish myofibrillar proteins. Radiation Physics and Chemistry. 2015;109:70–72. [Google Scholar]
- Szymczak M., Kołakowski E. Losses of nitrogen fractions from herring to brine during marinating. Food Chemistry. 2012;132(1):237–243. doi: 10.1016/j.foodchem.2011.10.062. [DOI] [PubMed] [Google Scholar]
- Szymczak M., Kołakowski E., Felisiak K. Influence of salt concentration on properties of marinated meat from fresh and frozen herring (Clupea harengus L.) International Journal of Food Science and Technology. 2012;47(2):282–289. [Google Scholar]
- Thorarinsdottir K.A., Arason S., Geirsdottir M., Bogason S.G., Kristbergsson K. Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chemistry. 2002;77(3):377–385. [Google Scholar]
- Thorarinsdottir K.A., Arason S., Sigurgisladottir S., Valsdottir T., Tornberg E. Effects of different pre-salting methods on protein aggregation during heavy salting of cod fillets. Food Chemistry. 2011;124(1):7–14. [Google Scholar]
- Thorarinsdottir K.A., Arason S., Sigurgisladottir S., Gunnlaugsson V.N., Johannsodottir J., Tornberg E. The effects of salt-curing and salting procedures on the microstructure of cod (Gadus morhua) muscle. Food Chemistry. 2011;126(1):109–115. [Google Scholar]
- Van Nguyen M., Arason S., Thorarinsdottir K.A., Thorkelsson G., Gudmundsdottir A. Influence of salt concentration on the salting kinetics of cod loin (Gadus morhua) during brine salting. Journal of Food Engineering. 2010;100(2):225–231. [Google Scholar]
- Vyncke W. Evaluation of the direct thiobarbituric acid extraction method for determining oxidative rancidity in mackerel (Scomber scombrus L.) European Journal of Lipid Science and Technology. 1975;77(6):239–240. [Google Scholar]
- Wong L.W., Loke X.J., Chang C.K., Ko W.C., Hou C.Y., Hsieh C.W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film for the preservation of tilapia (Orechromis niloticus) fillets. Food Chemistry. 2020;329 doi: 10.1016/j.foodchem.2020.126989. [DOI] [PubMed] [Google Scholar]
- World Organization for Animal Health. Welfare aspects of stunning and killing of farmed fish for human consumption. In Aquatic animal health code. (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.