Abstract
Introduction
Ventilator-associated pneumonia (VAP) remains the leading cause of infections treated in the intensive care units (ICU). In a personalised care approach, we hypothesise that the duration of treatment of VAP can be reduced in function of the response to treatment.
Methods and analysis
The Antimicrobial Stewardship for Ventilator-Associated Pneumonia in Intensive Care (ASPIC) trial is a pragmatic national multicentre, phase III, non-inferiority, comparative randomised (1:1) single-blinded clinical trial. Five hundred and ninety adult patients hospitalised in 24 French ICU with a microbiologically confirmed first episode of VAP that received appropriate empirical antibiotic therapy will be included. They will be randomly allocated to standard management with duration of appropriate antibiotic fixed for 7 days according to international guidelines or antimicrobial stewardship based on daily clinical assessment of clinical cure. The assessment of clinical cure will be repeated daily until at least three criteria of clinical cure are met, allowing the discontinuation of antibiotic therapy in experimental group. The primary endpoint is a composite endpoint combining of all-cause mortality measured at day 28, treatment failure or new episode of microbiologically confirmed VAP until day 28.
The aim of the study is to demonstrate that a strategy to reduce the duration of antibiotic therapy for VAP based on clinical assessment is safe could lead to changes in practice as part of a personalised therapeutic approach, by reducing exposure to antibiotics and their side effects.
Ethics and dissemination
The ASPIC trial has been approved by the French regulatory agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM; EUDRACT number 2021-002197-78, 19 August 2021) and an independent ethics committee the Comité de Protection des Personnes Ile-de-France III (CNRIPH : 21.03.25.60729, 10 October 2021) for the study protocol (version ASPIC−1.3; 03 September 2021) for all study centres. Participant recruitment is scheduled to begin in 2022. Results will be published in international peer-reviewed medical journals.
Trial registration number
Keywords: Adult intensive & critical care, Infection control, Respiratory infections
Strengths and limitations of this study.
High-quality methodology using randomised controlled trial (RCT) design that will provide a high-level of evidence on antimicrobial stewardship for management of ventilator-associated pneumonia antibiotic strategy.
First RCT conducting in Europe assessing the value of clinical cure criteria (‘STOP criteria’) supported by an international expert panel to develop an antimicrobial stewardship strategy.
Class of antibiotics prescription not imposed by the protocol, in a pragmatic approach and in order to maximise the external validity of the results.
Risk of poor adherence of investigator team to experimental strategy, which could lead to absence of antibiotic discontinuation even if ‘STOP’ criteria are met.
Introduction
Reduction of use of antibiotics is a major point to control antimicrobial resistance in intensive care unit (ICU).1 Ventilator-associated pneumonia (VAP) is the first cause of healthcare-associated infections in ICU and more than half of antibiotics prescriptions in ICU are due to respiratory tract infections.2 3 The association between increase in antibiotic consumption and resistance emergence has been well documented for all patients admitted to the ICU who received antibiotic treatment and for patients treated for VAP.4
In the last few years, the concept of antimicrobial stewardship (ASP) has been developed. It refers to programmes, education, interventions that aim to optimise antibiotic use.5 The review by Dyar et al reports different definitions of ASP used in the literature.6 ASP refers to the responsible use of antimicrobials by healthcare professionals, and more specifically, to selection of the most appropriate antibiotic, duration, dose and route of administration for a given patient with a demonstrated or suspected infection.7 8
For VAP treatment, international guidelines9–11 strongly recommend a 7-day course of antibiotic therapy rather than a longer duration but underline that ‘there are situations in which a shorter or longer duration of antibiotics may be indicated, depending on the rate of improvement of clinical, radiologic and laboratory parameters’. In the absence of very specific situations (severe immunodepression, abscessed pneumonia, necrotising pneumonia), it is recommended not to exceed the duration of antibiotic therapy by more than 7–8 days. These recommendations are based on the concordant results of two meta-analyses that compared two treatment durations: 7–8 days versus longer durations.12 13
Recently, Weiss et al 14 poled a panel of international experts to develop consensus criteria to evaluate the clinical response to antibiotic treatment for hospital-acquired pneumonia and VAP. In this work, various innovative concepts are developed. First, the experts agree that the criteria usually used in the literature to characterise the suspicion of VAP are weighted differently. According to the experts, among nine selected criteria, the first four criteria with the most significant impact were: (1) worsening of gas exchange, (2) hypotension/vasopressor requirement, (3) temperature abnormalities (fever or hypothermia), (4) purulent tracheal secretions (rated ex-aequo with temperature abnormalities). Logically, less specific signs (hyperleukocytosis, encephalopathy, auscultatory abnormalities) were ranked lower.
According to the experts, when these criteria regress or disappear, they are, therefore, considered associated with clinical cure of VAP. Considering the small differences in the relative weights of each criterion, it seems reasonable to consider that the association of at least 3 of these criteria is necessary to consider a clinical cure. To date, no prospective evaluation of the robustness of these criteria to guide antimicrobial treatment duration has been performed.
The Antimicrobial Stewardship for Ventilator Associated Pneumonia in Intensive Care (ASPIC) study aims at investigating whether an ASP for microbiologically proven VAP based on daily assessment of clinical cure and antimicrobial discontinuation, if it is obtained, would be non-inferior to standard management in terms of all-cause mortality (ACM), treatment failure or occurrence of new episode of VAP before day 28.
Methods and analysis
Study design
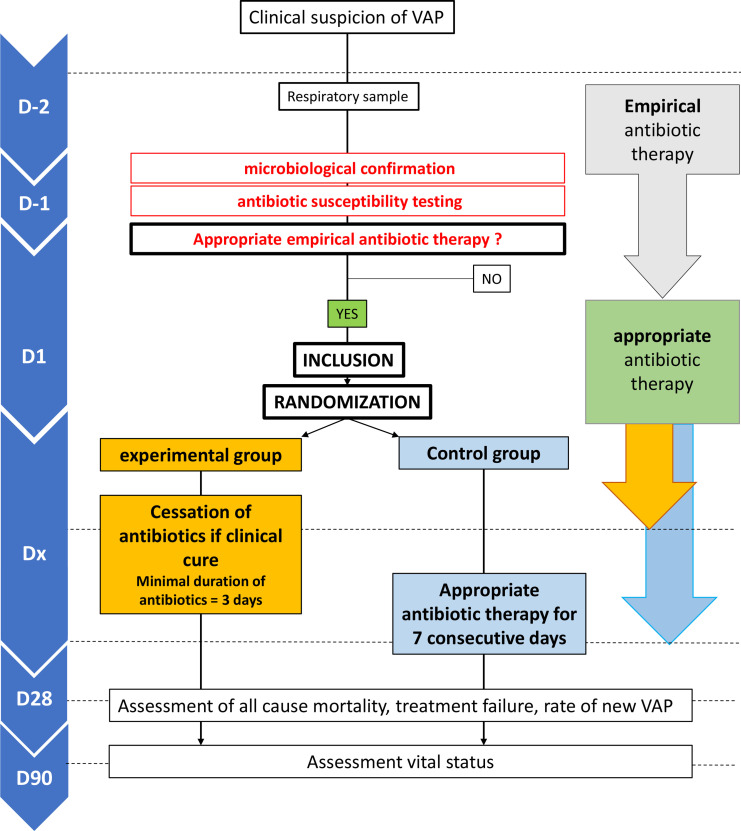
This study is a pragmatic, national, multicentre, phase III, single-blinded, non-inferiority comparative randomised clinical trial comparing two therapeutic strategies for microbiologically proven VAP on the basis of two parallel arms:
Experimental group: ASP based on daily clinical assessment of clinical cure. Discontinuation of appropriate antibiotic therapy is made if clinical cure (daily assessment) criteria of VAP are met.
Control group: standard management: duration of 7 full days (7 times consecutive 24 hours) of appropriate antibiotic therapy according to VAP guidelines. In the control group, clinical cure assessment will be performed daily by the intensivist in charge of the patient but the antibiotic therapy will not be discontinued until 7 days whatever the clinical cure.
The trial overview is summarised in figure 1. We report here the study protocol according to theStandard Protocol Items: Recommendations for interventional Trials statement.15
Figure 1.
General flowchart of the study. VAP, ventilator-associated pneumonia.
Definitions
Appropriate empirical antibiotic therapy: the empirical antibiotic therapy is defined as appropriate if all the VAP causative pathogens are susceptible (in vitro) to at least one molecule of the empirical treatment. Empirical antibiotic therapy is defined as inappropriate if at least one causative bacteria is resistant (in vitro) to the empirical treatment.
Definitive diagnosis of VAP is defined, in accordance with international guidelines, by the association of:
Mechanical ventilation (MV) requirement for more than 48 hours.
New pulmonary infiltrate of strongly suspected infectious origin.
Worsening oxygenation.
Purulent tracheal secretions and at least 1 of the following criteria within the 24 hours prior to the first dose of antibiotic therapy: (1) fever (body temperature >38.3°C) or hypothermia (body temperature <35°C), (2) white blood cell count >10 000 cells/mm3 or <4000 cells/mm3.
microbiological criteria (positive quantitative culture of a lower respiratory tract (LRT): bronchoalveolar lavage fluid (positivity threshold ≥104 colony-forming units/mL) or plugged telescopic catheter (PTC) (threshold ≥103 colony-forming units/mL) or quantitative endotracheal aspirate distal pulmonary secretion samples (significant threshold ≥105 colony-forming units/mL).
Clinical cure
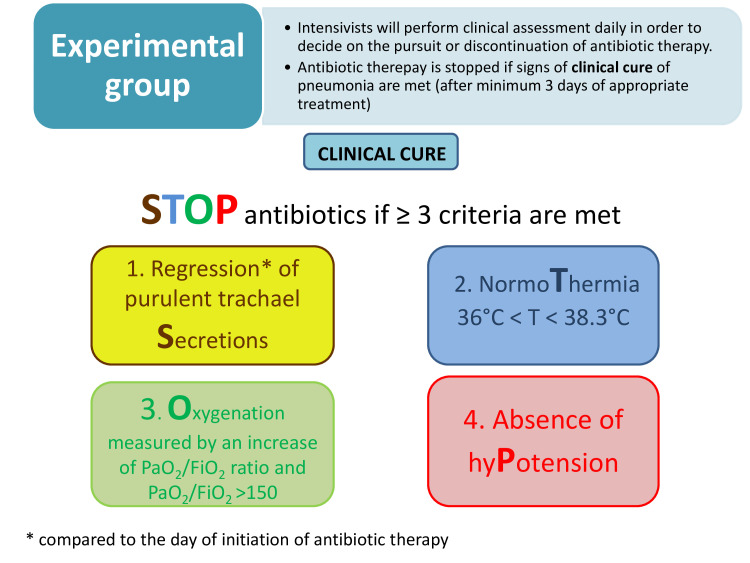
Complete resolution of at least three or four clinical signs and/of symptoms of VAP, according the STOP algorithm (items: purulent Secretions, body Temperature, Oxygenation, systolic blood Pressure—see figure 2). AND
No additional antibiotic therapy required for VAP treatment AND.
Patient is alive.
Figure 2.
Criteria of clinical cure and criteria for discontinuation of antibiotic therapy in experimental arm. *Compared to the day of initiation of antibiotic therapy. VAP: ventilator-associated pneumonia.
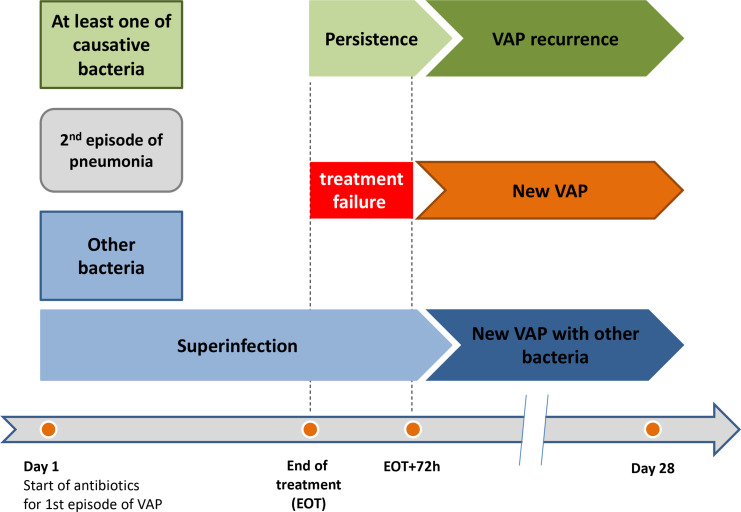
Treatment failure defined by signs of VAP within 72 hours after the end antibiotic treatment at the test of cure visit
Superinfection: isolation of a pathogen, other than the causative baseline pathogen, from an LRT specimen obtained in a subject with signs and symptoms of VAP developed during antibiotic treatment.
Persistence: continued presence of the original causative baseline pathogen(s) from an LRT culture obtained between EOT and 72 after EOT.
New VAP: new episode of microbiologically documented VAP from 72 hours after the EOT to day 28.
VAP-Recurrence: new VAP due to at least one of the original causative pathogen(s) found at baseline.
Definitions of treatment failure, persistence, superinfection, persistence, VAP recurrence and new VAP are summarised in figure 3.
Figure 3.
Description of microbiological categories of outcomes in relation to time of occurrence of new episode of VAP after inclusion adapted from Bai et al.20 VAP: ventilator-associated pneumonia.
Setting
This will be a French multicentre study involving 24 centres. Participants will be recruited in ICU wards during their hospital stay.
Study population
Participants in ICU wards will be eligible if they fulfilled following criteria:
Inclusion criteria:
Aged 18 years or more.
Patient under MV.
Microbiologically confirmed diagnosis of first episode of VAP (see the Definition section).
Initial appropriate (see definitions section) antibiotic therapy (whether empirical or not).
Written informed consent from the patient or a legal representative if appropriate. If absence of a legal representative, the patient can be included following an emergency procedure.
Exclusion criteria:
Patient under selective bowel decontamination.
Concomitant extra-respiratory infection requiring antibiotic therapy at inclusion.
Inclusion in another experimental study on ASP.
Moribund at admission SAPS>80).
Thoracic trauma with Abbreviated Injury Scale thorax ≥3.
Severely immunocompromised patients: congenital immunodeficiency, neutropenia (<0.5 G/L), leucopenia (<1 G/L), acute hematologic malignancy or stem cell transplant, HIV infection with CD4 count below 200 /mm3, immunosuppressive therapy or long-term corticosteroid therapy >0.5 mg/kg.
VAP due to: Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter spp, carbapenem-resistant Enterobacterales.
Bacterial VAP occurring in the context of coinfection of COVID-19 or other viral VAP (confirmed by reverse transcription-PCR).
Patients with empyema, necrotising and abscessed pneumonia.
Pregnant women.
No health insurance coverage.
Recruitment
The screening will aim at identifying patients hospitalised in ICU who underwent an LRT sample because a VAP was suspected. During this period, management of patients is similar to usual care with clinical, biological and radiological assessments. After microbiological diagnosis confirmation and reception of antibiotic susceptibility testing (AST) proving that the initial empirical antibiotic therapy was appropriate, eligible patient will be offered participation in the trial. Written informed consent would be obtained by the investigator or by a physician representing the investigator, from all patients, their next of kin, as appropriate, accordingly to French regulatory agency authorisation (see Methods section for obtaining information and consent from research participants).
Treatment allocation and randomisation
Participants will be randomised (day 1) with a 1:1 ratio to either ASP-guided antibiotic therapy strategy (experimental group) or standard management (control group) using a computer-generated randomisation scheme of various-sized blocks, through an internet centralised randomisation service running 24 hours/24 hours. Random block sizes proportional to the number of groups will be generated using a prespecified maximum blindly from the investigators. Permuted block technique will be used to assign treatment within the various-sized blocks. Randomisation will be stratified by centre. The randomisation scheme will be generated by a statistician who is not involved in any other aspect of the study, and all researchers will be blinded to block size(s) and randomisation list to avoid prediction of future patient’s allocation. Allocation concealment will be ensured, as the service will not release the randomisation code until the patient has been recruited into the trial.
Blinding
This study will be single blinded. Participants will not be informed of their group allocation. Blinding will be ensured as most patients will be either sedated (within standard of care) or unable to have appropriate discussions with investigational team for the duration of the experimental at study. The statistician conducting the data analysis will also be blinded to group allocation. The medical staff cannot be blinded to the randomisation arm due to the nature of experimental design and our choice to evaluate this strategy in real-life clinical practice conditions. If the patient is transferred to another clinical ward or leave the hospital during the 3-month follow-up, other healthcare professionals involved in their management will not be made aware of the randomisation arm.
Study procedures
A pragmatic approach will be followed and usual patient management recommended by international guidelines9–11 will be provided in participating ICUs. In particular, the choice of antibiotic therapy will be left at investigator discretion (according to current French guidelines,11 table 1).
Table 1.
Choice of empirical antibiotic therapy according to current French guidelines11
| Situations | Therapeutic agent |
|
Early VAP
≤5th day after admission and absence of:
|
Amoxillin+clavulanic acid OR 3rd cephalosporin |
|
Early VAP
≤5th day after admission AND
|
Amoxillicin+clavulanic acid OR 3rd cephalosporin AND aminosid |
|
Delayed VAP
>5th day of admission Or other risk factor* non-fermenting GNB |
Ceftazidim OR cefepim OR piperacillin+tazobactam (in absence of known carriage of MDR) OR imipenem or meropenem (if known carriage of MDR) AND amikacin or ciprofloxacin |
| Risk factor† of SAMR | Vancomycin OR linezolid |
*Prior intravenous antibiotic use within 90 day, septic shock at time of VAP, ARDS preceding pneumonia, 5 or more days of hospitalisation prior to the occurrence of VAP, acute renal replacement therapy prior to VAP onset.
†If local prevalence of SAMR is elevated, recent colonisation to SAMR, chronic cutaneous lesion, chronic dialysis.
ARDS, Acute Respiratory Distress Syndrome; GNB, Gram-negative Bacilli; SAMR, Staphyococcus aureus methicillin resistant; VAP, ventilator-associated pneumonia.
In the experimental group, the ICU physician will discontinue the antibiotic therapy as soon as clinical cure criteria of VAP are met. Minimal duration of appropriate antibiotic treatment will be 3 days (including empirical antibiotic therapy). After 72 hours (usual delay to receive AST results) of appropriate antibiotic treatment, the assessment of clinical cure will be performed daily based on four criteria:
Regression or the decreased abundance of purulent tracheal secretions.
Absence of fever or hypothermia.
Improvement of oxygenation (assessed by increase of PaO2/FiO2 ratio and PaO2/FiO2 >150).
Absence of arterial hypotension (hypotension is defined by mean arterial pressure <70 mm Hg16 17) or decreased need for epinephrine or norepinephrine by at least 0.1 µg/kg/min compared with baseline (day of inclusion).
This assessment will be repeated daily until at least 3 of the 4 criteria are met, that is, the patient is considered clinically cured, thereby allowing the discontinuation of antibiotic therapy.
A daily phone hotline, provided by coordinating investigator’s team, will be accessible to investigators for multidisciplinary validation of antibiotic discontinuation in patients included in the experimental group.
For control group, duration of antibiotic therapy will be at least seven full days (since the initiation of empirical antibiotic therapy), whatever clinical assessment.
For both groups, in case of non-clinical recovery after seven full days (treatment failure) and/or in case of suspicion of new VAP during treatment (superinfection), a new LRT sampling will be performed, and a new antibiotic therapy will be initiated. In case of new VAP, patients will be treated according to the usual practices of the centre.
Following data will be collected daily from day 2 to day 28 or to ICU discharge in participants from both arms: vital status, ventilation status, PaO2 and FiO2 (if ventilated), temperature, tracheal secretions, blood pressure, use and dose of vasopressors, data on any infection throughout study period (infection site, bacteriological documentation, number of days of antibiotic therapy), antibiotic use (molecule; dosage; duration of treatment).
Additional data will be collected daily from day 2 to day 8: clinical assessment, focused pulmonary examination, laboratory assessment (usual tests, biochemical, haematological), radiological evaluation (chest X-ray/CT scan), if performed as part of usual care.
Rectal swabbing for collection of data on colonisation or acquisition of multidrug resistant (MDR) bacteria will be performed at ICU admission and weekly until ICU discharge as part as usual care.
All participants will be followed up to day 90 with vital status assessment.
Outcomes
The primary endpoint will be a composite of:
ACM measured at day 28 after initiation of therapy OR.
Treatment failure defined by signs of VAP within 72 hours after the end antibiotic treatment at the test of cure visit OR.
New episode of microbiologically confirmed VAP from 72 hours after the end of antibiotic treatment to day 28 after initiation of VAP antibiotic treatment.
To avoid interpretation bias for the primary outcome, clinical and microbiological records of all participants will be reviewed by adjudication committee composed with two experts in order to evaluate the presence of (1) clinical cure, (2) treatment failure and (3) new episode of VAP. This evaluation will be performed blindly from the randomisation group and from the interpretation of the investigation team, according to predefined criteria (see the Definition section). The adjudication committee will be composed of study investigators (including scientific committee of ASPIC). Each member will review the primary endpoints criteria of a subgroup of patients that were not enrolled in its centre.
Secondary endpoints will be: day 28 ACM, proportion of treatment failure and of new episode of VAP; the number of antibiotic free days alive from initiation of VAP antibiotic therapy to day 28; the duration of invasive MV; the length of ICU stay, defined by the number of days between inclusion and ICU discharge or in-ICU death; the proportion of VAP recurrence assessed by the intensivist; the antibiotic related side effects; the proportion of acquisition of MDR bacteria (defined as the identification of an MDR bacteria carriage not present at admission); the proportion of protocol deviation, that is, lack of antibiotic therapy discontinuation despite a fulfilment of clinical cure definition in the experimental group; the total cumulative costs of antibiotics and incremental cost-effectiveness ratio and the Desirability of Outcome Ranking (DOOR) and the Response Adjusted for Duration of Antibiotic Risk (RADAR) for each strategy (experimental and control groups).18
All trial participants will be ranked with respect to the desirability of their overall outcome and the distributions of DOORs will be compared between strategies. Overall clinical outcomes at day 28 will be ranked from most to least desirable as followed:
Survival, clinical cure.
Survival, new pulmonary infection.
Death.
In RADAR analyses, patients will be ranked overall clinical outcome, but in case of ex-aequo, the patient with a shorter duration of antibiotic use will receive a higher rank.
Sample size justification
Assuming that 25% of the patients will encountered ACM, treatment failure or occurrence of new episode of VAP before day 28 in the control arm,19 590 subjects (295 per arm) are needed to establish non-inferiority with the absolute difference of death, treatment failure or occurrence of new episode of VAP does not exceed 10% (non-inferiority margin) between experimental and control arms with a power of 80%, a type I error (alpha) of 2.5%.
A non-inferiority margin of 10% was chosen taking into account the methodological data applied to the randomised controlled trials dedicated to VAP. According to European medicines Agency (https://www.ema.europa.eu/en/addendum-note-guidance-evaluation-medicinal-products-indicated-treatment-bacterial-infections-0), the suggested non-inferiority margin should not exceed—12.5% for clinical outcome documented at a Test-of-Cure visit. In this recommendation, the margin of 12.5% does not include mortality.
In a published study (ASPECT)20 designed to show non-inferiority for the primary endpoint in the intention-to-treat population, with a 10% non-inferiority margin to achieve 90% power at a one-sided significance level of 0.025 (based on regulatory agency guidance and assuming a 28-day ACM rate of 20% in both groups).
Data analysis plan
The primary analysis will be performed on the intention-to-treat population.22 The 95% CI of the difference in proportions of all-cause death, treatment failure or occurrence of new episode of VAP observed between the two groups will be estimated. This CI will be compared with the non-inferiority margin of 10%. If the lower limit of the CI of the difference in proportions is less than or equal to −10%, then we cannot conclude that the ASP-based strategy is non-inferior to the reference strategy. In the opposite case, if the lower limit of the CI is strictly greater than 10%, then we will conclude that the ASP-based strategy is non-inferior on ACM, treatment failure or occurrence of new episode of VAP at day 28 after inclusion. Sensitivity analysis on per-protocol population will be performed. All tests of superiority (secondary objectives) will be two sided with type I error of 5% and tests of non-inferiority will be one sided with type I error, 2.5%. All statistical analyses will be performed using SAS software (SAS Institute, Cary, North Carolina) V.9.4 or later, or R software (R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/) V.4.0 or later.
The primary analysis will also be performed in the following subgroups of patients:
Those whose baseline bacteriological samples were assessed by rapid microbiological technique (germ identification and AST)
Patients admitted to ICU for trauma versus other reasons of admission.
Patients with early onset VAP (<5 days after ICU admission) vs late-onset VAP (≥5 days after ICU admission).
No strategy of imputation is forecasted in case of missing data for the primary assessment criterion. Information available at time of last follow-up will be taken into account.
Data collection and management
Data collection will be performed in electronic format. The statistical software used for data entry will be CleanWeb; it will fulfil the regulatory requirements and security norms. Data will be handled according to the French law. All original records (including consent forms, reports of suspected unexpected serious adverse reactions and relevant correspondences) will be archived at trial sites for 15 years. The cleaned trial database file will be anonymised and maintained for 15 years. Data on primary and secondary endpoints will be collected, as detailed in the Study procedures section and table 2. The data of this study will be available on reasonable request from the corresponding author, but it will not be publicly available due to privacy or ethical restrictions.
Table 2.
Chronology of the study and procedures
| Actions | D2 to D1 | D1 | D2 to D8 | D9 to D27 or discharge of hospital | D28 | D90 |
| Inclusion visit |
|
XR |
|
|
|
|
| Verification of inclusion and non-inclusion criteria |
|
XR |
|
|
|
|
| Information |
|
XR |
|
|
|
|
| Written informed consent |
|
XR |
|
|
|
|
| Randomisation |
|
XR |
|
|
|
|
| Pregnancy test |
|
XR |
|
|
|
|
| Medical history | XC | XC |
|
|
|
|
| Physical examination | XC | XC | XC | XC | XR |
|
| Phone call |
|
|
|
|
|
XR |
| Chest X-ray/CT-scan | XC | XC | XC |
|
|
|
| PaO2/FiO2 ratio | XC | XC | XC | XC |
|
|
| Assessment of clinical symptoms of VAP | XC | XC |
|
|
|
|
| Assessment of clinical recovery of VAP |
|
|
Xc |
|
|
|
| Start antibiotics |
|
XC |
|
|
|
|
| Antibiotics |
|
XC | XC | XC |
|
|
| Rectal swab |
|
|
XC | XC |
|
|
| Serum creatinin and calculated creatinin clearance |
|
XC* |
|
|
|
|
| White blood count | XC | XC | XC | XC |
|
|
| SCORE |
|
|
|
|
|
|
| ISS | XC |
|
|
|
|
|
| SOFA | XC | XR |
|
|
|
|
| SAPS II | XC |
|
|
|
|
|
| Assessment of rate of treatment failure and new episode of VAP |
|
|
|
|
XR |
|
| Antibiotic free days |
|
|
|
|
XR |
|
| Vital status |
|
|
|
|
XR | XR |
| Adverse events |
|
XR | XR | XR | XR | XR |
| Hospital admissions |
|
|
XC | XC | XR | XR |
*Creatinin clearance may be performed (Clcr= (urinary creatinin/serum creatinin)*urine volume24h) as frequently as clinically indicated to guide appropriate antibiotic therapy in subjects with renal impair.
ISS, Injury Severity Score; SAPS II, Severity Acute Physiology Score; SOFA, Sepsis-related Organ Failure Assessment; VAP, ventilator-associated pneumonia; XC, made in usual care; XR, acts added for research.
Trial status
Recruitment of participants started in October 2022 and the estimated completion date for inclusions is September 2025.
Ethics and dissemination
Legal obligations and approval
Sponsorship has been agreed by Assistance Publique—Hôpitaux de Paris (AP-HP, Clinical Research and Innovation Department) for this interventional research protocol involving human participants concerning a health product. AP-HP has obtained the approval of the French medicine regulatory agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM; EUDRACT number 2021-002197-78, 19 August 2021) and of the ethics committee (Comité de Protection des Personnes (CPP) Ile-de-France III (CNRIPH: 21.03.25.60729, 10 October 2021)) for the study protocol (version ASPIC−1.3; 03 September 2021). The trial will be carried out in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. Any substantial modification to the protocol will be sent to the sponsor, and then to the ANSM and the CPP for approval before the amendment can be implemented. The information sheet and the consent form can be revised if necessary, particularly if there is a substantial amendment to the study or if adverse reactions occur. AP-HP is the owner of the data. The data cannot be used or disclosed to a third party without its prior permission.
Methods for obtaining information and consent from research participants
In accordance with Article L.1122-1-1 of the French Public Health Code, no research will be carried out without patient free and informed consent, obtained in writing after the person has been given the information specified in Article L.1122–1 of said Code. Written informed consent will be obtained from all patients, their next of kin, as appropriate. If patients are unable to provide informed consent and if neither their next of kin nor other designated person is available, a procedure for inclusion in the study in emergency situations would be applied. A definitive post hoc consent form would be ultimately obtained from patients who survived but had been initially treated on the basis of the emergency consent. These procedures have been approved for the ASPIC trial by the French Commission nationale de l'informatique et des libertés (CNIL, ref MLD/MFI/AR2111748, 18 October 2021).
Patient and public involvement
The patient’s (or next of kin’s) free and informed written consent will be obtained after a reflection period of at least 15 min after information, by the investigator or by a doctor representing the investigator, before enrolment in the trial, during the baseline visit.
The investigator will specify in the research participant’s medical file the methods used for obtaining their consent as well as the methods used for providing information with a view to obtaining consent. The investigator will retain the original signed and dated consent form.
Subjects may exit the study at any time and for any reason.
Data deposition, quality control and curation
The persons responsible for the quality control of clinical matters will take all necessary precautions to ensure the confidentiality of information related to the study participants. These persons, as well as the investigators themselves, are bound by professional confidentiality. During or after the research, all data collected about the participants and sent to the sponsor by the investigators (or any other specialised collaborators) will be anonymised.
In any case of premature withdrawals and exits, the investigator must provide their reason(s) and try to collect primary endpoint, secondary endpoints and safety assessment, if the participant agrees. If a participant exits the study prematurely or withdraws consent, any data collected prior to the date of premature exit may still be used excepted if the participant refuses it in writing.
The research data will be collected and monitored using an electronic Case Report Form e(CRF) through CleanWEB Electronic Observation Book and will be centralised on a server hosted by the AP-HP Operation Department.
Research staff of the Clinical Trial Unit will work with local investigators to obtain data that are as complete and accurate as possible. An independent Clinical Research Associate appointed by the sponsor will be responsible for the proper running of the study, for collecting, documenting, recording and reporting all handwritten data, in accordance with the Standard Operating Procedures applied within the Clinical Research and Innovation Department of AP-HP. The investigators agree to accept the quality assurance audits carried out by the sponsor as well as the inspections carried out by the competent authorities. All data, documents and reports may be subjected to regulatory audits. These audits and inspections cannot be refused on the grounds of medical secrecy. An audit can be carried out at any time by independent individuals appointed by the sponsor, aiming at ensuring the quality of the study, the validity of the results and compliance with the legislation and regulations in force. The persons who manage and monitor the study agree to comply with the sponsor’s audit requirements. The audit may encompass all stages of the study, from the development of the protocol to the publication of the results and the storage of the data used or produced as part of the study. Sponsor is responsible for access to the study database.
The investigator will assess the seriousness of each adverse event, report all serious and non-serious adverse events in the case report form and assess the causal relationship of serious adverse events with the study procedures according to the WHO method.
A data monitoring committee is not needed for this trial as the expected risk for the participant is minimal.
Publication plan
Results will be published in international peer-reviewed medical journals. Scientific presentations and reports will be written under the responsibility of the coordinating investigator of the study with the agreement of the principal investigators and the methodologist. The coauthors of the reports and publications will be the investigators and clinicians involved, on a pro rata basis of their contribution in the study as well as the biostatistician and associated researchers. Rules on publication will follow international recommendations.21
Supplementary Material
Footnotes
Collaborators: Jean-Michel Constantin, CHU Pitié-Salpétrière, AP-HP; Vincent Degos, CHU Pitié-Salpétrière, AP-HP; Guillaume Voiriot, CHU Tenon, AP-HP; Etienne Gayat, CHU Lariboisière-Saint-Louis, AP-HP; Frank Verdonk, CHU Saint-Antoine, AP-HP; Stephane Gaudry, CHU Avicenne, AP-HP; Antoine Vieillard-Baron, CHU Ambroise Paré, AP-HP; Jean-Damien Ricard, CHU Louis Mourier, AP-HP; Gaëtan Plantefeve, CH Victor Dupouy, Argenteuil, Daniel Da Silva, CH Delafontaine, Saint-Denis, Matthieu Biais, CHU Bordeaux, Pierre Bouzat, CHU Grenoble-Alpes, Thomas Geeraerts, CHU Toulouse Julien Pottecher, CHRU Strasbourg Marie Werner, CHU du Kremlin-Bicêtre, AP-HP; Eric Kipnis, CHU de Lille; Claire Dahyot-Fizelier, CHU de Poitiers; Sigismond Lasocki, CHU Anger, François Barbier, CHU Orléans; Adrien Bouglé, CHU Pitié-Salpétrière, AP-HP, Paër-Selim Abback, CHRU de Tours, Karim Lakhal, Stéphanie Ruiz, CHU de Toulouse, Antoine Monsel, CHU Pitié-Salpétrière, AP-HP
Contributors: AF and EW contributed to the conception and design of the research protocol, assisted by DB and PE. AR, IM-L, PM, J-FT, AB and J-RZ provided critical input pertaining to the design of the trial interventions and procedures. AF wrote the first draft of the protocol and this manuscript. DB and PE designed the statistical analysis plan. All authors critically revised and modified the protocol and the article. They all approved the final version to be published.
Funding: This work was supported by Programme Hospitalier de Recherche Clinique—PHRC 2019 (Ministère de la Santé) grant number 2020-A02837-32.
Competing interests: AF, DB, AB, PE: declare no competing interest. AR: grants from bioMerieux and Merck. IM-L: board on PFIZER, MSD, GILEAD. J-FT directly related to the protocol: none, participation to scientific advisory boards: Pfizer, Gilead, Merck, BD, Shionoghi; readings: Merck, Biomerieux, Pfizer, Shionogi; research grants to my research unit: Thermo Fischer, Pfizer, Merck. J-RZ: consulting fees from MSD, Pfizer, speaker fees from MSD, Pfizer, Shionogi, Correvio and Eumedica. EW: Speaker fees from MSD, Akcea therapeutics and LFB, support for attending meeting/travel: LFB and Akcea therapeutics.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med 2018;44:189–96. 10.1007/s00134-017-5036-1 [DOI] [PubMed] [Google Scholar]
- 2. Ashraf M, Ostrosky-Zeichner L. Ventilator-associated pneumonia: a review. Hosp Pract (1995) 2012;40:93–105. 10.3810/hp.2012.02.950 [DOI] [PubMed] [Google Scholar]
- 3. Bergmans DC, Bonten MJ, Gaillard CA, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother 1997;39:527–35. 10.1093/jac/39.4.527 [DOI] [PubMed] [Google Scholar]
- 4. Fihman V, Messika J, Hajage D, et al. Five-year trends for ventilator-associated pneumonia: correlation between microbiological findings and antimicrobial drug consumption. Int J Antimicrob Agents 2015;46:518–25. 10.1016/j.ijantimicag.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 5. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases Society of America and the Society for healthcare epidemiology of America. Clin Infect Dis 2016;62:e51–77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol Infect 2017;23:793–8. 10.1016/j.cmi.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 7. Nicolau DP. Current challenges in the management of the infected patient. Curr Opin Infect Dis 2011;24 Suppl 1:S1–10. 10.1097/01.qco.0000393483.10270.ff [DOI] [PubMed] [Google Scholar]
- 8. Timsit J-F, Bassetti M, Cremer O, et al. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med 2019;45:172–89. 10.1007/s00134-019-05520-5 [DOI] [PubMed] [Google Scholar]
- 9. Kalil AC, Metersky ML, Klompas M, et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American thoracic Society. Clin Infect Dis 2016;63:575–82. 10.1093/cid/ciw504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European respiratory society (ERS), European society of intensive care medicine (ESICM), European society of clinical microbiology and infectious diseases (ESCMID) and asociación latinoamericana del Tórax (ALAT). Eur Respir J 2017;50:1700582. 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- 11. Leone M, Bouadma L, Bouhemad B, et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med 2018;37:83–98. 10.1016/j.accpm.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 12. Dimopoulos G, Poulakou G, Pneumatikos IA, et al. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 2013;144:1759–67. 10.1378/chest.13-0076 [DOI] [PubMed] [Google Scholar]
- 13. Pugh R, Grant C, Cooke RPD, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015;2015:CD007577. 10.1002/14651858.CD007577.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss E, Zahar J-R, Alder J, et al. Elaboration of consensus clinical endpoints to evaluate antimicrobial treatment efficacy in future hospital-acquired/ventilator-associated bacterial pneumonia clinical trials. Clin Infect Dis 2019;69:1912–8. 10.1093/cid/ciz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18. Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (door) and response adjusted for duration of antibiotic risk (radar). Clin Infect Dis 2015;61:800–6. 10.1093/cid/civ495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 2018;18:285–95. 10.1016/S1473-3099(17)30747-8 [DOI] [PubMed] [Google Scholar]
- 20. Kollef MH, Nováček M, Kivistik Ü, et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2019;19:1299–311. 10.1016/S1473-3099(19)30403-7 [DOI] [PubMed] [Google Scholar]
- 21. International Committee of Medical Journal Editors . Uniform requirements for manuscripts submitted to biomedical journals. N Engl J Med 1997;336:309–15. 10.1056/NEJM199701233360422 [DOI] [PubMed] [Google Scholar]
- 22. Bai AD, Komorowski AS, Lo CKL, et al. Intention-to-treat analysis may be more conservative than per protocol analysis in antibiotic non-inferiority trials: a systematic review. BMC Med Res Methodol 2021;21:75. 10.1186/s12874-021-01260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.