Abstract
Problem of research
Candida spp. biofilms are complex microbial communities that have been associated with increasing resistance to clinically available antifungal drugs. Hence, novel pharmacological approaches with ability to inhibit biofilm formation have been investigated.
Aim of study
The aim was to analyze in vitro antifungal activity of Euterpe oleracea Mart. (açaí berry) extract on biofilm strains of Candida albicans, C. parapsilosis, and C. tropicalis that were formed on abiotic surfaces.
Remarkable methodology
Biofilms of C. albicans, C. parapsilosis, and C. tropicalis were grown in vitro. They were then treated with E. oleracea Mart. extract at different concentrations (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 μg/mL) for evaluation of both biofilm removal and anti-biofilm activity.
Remarkable results
All Candida species analyzed formed biofilms on abiotic surfaces. Yet, increased biofilm formation was displayed for C. tropicalis in comparison with the other two species. E. oleracea Mart. extract was shown to inhibit biofilm formation at all concentrations used when compared to no treatment (p < 0.05).
Significance of the study
In the current study, the extract of E. oleracea Mart. demonstrated antifungal activity against Candida albicans, C. parapsilosis, and C. tropicalis biofilms, regardless of the dose utilized. These results are important to evaluate a natural product as antifungal for Candida species.
Keywords: Euterpe oleracea, Candida, Biofilms, Antifungal activity
Introduction
Infections by Candida species, such as Candida albicans, C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis, are increasingly frequent [1–3]. They often occur due to host immunosuppression and/or virulence factors expressed by these yeasts, which contribute to their ability to colonize, penetrate, and invade tissues [4]. Such strains have also the ability to form biofilm.
According to Costerton (1999), biofilm consists of cells that attach to surfaces aggregate in a hydrated polymeric matrix of their own synthesis to form biofilms [5]. Importantly, biofilms may hinder the penetration of most antifungal agents, thereby leading to a poor response to treatment and drug resistance [6–9]. The formation and development of distinct phases of biofilm (adhesion/colonization, maturation, and dispersion) are mediated by regulatory genetic alterations and complex molecular events [10].
Because they consist in a matrix of microorganisms, biofilms can be considered a defense strategy of pathogens, affecting either biotic or abiotic surfaces such as medical devices (e.g., catheters, bladder probes) [11–13].
Moreover, biofilms have been increasingly linked to both mucosal infections such as candidiasis, which is facilitated by virulent factors from Candida species, including their capacity to form biofilms and the transition to filamentous or hyphal form [14].
In this context, natural products with ability to inhibit or disrupt biofilms have been investigated as a potential source of novel antifungals [15, 16]. Al-Sokari and Sheikha (2015) evidenced that crude extracts of Ruta graveolens L have good inhibition zone against Escherichia coli and Pseudomonas aeruginosa. Latex of Ficus carica Linn plants showed that it is a good inhibitor for Candida albicans [17].
Khan and Ahmad had studied the inhibitory effect of essential oil of Cymbopogan citratus and Syzygium aromaticum on the biofilm of drug-resistant Candida from clinical origin. C. citratus was capable to inhibit the biofilm formation of approximately 88% in C. albicans 04 and 82% in C. albicans SC5314 and S. aromaticum inhibited 52% and 57% biofilm in above-mentioned test strains at the same concentration [18].
Açaí (Euterpe oleracea Mart.) is a native plant from Amazonian region, and it has been used despite of its high antioxidant activity as antimicrobial. Euterpe genus includes over 28 species distributed throughout the Amazon region in Latin America, where E. oleracea, E. precatoria, and E. edulis are the most frequent species.
The phytochemical composition of the fruit known as “açaí berry” has been well characterized. It includes phenolic acids, anthocyanins (e.g., cyanidin-3-rutinoside and cyanidin-O-glucoside), proanthocyanidins, lignans (e.g., aryltetrahydronaphthalene, dihydrobenzofuran, furofuran, 8-O-4′-neolignan, and tetrahydrofuran), and polyphenolic constituents (e.g., epicatechin, the catechin homoorientin, orientin, isovitexin, and taxifolin deoxyhexose) [19].
Hence, the present study was aimed at investigating in vitro whether the treatment with E. oleracea Mart. extract would have the ability to inhibit or disrupt biofilms from C. albicans, C. parapsilosis, and C. tropicalis formed on abiotic surfaces.
It is important to study natural products as antifungal agents, considering the remarkable resistance of Candida species to imidazoles.
This is relevant to have alternative treatments because the increasing incidence of drug-resistant pathogens and the toxicity of existing antifungal compounds have increased interest in the antifungal properties of natural products. Furthermore, most of the available antifungals are either ineffective against Candida biofilms or exhibit their inhibitory activity at high concentrations.
Methods
Microbial strain identification
Commercially available strains of C. albicans (ATCC 10,231), C. tropicalis (ATCC 1369), and C. parapsilosis (ATCC 22,019) were obtained from Plast Labor (Rio de Janeiro, RJ, Brazil) and kept under refrigeration until use. Field experiments have been approved by the Brazilian Ministry of the Environment, Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio (approval number: 57805–1).
The strains were kept on Sabouraud Dextrose Agar with 4% chloramphenicol. The fungal suspension for the experiments was prepared from 24 h colonies diluted in 0.85% saline according to the 0.5 MacFarland scale (1–5 × 106 cells/mL).
Preparation of E. oleracea Mart. extract
The fruits of E. oleracea Mart. used in this study were collected from Juçara Park, located in São Luís, MA, Brazil (latitude: 02° 31′ 47″ S, longitude: 44° 18′ 10″ W, altitude: 24 m). Approval was obtained through the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SisGen, protocol A91B0BA). The extract was prepared using a protocol previously utilized with some adaptations [20]. Briefly, fruits were thawed and washed three times with distilled water, then soaked in warm water for 1 h. Subsequently, 365 g of whole fruit extract was grinded and mixed with 400 mL of ethyl alcohol p.a. For 10 days, the mix was shaken for 2 h/day, followed by vacuum filtration. The solvent was removed by rotary evaporation, lyophilized, aliquoted, and then refrigerated until use. After the maceration period, the extract was concentrated in rotoevaporator, lyophilized, and sent for identification of chemical compounds through mass spectrometry analysis.
Mass spectrometry analysis
Mass spectrometry analysis was performed using an electrospray ionization (ESI) source and a cyclotron analyzer coupled to a Fourier transform (ESI-FT-ICR MS). Samples were diluted with 0.1% acetic acid for positive analysis, and the resulting solution was then infused directly into the SOLARIX 9.4 T mass spectrometer (Bruker Daltonics, Bremen, Germany), operating in a range of 100–1000 m/z. The general conditions for EIS analysis were gas pressure of 0.3 psi, capillary voltage of 4.5 kV, and 220 °C for the ion transfer capillary temperature. The ESI ( +)—FT—ICR MS spectra were acquired and processed using the Compass Data Analysis software (Bruker Daltonics).
Evaluation of biofilm formation
Biofilm formation was evaluated in 96-well polystyrene microplates as previously described [21]. First, Candida spp. strains were cultivated in Sabouraud Dextrose Agar and incubated at 37 °C for 24 h. Next, isolates were diluted in saline solution to match the 0.5 McFarland turbidity standard, corresponding to 1 × 106 to 5 × 106 cells per mL [22]. The wells of microplates were filled sequentially in triplicates. In the negative control group, only 200 μL of BHI with 6% glucose were added. In the remaining wells, 180 μL of BHI with 6% glucose plus 20 μL of the suspension of each Candida species in saline solution was added. The microplates were incubated (37 °C, 24 h) and, subsequently, washed three times with sterile distilled water, and received 200 μL of crystal violet dye each well for 5 min. They were then washed three times with sterile distilled water, and lastly, 200 μL of sterile distilled water was added to each well for spectrophotometric analysis at 570 nm wavelength absorbance.
Adhesion and antibiofilm activity of E. oleracea Mart. extract on Candida spp.
Biofilms in 96-well polystyrene microplates were formed by inoculum of C. albicans, C. parapsilosis and C. tropicalis (100 μL), incubated for 1 h and 30 min at 37 °C, which corresponds to adhesion phase of yeasts to the abiotic surface [21].
Subsequently, the medium was aspirated, the wells were washed three times with PBS 1X and 200 μL of different concentrations (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 μg/mL) diluted in BHI with 6% glucose were added. The wells were washed with sterile PBS 1X 3 times, fixed with PA ethanol for 2 min and stained with 1% violet crystal solution for 5 min and again washed with sterile 1X PBS 5 times to remove excess dye.
After this step, 200 μL of sterile 1X PBS was added to each well and the reading was performed on the Epoch microplate reader with a wavelength of 570 nm. In columns A1, A2, and A3 the positive control was inoculated adding only the culture medium.
Biofilm removal activity of E. oleracea Mart. extract on Candida spp. biofilms
To analyze in vitro biofilm removal activity of E. oleracea Mart. extract on biofilms formed by C. albicans, C. parapsilosis, and C. tropicalis on abiotic surfaces of microplates (TPP) after 72 h, different concentrations (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 μg/mL) of E. oleracea Mart. extract (200 μL) were added in each well over the mature biofilm [21].
Subsequently, the wells were washed with sterile 1X PBS 3 times, fixed with PA ethanol for 2 min and stained with 1% violet crystal solution for 5 min and again washed 5 times with 1X PBS to remove excess dye. After this step, 200 μL of sterile 1X PBS was added to each well and the reading was performed on the Epoch microplate reader with a wavelength of 570 nm.
Antibiofilm activity of E. oleracea Mart. extract in coverslips
The biofilm evaluation in coverslips was performed using protocols previously reported with some adaptations [23–25]. C. albicans, C. tropicalis, and C. parapsilopsis yeasts were grown in a 24-well plate containing 13 cm round glass coverslip in 1-mL 0.85% saline.
The fungal samples were seeded in Sabouraud Dextrose Agar and incubated at 37 °C for 24 h. Next, isolates were diluted in saline solution to match the 0.5 McFarland turbidity standard, corresponding to 1 × 106 to 5 × 106 cells per mL [22]. In each well, 100 μL of the fungal suspension was added and placed to adhere for 6 h. The wells were subsequently washed three times with 0.85% saline solution and then 1 mL of each concentration of the extract (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 μg/mL) was added, and plates were incubated for 48 h.
Next, the plates were incubated at 37 °C for 48 h in a BOD oven. After that, the coverslips were gently removed, washed with PBS 1X 3 times, dried at room temperature, fixed with PA ethanol, stained with 1% violet crystal solution. The coverslips were glued with mesh onto the surface of a glass slide and analyzed under the Ninkon optical microscope.
Cells adhered to the coverslip were counted and classified according to cell arrangement patterns adhered to glass coverslips as: diffuse pattern, when yeast cells adhered to entire surface of the glass coverslip without forming cell groups; localized adhesion, when involving groups of yeast that adhered to localized regions of the coverslip; aggregative, which is characterized by yeast clumps arranged as “stacked bricks” or “grape clusters” that attached to the glass slide surface. The formation of filaments or pseudohyphae along the surface of the coverslip characterized the filamentous or pseudohyphal pattern.
Statistical analysis
Data were presented as means ± standard deviations or as medians and interquatile ranges. Normality of variables was analyzed using the Shapiro–Wilk test. Comparisons were performed using the Kruskal–Wallis or Wilcoxon tests. A p value ≤ 0.05 was considered to be statistically significant. Statistical analyzes were performed using STATA (StataCorp, Release 14; College Station, TX, USA).
Results
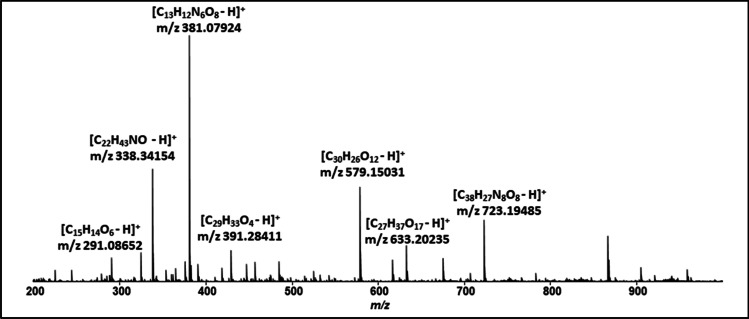
Figure 1 displays the chemical characterization of crude ethanolic extract of açaí berry fruit by mass spectrometry analysis using the positive method. The extract was shown to be rich in polyphenols, and the compounds identified are exhibited in Table 1.
Fig. 1.
Mass spectrometry from Euterpe oleracea Mart extract
Table 1.
Compounds isolated from Euterpe oleracea Mart extract
| m/z | Compounds |
|---|---|
| 291,08,652 | Epicatechin |
| 338,34,154 | Erucamide |
| 381,07,924 | N-(3-methoxy-5-nitrophenyl)-2-(5-methyl-3,4-dinitro-1H-pyrazol-1-yl) acetamide |
| 391,28,411 | Not identidied |
| 579,15,031 | Procyanidin B3 |
| 633,20,235 | (4-Acetoxy-5-((2-((4.5-dihyroxytetrahydro-2H-pyran-2-yl) oxy)-4.5-dilydroxytetrahydro-2H-pyran-3-yl) oxy)-3-hydroxytetrahydrofuran-2-yl) methyl (E)-3-(4-hydroxy-3-methoxyphenyl) acrylate |
| 723,19,485 | Not identified |
Further, Candida spp. used in this study had the ability to form biofilms on abiotic surfaces. Higher biomass formation on abiotic surfaces was observed in C. tropicalis (2.397 ± 0.23) and C. parapsilosis (1.176 ± 0.37) biofilms, whereas lower biomass was shown for C. albicans biofilm (0.53 ± 0.07).
A variation in biomass formed between Candida species was observed, where C. tropicalis was the most adherent, thereby producing more biofilm. Table 2 shows the absorbance before and after the addition of E. oleracea Mart. extract to surfaces containing biofilms formed by each Candida species analyzed. It was found a statistically significant difference between the median absorbance measured before and after treatment with E. oleracea Mart. extract for all Candida species evaluated (p < 0.001).
Table 2.
Median absorbance (570 nm) of Candida albicans, Candida parapsilosis, and Candida tropicalis species during biofilm formation, pre- and postuse of E. oleracea Mart extract
| Strains | Absorbance | p value** | |
|---|---|---|---|
| Pre (n = 72) | Post (n = 72) | ||
| Median [IQR*] | Median [IQR*] | ||
| C. albicans | 0.773 [0.625–1.072] | 0.058[0.049–0.081] | < 0.001 |
| C. parapsilosis | 1.504 [1.113–1.680] | 0.049[0.047–0.053] | < 0.001 |
| C. tropicalis | 2.500 [2.332–2.587] | 0.125 [0.056–0.333] | < 0.001 |
*Interquartile range (p25– p75)
**Wilcoxon test for paired samples
Since the treatment with E. oleracea Mart. extract decreased Candida spp. biomass formation, we further tested different concentrations of the extract. Table 3 displays significant differences in medians from before and after the addition of the extract at all concentrations (p < 0.05). Therefore, regardless of concentration, antibiofilm activity of açaí berry extract was maintained. Finally, when the removal activity of E. oleracea Mart. extract at the different concentrations was analyzed, there was a statistically significant difference between the values obtained for C. albicans, C. parapsilosis, and C. tropicalis (Table 4).
Table 3.
Absorbance for different concentrations of E. oleracea Mart extract, pre- and postuse, on biofilms of C. albicans, C. parapsilosis, and C. tropicalis on abiotic surfaces
| Concentration (μg/mL) | Absorbance | p value** | |
|---|---|---|---|
| Pre (n = 72) | Post (n = 72) | ||
| Median [IIQ*] | Median [IIQ] | ||
| 7.8 | 1.341[0.765–2.538] | 0.047 [0.046–0.055] | 0.019 |
| 15.6 | 1.112 [0.812–2.489] | 0.051 [0.049–0.323] | 0.007 |
| 31.2 | 1.614 [0.964–2.379] | 0.077 [0.053–0.146] | 0.007 |
| 62.5 | 1.631[1.123–2.511] | 0.085[0.049–0.253] | 0.007 |
| 125 | 0.709 [0.518–1.969] | 0.058 [0.047–0.386] | 0.007 |
| 250 | 1.644 [1.274–1.664] | 0.052 [0.049–0.066] | 0.007 |
| 500 | 1.688[0.843–2.479] | 0.053 [0.050–0.063] | 0.007 |
| 1000 | 1.579 [1.462–2.462] | 0.055 [0.053–0.086] | 0.007 |
*Interquartile range (p25– p75)
**Wilcoxon test for paired samples
Table 4.
Evaluation of the removal activity in C. albicans, C. parapsilosis, and C. tropicalis specimens in relation to the concentration of E. oleracea Mart extract
| Concentration (μg/mL) | Removal activity | ||
|---|---|---|---|
| C. albicans | C. parapsilosis | C. tropicalis | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 7.8 | 0.607 ± 0.096 | 0.154 ± 0.021 | 0.440 ± 0.204 |
| 15.6 | 0.504 ± 0.064 | 0.373 ± 0.213 | 0.665 ± 0.282 |
| 31.2 | 0.656 ± 0.058 | 0.196 ± 0.007 | 0.658 ± 0.168 |
| 62.5 | 0.844 ± 0.161 | 0.344 ± 0.254 | 0.805 ± 0.101 |
| 125 | 0.715 ± 0.140 | 0.497 ± 0.073 | 0.589 ± 0.065 |
| 250 | 0.962 ± 0.549 | 0.471 ± 0.060 | 0.807 ± 0.110 |
| 500 | 0.251 ± 0.112 | 0.660 ± 0.074 | 0.648 ± 0.068 |
| 1000 | 1.275 ± 0.279 | 1.083 ± 0.005 | 1.640 ± 0.093 |
| p value* | 0.016 | 0.010 | 0.034 |
*Kruskal–Wallis test
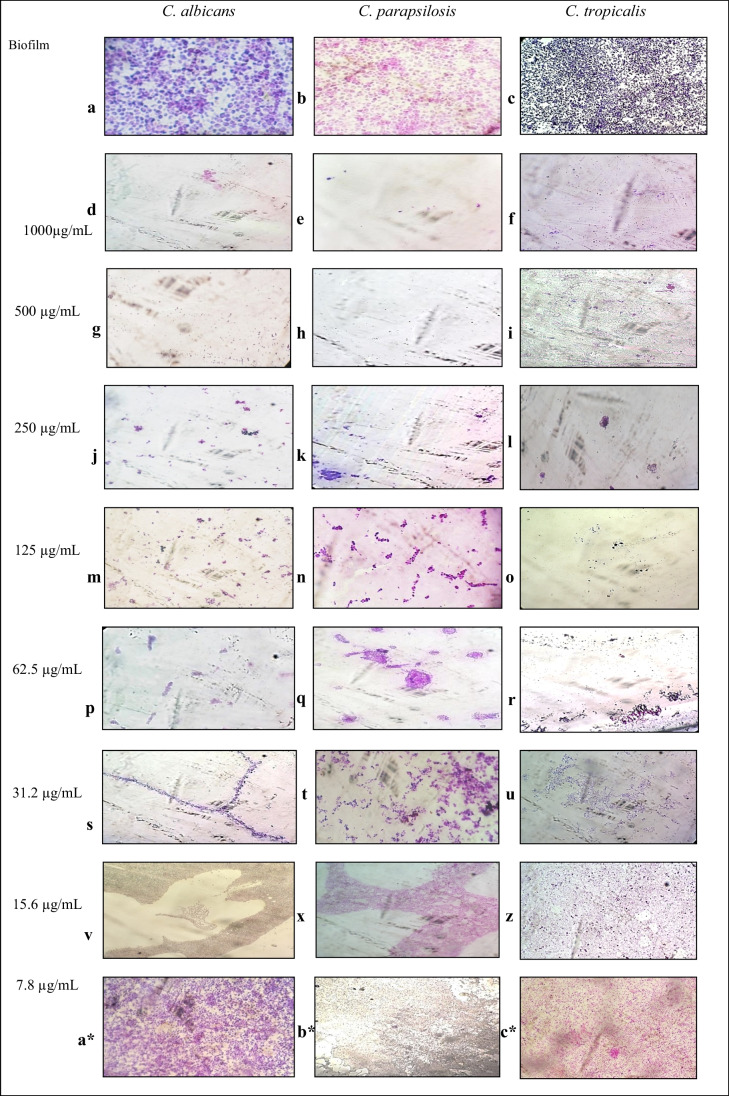
These findings corroborate with those obtained for the antibiofilm activity of E. oleracea Mart. extract on glass surface, which was found to prevent biofilm formation. Isolated cells (4 to 6 cells isolated per field) and 99% absence of biofilm (Fig. 2a and b) and 39 isolated cells and absence of biofilm at concentrations of 250, 500, and 1000 μg/mL (Fig. 2c) were observed, preventing biofilm development on glass coverslips. At concentrations of 31.2 to 125 μg/mL, there was also no biofilm formation; however, the presence of aggregative arrangements was observed (Fig. 2j–u). Notably, concentrations 7.8 (a–c*) and 15.6 (v–z) μg/mL were not able to prevent biofilm formation.
Fig. 2.
Antibiofilm effect of different concentration of the Euterpe oleracea ethanolic extract (μg/mL) on biofilm of C. albicans, C. parapsilosis, and C. tropicalis
Discussion
In the current study, the ability of C. albicans, C. parapsilosis, and C. tropicalis to form biofilms on abiotic surfaces was demonstrated, and the biomass produced varied according to each species. C. tropicalis adhered more easily to the abiotic material and was thus associated with greater biofilm formation.
The increasing incidence of drug-resistant pathogens and the toxicity of existing antifungal compounds have increased the interest from antifungal properties of natural products [26]. Several studies have been conducted using natural products to evaluate interference in C. albicans biofilm and anticandidal activity on planktonic and biofilm cultures of the C. parapsilosis complex [27, 28].
Most of the available antifungals are either ineffective against Candida biofilms or exhibit activity at very high concentrations [29, 30]. Plants are rich sources of bioactive molecules exhibiting various biological and pharmaceutical properties. Various phytochemicals are known to possess strong antimicrobial/antifungal activities [31]. Use of these phytochemicals against biofilms could be an excellent strategy [32, 33].
Cannas et al. (2014) evidenced a considerable activity of essential oil of Myrtus communis L. against C. albicans and C. parapsilosis after 24–48 h [34].
Borges et al. [35] demonstrated an increased adhesion of C. parapsilosis, which formed biofilm in copper fragments after 6 and 24 h of incubation, corroborating to our findings. In addition, açaí berry extract, in contact with abiotic surfaces containing biofilms formed by C. albicans, C. parapsilosis, and C. tropicalis, presented with a biofilm removal effect, whereby medians from before and after the treatment with the extract varied significantly in this study.
Several plant extracts, essential oils, and phytomolecules have been found to inhibit biofilm formation by Candida spp. [36]. Nair et al. [37] analyzed several phytochemicals and identified plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), a phytochemical of Plumbago species, as a potent antifungal agent against C. albicans, with a low minimum inhibitory concentration that was effective at preventing and dispersing biofilms in catheters formed by C. albicans. Therefore, in vivo and in vivo evaluation as well as clinical trials is required to further investigate the use of phytochemicals as candidate molecules for anti-biofilm drugs.
Polyphenols and flavonoids are the main chemical compounds from açaí. Polyphenols display excellent biofilm inhibitory activities in C. albicans. Studies showed that curcumin, pyrogallol and pyrocatechol possess anti-Candida biofilm activity [38]. Epigallocatechin-3-gallate extracted from green tea prevented biofilm formation by C. albicans [39].
Epigallocatechin-3-gallate (ECGC) has antifungal activity against human-pathogenic yeasts like Candida albicans. Although the mechanistic effects of EGCG are not fully understood, there are results indicating that EGCG binds to lipid membranes and affects the folic acid metabolism of bacteria and fungi by inhibiting the cytoplasmic enzyme dihydrofolate reductase [40].
In the current study, data from before and after the addition of açaí berry extract on biofilms of C. albicans, C. parapsilosis, and C. tropicalis on abiotic surfaces demonstrated a significant difference in biofilm formation at both the lowest (7.8 μg/mL) and the highest (1000 μg/mL) concentrations of the extract. It is worth mentioning that anti-biofilm activity of E. oleracea Mart. extract was maintained at all concentrations tested, suggesting that even at low doses, açaí berry extract shows an antifungal effect against Candida spp. biofilm. Nadaf et al. [41] observed that Hymenocallis littoralis leaf extract at concentrations of up to 70 μg/mL presented with anti-biofilm properties, reducing biomass production by C. albicans through interaction with active site residues of adhesin proteins.
The pathogenicity of Candida species through various virulence factors, such as adhesion to host surfaces, formation of biofilms and secretion of hydrolytic enzymes has been demonstrated [10]. The apparent increase in the emergence of C. glabrata, C. tropicalis, and C. parapsilosis species can be attributed to better identification methods and has also been associated with clinical impairment, interventions performed, and pharmacological therapy. Although studies to identify virulence factors, particularly in C. albicans, are frequent, relatively little is known regarding non-albicans Candida species. Millot et al. [42] analyzed several lichen extracts towards identifying their potential activity against C. albicans biofilm, eleven of which were found to inhibit biofilm maturation by C. albicans.
In relation to the dosage of E. oleracea Mart. extract utilized, the greatest inhibitory action on biofilm formation in the species analyzed in this study was obtained at a concentration of 250 μg/mL. Dias-Souza et al. [43] evaluated the effects of different doses of E. oleracea Mart. against Staphylococcus aureus biofilm and found a minimum biofilm eradication concentration of 250 μg/mL. This indicates that low concentrations are required to obtain an antibiofilm activity of açaí berry extract against Candida spp. biomass production.
Conclusion
In summary, an extract obtained from E. oleracea Mart. presented with both anti-biofilm activity and removal effect against Candida albicans, C. parapsilosis, and C. tropicalis biofilms, even when low concentrations were used. These results are important for the development of a new antifungal from a natural product. Further in vitro investigation is required to determine which compounds from açaí berry extract are responsible for the actions observed in the present study before developing in vivo analysis and clinical trials in this regard.
Acknowledgements
The authors thank the Center for Basic and Applied Immunology at the Federal University of Maranhão for the infrastructure provided for development of the current study. Funding for this study was provided by Maranhão State Research Support Foundation (Brazil, grant number 00900/17). The funding source had no role in the study conception and design, data collection and interpretation, manuscript draft, and approval of the final version to be published.
Abbreviations
- ATCC
Anatomical Therapeutic Chemical Code
- BHI
Brains heart infusion
- ESI
Electrospray ionization
- ESI-FT-ICR MS
Cyclotron analyzer coupled to a Fourier transform
- ICMBio
Instituto Chico Mendes de Conservação da Biodiversidade
- Sisgen
Sistema Nacional de Gestão do Patrimônio Genético
Data Availability
All data is included in the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krcmery V, Barnes AJ. Non-albicans Candida spp causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50(4):243–260. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 2.Arendrup MC. Candida and Candidaemia: susceptibility and epidemiology. Dan Med J. 2013;60(11):1–32. [PubMed] [Google Scholar]
- 3.Guinea J. Global trends in the distribution of candida species candidemia. Clinical Microbiology and infection. Clin Microbiol Infect. 2014;6:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 4.Tamura NK, Fernanda M, Negri N. Fatores de virulência de Candida spp. isoladas de cateteres venosos e mãos de servidores hospitalares. Revista da Sociedade Brasileira de Medicina Tropical. 2007;40(1):91–93. doi: 10.1590/S0037-86822007000100021. [DOI] [PubMed] [Google Scholar]
- 5.Lu YSUC, Wang A, Liu H. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol. 2017;7:e1001105. doi: 10.1371/journal.pbio.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Panizo MM, Revia Kina V, Dolande M, Selgrad S. Candida spp. in vitro susceptibility profile to four antifungal agents. Resistance surveillance study in Venezuelan strains. Med Mycol. 2009;2:137–43. doi: 10.1080/13693780802144339. [DOI] [PubMed] [Google Scholar]
- 8.Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18(5):310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araújo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;1:62–75. doi: 10.1016/j.tim.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Silva S, Negri M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemilogy, phatogenecity and antifungal resistence. FEMS Microbiol Rev. 2012;2:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 11.Cl Seabra, Cm Botelho, Henriques M, Oliveira R. Differential adherence and expression of virulence traits by Candida albicans and Candida parapsilosis in mono- and dual-species cultures in artificial saliva. Mycopathologia. 2013;176(1-2):33–40. doi: 10.1007/s11046-013-9661-0. [DOI] [PubMed] [Google Scholar]
- 12.Treviño-Rangel RJ, Rodriguez-Sánchez IPR, et al. Biofilm formation and genetic variability of BCR1 gene in the Candida parapsilosis complex. Rev Iberoam Micol. 2015;3:180–184. doi: 10.1016/j.riam.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Goel S, Mittal S, Chaudhary U. Role of non Albicans Candida Spp. and biofilm in neonatal ICU. Infect Disord Drug Targets. 2016;3:192–198. doi: 10.2174/1871526516666160818150148. [DOI] [PubMed] [Google Scholar]
- 14.Raut JS, Shinde RB, Chauhan NM, Karuppayil SM. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29(1):87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- 15.Zacchino SA, Butassi E, Cordisco E, Svetaz LA. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine. 2017;37:14–26. doi: 10.1016/j.phymed.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Sardi JC, Freires IA, et al. Unexplored endemic fruit species from Brazil: antibiofilm properties, insights into mode of action, and systemic toxicity of four Eugenia spp. Microb Pathog. 2017;105:280–287. doi: 10.1016/j.micpath.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Al-Sokari SS, El Sheikha AF. In vitro antimicrobial activity of crude extracts of some medicinal plants from Al-Baha Region in Saudi Arabia. J Food Nutr Scie. 2015;3(1–2):74–78. [Google Scholar]
- 18.Khan MSA, Ahmad I. Biofilm inhibition by Cymbopogon citratus and Syzygium aromaticum essential oils in the strains of Candida albicans. J Ethnopharmacol. 2012;140(2):416–423. doi: 10.1016/j.jep.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi KK, Pereira LF, Lamarão CV, Lima ES, da Veiga-Junior VF. Amazon acai: chemistry and biological activities: a review. Food Chem. 2015;179:137–151. doi: 10.1016/j.foodchem.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 20.de Moura RS, Pires KM, Santos Ferreira T, Lopes AA, Nesi RT, Resende AC, et al. Addition of acai (Euterpe oleracea) to cigarettes has a protective effect against emphysema in mice. Food Chem Toxicol. 2011;49(4):855–863. doi: 10.1016/j.fct.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, et al. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40(4):1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd edn. Document M27-AL3, Wayne, PA. CLSI, Pennsylvania, Clinical and Laboratory Standard Institute
- 23.Biasoli MS, Tosello ME, Magaró HM. Adherence of Candida strains isolated from the human gastrointestinal tract. Mycoses. 2002;45(11–12):465–469. doi: 10.1046/j.1439-0507.2002.00793.x. [DOI] [PubMed] [Google Scholar]
- 24.Ben Abdeljelil J, Saghrouni F, Emira N, Valentin-Gomez E, Chatti N, Boukadida J, Ben Saïd M, Del Castillo AL. Molecular typing of Candida albicans isolates from patients and health care workers in a neonatal intensive care unit. J Appl Microbiol. 2011;111(5):1235–1249. doi: 10.1111/j.1365-2672.2011.05121.x. [DOI] [PubMed] [Google Scholar]
- 25.Menezes EA, VasconcelosJúnior AA, Ângelo MR, Cunha Mda C, Cunha FA. Correlation between microdilution, Etest, and disk diffusion methods for antifungal susceptibility testing of fluconazole against Candida sp. blood isolates. Rev Soc Bras Med Trop. 2013;46(1):106–7. doi: 10.1590/0037-868210502013. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira LF, Jorge AO, Dos Santos SS. In vitro minocycline activity on superinfecting microorganisms isolated from chronic periodontitis patients. Braz Oral Res. 2006;20:202–206. doi: 10.1590/S1806-83242006000300004. [DOI] [PubMed] [Google Scholar]
- 27.Furletti VF, Teixeira IP, Obando-Pereda G, Mardegan RC, Sartoratto A, Figueira GM, Duarte RM, Rehder VL, Duarte MC, Hofling JF (2011) Action of coriandrum sativum L. essential oil upon oral Can-dida albicans biofilm formation. Evid Based Complement Altern Med 2011:985832 [DOI] [PMC free article] [PubMed]
- 28.Pires RH, Montanari LB, Martins CH, Zaia JE, Almeida AM, Matsumoto MT, Mendes-Giannini MJ. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia. 2011;172:453–464. doi: 10.1007/s11046-011-9448-0. [DOI] [PubMed] [Google Scholar]
- 29.Shinde RB, Raut JS, Karuppayil MS. Biofilm formation by Can- dida albicans on various prosthetic materials and its fluconazole sensitivity: a kinetic study. Mycoscience. 2012;53(3):220–226. doi: 10.1007/S10267-011-0155-Y. [DOI] [Google Scholar]
- 30.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Inf Immun. 2003;71(8):4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bink A, Pellens K, Cammue BP, Thevissen K. Anti-biofilm strategies: how to eradicate Candida biofilms? Open Mycol. 2011;J5:29–38. doi: 10.2174/1874437001105010029. [DOI] [Google Scholar]
- 33.Raut JS, Shinde RB, Chauhan NM, Mohan KS. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29(1):87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- 34.Cannas S, Molicotti P, Usai D, Maxia A, Zanetti S (2014) Antifungal, anti-biofilm and adhesion activity of the essential oil of Myrtus communis L. against Candida species. Nat Prod Res 28(23):2173–7 [DOI] [PubMed]
- 35.Borges KRA, Pimentel IV, Lucena LCLDS, Silva MACND, Monteiro SG, Monteiro CA, et al. Adhesion and biofilm formation of Candida parapsilosis isolated from vaginal secretions to copper intrauterine devices. Rev Inst Med Trop Sao Paulo. 2018;22(60):e59. doi: 10.1590/S1678-9946201860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raut JS, Karuppayil SM. Phytochemicals as Inhibitors of Candida Biofilm. Curr Pharm Des. 2016;27:1–24. doi: 10.2174/1381612822666160601104721. [DOI] [PubMed] [Google Scholar]
- 37.Nair SV, Baranwal G, Chatterjee M, Sachu A, Vasudevan AK, Bose C, et al. Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int J Med Microbiol. 2016;306(4):237–248. doi: 10.1016/j.ijmm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Shahzad M, Sherry L, Rajendran R, Edwards CA, Combet E, Ram- age G. Utilising polyphenols for the clinical management of Can- dida albicans biofilms. Int J Antimicrob Agents. 2014;44(3):269–73. doi: 10.1016/j.ijantimicag.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Evensen NA, Braun PC. The effects of tea polyphenols on Candida albicans: inhibition of biofilm formation and proteasome inactivation. Can J Microbiol. 2009;55(9):1033–1039. doi: 10.1139/W09-058. [DOI] [PubMed] [Google Scholar]
- 40.Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nadaf NH, Parulekar RS, Patil RS, Gade TK, Momin AA, Waghmare SR, et al. Biofilm inhibition mechanism from extract of Hymenocallis littoralis leaves. J Ethnopharmacol. 2018;10(222):121–132. doi: 10.1016/j.jep.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Millot M, Girardot M, Dutreix L, Mambu L, Imbert C. Antifungal and anti-biofilm activities of acetone lichen extracts against Candida albicans. Molecules. 2017;22(4):pii: E651. doi: 10.3390/molecules22040651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dias-Souza MV, Dos Santos RM, Cerávolo IP, Cosenza G, Ferreira Marçal PH, Figueiredo FJB. Euterpe oleracea pulp extract: chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb Pathog. 2018;114:29–35. doi: 10.1016/j.micpath.2017.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is included in the manuscript.