Abstract
Objectives
Frailty is a multidimensional syndrome of loss of reserves in energy, physical ability, cognition and general health. Primary care is key in preventing and managing frailty, mindful of the social dimensions that contribute to its risk, prognosis and appropriate patient support. We studied associations between frailty levels and both chronic conditions and socioeconomic status (SES).
Design
Cross-sectional cohort study
Setting
A practice-based research network (PBRN) in Ontario, Canada, providing primary care to 38 000 patients. The PBRN hosts a regularly updated database containing deidentified, longitudinal, primary care practice data.
Participants
Patients aged 65 years or older, with a recent encounter, rostered to family physicians at the PBRN.
Intervention
Physicians assigned a frailty score to patients using the 9-point Clinical Frailty Scale. We linked frailty scores to chronic conditions and neighbourhood-level SES to examine associations between these three domains.
Results
Among 2043 patients assessed, the prevalence of low (scoring 1–3), medium (scoring 4–6) and high (scoring 7–9) frailty was 55.8%, 40.3%, and 3.8%, respectively. The prevalence of five or more chronic diseases was 11% among low-frailty, 26% among medium-frailty and 44% among high-frailty groups (χ2=137.92, df 2, p<0.001). More disabling conditions appeared in the top 50% of conditions in the highest-frailty group compared with the low and medium groups. Increasing frailty was significantly associated with lower neighbourhood income (χ2=61.42, df 8, p<0.001) and higher neighbourhood material deprivation (χ2=55.24, df 8, p<0.001).
Conclusion
This study demonstrates the triple disadvantage of frailty, disease burden and socioeconomic disadvantage. Frailty care needs a health equity approach: we demonstrate the utility and feasibility of collecting patient-level data within primary care. Such data can relate social risk factors, frailty and chronic disease towards flagging patients with the greatest need and creating targeted interventions.
Keywords: PRIMARY CARE, EPIDEMIOLOGY, Information management
Strengths and limitations of this study.
Data collection was facilitated with clinical championing, contributing to a high Clinical Frailty Scale scoring completion rate (77%) of among eligible patients, supporting internal and external validity.
A primary care practice-based research network was the source of both the prospective frailty assessment and retrospective clinical electronic medical record data demonstrating the research capacity of practice-based research network data.
Socioeconomic status (SES) was linked to patients through Canadian Census data.
This study is cross-sectional; therefore, we cannot deduce the direction of causation of the associations between SES, multimorbidity and level of frailty.
We used neighbourhood-level income and deprivation status in the absence of directly collected, patient-level SES, which is not collected in the primary care electronic medical record.
Introduction
Frailty is considered a multidimensional syndrome of loss of reserves in energy, physical ability, cognition and general health. There are approximately 1.6 million people in Canada living with frailty.1 Frail patients are complex and more susceptible to adverse health outcomes compared with non-frail people of the same chronological age.2 Left unchecked, frail patients experience diminished quality of life, high risk of hospitalisation and long-term care admission and increased mortality.3–5
Primary care, as the medical home for patients, plays a central role in chronic condition prevention and management, and care coordination.6 Evidence clearly shows that stronger primary care is associated with better population outcomes, achieved through the key mechanisms of access (first contact), comprehensiveness, coordination and continuity (longitudinal care) and, as such, has the potential to be the most equity-enhancing aspect of clinical care.7 On this premise, primary care plays a key role in preventing, identifying and managing frailty8 while taking into consideration the social dimensions that contribute to its associated risk, prognosis and facilitators or barriers to clinical intervention and support.9
While frailty is not fully explained by the presence of specific health conditions, a large proportion of people experiencing frailty also have multiple chronic medical conditions (multimorbidity).10–12 Multimorbidity negatively affects patient-important outcomes like disability, social participation, and self-rated physical and mental health, and it contributes to care burden13 14 and the need for acute care services.12 The combination of frailty and multimorbidity is likely to exacerbate their respective load of associated issues.
Low socioeconomic status (SES) is associated with multimorbidity, health risks, poorer prognoses and challenges in accessing equitable care.15–18 Lower socioeconomic status also reduces access to primary care.16 19 The addition of a socioeconomic lens on the multimorbid and frail population can help focus effective interventions and distribute resources more equitably. In primary care, the longitudinal comprehensive primary care framework can be leveraged with clinicians identifying their frail patients, guiding them to appropriate goal-directed care, and helping them to proactively manage their complex health and social needs.
Reliable screening tools for frailty are easy to use in primary care settings.20 21 Identification of prefrail and frail patients in primary care creates the opportunity to develop targeted interventions that address quality of life, burden of unnecessary treatment and assess barriers.2 3 22–24 Such approaches are likely to improve the individual patient’s health and experience of equitably delivered healthcare and to ameliorate resource use. Planning and evaluating programmes in primary care designed to meet the needs of all frail older adults would be better guided with an understanding of how chronic disease patterns and social factors (SES) intersect and are patterned across prefrail and frail states.22 We found little evidence that examined the intersection of frailty, multimorbidity and SES, and with this study, we sought to explore their relationship.
We hypothesised that patients with a greater number of chronic conditions, especially disabling conditions, and with lower SES would be more likely to have a higher clinical frailty score. Our aim was to describe the distribution of frailty among older adult patients in primary care, and to describe the association between chronic conditions, SES and frailty. This study adds a unique analysis of this association within a primary care population using directly collected clinical frailty scores, not routinely applied in this population.
Methods
We conducted a cross-sectional study of a cohort of older adults, created using electronic medical records from a primary care practice-based research network (PBRN). Clinical frailty assessments and neighbourhood-level income and deprivation data were linked to the patient’s clinical data available from their electronic medical records.
Setting
The McMaster University Sentinel and Information Collaboration (MUSIC) Network is a PBRN in Hamilton, Ontario, Canada. Data holdings include a regularly updated, deidentified, longitudinal database containing primary care practice data. There are 55 family physicians associated with the MUSIC Network. This study was based on a subset of the 37 physicians in the MUSIC PBRN, and their previously described 38 000 patients represent a broad cross section of patients.25 26
Participants
Patients aged 65 years or older, currently rostered (as of January 2020) to family physicians of the MUSIC network subgroup described previously, were eligible to be included in the cohort.
Patient and public involvement
Patients and the public were not involved in this study.
Data collection and preparation
Frailty score assignment
The Clinical Frailty Scale (CFS) is a frailty screening tool that applies clinical judgement for scoring personal capacity and independence related to fitness and self-management of health. It can be reliably used to predict outcomes related to mortality, comorbidity, mobility and functional and cognitive decline. The CFS features a clinically validated 9-point scale ranging from 1 (very fit) to 9 (terminally ill) with scores of ≥5 indicating a frail state.20 27 With low administration time (less than 1 min per patient assessment) and low cost, the CFS has seen high utility across many clinical practice domains.20 28 29 The CFS scoring instrument has been validated in a variety of healthcare settings including primary care, and patients do not need to be seen in person for the physician to form an accurate score.21 The CFS has good inter-rater agreement between physicians and multidisciplinary teams and correlates well with other frailty scoring instruments.28 29
Physicians were approached personally by the network leadership to discuss the rationale for the study and the utility of its results, to assess their support and perception of clinical relevance, before formally engaging in the study. The participating physicians’ electronic medical record (EMR) was used to identify rostered patients over the age of 65 and who had attended a clinical encounter within the last 6 months. Rostered patients are patients who are attached to a particular family physician who provides longitudinal primary care. The family physicians were provided with a list of eligible patients in their care, accompanied by the CFS scoring guide (online supplemental appendix A). Physicians were asked to complete frailty assessments (selecting a CFS score between 1 and 9) for patients for whom they were confident that they could assess, based on their overall knowledge of the patient. This provided a reasonable, current assessment across the study population. There were no other specific exclusion criteria.
bmjopen-2022-066269supp001.pdf (1.4MB, pdf)
Scored lists were collected from each provider and securely entered within a research database, replacing all patient identifiers with the MUSIC patient ID. Scores were completed for 77% of eligible patients.
Cohort creation and data linkage
The final cohort for this study included 2043 patients whose physician had assessed their frailty level and who had (1) a postal code in Hamilton that could accurately be linked to neighbourhood-level information (described further) and (2) accessible disease diagnoses through the MUSIC-PBRN database.
To capture conditions, we used the International Classification of Diseases, Ninth Revision (ICD-9) disease codes which are recorded by physicians, within the disease registry module of the EMR. Coded conditions include disorders such as chronic obstructive pulmonary disease (COPD) and heart failure as well as risk factors such as hypertension and dyslipidaemia. Previous work that improved the quantity and the consistency of chronic disease codes recorded within the MUSIC PBRN EMR featured quick-pick lists of ‘preferred terms’ for common and discrete primary care conditions such as unipolar depression (311) and bipolar depression (296), both with distinct codes.25 For this study, certain conditions considered to be similar (ie, variations of the same base condition) were grouped, for example, dementia (ICD-9 290) and Alzheimer’s disease (ICD-9 331.0), in order to form meaningful groupings for frequency analysis.
We used the Postal Code Conversion File, complementary to the 2016 Canadian Census,30 to translate patients’ postal codes to the geographical census unit of dissemination area (DA). A DA is a small geographical census area composed of one or more neighbouring blocks, with a population of 400–700 persons.30 In this study, the cohort was limited to only patients having a 1:1 mapping between their postal code and matching DA to facilitate a simple and direct means of linking DA to SES data.
The Canadian Socio-economic Information Management System database was accessed via the Computing in the Humanities and Social Sciences Canadian Census Analyser31 to retrieve economic family after-tax income decile group data.32 Economic family after-tax neighbourhood income decile groups can provide a rough ranking of an individual’s relative economic position. These income decile data were organised by DA geographical units. We calculated the median decile for each DA unit represented in our cohort and linked this value to each patient via the postal code to DA map. We further collapsed the decile groups into quintiles to achieve reasonable category sizes for statistical analysis.
A second set of socioeconomic data from the Ontario Marginalisation Index33 was also linked to the dataset using the same postal code to DA translation. The index provides a measure of material deprivation, an estimate of the inability for individuals and communities to access and attain basic material needs using indicators of income, quality of housing, educational attainment and family structure characteristics.
Data analysis
We described the distribution of patient demographic characteristics, frailty, chronic conditions and SES variables using simple descriptive analyses (means, median, frequencies and proportions, as appropriate). A review of the distribution of the CFS scores showed some score categories had very small numbers; therefore, for analysis purposes, we further grouped the cohort’s frailty scores as low (scored 1–3), medium (scored 4–6) and high (scored 7–9).
We examined bivariate associations between frailty and other patient characteristics (demographics, multimorbidity and SES). Age was categorised as 65–69, 70–74, 75–79, 80–84, 85–89 and >90 years. Male and female captured sex groupings. Income quintiles included scores of 1 (lowest income) to 5 (highest income). Material deprivation quintiles included groupings of 1 (least deprived) to 5 (most deprived). We examined chronic conditions (disorders and risk factors) in two ways: first, dichotomising as having one or more conditions versus none and second, as 0, 1, 2-4, 5+ conditions.
We examined associations using χ2, and analysis of variance (ANOVA) as appropriate. χ2 test was used for association between frailty level grouping and other factors for all variables except age, which was a continuous variable, where we used ANOVA.
Statistical significance was set at alpha <0.05 (two-tailed). The data were analysed using IBM SPSS Statistics V.28.
Results
Demographics
The mean age of the patients (n=2043) was 76 years (as of February 2020) and 60.5% (1236/2043) were female. Two-thirds of the patients (63.5%, 1296/2043) had two or more chronic conditions (table 1).
Table 1.
Associations between frailty score and demographic characteristics, deprivation indicators and multimorbidity (N=2043)
| Characteristics | Overall | Low frailty, n (%) |
Medium frailty, n (%) |
High frailty, n (%) | P value |
| Frailty group | 2043 | 1141 (55.9) | 824 (40.3) | 78 (3.8) | |
| Demographics | |||||
| Mean age (SD) | 76 (7.8) | 73.3 (5.9) | 79.1 (8.2) | 85.2 (9.0) | <0.001* |
| Median age (range) | 74 (65–103) | 72 (65–99) | 78 (65–103) | 86 (66–102) | <0.001† |
| Age grouping (years) | |||||
| 65–69 | 589 (29) | 440 (39) | 143 (17) | 6 (8) | <0.001‡ |
| 70–74 | 540 (26) | 355 (31) | 176 (21) | 9 (12) | |
| 75–79 | 374 (18) | 210 (18) | 156 (19) | 8 (10) | |
| 80–84 | 247 (12) | 84 (7) | 148 (18) | 15 (19) | |
| 85–89 | 173 (9) | 40 (4) | 120 (15) | 13 (17) | |
| >90 | 120 (6) | 12 (1) | 81 (10) | 27 (35) | |
| Female, n (%) | 1236 (61) | 647 (57) | 535 (65) | 54 (69) | <0.001‡ |
| Male, n (%) | 807 (40) | 494 (43) | 289 (35) | 24 (31) | |
| Median income quintile§ | |||||
| 1 (low) | 104 (5) | 35 (3) | 65 (8) | 4 (5) | <0.001‡ |
| 2 | 545 (27) | 269 (24) | 250 (30) | 26 (33) | |
| 3 | 783 (38) | 429 (38) | 324 (39) | 30 (39) | |
| 4 | 520 (26) | 348 (31) | 157 (19) | 15 (19) | |
| 5 (high) | 91 (5) | 60 (5) | 28 (3) | 3 (4) | |
| Deprivation quintile¶ | |||||
| 1 (least deprived) | 411 (20) | 286 (25) | 113 (14) | 12 (15) | <0.001‡ |
| 2 | 302 (15) | 182 (16) | 106 (13) | 14 (18) | |
| 3 | 348 (17) | 179 (16) | 156 (19) | 13 (17) | |
| 4 | 530 (26) | 277 (24) | 228 (28) | 25 (32) | |
| 5 (most deprived) | 452 (22) | 217 (19) | 221 (27) | 14 (18) | |
| Multimorbidity** | |||||
| Chronic conditions, n** | |||||
| 0 | 338 (17) | 261 (23) | 73 (9) | 4 (5) | |
| 1+ | 1705 (84) | 880 (77) | 751 (91) | 74 (95) | <0.001‡ |
| 1 | 409 (20) | 267 (23) | 133 (16) | 9 (12) | <0.001‡ |
| 2–4 | 923 (45) | 487 (43) | 405 (49) | 31 (40) | |
| 5+ | 373 (18) | 126 (11) | 213 (26) | 34 (44) |
*P value from analysis of variance test.
†P value from Kruskal-Wallis test.
‡P value from χ2 test for categorical data.
§Mean value of economic family after-tax income decile group data at DA level based on Statistics Canada 2016 Census data.
¶Deprivation score categories from the Ontario Marginalisation Index at DA level.
**Chronic disorders and risk factor conditions coded by physicians with ICD-9.
DA, dissemination area.
Frailty distribution
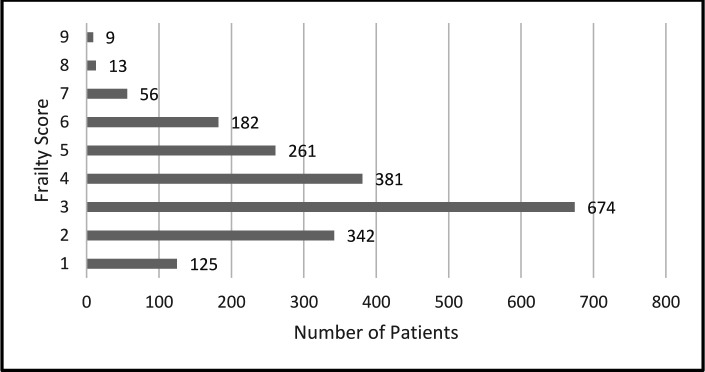
Figure 1 shows the frailty distribution over all nine CFS categories with a slightly skewed distribution with fewer patients in the frailest categories and the highest proportion of patients scored as 3.
Figure 1.
Frailty score distribution among the 2043 primary care patients. Frailty scored on a 9-point scale ranging from 1 (very fit) to 9 (terminally ill), with scores of ≥5 indicating a frail state.20 27
Median income distribution
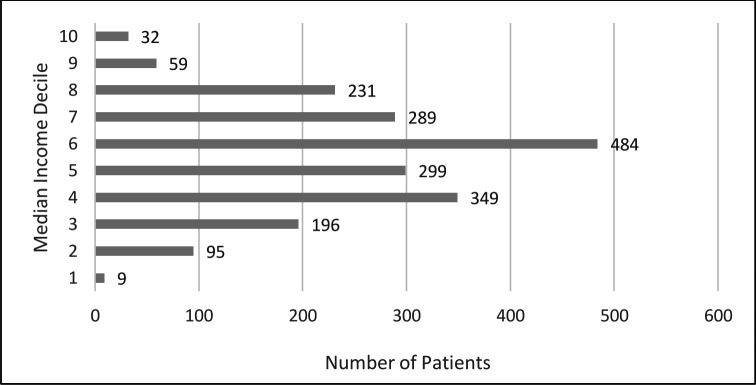
Figure 2 shows the full distribution of median economic family after-tax income deciles (1 as lowest income decile and 10 as highest income decile). There was a near-normal distribution with the highest proportion of patients in decile 6. Grouping deciles into quintiles with 1 designated as lower income and 5 as higher income, results showed that most patients were in the middle quintile (3) (38.3%) with the smallest proportions in the lowest (5.1%) and highest income (4.5%) quintiles (table 1).
Figure 2.
Median family after-tax neighbourhood income decile distribution among the 2043 patients. Scale: one as lowest income decile, 10 as highest income decile.
Associations between frailty level and demographic characteristics, SES and multimorbidity
Mean age ascended across frailty groups at 73 years in the low-frailty group, 79 years in the medium-frailty group and 85 years in the high-frailty group (ANOVA, F=231.62, df 2, p<0.0001) (table 1). The proportion of patients who were female increased across low, medium and high-frailty groups (57%, 65%, 69% respectively) (χ2=16.12, df=3, p<0.001).
The distribution of income quintiles was significantly different across the frailty groups (χ2=61.42, df=8, p<0.001). There is some trend of moderate-frailty and higher-frailty patients associated with lower income quintiles compared with patients with low frailty (table 1).
There were significant differences in proportions of patients in different frailty groups across the material deprivation quintile groupings (χ2=55.243, df=8, p<0.001). Table 1 also shows certain trending of the higher-frailty group associated with greater deprivation, compared with the moderate-frailty and low-frailty groups that show less deprivation.
Patients with higher frailty were more likely to have at least one coded condition; 95%, 91% and 77% in the high-frailty, medium-frailty and low-frailty groupings, respectively (χ2=75.7254, df 2, p<0.0001). Furthermore, the accumulation of chronic disease (1, 2–4 or 5+ conditions) differed across frailty groups (χ2=169.6 df 6, p<0.001). The data show a trend of more multimorbidity burden in the high-frailty group (12%, 40%, 44% for 1, 2–4 and 5+ conditions), whereas the low-frailty and medium-frailty groups showed higher proportions in the 2–4 conditions category (43% and 49% for low frailty and medium frailty, respectively) (p<0.001).
Chronic conditions patterns
Table 2 shows the most frequent chronic conditions and risk factors (eg, hypertension and hyperlipidaemia) in each frailty grouping. A core set of high-frequency conditions composed at least 50% of all conditions in all three frailty groups but a broader range of conditions were included in the top 50% as frailty increased. In the low-frailty group, five conditions including the risk factors hypertension and hyperlipidaemia and the disorders diabetes, osteoarthritis/joint pain and depression-unipolar composed 52% of their conditions. These five conditions persisted in the medium-frailty group with the addition of two more conditions (back, neck pain and sciatica and cardiac dysrhythmia) which together, then compoed 51% of conditions. In the high-scored frailty group, nine conditions composed 52% of the conditions, with dementia/Alzheimer’s disease, congestive heart failure and acute and chronic coronary artery disease appearing uniquely and displacing back, neck pain and sciatica. Among other burdensome diseases, COPD was a relatively common condition in the high-frailty and medium-frailty groups (falling within 54% of all conditions for both) but less so in the low-frailty group (falling within the top 70% of all conditions).
Table 2.
Array and proportion of conditions (disorders and risk factors*) in each frailty grouping among the 2043 primary care patients
| Frailty score 1–3 | Proportion of grouping diagnoses (%) | Frailty score 4–6 | Proportion of grouping diagnoses (%) | Frailty score 7–9 | Proportion of Grouping diagnoses (%) |
| Hypertension* | 19 | Hypertension* | 14.9 | Hypertension* | 11.8 |
| Diabetes | 12 | Diabetes | 9.7 | Osteoarthritis and joint pain | 7.3 |
| Hyperlipidaemia/ dyslipidaemia* |
8.3 | Osteoarthritis and joint pain | 7.7 | Diabetes | 6.4 |
| Osteoarthritis and joint pain | 7.2 | Hyperlipidaemia/ dyslipidaemia* |
5.8 | Dementia/ Alzheimer’s disease† |
5.6 |
| Depression (unipolar) | 5.9 | Depression (unipolar) | 5.5 | Cardiac dysrhythmia | 5.6 |
| Back, neck pain and sciatica‡ | 4 | Depression | 4.5 | ||
| Cardiac dysrhythmia | 3.3 | Congestive heart failure† | 3.6 | ||
| Hyperlipidaemia/ dyslipidaemia* |
3.4 | ||||
| Acute and chronic coronary artery disease† | 3.40 | ||||
| Total proportion | 52.3 | 50.7 | 51.5 |
*Risk factor type among conditions.
†First appears in high-frailty group.
‡First appears in medium-frailty group.
Discussion
In this study of older adult patients in primary care, almost half of the patients evaluated by their family physician had a moderate or high level of frailty. Higher frailty level was associated with older age, being female and having more chronic conditions, especially life-limiting or disabling conditions such as cardiovascular diseases and dementia. Higher frailty level was also associated with indicators of lower SES.
The usability of the CFS in primary care was evidenced in our study with frailty scores completed for 77% of eligible patients. This directly collected data was a strength of the study, as was the study setting within a PBRN where disease coding in the EMR has been strengthened through previous initiatives.25 We attribute the high scoring completion rate to a combination of factors: clinicians were engaged prior to deciding to go forward with the study; the study aligned with physicians’ interests in supporting frail patients as well as effective leadership and clinical championing of this study and its data collection requirements.
The cohort showed a near normal distribution across high-frailty, medium-frailty and low-frailty groups, with the most common scores lying in the middle quintile. As expected in a community-dwelling cohort, there were fewer older adults in the most frail group as these patients are more likely to need long-term care accommodation support.4
We have identified shifts in the patterns of the most common conditions that affect the older adult primary care population across categories of increasing frailty. Furthermore, in those experiencing high-frailty conditions, substantial and life-limiting morbidity appears more commonly, and so does their burdensome management requirements, greater risk of hospitalisation and lowered quality of life.12–14 34
We found that living in neighbourhoods of lower income and higher social material deprivation was more common among patients with moderate and high frailty. These differences have important implications at population level and may reflect large numbers of patients at risk for negative health outcomes. Socioeconomic disadvantage may be compounded by costs associated with living with frailty (eg, need for mobility aids) in addition to the costs of managing the individual health conditions.5 12
A health-equity approach involves specific targeting of programmes or resources to those most disadvantaged. The associations among frailty, chronic disease and SES, as shown in this study, can support a health equity approach in planning healthcare for older adults within primary care. Ideally, the availability of all three data pieces would enable focused targeting of programmes or resources to older adults who are most disadvantaged in primary care, but the unique disease patterns we identified in higher-frailty groups also provides a marker of likely triple disadvantage.
We have also shown the utility and feasibility of collecting frailty data in a PBRN. The MUSIC Network immediately used these data in a targeted approach to COVID-19 remote care monitoring during Ontario’s first and most serious wave of the pandemic. As it was not practically possible to contact all older adults, the MUSIC clinical teams used prepared digital files that sorted older adults by frailty score, with chronic conditions information. This enabled the family health teams to take an equity-focused approach, prioritising contact with a subgroup of older adult patients who were scored higher for frailty to provide COVID-19 education and to ensure food and medication security.
The high uptake of CFS coding shows that frailty measures could become routine data points that are scored regularly and recorded within the EMR. Future work should also be directed at systematically and standardly collecting and integrating, as a first step, neighbourhood-level SES data available from census data, and then ideally moving to patient level SES data within the primary care EMR. SES, frailty and chronic disease markers could be automatically combined within the EMR to identify patients at risk of a poor prognosis. Patient-level EMR flags or practice-level dashboard could alert providers or quality specialists, respectively, to the need for interventions for addressing social and clinical risk factors for these patients, and to allow programme development within clinics of larger groupings to direct resources to those with greatest need.
Limitations
The study design is cross-sectional; therefore, we cannot deduce the direction of causation of the associations between SES, multimorbidity and level of frailty.
Information on individual SES is not routinely collected in primary care. We have assumed neighbourhood-level income and deprivation status provide reasonable accuracy with respect to the actual SES of individual patients within our frailty cohort. Neighbourhood-level information on indicators such as income may not reflect actual wealth among older adults who are more likely to have left the workforce due to retirement.
We limited the cohort to patients for whom a single DA code mapped to their postal code. This affects sample size, but there is no reason to think it would affect the associations found. Similarly, the limitation of the cohort to those with a recent encounter for whom the clinician had enough knowledge to complete a CFS score, should not affect the associations seen.
Conclusions
Awareness of the triple disadvantage of frailty, disease burden and socioeconomic disadvantage, as well as specific disease patterns, can support a health equity approach to care for older adults within primary care.
The confluence of health and social disadvantage and increasing frailty highlights the need for targeted health and social care approaches for achieving improved health equity. Our findings also underscore the need to anticipate required healthcare services and to use finite resources most effectively. Grounding this approach in primary care with the appropriate data support is highly appropriate as evidence shows primary care is a strong mechanism for reducing health inequity, fostering access to comprehensive longitudinal care as well as targeted clinical and programme innovations that are effective in reducing modifiable health inequity.7
Supplementary Material
Acknowledgments
The authors would like to thank the primary care clinicians and patients of the MUSIC PBRN who contribute their data to the MUSIC Network through which the study data were generated for this project. We also acknowledge Krzysztof Adamczyk, the information technology lead for the MUSIC network and Vivek Jadon from McMaster Libraries for guidance to Statistics Canada data and Jennifer Salerno for statistical support. We thank Kathy De Caire, Kati Ivanyi, Doug Oliver and Jill Berridge for their executive support in the implementation of this project. We acknowledge the support of the McMaster University, Department of Family Medicine for this PBRN. We recognise the work of the Canadian Primary Care Sentinel Surveillance Network partnering with the Canadian Frailty Network in the original implementation of the CFS across many of its networks.
Footnotes
Twitter: @DeeMangin, @risdonc, @mhoward101
Contributors: Substantial contributions to the conception or design of the work, analysis or interpretation of data for the work: DM, JL and MH. Acquisition of data: DM and JL. Drafting the work or revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: DM, JL, CR, HS, TP, SW and MH. Guarantor: DM.
Funding: This work was supported by the INSPIRE-PHC Program, Applied Health Research Question, Ontario Ministry of Health (grant number 6023175, total funding: $31 680).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The dataset is available by contact with the corresponding author, DM (mangind@mcmaster.ca).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Hamilton Integrated Research Ethics Board (reference number 10683-C). The ethics board that approved this study (HiREB) granted a waiver for the need for patient consent, acknowledging the impracticality of obtaining patient consent for epidemiological, retrospective research. Also, the physicians who collected the frailty scores of their patients are members of the MUSIC Network, a practice-based research network, and physician members have agreed to the use of their patient data for approved MUSIC Network-related research, into which this study falls.
References
- 1.Canadian Frailty Network . Frailty matters. Available: https://www.cfn-nce.ca/frailty-matters [Accessed Jan 2022].
- 2.Lacas A, Rockwood K. Frailty in primary care: a review of its conceptualization and implications for practice. BMC Med 2012;10:4. 10.1186/1741-7015-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker TF, Brown L, Clegg A, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 2019;28:2041–56. 10.1007/s11136-019-02149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima G. Frailty as a predictor of nursing home placement among community-dwelling older adults: a systematic review and meta-analysis. J Geriatr Phys Ther 2018;41:42–8. 10.1519/JPT.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 5.Kojima G. Increased healthcare costs associated with frailty among community-dwelling older people: a systematic review and meta-analysis. Arch Gerontol Geriatr 2019;84:103898. 10.1016/j.archger.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Bodenheimer T, Pham HH. Primary care: current problems and proposed solutions. Health Aff (Millwood) 2010;29:799–805. 10.1377/hlthaff.2010.0026 [DOI] [PubMed] [Google Scholar]
- 7.Starfield B, Gérvas J, Mangin D. Clinical care and health disparities. Annu Rev Public Health 2012;33:89–106. 10.1146/annurev-publhealth-031811-124528 [DOI] [PubMed] [Google Scholar]
- 8.Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017;46:401–7. 10.1093/ageing/afw242 [DOI] [PubMed] [Google Scholar]
- 9.Andrew MK, Dupuis-Blanchard S, Maxwell C, et al. Social and societal implications of frailty, including impact on Canadian healthcare systems. J Frailty Aging 2018;7:217–23. 10.14283/jfa.2018.30 [DOI] [PubMed] [Google Scholar]
- 10.Yarnall AJ, Sayer AA, Clegg A, et al. New horizons in multimorbidity in older adults. Age Ageing 2017;46:882–8. 10.1093/ageing/afx150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2019;74:659–66. 10.1093/gerona/gly110 [DOI] [PubMed] [Google Scholar]
- 12.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162:2269–76. 10.1001/archinte.162.20.2269 [DOI] [PubMed] [Google Scholar]
- 13.Griffith LE, Gilsing A, Mangin D, et al. Multimorbidity frameworks impact prevalence and relationships with patient-important outcomes. J Am Geriatr Soc 2019;67:1632–40. 10.1111/jgs.15921 [DOI] [PubMed] [Google Scholar]
- 14.Fortin M, Dubois MF, Hudon C, et al. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes 2007;5:52. 10.1186/1477-7525-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer SW, Watt GCM. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med 2007;5:503–10. 10.1370/afm.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercer SW, Zhou Y, Humphris GM, et al. Multimorbidity and socioeconomic deprivation in primary care consultations. Ann Fam Med 2018;16:127–31. 10.1370/afm.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahrouge S, Hogg W, Muggah E, et al. Equity of primary care service delivery for low income “ sicker ” adults across 10 OECD countries. Int J Equity Health 2018;17:182. 10.1186/s12939-018-0892-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011;61:e12–21. 10.3399/bjgp11X548929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien R, Wyke S, Guthrie B, et al. An “ endless struggle ”: a qualitative study of general practitione’s' and practice nurs’s' experiences of managing multimorbidity in socio-economically deprived areas of Scotland. Chronic Illn 2011;7:45–59. 10.1177/1742395310382461 [DOI] [PubMed] [Google Scholar]
- 20.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thandi M, Wong ST, Aponte-Hao S, et al. Strategies for working across Canadian practice-based research and learning networks (pbrlns) in primary care: focus on frailty. BMC Fam Pract 2021;22:220. 10.1186/s12875-021-01573-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee L, Heckman G, Molnar FJ. Frailty: identifying elderly patients at high risk of poor outcomes. Can Fam Physician 2015;61:227–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Theou O, Park GH, Garm A, et al. Reversing frailty levels in primary care using the cares model. Can Geriatr J 2017;20:105–11. 10.5770/cgj.20.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson B, Sampalli T, Warner G, et al. Improving care for the frail in nova Scotia: an implementation evaluation of a frailty portal in primary care practice. Int J Health Policy Manag 2019;8:112–23. 10.15171/ijhpm.2018.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangin D, Lawson J, Adamczyk K, et al. Embedding “smart” disease coding within routine electronic medical record workflow: prospective single-arm trial. JMIR Med Inform 2020;8:e16764. 10.2196/16764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangin D, Lawson J, Cuppage J, et al. Legacy drug-prescribing patterns in primary care. Ann Fam Med 2018;16:515–20. 10.1370/afm.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J 2020;23:210–5. 10.5770/cgj.23.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church S, Rogers E, Rockwood K, et al. A scoping review of the clinical frailty scale. BMC Geriatr 2020;20:393. 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oviedo-Briones M, Laso ÁR, Carnicero JA, et al. A comparison of frailty assessment instruments in different clinical and social care settings: the frailtools project. J Am Med Dir Assoc 2021;22:607. 10.1016/j.jamda.2020.09.024 [DOI] [PubMed] [Google Scholar]
- 30.Statistics Canada . Postal codeom conversion file (PCCF), reference guide. Available: https://www150.statcan.gc.ca/n1/pub/92-154-g/92-154-g2017001-eng.htm [Accessed Mar 2020].
- 31.University of Toronto . CHASS canadian census analyser. Available: http://dc1.chass.utoronto.ca/census/index.html [Accessed Mar 2020].
- 32.Statistics Canada . Dictionary, census of population, 2016 - economic family after-tax income decile group. Available: https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/pop166-eng.cfm [Accessed Sep 2020].
- 33.Matheson FI, van T. Ontario marginalization index. Toronto, ON: St. Michael’s Hospital, Joint publication with Public Health Ontario, 2018. [Google Scholar]
- 34.Megari K. Quality of life in chronic disease patients. Health Psychol Res 2013;1:e27. 10.4081/hpr.2013.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066269supp001.pdf (1.4MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The dataset is available by contact with the corresponding author, DM (mangind@mcmaster.ca).