Abstract
Background
Murine chimeric antigen receptor T (CAR-T) cell therapy has demonstrated clinical benefit in patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). However, the potential immunogenicity of the murine single-chain variable fragment domain may limit the persistence of CAR-T cell, leading to relapse.
Methods
We performed a clinical trial to determine the safety and efficacy of autologous and allogeneic humanized CD19-targeted CAR-T cell (hCART19) for R/R B-ALL. Fifty-eight patients (aged 13–74 years) were enrolled and treated between February 2020 and March 2022. The endpoints were complete remission (CR) rate, overall survival (OS), event-free survival (EFS), and safety.
Results
Overall, 93.1% (54/58) of patients achieved CR or CR with incomplete count recovery (CRi) by day 28, with 53 patients having minimal residual disease negativity. With a median follow-up of 13.5 months, the estimated 1-year OS and EFS were 73.6% (95% CI 62.1% to 87.4%) and 46.0% (95% CI 33.7% to 62.8%), with a median OS and EFS of 21.5 months and 9.5 months, respectively. No significant increase in human antimouse antibodies was observed following infusion (p=0.78). Duration of B-cell aplasia in the blood was observed for as long as 616 days, which was longer than that in our prior mCART19 trial. All toxicities were reversible, including severe cytokine release syndrome, which developed in 36% (21/58) of patients and severe neurotoxicity, which developed in 5% (3/58) of patients. Compared with our prior mCART19 trial, patients treated with hCART19 had longer EFS without increased toxicity. Additionally, our data also suggest that patients treated with consolidation therapy, including allogeneic hematopoietic stem cell transplantation or CD22-targeted CAR-T cell, following hCART19 therapy had a longer EFS than those without consolidation therapy.
Conclusion
hCART19 has good short-term efficacy and manageable toxicity in R/R B-ALL patients.
Trial registration number
Keywords: Clinical Trials as Topic; Cytotoxicity, Immunologic; Hematologic Neoplasms; Immunity, Cellular; Immunotherapy, Adoptive
WHAT IS ALREADY KNOWN ON THIS TOPIC
No significant treatment-induced humoral immunogenicity was observed in patients’ serum following hCART19 therapy.
WHAT THIS STUDY ADDS
hCART19 had a longer persistence and event-free survival (EFS) compared with our previous murine CD19 CAR-T trial.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Patients treated with consolidation therapy, including allogeneic hematopoietic stem cell transplantation or CD22-targeted CAR-T cell, following hCART19 therapy have a longer EFS than those without consolidation therapy.
Introduction
In recent years, multiple therapies, including chemotherapy, hematopoietic stem cell transplantation (HSCT), and targeted drug therapy, have significantly increased the survival of patients with B-cell acute lymphoblastic leukemia (B-ALL). Particularly the emergence of Chimeric antigen receptor T (CAR-T) cells therapy has provided a new treatment strategy for relapsed or refractory (R/R) patients. Autologous and, occasionally, allogeneic (HSCT donor-derived) CAR-T cells have been successfully manufactured and used clinically.1–3 And four successful CAR-T cells products targeting the B-cell lineage antigen CD19 have received FDA approval (tisagenlecleucel, axicabtagene ciloleucel, lisocabtagene maraleucel and brexucabtagene autoleucel). Many studies have shown that the complete remission (CR) rate of patients with R/R B-ALL treated with murine CD19-targeted CAR-T cells (mCART19) is 70%–90%.4–6 Despite this achievement, the high recurrence rate and high rate of severe cytokine release syndrome (CRS) complicate current therapy.
The toxicity and antitumor efficacy of CAR-T cells are largely determined by their structure. Therefore, it is necessary to optimize the structure of CAR-T cells to increase these two parameters. Research has shown that a murine single-chain variable fragment (scFv) in the CAR structure causes an HLA-restricted T-cell-mediated immune response,7 resulting in the disappearance or loss of CAR-T cells. A preclinical study by Dwivedi et al8 revealed that a humanized scFv generated by altering the CAR framework or non-complementarity-determining region reduced the immunogenicity of CAR, thereby reducing cytokine release and enhancing antitumor efficacy. However, the immunity to CAR is associated with treatment failure in some, not all clinical trials.7 9–12 Its possible effects on CAR-T cells persistence and function are currently poorly understood.
Here, we report the results of 58 patients with R/R B-ALL with a median follow-up of 1.1 years who received humanized CD19-targeted CAR-T cells (hCART19). Additionally, 52% (30/58) of the patients received allogeneic HSCT (allo-HSCT) or CD22-targeted CAR-T cells following hCART19 therapy, providing an opportunity to evaluate the role of consolidation therapy after hCART19 treatment.
Methods
Study design and CAR-T cell manufacture
This clinical trial was designed to assess the safety and efficacy of infusing hCART19 into 58 patients with R/R CD19+ B-ALL treated in the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China. The report incorporates data from all patients with B-ALL who were treated with hCART19 from February 2020 through March 2022 with a data cut-off of May 1, 2022.
The human-mouse hCART19 with murine components was used in this study. It was manufactured as described before.13 Briefly, autologous peripheral blood mononuclear cells (PBMCs) or allogeneic PBMCs apheresed directly from the allo-HSCT donors were stimulated with magnetic beads coated with anti-CD3/CD28 antibodies (Thermo Fisher Scientific) overnight. The next day, transduction via a lentiviral vector was performed at a multiplicity of infection 1:10 ratio. Transduced cells were cultured in X-VIVO 15, a serum-free medium (Lonza) with 300 IU/mL interleukin-2, for the duration of cell culture (5–8 days).
Toxicity and efficacy evaluations
CRS was graded using the Lee scale.14 Severe CRS was defined as grade ≥3 CRS. Neurotoxicity and other adverse events were identified according to the National Cancer Institute Common Terminology Criteria for Adverse Events V.5.0. Severe neurotoxicity was defined as any seizure or grade ≥3 toxicity of the nervous system. Acute graft-versus-host disease (aGVHD) was graded using the modified Glucksberg grading standard.
CR, no-response (NR), and CR with incomplete count recovery (CRi) were defined according to the National Comprehensive Cancer Network guidelines, V.1.2016. Minimal residual disease (MRD) negativity was defined as <0.01% abnormal B-cell (aberrant immunophenotypes) in the bone marrow (BM), which was determined by flow cytometry (FACS).
Assessment of hCART19 expansion and persistence
Initial response evaluations were performed on day 28 post-CAR-T cell infusion with routine surveillance thereafter. Blood samples were collected from patients for evaluation of the postinfusion percentages of CAR+/CD3+ cells via FACS. Circulating hCART19 numbers per microliter were calculated based on absolute CD3+ T lymphocyte counts. Cellular kinetics exposure parameters included maximal expansion of CAR-T cell levels in vivo postinfusion (Cmax), time to maximal expansion, and the median area under the curve (AUC) in a plot of CAR-T cell in peripheral blood between days 0 and 28 (AUC0-28). B-cell aplasia (BCA) was defined as CD19+ B-cell <3% of peripheral blood or bone marrow mononuclear cells, censored at the time of HSCT.
Assessment of cytokine and humoral immunogenicity
The serum concentrations of the cytokines IL-2, IL-6, IL-10, IL-17A, IFN-γ, TNF-α and human-anti-mouse antibodies (HAMA)were measured by ELISA (R&D Systems, Minneapolis, USA; Biochannel Biotechnology, Nanjing, China).
Statistical analysis
Descriptive statistics were computed to summarize patient and disease characteristics. The comparison of continuous variables was performed using the t-test or Wilcoxon test; categorical variables were compared by the or Fisher’s exact test. The Kaplan-Meier and Gehan-Wilcoxon test was employed to perform time-to-event analyses. If the survival curve was crossed, the Tarone-Ware test was employed. Pearson correlation analysis was applied to calculate the correlation between CRS grade and serum indicators. The CAR-T cell concentration-time profile differences between different CRS grades and different treatment responses were evaluated using two-way repeated-measures analysis of variance (ANOVA). The optimal cut-off value was determined by the ‘surv-cutpoint {survminer}’ function in R, which is an outcome-oriented method providing the value of the cutpoint that corresponds to the most significant relation with the outcome, using the maximally selected rank statistics. Clinical parameters on the overall survival (OS) were initially identified by univariate analysis; the duration of BCA, the level of serum LDH before infusion and the expansion threshold on day 14 (>50.4%) were evaluated for their joint effect using multivariable Cox proportional hazards analysis. Subgroup analysis was performed in patients who received hCART19 alone to develop predictors of persistence. All statistical analyses were performed with the Statistical Package for R software V.4.0.3, and p values less than 0.05 were considered significant.χ2
Results
CAR-T cell characteristics and infusion
The mean lentiviral CAR gene transfer efficiency was 48.9% (range 8.2%–89.7%) for all products manufactured. Compared with autologous CAR-T cell, allogeneic CAR-T cell had a higher transduction efficiency (55.2% (range: 8.16%–89.7%) vs 68.1% (range: 38.1%–87.1%); p=0.038). Compare to the autologous CAR-T cell product, there was a higher CD4:CD8 ratio (5.91 vs 4.81++; p=0.002) (online supplemental figure S1). More detailed data on differences between the autologous and allogeneic hCART19 was shown in thesupplementary appendix. Following lymphocyte-depleting chemotherapy with a fludarabine and cyclophosphamide (FC) regimen (fludarabine at 30 mg/m2 on days −4 to −2 and cyclophosphamide at 500 mg/m2 on days −3 to −2), all patients received a single dose of infusedhCART19 at a median of 2.24 (0.83–3.64) ×106 CAR-T cells/kg on day 0. Patients who had previously received allo-HSCT were infused with allogeneic (HSCT donor-derived) hCART19, except for patients whose donor cells were not available. As a result, nine patients received allogeneic hCART19, and the rest received autologous hCART19.
jitc-2022-005701supp001.pdf (323.2KB, pdf)
Patient characteristics
A total of 58 patients with pathologically confirmed CD19+ R/R B-ALL aged between 13 and 74 years and with a median age of 44.5 years were recruited for the study. All patients were required to have >95% CD19 and CD22 expression prior to treatment, and no patient had been previously treated with blinatumomab or inotuzumab. Of these patients, 12 had received prior allo-HSCT, 1 had received prior auto-HSCT, and 2 had received prior mCART19 therapy. Forty-one per cent of patients (24/58) had a high-risk cytogenetic and molecular profile, of which 14 had Ph+ ALL (ABL T315I mutation in 11 patients) and were previously treated with tyrosine kinase inhibitors. Sixteen patients had previous extramedullary relapse, including 5 patients with central nervous system leukemia (CNSL). The median number of chemotherapy cycles before CAR-T cell infusion was 5 (range: 2–16). The median leukemia burden before hCART19 infusion was 59% (range: 0%–94%) of marrow blasts. The detailed baseline characteristics of the patients are shown in table 1.
Table 1.
Baseline and treatment characteristics
| Characteristics | Whole cohort | CART1 | CART2 | CART+HSCT | P value | ||
| N=58 | N=24 | N=14 | N=16 | a | b | c | |
| Age at infusion, years (range) | 44.5 (13–74) | 46.5 (14–74) | 59 (26–68) | 32 (13–63) | 0.004 | 0.525 | 0.003 |
| Male, n (%) | 32 (55) | 12 (50) | 8 (57) | 9 (56) | 0.688 | 0.714 | 0.981 |
| Number of previous lines of treatment (n, range) | 5 (2–16) | 5 (2–16) | 7 (2–13) | 4.5 (3–13) | 0.196 | 0.967 | 0.154 |
| BM blasts before infusion, % (range) | 59 (0–94) | 52 (0–94) | 39.6 (0–88) | 71.5 (1–94) | 0.797 | 0.151 | 0.092 |
| Extramedullary disease, n (%) | 17 (29) | 5 (21) | 5 (35) | 4 (25) | 0.289 | 0.761 | 0.502 |
| non-CNS | 12 (20) | 4 (17) | 3 (21) | 3 (19) | |||
| CNS | 5 (9) | 1 (4) | 2 (14) | 1 (6) | |||
| High-risk cytogenetic abnormalities, n (%) | 24 (41) | 12 (50) | 5 (36) | 7 (44) | 0.409 | 0.714 | 0.677 |
| Prior HSCT, n (%) | 12 (22) | 9 (37) | 2 (14) | 0 (0) | 0.151 | 0.007 | 0.136 |
| Autologous | 1 (2) | 1 (4) | 0 (0) | 0 (0) | |||
| Allogeneic | 11 (19) | 8 (33) | 2 (14) | 0 (0) | |||
| Prior murine CD19 CAR-T, n (%) | 2 (3) | 1 (4) | 0 (0) | 1 (6) | 0.478 | 0.798 | 0.385 |
| Prior TKI | 14(24) | 7 (29) | 4 (29) | 3 (19) | 0.985 | 0.473 | 0.551 |
| CAR-T cell source, n (%) | 0.118 | 0.02 | 0.316 | ||||
| Autologous | 49 (84) | 17 (71) | 13 (93) | 16 (100) | |||
| Allogeneic | 9 (16) | 7 (29) | 1 (7) | 0 (0) | |||
| Disease response at consolidative therapy, n (%) | 0.21 | 1 | 0.316 | ||||
| MRD CR- | 58 (100) | 24 (100) | 13 (93) | 16 (100) | |||
| MRD CR+ | 1 (2) | 0 (0) | 1 (7) | 0 (0) | |||
| Onset of CD19 B-+cell Aplasia, day (range) | 1 (1–14) | 1 (1–12) | 1 (1–9) | 2.5 (1–14) | 0.957 | 0.424 | 0.555 |
| Duration of B-cell aplasia, days (range) | 85 (3–616) | 100.5 (28–616) | 109 (41–152) | 67.5 (47–101) | 0.431 | 0.024 | 0.0.003 |
Bold values indicates that P values were considered significant.
a, CART1 vs CART2; b, CART1 vs CART+HSCT; BM, bone marrow; c, CART2 vs CART+HSCT; CAR-T, chimeric antigen receptor T cell; CNS, central nervous system; CR, complete remission; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease; TKI, tyrosine kinase inhibitor.
Efficacy
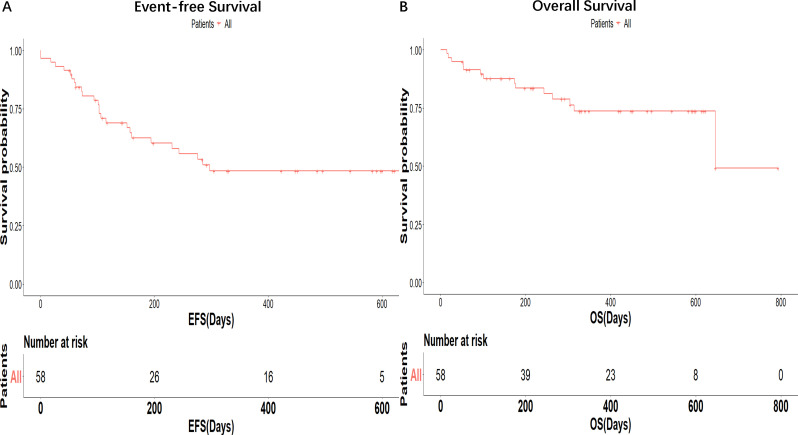
All patients were included in the efficacy analysis. Patients were evaluated for the response on day 28 after receiving hCART19 infusion. The overall CR/CRi rate was 93.1% (54/58), with 53 patients achieving MRD negativity. Two patients who were treated with hCART19 after previous non-response to murine CD19 CAR-T cells achieved MRD- CR. The two patients received the hCART19 treatment 32 and 41 days, respectively, after the mCART19 treatment. One of them received intercurrent therapy with COP (cyclophosphamide 1.2 g day 1, vindesine 4 mg day 1, dexamethasone 10 mg days 1–5) and the other patient did not receive intercurrent therapy. One patient with NR to the first hCART19 infusion because of poor CAR T-cell amplification received a second infusion of hCART19 1 month later and achieved CR. With a median follow-up of 13.5 months, the estimated 1-year OS and event-free survival (EFS) were 73.6% (95% CI 62.1% to 87.4%) and 46.0% (95% CI 33.7% to 62.8%), with a median OS and EFS of 21.5 months and 9.5 months, respectively (figure 1A, B).
Figure 1.
Survival outcomes. Event-free survival (EFS) (A) and overall survival (OS) (B).
Safety
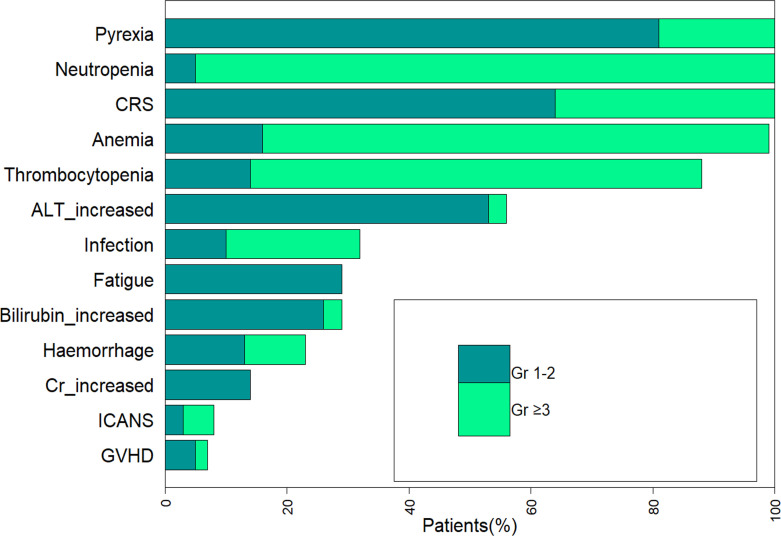
The most common treatment-emergent adverse events of any grade were fever (100%), CRS (100%) and decreased neutrophil count (100%; figure 2). The most common grade III/IV toxicities were decreased neutrophil count (95%), anemia (83%), and thrombocytopenia (74%; figure 2). The median duration of severe neutropenia, severe anemia and severe thrombocytopenia, when occurred, was 18 days (range: 4–56 days), 30 days (range: 6–105 days), and 31 days (range: 2–143 days), respectively.
Figure 2.
Treatment-emergent adverse events. ALT, aminotransferase; CRS, cytokine release syndrome; Cr, creatinine; GVHD, graft-versus-host disease.
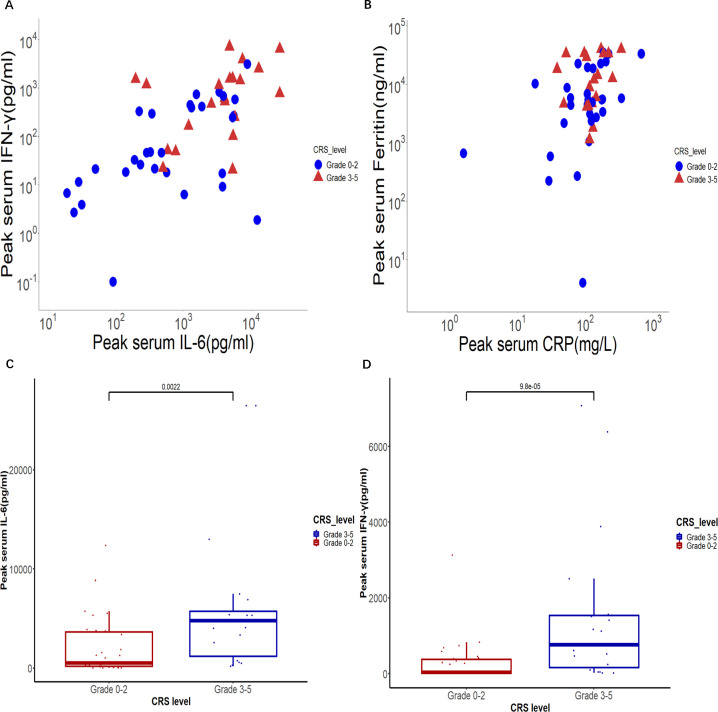
CRS of any grade occurred in all 58 patients, with severe CRS occurring in 36% (21/58) of patients treated, and no patients died from CRS. The median time to onset of CRS after the infusion was 3 days (range: 1–10 days), and the median event duration was 7 days. The majority of patients mainly showed fever and fatigue, and the symptoms could be alleviated with supportive care alone. Tocilizumab and steroids were administered in 58% of patients (35/58) and 69% of patients (40/58), respectively. Five patients with grades 1–2 CRS concurrent withneurotoxicity received steroids without tocilizumab. CRS is a systemic inflammatory response triggered by the overactivation of effector cells, characterized by supraphysiological levels of various proinflammatory biomarkers, including C reactive protein (CRP), ferritin, IL-6, and so on. Our study found that the peak levels of IL-6 and IFN-γ in the peripheral blood of patients with grade ≥3 CRS were significantly higher than those of patients with grades 0–2 CRS (p<0.01 and p<0.01, respectively; figure 3A–D). However, compared with serum cytokines, serum CRP and ferritin, which are more easily detected in clinical laboratories, are significantly increased but not correlated with severe CRS.
Figure 3.
Serum cytokines associated with CRS. The peak IFN-γ and IL-6 concentrations (A) and ferritin and CRP levels (B) in serum after infusion in patients with grade 0–2 CRS and grade 3–5 CRS are shown. Each point represents a value from one patient. The peak serum levels of IL-6 (C) and IFN-γ (D) in patients who developed grades 3–5 CRS are compared with those in patients who developed grades 0–2 CRS. CRP, C reactive protein; CRS, cytokine release syndrome.
Five patients developed neurotoxicity, including two with grade 2, two with grade 3, and one with grade 4. Three of the five patients with neurotoxicity had previous CNSL. The most common clinical manifestations were headache, dizziness, disorientation, limb weakness, and a depressed level of consciousness. The symptoms were reversible in all patients after supportive care and corticosteroids.
aGVHD occurred in four patients treated with allogeneic hCART19, involving the intestine, liver, and skin, including two patients with grade I, one patient with grade II, and one patient with grade III. None of the four had a history of GVHD. The median time developing aGVHD following hCART19 was 13 days (range: 7–15 days). Two of the four patients receive glucocorticoids and ruxolitinib, and the other two receive glucocorticoids alone. aGVHD was controlled in all the patients after treatment.
Follow-up and additional treatment
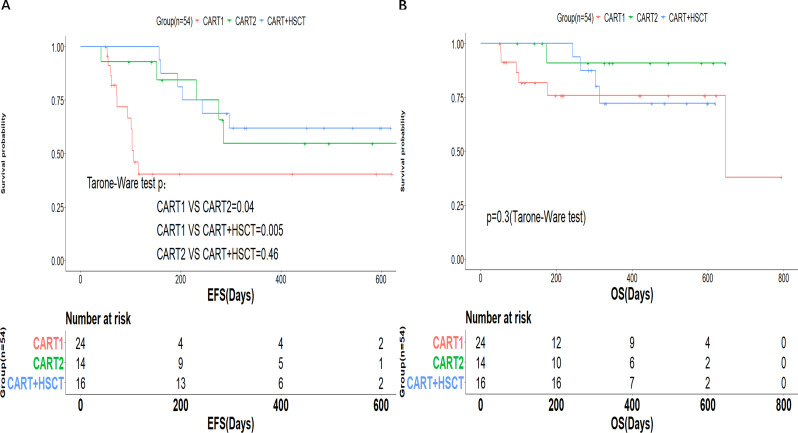
Of the 54 patients with CR, 56% (30/54) proceeded to consolidation therapy with allo-HSCT or CD22-targeted CAR-T cell. All patients went to planned consolidative therapy following the early loss of BCA, while still in primary hCART19-mediated remission. The median time from hCART19 infusion to HSCT and CD22 CAR-T cell was 73.5 days (range: 53–107 days) and 116.5 days (range: 53–433 days), respectively. Allo-HSCT consolidation therapy was performed for all patients who did not have prior HSCT and elected for HSCT, and CD22 CAR-T cell was an alternative if a suitable HSCT donor was not available. The CD22 CAR-T cell was humanized. PBMCs for CD19 CAR-T and CD22 CAR-T were collected before hCART19 treatment. As a result, 16 patients received allo-HSCT, 14 patients received CD22 CAR-T cell sequential therapy, and 24 patients did not receive consolidation therapy. Of patients proceeding to CD22 CAR-T therapy, three patients received allo-HSCT donor-derived CD22 CAR-T cell, and others receive autologous cells. According to consolidation therapy, patients were divided into three groups: initial infusion of hCART19 without transplant or infusion of CD22 CAR-T cell (CART1 group), infusion of CD22 CAR-T cell after hCART19 infusion (CART2 group), and initial infusion of hCART19 followed by allo-HSCT (CART+HSCT group). Except for age and number of patients who had prior HSCT, there were no statistical differences in baseline characteristics between the three groups (table 1). The two cohorts receiving consolidation therapy had a longer EFS than those who did not receive consolidation therapy (1-year EFS: CART1 40.4% (95% CI 23.5% to 69.4%) vs CART2 54.7% (95% CI 31.5% to 95.1%) vs CART+HSCT 61.9% (95% CI 41.9% to 91%); p value: CART1 vs CART2 0.04, CART1 vs CART+HSCT 0.005) (figure 4A). However, there was no difference in EFS between the CART+HSCT group and the CART2 group (p=0.46). No significant differences in terms of OS were observed between the three cohorts, with a 1-year OS of 75.9% (95% CI 59.3% to 97.1%), 90.9% (95% CI 75.4% to 100.0%), and 72.2% (95% CI 52.1% to 100.0%) in the CART1, CART2, and CART+HSCT groups, respectively (p=0.3) (figure 4B). Subgroup analysis for the patients treated with hCART19 alone (n=24) showed that females, patients who had prior HSCT and patients infusing allogeneic hCART19 were more likely to achieve durable remissions (online supplemental figure S2).
Figure 4.
Prognosis of patients after hCART19 therapy. Event-free survival (EFS) (A) and overall survival (OS) (B) of complete remission (CR) patients in the three groups.
Thirty-nine per cent (21/54) of patients had relapsed at a median of 169 days (range: 41–297 days) after hCART19 therapy, and all were CD19 negative. Among the patients with recurrence, three patients underwent allo-HSCT after salvage chemotherapy, six patients underwent CD22 CAR-T cell therapy, two patients received salvage chemotherapy alone, and the remaining patients did not receive further treatment. CR rate following salvage therapy following relapse was 67% (7/11). Notably, of nine patients treated with CAR-T cell derived from HSCT donors, except one patient who died of cerebral hemorrhage, eight patients did not relapse by the end of follow-up. The EFS of the first two patients treated was 20.7 months. At the end of follow-up, 14 patients out of 58 patients had died, among whom 3 patients died from severe massive hemorrhage, including diffuse alveolar hemorrhage, gastrointestinal hemorrhage and cerebral hemorrhage, and 11 patients died due to disease progression.
CAR-T cell proliferation and persistence
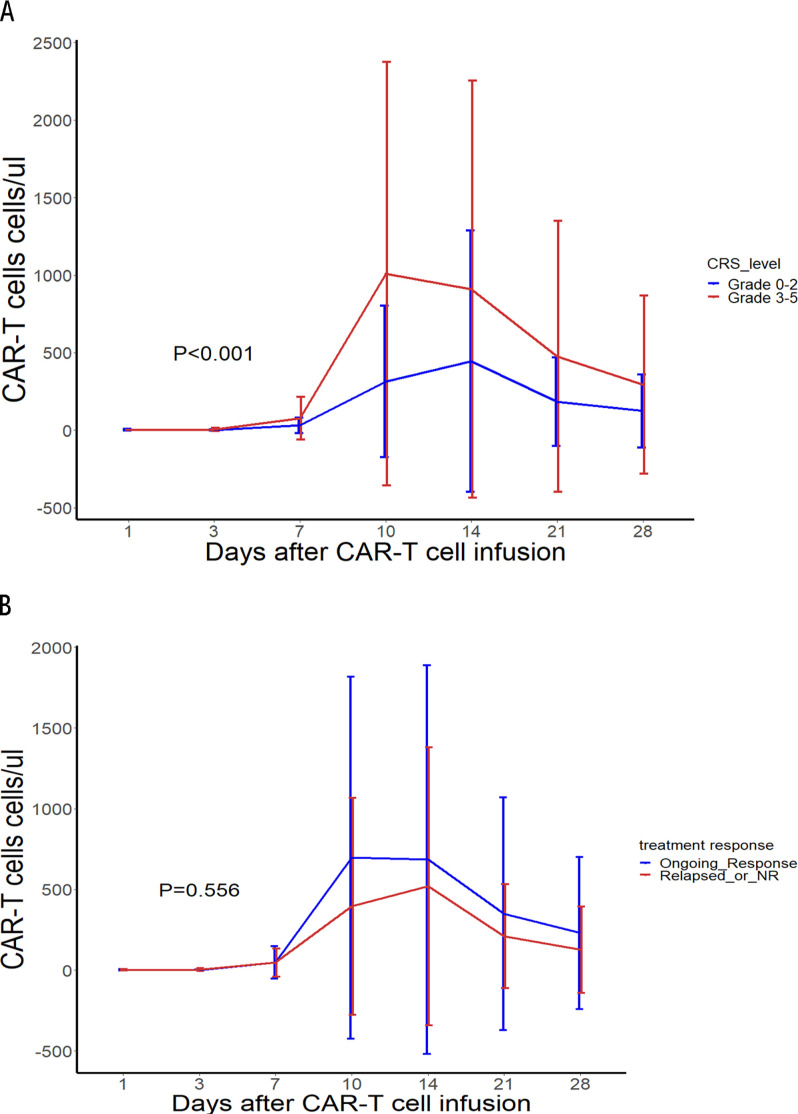
The time to maximal expansion was 14 days (range: 7–28 days), with a significant decrease in CAR-T cell proliferation after 28 days. The median Cmax was 51.3%, and the AUC0-28 was 32,509.82 cells µL–1×days. The CAR-T cell expansion among patients with grades 3–5 CRS was significantly higher than that among patients with grades 0–2 CRS (p<0.01) (figure 5A). The CAR-T cell expansion among patients who relapsed or did not respond was lower but not significantly lower than that among patients who had an ongoing response as of the data cut-off date (p=0.556) (figure 5B). For subsequent analysis, 50.4% CAR+/CD3+ cells in peripheral blood as determined on day 14 postinfusion was selected as the cut-off value related to survival. Compared with those with poor expansion (≤50.4%), those with good expansion (>50.4%) had longer OS (p=0.04) (online supplemental figure S3). But multivariable analysis showed that the expansion threshold on day 14 (>50.4%) was not an independent protective factor for OS (online supplemental table S3).
Figure 5.
hCART19 expansion and persistence in vivo as assessed via flow cytometry. (A) The postinfusion percentage of CAR+/CD3+cells in peripheral blood in patients with grades 0–2 CRS and grades 3–5 CRS. (B) The postinfusion percentage of CAR+/CD3+cells in peripheral blood in patients who relapsed or did not respond (NR) or had an ongoing response. CAR-T, chimeric antigen receptor T; CRS, cytokine release syndrome; CAR T, chimeric antigen receptor T.
Multivariable analysis showed that BCA, a surrogate for persistence, was an independent protective factor for OS (online supplemental table S3). All patients with a response to treatment had BCA, and consolidation therapy was performed at the time of loss of BCA. The median onset of BCA was day 1 (range: 1–14); the median duration of BCA was 85 days (range: 3–616 days). The CART+HSCT group had a shorter duration of BCA than the other two groups (table 1).
Humoral immunogenicity
The median HAMA titers before and after hCART19 infusing were 204.6 ng/L (range:165.6–245.0 ng/L) and 209.0 ng/L (range: 166.5–248.0 ng/L), respectively. No significant increase in HAMA was observed following infusion (p=0.78).
Clinical outcomes compared with mCART19 treatment
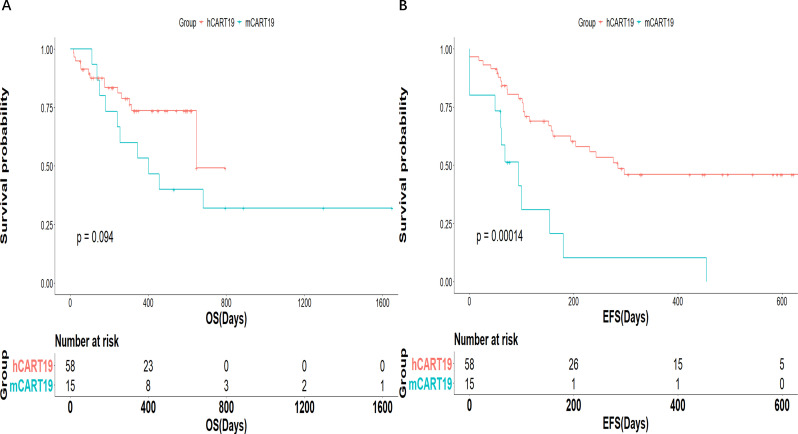
We compared the clinical outcomes, toxicity and persistence to our prior murine mCART19 trial (n=15). There were no significant differences in the CR rate, OS, and incidence of CRS, neurotoxicity, and aGVHD (online supplemental table S4). However, the duration of BCA was 112 days vs 49 days for patients treated with hCART19 compared with mCART19, respectively (p<0.001; online supplemental table S4). EFS was also improved after hCART19 infusion compared with after mCART19 infusion (1-year EFS: 46% vs 10.3%; p<0.001; figure 6 and online supplemental table S4).
Figure 6.
Survival according to CAR T product. Event-free survival (EFS) (A) and overall survival (OS) (B). CAR T, chimeric antigen receptor T.
Discussion
CD19 CAR-T cell therapy has a CR rate of 70%–90%4–6 and has played an important role in R/R ALL treatment since it was introduced into the clinic. In this study, the CR/CRi rate is better than those of previous studies. Notably, the two patients who were treated with hCART19 after the previous non-response to mCART19 achieved MRD- CR. This finding indicates that hCART19 could serve as an effective treatment for patients without response or with recurrence after mCART19 treatment. Cao et al15 also demonstrated that patients who relapsed after infusion of mCART19 still benefited from hCART19 infusion.
In this study, toxicities includingCRS andneurotoxicity were largely consistent with those observed with mCART19 treatment. But a subset of patients developed aGVHD and a surprizing proportion had hemorrhage events. aGVHD after infusing allogeneic CAR-T cell has been reported in previous studies,16 17 mainly involving the intestine, liver, and skin. In this study, the four patients with aGVHD were all patients who received CAR-T cell collected directly from the HSCT donor (4/9). The incidence is higher than in a systematic review of donor-derived CAR-T cell18 but lower than in a study of donor-derived hCART19.19 Several risk factors might attribute to the higher incidence of aGVHD in donor-derived CAR-T cell: (1) patients had preexisting GVHD before CAR-T cell therapy; (2) patients underwent HLA-haploidentical instead of HLA-matched transplantation20; (3) the costimulator of CARs was 4-1-BB rather than CD28.21 In this study, 75% (3/4) of patients with aGVHD had prior HLA-haploidentical transplantation. Similarly, the costimulator of hCART19 was 4-1-BB. Another interesting explanation is that the persistence of CD19 CAR-T cell was limited to <4 weeks in previous studies, while aGVHD occurred with a median of 4 weeks after the donor leucocyte infusion.22 Whether the higher incidence of aGVHD in patients treated with allogeneic CAR-T cell indicates its better persistence? However, there was no difference in BCA between autologous and allogeneic hCART19 cohorts. It needs to be expanded using more studies. Bleeding events occurred in 22% (13/58) of patients in this study, higher than in previous studies with similar construct/manufacture from murine CAR.23 24 Prior data support an association with low pretreatment platelet counts and severe neurotoxicity.23 In our study, 2 of 13 patients who experienced bleeding complications had respective grades 3 and 4 neurotoxicity and 8/13 had low pretreatment platelets, of which 46% (6/13) were ≥grade 3. It remains unclear whether the CAR product itself contributed to higher-than-expected rates of neurotoxicity, but will be closely monitored and investigated in the future. Moreover, Johnsrud et al23 observed that bleeding events coincided with the onset of thrombocytopenia and hypofibrinogenemia, suggesting that systemic coagulopathy/DIC-like picture may be associated with bleeding complications after CAR-T therapy. In this study, all 13 patients had grade 4 thrombocytopenia after infusion, but only a mild reduction in fibrinogen. Regrettably, we did not proceed to further investigate, such as autopsies in patients who died of hemorrhage. The mechanism of systemic coagulopathy in CAR-T therapy should be evaluated in a larger cohort in the future.
Although a high CR rate has been shown in R/R B-ALL patients treated with CD19 CAR-T cell, 30%–50% of patients relapse within 1 year after infusion,25 26 which has been attributed to loss of CD19 antigen epitopes27 28 and the limited persistence of CAR-T cell in vivo.29 The recurrence rate in our study (39%) is similar to prior reports. The difference is that all recurrent cases were CD19 negative. Pillai et al30 revealed that CD19- relapse was associated with continued CAR-T cell function. All relapses post-hCART19 therapy were CD19 negative in this study, which may support increased persistence and efficacy in eradicating CD19+ cells. As was shown in our study, duration of primary CAR-mediated BCA was observed for as long as 616 days, which was longer than that in our prior mCART19 trial. Furthermore, we found that there was no significant difference between expansion in patients who recurred or did not respond and those who responded and remained in remission. It also suggested that antigen loss was the dominant escape mechanism rather than lack of persistence with hCART19. Additionally, high preinfusion disease burden and 4-1BB CAR construct were considered associated with CD19- relapses.31 In our study, the median leukemia burden before hCART19 constructed with the 4-1BB costimulatory domain infusion was 59% (range: 0%–94%) of marrow blasts.
In this study, the 1-year OS and EFS were similar to outcomes in ELIANA,25 but the younger age of patients in ELIANA should be taken into account. Compared with our prior murine CD19 CAR-T trial, the EFS was longer in the hCART19 trial. It may be attributed to the high efficiency and persistence of hCART19. Nonetheless, the comparator arm of our prior murine-CAR has a very small sample size (n=15) and the EFS/survival outcomes were far inferior to that reported in the broader CD19- murine-based CAR historic experience. Therefore, a head-to-head study in a larger cohort is needed to further compare the efficacy of hCART19 with that of mCART19. Furthermore, the role of timely consolidation therapy in patients who were still in primary hCART19-mediated remission with loss of BCA should not be overlooked. The two cohorts receiving consolidation therapy had higher EFS than those that did not receive consolidation therapy. Hu et al32 and Jiang et al33 showed that bridging to HSCT with CD19 CAR-T cell can effectively prolong EFS in R/R B-ALL. Similarly, Pan et al34 showed that sequential infusion of CD19 and CD22 CAR-T cell in R/R B-ALL can prolong the duration of remission by preventing antigen escape and prolonging the persistence of CAR-T cell in vivo. Additionally, our study showed that females, patients who had prior HSCT and patients infusing allogeneic hCART19 were more likely to achieve durable remissions with hCART19 treatment alone. It remains to be determined which consolidation therapy is better for patients with CR after hCART19s therapy. No relevant studies have been conducted yet. But a retrospective study35 showed that after hCART19 treatment, patients who received HSCT had longer OS and leukemia-free survival (LFS) than those who received a second round of hCART19 treatment. However, no significant difference in EFS was seen between the CART+HSCT group and the CART2 group in our study. This contradictory result may be explained by the fact that in the study of Chen et al,35 the timing of the second hCART19 treatment and HSCT following hCART19 treatment was postrelapse and remission, respectively. However, in our study, all patients going on to CD22 CAR-T or HSCT went to planned consolidative therapy while still in primary CAR-mediated remission.
In conclusion, hCART19 has good short-term efficacy and controllable toxicity in R/R B-ALL patients. However, due to the limited sample size and short follow-up time in this study, the long-term efficacy needs to be further assessed.
Acknowledgments
We thank the patients, their families and caregivers for participating in the trial and all site personnel, clinical pharmacists and clinical staff for clinical trial support in this trial. Shanghai YaKe Biotechnology Ltd provided the CAR-T cells.
Footnotes
Contributors: Concept and design: GW and HH. Acquisition, analysis, or interpretation of data: FS and YH. Drafting of the manuscript: FS. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: FS, TY and YH. Administrative, technical, or material support: HX, YZ, AHC, MZ, SH and WW. Supervision: GW, HH and YH. GW accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: Key projects of the National Natural Science Foundation of China (81730008; 82130003); General Project of the National Natural Science Foundation of China (81870153); Key R&D Program of Zhejiang Province (2019C03016;2018C03016-2).
Competing interests: FS, YH, MZ, TY, WW, SH, HX, HH and GW declare that they have no competing interests. YZ and AHC are employees of Shanghai YaKe Biotechnology.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available on reasonable request. Thedata sets used and/or analyzed during the current study are available from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the 1st Affiliated Hospital, Zhejiang University School of Medicine Institutional Review Board (IIT20200058C). All study participants or their legal guardians provided written informed consent.
References
- 1.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-Long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012;4:132ra53. 10.1126/scitranslmed.3003761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsallab M, Levine BL, Wayne AS, et al. Car T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol 2020;21:e104–16. 10.1016/S1470-2045(19)30729-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKay M, Afshinnekoo E, Rub J, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol 2020;38:233–44. 10.1038/s41587-019-0329-2 [DOI] [PubMed] [Google Scholar]
- 4.Pan J, Yang JF, Deng BP, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017;31:2587–93. 10.1038/leu.2017.145 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Lu X-A, Yang J, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv 2020;4:2325–38. 10.1182/bloodadvances.2020001466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anagnostou T, Riaz IB, Hashmi SK, et al. Anti-Cd19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. The Lancet Haematology 2020;7:e816–26. 10.1016/S2352-3026(20)30277-5 [DOI] [PubMed] [Google Scholar]
- 7.Lamers CHJ, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo–engineered T cells. Blood 2011;117:72–82. 10.1182/blood-2010-07-294520 [DOI] [PubMed] [Google Scholar]
- 8.Dwivedi A, Karulkar A, Ghosh S, et al. Robust antitumor activity and low cytokine production by novel humanized anti-CD19 CAR T cells. Mol Cancer Ther 2021;20:846–58. 10.1158/1535-7163.MCT-20-0476 [DOI] [PubMed] [Google Scholar]
- 9.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010;16:1245–56. 10.1016/j.bbmt.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi L-A, Berger C, et al. Immunotherapy of non-hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116. 10.1126/scitranslmed.aaf8621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turtle CJ, Hanafi L-A, Berger C, et al. Cd19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell all patients. J Clin Invest 2016;126:2123–38. 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An L, Lin Y, Deng B, et al. Humanized CD19 CAR-T cells in relapsed/refractory B-ALL patients who relapsed after or failed murine CD19 CAR-T therapy. BMC Cancer 2022;22:393. 10.1186/s12885-022-09489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma F, Ho J-Y, Du H, et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor-modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol Oncol 2019;37:601–8. 10.1002/hon.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao J, Wang G, Cheng H, et al. Potent anti-leukemia activities of humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol 2018;93:851–8. 10.1002/ajh.25108 [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Wang J, Wei G, et al. A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplant 2019;54:1208–17. 10.1038/s41409-018-0403-2 [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Cheng Y, Suo P, et al. Donor-Derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol 2017;179:598–605. 10.1111/bjh.14923 [DOI] [PubMed] [Google Scholar]
- 18.Anwer F, Shaukat A-A, Zahid U, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy 2017;9:123–30. 10.2217/imt-2016-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Liu M, Lyu C, et al. Acute graft-versus-host disease after humanized anti-CD19-CAR T therapy in relapsed B-ALL patients after allogeneic hematopoietic stem cell transplant. Front Oncol 2020;10:573822. 10.3389/fonc.2020.573822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Deng B, Yin Z, et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol 2021;96:671–9. 10.1002/ajh.26160 [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A, Smith M, James SE, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med 2017;23:242–9. 10.1038/nm.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins RH, Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant 2000;26:511–6. 10.1038/sj.bmt.1702555 [DOI] [PubMed] [Google Scholar]
- 23.Johnsrud A, Craig J, Baird J, et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv 2021;5:4465–75. 10.1182/bloodadvances.2021004716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buechner J, Grupp SA, Hiramatsu H, et al. Practical guidelines for monitoring and management of coagulopathy following tisagenlecleucel CAR T-cell therapy. Blood Adv 2021;5:593–601. 10.1182/bloodadvances.2020002757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Rivière I, Gonen M, et al. Long-Term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449–59. 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018;24:1504–6. 10.1038/s41591-018-0146-z [DOI] [PubMed] [Google Scholar]
- 28.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016;127:2406–10. 10.1182/blood-2015-08-665547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017;130:2317–25. 10.1182/blood-2017-06-786129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillai V, Muralidharan K, Meng W, et al. Car T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv 2019;3:3539–49. 10.1182/bloodadvances.2019000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamble A, Myers RM, Taraseviciute A, et al. Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv 2022:bloodadvances.2022007423. 10.1182/bloodadvances.2022007423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L, Charwudzi A, Li Q, et al. Anti-Cd19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: a systematic review and meta-analysis. Ann Hematol 2021;100:1003–12. 10.1007/s00277-021-04451-w [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Li C, Yin P, et al. Anti-Cd19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: an open-label pragmatic clinical trial. Am J Hematol 2019;94:1113–22. 10.1002/ajh.25582 [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Zuo S, Deng B, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 2020;135:387–91. 10.1182/blood.2019003293 [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Ma Y, Shen Z, et al. Humanized anti-CD19 CAR-T cell therapy and sequential allogeneic hematopoietic stem cell transplantation achieved long-term survival in refractory and relapsed B lymphocytic leukemia: a retrospective study of CAR-T cell therapy. Front Immunol 2021;12:755549. 10.3389/fimmu.2021.755549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005701supp001.pdf (323.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available on reasonable request. Thedata sets used and/or analyzed during the current study are available from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.