Highlights
-
•
Aroma characteristic of Chinese Dornfelder wine was featured for the first time.
-
•
61 volatiles determined by ‘sensomics’ could reconstruct Dornfelder wine aroma.
-
•
Difference in aroma profiles of Dornfleder wines from three regions was revealed.
-
•
Terpenoids were key volatiles contributing to floral note in Dornfelder wine.
-
•
Perceptual interaction existed between terpenoids and volatile phenols.
Keywords: Dornfelder wine, Aroma, Chromatography-olfactometry/mass spectrometry, Sensory interaction, Terpenoids
Abstract
In this study, aroma characteristics and odor-active compounds in Dornfelder wines from three main production regions of China were comprehensively investigated for the first time. The leading features of Chinese Dornfelder wines were black fruit, violet, acacia/lilac, red fruit, spice, dried plum, honey, and hay based on check-all-that-apply. Wines from the Northern Foothills of Tianshan Mountains and Eastern Foothills of Helan Mountains were dominated by floral and fruity aromas, while wines from the Jiaodong Peninsula were characterized by mushroom/earth, hay, and medicinal material notes. Aroma profiles of Dornfelder wines in three regions were successfully reconstructed with 61 volatiles determined by AEDA-GC-O/MS and OAV. Through aroma reconstitution, omission tests, and descriptive analysis, terpenoids could be regarded as varietal characteristic compounds directly contributing to floral perception in Dornfelder wines. Guaiacol, eugenol, and isoeugenol were further revealed to have a synergistic effect with linalool and geraniol on violet, acacia/lilac, spice, and black fruit.
1. Introduction
Dornfelder (Vitis vinifera L.) is a new red grape variety that originated in Germany, which was a hybrid between Helfensteiner (V. vinifera L.) and Heroldrebe (V. vinifera L.). The wines made from Dornfelder are recognized as having deep color, medium tannins, moderate acidity, and rich aromas (Fischer, Strasser, & Gutzler, 2000). Many researchers have focused on its protein components (Jaeckels et al., 2013, Riebel et al., 2017), polyphenol oxidase (Fronk et al., 2015), phenolic compounds and color quality (Wojdylo, Samoticha, & Chmielewska, 2021) in Dornfelder wines. Meanwhile, the investigation of aroma characteristics and odor-active compounds in Dornfelder wines is gaining more and more attention due to the distinct aroma typicality of this variety. In Germany, Dornfelder wine from Rheinhessen region was described as fruity, floral, smoky, and cooked-apple, along with (S)-2,3-dimethyl-1-butanol, phenethyl alcohol, and sotolon being identified as the most active aroma compounds (FD ≥ 8192) through gas chromatography–olfactometry (GC-O) analysis (Frank, Wollmann, Schieberle, & Hofmann, 2011). On the contrary, in Romania, Dornfelder wine was identified as a quite simple wine with the perception of predominant sour cherry notes, mineral, violets, and vegetal aroma (Antoce & Cojocaru, 2015), indicating that the aroma performance of Dornfelder wines might be various in different regions.
In the early 21st century, Dornfelder was introduced to China and presented good adaptability (He, Li, Li, & Wang, 2015). It has been cultivated in several regions in China with the planting areas expanding, such as in the region of Northern of Tianshan Mountains, Eastern Foothills of Helan Mountains, and Jiaodong Peninsula. The diversity of regional climate, soil, geology, human management measures, and their interactions in a vineyard could bring a specific style to grapes and wines (Fabres, Collins, Cavagnaro, & Rodríguez López, 2017). Different influences of terroir in various wine-producing regions in China on the chemical profiles and sensory characteristics of wines have also been investigated, such as studies on Marselan red wines (Lan, Liu, Zhang, Li, Shi, & Duan, 2022), and Cabernet Sauvignon and Merlot red wines (Jiang, Xi, Luo, & Zhang, 2013). Previous investigations on Dornfelder in China only focused on its varietal and oenological characteristics in comparison with other grape varieties, such as the varietal distinction between Dornfelder, Tannat, and Tempranillo from the Jiaodong Peninsula (He, Ren, & Qu, 2008), and the difference of phenolic composition in Dornfelder, Marselan, Tempranillo, and Montepulciano wines from the Northern of Tianshan Mountains (Wei, Lu, Jia, Chen, Li, & He, 2020). An integrated analysis of regional variation of aroma characteristics in Chinese Dornfelder wine and the sensory contribution of its key odor-active compounds is urgently needed.
With the development of molecular sensory science, namely the ‘sensomics’ approach which is regarded as a multidisciplinary and integrated technique for studying the sensory quality of food at the molecular level (Veronika & Peter, 2007), the investigation of odor-active compounds in wines and their perceptual interaction is getting more in-depth and thorough. To be more specific, GC-O combined with aroma extract dilution analysis (AEDA) is widely used to identify key odor-active compounds through the calculation of flavor dilution (FD), followed by aroma reconstitution and omission tests to verify the contribution of key volatile compounds to the overall aroma (Villière, Le Roy, Fillonneau, & Prost, 2018). Some rapid descriptive sensory methods have also emerged to replenish sensory research, such as Napping, Flash profile (FP), Pivot© profile (PP), Check-all-that-apply (CATA), and Rate-all-that-apply (RATA). Among them, CATA is widely used in the study of wine color, aroma, and taste (Nanou et al., 2020, Veríssimo et al., 2021) by providing a list of sensory vocabulary to the evaluator to select all of the terms that apply to the sample (Jaeger et al., 2015). The combination of traditional ‘sensomics’ methods with rapid descriptive sensory methods could demonstrate a more comprehensive analysis of wine aroma profiles.
In this study, the aroma profiles of Dornfelder wines from three premium regions of China, the Northern Foothills of Tianshan Mountains, the Eastern Foothills of Helan Mountains, and the Jiaodong Peninsula were investigated. The odor-active compounds in Dornfelder wines were identified by AEDA-GC-O/mass spectrometry (MS) and the analysis of odor activity values (OAVs). Combined with sensory analysis, the influence of environmental conditions in three regions on the aroma profiles of Dornfelder wines was revealed. Furthermore, the sensory contribution and interaction of key odor-active compounds were investigated through reconstitution and omission tests.
2. Material and methods
2.1. Wine samples
Three monovarietal Dornfelder wines were made in the vintage of 2020 in CITIC Guoan Winery (Changji, Xinjiang province), Chateau Yuanshi (Yinchuan, Ningxia province), and Chateau Junding (Yantai, Shandong province), respectively, which located in three premium wine-producing regions in China, namely the Northern Foothills of Tianshan Mountains (NFTM), the Eastern Foothills of Helan Mountains (EFHM), and the Jiaodong Peninsula (JP). All wines were made strictly under the local standard winemaking procedures and considered to represent the regional characteristics in Dornfelder wines from each area. After malolactic fermentation, six 750 mL bottles of wine samples from each region were collected. The physicochemical parameters of wine samples are shown in Table S1.
2.2. Reagents and standards
Analytical grade NaCl, NaOH, anhydrous Na2SO4, glucose, and tartaric acid were supplied by Beijing Chemical Reagent Company (Beijing, China). The solid phase extraction (SPE) column Cleanert PEP-SPE (500 mg/6 mL) was supplied by Tianjin Bonna-Agela Technologies (Tianjin, China). The GC-grade solvents, including methanol, ethanol, and dichloromethane were purchased from Honeywell (Marris Township, NJ, USA). The reference standards of C7-C30 n-alkanes, as well as aroma compounds used for identification, quantification, aroma reconstitution, and omission tests, were supplied by Sigma-Aldrich (St. Louis, MO, USA).
2.3. Gas chromatography–olfactometry/mass spectrometry analysis (GC-O/MS)
2.3.1. Volatile extraction
The aroma compounds were extracted by liquid–liquid-extraction (LLE) combined with solvent-assisted flavor evaporation (SAFE) distillation (Glasbläserei Bahr, Freising, Germany) according to our previous method with slight modifications (Lan et al., 2019). A 150 mL of wine sample with the addition of NaCl to saturation was extracted three times with 80 mL, 40 mL, and 40 mL of dichloromethane, respectively, in a 500 mL flask with magnetic stirring for 10 min each time at room temperature. The organic and aqueous phases were separated by a separatory funnel and then processed in sequence by the SAFE distillation coupled with a high-vacuum pump (KYKY Technology Co. ltd, Beijing, China) to separate volatiles from the nonvolatile matrix (Engel, Bahr, & Schieberle, 1999). The apparatus was thermostated at 35 °C and kept under a high vacuum (10−3 Pa), and the sample was added dropwise into the evaporation flask slowly to maintain the high vacuum. After the completeness of SAFE distillation and an additional 5 min, the vacuum was released. The SAFE distillate was then naturally thawed at room temperature, and dried with 10 g of anhydrous Na2SO4. After storage at 4 ℃ for 12 h, the organic phase was concentrated to a final volume of 1 mL by a steam of nitrogen, to obtain a concentration of volatile components for GC-O/MS analysis.
2.3.2. Aroma extraction dilution analysis (AEDA)
The concentrated extract of the aroma compounds was gradually diluted with dichloromethane in the proportions of 1:1, 1:3, 1:9, 1:27, 1:81, …, and 1:2187, and analyzed by GC-O/MS, respectively. The undiluted extract was evaluated by five experienced judges (panel 1, two males and three females, aged 24–30) to come to a consensus on odor-active zones. Each dilution was then analyzed by three judges (panel 2, one male and two females, aged 24–28) who have been trained before the formal analysis to identify all odor active zones by sniffing the undiluted extract and solutions of reference standards through the same GC-O/MS condition. The reference standards used for training were prepared in 1 mL dichloromethane solution as listed in Table S2. The FD factor was defined as the maximum dilution in which at least two or more judges could still perceive the odorant.
2.3.3. GC-O/MS conditions
GC-O/MS analysis was conducted using an Agilent 7890B GC coupled with an Agilent 5975C MS and an olfactory detection port (ODP, Gerstel, Germany) on an HP-INNOWAX capillary column (60 m × 0.25 mm id, 0.25 μm film thickness; J&W Scientific, CA, USA). The injection volume was 1 μL and the temperature of the injector was set at 250 ℃ in splitless mode. The initial oven temperature was held at 40 ℃ for 2 min and increased to 230 ℃ at 4 ℃/min held for 15 min. The GC effluent was split into the mass selective detector (MSD) and ODP at a ratio of 1:1. The flow rate of the helium carrier gas was maintained at 2 mL/min. The mass spectrometer ion source was an electron ionization (EI) source with an electron energy of 70 eV, and the mass scanning range was 30–350 m/z. The temperatures of both the MSD and the ODP transfer line heater were set at 250 ℃, and the temperatures of the ion source and quadrupole were set at 230 ℃ and 150 ℃, respectively.
2.3.4. Identification of volatiles
The identification of volatile compounds was achieved by comparing the obtained mass spectra and retention indices (RI) with those of reference standards and compounds in the NIST 2014 mass spectrometry database through the automatic mass spectral deconvolution and identification system (AMDIS).
2.4. Detection and quantification of aroma compounds
2.4.1. Solid phase extraction-gas chromatography-triple quadrupole tandem mass spectrometry (SPE-GC-QqQ-MS/MS)
Methional, methionol, butyrolactone, trans-whiskey lactone, γ-nonalactone, guaiacol, eugenol, 4-ethylphenol, syringol, phenol, 2-methylphenol, 4-methylguaiacol, 4-ethylguaiacol, 4-vinylguaiacol, 4-propylguaiacol, vanillin, and isoeugenol were extracted and quantified by the targeted SPE-GC-QqQ-MS/MS method with slight modification (Qian et al., 2020, Yang et al., 2022). Cleanert PEP-SPE column was placed in Fotector Plus automatic SPE system (RayKol Group Corp., ltd., Xiamen, China) and was activated with 10 mL dichloromethane, 10 mL methanol, and 10 mL Milli-Q water in sequence. A 20 mL wine sample mixed with 10 μL of l-menthol (internal standard, 580 mg/L) passed through the activated SPE column at a flow rate of 2 mL/min. Then 5 mL of Milli-Q water was added to rinse the extraction column to remove polar substances, such as pigments, sugars, and acids. Finally, 15 mL of dichloromethane was added to elute the target compounds. The organic phase containing target compounds was dried with 1.5 g of anhydrous Na2SO4, and concentrated to 500 μL by a gentle stream of nitrogen, followed by being filtered with a 0.22 μm organic microporous filter membrane.
An Agilent 7890B GC equipped with an Agilent 7000D triple quadrupole mass spectrometer (Agilent Technologies, Inc. CA, USA) was used for detection. The separation was carried out on an HP-5MS UI capillary column (30 m × 0.25 mm id, 0.25 μm film thickness; J&W Scientific, CA, USA), and the flow rate of the helium carrier gas was 1 mL/min. The injection volume was 1 μL and the temperature of the injector was set at 250 ℃ in splitless mode. The initial column temperature was held at 40 ℃ for 1 min and then increased to 160 ℃ at 5 ℃/min, finally, increased to 220 ℃ at 30 ℃/min, held for 1 min. After each analytical run, a post-run was performed at 280 ℃ for 2 min. The mass spectrometer was operated in electron ionization (EI) mode at 70 eV with MRM. The quadrupole temperatures of MS1 and MS2 were both set at 150 ℃, and the temperatures of the ion source and auxiliary heater were set at 230 ℃ and 300 ℃, respectively. The quenching gas was helium with a flow rate of 2.25 mL/min, and the collision gas was nitrogen with a flow rate of 1.5 mL/min. Calibration curves of each reference standard prepared in the model wine matrix (13 % alcohol with 2 g/L glucose and 6 g/L tartaric acid, pH 3.5) were used for quantification.
2.4.2. Headspace solid-phase microextraction-GC–MS (HS-SPME-GC–MS)
The other aroma compounds, mainly including esters, higher alcohols, terpenoids, and C13-norisoprenoids (Table S3), were extracted and quantified by the HS-SPME-GC–MS method (Ling et al., 2022). A wine sample (5 mL) was placed in a 20 mL vial with the addition of 1.5 g NaCl and 10 μL 4-methyl-2-pentanol (internal standard, 1.018 g/L) capped with a PTFE-silicon septum. The equilibration was conducted at 40 °C for 30 min and then the activated 2 cm DVB/CAR/PDMS 50/30 μm SPME fiber (Supelco, Bellefonte, PA, USA) was inserted into the headspace of the vial for 30 min extraction. The injection was at a split ratio of 5:1. The temperature of the injector was set at 250 ℃. The initial column temperature was held at 50 ℃ for 1 min, then, increased to 220 ℃ at 3 ℃/min, held for 3 min, finally, increased to 240 ℃ at 10 ℃/min, and held for 5 min. The MS conditions were the same as that of GC-O/MS.
2.5. Sensory analysis
2.5.1. General conditions
Sensory analysis was performed in the sensory laboratory equipped with individual booths at a controlled room temperature (20 ℃). Wine samples were presented in the tasting glass (ISO 3591:1977) of the international organization for standardization (ISO). All panelists were recruited from the Center of Viticulture and Enology (CFVE) and received sensory training for more than half a year.
2.5.2. Check-all-that-apply (CATA)
The aroma profiles of Dornfelder wines were analyzed by the CATA experiment. In the first session, eight experienced judges (panel 3, three males and five females, aged 24–30) discussed the aroma characteristics of Dornfelder wines and established a list of aroma attributes, including black fruit (blueberry/blackberry/mulberry), red fruit (hawthorn/cherry), violet, acacia/lilac, spice, dried plum, honey, hay, peach, mushroom/earth, green pepper, caramel, strawberry, apple/pear, mint, banana, cooked potato, smoky, medicinal material (the mixed perception of some Chinese medicinal herbs, such as liquorice and clove), and orange. The reference standards were prepared by the ‘Le nez du vin’ (Jean Lenoir, Provence, France), model wine solution mixed with aroma standards, or natural materials, which were used for identification training of each aroma attribute before formal sensory analysis in this study (Table S4). Then, panel 4 (nine males and 22 females, aged 19–29) were asked to select descriptors from the list of aroma attributes with no limitation to the number of descriptors. Cochran’s Q test was conducted for CATA data to identify descriptors with significant differences (P < 0.05) in Dornfelder wines from three regions.
2.5.3. Aroma reconstitution
Three aroma reconstitutions were prepared through 61 key odor-active compounds dissolved in the odorless model wine matrix (13 % alcohol with 2 g/L glucose and 6 g/L tartaric acid, pH 3.5) in the concentrations in three Dornfelder wines (NFTM, EFHM, and JP), respectively (Table 3). According to the CATA results, eight aroma attributes with high frequencies were selected and confirmed by all panelists through discussions, which were used to describe the overall aroma of Dornfelder wines, including black fruit, red fruit, violet, acacia/lilac, spice, dried plum, honey, and hay. Panel 5 (five males and nine females, aged 23–27) were asked to evaluate the aroma intensity of Dornfelder original wines and reconstitution models. The intensity of each aroma attribute was scored on an 11-point scale (0 = very low intensity, 10 = strong intensity). The significance analysis of the sensory data was carried out in PanelCheck (Nofima, Tromso, Norway), and the judges with large differences were eliminated through Friedman’s test with an F value. The statistically significant level was 5 % (P ≤ 0.05).
Table 3.
Concentration and OAV analysis of key odor-active compounds in Dornfelder wines.
| Compound a | Concentration (μg/L) b |
OAV d |
||||
|---|---|---|---|---|---|---|
| NFTM c | EFHM | JP | NFTM c | EFHM | JP | |
| Ethyl isobutanoate | 98.37 ± 1.91b | 83.63 ± 2.20c | 120.72 ± 2.70 a | 6.56 | 5.58 | 8.05 |
| Isobutyl acetate | 193.81 ± 2.92 a | 84.29 ± 3.69b | 68.22 ± 1.28c | 0.12 | 0.05 | 0.04 |
| Ethyl butanoate | 389.70 ± 7.32 a | 304.36 ± 12.60b | 156.57 ± 3.38c | 19.48 | 15.22 | 7.83 |
| Butyl acetate | 6.36 ± 0.21 a | 1.30 ± 0.03b | 0.38 ± 0.04c | 0.00 | 0.00 | 0.00 |
| Isoamyl acetate | 6415.26 ± 122.98 a | 1594.68 ± 80.78b | 1268.69 ± 28.10c | 213.84 | 53.16 | 42.29 |
| Ethyl hexanoate | 466.24 ± 12.49 a | 478.01 ± 30.80 a | 431.91 ± 11.04 a | 93.25 | 95.6 | 86.38 |
| Ethyl lactate | 55569.01 ± 886.04 a | 49439.61 ± 5385.60 a | 56750.07 ± 920.32 a | 0.56 | 0.49 | 0.57 |
| Methyl octanoate | 4.46 ± 0.08b | 4.65 ± 0.27b | 5.72 ± 0.18 a | 0.02 | 0.02 | 0.03 |
| Ethyl octanoate | 774.83 ± 16.86b | 770.31 ± 41.43b | 926.44 ± 27.87 a | 1.34 | 1.33 | 1.60 |
| Ethyl 3-hydroxybutyrate | 615.80 ± 78.78b | 1009.85 ± 230.23 a | 209.63 ± 10.33c | 0.03 | 0.05 | 0.01 |
| Ethyl 2-hydroxy-4-methylpentanoate | 90.45 ± 1.91 a | 50.36 ± 3.05c | 68.52 ± 0.85b | 1.77 | 0.99 | 1.34 |
| Diethyl succinate | 5606.23 ± 624.54b | 4555.85 ± 527.44b | 11060.55 ± 196.34 a | 0.06 | 0.05 | 0.11 |
| Phenethyl acetate | 139.27 ± 7.97 a | 56.89 ± 5.46b | 55.54 ± 0.17b | 0.56 | 0.23 | 0.22 |
| Total esters | 70369.79 ± 1764.00 a | 58433.77 ± 6323.57b | 71122.95 ± 1202.59 a | |||
| 2,3-Butanedione | 13621.96 ± 503.52 a | 6465.80 ± 385.37b | 2272.09 ± 136.80c | 136.22 | 64.66 | 22.72 |
| Acetoin | 14322.61 ± 761.84 a | 8686.61 ± 2178.50b | 2749.40 ± 310.57c | 0.10 | 0.06 | 0.02 |
| Phenylacetaldehyde | 8.74 ± 0.25 a | 8.68 ± 1.23 a | 4.93 ± 0.35b | 8.74 | 8.68 | 4.93 |
| Total aldehydes and ketones | 27953.31 ± 1265.62 a | 15161.10 ± 2565.10b | 5026.42 ± 447.72c | |||
| 1-Propanol | 44343.86 ± 824.22 a | 33749.43 ± 3115.18b | 26543.53 ± 628.54c | 0.14 | 0.11 | 0.09 |
| 2-Methyl-1-propanol | 44540.37 ± 415.47c | 65006.64 ± 4491.07 a | 56961.05 ± 714.22b | 1.11 | 1.63 | 1.42 |
| 3-Methyl-1-butanol | 204530.76 ± 2603.74 a | 207265.23 ± 5329.95 a | 188308.43 ± 2556.27b | 3.15 | 3.19 | 2.90 |
| 2,3-Butanediol | 2519993.81 ± 10409.62 a | 2467577.15 ± 1039129.17 a | 710224.82 ± 159990.10b | 21.00 | 20.56 | 5.92 |
| Phenylethyl alcohol | 24322.67 ± 4088.29 a | 33819.49 ± 7090.20 a | 35543.18 ± 3074.90 a | 2.43 | 3.38 | 3.55 |
| Total higher alcohols | 2837731.47 ± 18341.33 a | 2807417.95 ± 1059155.57 a | 1017581.01 ± 166964.03b | |||
| 1-Hexanol | 1257.33 ± 23.84b | 1536.40 ± 34.59 a | 708.24 ± 19.40c | 0.16 | 0.19 | 0.09 |
| (E)-3-Hexenol | 63.99 ± 1.68 a | 49.39 ± 2.11b | 25.98 ± 0.20c | 0.06 | 0.05 | 0.03 |
| (Z)-3-Hexenol | 53.92 ± 4.57 a | 55.48 ± 1.18 a | 49.45 ± 5.03 a | 0.05 | 0.06 | 0.05 |
| (E)-2-Hexenol | 12.40 ± 0.30b | 16.48 ± 1.83 a | 10.54 ± 0.28b | 0.00 | 0.00 | 0.00 |
| (Z)-2-Hexenol | 11.04 ± 0.78b | 20.39 ± 0.79 a | 11.21 ± 0.40b | – | – | – |
| Total C6 alcohols | 1398.68 ± 31.17b | 1678.14 ± 40.50 a | 805.42 ± 25.31c | |||
| Acetic acid | 319478.52 ± 7530.18 a | 340344.50 ± 53610.61 a | 132516.75 ± 13707.63b | 1.60 | 1.70 | 0.66 |
| Isobutanoic acid | 1777.09 ± 57.31b | 1629.08 ± 132.99b | 2128.58 ± 83.90 a | 0.77 | 0.71 | 0.93 |
| Butanoic acid | 787.68 ± 32.23b | 910.99 ± 76.99 a | 604.29 ± 6.16c | 0.08 | 0.09 | 0.06 |
| Hexanoic acid | 1465.95 ± 262.59 a | 1864.46 ± 325.70 a | 1735.70 ± 103.93 a | 3.49 | 4.44 | 4.13 |
| 3-Methylbutanoic acid | 946.13 ± 19.25 a | 651.26 ± 52.51c | 852.32 ± 20.94b | 0.32 | 0.22 | 0.28 |
| (E)-2-Hexenoic acid | 69.69 ± 11.53 a | 34.91 ± 3.41b | 7.61 ± 0.45c | – | – | – |
| Octanoic acid | 1938.21 ± 321.39 a | 2391.83 ± 105.90 a | 2660.76 ± 215.68 a | 3.88 | 4.78 | 5.32 |
| Total fatty acids | 326463.27 ± 8234.49 a | 347827.03 ± 54308.12 a | 140506.01 ± 14138.69b | |||
| Methional | 1.12 ± 0.05 a | 1.21 ± 0.36 a | 1.31 ± 0.51 a | 2.24 | 2.43 | 2.62 |
| Methionol | 122.03 ± 11.87b | 91.88 ± 15.38b | 1754.34 ± 58.89 a | 0.24 | 0.18 | 3.51 |
| Total sulfur compounds | 123.15 ± 11.92b | 93.09 ± 15.73b | 1755.66 ± 59.41 a | |||
| Furfural | Nd | Nd | 39.09 ± 1.45 | – | – | 0.00 |
| 2-Furanmethanol | 191.78 ± 25.68b | 116.51 ± 12.94b | 667.49 ± 29.45 a | 0.10 | 0.06 | 0.33 |
| Total furfurals | 191.78 ± 25.68b | 116.51 ± 12.94c | 706.57 ± 30.90 a | |||
| Butyrolactone | 3055.23 ± 151.24b | 3935.28 ± 415.43 a | 3232.78 ± 221.68b | 0.15 | 0.20 | 0.16 |
| γ-Nonalactone | 11.98 ± 0.48 a | 8.23 ± 0.56b | 5.06 ± 0.22c | 0.40 | 0.27 | 0.17 |
| trans-Whiskey lactone | 17.42 ± 0.09 a | 14.95 ± 0.59b | 14.27 ± 1.08b | 0.14 | 0.12 | 0.12 |
| Total lactones | 3084.63 ± 151.80b | 3958.46 ± 416.58 a | 3252.11 ± 222.98b | |||
| β-Damascenone | 9.64 ± 0.51b | 10.96 ± 1.09b | 19.42 ± 0.53 a | 192.73 | 219.19 | 388.37 |
| Total C13-norisoprenes | 9.64 ± 0.51b | 10.96 ± 1.09b | 19.42 ± 0.53 a | |||
| Linalool | 30.10 ± 0.34b | 98.04 ± 3.57 a | 8.67 ± 0.25c | 2.01 | 6.54 | 0.58 |
| Geraniol | 16.43 ± 2.31b | 22.91 ± 3.65 a | 9.54 ± 0.27c | 0.55 | 0.76 | 0.32 |
| d-Limonene* | 2.18 ± 0.09b | 4.41 ± 0.40 a | Nd | 0.22 | 0.44 | – |
| (E)-Rose oxide* | 0.06 ± 0.00b | 0.17 ± 0.01 a | 0.05 ± 0.00b | 0.11 | 0.33 | 0.11 |
| Terpinolene* | 6.13 ± 0.06b | 8.12 ± 0.19 a | 5.01 ± 0.02c | 0.03 | 0.04 | 0.03 |
| α-Terpineol | 9.38 ± 1.12b | 34.36 ± 4.40 a | 2.48 ± 0.11c | 0.04 | 0.14 | 0.01 |
| Citronellol* | 7.37 ± 0.75c | 22.64 ± 2.84 a | 12.67 ± 0.16b | 0.18 | 0.57 | 0.32 |
| (Z)-Nerol* | 9.78 ± 0.84b | 12.16 ± 1.11 a | 6.68 ± 0.14c | 0.03 | 0.04 | 0.02 |
| Total terpenoids | 55.91 ± 3.77b | 155.31 ± 11.62 a | 20.69 ± 0.62c | |||
| Guaiacol | 12.26 ± 0.41 a | 9.19 ± 0.23b | 10.23 ± 0.23b | 1.23 | 0.92 | 1.02 |
| Eugenol | 27.94 ± 0.65 a | 16.62 ± 0.58b | 28.41 ± 0.46 a | 5.59 | 3.32 | 5.68 |
| 4-Ethylphenol | 60.25 ± 2.54 a | 4.04 ± 0.75c | 48.36 ± 0.22b | 0.14 | 0.01 | 0.11 |
| Syringol | 63.65 ± 2.44 a | 38.51 ± 1.49b | 36.62 ± 2.63b | 0.11 | 0.07 | 0.06 |
| Phenol | 24.87 ± 1.52b | 22.15 ± 1.06b | 133.96 ± 7.97 a | – | – | – |
| 2-Methylphenol* | 2.45 ± 0.25b | 2.95 ± 0.21 a | 2.52 ± 0.07 ab | 0.08 | 0.10 | 0.08 |
| 4-Methylguaiacol* | 5.68 ± 0.02b | 5.28 ± 0.04b | 13.28 ± 0.48 a | 0.09 | 0.08 | 0.20 |
| 4-Ethylguaiacol* | 6.51 ± 0.25b | 4.11 ± 0.03c | 7.39 ± 0.38 a | 0.20 | 0.12 | 0.22 |
| 4-Vinylguaiacol* | 291.56 ± 1.60 a | 168.12 ± 18.71b | 206.67 ± 18.18b | 0.27 | 0.15 | 0.19 |
| 4-Propylguaiacol* | 6.06 ± 0.00b | 6.05 ± 0.00c | 6.09 ± 0.00 a | – | – | – |
| Vanillin* | 13.46 ± 0.17c | 20.46 ± 2.04b | 104.06 ± 0.88 a | 0.07 | 0.10 | 0.52 |
| Isoeugenol* | 19.86 ± 0.01 a | 18.30 ± 0.12b | 20.22 ± 0.22 a | 3.31 | 3.05 | 3.37 |
| Total volatile phenols | 534.49 ± 8.34b | 315.77 ± 24.16c | 617.82 ± 23.66 a | |||
* Represents the terpenoids and volatile phenols being detected for quantification but not identified in the GC-O analysis.
Different letters in the same row indicate significant differences by Duncan’s test (P < 0.05). ‘Nd’ represents ‘not detected’.
NFTM: Wines from the Northern Foothills of Tianshan Mountains; EFHM: Wines from the Eastern Foothills of Helan Mountains; JP: Wines from the Jiaodong Peninsula.
Threshold of each compound is listed in Table S3.
2.5.4. Aroma omission tests
The omission models were prepared by omitting targeted compounds from the complete reconstitution models. Panel 6 (seven males and 11 females, aged 21–27) were asked to identify the distinctive sample through a triangle test with samples provided in random order. To further verify the contribution and sensory interaction of key odor-active compounds of Dornfelder wines, quantitative sensory analysis of the complete reconstitution and omission models were then carried out by the panel 5 to evaluate those eight aroma attributes (black fruit, red fruit, violet, acacia/lilac, spice, dried plum, honey, and hay). The difference in the intensity of each attribute between the complete reconstitution model and the omission model was evaluated by independent sample t-test.
2.6. Statistical analysis
Cochran’s Q test was performed by XLSTAT (Addinsoft, Paris, France). One-way analysis of variance (ANOVA) and independent sample t-test were conducted with SPSS 20.0 (SPSS, Chicago, IL, USA). The sensory radar chart of the original wine and the reconstructed wine was prepared by OriginPro 2019b (Microcal, Northampton, MA, USA).
3. Results and discussion
3.1. Different aroma characteristics of Dornfelder wines among three regions based on CATA analysis
The frequency of choice of 20 descriptors by 31 panelists was sorted out (Table 1). In general, black fruit had the highest total frequency, followed by violet, acacia/lilac, red fruit, spice, dried plum, honey, and hay, which were regarded as the main descriptors to depict Chinese Dornfelder wines. After the consistency reached by panelists, these descriptors were also used for subsequent aroma intensity analysis.
Table 1.
Results of CATA experiments in Dornfelder wines from three regions of China.
| NO | Attributes a | Frequency in each sample b |
Total frequency | ||
|---|---|---|---|---|---|
| NFTM c | EFHM | JP | |||
| 1 | Black fruit (Blueberry/Blackberry/Mulberry) | 21 | 25 | 21 | 67 |
| 2 | Violet | 21 | 22 | 19 | 62 |
| 3 | Acacia/lilac | 20 | 16 | 15 | 51 |
| 4 | Red fruit (Hawthorn/Cherry) | 14 | 16 | 20 | 50 |
| 5 | Spice | 18 | 16 | 14 | 48 |
| 6 | Dried plum | 15 | 13 | 17 | 45 |
| 7 | Honey* | 19 | 10 | 10 | 39 |
| 8 | Hay* | 6 | 13 | 20 | 39 |
| 9 | Peach* | 16 | 13 | 7 | 36 |
| 10 | Mushroom/Earth* | 10 | 7 | 19 | 36 |
| 11 | Green pepper | 14 | 11 | 11 | 36 |
| 12 | Caramel | 11 | 13 | 11 | 35 |
| 13 | Strawberry | 11 | 14 | 9 | 34 |
| 14 | Apple/Pear | 13 | 9 | 10 | 32 |
| 15 | Mint | 10 | 13 | 8 | 31 |
| 16 | Banana* | 13 | 11 | 6 | 30 |
| 17 | Cooked potato | 9 | 9 | 10 | 28 |
| 18 | Smoky | 7 | 8 | 13 | 28 |
| 19 | Medicinal material* | 2 | 8 | 12 | 22 |
| 20 | Orange | 10 | 7 | 4 | 21 |
* Significant difference by Cochran’s Q test (P < 0.05) in the three regions.
The descriptors of Dornfelder wines were sorted according to the total frequency by 31 panelists.
NFTM: Wines from the Northern Foothills of Tianshan Mountains; EFHM: Wines from the Eastern Foothills of Helan Mountains; JP: Wines from the Jiaodong Peninsula.
As for the regional difference, honey, banana, peach, mushroom/earth, hay, and medicinal material had significant differences in the frequency among wines from three regions (P < 0.05), which were labeled in Table 1. The description of mushroom/earth, hay, and medicinal material were recognized by more panelists in wines from the Jiaodong Peninsula (JP). On the contrary, wines from the Northern Foothills of Tianshan Mountains (NFTM) were found with more descriptions of honey, peach, and banana, followed by wines from the Eastern Foothills of Helan Mountains (EFHM). The frequency of violet, acacia/lilac, and spice were also found higher in the NFTM and EFHM wines, which was consistent with the aroma description of Dornfelder wines in the previous studies (He et al., 2015, Wei et al., 2020). The region-driven difference of sensory characteristics in wines has also been investigated by a wide range of studies through the application of rapid sensory analysis methods (e.g., Flash profile and Pivot© profile), such as the studies on Garnacha (Alegre, Sáenz-Navajas, Hernández-Orte, & Ferreira, 2020), Shiraz (Pearson, Schmidtke, Francis, Carr, & Blackman, 2020), Malagousia and Roditis wines (Nanou et al., 2020). The chemical basis of the aroma variation in Chinese Dornfelder wines from three regions (NFTM, EFHM, and JP) was further investigated by GC-O analysis.
3.2. GC-O analysis
According to the GC-O results, a total of 59 odor-active zones were detected, among which 49 odor-active zones could be identified, especially for some floral and spice zones (Table 2). The aroma compounds with FD ≥ 3 were further summarized and discussed according to their origins and chemical categories.
Table 2.
Key odor-active compounds identified in Dornfelder wines by AEDA-GC-O/MS.
| NO | RI a | CAS | Odor description | Compounds b | FD factor |
Qualitative method d | ||
|---|---|---|---|---|---|---|---|---|
| NFTM c | EFHM | JP | ||||||
| 1 | 1022 | 97–62-1 | Fruity (pineapple) | Ethyl isobutanoate | 729 | 243 | 243 | RI, MS, O |
| 2 | 1025 | 431–03-8 | Buttery | 2,3-Butanedione | 729 | 729 | 729 | RI, MS, O |
| 3 | 1037 | 110–19-0 | Fruity | Isobutyl acetate | 243 | 81 | 81 | RI, MS, O |
| 4 | 1057 | 71–23-8 | Chemical, solvent | 1-Propanol | 9 | 9 | 3 | RI, MS, O |
| 5 | 1060 | 105–54-4 | Fruity | Ethyl butanoate | 729 | 27 | 81 | RI, MS, O |
| 6 | 1091 | 123–86-4 | Fruity | Butyl acetate | 81 | 27 | 9 | RI, MS, O |
| 7 | 1099 | 78–83-1 | Chemical, solvent | 2-Methyl-1-propanol | 243 | 81 | 243 | RI, MS, O |
| 8 | 1137 | 123–92-2 | Fruity (Banana) | Isoamyl acetate | 729 | 81 | 81 | RI, MS, O |
| 9 | 1222 | 123–51-3 | Chemical, solvent | 3-Methyl-1-butanol | > 2187 | > 2187 | > 2187 | RI, MS, O |
| 10 | 1245 | 123–66-0 | Fruity | Ethyl hexanoate | 243 | 81 | 243 | RI, MS, O |
| 11 | 1302 | 513–86-0 | Buttery | Acetoin | 81 | 81 | 81 | RI, MS, O |
| 12 | 1357 | 97–64-3 | Sweet, fruity, creamy | Ethyl lactate | > 2187 | 243 | 243 | RI, MS, O |
| 13 | 1358 | 111–27-3 | Herbaceous | 1-Hexanol | 3 | 3 | 3 | RI, MS, O |
| 14 | 1369 | 928–97-2 | Herbaceous | (E)-3-Hexenol | 3 | 3 | 3 | RI, MS, O |
| 15 | 1391 | 928–96-1 | Herbaceous | (Z)-3-Hexenol | 81 | 27 | 27 | RI, MS, O |
| 16 | 1401 | 111–11-5 | Fruity | Methyl octanoate | 81 | 81 | 9 | RI, MS, O |
| 17 | 1412 | 928–95-0 | Herbaceous | (E)-2-Hexenol | 9 | 3 | 3 | RI, MS, O |
| 18 | 1421 | 928–94-9 | Herbaceous | (Z)-2-Hexenol | 3 | 3 | 3 | RI, MS, O |
| 19 | 1443 | 106–32-1 | Fruity | Ethyl octanoate | 27 | 27 | 81 | RI, MS, O |
| 20 | 1467 | 64–19-7 | Vinegar | Acetic acid | 27 | 27 | 27 | RI, MS, O |
| 21 | 1473 | 3268–49-3 | Cooked potato | Methional | 27 | 243 | 729 | RI, MS, O |
| 22 | 1480 | 98–01-1 | Caramel, almond, sweet | Furfural | 9 | 9 | 27 | RI, MS, O |
| 23 | 1528 | 5405–41-4 | Fruity | Ethyl 3-hydroxybutyrate | 243 | 243 | 243 | RI, MS, O |
| 24 | 1544 | 19132–06-0 | Caramel, almond | 2,3-Butanediol | 27 | 27 | 81 | RI, MS, O |
| 25 | 1552 | 78–70-6 | Floral | Linalool | 729 | 729 | > 2187 | RI, MS, O |
| 26 | 1553 | 10348–47-7 | Fruity | Ethyl 2-hydroxy-4-methylpentanoate | 729 | 243 | 81 | RI, MS, O |
| 27 | 1586 | 79–31-2 | Cheese | Isobutanoic acid | 243 | 27 | 81 | RI, MS, O |
| 28 | 1596 | – | Sweet | Unknown 1596 | 243 | 27 | 9 | O |
| 29 | 1647 | 107–92-6 | Cheese | Butanoic acid | 243 | 243 | 243 | RI, MS, O |
| 30 | 1652 | 96–48-0 | Caramel, sweet | Butyrolactone | 243 | 243 | 81 | RI, MS, O |
| 31 | 1663 | 122–78-1 | Floral | Phenylacetaldehyde | 3 | 9 | 9 | RI, MS, O |
| 32 | 1671 | 98–00-0 | Sweet, solvent | 2-Furanmethanol | 81 | 81 | 81 | RI, MS, O |
| 33 | 1684 | 503–74-2 | Cheese | 3-Methylbutanoic acid | 27 | 243 | 729 | RI, MS, O |
| 34 | 1686 | 123–25-1 | Wine, fruit | Diethyl succinate | 729 | 243 | 729 | RI, MS, O |
| 35 | 1707 | 98–55-5 | Floral | α-Terpineol | 729 | 729 | 729 | RI, MS, O |
| 36 | 1729 | 505–10-2 | Cooked potato | Methionol | 27 | 243 | 729 | RI, MS, O |
| 37 | 1785 | – | Fruity | Unknown 1785 | 243 | 27 | 9 | O |
| 38 | 1812 | – | Fruity | Unknown 1812 | 81 | 729 | 81 | O |
| 39 | 1832 | 103–45-7 | Rose, sweet | Phenethyl acetate | 243 | 81 | 243 | RI, MS, O |
| 40 | 1837 | 23726–93-4 | Sweet, honey | β-Damascenone | > 2187 | 729 | > 2187 | RI, MS, O |
| 41 | 1853 | 106–24-1 | Rose | Geraniol | 729 | 729 | 729 | RI, MS, O |
| 42 | 1863 | 142–62-1 | Cheese | Hexanoic acid | 81 | 81 | 243 | RI, MS, O |
| 43 | 1878 | 90–05-1 | Smoky, medicinal | Guaiacol | 729 | 729 | 243 | RI, MS, O |
| 44 | 1928 | 60–12-8 | Rose | Phenylethyl Alcohol | > 2187 | > 2187 | > 2187 | RI, MS, O |
| 45 | 1978 | 55013–32-6 | Coconut | trans-Whiskey lactone | 9 | 27 | 243 | RI, MS, O |
| 46 | 1997 | 13419–69-7 | Fruity, sweet | (E)-2-Hexenoic acid | 9 | 27 | 81 | RI, MS, O |
| 47 | 2024 | 108–95-2 | Plastic | Phenol | 243 | 81 | 27 | RI, MS, O |
| 48 | 2050 | 104–61-0 | Coconut, sweet | γ-Nonalactone | 81 | 81 | 81 | RI, MS, O |
| 49 | 2078 | 124–07-2 | Cheese | Octanoic acid | 729 | 243 | 243 | RI, MS, O |
| 50 | 2114 | – | Caramel, Spice | Unknown 2114 | 81 | 243 | 81 | O |
| 51 | 2140 | – | Spice | Unknown 2140 | 243 | 243 | 81 | O |
| 52 | 2175 | – | Caramel | Unknown 2175 | 81 | 81 | 81 | O |
| 53 | 2185 | 97–53-0 | Clove, smoky | Eugenol | 729 | 729 | > 2187 | RI, MS, O |
| 54 | 2193 | 123–07-9 | Smoky | 4-Ethylphenol | 243 | 81 | 81 | RI, MS, O |
| 55 | 2254 | – | Caramel | Unknown 2254 | 27 | 243 | 729 | O |
| 56 | 2283 | 91–10-1 | Clove, smoky | Syringol | 243 | 243 | 81 | RI, MS, O |
| 57 | 2322 | – | Smoky | Unknown 2322 | 27 | 243 | 27 | O |
| 58 | 2351 | – | Smoky, oaky | Unknown 2351 | 729 | 729 | 729 | O |
| 59 | 2404 | – | Smoky, medical | Unknown 2404 | 729 | > 2187 | 729 | O |
The retention index (RI) on HP-INNOWAX capillary column (60 m × 0.25 mm × 0.25 μm).
Unknown, not identified compounds.
NFTM: Wines from the Northern Foothills of Tianshan Mountains; EFHM: Wines from the Eastern Foothills of Helan Mountains; JP: Wines from the Jiaodong Peninsula.
Qualitative way of compounds. RI: Identification based on RI; MS: Identification based on mass spectrometry compared with NIST database and standard compound; O: Identification by aroma description.
Among those compounds, terpenoids are mainly derived from grape berries and can be generated through the biological action of enzymes and microorganisms or chemical reactions under acidic and heating conditions during the fermentation process (Juega et al., 2012, Zhang and Pan, 2021). In this study, three terpenoids (including linalool, α-terpineol, and geraniol) were identified with relatively high FD factors (FD ≥ 729). It was inconsistent with the previous GC-O study on Dornfelder wines in the Rheinhessen region (Germany), in which the authors did not identify any terpenoids through GC-O study, although they also used the term ‘flowery’ to describe Dornfelder wines (Frank et al., 2011). However, in their recent study, linalool was identified in both Dornfelder juice and wines (Frank, & Schieberle, 2022). It can be speculated that terpenoids in Chinese Dornfelder wines might be the reason for the floral note described above. As for norisoprenoids, the secondary metabolites degraded from carotenoids in grape berries, β-damascenone had high FD factors in all three Dornfelder wines (FD ≥ 729), which mainly contributed to honey and sweetness in wines and was identified as a ubiquitous key odor-active compound in various styles of wines, such as icewines (Lan et al., 2019, Ma et al., 2017), botrytized wines (Sarrazin, Dubourdieu, & Darriet, 2007), and late harvest wines (Genovese, Gambuti, Piombino, & Moio, 2007).
Volatile phenols can not only be extracted from oak wood during barrel aging, but are also derived from the metabolic activities of grape and yeast (Krstic, Johnson, & Herderich, 2015). In this study, five volatile phenols were identified in Dornfelder wines not being aged in oak barrels through GC-O analysis, including guaiacol, phenol, eugenol, 4-ethylphenol, and syringol. Among them, eugenol had the largest FD factor (FD ≥ 729). It was similar to the result of the research on Dornfelder wines from the Rheinhessen region of Germany (Frank et al., 2011). The authors found that eugenol presented the largest FD factor among the volatile phenols contributing to a clove-like aroma, which was considered to be derived from grape berries. Besides eugenol, 4-ethylphenol and guaiacol were also identified by GC-O in their study and described as phenolic and smoky odors, respectively. As shown in Table 2, the FD factor of 4-ethylphenol in the NFTM wine was the highest (FD = 243), while guaiacol in the JP wine had the lowest FD factor (FD = 243). Meanwhile, phenol and syringol also had higher FD factors in the NFTM and EFHM wines than those in the JP wine.
Esters, higher alcohols, and fatty acids mainly originate from the fermentation process. A total of 13 esters were identified through GC-O analysis, most of which were higher in the NFTM wine compared to other two wines, such as ethyl 2-methylpropanoate, isobutyl acetate, ethyl butanoate, isoamyl acetate, ethyl 2-hydroxy-4-methylpentanoate, and ethyl lactate. According to the results of AEDA-GC-O/MS (Table 2), 3-methyl-1-butanol (identified as ‘chemical, solvent’ note) and phenylethyl alcohol (identified as ‘floral’ note) had the largest FD factors (FD = 2187) in wines from three regions, but the remaining higher alcohols in the EFHM wine had lower FD factors than those in other two wines. Five C6 alcohols were identified in Dornfelder wine, of which the FD factor was higher in the NFTM wine. The FD factors of octanoic acid in three wines were > 243, which might be a potential key aroma compound in Dornfelder wines contributing to the ‘cheese’ note. 3-Methylbutanoic acid, which was also identified as a ‘cheese’ note, had the largest FD factor (FD = 729) in the JP wine, while its FD factor in the NFTM wine was only 27.
Sulfur compounds, lactones, furans, aldehydes, and ketones were also identified through GC-O analysis. Methional and methionol had higher FD factors in the JP wine (FD = 729) than those in the EFHM and NFTM wines. γ-Nonalactone had the FD factors of 81 in wines from three regions described as having coconut and sweet aromas. Butyrolactone had higher FD factors in the EFHM and NFTM wines, while trans-whiskey lactone had higher FD factors in the JP wine. The FD factor of 2-furanmethanol in wines from three regions was 81 higher than that of furfural. 2,3-Butanedione was one of the most important ketones mainly contributing butter and sweet aromas to wines and had the FD factors of 729 in wines from three regions.
3.3. Difference of key volatile compounds in Dornfelder wines among three regions
Some odor-active zones couldn’t be identified by the GC-O analysis, mainly due to the weak signal intensity of the peak. Among them, several unknown compounds were speculated to be terpenoids and volatile phenols in consideration of the perception of obvious floral and spice aromas (Table 2). Therefore, in addition to 49 compounds identified through GC-O, other qualitative terpenoids (d-limonene, (E)-rose oxide, terpinolene, citronellol, and (Z)-nerol) and volatile phenols (2-methylphenol, 4-methylguaiacol, 4-ethylguaiacol, 4-vinylguaiacol, 4-propylguaiacol, vanillin, and isoeugenol) in Dornfelder wines were also quantified and conducted OAV analysis for further investigation (Table 3).
The total concentration of terpenoids in Chinese Dornfelder wines from three regions ranged from 20.69 μg/L to 155.31 μg/L. Although the concentration of terpenoids detected in Dornfelder grape berries was lower than that of Muscat grapes such as Morio Muskat and Siegerrebe, it was still higher than that of common non-aromatic varieties, such as Chardonnay and Pinot Noir (Nitsch, Hey, Rühl, & Bitz, 2014). The distinct floral aroma in Chinese Dornfelder wines might be ascribed to those grape-derived terpenoids, especially linalool with the highest OAV among eight terpenoids quantified in wines from three regions. It was consistent with a recent study that the concentration of linalool in Dornfleder grape juice, must, and young and oak wood-aged wines taken from the same batch of grapes from Germany ranged from 5.2 to 50 μg/L, even reaching a high concentration of 71 μg/L (Frank et al., 2022). Except for being regulated by internal genes, terpenoids were also reported to be affected by external climate factors (Mele, Kang, Lee, & Islam, 2021). There is a distinct climate difference among these three premium Dornfleder wine-producing regions in China. The Northern Foothills of Tianshan Mountains (NFTM) and the Eastern Foothills of Helan Mountains (EFHM) are both continental semi-arid climate with longer sunlight duration, higher diurnal temperature range, and photosynthetically active radiation (PAR) compared to the Jiaodong Peninsula (JP), which is located in the eastern coast of China and has a temperate monsoon climate with more rainfall (Lan et al., 2022, Jiang et al., 2013). Meanwhile, slightly shorter sunlight duration and PAR but higher average temperature and rainfall were found in the EFHM region than the NFTM region. As shown in Table 3, the concentration of total terpenoids was the highest in the EFHM wine, followed by the NFTM wine and the JP wine. The accumulation of terpenoids could be influenced by sun exposure, especially for linalool (98.04 μg/L in the EFHM wine), which was reported to be the most light-sensitive terpenoid representing higher content in semi-shaded (50 %) treatment than 20 % and 100 % exposure to sunlight (González-Barreiro, Rial-Otero, Cancho-Grande, & Simal-Gándara, 2015). The higher temperature in the EFHM region might also increase the contents of terpenoids, as previous research found higher terpenoids in ‘Muscat Blanc a Petits Grains’ grapes in the Changli region with a warmer climate than that in Gaotai region in China (Wen, Zhong, Gao, Lan, Duan, & Pan, 2015). Therefore, the significantly high level of terpenoids in the EFHM wine might be due to the integrated environmental conditions, of which the influence on the terpenoid metabolism in grapes is still a worthwhile issue.
As for C13-norisoprenoids derived from grape carotenoids, β-damascenone was the most important compound with high OAV ranging from 192.73 to 388.37 in Chinese Dornfelder wines, of which the highest concentration was found in the JP wine (Table 3). It was consistent with the study of Marselan red wines that a higher concentration of β-damascenone was found in wines from the JP region than in wines from the NFTM region (Lan et al., 2022). Another study of Cabernet Sauvignon from different Chinese viticulture regions also found that grape berries from the JP region (Yantai City) had higher contents of C13-norisoprenoids than those from the NFTM region (Wujiaqu County) and the EFHM region (Yongning County) (Xie, Lei, Wang, Wang, Ren, & Zhang, 2018). Many viticultural trials have revealed that the factors of temperature, sun exposure, water stress, and soil type could have influence on the accumulation of C13-norisoprenoids (González-Barreiro et al., 2015, Mele et al., 2021). However, the terroir influence from different regions could not be directly interpreted by the findings from the viticultural trials related to the modulation of vineyard microclimate, which needed further investigation.
Total volatile phenols in Chinese Dornfelder wines reached the concentration of 315.77 ∼ 617.82 μg/L (Table 3), which were higher than those un-barrel-aged wines made from V. vinifera varieties (Jiang et al., 2013). Except for the factors like oak chips and tannin additions, the smoke exposure of vineyards, and the effect of yeast and malolactic bacteria, volatile phenols were also reported to differ among grape cultivars (Krstic et al., 2015). Therefore, the high level of volatile phenols which were derived from the grape berries could be considered as the varietal aroma compounds of Dornfelder wines. Among the volatile phenols, guaiacol, eugenol, and isoeugenol had high OAVs above 1.0 in wines from at least one region. In addition, the concentration of volatile phenols in three Dornfelder wines existed obvious differences. Eugenol, phenol, 4-methylguaiacol, 4-ethylguaiacol, 4-propylguaiacol, vanillin, and isoeugenol had higher concentrations in the JP wine. Conversely, the concentrations of guaiacol, 4-ethylphenol, syringol, 4-vinylguaiacol, and 2-methylphenol were much higher in the NFTM and EFHM wines. The difference in volatile phenols might also be related to the different climate traits among three regions, especially being influenced by the factor of sunlight and temperature as reported in the previous research (González-Barreiro et al., 2015).
Except for linalool, β-damascenone, guaiacol, eugenol, and isoeugenol, other 17 compounds were identified as having OAV > 1.0 in wines from at least one region, which were regarded to have a great contribution to the aroma of Chinese Dornfelder wines. Six esters were included, among which isoamyl acetate was found high values of OAVs (42.29 ∼ 213.84) and had the highest concentration in the NFTM wine. The concentration of total esters was also higher in the NFTM wine, which might be the reason for its prominent fruity notes. Four higher alcohols and three fatty acids were identified, of which the difference among three regions were was quite limited, except that 2,3-butanediol presented the lowest concentration in the JP wine. As for other compounds, the concentrations of phenylacetaldehyde and 2,3-butanedione were much higher in the NFTM and EFHM wines, while the concentrations of methional and methionol were higher in the JP wine.
3.4. Aroma reconstitution
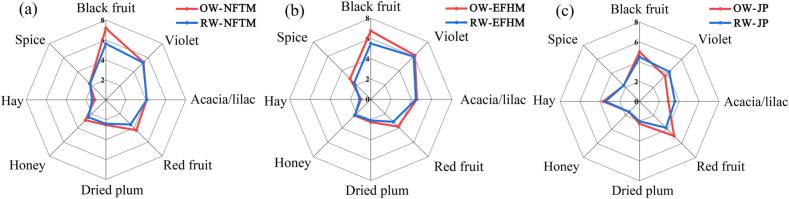
To demonstrate the volatile compounds contributing to the overall aroma of Chinese Dornfelder wines, aroma reconstitution experiment was carried out. The reconstitution model consisted of 61 key odor-active compounds, including 49 volatile compounds with FD ≥ 3 identified by GC-O analysis, and the other five terpenoids and seven volatile phenols discussed in the 3.3 section (Table 3). As shown in Fig. 1, the aroma profiles of the reconstitution models were similar to those of the original wines, confirming the contribution of 61 key odor-active compounds. The aroma profiles of Dornfelder wines among three regions showed distinct differences. The intensities of black fruit, violet, acacia/lilac, and honey were higher in the NFTM and EFHM wines, while the intensity of hay was higher in the JP wine. Combined with the results of CATA (Table 1), it could be summarized that Dornfelder wines from the Northern Foothills of Tianshan Mountains and the Eastern Foothills of Helan Mountains were dominated by floral and fruity notes, while wines from the Jiaodong Peninsula was characterized by pronounced mushroom/earth, hay, and medicinal material aromas. The different performance of Dornfelder wines from three regions might be due to the distinct terroir expression of the variety, which was also evidenced by previous studies that the aroma characteristics of Dornfelder wines from Germany and Romania were different (Antoce and Cojocaru, 2015, Frank et al., 2011).
Fig. 1.
Aroma profile analysis of complete reconstitution models (RW) and Dornfelder wine samples (OW) from the Northern Foothills of Tianshan Mountains (a), the Eastern Foothills of Helan Mountains (b), and the Jiaodong Peninsula (c).
3.5. Identification of odor-active compounds and their sensory interaction by omission test
After the establishment of aroma reconstitution, omission models were prepared to explore the contribution of eight categories of volatile compounds (A1-A8) in Dornfelder wines (Table 4). The spiked concentration of each aroma compound was consistent with the concentration determined in the wine from the Eastern Foothills of Helan Mountains, which was considered as the typical wine to well express the varietal character of Dornfelder. Each omission model was presented in comparison with the complete reconstitution model by triangle tests. The omission of esters, terpenoids, volatile phenols, and C13-norisoprenoids was successfully perceived by > 12 out of 18 panelists, showing a significant influence on the aroma perception of Dornfelder wines. In comparison, the omission of aldehydes and ketones, and fatty acids had relatively fewer effects which were perceived by the panelists at 0.05 significance level.
Table 4.
Triangle test of the omission of volatile compounds from the complete reconstitution model.
| NO a | Omitted compounds | Correct number in all | Significance b |
|---|---|---|---|
| A1 | Esters | 13/18 | *** |
| A2 | Terpenoids | 12/18 | ** |
| A3 | C13-norisoprenes | 12/18 | ** |
| A4 | C6 alcohols | 6/18 | ns. |
| A5 | Higher alcohols | 9/18 | ns. |
| A6 | Aldehydes and ketones | 11/18 | * |
| A7 | Fatty acids | 10/18 | * |
| A8 | Volatile phenols | 12/18 | ** |
| B1 | Linalool | 7/18 | ns. |
| B2 | Geraniol | 7/18 | ns. |
| B3 | Eugenol | 8/18 | ns. |
| B4 | Isoeugenol | 7/18 | ns. |
| B5 | Guaiacol | 6/18 | ns. |
| B6 | Linalool, geraniol | 12/18 | ** |
| B7 | Eugenol, isoeugenol | 15/18 | *** |
| B8 | Eugenol, guaiacol | 10/18 | * |
| B9 | Isoeugenol, guaiacol | 10/18 | * |
| B10 | Linalool, eugenol | 12/18 | ** |
| B11 | Linalool, isoeugenol | 11/18 | * |
| B12 | Linalool, guaiacol | 13/18 | *** |
| B13 | Geraniol, eugenol | 6/18 | ns. |
| B14 | Geraniol, isoeugenol | 10/18 | * |
| B15 | Geraniol, guaiacol | 12/18 | ** |
A1-A8 were the omission of each category of volatile compounds from the complete reconstitution model. B1-B15 were the onefold/binary omission of terpenoids and volatile phenols from the complete reconstitution model.
‘ns.’ represents no significant difference; *: represents 0.05 significance level; **: represents 0.01 significance level; ***: represents 0.001 significance level.
According to the results of the CATA experiment, the aroma characteristic of typical Dornfelder wines was regarded as a prominent floral aroma with the perception of spice note which might be mostly related to high concentrations of terpenoids and volatile phenols, respectively (Ferreira and Lopez, 2019, Frank and Schieberle, 2022, Frank et al., 2011). Combined with the results of GC-O and OAV analysis (Table 2, Table 3), linalool (FD = 729, OAV > 1.0), geraniol (FD = 729, OAV = 0.76), eugenol (FD = 729, OAV > 1.0), isoeugenol (OAV > 1.0), and guaiacol (FD = 729, OAV = 0.92) were speculated to highly contribute to the typical aroma of Dornfelder wines. To further confirm this hypothesis, the contribution of individual terpenoids and volatile phenols, and their perceptual interaction on aroma expression were further investigated through 15 aroma omission models (B1-B15) (Table 4). Results showed that the omission of a single compound (B1-B5) couldn’t lead to a recognizable perception compared to the complete reconstitution model. However, in the binary mixture omission group (B6-B15), except for the omission of geraniol and eugenol (B13), the omission of other combinations had a clear impact on the aroma perception of Dornfelder wines. It indicated that sensory interaction was not only found in the compounds from the same chemical category, but also in the compounds with different aroma descriptions, namely, there might exist a more complex contribution of terpenoids and volatile phenols on the perception of Chinese Dornfelder wine aroma.
Descriptive analysis was used in the omission and reconstructed models for further exploration. As shown in Table 5, the absence of linalool and geraniol (RW-T) had a significantly lower intensity of blank fruit (P < 0.05), violet (P < 0.001), and acacia/lilac (P < 0.01) than the complete reconstitution model (RW), among which those floral-like perceptions were corresponding to the recognition of these two terpenoids in GC-O analysis (Table 2), indicating their direct contribution on violet and acacia/lilac aroma in Dornfelder wines. The absence of guaiacol, eugenol, and isoeugenol (RW-V) also had a lower intensity of violet than RW (P < 0.05). Although the difference of spice between RW-V and RW was not significant, the simultaneous absence of terpenoids and volatile phenols (RW-T-V) had an obviously lower intensity of spice than RW (P < 0.05). Besides, the aroma intensities of black fruit, violet, and acacia/lilac were significantly reduced in RW-T-V compared to RW, of which the intensities were also lower than in RW-T and RW-V, further confirming that there might exist a synergistic effect between terpenoids and volatile phenols. Similar results were reported that volatile phenols would interact with terpenoids and some unknown compounds to produce a note of herb perception and benefit aroma perception in Moutai liquor (Wang, Hu, Lei, Lin, Wang, & Wu, 2015), or interact with lactones and contribute to the typical aroma of overripe oranges aroma in Bordeaux liqueurs (Stamatopoulos, Frérot, Tempère, Pons, & Darriet, 2014). Further research could focus on the sensory interaction of other kinds of terpenoids and volatile phenols in wines on the molecular level.
Table 5.
Olfactory impact of terpenoids and volatile phenols by quantitative descriptive analysis of omission models.
| Omission model a | △I = I omission model - I reconstitution model b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Black fruit | Violet | Acacia/lilac | Red fruit | Dried plum | Honey | Hay | Spice | |
| RW-T | −0.479* | −1.786*** | −1.074** | 0.117 | 0.082 | −0.144 | 0.096 | −0.377 |
| RW-V | −0.114 | −0.671* | −0.346 | 0.053 | 0.039 | 0.085 | −0.111 | −0.413 |
| RW-T-V | −0.607* | −2.250*** | −1.482*** | −0.140 | −0.226 | −0.401 | 0.246 | −0.685* |
RW: Reconstitution model; RW-T: Reconstitution model in the absence of two terpenoids (linalool, geraniol); RW-V: Reconstitution model in the absence of three volatile phenols (eugenol, isoeugenol, and guaiacol); RW-T-V: Reconstitution model in the absence of two terpenoids and three volatile phenols.
‘I’ represents the intensity of each aroma attribute. Significance is based on independent sample t-test in the reconstitution model and each omission model (namely RW vs RW-T, RW vs RW-V, and RW vs RW-T-V). *: P < 0.05, **: P < 0.01, ***: P < 0.001.
4. Conclusion
With multiple approaches of molecular sensory science, the aroma of Dornfelder wines from three regions of China with different environmental conditions was comprehensively investigated. In general, Chinese Dornfelder wines were described as black fruit, violet, acacia/lilac, red fruit, spice, dried plum, hay, and honey. As for the regional difference, wines from the Eastern Foothills of Helan Mountains (EFHM) had the highest level of terpenoids and were considered the typical wine with a prominent perception of violet and acacia/lilac to well express the varietal character of Dornfelder. Wines from the Northern Foothills of Tianshan Mountains (NFTM) had a similar aroma profile to the EFHM wine with higher intensity of black/red fruit and dried plum notes mainly due to higher levels of esters. Unlike those two regions with a continental semi-arid climate, wines from the Jiaodong Peninsula (JP) with a temperate monsoon climate were recognized as mushroom/earth, hay, and medicinal material aromas, with relatively higher concentrations of some volatile phenols and C13-norisoprenoids. Based on AEDA-GC-O/MS and OAV analysis, 61 key odor-active compounds were selected to successfully reconstruct aroma profiles in wines from three regions, among which terpenoids and volatile phenols could be regarded as varietal characteristic compounds for Dornfelder wines. The results of omission tests and descriptive analysis further indicated that linalool and geraniol directly contributed to the floral aroma, while guaiacol, eugenol, and isoeugenol could have a synergistic effect with terpenoids, especially on the notes of violet, acacia/lilac, spice, and black fruit.
CRediT authorship contribution statement
Mengqi Ling: Formal analysis, Investigation, Visualization, Writing – original draft. Ruixue Chai: Formal analysis, Investigation, Writing – original draft. Xiaofeng Xiang: Visualization, Writing – review & editing. Jin Li: Investigation, Resources. Penghui Zhou: Investigation, Resources. Ying Shi: Supervision, Project administration. Changqing Duan: Conceptualization, Supervision, Project administration. Yibin Lan: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant NO. 32102116), the Key Project of R&D Program of Ningxia Hui Autonomous Region, China (Grant NO. 2020BCF01003), the Major Project of Science and Technology of Shandong Province, China (Grant NO. 2022CXGC010605), and China Agriculture Research System of MOF and MARA (CARS-29). We are grateful to the Center of Viticulture and Enology (CFVE) trained panelists for their time and effort with the sensory evaluation. We also thank CITIC Guoan Winery, Chateau Zhihui Yuanshi, and Chateau Junding for providing the wine samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100598.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alegre Y., Sáenz-Navajas M.P., Hernández-Orte P., Ferreira V. Sensory, olfactometric and chemical characterization of the aroma potential of Garnacha and Tempranillo winemaking grapes. Food Chemistry. 2020;30(331) doi: 10.1016/j.foodchem.2020.127207. [DOI] [PubMed] [Google Scholar]
- Antoce A.O., Cojocaru G.A. Technological approaches to the vinification of Dornfelder grape variety cultivated in Romania. BIO Web of Conferences. 2015;5:1–9. doi: 10.1051/bioconf/20150502009. [DOI] [Google Scholar]
- Engel W., Bahr W., Schieberle P. Solvent assisted flavour evaporation - a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. European Food Research and Technology. 1999;209(3):237–241. doi: 10.1007/s002170050486. [DOI] [Google Scholar]
- Fabres P.J., Collins C., Cavagnaro T.R., Rodríguez López C.M. A concise review on multi-omics data integration for terroir analysis in Vitis vinifera. Frontiers in Plant Science. 2017;8:1065–1072. doi: 10.3389/fpls.2017.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V., Lopez R. The actual and potential aroma of winemaking grapes. Biomolecules. 2019;9(12):818–853. doi: 10.3390/biom9120818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Strasser M., Gutzler K. Impact of fermentation technology on the phenolic and volatile composition of German red wines. International Journal of Food Science and Technology. 2000;35:81–94. doi: 10.1046/j.1365-2621.2000.00365.x. [DOI] [Google Scholar]
- Frank S., Schieberle P. Changes in the major odorants of grape juice during manufacturing of Dornfelder red wine. Journal of Agricultural and Food Chemistry. 2022;70(43):13979–13986. doi: 10.1021/acs.jafc.2c06234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Wollmann N., Schieberle P., Hofmann T. Reconstitution of the flavor signature of Dornfelder red wine on the basis of the natural concentrations of its key aroma and taste compounds. Journal of Agricultural and Food Chemistry. 2011;59(16):8866–8874. doi: 10.1021/jf202169h. [DOI] [PubMed] [Google Scholar]
- Fronk P., Hartmann H., Bauer M., Solem E., Jaenicke E., Tenzer S., Decker H. Polyphenoloxidase from Riesling and Dornfelder wine grapes (Vitis vinifera) is a tyrosinase. Food Chemistry. 2015;183:49–57. doi: 10.1016/j.foodchem.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Genovese A., Gambuti A., Piombino P., Moio L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chemistry. 2007;103(4):1228–1236. doi: 10.1016/j.foodchem.2006.10.027. [DOI] [Google Scholar]
- González-Barreiro C., Rial-Otero R., Cancho-Grande B., Simal-Gándara J. Wine aroma compounds in grapes: A critical review. Critical Reviews in Food Science and Nutrition. 2015;55(2):202–218. doi: 10.1080/10408398.2011.650336. [DOI] [PubMed] [Google Scholar]
- He W.-H., Ren Y.-H., Qu L.-H., Wang J.-X., Zhang X.-J., Wang X.-W. Preliminary report on the performance of three wine grape varieties in Penglai, Shandong. China. Fruits. 2008;1:32–34. doi: 10.16626/j.cnki. [DOI] [Google Scholar]
- He Y.-Y., Li H., Li Y., Wang H. Study on variety characteristics of wine grape Dornfelder. Northern. Horticulture. 2015;24:1–4. doi: 10.11937/bfyy.201524001. [DOI] [Google Scholar]
- Jaeckels N., Tenzer S., Rosfa S., Schild H., Decker H., Wigand P. Purification and structural characterisation of lipid transfer protein from red wine and grapes. Food Chemistry. 2013;138(1):263–269. doi: 10.1016/j.foodchem.2012.09.113. [DOI] [PubMed] [Google Scholar]
- Jaeger S.R., Beresford M.K., Paisley A.G., Antúnez L., Vidal L., Cadena R.S., Giménez A., Ares G. Check-all-that-apply (CATA) questions for sensory product characterization by consumers: Investigations into the number of terms used in CATA questions. Food Quality and Preference. 2015;42:154–164. doi: 10.1016/j.foodqual.2015.02.003. [DOI] [Google Scholar]
- Jiang B., Xi Z.-M., Luo M.-J., Zhang Z.-W. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Research International. 2013;51(2):482–489. doi: 10.1016/j.foodres.2013.01.001. [DOI] [Google Scholar]
- Juega M., Nunez Y.P., Carrascosa A.V., Martinez-Rodriguez A.J. Influence of yeast mannoproteins in the aroma improvement of white wines. Journal of Food Science. 2012;77(8):M499–M504. doi: 10.1111/j.1750-3841.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- Krstic M.P., Johnson D.L., Herderich M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Australian Journal of Grape and Wine Research. 2015;21:537–553. doi: 10.1111/ajgw.12183. [DOI] [Google Scholar]
- Lan Y., Liu M., Zhang X., Li S., Shi Y., Duan C. Regional variation of chemical characteristics in young Marselan (Vitis vinifera L.) red wines from five regions of China. Foods. 2022;11(6):787–804. doi: 10.3390/foods11060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y.-B., Xiang X.-F., Qian X., Wang J.-M., Ling M.-Q., Zhu B.-Q., Liu T., Sun L.-B., Shi Y., Reynolds A.G., Duan C.-Q. Characterization and differentiation of key odor-active compounds of 'Beibinghong' icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chemistry. 2019;287:186–196. doi: 10.1016/j.foodchem.2019.02.074. [DOI] [PubMed] [Google Scholar]
- Ling M., Qi M., Li S., Shi Y., Pan Q., Cheng C.…Duan C. The influence of polyphenol supplementation on ester formation during red wine alcoholic fermentation. Food Chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.131961. [DOI] [PubMed] [Google Scholar]
- Ma Y., Tang K., Xu Y., Li J.-M. Characterization of the key aroma compounds in Chinese Vidal icewine by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission tests. Journal of Agricultural and Food Chemistry. 2017;65(2):394–401. doi: 10.1021/acs.jafc.6b04509. [DOI] [PubMed] [Google Scholar]
- Mele M.A., Kang H.M., Lee Y.T., Islam M.Z. Grape terpenoids: Flavor importance, genetic regulation, and future potential. Critical Reviews in Food Science and Nutrition. 2021;61(9):1429–1447. doi: 10.1080/10408398.2020.1760203. [DOI] [PubMed] [Google Scholar]
- Nanou E., Mavridou E., Milienos F.S., Papadopoulos G., Tempere S., Kotseridis Y. Odor characterization of white wines produced from indigenous Greek grape varieties using the frequency of attribute citation method with trained assessors. Foods. 2020;9(10):1396–1415. doi: 10.3390/foods9101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch, M., Hey, M., Rühl, E. H., & Bitz, O. (2014). Monoterpene levels in different grapevine (Vitis vinifera L.) cultivars and clones during fruit ripening. Acta Horticulturae, 1046, 471-476. 10.17660/ActaHortic.2014.1046.64.
- Pearson W., Schmidtke L.M., Francis I.L., Carr B.T., Blackman J.W. Characterising inter- and intra-regional variation in sensory profiles of Australian Shiraz wines from six regions. Australian Journal of Grape and Wine Research. 2020;26(4):372–384. doi: 10.1111/ajgw.12455. [DOI] [Google Scholar]
- Qian X., Lan Y.-B., Han S., Liang N.-N., Zhu B.-Q., Shi Y., Duan C.-Q. Comprehensive investigation of lactones and furanones in icewines and dry wines using gas chromatography-triple quadrupole mass spectrometry. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109650. [DOI] [PubMed] [Google Scholar]
- Riebel M., Fronk P., Distler U., Tenzer S., Decker H. Proteomic profiling of German Dornfelder grape berries using data-independent acquisition. Plant Physiology and Biochemistry. 2017;118:64–70. doi: 10.1016/j.plaphy.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Sarrazin E., Dubourdieu D., Darriet P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chemistry. 2007;103(2):536–545. doi: 10.1016/j.foodchem.2006.08.026. [DOI] [Google Scholar]
- Stamatopoulos P., Frérot E., Tempère S., Pons A., Darriet P. Identification of a new lactone contributing to overripe orange aroma in Bordeaux dessert wines via perceptual interaction phenomena. Journal of Agricultural and Food Chemistry. 2014;62(12):2469–2478. doi: 10.1021/jf405397c. [DOI] [PubMed] [Google Scholar]
- Veríssimo C.M., Alcântara R.L., Lima L.L.d.A., Pereira G.E., Maciel M.I.S. Impact of chemical profile on sensory evaluation of tropical red wines. International Journal of Food Science & Technology. 2021;56(7):3588–3599. doi: 10.1111/ijfs.14987. [DOI] [Google Scholar]
- Veronika G., Peter S. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. Journal of Agricultural and Food Chemistry. 2007;55:5221–5228. doi: 10.1021/jf0705015. [DOI] [PubMed] [Google Scholar]
- Villière A., Le Roy S., Fillonneau C., Prost C. InnOscent system: Advancing flavor analysis using an original gas chromatographic analytical device. Journal of Chromatography A. 2018;1535:129–140. doi: 10.1016/j.chroma.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Wang L., Hu G.-Y., Lei L.-B., Lin L., Wang D.-Q., Wu J.-X. Identification and aroma impact of volatile terpenes in Moutai liquor. International Journal of Food Properties. 2015;19(6):1335–1352. doi: 10.1080/10942912.2015.1064442. [DOI] [Google Scholar]
- Wei, W., Lu, H.-C., Jia, Q.-Q., Chen, W., Li, S.-D., & He, F. (2020). Study on winemaking characteristics of four grape varieties in northern foothills of Tianshan mountains. Sino-overseas Grapevine & Wine, (02), 1-8. 10.13414/j.cnki.zwpp.2020.02.001.
- Wen Y.-Q., Zhong G.-Y., Gao Y., Lan Y.-B., Duan C.-Q., Pan Q.-H. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biology. 2015;15:240–261. doi: 10.1186/s12870-015-0631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdylo A., Samoticha J., Chmielewska J. Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.127888. [DOI] [PubMed] [Google Scholar]
- Xie S., Lei Y.-J., Wang Y.-J., Wang X.-Q., Ren R.-H., Zhang Z.-W. Influence of continental climates on the volatile profile of Cabernet Sauvignon grapes from five Chinese viticulture regions. Plant Growth Regulation. 2018;87(1):83–92. doi: 10.1007/s10725-018-0455-8. [DOI] [Google Scholar]
- Yang W.-X., Zhang S.-Q., Xiang X.-F., Shi Y., Duan C.-Q., Lan Y.-B. Determination of volatile phenols in grape and wine by gas chromatography-triple quadrupole mass spectrometry. Food Science. 2022;43(12):252–259. doi: 10.7506/spkx1002-6630-20210731-380. [DOI] [Google Scholar]
- Zhang H., Pan Q. Advances in understanding the formation mechanism of terpenoids during winemaking and factors influencing it. Food Science. 2021;42(13):249–258. doi: 10.7506/spkx1002-6630-20200802-024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.