Abstract
Parkinson's disease (PD) is one of the most common neurodegenerative illnesses, and is a major healthcare burden with prodigious consequences on life-quality, morbidity, and survival. Cardiovascular diseases are the leading cause of mortality worldwide and growing evidence frequently reports their co-existence with PD. Cardiac dysautonomia due to autonomic nervous system malfunction is the most prevalent type of cardiovascular manifestation in these patients, comprising orthostatic and postprandial hypotension, along with supine and postural hypertension. Moreover, many studies have endorsed the risk of patients with PD to develop ischemic heart disease, heart failure and even arrhythmias, but the underlying mechanisms are not entirely clear. As importantly, the medication used in treating PD, such as levodopa, dopamine agonists or anticholinergic agents, is also responsible for cardiovascular adverse reactions, but further studies are required to elucidate the underlying mechanisms. The purpose of this review was to provide a comprehensive overview of current available data regarding the overlapping cardiovascular disease in patients with PD.
Keywords: Parkinson's disease, cardiovascular disease, cardiac dysautonomia, heart disease, heart failure, levodopa
1. Background
Parkinson's disease (PD) has a great negative impact on life quality and countless more comorbidities, significantly affecting both physical and psychological health. Overall, the most important unfavorable outcome of PD encompasses lack of dexterity and ambulation, along with disturbed cognition (1). Epidemiological studies have reported that, with the increasing life span, the prevalence of PD is facing a significant increase in most countries of the world, thus representing a healthcare burden not to be neglected (2). Advanced aging is also related with the increasing prevalence of cardiovascular diseases and, therefore, investigating possible associations between the two and PD is quite justified.
Furthermore, aging, diabetes mellitus and the male sex have been shown to be notable risk factors for both cardiovascular illnesses and PD (3). Studies have shown that various metabolic dysregulations which are found in cardiovascular diseases, are present in PD, as well. Increased glycemia values and insulin-resistance have been revealed to be associated with PD, while inflammatory markers, such as reactive oxygen species, lipid degradation products or C-reactive protein (CRP), have also been revealed to be associated with this disease (2). Consequently, these patients have significantly higher risk to develop cardiovascular disease, high blood pressure or diabetes mellitus. Additionally, another link between these two illnesses is represented by the molecular changes within the structures of cells, including storage of protein agglomerates, dysfunctional clearing process of proteins, mitochondrial involvement, inflammation within the nervous system and various gene mutations, all of which are responsible for the phenotype and severity of a disease (4,5).
By and large, the cardiovascular involvement in patients with PD is represented on the one hand by cardiovascular dysautonomia and, on the other hand, by structural cardiovascular illnesses. The most common cardiovascular abnormalities found in patients with PD are related to dysautonomia, while structural cardiac diseases are more scarcely identified in this category of patients. Nevertheless, previous research has demonstrated a positive link between cardiovascular illnesses and PD (6). Autonomic nervous system dysregulation usually leads to the occurrence of orthostatic or postprandial hypotension, either nocturnal or supine hypertension (2). Moreover, either independent or in conjunction to dysautonomia, patients with PD have increased risk of developing structural and functional cardiovascular illnesses, such as left ventricular hypertrophy and diastolic dysfunction, in addition to, as the diseases progress, heart failure, ischemic heart disease and even ventricular tachyarrhythmias (2).
Furthermore, patients with PD are at risk of developing heart disease secondary to PD medication. While levodopa potentiates orthostatic hypotension, dopamine agonists are responsible for restrictive valvular heart disease (7,8). As for anticholinergic agents, donepezil has been reported to cause ventricular tachyarrhythmias, which were reversible after treatment cessation (9).
In the present state-of-the-art review, a unique and novel approach of presenting cardiovascular involvement in patients with PD is provided. To the best of our knowledge, to date, there is no research that has been published which covers the entire aspect of cardiovascular disease in patients with PD. For this purpose, the latest available articles were reviewed and reported, providing on the one hand a clear and comprehensive perspective on the matter and, on the other hand, starting from current findings, future research perspectives in terms of cardiovascular protection and risk assessment (2), along with specific possible future biomarkers. Another strength of the present review is its clinical viewpoint and the fact that it is addressed not only to experimental researchers, but also to clinical researchers and doctors as well (7,8). Thus, the present article is considered to be a comprehensive and useful review, which reveals several aspects in the field of PD and cardiovascular involvement.
2. Cardiovascular dysautonomia in patients with PD
Autonomic nervous system dysfunction represents a non-motor involvement in PD, which occurs early in disease progression and growing evidence suggests that it may predict the diagnosis long before the appearance of standard motor signs and symptoms (10,11). With regard to the mechanisms responsible for these manifestations, it has been considered that α-synuclein and autonomic nerve denervation were the keystones of cardiac dysautonomia, however recent research has shown that there is more than meets the eye in terms of pathogenesis (12). Amongst all, autonomic nervous system dysregulation is responsible for cardiovascular dysautonomia in >80% of cases, including orthostatic and postprandial arterial hypotension, supine arterial hypertension, as well as the presence of nocturnal nondipping profile in this category of subjects (Fig. 1) (13,14). In a meta-analysis conducted by Velseboer et al, it was concluded that orthostatic and postprandial hypotension usually occur in up to one third of patients with PD (15), with postprandial hypotension being more frequently observed in patients with orthostatic hypotension (16). Moreover, orthostatic hypotension has been shown to significantly impact the quality of life of sufferers and PD progression (12).
Figure 1.
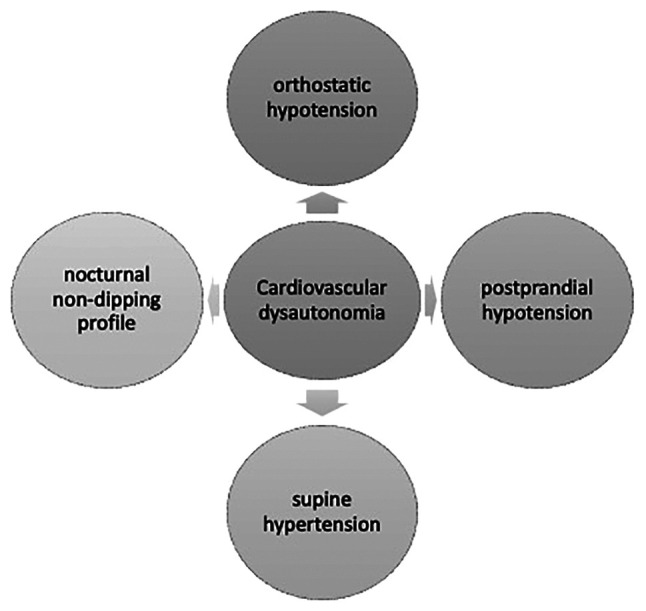
Subtypes of cardiac dysautonomia in patients with PD.
Orthostatic hypotension is one of the major cardiovascular illnesses, which occurs in patients with PD, requiring watchful and careful monitoring due to the patient's risk of falling and social isolation. Specific medications, comprising synthetic mineralocorticoids and pressor agents, along with general measurements, such as proper hydration, siting immediately after feeling lightheaded upon standing, external compression, physical counter-maneuvers that enhance venous return, are required (16,17). According to Jain et al, 30-40% of patients with PD have orthostatic hypotension, while others have suggested a percentage of >50% (18). In a study by Scorza et al, the incidence of various cardiovascular diseases was low, under 3-5% for myocardial infarction, with only one case of takotsubo cardiomyopathy reported. To date, there are no epidemiological studies that may clarify the true proportions of various cardiac diseases in patients with PD (6). Senard et al revealed that 58.2% of patients with PD have concomitant orthostatic hypotension and one third of them are asymptomatic at the moment of diagnosis (19). In a study by Blaho et al conducted in patients with PD, it was demonstrated that decreased baroreflex response was closely associated with supine hypertension and orthostatic hypotension (20). Similarly, the adaptability of systemic peripheral resistance was revealed to be severely dysregulated due to sympathetic nerve degeneration, thus promoting orthostatic hypotension (21). In addition, it was reported that parasympathetic nerve fibers are degenerated, thus contributing to orthostatic hypotension (22).
Another interesting aspect of orthostatic hypotension derives from the differences with Parkinson-like syndromes, such as multiple system atrophy (MSA) in terms of pathogenetic mechanisms. If in PD, the main mechanisms of orthostatic hypotension are neurological lesions of the brainstem, sympathetic denervation and postganglionic affliction, in MSA, central nervous system damages are exclusively responsible for this medical condition (23,24). Furthermore, in PD, the histological lesions are represented by the accumulation of Lewy bodies, while in MSA, α-synuclein aggregates are found in neurons and oligodendrogial cytoplasmatic inclusions (25,26). Moreover, in a recently published study by Cuoco et al, patients with MSA who also suffered from orthostatic hypotension had a significantly increased risk of cognitive degradation at 1-year follow-up (27).
As for the nocturnal nondipping profile of patients with PD, studies have shown that almost 90% were nondippers (12). In a study by Sommer et al, which comprehensively characterized the presence of a nondipping profile in a cohort of patients with PD, it was concluded that up to 95% of patients with orthostatic hypotension are nocturnal nondippers, however the underlying pathogenetic mechanism is quite independent of this because up to 80% of patients who did not present with orthostatic hypotension also had a nondipping profile at 24 h ambulatory blood pressure monitoring (14). This aspect represents a paramount link between cardiovascular risk and PD because, as demonstrated by de la Sierra et al, nondipping profiles are strongly related to cardiovascular illnesses (28).
Supine hypertension is considerably frequent in patients with PD, and it has been shown that this category of cardiovascular dysautonomia is associated with general cardiovascular risk, the occurrence of myocardial infarction and ischemic stroke (12). Likewise, supine hypertension has been strongly associated with left ventricular hypertrophy and, in the long term, diastolic dysfunction, and heart failure (29). Moreover, a recent study conducted by Shin et al sought to investigate the clinical risk factors of the dilated perivascular space-cognition-motor symptom axis. The study concluded that basal ganglia-dilated perivascular space secondary to supine hypertension is a major contributor to cognitive failure due to cerebral white matter hyperintensities and is responsible for more pronounced motor symptoms in patients with PD (30).
Another keystone component in PD is α-synuclein, the main constituent of Lewy bodies, which has significant roles in disease development. Recent technological advances in the field of molecular biochemistry and proteomics have led to a better understanding of the morphological and functional hallmarks of α-synuclein, thus providing even more enlightenment in PD (31). Furthermore, a recent study published by Javanshiri et al revealed that up to 82% of patients with α-synucleinopathies presented intracardiac α-synuclein at pathological examination, as compared to those without α-synucleinopathies for whom cardiac α-synuclein was completely absent (32). In addition, Isonaka et al demonstrated that in patients with neurogenic orthostatic hypotension, the accumulation of α-synuclein within the sympathetic ganglions, especially in the noradrenergic nerve fibers, is considerably correlated with the loss of cardiac noradrenaline, along with its neuronal storage. These findings may be in favor of a possible pathogenetic link between α-synuclein deposition, Lewy bodies and retrogression of cardiac sympathetic nerve fibers, although further research needs to be conducted (33). Furthermore, it has been shown that 6-hydroxydopamine, a neurotoxin with major implications in the pathogenesis of PD, plays significant roles in reducing cardiac sympathetic nerve fibers (12,34).
Notwithstanding, several specific gene mutations have been shown to be associated with autonomic nervous system dysfunction. Tijero et al revealed that E46K mutation within the α-synuclein gene was notably associated with significant degeneration of cardiac sympathetic nerves in both symptomatic and asymptomatic patients with PD (35). Moreover, another specific mutation, namely LRRK2, led to increased myocardial uptake of I-meta-iodobenzylguanidine (MIBG) at scintigraphy (36).
3. Appraising the risk of heart disease in patients with PD
In addition to cardiac dysautonomia, patients with PD commonly develop structural heart disease such as ischemic heart disease or heart failure, representing a paramount additive factor to their morbidity and mortality. As recently suggested by Park et al, patients with PD are at a significantly higher risk to develop cardiovascular disease, even though the exact pathogenetic mechanisms are not fully understood and further research is required in order to elucidate them (37).
In a recently published systematic review that sought to establish pathogenetic links between PD and heart disease, Suri et al emphasized the role of atherosclerosis in cardiovascular and cerebrovascular disease in this category of patients, endorsing the importance of constant evaluation for cardiovascular diseases (38). Notably, Driver et al conducted a prospective study on 330 patients who succumbed to PD and had similar comorbidities. They concluded that mortality was independent of aging and smoking in patients with PD (39).
Furthermore, Nam et al revealed that subjects with metabolic syndrome were at an increased risk of developing PD than healthy individuals. Moreover, the incidence of PD slightly increased with the number of metabolic syndrome components, especially in individuals who were ≥65 years old (40). In addition, a slight increase in arterial pressure in high-normal blood pressure individuals was revealed to significantly increase the risk of PD (41). Moreover, Liang et al conducted a case-control study on 3,211 patients with PD and a similar number of controls who underwent a 3-year follow-up. In the diseased group, a significantly increased risk of developing acute myocardial infarction, either fatal or not, was observed (42). Interestingly, a recently published study revealed that statins were significantly associated with increased risk of PD, while serum hypercholesterolemia slightly prevented the occurrence of the disease (43). Conversely, Vikdahl et al revealed that hypercholesterolemia, smoking, and obesity were slightly associated with increased risk of acquiring PD, while physical activity was a protective factor for this disease (44).
Similar to cardiovascular diseases, in PD there is a high grade of inflammation, insulin resistance, dyslipidemia and oxidative stress. Physical exertion and coffee consumption are inversely associated with the risk of both PD and cardiovascular diseases, along with optimal diabetes and hypertension therapy (43). Recently, Scorza et al revealed that in addition to ischemic heart disease, patients with PD may also develop cardiomyopathies, arrhythmias, or sudden cardiac death, although their real incidence is a topic of debate (6). Interestingly, Chohan et al performed a meta-analysis in which they sought to establish the link between diabetes mellitus and PD. The study revealed a strong relationship between the presence of diabetes mellitus and the development of PD, showing a 21% increased risk to develop PD in diabetic patients (45). These findings may be elucidated by similar pathogenetic mechanisms, including mitochondrial malfunction, increased oxidative stress, insulin resistance and hyperglycemia. Moreover, reported data revealed a notable association even with the progression of PD and, conversely, the positive effects of anti-diabetic medication on halting PD were identified (46). In addition to diabetes, insulin resistance itself has been shown to be associated with PD. Insulin passes into the central nervous system and regulates neuronal development and apoptosis, dopaminergic transmission, and synapses functionality, while it may also promote α-synuclein synthesis (47).
Furthermore, previously reported data support the role of vitamin D in the progression of PD, specifically through its own receptor, and also through various molecules which are involved in different pathogenetic pathways. Therefore, several genes which are associated with vitamin D, including type II major histocompatibility complex, cytochrome P4502D6, chromosome 22, the renin-angiotensin system, heme oxygenase-1, poly-(ADP-ribose) polymerase-1 gene, neurotrophic factor, and Sp1 transcription factor, have been demonstrated to be strongly associated with PD. In addition, various inflammatory markers, such as L-type voltage-sensitive calcium channels, nerve growth factor, matrix metalloproteinases, prostaglandins and cyclooxygenase-2, have been revealed to be associated with PD via vitamin D (48-53). A possible pertinent explanation for these findings may be that vitamin D and its activating enzyme called alpha1-hydroxylase are considerably found in the substantia nigra. In a recently published review of the literature, Fullard et al concluded that sera vitamin D levels are closely related to the severity of motor signs, memory, psychological status, orthostatic hypotension, and loss of smell (54). It has recently been shown that decreased levels of vitamin D leads to apoptosis of dopaminergic neurons, while its systemic administration counterbalances the process (55). In addition, growing evidence suggests that vitamin D may become a feasible biomarker for PD, but larger cohort studies are required (56).
Moreover, low serum levels of vitamin D have been strongly associated with increased risk of numerous cardiovascular illnesses. Patients who experienced stable coronary artery disease and acute myocardial infarction were demonstrated to have decreased levels of vitamin D (57). Likewise, a meta-analysis which evaluated the relationship between this molecule and the risk of atrial fibrillation concluded that vitamin D deficiency significantly increases the risk of atrial fibrillation (58). Conversely, in a meta-analysis which included ~83,000 patients, exogenic supplementation with vitamin D did not reduce the risk of cardiovascular disease of any kind (59). Thus, based on these findings, vitamin D may become a useful marker of cardiovascular dysfunction in patients with PD.
As for biomarkers of inflammation, it has been proposed that CRP may become a valuable marker of disease progression in chronic inflammatory and neurodegenerative diseases, such as PD (60). Qiu et al conducted an interesting meta-analysis which compared patients with PD and healthy individuals and revealed that increased levels of CRP were identified in the diseased group, thus suggesting that this biomarker may become a risk factor for PD (61). Moreover, Sawada et al revealed that initial CRP levels were associated with mortality and prognosis prediction of patients with PD, independent of disease duration, severity, age or other confounders (62). Furthermore, urate is another important serum biomarker in patients with PD, having a multitude of anti-inflammatory effects within human cells and promoting reactive oxygen species within neurons (63,64). Clinical studies have shown that increased serum levels of urate were associated with a considerable decrease in the progression of PD and a lower unified PD rating scale score, promoting its protective effects in this category of patients (65,66). In addition to the potential role of urate as a biomarker of disease progression, its therapeutic ability to halt the development of PD has been recently assessed. In a recent clinical trial, it was determined whether high plasmatic levels of urate induced by inosine produced protective effects in patients with early PD. The results revealed that compared with placebo, patients with PD who were treated with inosine did not benefit in terms of clinical disease progression (67). Moreover, urate was also revealed to be associated with increased risk of cardiovascular diseases. In a recently published review, high levels of urate were closely associated with various cardiovascular diseases, increasing the risk of arterial hypertension, metabolic syndrome and intrinsic cardiac diseases, mainly by promoting vascular endothelial dysfunction, atherogenesis and lipid oxidation. Additionally, increased levels of urate have also been revealed to be associated with cardiovascular outcome and heart failure (68). These findings suggest an indirect, but strong association between PD and cardiovascular disease. Thus, although increased serum levels of urate are associated with a decreased progression of PD, they conversely, significantly increase the risk of cardiovascular diseases.
Another important serum marker in patients with PD is protein DJ-1, which is also a neuroprotector with significant impact in neurodegenerative diseases (69). Initially, Waragai et al revealed that this protein is increased in blood and cerebrospinal fluid in patients with PD (70). Furthermore, various mutations in genes of the DJ-1 protein have been associated with significantly increased risk of PD (71), and in a recently published review, its possible role as a biomarker of PD progression and even its ability to serve as a therapeutic target were suggested, however further studies still need to be conducted (72). Moreover, it has been shown that protein DJ-1 is an endogenous protective molecule that halts glycative stress, thus preventing heart failure induced by myocardial ischemia (73). In addition, protein DJ-1 may exert protective effects on the cardiovascular system, especially in patients with heart failure, pulmonary hypertension or those who have undergone coronary artery by-pass, by promoting antioxidant gene expression (74). These findings suggest that protein DJ-1 may become another pivotal biomarker which could mediate the association between PD and cardiovascular illnesses.
Furthermore, other serum biomarkers which are closely related with both PD and cardiovascular disease include coenzyme Q10, homocysteine and advanced oxidized protein products. These molecules are involved in the pathogenesis of cardiac diseases by promoting inflammation, myocyte disruption, atherogenesis, and heart failure, and thus may become biomarkers of PD progression (Fig. 2) in the near future and are presented in Table I (75).
Figure 2.
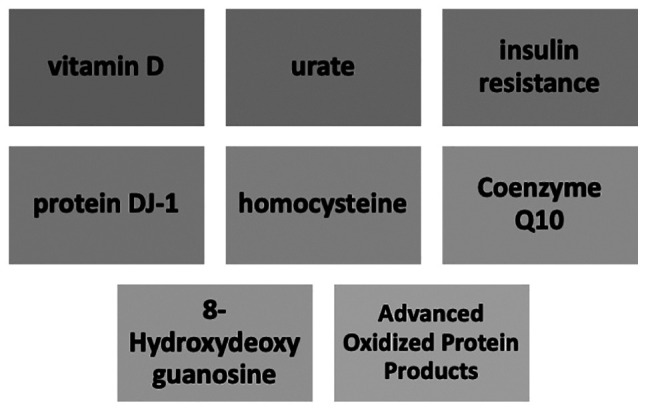
Biological factors that may aid cardiovascular risk stratification in patients with PD.
Table I.
Potential future biomarkers in PD.
| Biomarker | Neurodegenerative effects |
|---|---|
| Urate | • Antioxidant effects |
| • May prevent neurodegeneration of substantia nigra | |
| • Reduces mitochondrial oxidation | |
| Protein DJ-1 | • Neuroprotective role in oxidative stress |
| • Reduces neurodegeneration | |
| • Modulates chaperone proteins | |
| • Reduces mitochondrial oxidation | |
| Coenzyme Q10 | • Destabilizes the redox equilibrium |
| • Neuronal toxicity | |
| • Mitochondrial defects | |
| Homocysteine | • Substantia nigra toxicity |
| • Neurotoxicity, especially in dopaminergic neurons | |
| • Increases sera levels of amyloid β | |
| 8-Hydroxydeoxyguanosine | • Promotes reactive oxygen species synthesis |
| • Substantia nigra toxicity | |
| Advanced oxidized protein products | • Protein halogenation |
| • Increases phagocytosis |
Despite the fact that there are only a handful of studies demonstrating significant associations between PD and structural heart disease, there is significant evidence to endorse further studies in this direction.
4. PD therapy related to cardiovascular disease
The most used medications in patients with PD include levodopa, dopamine agonists, inhibitors of monoaminoxidase B, inhibitors of catechol-O-methyltransferase (COMT), amantadine, and anticholinergic agents. Growing evidence has revealed the negative impact of PD therapy on the cardiovascular system, with research demonstrating a close association between this category of treatment and the development and progression of heart disease (76).
Levodopa
Considered to be the most efficient and extensively used for the treatment of PD, levodopa has major positive effects on survival, morbidity, quality of life, and significantly improving symptoms of PD (77). Previously reported data have indicated that levodopa is associated with increased stiffness within the aorta as well as hypertension, and that it promotes left ventricular diastolic dysfunction (7,78). It is also responsible for promoting orthostatic hypotension, however the pathogenetic mechanism for this, either vasodepressor or cardioinhibitory effect, is not fully elucidated, although certain data are in favor of a negative inotropic mechanism (7). In addition, levodopa is also responsible for increased sera levels of homocysteine due to its methylation through the COMT pathway (79). In a study by Kocer et al, which sought to evaluate the ability of a COMT inhibitor to prevent hyperhomocysteinemia secondary to levodopa therapy, the study failed to identify a protective effect of entacapone on preventing hyperhomocysteinemia (80). Furthermore, in a study by O'Suilleabhain et al, it was demonstrated that hyperhomocysteinemia in patients with PD was significantly associated with depression and poorer neuropsychometric activity, as compared to individuals with normal sera levels of homocysteine (81).
Ergot-derived agonists
Another pharmacological class of anti-PD drugs represented by ergot-derived dopamine agonists, such as bromocriptine and cabergoline, are even more incriminated in cardiovascular involvement. Initially, Van Camp et al reported two cases of PD with restrictive valvular heart disease and clinical signs of significant heart failure due to daily doses of pergolide (82). Subsequently, the same research team evaluated the incidence of restrictive mitral stenosis in a cohort of 78 patients with PD treated with pergolide. The results revealed that 33% of patients developed restrictive mitral stenosis, of whom 19% had a severe form of this illness, thus suggesting that this complication is not rare in patients treated with this medication (8). Similarly, Horvath et al also identified restrictive valvular heart disease and suggested a comprehensive echocardiographic evaluation at the beginning and during treatment with pergolide and cabergoline (83). As for non-ergot-based dopamine agonists, pramipexole has scientific evidence for being responsible for heart failure. In a systematic review published by Tran et al, it was revealed that this drug was significantly associated with the risk of heart failure (84). However, further studies are required to elucidate the underlying mechanisms.
Anticholinergic agents
With regard to anticholinergic agents, donepezil has been found to prolong the QT interval in long-term administration. Additionally, a close monitoring strategy should be applied when this drug is used in combination with tricyclic antidepressants (85). Recently, Kho et al demonstrated that, due to increased sera levels of acetylcholine which blocks the potassium ion channel from the heart, donepezil may be responsible for adverse tachyarrhythmias, namely polymorphic ventricular tachycardia with oscillatory changes in amplitude of the QRS complexes around the isoelectric line (9).
Dexrazoxane, a potential cardioprotective agent in patients with PD
To date, there is no drug used in patients with PD that does not carry any cardiovascular risk. A noteworthy pharmacological agent which may benefit PD patients and also exert protective cardiovascular effects is dexrazoxane. Dexrazoxane was recently approved for the prevention of chemotherapy-induced cardiotoxicity in both adults and children who received anthracycline therapeutic regimens (86). A previous experimental study has suggested that dexrazoxane may act as protective agent against neurodegeneration, however further studies need to be conducted to reveal its potential clinical effects in patients with PD (87).
5. Conclusion
Compelling evidence supports a cause-effect relationship between PD and cardiovascular involvement. PD leads to cardiac dysautonomia and promotes ischemic heart, heart failure and arterial hypertension. Additionally, several drugs used to treat PD, such as levodopa, dopamine agonists and anticholinergic agents, are responsible for heart disease, as well. Nevertheless, there is not enough evidence available to identify the underlying mechanisms of these links. Further studies are required to provide appropriate data regarding the pathogenetic and clinical associations between PD and cardiovascular disease in order to deploy therapeutic options.
Acknowledgements
This work was supported by the Internal Doctoral Fellowship of Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Funding Statement
Funding: No funding was received.
Availability of data and material
Not applicable.
Authors's contributions
LG, AIG, and AZ researched data for the article and wrote the manuscript. LG, DC and AIG discussed the content of the review, and LG and LPD reviewed and edited the manuscript before submission. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pohar SL, Allyson Jones C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr. 2009;49:317–321. doi: 10.1016/j.archger.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, Wang Z. Global trends in the incidence, prevalence, and years lived with disability of Parkinson's disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9(776847) doi: 10.3389/fpubh.2021.776847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG, et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanneveich M, Moisan F, Jacqmin-Gadda H, Elbaz A, Joly P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson's disease (2010-2030) in France. Mov Disord. 2018;33:1449–1455. doi: 10.1002/mds.27447. [DOI] [PubMed] [Google Scholar]
- 6.Scorza FA, Fiorini AC, Scorza CA, Finsterer J. Cardiac abnormalities in Parkinson's disease and Parkinsonism. J Clin Neurosci. 2018;53:1–5. doi: 10.1016/j.jocn.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Noack C, Schroeder C, Heusser K, Lipp A. Cardiovascular effects of levodopa in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:815–818. doi: 10.1016/j.parkreldis.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, Van Zandijcke M, De Sutter J, Santens P, Decoodt P, Moerman C, Schoors D. Treatment of Parkinson's disease with pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363:1179–1183. doi: 10.1016/S0140-6736(04)15945-X. [DOI] [PubMed] [Google Scholar]
- 9.Kho J, Ioannou A, Mandal AKJ, Missouris CG. Donepezil induces ventricular arrhythmias by delayed repolarisation. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:559–560. doi: 10.1007/s00210-020-02028-4. [DOI] [PubMed] [Google Scholar]
- 10.Palma JA, Kaufmann H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord. 2018;33:372–390. doi: 10.1002/mds.27344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coon EA, Cutsforth-Gregory JK, Benarroch EE. Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord. 2018;33:349–358. doi: 10.1002/mds.27186. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson's disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134(104700) doi: 10.1016/j.nbd.2019.104700. [DOI] [PubMed] [Google Scholar]
- 13.Arici Duz O, Helvaci Yilmaz N. Nocturnal blood pressure changes in Parkinson's disease: Correlation with autonomic dysfunction and vitamin D levels. Acta Neurol Belg. 2020;120:915–920. doi: 10.1007/s13760-019-01113-7. [DOI] [PubMed] [Google Scholar]
- 14.Sommer S, Aral-Becher B, Jost W. Nondipping in Parkinson's disease. Parkinsons Dis. 2011;2011(897586) doi: 10.4061/2011/897586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RMA. Prevalence of orthostatic hypotension in Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:724–729. doi: 10.1016/j.parkreldis.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yalcin A, Atmis V, Cengiz OK, Cinar E, Aras S, Varli M, Atli T. Evaluation of cardiac autonomic functions in older Parkinson's disease patients: A cross-sectional study. Aging Dis. 2016;7:28–35. doi: 10.14336/AD.2015.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palma JA, Kaufmann H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pract. 2017;4:298–308. doi: 10.1002/mdc3.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: From pathophysiology to pathogenesis. Neurobiol Dis. 2012;46:572–580. doi: 10.1016/j.nbd.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senard JM, Raï S, Lapeyre-Mestre M, Brefel C, Rascol O, Rascol A, Montastruc JL. Prevalence of orthostatic hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63:584–589. doi: 10.1136/jnnp.63.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaho A, Šutovský S, Valkovič P, Šiarnik P, Sýkora M, Turčáni P. Decreased baroreflex sensitivity in Parkinson's disease is associated with orthostatic hypotension. J Neurol Sci. 2017;377:207–211. doi: 10.1016/j.jns.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Hirayama M, Hara T, Mizutani Y, Suzuki J, Watanabe H, Sobue G. Role of cardiac sympathetic nerves in preventing orthostatic hypotension in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:409–414. doi: 10.1016/j.parkreldis.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Shibata M, Morita Y, Shimizu T, Takahashi K, Suzuki N. Cardiac parasympathetic dysfunction concurrent with cardiac sympathetic denervation in Parkinson's disease. J Neurol Sci. 2009;276:79–83. doi: 10.1016/j.jns.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DS. Dysautonomia in Parkinson's disease: Neurocardiological abnormalities. Lancet Neurol. 2003;2:669–676. doi: 10.1016/s1474-4422(03)00555-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42:136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- 25.Galvin JE, Lee VMY, Trojanowski JQ. Synucleinopathies: Clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 26.Iodice V, Low DA, Vichayanrat E, Mathias CJ. Cardiovascular autonomic dysfunction in MSA and Parkinson's disease: Similarities and differences. J Neurol Sci. 2011;310:133–138. doi: 10.1016/j.jns.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Cuoco S, Carotenuto I, Cappiello A, Scannapieco S, Russillo MC, Andreozzi V, Forino L, Amboni M, Picillo M, Erro R, et al. Relationship between orthostatic hypotension and cognitive functions in multiple system atrophy: A longitudinal study. Front Neurol. 2021;12(711358) doi: 10.3389/fneur.2021.711358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: Which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27:680–687. doi: 10.1093/ajh/hpt175. [DOI] [PubMed] [Google Scholar]
- 29.Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: Prioritisation of treatment targets. Lancet Neurol. 2016;15:954–966. doi: 10.1016/S1474-4422(16)30079-5. [DOI] [PubMed] [Google Scholar]
- 30.Shin NY, Park YW, Yoo SW, Yoo JY, Choi Y, Jang J, Ahn KJ, Kim BS, Kim JS. Adverse effects of hypertension, supine hypertension, and perivascular space on cognition and motor function in PD. NPJ Parkinsons Dis. 2021;7(69) doi: 10.1038/s41531-021-00214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meade RM, Fairlie DP, Mason JM. Alpha-synuclein structure and Parkinson's disease-lessons and emerging principles. Mol Neurodegener. 2019;14(29) doi: 10.1186/s13024-019-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javanshiri K, Drakenberg T, Haglund M, Englund E. Cardiac alpha-synuclein is Present in alpha-synucleinopathies. J Parkinsons Dis. 2022;12:1125–1131. doi: 10.3233/JPD-223161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isonaka R, Rosenberg AZ, Sullivan P, Corrales A, Holmes C, Sharabi Y, Goldstein DS. Alpha-synuclein deposition within sympathetic noradrenergic neurons is associated with myocardial noradrenergic deficiency in neurogenic orthostatic hypotension. Hypertension. 2019;73:910–918. doi: 10.1161/HYPERTENSIONAHA.118.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues LD, Oliveira LF, Shinoda L, Scorza CA, Faber J, Ferraz HB, Britto LRG, Scorza FA. Cardiovascular alterations in rats with Parkinsonism induced by 6-OHDA and treated with Domperidone. Sci Rep. 2019;9(8965) doi: 10.1038/s41598-019-45518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tijero B, Gómez-Esteban JC, Lezcano E, Fernández-González C, Somme J, Llorens V, Martínez A, Ruiz-Martínez J, Foncea N, Escalza I, et al. Cardiac sympathetic denervation in symptomatic and asymptomatic carriers of the E46K mutation in the α synuclein gene. Parkinsonism Relat Disord. 2013;19:95–100. doi: 10.1016/j.parkreldis.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Tijero B, Gómez Esteban JC, Somme J, Llorens V, Lezcano E, Martinez A, Rodríguez T, Berganzo K, Zarranz JJ. Autonomic dysfunction in parkinsonian LRRK2 mutation carriers. Parkinsonism Relat Disord. 2013;19:906–909. doi: 10.1016/j.parkreldis.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Kim DH, Park YG, Kwon DY, Choi M, Jung JH, Han K. Association of Parkinson disease with risk of cardiovascular disease and all-cause mortality: A nationwide, population-based cohort study. Circulation. 2020;141:1205–1207. doi: 10.1161/CIRCULATIONAHA.119.044948. [DOI] [PubMed] [Google Scholar]
- 38.Suri JS, Paul S, Maindarkar MA, Puvvula A, Saxena S, Saba L, Turk M, Laird JR, Khanna NN, Viskovic K, et al. Cardiovascular/stroke risk stratification in Parkinson's disease patients using atherosclerosis pathway and artificial intelligence paradigm: A systematic review. Metabolites. 2022;12(312) doi: 10.3390/metabo12040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: A prospective comorbidity-matched cohort study. Neurology. 2008;70:1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 40.Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, Han B, Cho SJ, Yu JH, Park YG, Choi KM. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018;15(e1002640) doi: 10.1371/journal.pmed.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu C, Hu G, Kivipelto M, Laatikainen T, Antikainen R, Fratiglioni L, Jousilahti P, Tuomilehto J. Association of blood pressure and hypertension with the risk of Parkinson disease: The national FINRISK study. Hypertension. 2011;57:1094–1100. doi: 10.1161/HYPERTENSIONAHA.111.171249. [DOI] [PubMed] [Google Scholar]
- 42.Liang HW, Huang YP, Pan SL. Parkinson disease and risk of acute myocardial infarction: A population-based, propensity score-matched, longitudinal follow-up study. Am Heart J. 2015;169:508–514. doi: 10.1016/j.ahj.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Potashkin J, Huang X, Becker C, Chen H, Foltynie T, Marras C. Understanding the links between cardiovascular disease and Parkinson's disease. Mov Disord. 2020;35:55–74. doi: 10.1002/mds.27836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vikdahl M, Carlsson M, Linder J, Forsgren L, Håglin L. Weight gain and increased central obesity in the early phase of Parkinson's disease. Clin Nutr. 2014;33:1132–1139. doi: 10.1016/j.clnu.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Chohan H, Senkevich K, Patel RK, Bestwick JP, Jacobs BM, Bandres Ciga S, Gan-Or Z, Noyce AJ. Type 2 diabetes as a determinant of Parkinson's disease risk and progression. Mov Disord. 2021;36:1420–1429. doi: 10.1002/mds.28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan A, Sharma Kandel R, Mishra R, Gautam J, Alaref A, Jahan N. Diabetes mellitus and Parkinson's disease: Shared pathophysiological links and possible therapeutic implications. Cureus. 2020;12(e9853) doi: 10.7759/cureus.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Athauda D, Foltynie T. Insulin resistance and Parkinson's disease: A new target for disease modification? Prog Neurobiol. 2016;145-146:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Lương K, Nguyễn L. Role of vitamin D in Parkinson's disease. ISRN Neurol. 2012;2012(134289) doi: 10.5402/2012/134289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JS, Kim YI, Song C, Yoon I, Park JW, Choi YB, Kim HT, Lee KS. Association of vitamin D receptor gene polymorphism and Parkinson's disease in Koreans. J Korean Med Sci. 2005;20:495–498. doi: 10.3346/jkms.2005.20.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pascale E, Purcaro C, Passarelli E, Guglielmi R, Vestri AR, Passarelli F, Meco G. Genetic polymorphism of angiotensin-converting enzyme is not associated with the development of Parkinson's disease and of L-dopa-induced adverse effects. J Neurol Sci. 2009;276:18–21. doi: 10.1016/j.jns.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Castellani R, Smith MA, Richey GL, Perry G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996;737:195–200. doi: 10.1016/0006-8993(96)00729-9. [DOI] [PubMed] [Google Scholar]
- 52.Soós J, Engelhardt JI, Siklós L, Havas L, Majtényi K. The expression of PARP, NF-kappa B and parvalbumin is increased in Parkinson disease. Neuroreport. 2004;15:1715–1718. doi: 10.1097/01.wnr.0000136175.51954.ce. [DOI] [PubMed] [Google Scholar]
- 53.Choi JM, Hong JH, Chae MJ, Hung NP, Kang HS, Ma HI, Kim YJ. Analysis of mutations and the association between polymorphisms in the cerebral dopamine neurotrophic factor (CDNF) gene and Parkinson disease. Neurosci Lett. 2011;493:97–101. doi: 10.1016/j.neulet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Fullard ME, Duda JE. A review of the relationship between vitamin D and Parkinson disease symptoms. Front Neurol. 2020;11(454) doi: 10.3389/fneur.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pignolo A, Mastrilli S, Davì C, Arnao V, Aridon P, Dos Santos Mendes FA, Gagliardo C, D'Amelio M. Vitamin D and Parkinson's disease. Nutrients. 2022;14(1220) doi: 10.3390/nu14061220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barichella M, Garrì F, Caronni S, Bolliri C, Zocchi L, Macchione MC, Ferri V, Calandrella D, Pezzoli G. Vitamin D status and Parkinson's disease. Brain Sci. 2022;12(790) doi: 10.3390/brainsci12060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzoni D, Rizzoni M, Nardin M. Vitamin D and ischaemic heart disease: A casual or A causal association?: Commentary on: ‘Raslan E et al. Association of vitamin D deficiency with chronic stable angina: A case-control study’. High Blood Press Cardiovasc Prev. 2019;26:151–155. doi: 10.1007/s40292-019-00302-y. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Wang W, Tan Z, Zhu X, Liu M, Wan R, Hong K. The relationship between vitamin D and risk of atrial fibrillation: A dose-response analysis of observational studies. Nutr J. 2019;18(73) doi: 10.1186/s12937-019-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, Yelangi A, Sundus S, Bachuwa G, Alkotob ML, Manson JE. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: A Meta-analysis. JAMA Cardiol. 2019;4:765–776. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luan Y, Yao Y. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. 2018;9(1302) doi: 10.3389/fimmu.2018.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu X, Xiao Y, Wu J, Gan L, Huang Y, Wang J. C-reactive protein and risk of Parkinson's disease: A systematic review and meta-analysis. Front Neurol. 2019;10(384) doi: 10.3389/fneur.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawada H, Oeda T, Umemura A, Tomita S, Kohsaka M, Park K, Yamamoto K, Sugiyama H. Baseline C-reactive protein levels and life prognosis in Parkinson disease. PLoS One. 2015;10(e0134118) doi: 10.1371/journal.pone.0134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang O. Role of oxidative stress in Parkinson's disease. Exp Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med. 2012;53:1791–1806. doi: 10.1016/j.freeradbiomed.2012.08.569. [DOI] [PubMed] [Google Scholar]
- 65.Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, Ascherio A. Hyson C, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. Parkinson Study Group PRECEPT Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bluett B, Togasaki DM, Mihaila D, Evatt M, Rezak M, Jain S, Schwarzschild MA, Ascherio A, Casaceli C, Curhan GC, et al. Effect of urate-elevating inosine on early Parkinson disease progression: The SURE-PD3 randomized clinical trial. JAMA. 2021;326:926–939. doi: 10.1001/jama.2021.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borghi C, Piani F. Uric acid and risk of cardiovascular disease: A question of start and finish. Hypertension. 2021;78:1219–1221. doi: 10.1161/HYPERTENSIONAHA.121.17631. [DOI] [PubMed] [Google Scholar]
- 69.Saito Y, Shioya A, Sano T, Sumikura H, Murata M, Murayama S. Lewy body pathology involves the olfactory cells in Parkinson's disease and related disorders. Mov Disord. 2016;31:135–138. doi: 10.1002/mds.26463. [DOI] [PubMed] [Google Scholar]
- 70.Waragai M, Nakai M, Wei J, Fujita M, Mizuno H, Ho G, Masliah E, Akatsu H, Yokochi F, Hashimoto M. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson's disease. Neurosci Lett. 2007;425:18–22. doi: 10.1016/j.neulet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Lev N, Roncevich D, Ickowicz D, Melamed E, Offen D. Role of DJ-1 in Parkinson's disease. J Mol Neurosci. 2006;29:215–226. doi: 10.1385/jmn:29:3:215. [DOI] [PubMed] [Google Scholar]
- 72.Repici M, Giorgini F. DJ-1 in Parkinson's disease: Clinical insights and therapeutic perspectives. J Clin Med. 2019;8(1377) doi: 10.3390/jcm8091377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimizu Y, Nicholson CK, Polavarapu R, Pantner Y, Husain A, Naqvi N, Chin LS, Li L, Calvert JW. Role of DJ-1 in modulating glycative stress in heart failure. J Am Heart Assoc. 2020;9(e014691) doi: 10.1161/JAHA.119.014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsoporis JN, Drosatos IA, Gupta S, Amatullah H, Izhar S, Dos Santos CC, Salpeas V, Rigopoulos AG, Toumpoulis IK, Triantafyllis AS, et al. Cytoprotective mechanisms of DJ-1: Implications in cardiac pathophysiology. Molecules. 2021;26(3795) doi: 10.3390/molecules26133795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He R, Yan X, Guo J, Xu Q, Tang B, Sun Q. Recent advances in biomarkers for Parkinson's disease. Front Aging Neurosci. 2018;10(305) doi: 10.3389/fnagi.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuenca-Bermejo L, Almela P, Navarro-Zaragoza J, Fernández Villalba E, González-Cuello AM, Laorden ML, Herrero MT. Cardiac changes in Parkinson's disease: Lessons from clinical and experimental evidence. Int J Mol Sci. 2021;22(13488) doi: 10.3390/ijms222413488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhall R, Kreitzman DL. Advances in levodopa therapy for Parkinson disease: Review of RYTARY (carbidopa and levodopa) clinical efficacy and safety. Neurology. 2016;86 (14 Suppl 1):S13–S24. doi: 10.1212/WNL.0000000000002510. [DOI] [PubMed] [Google Scholar]
- 78.Günaydin ZY, Özer FF, Karagöz A, Bektaş O, Karataş MB, Vural A, Bayramoğlu A, Çelik A, Yaman M. Evaluation of cardiovascular risk in patients with Parkinson disease under levodopa treatment. J Geriatr Cardiol. 2016;13:75–80. doi: 10.11909/j.issn.1671-5411.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martignoni E, Tassorelli C, Nappi G, Zangaglia R, Pacchetti C, Blandini F. Homocysteine and Parkinson's disease: A dangerous liaison? J Neurol Sci. 2007;257:31–37. doi: 10.1016/j.jns.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 80.Kocer B, Guven H, Comoglu SS. Homocysteine levels in Parkinson's disease: Is entacapone effective? Biomed Res Int. 2016;2016(7563705) doi: 10.1155/2016/7563705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Suilleabhain PE, Sung V, Hernandez C, Lacritz L, Dewey RB Jr, Bottiglieri T, Diaz-Arrastia R. Elevated plasma homocysteine level in patients with Parkinson disease: Motor, affective, and cognitive associations. Arch Neurol. 2004;61:865–868. doi: 10.1001/archneur.61.6.865. [DOI] [PubMed] [Google Scholar]
- 82.van Camp G, Flamez A, Cosyns B, Goldstein J, Perdaens C, Schoors D. Heart valvular disease in patients with Parkinson's disease treated with high-dose pergolide. Neurology. 2003;61:859–861. doi: 10.1212/01.wnl.0000083985.00343.f2. [DOI] [PubMed] [Google Scholar]
- 83.Horvath J, Fross RD, Kleiner-Fisman G, Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski H, Pache JC, et al. Severe multivalvular heart disease: A new complication of the ergot derivative dopamine agonists. Mov Disord. 2004;19:656–662. doi: 10.1002/mds.20201. [DOI] [PubMed] [Google Scholar]
- 84.Tran T, Brophy JM, Suissa S, Renoux C. Risks of cardiac valve regurgitation and heart failure associated with ergot- and non-ergot-derived dopamine agonist use in patients with Parkinson's disease: A systematic review of observational studies. CNS Drugs. 2015;29:985–998. doi: 10.1007/s40263-015-0293-4. [DOI] [PubMed] [Google Scholar]
- 85.Kho J, Ioannou A, Mandal AKJ, Cox A, Nasim A, Metaxa S, Missouris CG. Long term use of donepezil and QTc prolongation. Clin Toxicol (Phila) 2021;59:208–214. doi: 10.1080/15563650.2020.1788054. [DOI] [PubMed] [Google Scholar]
- 86.de Baat EC, Mulder RL, Armenian S, Feijen EA, Grotenhuis H, Hudson MM, Mavinkurve-Groothuis AM, Kremer LC, van Dalen EC. Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines. Cochrane Database Syst Rev. 2022;9(CD014638) doi: 10.1002/14651858.CD014638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mei M, Zhou Y, Liu M, Zhao F, Wang C, Ding J, Lu M, Hu G. Antioxidant and anti-inflammatory effects of dexrazoxane on dopaminergic neuron degeneration in rodent models of Parkinson's disease. Neuropharmacology. 2019;160(107758) doi: 10.1016/j.neuropharm.2019.107758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


