To the Editor: Booster doses of bivalent messenger RNA (mRNA) vaccines containing the ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the omicron B.1.1.529 (BA.4 and BA.5) variant spike sequences have been strongly recommended for persons at risk for severe sequelae of coronavirus disease 2019 (Covid-19). Patients undergoing hemodialysis have shown inferior humoral immunity against variants despite the receipt of four monovalent vaccine doses.1,2
In this case series, 55 patients undergoing hemodialysis who had received four previous SARS-CoV-2 vaccinations were monitored 6 and 2 weeks before and 2 and 4 weeks after a fifth vaccination with a bivalent mRNA vaccine (see the Supplementary Appendix, available with the full text of this letter at NEJM.org). Before the fifth vaccination, 18 patients had had previous omicron breakthrough infection that had been confirmed by polymerase-chain-reaction testing, whereas 37 had not had previous omicron breakthrough infection.
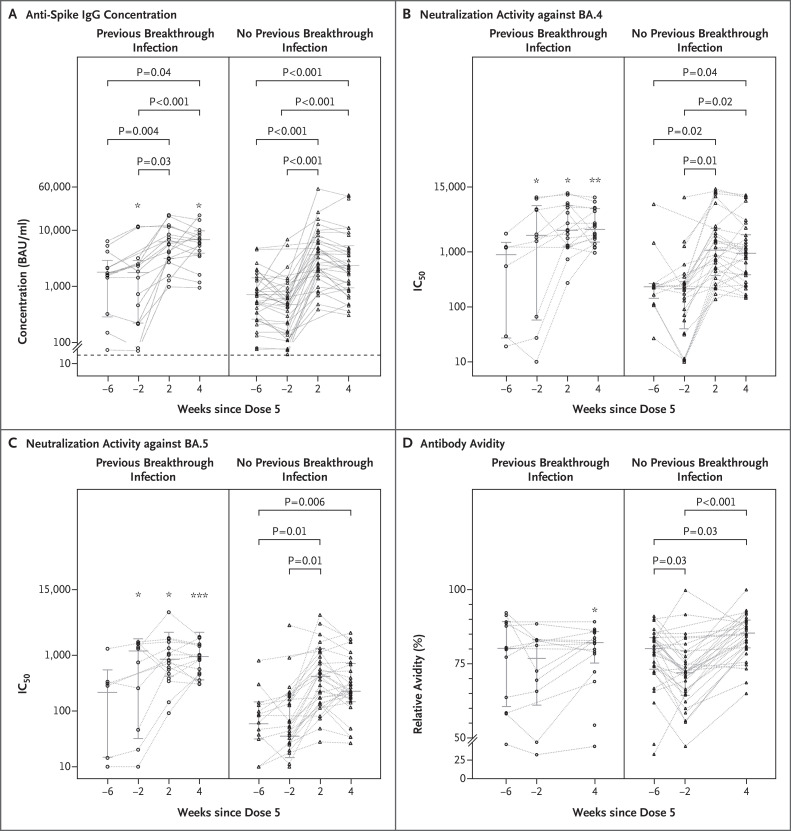
From 2 weeks before to 2 weeks after the fifth vaccination, a significant increase by a factor of 2.5 in anti-spike IgG concentrations was stimulated in the patients who had had previous omicron breakthrough infection, and an increase by a factor of 7.3 was stimulated in those who had not had previous omicron breakthrough infection (Figure 1A). Patients with previous breakthrough infection had significantly higher anti-spike IgG concentrations and neutralization activities (expressed as 50% inhibitory concentrations)3,4 against BA.4 (Figure 1B) and BA.5 (Figure 1C) than those without previous breakthrough infection, both before and after the fifth vaccination. Patients without previous breakthrough infection had significantly increased infection-neutralizing capacity by a factor of 4.9 against BA.4 and by a factor of 3.3 against BA.5 at 2 weeks after vaccination and increased anti-spike IgG avidity by 17% at 4 weeks after vaccination (Figure 1D). After the fifth vaccination, neutralization activities against both omicron BA.4 and BA.5 in both groups — those with and those without previous breakthrough infection — positively correlated with anti-spike IgG concentrations, antibody avidity, and neutralization activity before the fifth vaccination (Figs. S2 and S3 in the Supplementary Appendix), whereas age did not significantly influence the results. A regression analysis that included all study patients showed that the anti-spike IgG concentration after booster vaccination was the most important indicator of high neutralization activity against BA.4 (P=0.008) and BA.5 (P=0.005).
Figure 1. Anti-Spike IgG Responses against Omicron BA.4 and BA.5 Subvariants before and after Bivalent mRNA Booster in Patients Undergoing Hemodialysis.
Anti-spike IgG concentrations 6 and 2 weeks before, as well as 2 and 4 weeks after, fifth messenger RNA (mRNA) vaccination in patients with previous B.1.1.529 (omicron) breakthrough infection (open circles) and those with no previous omicron breakthrough infection (triangles) are shown (Panel A). Connecting lines between the time points indicate matched serum samples. The dashed horizontal line indicates the lower limit of detection (31 binding antibody units [BAU] per milliliter) in the detection system. Neutralizing activities are presented as serum dilutions for half-maximal infection-neutralization capacities normalized to 107 viral RNA copies (50% inhibitory concentration [IC50]) for omicron BA.4 (Panel B) and BA.5 (Panel C). The anti-spike IgG antibody avidities are shown as relative percentages for three time points (Panel D). The median value (horizontal line) and interquartile range (whiskers) are shown. P values are given for comparison of the dependent variables between different time points for each group. For comparison between patients with previous omicron breakthrough infection and those with no previous omicron breakthrough infection at each time point, one asterisk denotes P<0.05, two asterisks P<0.01, and three asterisks P<0.001.
The findings are in accordance with results from clinical studies of bivalent mRNA vaccines in healthy persons that showed a significant rise in anti-spike IgG concentrations.5 In particular, patients who did not have previous breakthrough infection immunologically benefited from a bivalent mRNA booster: despite having lower anti-spike IgG concentrations before the fifth vaccination, they had a significant increase after the fifth vaccination, such that their concentrations matched those in persons with hybrid immunity due to omicron breakthrough infection. The high dependency of neutralization activity against omicron BA.4 and BA.5 on baseline anti-spike IgG concentrations and binding activity before booster vaccination corroborates the concept that antibodies should be maintained at high levels by means of periodic vaccinations. The results may be of particular importance for persons at risk for severe disease such as patients undergoing hemodialysis and support recommendations that these patients should receive booster doses of bivalent mRNA vaccines, particularly if they have no hybrid immunity from an omicron breakthrough infection.
Supplementary Appendix
Disclosure Forms
This letter was published on February 15, 2023, at NEJM.org.
Footnotes
Supported by the Department of Pediatrics, University Hospital Würzburg, Würzburg, Germany, and by the Bay-VOC initiative funded by the Free State of Bavaria.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Anft M, Blazquez-Navarro A, Frahnert M, et al. Inferior cellular and humoral immunity against omicron and delta variants of concern compared with SARS-CoV-2 wild type in hemodialysis patients immunized with 4 SARS-CoV-2 vaccine doses. Kidney Int 2022;102:207-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr EJ, Wu M, Harvey R, et al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet 2022;399:800-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med 2022;28:496-503. [DOI] [PubMed] [Google Scholar]
- 4.Keppler-Hafkemeyer A, Greil C, Wratil PR, et al. Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma. Nat Cancer 2023;4:81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.