Abstract
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), causes increasing physical impairment and disability. People with ALS/MND face huge physical challenges, and the diagnosis can be a source of great psychological distress for both people with ALS/MND and their carers. In such a context, how news of the diagnosis is broken is important. At present, there are no systematic reviews of methods for informing people with ALS/MND of their diagnosis.
Objectives
To examine the effects and effectiveness of different methods for informing people of a diagnosis of amyotrophic lateral sclerosis/motor neuron disease (ALS/MND), including effects on the person's knowledge and understanding of their disease, its treatment, and care; and on coping and adjustment to the effects of ALS/MND, its treatment, and care.
Search methods
We searched the Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, and two trials registers (February 2022). We contacted individuals or organisations to locate studies. We contacted study authors to obtain additional unpublished data.
Selection criteria
We planned to include randomised controlled trials (RCTs) and quasi‐RCTs of techniques for informing people with ALS/MND of their diagnosis. We planned to include adults (aged 17 years or over) with ALS/MND, according to the El Escorial criteria.
Data collection and analysis
Three review authors independently reviewed the results of the search to identify RCTs, and three review authors identified non‐randomised studies to include in the discussion section. We planned that two review authors would independently extract data, and three would assess the risk of bias in any included trials.
Main results
We did not identify any RCTs that met our inclusion criteria.
Authors' conclusions
There are no RCTs that evaluate different communication strategies for breaking the bad news for people diagnosed with ALS/MND.
Focused research studies are needed to assess the effectiveness and efficacy of different communication methods.
Keywords: Adult, Humans, Amyotrophic Lateral Sclerosis, Motor Neuron Disease, Motor Neuron Disease/psychology, Motor Neuron Disease/therapy
Plain language summary
Methods for informing people with amyotrophic lateral sclerosis/motor neuron disease of their diagnosis
Review question
We planned to assess the evidence about communicating the diagnosis of amyotrophic lateral sclerosis (ALS) or motor neuron disease (MND).
Background
ALS, which is also known as MND, is an illness affecting the nerves that control movement. It causes increasing disability, including limb weakness, difficulty with breathing, and speech and swallowing problems. Most importantly, people with ALS/MND have to cope with the fact that ALS/MND usually leads to death within three to five years of onset. At diagnosis, people with ALS/MND, relatives, and carers can experience enormous distress, therefore, it is important to understand how to tell them the diagnosis. We carried out a wide search for randomised controlled trials (RCTs) of methods of communicating the diagnosis of ALS/MND.
Results
We found no RCTs of methods for giving a diagnosis of ALS/MND.
We searched to February 2022.
Background
Description of the condition
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a life‐limiting illness that affects the motor neurons. The condition causes increasing disability, with limb weakness, dyspnoea, speech, and swallowing difficulties (Borasio 1998; Chiò 2004; GBD 2016 Motor Neuron Disease Collaborators 2018). The worldwide prevalence is approximately 4.5 per 100,000 people; the annual incidence is 2 per 100,000. The mean age of onset is between 55 and 65 years, with a male preponderance.
Diagnosis of ALS/MND is primarily clinical, that is, based on the presence of characteristic symptoms and signs. Subsequent imaging, laboratory tests, and other investigations are performed to rule out other possible diagnoses.
Because of the progressive loss of fundamental abilities and the poor prognosis, people with ALS/MND and their carers face enormous physical and psychological distress (Borasio 1997; Borasio 1998; GBD 2016 Motor Neuron Disease Collaborators 2018; McCluskey 2004; Silani 1999). Most importantly, people with ALS/MND must cope with the fact that the condition usually leads to death within three to five years of onset.
Description of the intervention
Awareness of ALS/MND is not widespread in the general population or amongst general practitioners. People who receive a diagnosis of ALS/MND and their families often have little or no prior knowledge of the condition.
The importance of knowing how best to break news of the diagnosis in this context is obvious (Anestis 2020; Johnston 1996; Meininger 1993; Rudnick 2000; Silverstein 1991). We have evidence from research in other life‐threatening diseases on the impact of receiving bad news on anxiety, mood, and quality of life (Greer 1991; Lockhart 2007; Mirza 2019).
In ALS/MND, communicating the diagnosis is an important step in delivering comprehensive care (Hirayama 2021). The goal is to gradually provide the person and their caregiver(s) with all the information they need to foster coping efforts and make informed, timely decisions (Sakellariou 2013). It is essential to take into account the person's and caregiver(s)' psychological reactions to such bad news (Aoun 2017; O'Connor 2018).
The physician usually breaks the news during interactions with the person over the course of reaching a diagnosis. The timing depends on the person's and their caregiver(s)' coping styles, and on the disease progression (Aoun 2018).
Particular features of ALS/MND (for example, relentless progression of symptoms, partial efficacy of symptomatic treatment, and the absence of effective curative treatment) deserve special attention. Communicating the diagnosis rarely occurs as a single event, but is frequently a long process that follows the course of the illness (Anestis 2021; Borasio 1998; Chiò 2004; Silani 1999; Silverstein 1991). In other words, the process provides various pieces of information over time, rather than in a single disclosure (Eggly 2006).
Two aspects of diagnostic communication are relevant: the process of how the information is delivered, and the content delivered (Chiò 2004). The former includes the style of the communication and the context of the discussion, both of which can have a profound impact on the person's satisfaction. The latter encompasses the name of the disease, its course, outcome, care and treatment, and ongoing research. The delay in getting a diagnosis, and misdiagnoses along the way, are relevant factors that must be taken into account when breaking bad news in ALS/MND. A survey performed in the Turin ALS Centre addressed the information needs of people with ALS/MND. People with ALS/MND, their caregivers, and physicians all indicated that the three most important information components were: the course and outcome of the disease, therapies that can modify the course of the disease, and research findings (Chiò 2004).
There are both interactive and passive interventions, for example written information to complement face‐to‐face discussion and reinforce the communication. People may not be ready to receive an information booklet initially, but may prefer to receive the material at a later stage. A small study on people with ALS/MND found that their perceived level of satisfaction with information on the diagnosis rose from 51% to 97% after receiving an ALS brochure; their caregiver(s)' satisfaction rose from 47% to 95% (Borasio 1998).
How the intervention might work
Elements likely to determine the outcome of different types of communication include person‐centeredness versus disease‐centeredness, communication pacing, and completeness of information (Vail 2011).
Interventions might aim to improve routine communication between health professionals and people with ALS/MND, or they might be in addition to routine communication (e.g. additional discussion sessions or educational social programmes). They can be directed at people with ALS/MND and their caregivers, and health professionals (e.g. training courses).
Studies in various clinical conditions highlight the importance of effectively communicating the diagnosis to maintain satisfactory communication between physicians and the people they are treating, thus improving adherence to treatment protocols (Lockhart 2007; Mujezinovic 2010; Ryan 2011; Sakellariou 2013). For example, In the field of oncology, there is evidence that the way the diagnosis is communicated affects how the person comprehends the information delivered, and adjusts to the illness (Maynard 1996). Such evidence led to the creation of specific and focused methods for learning proper communication skills (Roberts 1994).
Johnston and colleagues summarised the results of interviews with 50 people about their experiences of receiving an ALS diagnosis. Study participants referred to the positive effect of receiving clear information about the disease, at least in providing a label for their condition. Negative aspects reported were a lack of privacy, lack of opportunity to attend with a family member or friend when the diagnosis was given, delay in diagnosis because of clinical investigations, the use of vague terms, and too much information given all at once (Johnston 1996).
In a study by McCluskey and colleagues, 144 people with ALS and 113 caregivers were asked to rate the quality of the communication of the diagnosis. Almost 56% of the people with ALS rated it as average or worse: 30.7% rated it as average. 8.6% below average, and 16.4% as poor. Forty‐eight per cent of the caregivers considered the communication average or worse: 28.8% as average, 4.8% below average, and 14.4% as poor (McCluskey 2004). The time spent discussing the diagnosis, as well as specific efforts towards effective communication, were correlated with higher satisfaction by people with ALS and their caregivers; unfortunately, as other available surveys of people with ALS and their caregivers have also shown, the diagnosis is too often communicated quickly, and in an unsatisfactory manner (Borasio 1998; Borasio 2001). In a recent survey, neurologists themselves emphasised the importance of having enough time for this kind of communication and the need for professional training (Anestis 2021). Those delivering the diagnosis need sufficient time to assess how much information the person is ready to handle, and their anxiety level (Hirayama 2021).
In the Netherlands, Seeber and colleagues tested a two‐phase appointment method for delivering the diagnosis, and evaluated participants' satisfaction by qualitative analysis (Seeber 2016). The evaluation suggested that this method was successful in providing structure for the period immediately after the diagnosis. The first appointment allowed an initial opportunity to re‐orient the participants to their changing life perspectives, and the second appointment allowed further discussion of details of the disease and a treatment plan.
Why it is important to do this review
We know of no other systematic review on methods for informing people with ALS/MND of their diagnosis. The value of this review would be in highlighting the research gaps and identifying features of interventions that could inform systematic and focused studies about this topic.
Objectives
To examine the effects and effectiveness of different methods for informing people of a diagnosis of amyotrophic lateral sclerosis/motor neuron disease (ALS/MND), including effects on the person's knowledge and understanding of their disease, its treatment, and care; and on coping and adjustment to the effects of ALS/MND, its treatment, and care.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised and quasi‐randomised, controlled before‐after clinical studies evaluating techniques to inform people with amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) of their diagnosis.
Since these study designs are rare in this topic area, we also considered and summarised findings from non‐randomised studies in a thematic synthesis in the Discussion.
Studies were eligible regardless of language or publication status.
Cross‐over trials and cluster‐randomised trials were not eligible.
Types of participants
Adults (aged ≥ 17 years) with a diagnosis of ALS/MND according to the El Escorial Criteria (Brooks 2000). Had we found a study of people with various conditions, some of whom had ALS/MND, we would have included the study only if data for participants with ALS/MND were reported separately.
Types of interventions
We considered interventions in any setting (e.g. hospital, home, community), designed to communicate the diagnosis of ALS/MND, by health professionals, to people with ALS/MND, their caregivers, or family members, by any format or medium. Since we considered it likely that different studies would categorise aspects of the communication process and communication content differently, it was difficult to explicitly state different types of intervention in advance. At a minimum, we planned to distinguish between interactive (e.g. face‐to‐face discussion) and passive (e.g. provision of written material) interventions.
Types of outcome measures
Our outcome measures concerned disease adjustment, and patient and carer satisfaction. These are the outcomes of interest in the review. We did not base study selection on outcome reporting.
Primary outcomes
Coping with, and adjustment to the effects of ALS/MND and its treatment and care (measured by coping scales, questionnaires, or both) among people with ALS/MND, up to two weeks after, and six months after communication of the diagnosis.
In the field of ALS/MND, both generic coping style inventories, such as the COPE questionnaire (Carver 1989), and disease‐specific instruments, such as the MND Coping Scale by Lee and colleagues, are commonly used (Lee 2001).
Secondary outcomes
Patients' perceived quality of relationships within the family, anxiety, and depression, measured up to two weeks after, and six months after communication of the diagnosis, by interviews and questionnaires, such as the State‐Trait Anxiety Inventory (Form Y; STAI‐Y (Spielberger 1983)), and Beck Depression Inventory (BDI‐II (Beck 1996))
Patients' health‐related quality of life, measured up to two weeks after, and six months after communication of the diagnosis, by quality of life rating scales, such as the Short Form‐36 (SF‐36) Health Survey (Ware 1992), and Schedule for the Evaluation of Individual Quality of Life (SEIQoL‐DW) semi‐structured interview (Wettergren 2009)
Patients' illness perception, knowledge of, and understanding about ALS/MND, and its treatment and care, assessed up to two weeks after, and six months after communication of the diagnosis, by interviews, and questionnaires, such as the Revised Illness Perception Questionnaire (IPQ‐R (Moss‐Morris 2002))
Carers' perceived quality of relationships within the family, burden, anxiety, and depression, assessed up to two weeks after, and six months after communication of the diagnosis, by interviews and questionnaires, such as the Glasgow Benefit Inventory (GBI (Novak 1989)), the STAI‐Y, and BDI‐II
Carers' knowledge and understanding about ALS/MND and its treatment and care, assessed up to two weeks after, and six months after communication of the diagnosis, by interviews
Patients' and carers' satisfaction with the communication process, assessed up to two weeks after, and six months after communication of the diagnosis, by interviews
Search methods for identification of studies
Electronic searches of databases and direct contacts for further information
Electronic searches
We searched the following databases:
the Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; searched 26 February 2022; see Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web (searched 26 February 2022; see Appendix 2);
MEDLINE Ovid (1946 to 26 February 2022; see Appendix 3);
Embase Ovid (1974 to 26 February 2022; see Appendix 4);
PsycINFO Ovid (1806 to 26 February 2022: see Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 February 2022; see Appendix 6);
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch; searched 26 February 2022; see Appendix 7).
We reviewed the identified non‐randomised evidence in the Discussion section. We suspected at the outset there would be no or few RCTs or quasi‐RCTs on this topic.
Searching other resources
We checked the reference lists of relevant papers to identify more studies. Had it been necessary, we planned to contact study authors for more information.
Data collection and analysis
Selection of studies
Three review authors independently checked all titles and abstracts identified by the searches to identify potentially relevant studies. Three review authors independently identified relevant non‐randomised studies from the search for RCTs, to discuss in the Discussion section. Each review author independently examined the full text of these papers to see whether they matched the inclusion criteria for the review. We resolved disagreements about whether to exclude or include studies by discussion among the review authors. We did not limit study selection by language or publication status.
Data extraction and management
We planned to produce a descriptive summary using a systematic approach to data extraction (containing clinical data, and information about interventions and outcomes) of evaluative studies of methods for informing people with ALS/MND of their diagnosis. We did not identify any studies to include in the review.
In future updates, if we identify RCTs for inclusion, two review authors will independently extract study data onto a specially designed data extraction form that we have piloted on one or more included studies; we will resolve disagreements by discussion with a third review author, if necessary. We will record details of study design, setting, and eligibility criteria. We will extract data about participants, including age, diagnosis, and stage of disease, and treatment. We will extract data about interventions, and will include aims, content, timing, and costs. We will extract outcome data that focuses on timing, type of outcome, and instruments used to measure the outcomes. We will try to obtain missing data by contacting the study authors. We will report study funding and conflicts of interest amongst investigators in the Characteristics of included studies table.
If we identify studies that require translation, the translator will either extract data into the data extraction form, or provide a translation from which the review authors will extract the data. When possible, the review authors will check the extraction of numerical data against the original report.
Assessment of risk of bias in included studies
In future updates, if we identify studies to include in the review, we will assess risk of bias in all included studies, and complete a risk of bias table according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). If we identify RCTs, we will assess them, using the RoB 1, for randomised sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. We will then make a judgement on each risk of bias criteria, judging them at high risk of bias, low risk of bias, or unclear risk of bias, where unclear means that there is insufficient information to make a judgement, or that the risk of bias is unknown. Three review authors will independently assess the risk of bias, and then reach agreement by consensus.
Measures of treatment effect
We planned to pool the outcome data with a meta‐analysis, using Review Manager 5, but we did not identify any relevant trials (Review Manager 2020). Since all our outcomes measures are expressed in continuous data, we planned to calculate them as mean differences (MD), with 95% confidence intervals (CI). Had we combined results from studies that measured the same outcome using different scales, we would have calculated the standardised mean difference (SMD) and 95% CI, and interpreted a value of 0.2 as a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). We planned to analyse all the primary and secondary outcomes.
Unit of analysis issues
Not applicable, as we did not identify any relevant RCTs. Had we had found multi‐arm studies, we would only have included data from arms that were eligible for the review, for pair‐wise comparisons. We would have followed methods to avoid double counting of participants, as outlined in Chapter 23 of the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2022b).
Dealing with missing data
We found no relevant RCTs. Had it been necessary, we would have contacted the trial authors in an attempt to obtain missing data for risk of bias assessment and analysis.
Assessment of heterogeneity
No studies were included.
If meta‐analysis is possible in future updates, we will use the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we identify substantial unexplained heterogeneity, we will report it and explore possible causes by prespecified subgroup analyses. We will use this rough guide to interpretation, as outlined in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We will avoid the use of absolute cut‐off values, but interpret I² in relation to the size and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi² test, or CI for I² (Deeks 2022)).
Assessment of reporting biases
It is unlikely that we will find sufficient studies to produce funnel plots (at least 10 in the same analysis (Page 2022)). If future searches identify trial protocols, clinical trial registrations, or abstracts indicating the existence of unpublished studies, we will attempt to determine their status by contacting the investigators.
Data synthesis
We found no relevant RCTs.
If we identify trials in future updates that are sufficiently similar to pool, we will perform meta‐analyses with a fixed‐effect model, using Review Manager 5 software (Review Manager 2020). We will use a random‐effects model if we identify moderate or greater heterogeneity. Our primary analysis will include all eligible trials.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses.
Sensitivity analysis
If meta‐analysis is possible in future updates, we will use a random‐effects model. If we find more than moderate heterogeneity, we will omit studies at high risk of bias and repeat the analysis, to see whether such studies account for heterogeneity. However, our primary analyses will include all eligible trials.
Summary of findings and assessment of the certainty of the evidence
If we identify studies during future updates, we will create a summary of findings table, using GRADEpro GDT software, for each comparison (GRADEpro GDT); we will summarise the results for the following outcomes:
Coping and adjustment to the effects of ALS/MND and its treatment and care among people with ALS/MND, up to two weeks after, and six months after communication of the diagnosis
Patients' perceived quality of relationships within the family, anxiety, and depression, measured up to two weeks after, and six months after communication of the diagnosis
Patients' health‐related quality of life, measured up to two weeks after, and six months after communication of the diagnosis
Patients' illness perception, knowledge of, and understanding about ALS/MND and its treatment and care, assessed up to two weeks after, and six months after communication of the diagnosis
Carers' perceived quality of relationships within the family, burden, anxiety, and depression, assessed up to two weeks after, and six months after communication of the diagnosis
Carers' knowledge and understanding about ALS/MND and its treatment and care, assessed up to two weeks after, and six months after communication of the diagnosis
Patients' and carers' satisfaction with the communication process, assessed up to two weeks after, and six months after communication of the diagnosis
If we identify studies during future updates, two review authors will use the five GRADE considerations (study limitations, no consistency of effect, imprecision, indirectness, and publication bias) to independently assess the certainty of the body of evidence for each outcome, based on the data from studies that contributed data for the outcome. We will use methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022). We will consider that RCTs provide high‐certainty evidence if the five factors above are not present to a serious degree, but may downgrade the certainty of the evidence to moderate, low, or very low, depending on the presence of the five factors. We will downgrade evidence once if a GRADE consideration is serious, and twice if very serious. We will justify all decisions to downgrade using footnotes, and make comments to aid readers' understanding of the review, where necessary. We will resolve disagreements by discussion, or by involving another review author.
Results
Description of studies
Results of the search
The current strategies, listed in the appendices, were run on 26 February 2022, and identified 57 reports. We identified no duplicates, so screened the titles and abstracts of all 57 reports. We reviewed the full text of 11 potentially relevant reports, and excluded all 11, with reasons. See the flowchart in Figure 1, and the Characteristics of excluded studies table for further details. We identified three surveys of participant and caregiver satisfaction for single, unstructured aspects of receiving communication of the disease, which we discussed in the Agreements and disagreements with other studies or reviews section (Hirayama 2021; Johnston 1996; McCluskey 2004).
1.
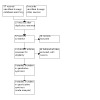
PRISMA flowchart of study selection
Included studies
We did not find any studies that met the inclusion criteria.
Excluded studies
We excluded 11 full‐text reports; none were experimental studies.
seven were theoretical and good‐practice articles, based on clinical expertise and a review of ALS/MND and other conditions in the literature (Borasio 1998; Brocq 2006; Campana‐Salort 2006; Chiò 2004; Corcia 2006; Couratier 2006; Meininger 1993)
two were post hoc surveys (Johnston 1996; McCluskey 2004)
one reported a qualitative analysis, based on videotaped sessions (Schellenberg 2014)
one reported a qualitative analysis, based on in‐depth interviews and non‐participant observations (Seeber 2016)
Risk of bias in included studies
We did not find any studies that met the inclusion criteria.
Effects of interventions
We did not find any studies that met the inclusion criteria.
Discussion
Summary of main results
We found no evidence from RCTs on different methods of communicating the diagnosis of amyotrophic lateral sclerosis/motor neuron disease (ALS/MND).
Overall completeness and applicability of evidence
Our review revealed the lack of studies with rigorous methodology that assessed the effectiveness of communication strategies in breaking the news in ALS/MND. Maybe this absence is at least partially due to ethical concerns. As evidenced in similar reviews, the randomisation of people with ALS/MND might be unethical, and consent difficult to obtain (Lockhart 2007). Breaking bad news is also inherently an interactive, and at least to some extent, an unpredictable process that is very difficult to reproduce in a controlled study without losing ecological validity.
Because a diagnosis of ALS/MND is often delivered incrementally, the effectiveness of communication may be influenced by variables that are difficult to control, such as ongoing family and social support, and individual coping strategies of people with ALS/MND and their caregivers.
Quality of the evidence
We found no evidence from RCTs on which to base practice when communicating the diagnosis of ALS/MND.
Potential biases in the review process
Based on our wide search strategies, we are confident that we found all relevant studies.
Agreements and disagreements with other studies or reviews
We were only able to perform a qualitative thematic synthesis, based on qualitative surveys and good practice protocols.
Although a standardised approach is unlikely to be either feasible or appropriate, it is important that neurologists develop communication skills and learn sensitive methods of breaking bad news (Anestis 2021; Chiò 2004; Johnston 1996; Meininger 1993); the American Academy of Neurology recognised this as an area for development (Miller 2009). A recent evaluation of the uptake of the European guidelines for the Diagnosis and Clinical Care of Patients with ALS/MND found that in a multicentric study, communication of the diagnosis was reported to be satisfactory in most cases, but could be improved in specific aspects (Marin 2016). For instance, the delivery of printed material for additional information was less frequent with older people with ALS/MND. Objective structured clinical examinations found a discrepancy between physicians' and examiners' scoring of breaking bad news sessions; physicians reported apprehension and dissatisfaction with their training on how to deliver the diagnosis (Schellenberg 2014). Although this study was based on a small sample of clinicians, it points to the importance of further, in‐depth examination of training and its evaluation.
According to most physicians and psychologists, gradual communication of an ALS/MND diagnosis, delivered at the pace of the person receiving the news, is considered the best option (Borasio 1998; Chiò 2004; Hirayama 2021; Johnston 1996; Meininger 1993; Silani 1999). Surveys have found that the specific personality traits of people with ALS/MND are not always taken into account when communicating the diagnosis (Hirayama 2021; Rudnick 2000; Silverstein 1991). When communicating the diagnosis, the physician may not always consider how much the person already knows, or how much they want to know (Borasio 1997; McCluskey 2004; Silverstein 1991). Given these differences, it is very difficult, if not impossible, to identify a 'perfect' or 'absolutely correct' way of breaking the news (Hirayama 2021; Rudnick 2000; Silverstein 1991). However, the effects of breaking bad news 'well' are seen as improved coping ability, and people with ALS/MND and their carers who adapt better to the diagnosis (Johnston 1996).
Despite the absence of focused and controlled studies on this topic, our overall perspective on the available literature is that there is a growing recognition that how a diagnosis of ALS/MND is communicated is an extremely important aspect of management. The space dedicated to this issue in the main references addressing good practice in the treatment of people with ALS/MND and caregivers testifies to this (Andersen 2005; Andersen 2012; Miller 1999; Miller 2009; Phukan 2009; Radunovic 2007; Simmons 2005). Adapting a general model suggested in Ptacek 1996, Miller and colleagues proposed the following key points for breaking the news in ALS (Miller 1999; Miller 2009).
The location should be quiet, comfortable, and private.
Information should be delivered at a convenient time for the person with ALS/MND.
Communication should be in person, and eye contact should be maintained.
Family members and a significant support network should be present.
The clinician should find out what the person already knows about the condition, and ascertain how much more information he/she wants to receive about ALS/MND; attention should be paid to the person's feedback and emotional expression, and there should be time for questions.
Information about prognosis and the course of symptom development should be precise and clear, but at the same time reassurance about the continuity of care, complications of treatment, and clinical trial opportunities are needed. The provision of information and printed material about organisations and useful websites are also important.
Information should be delivered with warmth, respect, and empathy.
The physician should be careful in the choice of words, be simple and direct, and avoid the use of euphemisms and medical jargon.
The good practice protocol, developed by the European Federation of Neurological Societies (EFNS) Task Force on the management of ALS, provided the same advice, and also underlined the importance of an early follow‐up visit (Andersen 2005; Andersen 2012). This creates the setting for ongoing care, and can reduce the person's perception of being abandoned. The qualitative study by Seeber and colleagues on a two‐tiered appointment supports the importance of this aspect (Seeber 2016). The EFNS guidelines agree that how the diagnosis is communicated can influence the quality of the relationship between the person with ALS/MND and health professionals. They note the need to balance clarity about the condition with the importance of not destroying hope. 'Honesty and hope' is the title of one of the first papers focused on breaking the news in ALS/MND, in which the authors recognise that communicating the diagnosis is a starting point towards a proper discussion of treatment options (Silani 1999). The article was preceded by a paper that influenced the American and European guidelines (Borasio 1998). Specifically, the authors emphasised communication skills, and giving information in a stepwise format, thus allowing the person receiving the diagnosis to provide more feedback, which guides the clinician on the appropriate pace of information delivery. In all these papers, the authors consider that providing an extensive description of available treatment options progressively, over time, might help to buffer the stress caused by the diagnosis, which in turn will play a significant role in functional psychological adjustment. This is an important point, given the influence that psychological well‐being has on treatment adherence and on the quality of life of people with ALS/MND and their caregivers. Although data on the prevalence of depression and anxiety in people with ALS/MND do not show that clinical affective disorders are widespread, the presence of more subtle signs of psychological distress has been recognised (Averill 2007; Goldstein 1998; Rabkin 2000; Rabkin 2009).
The psychological consequences of breaking bad news in ALS was the main topic in a special issue of the Revue Neurologique in 2006, in which leading French‐speaking clinicians discussed the implications and content of the communication process. In agreement with current trends and available guidelines, Brocq 2006 underlined the importance of emphasising what can be done to treat symptoms and complications, to prevent emotional reactions, and facilitate timely and coordinated care. Brocq 2006 also recommended that a multidisciplinary team be present during communication of the diagnosis, and that explicit and prompt information be given about the availability of psychological support. Couratier 2006 agreed with the common advice of giving information to both the person with ALS and family members, but emphasised the need to ask the person with ALS who they would like to be present during the consultation. Together with a review of the available literature, Campana‐Salort 2006 dedicated a specific paragraph to breaking the news in the case of familial ALS, with advice to family members to seek genetic and psychological counselling. Corcia 2006 noted that if they were not the first in the family to be affected, people diagnosed with familial ALS would already know about the course of the disease, a fact to be taken into account during the communication process.
Authors' conclusions
Implications for practice.
There are no randomised controlled trials on breaking bad news in amyotrophic lateral sclerosis (ALS) on which to base practice.
Implications for research.
Detailed and focused research studies are needed in order to assess the effectiveness and efficacy of different methods for informing people with amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) of their diagnosis.
| Using the EPICOT format to develop future research directions | |
| E ‐ Evidence | We did not find any randomised controlled trials (RCT) that assessed different methods of breaking the news in ALS/MND. Only post hoc surveys on patient and caregiver satisfaction with the communication of the diagnosis were available. Qualitative research into the interactive processes of communication should be conducted, given the importance assigned to the tailoring of information in breaking the news of an ALS/MND diagnosis. |
| P ‐ Population | People with ALS/MND |
| I ‐ Intervention | Further investigation is needed in developing and testing specific methods for informing people with ALS/MND of their diagnosis. Studies in other diseases might be of help in identifying specific skills and strategies. |
| C ‐ Comparison | If compatible with ethical concerns and standards, comparisons of different combinations of face‐to‐face communication, family member involvement, contextual factors, and delivery of written materials might be performed. Given the particular features of ALS/MND onset and diagnosis, the timing of when information is given should also be studied. |
| O ‐ Outcome | Different levels of outcome measures might be considered, e.g. surveys and standardised evaluations of patient and caregiver satisfaction with how the diagnosis is communicated, evaluations of coping strategies, quality of life, and psychological well‐being |
| T – Time stamp | February 2022 |
History
Protocol first published: Issue 1, 2009
Acknowledgements
The Managing Editor of Cochrane Neuromuscular, Ruth Brassington, drafted additional text for the methods section in accordance with current methodological standards (Higgins 2022a). The text was taken, in part, from a standard Cochrane Neuromuscular protocol, adapted from an original by Cochrane Airways.
We thank Victoria Pennick for copy editing the review.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRSWeb) search strategy
#1 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND INREGISTER #2 "motor neuron disease*" or "motor neurone disease*" AND INREGISTER #3 "motoneuron disease*" or "motoneurone disease*" AND INREGISTER #4 "motorneuron disease*" or "motorneurone disease*" AND INREGISTER #5 "charcot disease" AND INREGISTER #6 "amyotrophic lateral sclerosis" AND INREGISTER #7 als:ti or als:ab or nmd:ti or mnd:ab AND INREGISTER #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 AND INREGISTER #9 ((communicat* or advise or advice or counsel* or disclos* or educat* or discuss* or inform* or tell* or giv* or break*) near3 diagnosis) AND INREGISTER #10 ((communicat* or advise or advice or counsel* or disclos* or educat* or discuss* or inform* or tell* or giv* or break*) near3 news) AND INREGISTER #11 "bad news" AND INREGISTER #12 #9 OR #10 OR #11 AND INREGISTER #13 #8 AND #12 AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL; CRSWeb) search strategy
#1 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND CENTRAL:TARGET #2 "motor neuron disease*" or "motor neurone disease*" AND CENTRAL:TARGET #3 "motoneuron disease*" or "motoneurone disease*" AND CENTRAL:TARGET #4 "motorneuron disease*" or "motorneurone disease*" AND CENTRAL:TARGET #5 "charcot disease" AND CENTRAL:TARGET #6 "amyotrophic lateral sclerosis" AND CENTRAL:TARGET #7 als:ti or als:ab or nmd:ti or mnd:ab AND CENTRAL:TARGET #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 AND CENTRAL:TARGET #9 ((communicat* or advise or advice or counsel* or disclos* or educat* or discuss* or inform* or tell* or giv* or break*) near3 diagnosis) AND CENTRAL:TARGET #10 ((communicat* or advise or advice or counsel* or disclos* or educat* or discuss* or inform* or tell* or giv* or break*) near3 news) AND CENTRAL:TARGET #11 "bad news" AND CENTRAL:TARGET #12 #9 OR #10 OR #11 AND CENTRAL:TARGET #13 #8 AND #12 AND CENTRAL:TARGET
Appendix 3. MEDLINE OvidSP search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (456500) 2 controlled clinical trial.pt. (92271) 3 randomized.ab. (406149) 4 placebo.ab. (187405) 5 drug therapy.fs. (2003094) 6 randomly.ab. (287156) 7 trial.ab. (421778) 8 groups.ab. (1775972) 9 or/1‐8 (4165481) 10 exp animals/ not humans.sh. (4437662) 11 9 not 10 (3599389) 12 exp Motor Neuron Disease/ (24194) 13 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (8099) 14 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (172) 15 charcot disease.tw. (21) 16 Amyotrophic Lateral Sclerosis.mp. (23181) 17 or/12‐16 (32326) 18 ((communicat$ or advise or advice or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 diagnosis).mp. (22938) 19 ((communicat$ or advise or advice or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 news).mp. (1754) 20 (adaptation adj1 psychological).mp. or Adaptation, Psychological/ (86577) 21 (bad adj3 news).mp. (2016) 22 or/18‐21 (111903) 23 11 and 17 and 22 (40) 24 remove duplicates from 23 (40)
Appendix 4. Embase OvidSP search strategy
Database: Embase <1974 to 2018 June 11> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (54889) 2 double‐blind procedure.sh. (148021) 3 single‐blind procedure.sh. (30852) 4 randomized controlled trial.sh. (494977) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1499987) 6 trial.ti. (246080) 7 or/1‐6 (1670409) 8 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (25798892) 9 human/ or human cell/ or human tissue/ or normal human/ (19496028) 10 8 not 9 (6351377) 11 7 not 10 (1474023) 12 limit 11 to (conference abstracts or embase) (1238355) 13 motor neuron disease/ or amyotrophic lateral sclerosis/ (38681) 14 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (12666) 15 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (202) 16 charcot disease.tw. (27) 17 amyotrophic lateral sclerosis.tw. (26004) 18 or/13‐17 (42920) 19 adaptive behavior/ (53442) 20 (Adaptive Behavior or (adaptation adj3 psychological)).mp. (55854) 21 ((communicat$ or advise or advice or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 diagnosis).mp. (34469) 22 ((communicat$ or advise or advice or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 news).mp. (2147) 23 bad news.mp. (2682) 24 or/19‐23 (93437) 25 12 and 18 and 24 (8) 26 remove duplicates from 25 (8)
Appendix 5. PsycINFO OvidSP search strategy
Database: PsycINFO <1806 to June Week 1 2018> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (1286) 2 amyotrophic lateral sclerosis.mp. (4787) 3 1 or 2 (5455) 4 adjustment/ or (psychological adj3 (adjustment or adapt$)).mp. (22906) 5 ((communicat$ or advice or advise or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 diagnosis).mp. (8866) 6 ((communicat$ or advice or advise or counsel$ or disclos$ or educat$ or discuss$ or inform$ or tell$ or giv$3 or break$) adj3 news).mp. (1420) 7 bad news.mp. (989) 8 or/4‐7 (33617) 9 (random$ or rct or cct or trial$1).mp. (284144) 10 3 and 8 and 9 (3)
Appendix 6. ClinicalTrials.gov search strategy
Database: ClinicalTrials.gov 11 June 2018 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Amyotrophic Lateral Sclerosis or motor neuron disease (400)
2 communication of diagnosis (713)
3 bad news (7)
4 breaking bad news (2)
5 1 and 2 (5)
6 1 and 3 (0)
7 1 and 4 (0)
Appendix 7. WHO International Clinical Trials Registry search strategy
Database: WHO international Clinical trials Registry 11 June 2018 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Amyotrophic Lateral Sclerosis or motor neuron disease (1037)
2 communication of diagnosis (1)
3 bad news (7)
4 breaking bad news (6)
5 1 and 2 (0)
6 1 and 3 (0)
7 1 and 4 (0)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borasio 1998 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions literature; not an experimental study |
| Brocq 2006 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions literature; not an experimental study |
| Campana‐Salort 2006 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions literature; not an experimental study |
| Chiò 2004 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions literature; not an experimental study |
| Corcia 2006 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions in the literature. Not an experimental study. |
| Couratier 2006 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions in the literature; not an experimental study |
| Johnston 1996 | Post hoc survey; not an experimental study |
| McCluskey 2004 | Post hoc survey; not an experimental study |
| Meininger 1993 | Theoretical and good‐practice article based on clinical expertise and review of ALS/MND and other conditions in the literature; not an experimental study |
| Schellenberg 2014 | Qualitative analysis based on videotaped sessions; not an experimental study |
| Seeber 2016 | Qualitative analysis based on in‐depth interviews and non‐participating observations; not an experimental study |
Differences between protocol and review
This review has a published protocol (Bongioanni 2009).
We updated the risk of bias methodology in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, and described methods for summary of findings tables (Higgins 2017).
We reworded the objectives for consistency with the outcomes.
We provided examples of measurement tools for our prespecified outcomes and included measures of illness perception and caregiver burden.
For adherence to current standards, we (Higgins 2022a):
noted that cross‐over and cluster‐randomised trials were not eligible;
stated that we would include studies in which participants had various conditions only if results for people with ALS/MND were reported separately;
stated that we did not use outcomes in study selection, or limit selection by language or publication status;
stated that we would pilot our data extraction form;
noted that we would extract data about study design, conflicts of interest, and funding of included studies;
described methods for dealing with reports requiring translation;
noted that we would use standardised mean differences (SMDs) to combine results from studies using different scales for the same outcome, and stated how we would interpret SMDs;
included methods for dealing with unit‐of‐analysis issues, missing data, assessing heterogeneity, and assessing reporting bias.
KS joined the author team at the review stage.
Contributions of authors
Paolo Bongioanni wrote the first draft of the protocol; Karl Peter Kapitza added comments to the first draft; Francesco Tramonti integrated the comments into the second draft; Gian Domenico Borasio and David Oliver revised the second draft; Andrea Romagnoli wrote additional comments to the second draft; all the authors checked the final version of the protocol.
Paolo Bongioanni, Francesco Tramonti, and Andrea Romagnoli independently checked all titles and abstracts obtained from the searches to identify potentially relevant studies. Paolo Bongioanni and Francesco Tramonti wrote the first draft of the review; Karl Peter Kapitza, Gian Domenico Borasio, and David Oliver added comments and integrated them into the first draft; Andrea Romagnoli, Paolo Bongioanni, and Maria Chiara Carboncini wrote the second draft, and Francesco Tramonti integrated his own comments. Katie Sidle critically assessed drafts.
Sources of support
Internal sources
-
None to declare, Other
None
External sources
-
National Institute for Health Research (NIHR) Cochrane Infrastructure funding and Motor Neurone Disease Association, UK
This Project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health. Cochrane Neuromuscular Disease Group also receives support from the Motor Neurone Disease Association and the Queen Square Centre for Neuromuscular Disease.
Declarations of interest
PB: none known
GDB: none known
DJO: none known
FT: none known. FT works as a psychologist at Azienda USL Toscana Nordovest, Pisa, Italy (Public Health Sector).
AR: none known
KPK: none known
KS: none known
New
References
References to studies excluded from this review
Borasio 1998 {published data only}
- Borasio GD, Sloan R, Pongratz DE. Breaking the news in amyotrophic lateral sclerosis. Journal of the Neurological Sciences 1998;160(Suppl 1):S127-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brocq 2006 {published data only}
- Brocq H, Soriani MH, Desnuelle C. Psychological reactions to the announcement of a severe disease diagnosis: the amyotrophic lateral sclerosis example [Réactions psychologiques à l’annonce d’un diagnostic de maladie grave: spécificités de la SLA]. Revue Neurologique (Paris) 2006;162(Spec No 2):4S104-7. [PMID: ] [PubMed] [Google Scholar]
Campana‐Salort 2006 {published data only}
- Campana-Salort E. How should the diagnosis of amyotrophic lateral sclerosis be announced? [Comment dit-on le diagnostic de sclérose latérale amyotrophique (SLA)?]. Revue Neurologique (Paris) 2006;162(Spec No 2):4S113-21. [PMID: ] [PubMed] [Google Scholar]
Chiò 2004 {published data only}
- Chiò A, Borasio GD. Breaking the news in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2004;5(4):195-201. [DOI] [PubMed] [Google Scholar]
Corcia 2006 {published data only}
- Corcia P. Methods of the announcement of amyotrophic lateral sclerosis diagnosis in familial forms [Contenu et modalités de l’annonce du diagnostic de SLA dans un contexte familial]. Revue Neurologique (Paris) 2006;162(Spec No 2):4S122-6. [PMID: ] [PubMed] [Google Scholar]
Couratier 2006 {published data only}
- Couratier P, Desport JC, Torny F, Lacoste M. Announcement of amyotrophic lateral sclerosis diagnosis [Modalités et contenu de l’annonce du diagnostic de sclérose latérale amyotrophique sporadique]. Revue Neurologique (Paris) 2006;162(Spec No 2):4S108-12. [PMID: ] [PubMed] [Google Scholar]
Johnston 1996 {published data only}
- Johnston M, Earll L, Mitchell E, Morrison V, Wright S. Communicating the diagnosis of motor neurone disease. Palliative Medicine 1996;10(1):23-34. [DOI] [PubMed] [Google Scholar]
McCluskey 2004 {published data only}
- McCluskey L, Casarett D, Siderowf A. Breaking the news: a survey of ALS patients and their caregivers. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2004;5(3):131-5. [DOI] [PubMed] [Google Scholar]
Meininger 1993 {published data only}
- Meininger V. Breaking bad news in amyotrophic lateral sclerosis. Palliative Medicine 1993;7(4 Suppl):37-40. [DOI] [PubMed] [Google Scholar]
Schellenberg 2014 {published data only}
- Schellenberg KL, Schofield SJ, Fang S, Johnston WS. Breaking bad news in amyotrophic lateral sclerosis: the need for medical education. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2014;15:47-54. [DOI] [PubMed] [Google Scholar]
Seeber 2016 {published data only}
- Seeber AA, Pols AJ, Hijdra A, Grupstra HF, Willems DL, Visser M. Experiences and reflections of patients with motor neuron disease on breaking the news in a two-tired appointment: a qualitative study. BMJ Supportive & Palliative Care 2016 Feb 2 [Epub ahead of print]. [DOI: 10.1136/bmjspcare-2015-000977] [DOI] [PubMed]
Additional references
Andersen 2005
- Andersen PM, Borasio GD, Dengler R, Hardiman O, Kollewe K, Leigh PN, et al, EALSC working group. EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. European Journal of Neurology 2005;12(12):921-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Andersen 2012
- The EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis: Andersen PM, Abrahams S, Borasio GD, Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS) - revised report of an EFNS task force. European Journal of Neurology 2012;19(3):360-75. [DOI: 10.1111/j.1468-1331.2011.03501.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anestis 2020
- Anestis E, Eccles F, Fletcher I, French M, Simpson J. Giving and receiving a diagnosis of a progressive neurological condition: a scoping review of doctors' and patients' perspectives. Patient Education and Counselling 2020;103(9):1709-23. [DOI: 10.1016/j.pec.2020.03.023] [DOI] [PubMed] [Google Scholar]
Anestis 2021
- Anestis E, Eccles F, Fletcher I, Simpson J. Neurologists' current practice and perspectives on communicating the diagnosis of a motor neurodegenerative condition: a UK survey. BMC Neurology 2021;21(1):34. [DOI: 10.1186/s12883-021-02062-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoun 2017
- Aoun SM, Breen LJ, Oliver D, Henderson RD, Edis R, O'Connor M, et al. Family carers' experiences of receiving the news of a diagnosis of Motor Neurone Disease: a national survey. Journal of the Neurological Sciences 2017;372:144-51. [DOI: 10.1016/j.jns.2016.11.043] [DOI] [PubMed] [Google Scholar]
Aoun 2018
- Aoun SM, O'Brien MR, Breen LJ, O'Connor M. 'The shock of diagnosis': qualitative accounts from people with motor neurone disease reflecting the need for more person-centred care. Journal of the Neurological Sciences 2018;387:80-4. [DOI: 10.1016/j.jns.2018.01.026] [DOI] [PubMed] [Google Scholar]
Averill 2007
- Averill AJ, Kasarskis EJ, Segerstrom SC. Psychological health in patients with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders 2007;8(4):243-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
Borasio 1997
- Borasio GD, Pongratz DE. Considerations in patient education in amyotrophic lateral sclerosis (ALS) [Gedanken zur Aufklärung bei amyotropher Lateralsklerose (ALS)]. Der Nervenarzt 1997;68(12):1004-7. [DOI] [PubMed] [Google Scholar]
Borasio 2001
- Borasio GD, Shaw PJ, Hardiman O, Ludolph AC, Sale Luis ML, Silani V, European ALS Study Group. Standards of palliative care for patients with amyotrophic lateral sclerosis: results of a European survey. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders 2001;2(3):159-64. [DOI] [PubMed] [Google Scholar]
Brooks 2000
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(5):293-9. [DOI] [PubMed] [Google Scholar]
Carver 1989
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of Personality and Social Psychology 1989;56(2):267-83. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Eggly 2006
- Eggly S, Penner L, Albrecht TL, Cline RJW, Foster T, Naughton M, et al. Discussing bad news in the outpatient oncology clinic: rethinking current communication guidelines. Journal of Clinical Oncology 2006;24(4):716-9. [DOI] [PubMed] [Google Scholar]
GBD 2016 Motor Neuron Disease Collaborators 2018
- GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2018;17(12):1083-97. [DOI: 10.1016/S1474-4422(18)30404-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1998
- Goldstein LH, Adamson M, Jeffrey L, Down K, Barby T, Wilson C. The psychological impact of MND on patients and carers. Journal of the Neurological Sciences 1998;160(Suppl 1):S114-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 June 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Greer 1991
- Greer S. Psychological responses to cancer and survival. Psychological Medicine 1991;21:43-9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. training.cochrane.org/handbook/archive/v5.2.
Higgins 2022a
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological expectations of Cochrane intervention Reviews (MECIR), Cochrane: London, Version February 2022. Available from community.cochrane.org/mecir-manual.
Higgins 2022b
- Higgins JPT, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hirayama 2021
Lee 2001
- Lee JN, Rigby SA, Burchardt F, Thornton EW, Dougan C, Young CA. Quality of life issues in motor neurone disease: the development and validation of a coping strategies questionnaire, the MND Coping Scale. Journal of the Neurological Sciences 2001;191(1-2):79-85. [DOI] [PubMed] [Google Scholar]
Lockhart 2007
- Lockhart K, Dosser I, Cruickshank S, Kennedy C. Methods of communicating a primary diagnosis of breast cancer to patients. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006011. [DOI: 10.1002/14651858.CD006011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 2016
- Marin B, Beghi E, Vial C, Bernard E, Lautrette G, Clavelou P, et al, on behalf of EURECALS consortium. Evaluation of the application of the European guidelines for the diagnosis and clinical care of amyotrophic lateral sclerosis (ALS) patients in six French ALS centres. European Journal of Neurology 2016;23(4):787-95. [DOI] [PubMed] [Google Scholar]
Maynard 1996
- Maynard DW. On “realization” in everyday life: the forecasting of bad news as a social relation. American Sociology Review 1996;61:109-31. [Google Scholar]
Miller 1999
- Miller RG, Rosenberg JA, Gelinas DF, Mitsumoto H, Newman D, Sufit R. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology 1999;52(7):1311-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2009
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73(15):1227-33. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirza 2019
- Mirza RD, Ren M, Agarwal A, Guyatt GH. Assessing patient perspectives on receiving bad news: a survey of 1337 patients with life-changing diagnoses. AJOB Empirical Bioethics 2019;10(1):36-43. [DOI: 10.1080/23294515.2018.1543218] [DOI] [PubMed] [Google Scholar]
Moss‐Morris 2002
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised Illness Perception Questionnaire (IPQ-R). Psychology & Health 2002;17(1):1-16. [Google Scholar]
Mujezinovic 2010
- Mujezinovic F, Prosnik A, Alfirevic Z. Different communication strategies for disclosing results of diagnostic prenatal testing. Cochrane Database of Systematic Reviews 2010, Issue 11. Art. No: CD007750. [DOI: 10.1002/14651858.CD007750.pub2] [DOI] [PubMed] [Google Scholar]
Novak 1989
- Novak M, Guest CI. Application of a multidimensional Caregiver Burden Inventory. Gerontologist 1989;29(6):798-803. [DOI] [PubMed] [Google Scholar]
O'Connor 2018
- O'Connor M, Aoun SM, Breen LJ. Australian family carer responses when a loved one receives a diagnosis of Motor Neurone Disease – "our life has changed forever". Health and Social Care in the Community 2018;26(3):e415-21. [DOI: 10.1111/hsc.12541] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Phukan 2009
- Phukan J, Hardiman O. The management of amyotrophic lateral sclerosis. Journal of Neurology 2009;256(2):176-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ptacek 1996
- Ptacek JT, Eberhardt TL. Breaking bad news: a review of the literature. JAMA 1996;276(6):496-502. [PMID: ] [PubMed] [Google Scholar]
Rabkin 2000
- Rabkin JG, Wagner GJ, Del Bene M. Resilience and distress among amyotrophic lateral sclerosis patients and caregivers. Psychosomatic Medicine 2000;62(2):271-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rabkin 2009
- Rabkin JG, Albert SM, Rowland LP, Mitsumoto H. How common is depression among ALS caregivers? A longitudinal study. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders 2009;10(5-6):448-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radunovic 2007
- Radunović A, Mitsumoto H, Leigh PN. Clinical care of patients with amyotrophic lateral sclerosis. Journal of the American Medical Association 2007;6(10):913-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Cochrane Collaboration, 2020.
Roberts 1994
- Roberts CS, Cox CE, Reintgen DS, Baile WF, Gibertini M. Influence of physician communication on newly diagnosed breast cancer patients' psychologic adjustment and decision-making. Cancer 1994;74(1 Suppl):336-41. [DOI] [PubMed] [Google Scholar]
Rudnick 2000
- Rudnick A, Ezra Y, Melamed E. Breaking bad news and personality assessment. Patient Education and Counseling 2000;41(2):157-60. [DOI] [PubMed] [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Lowe D, Allen K, Taylor M, Mead C. Notification and support for people exposed to the risk of Creutzfeldt-Jakob disease (CJD) (or other prion diseases) through medical treatment (iatrogenically). Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD007578. [DOI: 10.1002/14651858.CD007578.pub2] [DOI] [PubMed] [Google Scholar]
Sakellariou 2013
- Sakellariou D, Boniface G, Brown P. Experiences of living with motor neurone disease: a review of qualitative research. Disability and Rehabilitation 2013;35(21):1765-73. [DOI: 10.3109/09638288.2012.753118] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Silani 1999
- Silani V, Borasio GD. Honesty and hope: announcement of diagnosis in ALS. Neurology 1999;53(8 Suppl 5):S37-9; discussion S40-2. [PMID: ] [PubMed] [Google Scholar]
Silverstein 1991
- Silverstein MD, Stocking CB, Antel JP, Beckwith J, Roos RP, Siegler M. Amyotrophic lateral sclerosis and life-sustaining therapy: patients' desires for information, participation in decision making, and life-sustaining therapy. Mayo Clinic Proceedings 1991;66(9):906-13. [DOI] [PubMed] [Google Scholar]
Simmons 2005
- Simmons Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. Neurologist 2005;11(5):257-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc., 1983. [Google Scholar]
Vail 2011
- Vail L, Sandhu H, Fisher J, Cooke H, Dale J, Barnett M. Hospital consultants breaking bad news with simulated patients: an analysis of communication using the Roter Interaction Analysis System. Patient Education and Counseling 2011;83(2):185-94. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30:473-83. [PubMed] [Google Scholar]
Wettergren 2009
- Wettergren L, Kettis-Lindblad A, Sprangers M, Ring L. The use, feasibility and psychometric properties of an individualised quality-of-life instrument: a systematic review of the SEIQoL-DW. Quality of Life Research 2009;18(6):737-46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bongioanni 2009
- Bongioanni P, Borasio GD, Oliver D, Tramonti F, Romagnoli A, Kapitza KP. Methods for informing people with amyotrophic lateral sclerosis/motor neuron disease of their diagnosis. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007593. [DOI: 10.1002/14651858.CD007593] [DOI] [PMC free article] [PubMed] [Google Scholar]


