Abstract
Transmission of the coronavirus disease 2019 is still ongoing despite mass vaccination, lockdowns, and other drastic measures to control the pandemic. This is due partly to our lack of understanding on the multiphase flow mechanics that control droplet transport and viral transmission dynamics. Various models of droplet evaporation have been reported, yet there is still limited knowledge about the influence of physicochemical parameters on the transport of respiratory droplets carrying the severe acute respiratory syndrome coronavirus 2. Here we review the effects of initial droplet size, environmental conditions, virus mutation, and non-volatile components on droplet evaporation and dispersion, and on virus stability. We present experimental and computational methods to analyze droplet transport, and factors controlling transport and evaporation. Methods include thermal manikins, flow techniques, aerosol-generating techniques, nucleic acid-based assays, antibody-based assays, polymerase chain reaction, loop-mediated isothermal amplification, field-effect transistor-based assay, and discrete and gas-phase modeling. Controlling factors include environmental conditions, turbulence, ventilation, ambient temperature, relative humidity, droplet size distribution, non-volatile components, evaporation and mutation. Current results show that medium-sized droplets, e.g., 50 µm, are sensitive to relative humidity. Medium-sized droplets experience delayed evaporation at high relative humidity, and increase airborne lifetime and travel distance. By contrast, at low relative humidity, medium-sized droplets quickly shrink to droplet nuclei and follow the cough jet. Virus inactivation within a few hours generally occurs at temperatures above 40 °C, and the presence of viral particles in aerosols impedes droplet evaporation.
Keywords: Expiratory aerosols, Virus transmission, Physical distancing, COVID-19, SARS-CoV-2, Airborne infection
Introduction
The first confirmed coronavirus disease 2019 (COVID-19) outbreak was reported in Wuhan, China at the end of 2019 (Chen and Zhao 2020; Phelan et al. 2020; Wang et al. 2020c; Zhu et al. 2020). The causative agent of the COVID-19 pandemic has been identified as the severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, a single-stranded RNA virus with several circulating variants (Gorbalenya et al. 2020; Lai et al. 2020). As of January 27, 2023, the World Health Organization reported over 752 million confirmed COVID-19 infections, including 6.8 million fatalities globally (WHO 2023). Volumes of studies have been published on previous epidemics caused by zoonotic respiratory pathogens, including the severe acute respiratory syndrome coronavirus, or SARS-CoV, and the Middle East respiratory syndrome coronavirus, or MERS-CoV (Liu et al. 2020). However, the airborne transmission of SARS-CoV-2 and its variants, particularly the Omicron variants, are poorly understood (CDC 2023; Wang and Han 2022; WHO 2021). The COVID-19 pandemic continues to threaten and impact human lives worldwide throughout 2022 (Akter et al. 2022; Li et al. 2020b; Ufnalska and Lichtfouse 2021).
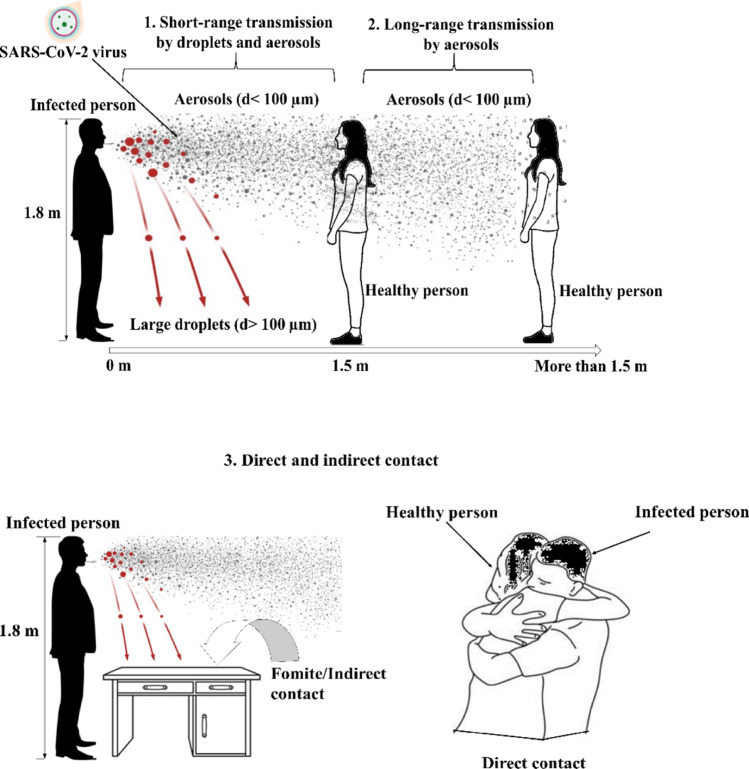
Discussions on COVID-19 transmission have focused on indoor settings with no or inadequate ventilation, emphasizing the need to implement infection prevention and control measures in buildings and other indoor environments. Human respiratory activities such as coughing, sneezing, speaking, and breathing are known to spread respiratory pathogens through the air, including SARS-CoV-2 (Asadi et al. 2020; Bourouiba 2020; Wang and Du 2020). Broadly, there are three pathways of transport for virus-laden respiratory droplets (Fig. 1), i.e., short-range transmission by inhaling virus-laden droplets or aerosols, long-range transmission either by inhaling aerosols or by contacting virus-contaminated surfaces, i.e., fomites (Asadi et al. 2020; Tellier et al. 2019). Here, aerosols refer to the suspensions of fine solid particles or liquid droplets in the air, which can linger in the air for significantly longer durations than larger droplets—the latter generally fall quickly under gravity. Droplet nuclei, for instance, are formed after the evaporation of respiratory droplets in the air and constitute an essential part of virus-laden aerosols originating from human respiratory activities (Nardell 2004; Wells 1934). While such classification is often conveniently used, the three transmission modes are not clearly distinguished, i.e., they overlap and sometimes cause misperceptions (Drossinos and Stilianakis 2020; Priyanka et al. 2020).
Fig. 1.
Main transmission modes of the severe acute respiratory syndrome coronavirus (SARS-CoV-2), based on the classifications by Li (2021) and Priyanka et al. (2020) 1. Susceptible individuals close to an infected person are prone to drop-spray and short-range airborne transmission. 2. Individuals beyond a certain physical distance, e.g., 1.5 m, are still prone to long-range airborne transmission, e.g., by aerosols. 3. Individuals who touch virus-contaminated inanimate objects, i.e., fomites, are prone to indirect contact transmission. The person who engages in direct physical contact with an infected person, e.g., hugging, hand shaking, or kissing, can also be at significant risk of infection by SARS-CoV-2
Although reviews on SARS-CoV-2 transmission already exist, there is still not enough understanding of the multiphase flow mechanics that control droplet transport and viral transmission dynamics. Various models of droplet evaporation have been reported, yet there is still limited knowledge on the influence of physicochemical parameters on the transport of respiratory droplets carrying the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Moreover, the multicomponent nature of viral droplets has not been clarified in detail in previous reviews since most computational investigations simplified a viral droplet as a single component. Overall, the impact of environmental factors, mutation, and non-volatile solutes like virus particles on droplet evaporation and virus stability remains unresolved. Here we review the transport and evaporation of exhaled respiratory particles from an infected person’s mouth to a vulnerable host. We discuss methods to analyze droplet transport, and factors controlling the transport and evaporation of exhaled droplets from the viewpoint of multiphase flow physics.
Experimental methods to analyze droplet transport
A detailed list of experimental methods for analyzing the size and transport of respiratory droplets is shown in Table 1. Existing methods include thermal manikins, flow techniques such as particle image velocimetry, schlieren imaging, laser light scattering, tracer gas techniques, and aerosol-generating techniques, e.g., collision nebulizer and cough machines.
Table 1.
Experimental methods for analyzing the size and transport of respiratory droplets
| Objective | Method | Respiratory activity | Main results | Investigator(s) |
|---|---|---|---|---|
| Size and number of respiratory droplets | Solid impaction with droplet spray directed at a slide | Sneeze, cough and speech |
Similar droplet size distribution: 95% of 2–100 µm; the overall size of 1–2000 µm Most common: 4–8 µm |
Duguid (1946) |
| Solid impaction with droplets collected inside a box | Cough and speech |
No significant difference in droplet size distribution (PSD) Average droplet sizes: 50–100 µm |
Xie et al. (2009) | |
| Interferometric Mie imaging (IMI) | Cough and speech | The speech geometric mean diameter (GMD) is 16 µm, and the cough is 13.5 µm | Chao et al. (2009) | |
| Scanning mobility particle sizing (SMPS) | Cough | The nominal size range is 0.01–10 µm | Lee et al. (2019) | |
| Laser light scattering | Speech | Droplet size ranges from 20 to 500 µm | Anfinrud et al. (2020) and Stadnytskyi et al. (2020) | |
| Laser diffraction droplet sizing | Cough and speech | The nominal size range is 1–2000 µm | Smith et al. (2020) | |
| Aerodynamic particle sizing (APS) | Breath, speech, singing | The nominal size range is 0.5–20 µm | Gregson et al. (2021) | |
| Velocities and ejection angles of the droplets | Particle image velocimetry (PIV) | Cough and speech |
3.9 m/s was the mean velocity for speech, while that for cough was 11.7 m/s The male volunteer’s peak airflow velocity is 13.2 m/s, whereas the female volunteer’s is 10.2 m/s |
Chao et al. (2009) |
| High-speed illumination and film-based cameras | Sneeze | The initial droplet velocity is about 46 m/s | Jennison (1941) | |
| Schlieren imaging | Cough | Airflow velocity of 8 m/s from a single cough | Tang et al. (2009) | |
| Particle image velocimetry (PIV) | Cough |
Initial velocity: 6–22 m/s Mean velocity: 11.2 m/s |
Zhu et al. (2006) | |
| Particle image velocimetry (PIV) | Cough | Peak velocities range from 1.5 to 28.8 m/s | VanSciver et al. (2011) | |
| Volumetric illumination and employed particle image velocimetry (PIV) | Cough and sneeze | The velocity of sneeze airflow is higher than 6 m/s | Nishimura et al. (2013) | |
| Shadowgraph imaging | Sneeze | A peak airflow velocity of 4.5 m/s | Tang et al. (2013) | |
| High-speed back-illuminated imaging | Sneeze | A peak velocity of 14 m/s for droplets and 35 m/s for ligaments | Scharfman et al. (2016) | |
| Particle image velocimetry (PIV) | Speech, cough and sneeze | An optimum airflow velocity of 6.25, 15.3, and 15.9 m/s for speech, cough and sneeze, respectively | Han et al. (2021b) | |
| Light-sheet illumination with particle tracking velocimetry (PTV) | Sneeze |
Most droplets had velocities lower than 5 m/s, while the minority had greater than 10 m/s The average droplet velocity is 2–5.4 m/s |
Bahl et al. 2020 | |
| Visualization of droplet spread and trajectories | High-speed videography | Cough and sneeze | The turbulent gas clouds from violent exhalations significantly affect the smaller droplets | Bourouiba et al. (2014) and Bourouiba (2020); Scharfman et al. (2016) |
Apart from the fluid mechanics tools used for studying droplet dynamics related to the SARS-CoV-2 virus, epidemiological tools are available to provide experimental insight into these viral droplets’ molecular structure and composition. Top-tier research facilities and private businesses currently employ three categories of SARS-CoV-2 detection methods: (i) molecular approaches for the detection of viral ribonucleic acid (RNA) sequences, (ii) rapid diagnostic tests (RDT) for the detection of the virus based on antigens or host antibodies, and (iii) imaging methods for the detection of lung changes (Nguyen et al. 2020). We have briefly explained the use of these fluid mechanics and biological tools in providing valuable tools in revealing the transport and evaporation phenomenon below. The subsections below discuss these techniques briefly, including their applications, advantages and current challenges.
Thermal manikins
Thermal manikins have been used in many studies involving thermal flows and the interactions of respiratory droplets, especially those from different subjects in indoor settings (Simova et al. 2021). Thermal manikins allow one to investigate the heat and mass transfer from a prototype human body to the environment. This technique is often inexpensive and rapid compared to human subjects (Simova et al. 2021). Thermal manikins are also helpful in assessing the spread of airborne particles in indoor environments. Their usage can even be extended to analyzing the influence of human body movements on droplet aerodynamics (Cao et al. 2017; Feng et al. 2021; Zhao et al. 2022b). The advantages of thermal manikins lie in their flexibility in controlling study parameters and use in hazardous environments compared with human subjects (Psikuta et al. 2016). The main challenges are using accurate models to simulate real-life events, effective operation of various sensors, and evaluation of thermal manikin results with experimental data (Nayak 2017).
Flow techniques
The main parameters studied on the airborne transport of respiratory droplets include droplet size, initial droplet velocity, duration of droplets remaining buoyant in air, dispersion and travel distance of droplets in the air, droplet evaporation and formation of droplet nuclei. Of these, droplet size measurements showed significant variations in data reported in existing studies, partly due to the measurement method employed and the neglect of droplet evaporation and condensation in some studies (Wei and Li 2016; Xie et al. 2009). The techniques for measuring the size distribution of expectorated droplets include solid impaction, high-speed photography, optical particle counter, aerodynamic particle sizing, and interferometric Mie imaging.
Impaction and microscopy
Solid impaction is one of the oldest techniques for measuring the size and duration of respiratory droplets and droplet nuclei (Duguid 1946). The microscope slide is used with the paper strip, and the celluloid-surfaced slide is held in front of the subject’s mouth for droplet impaction upon solid or liquid surfaces and sampling (Zhang et al. 2015). A microscope slide is then used for the analysis of the collected droplets. This method is limited by the need to insert dyes into the mouth, which can influence the secretion of saliva. Moreover, the technique leads to particle diffusion and splashing, which inevitably distorts the actual particle size. Solid impaction is mainly used to analyze droplets in the supermicron range, e.g., 4–8 µm, because droplets below the dye particle size and the droplets closer to the mouth region cannot be adequately captured.
Xie et al. (2009) used the solid impaction method to measure the particle size distribution for speech and cough droplets. They noticed a significant difference between their recorded droplet sizes of speech and cough droplets and those obtained earlier by Duguid (1946). The authors attributed the considerable variation in results to the disparity in droplet collection methods, i.e., droplets were collected in a box by Xie et al. (2009), and droplet sprays were directed onto a slide by Duguid (1946).
High-speed imaging
High-speed imaging provides a variety of high-speed cameras that may capture quickly moving objects or brief occurrences or phenomena for study and replay in slow motion. The particle size is then estimated by tracing the perimeter of the particle, identifying the region inside the perimeter, and determining the mean diameter from these pictures. Sphericity, as well as several other spray properties, are determined through image analysis. High-speed imaging or photography allowed researchers to overcome these issues, yet this method cannot measure droplet sizes smaller than 10 µm (Chao et al. 2009; Jennison 1941).
Optical techniques
The introduction of optical instruments such as the optical droplet counter (OPC), particle image velocimetry (PIV), and aerodynamic particle sizer (APS) led to a gradual improvement in instrument precision (Zhang et al. 2015). The basis for particle measurements made with optical equipment is that some light is scattered when a particle travels through a beam of light. The core of all such sensors is the detection of this dispersed light. Simply counting the dispersed light pulses that arrive at the detector allows one to determine the particle number. However, using optical scattering methods, more data may be gathered than just numbers. It is possible to quantify particle size by using the connection between scattered light intensity and the dispersed particle size.
Optical particle detection can detect droplet sizes in the submicrometric range (Papineni and Rosenthal 1997). Also, the aerodynamic particle sizer (APS) detects droplets in the 0.5–20 µm range (Asadi et al. 2019; Gregson et al. 2021; Morawska et al. 2009). The laser light scattering technique, which can detect droplets with sizes up to 500 µm, complements the aerodynamic particle sizer by extending the upper limit of its detecting range (20 µm) to larger droplets (Anfinrud et al. 2020; Stadnytskyi et al. 2020). The interferometric Mie imaging (IMI) technique uses de-focused images of droplets instead of focused images to overcome the limitation of high-speed photography.
Apart from the methods above, particle image velocimetry (PIV) has been widely used for measuring droplet velocity and ejection angle (Chao et al. 2009). While the technology showed good consistency in velocity measurements of cough droplets, some researchers observed significant discrepancies in their measurement data for various expiratory events using different measuring devices (Chao et al. 2009; Tang et al. 2009, 2013; Zhu et al. 2006). Inconsistencies reported on cough and sneeze droplet velocities may reflect the difficulty in measuring and analyzing such expiratory events at a specific time because it was not easy to naturally generate cough or sneeze on command compared with other expiratory events like talking and breathing. The inconsistencies in existing measurements by particle image velocimetry also provide a gap for further research and the potential to improve the reliability of the data recording.
High-speed videography
In earlier studies, the droplet spread and trajectory flow were visualized using dyed or flour solutions (Duguid 1946; Zhu et al. 2006). More recently, researchers employed high-speed videography to demonstrate that coughing and sneezing emit turbulent multiphase flows or gas clouds containing pathogen-laden expectorated droplets during violent respiratory events (Bourouiba et al. 2014; Bourouiba 2020; Scharfman et al. 2016). They also measured cough and sneezing durations as 0.3 s and 0.15–0.25 s, respectively. Gupta et al. (2009) reported similar durations for cough events, i.e., 0.3–0.8 s. High–speed videography is an improved technique for capturing and processing dynamic and high-speed scenes using a collection of precisely timed video cameras. To gain insight into the physics selecting the dominant droplet sizes emitted, high-speed videography directly records the emissions of droplets.
Bahl et al. (2020) recently employed a light-sheet illumination technique with particle tracking velocimetry to visualize sneeze droplets and understand the motion of expectorated droplets. This visualization approach allows particles to be located without overlap along the camera axis, resulting in more accurate droplet velocity measurements (Bahl et al. 2020). The light-sheet illumination technique uses a light sheet to illuminate the flow of expectorated droplets in a vertical plane. This 2D vertical plane can be illuminated using a halogen light source and a single high-speed motion camera to capture details of individual expectorated droplets (Bahl et al. 2020).
To conclude, the initial droplet size measurement is hugely affected by data measurement method, evaporation, and condensation. Recent technologies of droplet size measurements include aerodynamic particle sizing (APS), laser light scattering and interferometric Mie imaging (IMI) techniques. Particle image velocimetry (PIV) performs well in measuring droplet velocity. The inconsistencies reported in some studies provide a research gap in improving flow measurement techniques.
Tracer gas techniques
The tracer gas technique is a flow visualization procedure whereby surrogate gases are used to study the transport and trajectory of expectorated human droplets. These surrogate gases include CO2, sulfur hexafluoride (SF6), and ethane, mainly employed to measure airflow direction and flow rate (Jankovic et al. 2022). Several studies have reported using tracer gases to investigate indoor ventilation flows in residential apartments (Kang et al. 2020; Wang et al. 2022). Knibbs et al. (2011) used CO2 as a tracer gas to examine room ventilation's influence on the airborne transmission of pathogens in a large teaching hospital. Recent studies also investigated airflows in ventilated environments using sulfur hexafluoride (SF6) as a tracer gas (Linge et al. 2022; Wang et al. 2022). Luo et al. (2022) used ethane as a tracer gas to investigate respiratory droplet dispersion in a coach bus. The main advantage of tracer gas is the ease of visualization of droplets. However, the drawback is that the technique can only be adopted for visualizing the dispersion of fine droplets in the submicron range, e.g., 0.1–0.7 µm (Luo et al. 2022).
Aerosol-generating techniques
Aerosol-generating techniques, e.g., the collision nebulizer, also known as “pneumatic atomizer,” uses compressed air jets to atomize solutions or suspensions into fine droplets. Cough machines also use pressurized air or mechanical systems to produce a spray modeled as cough, sneeze or speech droplets. Several studies have employed them to investigate the lifetime and dispersion of expectorated viral droplets into the air (Bartels et al. 2022; Doremalen et al. 2020; Li et al. 2022b; Lordly et al. 2022). Aerosol-generating techniques provide the flexibility of producing desired droplet size ranges and controlling the injection velocity and other parameters. They make it possible to study various ventilation systems, disinfection methods, and protective equipment on aerosol clouds in a controlled environment (Lindsley et al. 2013). Limitations of these techniques include the inability to produce a full range of exhaled aerosols, the generation of warm aerosol clouds, and the fact that infectious agents carried on per unit volume of aerosols are often assumed to be the same (Lindsley et al. 2013).
Molecular biology-based diagnostic tools
Early in the COVID-19 pandemic, the whole SARS-CoV-2 genome became immediately available, which sped up the creation of specialized techniques and laboratory procedures for SARS-CoV-2 virus detection (Waris et al. 2020). These molecular biology-based techniques need data such as biomarkers, DNA, RNA, enzymes, and antigens in the target organism (Ilkhani et al. 2021). Several such tools have been used to detect the SARS-CoV-2 virus over the three years of this pandemic, and we have briefly explained the most common ones below.
Nucleic acid-based assays
For SARS-CoV-2 virus detection, the nucleic acid-based assays particularly recognize the ribonucleic acid (RNA) sequences that constitute the virus’s genetic makeup. The current primary approach for identifying COVID-19 is nucleic acid testing, which can accurately identify minute quantities of SARS-CoV-2 and are unlikely to give a false-negative result for the virus (CDC 2021). Reverse transcription polymerase chain reaction (RT-PCR) or other amplification techniques are used in nucleic acid amplification tests (NAATs) to find viral RNA (Arena et al. 2021).
Antibody-based assays
Another method for detecting COVID-19 is to test for specific antibodies in the blood, such as the immunoglobulin M (IgM) antibody against SARS-CoV-2, as it is well-accepted that IgM plays an essential role in the acute infection phase (Huang et al. 2020). The main drawback of immunoassays that look for antibodies to SARS-CoV-2 is that they cannot be used to diagnose infection; instead, they can only show that the immune system has already responded to the virus (Fulawka and Kuzan 2022). As a result, the ability to detect anti-SARS-CoV-2 immunoglobulins, also known as SARS-CoV-2 antibodies, can help the researcher identify sick patients and those who have recovered and can safely be released from isolation (Li et al. 2020c).
Polymerase chain reaction
The polymerase chain reaction (PCR) test for COVID-19 is a molecular test that examines your upper respiratory samples for genetic material (ribonucleic acid or RNA) of SARS-CoV-2, the virus that causes COVID-19. A positive COVID-19 PCR test indicates the presence of SARS-CoV-2. A negative result might suggest that the sample contained no virus or defective viral genetic material to identify. Real-time reverse transcription-PCR (RT-PCR) is one of the commercially available procedures for COVID-19 diagnosis (Ilkhani et al. 2021). The RT-PCR is widely used in SARS-CoV-2 detection because of its simplicity, high sensitivity, high accessibility and the fact that the tests can be quantitative (Wang et al. 2020b; Xiao et al. 2020).
Loop-mediated isothermal amplification
The loop-mediated isothermal amplification (LAMP), which amplifies DNA using DNA polymerase under isothermal conditions without the need for complicated lab equipment, is a straightforward, quick, selective, and effective viral detection approach (Zhao et al. 2020b). Recently, the reverse transcription LAMP (RT-LAMP) technology has been used for COVID-19 detection in several studies (Ali et al. 2022; Freire-Paspuel and Garcia-Bereguiain 2021). The amplification of viral DNA occurs in both PCR and LAMP techniques, and this process causes a change in color in the monitoring test. Because LAMP does not necessitate using such costly reagents and laboratory equipment as PCR, it is more economically efficient to perform (Cai et al. 2008).
Field-effect transistor-based assay
Field-effect transistor (FET)-based biosensors are one of the established methodologies among a large spectrum of biosensors due to their advantages, such as speedy, low cost, and easy detection (Ilkhani et al. 2021). FET-based biosensors are categorized into many types depending on the gate voltage application approach, design, gate material, and channel area. Seo et al. (2022) created a COVID-19 FET sensor that detects the SARS-CoV-2 virus in clinical samples by connecting the SARS-CoV-2 spike antibody to a graphene sheet that serves as the sensing region. These researchers emphasized that their FET sensor requires no model preprocessing or labeling.
To conclude, accurately measuring the initial droplet size distribution in indoor and outdoor environments is challenging. The inconsistencies reported in cough and sneeze velocities could reflect the complexity of measuring and analyzing such expiratory events at a specific time. Contradictions reported in the literature using various experimental methods show a research gap for further studies. Adequate knowledge of the size range of droplets exhaled from expiratory activities and their nuclei sizes before inhalation by susceptible individuals is vital to scientists and health workers in designing an appropriate intervention. In comparison, the molecular biology-based tools provide a valuable tool for the accurate and sensitive detection of the SARS-CoV-2 virus via their molecular properties. The fluid mechanics techniques offer insight into multiphase flow viral transmission.
Computational methods for modeling droplet transport
Early methods
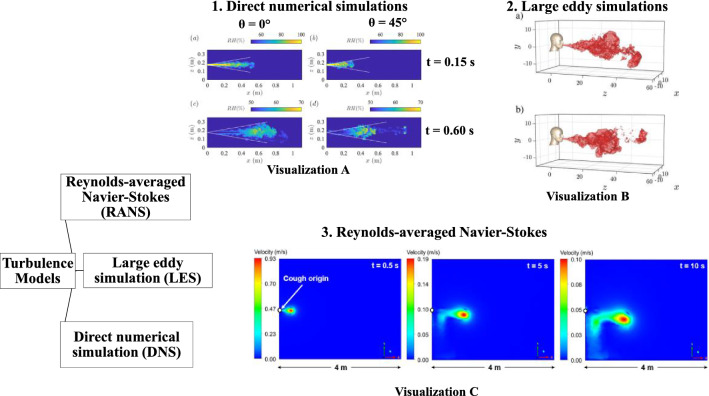
Since experimental techniques have practical limitations, many researchers performed numerical simulations to study the distribution of human expiratory droplets and the transmission of associated airborne pathogens throughout the last decade. This section presents an overview of previous computational methods used for studying droplet transport, emphasizing gas-phase modeling and discrete phase modeling of respiratory droplet dynamics in air. Figure 2 summarizes the recent years’ turbulence models used for numerical simulation. The turbulence models for gas-phase modeling of SARS-CoV-2 transport are presented in Table 2.
Fig. 2.
Computational turbulence models and profiles of cough turbulent jet/puff: a images extracted for direct numerical simulation (Li et al. 2022a), b large eddy simulation (Liu et al. 2021a), and c Reynolds-averaged Navier–Stokes model (Quiñones et al. 2022). (1) The direct numerical simulation gives a more precise presentation of the chaotic nature of the jet and puff. It also captures the detached chaotic vortexes in visualization A. (2) The large eddy simulation also captures more fluctuations of the cough jet in visualization B and the detached vortexes. (3) Finally, the Reynolds-averaged Navier–Stokes captures the cough jet’s less chaotic nature than the large eddy simulation in visualization C. Reprinted with permission of Elsevier and AIP publishing from Quiñones et al. (2022) and Liu et al. (2021a), respectively
Table 2.
Turbulence models that have been used to study the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). RANS: Reynolds-Averaged Navier–Stokes model
| Objective | Turbulence model | Main result | Investigator |
|---|---|---|---|
| To research vulnerable and infected SARS-CoV-2 people in general public locations | Monte Carlo modeling with large eddy simulation | The general turbulence strength and length scale inside the interior environment and the characteristic turbulent characteristics within the considered geometry define the inhaled aerosol cloud dilution time scale | Vuorinen et al. (2020) |
| To model the transitory movement and evaporation of SARS-CoV-2 virus-infected cough droplets | The Shear Stress Transport (SST) turbulence model based on RANS | The secondary flows formed by the recirculation of the two virtual people will be made more complex by the ambient wind | Feng et al. (2020) |
| To predict the overall direct maximum reach of tiny cough droplets by turbulence | Large eddy simulation | Micro-droplets are transported to greater distances by the turbulent gas puff | Pendar and Páscoa (2020) |
| To examine the buildup of respiratory droplets in the cold, humid air | Direct numerical simulation | The supersaturated vapor field favors droplet production, trapped and transferred within the humid puff | Ng et al. (2020) |
| To examine the fluid dynamics of a transient turbulent jet/puff with pathogen-carrying evaporating droplets as buoyancy | Direct numerical simulation | The model can simulate phase transition thermodynamics in a turbulent jet/plume | Diwan et al. (2020) |
| To study the vortex ring structure of a turbulent gas cloud | Direct numerical simulation |
High relative humidities also play a significant role in extending droplet lifetime The results support Bourouiba (2020) and co-worker’s experiment on turbulent cloud dynamics |
Diwan et al. (2020) and Chong et al. (2021) |
| To comprehensively understand virus transmission of sneeze droplets inside small indoor spaces | The realizable k-ε turbulence model based on RANS | The horizontal providing air conditioner increases the velocity of the droplets, causing them to spread wider | Wu et al. (2021) |
| To estimate aerosol transmission under a variety of situations | Large eddy simulation |
The tiny droplets of less than 100 µm stay suspended within the fast-moving-puff, transported to farther distances |
Liu et al. (2021b) |
| To study SARS-CoV-2 aerosol spread in a grocery store | Renormalization group (RNG) k-ε turbulence model based on RANS | Airflow and temperature drive viral aerosol dispersion within the store | Zhang et al. (2022) |
| To investigate how SARS-CoV-2 virus particles spread in a passenger car | Eulerian method coupled with the k-ε turbulence model based on RANS | SARS-CoV-2 virus particles can infect occupants in a poorly ventilated car within 6.38 min | Sarhan et al. (2022) |
The transmission of respiratory pathogens often involves a range of complex phenomena, such as liquid sheet fragmentation, air–droplet interaction, turbulence, droplet–droplet interaction, droplet evaporation, and deposition (Mittal et al. 2020). Analytical mathematical models were developed in early studies to predict human respiratory droplets’ transport. Wells (1934) was one of the first to study the evaporation and transport of exhaled droplets using analytical mathematical methods. The Wells model formed the basis of most evaporation and dispersion models. After over seventy years of use, researchers from the University of Hong Kong recently revisited the Wells model and made several modifications to improve the accuracy of the model (Xie et al. 2007). However, their tendency to overestimate droplet dispersion has contributed to the emergence and recent adoption of computational fluid dynamics (CFD) models in droplet transport simulation due to the higher accuracies of the latter. Some studies adopted mathematical models and incorporated them into computational fluid dynamics simulation tools. This technique is widely employed because it can effectively model droplet evaporation, droplet motion, turbulence, and particle tracking. Generally, the two-phase system is used to simulate the computational analysis of respiratory droplets in the air. In this scenario, respiratory droplets are modeled as discrete particles flowing in gas or continuous-phase medium. Below we provide a detailed discussion of the application of the discrete and gas-phase methods in existing studies.
Discrete phase modeling
The main approaches for modeling particle tracks include the Eulerian and the Lagrangian methods (Chao and Wan 2006; Zhang and Chen 2006, 2007). The Eulerian technique uses conservation equations of particles similar to the Navier–Stokes equations to model particles flowing through a control volume. One distinct feature of the Eulerian method is that the fluid and particle phases are treated as a continuum and modeled as continuous phases. The Lagrangian method treats the fluid phase and the particle phase differently. The particle phase is treated as a discrete phase, but the fluid phase is modeled as a continuous phase since it is considered a continuum. Individual discrete phase particles are tracked across the flow field by solving the motion equations of different forces acting on them.
The diffusion of dense particles in an enclosed space can be accurately captured using the Eulerian approach (Holmberg and Chen 2003; Murakami et al. 1992; Shimada et al. 1996; Zhao et al. 2004), while their pathway and transport can be tracked effectively using the Lagrangian technique (Beghein et al. 2005; Lu et al. 1996; Wei and Li 2015; Yan et al. 2019; Zhang and Chen 2006; Zhao et al. 2004). The main models used for simulating particle dispersion include the Lagrangian discrete random walk model (Liu et al. 2017; Wei and Li 2015) and two Eulerian models, i.e., the mixture model and the drift flux model (Chen et al. 2006; Gao and Niu 2007; Holmberg and Li 1998; Lai and Cheng 2007; Zhao and Guan 2007). The effectiveness of the abovementioned models for the simulation of particles in enclosed space has been comparatively studied (Zhang and Chen 2007; Zhao et al. 2008). The comparison showed that each method had its strength and weakness under steady-state conditions. However, it was noted that the Lagrangian method could predict the unsteady-state particle concentration distribution better than the Eulerian method.
Gas-phase modeling of droplet transport
As exhaled droplet is suspended in the air, various forces caused by the airflow and gravity play a decisive role in particle motions. As a result, it is critical to formulate a precise mathematical model of the airflow field. Table 2 briefly illustrates some of the airfield turbulence models used to study the transport and spread of human expiratory droplets over the years with their respective engineering applications. Generally, there are three computational approaches to model airflow, i.e., direct numerical simulation (DNS), large eddy simulation (LES), and Reynolds-averaged Navier–Stokes (RANS) (Fig. 2). The direct numerical simulation approach can simulate the entire flow field information by directly solving the three-dimensional unsteady Navier–Stokes equations without any turbulence model. In the large eddy simulation approach, small-scale turbulent structures are modeled by establishing sub-grid scale models, while large-scale turbulent systems are directly calculated. Finally, RANS can predict the characteristics of the mean flow field based on Reynolds’s time-average equation.
RANS and large eddy simulation have much higher computational efficiency than direct numerical simulation. In contrast to large eddy simulation and direct numerical simulation, RANS’s mesh size limitation is not strict. Thus, RANS is the most popular approach for tackling complex turbulence (Chao and Wan 2006; Feng et al. 2020; Ji et al. 2018; Li et al. 2018). For the calculation of the turbulent airflow, a comparison of five frequently used turbulence models was conducted by Chen (1995), i.e., the standard k-ε model, the low-Reynolds-number k-ε model, the two-scale k-ε model, the two-layer k-ε model and the renormalization group (RNG) k-ε turbulence model. And the results reveal that the renormalization group k-ε turbulence model is more precise than other turbulence models for predicting indoor airflow. Other studies have employed the renormalization group k-ε turbulence model in their RANS modeling of turbulence (Chao and Wan 2006; Li et al. 2018; Tian et al. 2007). The results were comparable to large eddy simulation, direct numerical simulation and experimental setups.
Furthermore, a zero-equation turbulence model was established by Chen and Xu (1998). Zhao et al. (2008) applied the renormalization k-ε model to investigate the turbulent flow in ventilated rooms. The results from the zero-equation turbulence model and renormalization k-ε model show that they can accurately predict air velocities in indoor airflow fields (Chen and Xu 1998; Zhao et al. 2008).
Figure 2 depicts the visualization-A for a cough jet at different injection angles and time stamps for direct numerical simulation (Li et al. 2022a), visualization-B for a turbulent puff of cough for two cases for large eddy simulation (LES) (Liu et al. 2021a) and visualization-C for a cough jet at different time stamps for RANS (Quiñones et al. 2022). We can confirm that these turbulence models display a distinct human cough jet/puff characteristic from the visualizations, whereas the RANS model captures fewer fluctuations than the large eddy simulation. It can be seen that the direct numerical simulation gives a more precise presentation of the chaotic nature of the jet and puff. The large eddy simulation model is an intermediate between the direct numerical simulation and RANS and captures many scalar field fluctuations. Typically, the direct numerical and large eddy simulations capture the detached chaotic vortexes in visualizations A and B, respectively. The application of either of these turbulence models lies in the computation cost and level of output the modeler desires. These observations can guide us in developing accurate models for predicting exhaled turbulent jets during coughs or sneezes.
Due to the limitation of RANS for the explicit estimates of turbulent motion, few numerical studies employed high-resolution large eddy simulation to simulate the transport of airborne viruses (Berrouk et al. 2010; Liu and You 2012; Tian et al. 2007; Vuorinen et al. 2020). Direct numerical simulation can resolve the turbulent mixing process’s small scales, although it is computationally the most expensive among the three methods. By fully coupling the temperature and humidity field surrounding the respiratory droplets to the Navier–Stokes equation, direct numerical simulation is sufficient to provide a much more accurate prediction of respiratory droplets’ transport and trajectories in a turbulent jet (Chong et al. 2021; Diwan et al. 2020; Ng et al. 2020).
Wei and Li (2015) discovered that exhaled particles could significantly experience widespread dispersion due to turbulent cough jet airflows. Recent studies have also revealed that violent exhalations are characterized by turbulent multiphase puffs of buoyant gas droplets (Bourouiba 2020, 2021). This observation demonstrated that resolving the turbulent eddies is critical in understanding how these puffs affect droplet transport and lifetime.
However, most earlier investigations were based on the Reynolds-averaged Navier–Stokes (RANS) technique, which does not explicitly resolve the turbulent motion. Turbulence effects on the mean flow field and aerosol dispersion have been modeled using RANS equations with significant errors utilizing approximate turbulence closure models (e.g., k-epsilon models). Although the performances of some models, like the renormalization group k-ε model, have been recognized, there is still a deficit in resolving turbulent eddies using RANS models (Chao and Wan 2006; Li et al. 2018; Tian et al. 2007). Typically, turbulent motion dominates indoor ventilation airflows. At the same time, compared to the impact of turbulent motion, the contribution of mean flow on the dispersion and dilution of an aerosol cloud may be minor. Hence, using a model that explicitly resolves most of the influential parts of a turbulent cloud is vital. The large eddy simulation is beneficial since it requires less computational resources than the direct numerical simulation. However, there have been few applications of large eddy simulation in respiratory virus transmission, which provides a research gap for further studies.
Factors controlling the transport and evaporation of exhaled droplets
The evaporation and dispersion of human exhaled droplets are highly influenced by the initial characteristics of expiratory particles, initial particle size distribution (PSD), ambient temperature, relative humidity, and ambient flow. The applications of various droplet evaporation models used to investigate the influencing factors in droplet spread and evaporation have been explained below (Table 3).
Table 3.
Droplet evaporation models used to study the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
| Country | Composition | Study parameter (s) | Model | Main Conclusions | Investigator |
|---|---|---|---|---|---|
| USA | NaCl | Ambient temperature, relative humidity, ambient flow | Single droplet: continuous random walk (CRW) model | Medium-sized (50 µm) droplets are very sensitive to the study parameters | Wang et al. (2020a) |
| USA | Pure water | Ambient temperature, relative humidity | One-dimensional droplet model | The droplet lifetime estimation is hugely dependent on the ambient relative humidity | Chen (2020) |
| Portugal | Newtonian fluid | Droplet size, injection angle, velocity, and environmental factors | A fully coupled eulerian–lagrangian model | Micro-droplets are transported to greater distances by turbulence and ambient conditions | Pendar and Páscoa (2020) |
| China | Pure water | Initial droplet size, ambient temperature, relative humidity | A new single droplet evaporation model | Droplet lifetime and transport distance get shortened with a decrease in initial droplet size and relative humidity but an increase in ambient temperature | Yin et al. (2022) |
| China | Pure water | Initial droplet size, ambient temperature, relative humidity, partition | A fully coupled eulerian–lagrangian model | Partitions effectively inhibit the spread of aerosol particles to another diner under quiescent indoor conditions | Zhao et al. (2022a) |
Environmental and turbulence influence on maximum horizontal spread
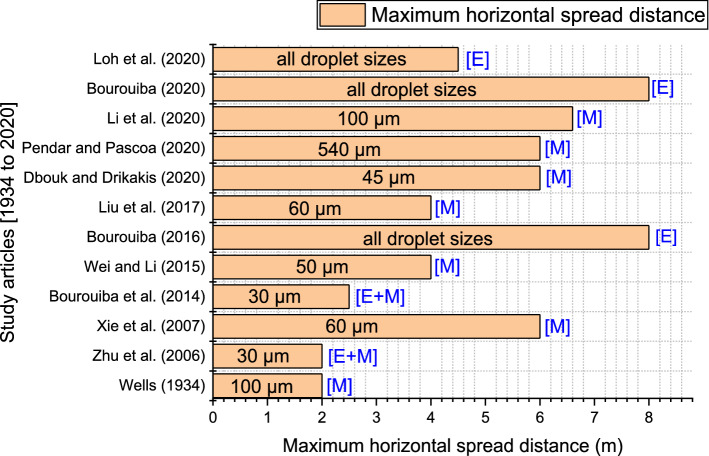
To examine the effect of numerous variables on droplet evaporation and dispersion/spread, researchers frequently utilize assessment indices such as “evaporation duration/time” and “critical distance.” This study defines the “critical distance” as the peak horizontal distance of infection caused by short-range transmission (Fig. 3). The “evaporation time” is the time it takes for a droplet to evaporate into a droplet nucleus fully. Based on the transmission paths of expiratory particles, control methods for respiratory viral infection can be divided into close contact at less than 1.5 m and long-distance at more than 1.5 m precautionary approaches. Consequently, when coughing and inhaling in this scenario, the 1.5 m distance is considered the average cutoff distance for droplet transmission (the peak traveling distance of large droplets) (Zhang et al. 2020).
Fig. 3.
Maximum horizontal distance of respiratory droplets. Note that E depicts an Experimental study, and M represents a Modeling numerical or mathematical approach. We note that a classical investigation by Wells (1934) predicted a maximum horizontal spread distance of 2 m. In contrast, new research has revealed that, when we consider turbulence and environmental factors, respiratory droplets can spread to even spaces beyond 6 m
The world health organization (WHO) and center for disease control and prevention (CDC) guidelines of 1 m and 2 m do not consider various factors such as ambient conditions, the nature of the expiratory event, and whether the event occurred indoors or outdoors. These guidelines were based on classical models proposed by Wells (1934). Moreover, prior research did not regard these expectorated droplets encapsulated in the turbulent puff (Wells 1934; Xie et al. 2007; Zhu et al. 2006). However, after revisiting the Wells’ evaporation curve and considering the effects of initial exhaled droplet speed and ambient conditions, Xie et al. (2007) recorded a maximum spread distance of 6 m. Dbouk and Drikakis (2020) and Pendar and Páscoa (2020) recorded similar maximum transmission distances as Xie et al. (2007) using the Euler–Lagrangian model. Wei and Li (2015) and Liu et al. (2017) also obtained similar results considering the effects of turbulence and evaporation on medium droplets. An experimental investigation by Loh et al. (2020) recorded a maximum cough distance of 4.5 m using a high-flow nasal cannula (HFNC). Although Li et al. (2020a) investigated a tropical environmental condition, they showed that 100 µm could spread to a distance of about 6.6 m.
The role of turbulence in violent exhalations has been extensively studied, and its role in droplet dispersion and spread cannot be ignored (Bourouiba et al. 2014; Bourouiba 2016, 2020, 2021). Bourouiba (2020) realized even greater maximum extremes because of violent, turbulent effects propelling the exhaled droplet to further distances. These turbulent puffs affect the evaporation rates and give the evaporating droplets additional momentum. A new study by Liu et al. (2021b) expounded on the chaotic growth of the ensuing droplet clouds in the air. They have discovered that droplet outliers occasionally occur as peel-off regions of the turbulent puff and need to be considered when considering the social distance rules for transmission risk evaluation. These fast-moving, peel-off sections of the puff can carry infectious droplets to longer distances. Based on an average ejected volume of 3 L, they determined a high-risk infection zone from 2.2 to 2.8 m, a medium-risk region from 3.4 to 3.6 m, and a low-risk area beyond 3.6 m.
Figure 3 below details the maximum horizontal distances captured by several investigations (Bourouiba et al. 2014; Bourouiba 2016, 2020; Dbouk and Drikakis 2020; Li et al. 2020a; Liu et al. 2017; Loh et al. 2020; Pendar and Páscoa 2020; Wei and Li 2015; Wells 1934; Xie et al. 2007; Zhu et al. 2006). Given recent developments, most results have proved that the current social distancing guidelines are insufficient. Although, a few inconsistencies reported by the various studies could still be evaluated in terms of the models used and the conditions under which such investigations were conducted.
We found out that the role of turbulence in violent exhalation events like coughing and sneezing cannot be ignored since the turbulent puff plays a significant part in droplet dispersion and spread. Also, occasional fast-moving droplet detachments occur, overshooting the turbulent puff to extreme distances. New studies should explore violent exhalations as a puff instead of simplifying them as jets to explore how ambient conditions influence the different droplet sizes encapsulated in the cloud.
Effects of ventilation on droplet transport and evaporation
A body of research supports the theory that most infections occur indoors compared to outdoor settings (Bulfone et al. 2021; Morawska et al. 2020; Nishiura et al. 2020). However, significant research gaps exist in this conclusion due to insufficient data and state-of-art analysis to bolster this hypothesis. Regardless of reasonable literature to support whether indoor or outdoor contagion was dominant, we still find the study on indoor infections significant (Chen et al. 2021).
When analyzing indoor contagions, the analyses of factors like ventilation airflow, relative humidity, droplet temperature, and ambient air influence on expectorated droplets’ dispersion are critical (Redrow et al. 2011; Wei and Li 2016). Redrow et al. (2011) are among the few to study the effects of environmental factors, chemical constituents and droplet temperature on complete sputum droplets. They found that droplets evaporated faster under lower relative humidity than at higher relative humidity. Wei and Li (2016) reviewed the literature on respiratory droplet transport and diffusion in the indoor environment. They found that human activities like locomotion and door opening could affect the droplets’ fate by transporting them to long-range areas (Wei and Li 2016). The human thermal plume also contributes to droplet dispersion in quiescent and indoor unidirectional airflow environments (Sun et al. 2021; Liu et al. 2022). This phenomenon shows that the human anatomy also interferes with indoor ambient flow dynamics, transporting the exhaled droplets (Khosronejad et al. 2020). A study in a poorly ventilated restaurant found that insufficient ventilation played a role in this outbreak of COVID-19 (Li et al. 2021).
The air conditioning or ventilation systems in public areas can either help control the pandemic or aggravate disease spread, depending on whether they are operated effectively (Jarvis 2020; Lu et al. 2020; Tellier et al. 2019; Valsamatzi-Panagiotou and Penchovsky 2022; Bhattacharyya et al. 2020). A study used computational fluid dynamics to explore aerodynamic dispersion and surface deposition (Abuhegazy et al. 2020). The room’s aerosol distribution was strongly influenced by the air conditioning layout and source location (Abuhegazy et al. 2020). Another study found that mixing ventilation could accelerate evaporation rates compared to displacement ventilation in indoor environments (Ji et al. 2018). Motamedi et al. (2022) also used a validated Eulerian–Lagrangian CFD to investigate the impact of different ventilation strategies like the cross, single, mechanical and no-ventilation systems, and found the single ventilation (SV) and no ventilation systems had the highest infection probability. A potential solution to reduce the risk of airborne transmission between sedentary passengers in public areas or transportation is personalized ventilation, which is effective in reducing the risk of airborne infection between occupants in close quarters with a modest amount of clean air supply (Liu et al. 2021c).
Can evaporation decrease droplet concentration and viral infectivity? A few studies have acknowledged a reduction in the viral titer of liquid droplet size during ambient flows and its aid in controlling the spread of infection, especially during high temperature and low humidity conditions (Dbouk and Drikakis 2020; Mao et al. 2020). In contrast, recent studies discovered that evaporation does not kill or deactivate the viruses in the droplets; thus, they remain airborne for a long time (Balachandar et al. 2020; Liu et al. 2021b). In addition, developing a mechanistic model that examines the combined effects of ambient temperature and relative humidity on SARS-CoV-2 viral stability is essential.
Moreover, several studies have adopted the Eulerian–Lagrangian technique to explore the effects of outdoor ventilation on droplet transport. For example, Dbouk and Drikakis (2020) studied how saliva droplet fate, spread, and evaporation are strongly affected by wind speed at 20 °C ambient temperature and 50% relative humidity. They discovered that droplets move less than 2 m in still air but travel up to 6 m when the velocity is increased from 4 to 15 km/h, with some droplets evaporating (Dbouk and Drikakis 2020). Another study also found that, although medical and non-medical face masks limited the spreading of expectorated droplets in an indoor environment, outdoor settings with just a unidirectional mild breeze could disperse the droplets quickly to the surrounding environment (Khosronejad et al. 2020). These events show that windy conditions provide a climate conducive to aerosol infections. Hence people should pay close attention to personal protection when engaging in outdoor activities.
Ventilation is essential in controlling infectious particles’ spread in indoor and outdoor spaces. Poor ventilation will lead to significant risks in crowded places, while sufficient ventilation can decrease the concentration of virus particles in enclosed spaces. Gaps exist, as indoor contagion studies have not fully accounted for the actual physical processes. Most existing studies oversimplify the complexity of realistic indoor environments.
Effect of ambient temperature and relative humidity on droplet transport and evaporation
Since COVID-19 was declared a global pandemic, there have been several theories surrounding whether weather changes could beneficially or adversely influence the fate of the virus transmission (Carlson et al. 2020; Lipsitch 2021). The growing cases reported worldwide have highlighted poorly understood environmental influences on this novel coronavirus.
According to recent research by Liu et al. (2021b), dry ambient conditions could enhance the quantity of airborne infectious bioaerosols more than four times due to delayed droplet settling under heightened evaporation. This event leaves a lot of potential viral droplet nuclei in the air, and ambient flows could easily transport these infectious bioaerosols to distances beyond 2 m (6 feet). This analysis could also explain why viral infections are more common or last longer during winter in cold regions when most people spend much of their time in central-heated rooms (Chen et al. 2021; Morawska et al. 2020). The relative humidity in indoor environments is essential, mainly during winter when a central heating system is used to warm enclosed spaces (Božič and Kanduč 2021). The central heating unit dries the cold air coming into the room, decreasing the relative humidity and increasing expectorated droplets’ evaporation rate (Božič and Kanduč 2021). This dry ambient condition can be a source of prolonged airborne respiratory infections. In contrast, humid ambient conditions can lower potentially airborne infectious bioaerosols due to enhanced droplet settling (Liu et al. 2021b).
A theoretical model by Liu et al. (2017) investigated ambient humidity, droplet composition, and turbulence effects on cough droplets’ evaporation and dispersion. They found that medium-sized droplets (e.g., 60 μm) were more considerably influenced by ambient relative humidity than larger droplets (greater or equal to 100 μm) as well as smaller droplets (Liu et al. 2017). They also found that droplets evaporated rapidly in dry air conditions, leading to a lengthy suspension of aerosol particles. Another study investigated the influence of ambient environmental temperature (0–40 °C), ambient relative humidity (0–90%), and ambient flow on the transmission of human speech droplets (Zhao et al. 2020a). They found that the droplets traveled three times farther under low temperatures and humid conditions. In addition, there was an increased aerosolization rate under high temperature and low humidity conditions. Another study collaborated on the above research showing that the medium-sized droplets (i.e., 30 μm–50 μm) used in their simulation were prone to environmental parameters such as ambient temperature, relative humidity, and ambient flows (Wang et al. 2020a). Studies have also shown that the thermal stratification and inhomogeneous humidity field could weaken or impede the evaporation of these medium droplets (Li et al. 2018; Liu et al. 2019).
We can infer from the above studies that environmental factors influence diverse droplet sizes differently. For example, with high relative humidity, the time required for the evaporation of a single droplet of varied sizes is more significant than at low relative humidity (Božič and Kanduč 2021). When dispersed in turbulent buoyant jets formed by coughing, small (20–30 μm) and large (100 μm) droplets are indifferent to relative humidity (Wei and Li 2015). On the other hand, relative humidity considerably influences medium-sized (50–60 μm) droplets (Liu et al. 2017; Wei and Li 2015). Hence, extensive insight into how the wide range of human respiratory droplets is affected is paramount to controlling the transmission of contagious infections.
Compared to the influence of relative humidity on droplet dynamics and evaporation, the role of ambient temperature drew less attention. Chen (2020) studied the potential effects of droplet size, ambient temperature, and relative humidity on the spread of COVID-19 through exhaled droplets. A one-dimensional evaporation model was formulated to predict the evaporation lifespan of droplets in the air. However, the droplet evaporation model used in this study was relatively simple, regarding the droplet size as a fixed value, ignoring the influence of droplet temperature, diameter, and velocity on water evaporation during droplet movement. The study also discovered a specific relative humidity threshold at which raising the ambient temperature did not consistently reduce droplet longevity but caused a rise, indicating that violating this critical relative humidity value led to longer droplet lifetimes (Chen 2020). Other studies by Chaudhuri et al. (2020) and Yin et al. (2022) also confirmed that droplet lifetime and transport distance get shortened with a decrease in initial droplet size and relative humidity but an increase in ambient temperature. More investigations are needed to fully understand how these threshold values help reduce the infectiousness of contagious diseases.
We need a detailed understanding of why medium-sized droplets tend to be more influenced by environmental factors like relative humidity and turbulence. Additionally, understanding what role these medium-sized droplets play in the droplet transport mechanism can aid us in developing non-pharmaceutical interventions to reduce the aerosolization of these droplets, which extends their spread distance. The collective influence of ambient temperature and relative humidity is still not comprehensive. Both computational and experimental studies can be performed across a wide range of temperatures and relative humidity to verify this threshold relative humidity and how it contributes to virus inactivation.
Effect of ambient temperature and relative humidity on virus stability
The effect of ambient temperature and relative humidity on the viral stability of SARS-CoV-2 in aerosols and surfaces has rarely been investigated (Table 4). The ability of respiratory viruses to propagate is regulated by virus stability under environmental conditions, which is influenced by the virus’s shape, whether or not it is enveloped, proteins, and other chemical elements (Schuit et al. 2020; Sharma et al. 2020; Vejerano and Marr 2018). One widely held misconception is that airborne viruses are unprotected particles that float through the air when virions are ejected from the host. Nevertheless, virions are encapsulated in a respiratory fluid.
Table 4.
Effects of environmental conditions on the stability of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
| Study focus | Duration of experiment | Room temperature, relative humidity | Conclusion | Investigator |
|---|---|---|---|---|
| SARS-CoV-2 aerosol and surface stability compared to SARS-CoV-1 |
Aerosol (3 h) Surface (7 days) |
Aerosol (21–23 °C; 65%) Surface (21–23 °C; 40%) |
SARS-CoV-2 was stable in the same way as SARS-CoV-1 was The aerosol stability of SARS-CoV-2 is about 3 h Surface stability (4 h to 72 h) |
Doremalen et al. (2020) |
| SARS-CoV-2 stability in various environmental settings | 7 days | 22 °C and at 65% |
Stability on porous surfaces (30 min to 2 days) Stability on non-porous surfaces (2 to 4 days) |
Chin et al. (2020) |
| SARS-CoV-2 inactivation on surfaces is accelerated by increasing temperature and relative humidity | 48 h | 24–35 °C; 20–40% | When humidity or temperature was increased, SARS-CoV-2 degraded faster | Biryukov et al. (2020) |
| SARS-CoV-2 aerosol survivability in artificial saliva and tissue culture media (TCM) at medium and high humidity was tested | 90 min | 19–22 °C; medium RH (40–60%) and high RH (68–88%) | The virus was more stable in tissue culture media at medium relative humidity than at higher relative humidity, but the converse was found in artificial saliva | Smither et al. (2020) |
| The effect of temperature on the persistence of SARS-CoV-2 on common surfaces | 28 days | 20–40 °C; 50% | Half-lives between 1.7 and 2.7 days at 20 °C, reducing to a few hours when the temperature was increased to 40 °C | Riddell et al. (2020) |
| SARS-CoV-2 persistence on critical personal protection equipment | 21 days | 20 °C; 35–50% | SARS-CoV-2 can remain viable for up to 2 weeks at room temperature on Tyvek | Kasloff et al. (2021) |
| The effect of temperature, humidity, and simulated sunlight on SARS-CoV-2 infectivity in aerosols | 20–60 min | 10–20 °C; 20–70% | Simulated sunlight and temperature have a more significant influence on decay than humidity across the tested range | Dabisch et al. (2021) |
| Effects of temperature and humidity on SARS-CoV-2 and other enveloped viruses’ inactivation | 4 days | 10–27 °C; 40–70% | SARS-CoV-2 has the best chance of surviving at low temperatures and relative humidity | Morris et al. (2021) |
Abbreviations: Relative humidity (RH); Room temperature (RT)
The viability of a virus is strongly related to its stability in diverse mediums such as aerosols, droplets, and on surfaces. It should also be mentioned that in scientific literature, the terminology used ranges between survival, presence, viability, stability, and persistence (Fernández-Raga et al. 2021). Ambient temperature and relative humidity can affect the risk of transmission by affecting the survival and persistence of respiratory viruses in droplets or on fomites. The chemical microenvironment in which viruses are embedded plays a vital role in determining their infectivity and stability (Oswin et al. 2022). Therefore, research on how environmental factors like ambient temperature and relative humidity influence the constituents of this microenvironment can provide knowledge on the infectivity loss mechanisms of the viruses.
A recent review article by Aboubakr et al. (2021) reported that coronaviruses (CoVs) had extended lifetimes in lower temperature and relative humidity environments. There is also a hypothesis concerning long-range carriers and transmission of SARS-CoV-2 via cold storage foods (Han et al. 2021a). These hypotheses showed the persistence of virus stability under cold temperature conditions. Although higher temperatures have favored viruses’ inactivation on fomites and aerosols, relative humidity’s role is often debated (Chin et al. 2020; Dabisch et al. 2021). At low relative humidity, the effects of relative humidity on viral inactivation, human immunology, and droplet settling may all combine, raising the chance of transmission (Morris et al. 2021). At high relative humidity, there may be reduced inactivation and droplet settling but better human immune responses; thus, the total impact on diffusion is unclear (Morris et al. 2021).
In addition, depending on the genetic makeup, different viruses may be distinctly affected by ambient temperature and relative humidity. For instance, although higher temperatures are often linked with decreased influenza virus stability, many researchers have revealed that the connection between influenza virus stability and relative humidity might be U-shaped (Leung 2021). A new investigation by Morris et al. (2021) on SARS-CoV-2 and other enveloped viruses combined the effects of ambient temperature and relative humidity using a mechanistic model and found the results to conform with the U-shaped influence of relative humidity on the influenza virus. Like other encapsulated viruses, the environmental stability of SARS-CoV-2 is considered to fluctuate as a function of the temperature and humidity (Biryukov et al. 2020; Matson et al. 2020). However, the combined influence of these two parameters is unknown.
Understanding the stability of viruses on surfaces or fomites is also essential to developing appropriate interventions. Although, this transmission mode has been controversial (Choi et al. 2021). Two highly cited studies by Chin et al. (2020) and Doremalen et al. (2020) have revealed the resilience of SARS-CoV-2 viruses on fomites for an extended duration under favorable environmental conditions. They also discovered that SARS-CoV-2 was less stable on printing and tissue papers, treated wood, cardboard, and copper but was more stable on smooth surfaces, including outer layers of plastic, stainless steel, glass, banknotes, and surgical masks (Chin et al. 2020; Doremalen et al. 2020; Liu et al. 2021d). Studies have also reported reduced viability of the SARS-CoV-2 virus on cotton compared to other materials (Kasloff et al. 2021; Liu et al. 2021d; Riddell et al. 2020).
However, there are currently conflicting findings on the survivability of SARS-CoV-2 on stainless steel, with data ranging from 3 to 14 days at ambient temperature and 3 to 21 days at room temperature for plastics (Chin et al. 2020; Doremalen et al. 2020; Kasloff et al. 2021). Riddell et al. (2020) even detected viable SARS-CoV-2 viruses on fomites after 28 days (20 °C), although the virus survived for only 24 h (40 °C) on some surfaces. SARS-CoV-2 viral inactivation on surfaces at higher temperatures has also been documented in several studies, as well as the efficiency of commonly used disinfectants in reducing fomite contamination (Biryukov et al. 2021; Chan et al. 2020; Chin et al. 2020; Riddell et al. 2020).
Various studies have been conducted to establish how long coronaviruses remain infectious in aerosols under different environmental conditions. According to current research, SARS-CoV-2 is relatively stable in aerosols under conditions similar to those expected in climate-controlled indoor environments (Doremalen et al. 2020; Fears et al. 2020; Schuit et al. 2020). A groundbreaking investigation on the aerosol and surface stability of the SARS-CoV-2 virus using a Collison nebulizer reveals that the virus can remain infectious and active in aerosols for up to 3 h (relative humidity of 65%, 21–23 °C) (Doremalen et al. 2020). Smither et al. (2020) hypothesized that aerosolized media containing the virus at variable relative humidity might alter viral stability. The virus was more stable in tissue culture media at medium relative humidity than at higher relative humidity, while the opposite was observed in artificial saliva. According to the author’s knowledge, there are no definitive data on the combined effective effects of ambient temperature and relative humidity in the inactivation of the SARS-CoV-2 virus in aerosols. The data on SARS-CoV-2 stability in aerosols can help calibrate numerical models and advise risk assessments and control actions.
Although human coronaviruses (CoVs) are shown to survive shorter under higher temperatures and higher relative humidity, a mechanistic model to study the combined influencing factors like temperature and relative humidity can provide scientific guidelines for developing efficient virus inactivation interventions in climatic-controlled environments (Aboubakr et al. 2021; Morris et al. 2021). Another potential research area is the relationship between SARS-CoV-2 virus seasonality spread and climatic conditions. There are currently no conclusive reports on how seasonality decline or surge of COVID-19 cases can be linked to climatic conditions across various parts of the world (Jamil et al. 2020). Table 4 summarizes some studies on the effects of ambient temperature and RH on virus survivability in aerosols and on surfaces.
Although these data should be interpreted with caution due to the sampling procedures used and other contributing variables, they imply that environmental circumstances significantly influence the duration of stability of these viruses. These contradictory results also allow further analysis into factors causing the variances. Although higher temperatures have favored viruses’ inactivation on fomites and aerosols, relative humidity’s influence is still not comprehensive.
Effect of initial droplet size distribution on droplet dispersion
Human respiratory droplet sizes range from a few micrometers to several thousand micrometers (Asadi et al. 2019; Morawska et al. 2009; Papineni and Rosenthal 1997; Xie et al. 2007; Zhang et al. 2015). The droplet threshold size helps us group these droplets as large droplets or bioaerosols (Wells 1934). Historically, the WHO recommended a 5 µm (World Health Organization 2014) critical droplet size, while some studies proposed a 10 µm (Liu et al. 2017; Xie et al. 2007). Recent literature supports using 50–100 µm or 100 µm as the threshold particle size for droplet or aerosol classification (Bourouiba 2020; Prather et al. 2020; Zhang et al. 2020). However, these critical sizes do not have a fixed value due to the continual fragmentation of large droplets into aerosols of diverse droplet size ranges making their analysis quite complicated. A conclusive resolution of the droplet threshold diameter can help develop accurate interventions and buttress public health policies.
We need to define the initial droplet size, in which we usually use the expectorated droplet diameter located just outside the mouth or nasal cavity of the human subject (Liu et al. 2017). These droplet sizes are preferred, and more reliable in the analysis as environmental factors showed no influence compared to interim diameters (i.e., located at the extensive droplet exposure) and final sizes (i.e., droplet nuclei). Dbouk and Drikakis (2020) pointed out that the maximum saliva droplet size is more critical in viral transmission investigation than the droplet diameter. The is 10% of the droplets, more diminutive than the initial droplet diameter and vital in expectorated droplets’ dispersion and evaporation time (Li et al. 2020a). For instance, a 50 μm droplet evaporates in 12.5 s, while a 4-μm droplet evaporates in 0.2 s (Li et al. 2020a).
Coughing and sneezing produce a turbulent multiphase cloud with polydisperse droplet sizes (Bourouiba et al. 2014; Bourouiba 2020). Since diverse droplet sizes are formed due to mucous fragmentation in the respiratory tract, the location within the respiratory tract plays a significant role in their formation (Jarvis 2020). Scharfman et al. (2016) refuted the idea that respiratory droplets were formed before ejection, first revealing that liquid droplet breakage occurs outside the respiratory system during vigorous exhalations. They also demonstrated that the ligaments trapped in the turbulent puff had an essential role in defining the ultimate droplet size of the ejection.
Several literature works have been analyzed, and significant discrepancies in values have been noticed in the droplet size distributions (Asadi et al. 2019; Chao et al. 2009; Duguid 1946; Morawska 2006; Stadnytskyi et al. 2020; Xie et al. 2009). Another study by Han et al. (2013) reported similar variations in previous sneeze droplet size distributions. These disparities in droplet size distributions are attributed to equipment or measurement error and evaporation and condensation effects (Xie et al. 2009). This is critical to controlling the infection in enclosed public areas like restaurants, classrooms, and buses because asymptomatic patients are likely to spread the disease via this route (Valsamatzi-Panagiotou and Penchovsky 2022).
The subject of initial droplet size distribution is not yet thoroughly exhausted, and more sophisticated instruments and measurement technologies can be used to quantify the distributions better. The vast range in size of aerosolized pathogen-carrying droplets and droplet nuclei from submicrometer to millimeter is vital in evaluating the size-dependent filtering performance of face masks. Therefore, an in-depth study in this area will provide insight for informed decisions like facemask design technology.
Effect of non-volatile components like the SARS-CoV-2 virus on droplet evaporation rate
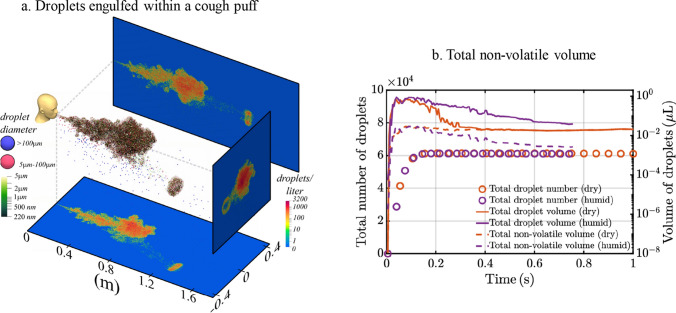
Human saliva is 99.5% water by volume, but it contains various organic and inorganic chemicals such as salt, proteins, peptides, mucins, enzymes, and other substances (Liu and Duan 2012). Despite having a total mass of less than 0.1 mg, an infected SARS-CoV-2 patient is expected to contain between 10 and 100 billion virions during peak infection (Sender et al. 2021). The non-volatile volume of viral droplets is indicated in (Fig. 4b) for both dry (dashed orange line) and humid (dashed purple line) conditions. These viral droplets are engulfed within the turbulent puff of the cough (Liu et al. 2021b) (Fig. 4a). The nearly constant volume of non-volatiles confirms complete evaporation in dry conditions (dash orange line) and its conformity with the overall volume (orange line) (Fig. 4b). Additionally, research shows that the potentially contagious viral components do not deactivate after evaporation.
Fig. 4.
a Entrapped droplets within a turbulent cough puff after 0.54 s time stamp. The ejected puff and droplets are at 35 °C, and the ambient temperature is at 20 °C. The large droplets overshoot the puff while smaller droplets remain afloat for extended durations. b The non-volatile volume of viral droplets is indicated for both dry (dashed orange line) and humid (dashed purple line) conditions. These viral droplets are engulfed within the turbulent puff of the cough. The nearly constant volume of non-volatiles confirms complete evaporation in dry conditions (dash orange line) and its conformity with the overall volume (orange line). Additionally, research shows that the potentially contagious viral components do not deactivate after evaporation. Reprinted with permission from Liu et al. (2021b).
Copyright © 2021, the Author(s), licensed under a Creative Commons Attribution (CC BY) license
The Spalding mass transfer number (), governed by the difference in vapor pressure on the droplet surface and that of ambient air, is directly proportional to the evaporation rate. This change can be affected based on the droplet constituents, thereby slowing the evaporation (Redrow et al. 2011). When insoluble components like virus particles are located in an evaporating saliva droplet, the water vapor concentration drops, thereby reducing the evaporation rate of the exhaled sputum (de Oliveira et al. 2021). Droplet evaporation is governed by droplet size, solute type and initial volume percentage, ambient temperature, moisture content, non-ideal effects of solute interactions inside the droplet, and internal droplet structure (Rezaei and Netz 2021).
Therefore, in modeling or transport mechanisms study on these droplets, we need to consider how these components affect the fate and survivability of the expectorated droplets in the air. The water content does not completely dry out in respiratory droplets, as the relative humidity determines the droplet’s final size (Liu et al. 2017; Nicas et al. 2010). A study explored expiratory droplets’ physio-chemical characteristics and found that different relative humidity influenced these droplets’ morphology, concentration and phase (Vejerano and Marr 2018). They concluded that these physiological properties could significantly affect the evaporation and diffusion of droplets or aerosols (Vejerano and Marr 2018). Since salt is one of the main compositions of mucosalivary fluid, most studies have explored its effects on droplet evaporation (Redrow et al. 2011).
Recent research by Trancossi et al. (2021) proposes looking at the thermo-electro-biochemical processes across viral cell membranes when interacting with their surroundings and how they affect virus survival and evolution. This knowledge can help develop the virus’s survival and mutation inhibitory control mechanisms. Besides, the virus’s viability within the droplet is highly associated with moisture content and temperature (Metz and Finn 2015; Weber and Stilianakis 2008). As a result, the evaporation of virus-laden droplets governs the droplet’s mobility and lifespan in the air and the viral aerosol’s survival rate and infectivity (Zuo et al. 2013). However, the research on droplet fate had been simplified to droplet nuclei in most numerical investigations, and droplet size variation and evaporation during transmission were neglected (Chen and Zhao 2010; Feng et al. 2020; Yan et al. 2019). Droplet evaporation is essential in dispersion, particularly for medium-size droplets (e.g., 50 µm) (Nicas et al. 2010; Wei and Li 2015; Wells 1934).
Hence, a complete understanding of how the internal constituents of viral droplets affect their evaporation and dispersion is paramount to developing an appropriate intervention. The composition of respiratory droplets is still a topic under research. We need to explore more complex saliva compositions from a SARS-CoV-2-infected person to understand how they influence droplet dynamics and evaporation.
Effect of evaporation on droplet trajectory of distinct droplet sizes
Although larger droplets are heavily influenced by gravity and fall to the ground nearby, evaporation of these droplets into bioaerosols can prolong their trajectory and spreading zone (Wells 1934) (Fig. 5). The evaporation process is affected by the binary diffusion coefficient and variation between the droplet surface’s saturated vapor pressure and ambient gas’s vapor pressure (Li et al. 2020a). This process reduces droplets’ size, transforming the droplet fate modification (Li et al. 2020a). Environmental factors like ambient temperature, relative humidity, and ambient flow also play a significant role in evaporation, although substantial research gaps exist (Netz and Eaton 2020). We have noticed from the literature that the combination of higher temperatures and lower relative humidity can increase the formation of bioaerosols by fast-tracking the evaporation rate (Li et al. 2020a; Wells 1934; Xie et al. 2007). However, much more study is needed to evaluate the influences of environmental settings on evaporation rates (Mittal et al. 2020). Moreover, high relative humidity conditions or the locally moist and warm atmosphere within the turbulent gas cloud of coughs can delay the evaporation of droplets trapped within it, extending their lifetimes from seconds to minutes (Bourouiba 2020).
Fig. 5.
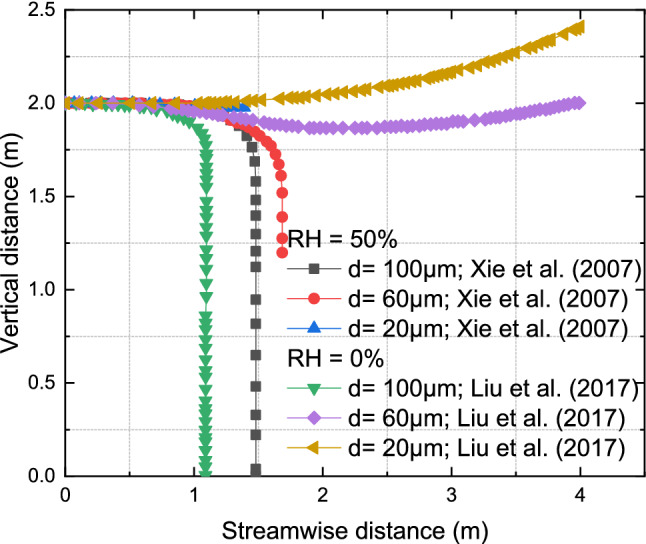
Effects of evaporation on spread distance of distinct droplets, i.e., 100 μm, 60 μm and 20 μm, under two relative humidities, i.e., RH = 0% and 50%, and initial salt mass fraction of 0.9%; Data were extracted from Liu et al. (2017) and Xie et al. (2007). Liu et al. (2017) reported that droplets greater than or equal to 80 µm, e.g., 100 µm in this case, always deposited around a horizontal distance of 1 m from the mouth, irrespective of the change in relative humidity, i.e., 0% and 90% in their case. Xie et al. (2007) also reported similar observations. We found that the 100 μm droplets of both studies did not travel beyond 1.5 m under both relative humidities. In contrast, the 60 μm droplet traveled close to 1.8 m at a relative humidity of 50% and beyond the computational domain of 4 m for 0%. For the small droplet of 20 μm, Xie et al. (2007) reported droplet only traveled 1.5 m at 50% relative humidity. In contrast, Liu et al. (2017) found droplets traveling beyond the 4 m computational domain at 0% and 90% relative humidity
The turbulent gas cloud also significantly influences droplets’ trajectory, evaporation rate, and dispersion (Dbouk and Drikakis 2020; Liu et al. 2017). According to recent research, large droplets with diameters greater than 100 μm can overreach the turbulent puff, allowing its transportation to greater distances and swiftly settle out of the puff (Liu et al. 2021b). The smaller droplets below 5 μm, or between 5 μm and 100 μm, follow the puff and remain afloat embedded in it.
Figure 5 shows the droplet flight dynamics of distinct droplet sizes (i.e., 100 μm, 60 μm, and 20 μm) at different ambient relative humidity and temperatures (Liu et al. 2017; Xie et al. 2007). The study by Xie et al. (2007) used a droplet temperature of 33 °C, an ambient temperature of 20 °C, RH of 50% and an initial velocity of 10 m/s. In comparison, Liu et al. (2017) used an ambient temperature of 25 °C, RH of 0%, an initial velocity of 10 m/s and an initial vertical height of the cough jet of 2 m (above the floor). The study also assumed a quiescent environment without turbulence (Liu et al. 2017).
Liu et al. (2017) reported that droplets greater than or equal to 80 µm (e.g., 100 µm) always deposited around a horizontal distance of 1 m from the mouth, irrespective of the change in relative humidity (i.e., 0% and 90%). Xie et al. (2007) also reported similar observations, stating that for the relative humidity range of 30–70% used in their analysis for the dynamics of droplets, the horizontal distance (about 1.5 m) remains unchanged for droplets greater than or equal to 80 µm (e.g., 100 µm). We believe that the slight variation in the horizontal distances (about 1 m or 1.5 m) reported by the two authors might be due to varied models and assumptions. We found that the 100 μm droplets of both studies did not travel beyond 1.5 m under both relative humidity ranges. In contrast, the 60 μm droplet traveled close to 1.8 m at a relative humidity of 50% and beyond the computational domain of 4 m for 0% relative humidity.
For the small droplet of 20 μm, Xie et al. (2007) reported droplet only traveled 1.5 m at a relative humidity of 50%. In contrast, Liu et al. (2017) found droplets traveling beyond the 4 m computational domain at 0% and 90% relative humidity. Thus, this analysis of the medium and small droplets (e.g., 60 μm and 20 μm) shows that evaporation under distinct relative humidities can significantly alter the dispersion range of the viral droplet. Besides, the contradictory results of the dynamics of medium and small droplets at distinct relative humidities provide a research gap for further analysis.
The effects of viral respiratory droplets’ initial non-volatile, e.g., SARS-CoV-2 virus particles, constituents on droplet nuclei formation are not well recognized. As a result, most CFD simulations resort to modeling saliva droplets as pure water, while others add salt, i.e., NaCl, as a non-volatile solute. The analysis of the medium and small droplets, e.g., 60 μm and 20 μm, in the studies above shows that the dispersion range of the viral droplet can be significantly altered by evaporation under distinct relative humidities.
Effect of SARS-CoV-2 virus mutation on transmission and control
The epidemiology of a disease is altered by the continual mutation of viruses, which creates new variants that are ultimately more contagious than the previous strains. Much work is being put into creating a vaccine to combat COVID-19, but novel coronaviruses like SARS-CoV-2 also quickly evolve because they spread similarly to other viruses. As a result, there is always a risk that the created antibodies will not remain effective over time and will be unable to stop the spiked protein from becoming more efficient at binding to cells. Therefore, it is critical to prevent the spread of infection. We must investigate the fluid dynamic nature of these variants of concern (VOCs) and their molecular properties to develop an effective intervention.
A study used a mathematical model to investigate the effectiveness of vaccination and limited physical distance and found that the possible dominance of a variant of concern in the future is mainly determined by its infectivity, which may not always result in a more significant public health burden (Le Rutte et al. 2022). They also demonstrate that highly immune-evading variants that become dominant would probably necessitate other methods to prevent stress on healthcare systems, such as improved physical distance precautions, innovative therapies, and second-generation vaccinations (Le Rutte et al. 2022). Another research that examined the environmental stability of the “Ancestral” strain of SARS-CoV-2 virus and other variants of concern (VOCs), such as the Alpha, Beta, Delta, and Omicron on surfaces made of plastic and human skin, revealed that the variants of concern had survival durations that were more than two times longer than those of the “original” strain and that they maintained infectivity for more than 16 h on the skin surface (Hirose et al. 2022). The research also revealed that the Omicron variant had the most robust environmental stability among variants of concern. Huang et al. (2022) also investigated the airborne transmission of the Delta variant in an auditorium and found that the viral emission rates were 30 times higher than those of the ancestral lineage.
The possibility that vaccinations will not be effective in preventing developing pandemic illnesses is a significant obstacle. A new vaccination is needed for every new strain (Das et al. 2021). A safe and effective immunization must be designed and mass produced over months or years. Hence, a comprehensive understanding of the fluid dynamics of these viral droplets is fundamental to designing non-pharmaceutical interventions to curb the spread of the infection.
Most mutations do not result in clinically significant alterations for particular infections. However, sporadic mutations might increase the virus’ infectiousness, worsen its illness, escape the protective effects of therapeutics or vaccinations, or alter the sensitivity or specificity of diagnostic tests. When mutations occur in regions where probes and primers may bind, the SARS-CoV-2 variants, particularly the variants of concern, may decrease the detection sensitivity of the real-time reverse transcription-PCR (RT-PCR)-based diagnostic methods (Jindal et al. 2021). When vaccinations for the variants of concern (VOCs) are unproductive or unobtainable, non-pharmaceutical interventions (NPIs) are among the most effective approaches to combat the COVID-19 epidemic.
Perspective
The research and scientific community are unclear on whether direct, indirect, droplet or aerosol transmission modalities play a significant role in the COVID-19 pandemic. One of the difficulties is that we need to employ a complex analysis to confidently ascertain whether an individual was infected through a particular droplet transmission route after the individual gets infected.
There is still no agreement in the research community on the definition of a droplet transport route based on the critical droplet size. Some studies use 100 μm (Wells 1934), while others take 10 μm (Liu et al. 2017) or 5 μm (WHO 2020) as the threshold droplet size. Knowledge of this can help in face design technology and ventilation control methods.
We need a detailed understanding of why medium-sized droplets tend to be more influenced by environmental factors like relative humidity and turbulence. Also, we found out that the role of turbulence in violent exhalation events like coughing and sneezing cannot be ignored since the turbulent puff plays a significant part in droplet dispersion and spread. Also, occasional fast-moving droplet detachments occur, overshooting the turbulent puff to extreme distances (Liu et al. 2021b).
Can evaporation decrease droplet concentration and viral infectivity? Unfortunately, few studies have acknowledged a reduction in the viral titer of liquid droplet size during ambient flows (Dbouk and Drikakis 2020) and its aid in controlling the spread of infection, especially during high temperature and low humidity conditions (Mao et al. 2020). In contrast, new studies (Balachandar et al. 2020; Liu et al. 2021b) discovered that evaporation does not deactivate the viruses in the droplets; thus, they remain airborne for a long time. In addition, a significant study is needed to develop a mechanistic model that examines the combined effects of ambient temperature and RH on SARS-CoV-2 viral stability.
The effects of viral respiratory droplets’ initial non-volatile, e.g., SARS-CoV-2 virus, constituents on droplet nuclei formation are not well recognized. As a result, most CFD simulations resort to modeling saliva droplets as pure water, while others add salt (i.e., NaCl) as a non-volatile solute. The efficacy of ventilation, especially the personalized ventilation (PV) systems, needs to be explored in detail since it has shown some practical applications in reducing the spread of COVID-19 to susceptible persons (Xu et al. 2022). Mixing and displacement ventilation applications in various indoor environments are still ongoing research.
It is now widely acknowledged that, aside from the mask, ventilation is the most effective way of reducing indoor airborne transmission. In the face of current variants of concern of the SARS-CoV-2 virus, we recommend non-pharmaceutical interventions like handwashing, disinfection, social distancing, mask-wearing, and sufficient ventilation in enclosed places. Since some of these variants of concern can evade vaccines and therapeutics, we must implement fluid dynamics interventions jointly with epidemiological therapeutics.
Conclusion
The world continues to explore the various side effects and effectiveness of the COVID-19 vaccine as one way to curtail the spread of the pandemic. Hence, a detailed understanding of the respiratory droplet spread distance, incorporating accurate multiphase flow physics in modeling, is critical to implementing efficient methodologies in curbing the COVID-19 pandemic. Here we reviewed the effects of turbulence, ventilation, environmental conditions, initial droplet size distribution and non-volatile components like virus particles on droplet evaporation, horizontal distance spread, dispersion, and virus stability on surfaces and in aerosols. The current results show that medium-sized droplets, e.g., 50 µm, are sensitive to relative humidity. The medium-sized droplets experienced delayed evaporation at high relative humidity and increased airborne lifetime and travel distance. In contrast, these droplets quickly shrink to droplet nuclei and follow the cough jet at low relative humidity. Virus inactivation is realized at higher temperatures, and the presence of viruses in aerosols impedes the evaporation rate. Also, we provided recommendations and discussed knowledge gaps to guide future research.
We also observed that higher relative humidities resulted in extended droplet lifetimes for droplets less than or equal to 60 µm, leading to lower infectious droplet nuclei formation due to slower evaporation and more rapid droplet settling. Studies reported contradictory results regarding the medium and small droplets, e.g., 60 μm and 20 μm. They showed that evaporation under distinct relative humidities could significantly alter the dispersion range of the viral droplets. In contrast, dry ambient conditions resulted in rapid evaporation and reduced droplet settling, enhancing more viral droplet nuclei formation. Evaporation of respiratory droplets does not dilute the virus’s viral concentration, but viruses remain within the droplet nuclei for extended durations. The presence of non-volatile components like virus particles could retard evaporation. Different droplet sizes, especially medium, e.g., 60 μm, are significantly affected by relative humidity. Virus inactivation is realized at higher temperatures beyond 40 °C, although the role of relative humidity is often debated. These results have expounded our knowledge regarding the fluid mechanics of infectious droplets and elaborated some scientific standpoints regarding public health guidelines like the 1 m and 2 m social distancing standards of WHO and CDC, respectively. Therefore, we recommend revisiting these guidelines based on scientific knowledge from the new developments.
Acknowledgements
We would like to acknowledge the support from the National Key Research and Development Program of China (2022YFB3803600), the national natural science foundation of China (52176163), the aerospace science funding (2019ZB070002) and the Fundamental Research Funds for the Central Universities. The corresponding author (Z.-F. Zhou) as a “Tang Scholar” greatly appreciates the support from the Cyrus Tang Foundation.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhi-Fu Zhou, Email: zfzhou@mail.xjtu.edu.cn.
Eric Lichtfouse, Email: eric.lichtfouse@gmail.com.
References
- Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg Dis. 2021;68:296–312. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhegazy M, Talaat K, Anderoglu O, Poroseva SV (2020) Numerical investigation of aerosol transport in a classroom with relevance to COVID-19. Phys Fluids 14 [DOI] [PMC free article] [PubMed]
- Akter S, Zakia MA, Mofijur M, et al. SARS-CoV-2 variants and environmental effects of lockdowns, masks and vaccination: a review. Environ Chem Lett. 2022;20:141–152. doi: 10.1007/s10311-021-01323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali WR, Zahra A, Rasheed H, et al (2022) Detection of SARS-CoV-2 by RT-LAMP assay in human COVID-19 patients. In: 2022 19th international Bhurban conference on applied sciences and technology (IBCAST). pp 381–385
- Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382:2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena F, Pollini S, Rossolini GM, Margaglione M. Summary of the available molecular methods for detection of SARS-CoV-2 during the ongoing pandemic. Int J Mol Sci. 2021;22:1298. doi: 10.3390/ijms22031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S, Wexler AS, Cappa CD, et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019 doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S, Bouvier N, Wexler AS, Ristenpart WD. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl P, de Silva CM, Chughtai AA, et al. An experimental framework to capture the flow dynamics of droplets expelled by a sneeze. Exp Fluids. 2020;61:176. doi: 10.1007/s00348-020-03008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar S, Zaleski S, Soldati A, et al. Host-to-host airborne transmission as a multiphase flow problem for science-based social distance guidelines. Int J Multiph Flow. 2020;132:103439. doi: 10.1016/j.ijmultiphaseflow.2020.103439. [DOI] [Google Scholar]
- Bartels J, Estill CF, Chen I-C, Neu D. Laboratory study of physical barrier efficiency for worker protection against SARS-CoV-2 while standing or sitting. Aerosol Sci Technol. 2022;56:295–303. doi: 10.1080/02786826.2021.2020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghein C, Jiang Y, Chen QY. Using large eddy simulation to study particle motions in a room. Indoor Air. 2005;15:281–290. doi: 10.1111/j.1600-0668.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- Berrouk AS, Lai ACK, Cheung ACT, Wong SL. Experimental measurements and large eddy simulation of expiratory droplet dispersion in a mechanically ventilated enclosure with thermal effects. Build Environ. 2010;45:371–379. doi: 10.1016/j.buildenv.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Dey K, Paul AR, Biswas R. A novel CFD analysis to minimize the spread of COVID-19 virus in hospital isolation room. Chaos, Solitons Fractals. 2020;139:110294. doi: 10.1016/j.chaos.2020.110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J, Boydston JA, Dunning RA, et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on Surfaces. mSphere. 2020;5:e0044120. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J, Boydston JA, Dunning RA, et al. SARS-CoV-2 is rapidly inactivated at high temperature. Environ Chem Lett. 2021;19:1773–1777. doi: 10.1007/s10311-021-01187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. The fluid dynamics of disease transmission. Annu Rev Fluid Mech. 2021;53:473–508. doi: 10.1146/annurev-fluid-060220-113712. [DOI] [Google Scholar]
- Bourouiba L, Dehandschoewercker E, Bush JWM. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- Bourouiba L (2016) A Sneeze. 10.1056/NEJMicm1501197
- Božič A, Kanduč M. Relative humidity in droplet and airborne transmission of disease. J Biol Phys. 2021 doi: 10.1007/s10867-020-09562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone TC, Malekinejad M, Rutherford GW, Razani N. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis. 2021;223:550–561. doi: 10.1093/infdis/jiaa742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Lou G, Yang J, et al. Development and evaluation of real-time loop-mediated isothermal amplification for hepatitis B virus DNA quantification: a new tool for HBV management. J Clin Virol. 2008;41:270–276. doi: 10.1016/j.jcv.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Cao S-J, Cen D, Zhang W, Feng Z. Study on the impacts of human walking on indoor particles dispersion using momentum theory method. Build Environ. 2017;126:195–206. doi: 10.1016/j.buildenv.2017.10.001. [DOI] [Google Scholar]
- Carlson CJ, Gomez ACR, Bansal S, Ryan SJ. Misconceptions about weather and seasonality must not misguide COVID-19 response. Nat Commun. 2020;11:4312. doi: 10.1038/s41467-020-18150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2021) Nucleic acid amplification tests (NAATs). In: Centers for disease control and prevention. https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html. Accessed 23 Jan 2023
- CDC (2023) COVID data tracker weekly review. In: Centers for disease control and prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/01062023.html. Accessed 30 Jan 2023
- Chan K-H, Sridhar S, Zhang RR, et al. Factors affecting stability and infectivity of SARS-CoV-2. J Hosp Infect. 2020;106:226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CYH, Wan MP. A study of the dispersion of expiratory aerosols in unidirectional downward and ceiling-return type airflows using a multiphase approach. Indoor Air. 2006;16:296–312. doi: 10.1111/j.1600-0668.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- Chao CYH, Wan MP, Morawska L, et al. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, Basu S, Kabi P, et al. Modeling ambient temperature and relative humidity sensitivity of respiratory droplets and their role in Covid-19 outbreaks. Phys Fluids. 2020;32:063309. doi: 10.1063/5.0015984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. Comparison of different k-ε models for indoor air flow computations. Numer Heat Transf Part b Fundam. 1995;28:353–369. doi: 10.1080/10407799508928838. [DOI] [Google Scholar]
- Chen L-D. Effects of ambient temperature and humidity on droplet lifetime—a perspective of exhalation sneeze droplets with COVID-19 virus transmission. Int J Hyg Environ Health. 2020;229:113568. doi: 10.1016/j.ijheh.2020.113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu W. A zero-equation turbulence model for indoor airflow simulation. Energy Build. 1998;28:137–144. doi: 10.1016/S0378-7788(98)00020-6. [DOI] [Google Scholar]
- Chen C, Zhao B. Some questions on dispersion of human exhaled droplets in ventilation room: answers from numerical investigation. Indoor Air. 2010 doi: 10.1111/j.1600-0668.2009.00626.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhao B. Makeshift hospitals for COVID-19 patients: where health-care workers and patients need sufficient ventilation for more protection. J Hosp Infect. 2020;105:98–99. doi: 10.1016/j.jhin.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Yu SCM, Lai ACK. Modeling particle distribution and deposition in indoor environments with a new drift–flux model. Atmos Environ. 2006;40:357–367. doi: 10.1016/j.atmosenv.2005.09.044. [DOI] [Google Scholar]
- Chen B, Jia P, Han J. Role of indoor aerosols for COVID-19 viral transmission: a review. Environ Chem Lett. 2021;19:1953–1970. doi: 10.1007/s10311-020-01174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Chatterjee P, Coppin JD, et al. Current understanding of the surface contamination and contact transmission of SARS-CoV-2 in healthcare settings. Environ Chem Lett. 2021;19:1935–1944. doi: 10.1007/s10311-021-01186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KL, Ng CS, Hori N, et al. Extended lifetime of respiratory droplets in a turbulent vapor puff and its implications on airborne disease transmission. Phys Rev Lett. 2021;126:034502. doi: 10.1103/PhysRevLett.126.034502. [DOI] [PubMed] [Google Scholar]
- Dabisch P, Schuit M, Herzog A, et al. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci Technol. 2021;55:142–153. doi: 10.1080/02786826.2020.1829536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ahmed R, Akhtar S, et al. An overview of basic molecular biology of SARS-CoV-2 and current COVID-19 prevention strategies. Gene Rep. 2021;23:101122. doi: 10.1016/j.genrep.2021.101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk T, Drikakis D. On coughing and airborne droplet transmission to humans. Phys Fluids. 2020;32:053310. doi: 10.1063/5.0011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira PM, Mesquita LCC, Gkantonas S, et al. Evolution of spray and aerosol from respiratory releases: theoretical estimates for insight on viral transmission. Proc R Soc Math Phys Eng Sci. 2021;477:20200584. doi: 10.1098/rspa.2020.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan SS, Ravichandran S, Govindarajan R, Narasimha R. Understanding transmission dynamics of COVID-19-type infections by direct numerical simulations of cough/sneeze flows. Trans Indian Natl Acad Eng. 2020;5:255–261. doi: 10.1007/s41403-020-00106-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremalen N van, Bushmaker T, Morris DH, et al (2020) Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med [DOI] [PMC free article] [PubMed]
- Drossinos Y, Stilianakis NI. What aerosol physics tells us about airborne pathogen transmission. Aerosol Sci Technol. 2020;54:639–643. doi: 10.1080/02786826.2020.1751055. [DOI] [Google Scholar]
- Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol Infect. 1946;44:471–479. doi: 10.1017/S0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil T, Alam I, Gojobori T, Duarte CM. No evidence for temperature-dependence of the COVID-19 epidemic. Front Public Health. 2020;8:436. doi: 10.3389/fpubh.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CS, Chong KL, Yang R, et al (2020) Growth of respiratory droplets in cold and humid air. Infectious Diseases (except HIV/AIDS)
- Simova I, Angelova RA, Markov D, et al (2021) Thermal Manikins – General Features and Applications. In: 2021 6th international symposium on environment-friendly energies and applications (EFEA). pp 1–5
- Hirose R, Itoh Y, Ikegaya H, et al (2022) Differences in environmental stability among SARS-CoV-2 variants of concern: omicron has higher stability. Microbiology [DOI] [PMC free article] [PubMed]
- Fears AC, Klimstra WB, Duprex P, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerging Infect Dis J CDC. 2020 doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Marchal T, Sperry T, Yi H. Influence of wind and relative humidity on the social distancing effectiveness to prevent COVID-19 airborne transmission: A numerical study. J Aerosol Sci. 2020;147:105585. doi: 10.1016/j.jaerosci.2020.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zeng F, Li R, et al. Influence of manikin movement on temperature stratification in a displacement ventilated room. Energy Build. 2021;234:110700. doi: 10.1016/j.enbuild.2020.110700. [DOI] [Google Scholar]
- Fernández-Raga M, Díaz-Marugán L, García Escolano M, et al. SARS-CoV-2 viability under different meteorological conditions, surfaces, fluids and transmission between animals. Environ Res. 2021;192:110293. doi: 10.1016/j.envres.2020.110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Paspuel B, Garcia-Bereguiain MA. Low clinical performance of the Isopollo COVID-19 detection kit (M Monitor, South Korea) for RT-LAMP SARS-CoV-2 diagnosis: a call for action against low quality products for developing countries. Int J Infect Dis. 2021;104:303–305. doi: 10.1016/j.ijid.2020.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulawka L, Kuzan A. Molecular Diagnostic Tools against SARS-CoV-2 in Poland in 2022. Biomedicines. 2022;10:3259. doi: 10.3390/biomedicines10123259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao NP, Niu JL. Modeling particle dispersion and deposition in indoor environments. Atmos Environ. 2007;41:3862–3876. doi: 10.1016/j.atmosenv.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome-related coronavirus : classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson FKA, Watson NA, Orton CM, et al. Comparing aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci Technol. 2021;55:681–691. doi: 10.1080/02786826.2021.1883544. [DOI] [Google Scholar]
- Gupta JK, Lin C-H, Chen Q. Flow dynamics and characterization of a cough. Indoor Air. 2009;19:517–525. doi: 10.1111/j.1600-0668.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- Han ZY, Weng WG, Huang QY. Characterizations of particle size distribution of the droplets exhaled by sneeze. J R Soc Interface. 2013;10:20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhang X, He S, Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2021;19:5–16. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Ooka R, Kikumoto H, et al. Measurements of exhaled airflow velocity through human coughs using particle image velocimetry. Build Environ. 2021;202:108020. doi: 10.1016/j.buildenv.2021.108020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S, Chen Q. Air flow and particle control with different ventilation systems in a classroom. Indoor Air. 2003;13:200–204. doi: 10.1034/j.1600-0668.2003.00186.x. [DOI] [PubMed] [Google Scholar]
- Holmberg S, Li Y. Modelling of the indoor environment: particle dispersion and deposition. Indoor Air. 1998;8:113–122. doi: 10.1111/j.1600-0668.1998.t01-2-00006.x. [DOI] [Google Scholar]
- Huang C, Wen T, Shi F-J, et al. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega. 2020;5:12550–12556. doi: 10.1021/acsomega.0c01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hao T, Liu X, et al. Airborne transmission of the Delta variant of SARS-CoV-2 in an auditorium. Build Environ. 2022;219:109212. doi: 10.1016/j.buildenv.2022.109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilkhani H, Hedayat N, Farhad S. Novel approaches for rapid detection of COVID-19 during the pandemic: a review. Analy Biochem. 2021;634:114362. doi: 10.1016/j.ab.2021.114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic A, Gennaro G, Chaudhary G, et al. Tracer gas techniques for airflow characterization in double skin facades. Build Environ. 2022;212:108803. doi: 10.1016/j.buildenv.2022.108803. [DOI] [Google Scholar]
- Jarvis MC. Aerosol transmission of SARS-CoV-2: physical principles and implications. Front Public Health. 2020;8:590041. doi: 10.3389/fpubh.2020.590041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison MW (1941) Atomizing of mouth and nose secretions into the air as revealed by high-speed photography
- Ji Y, Qian H, Ye J, Zheng X. The impact of ambient humidity on the evaporation and dispersion of exhaled breathing droplets: a numerical investigation. J Aerosol Sci. 2018;115:164–172. doi: 10.1016/j.jaerosci.2017.10.009. [DOI] [Google Scholar]
- Jindal H, Jain S, Suvvari TK, et al. False-negative RT-PCR findings and double mutant variant as factors of an overwhelming second wave of COVID-19 in India: an emerging global health disaster. SN Compr Clin Med. 2021;3:2383–2388. doi: 10.1007/s42399-021-01059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Wei J, Yuan J, et al. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann Intern Med. 2020;173:974–980. doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasloff SB, Leung A, Strong JE, et al. Stability of SARS-CoV-2 on critical personal protective equipment. Sci Rep. 2021;11:984. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosronejad A, Santoni C, Flora K, et al. Fluid dynamics simulations show that facial masks can suppress the spread of COVID-19 in indoor environments. AIP Adv. 2020;10:125109. doi: 10.1063/5.0035414. [DOI] [Google Scholar]
- Knibbs LD, Morawska L, Bell SC, Grzybowski P. Room ventilation and the risk of airborne infection transmission in 3 health care settings within a large teaching hospital. Am J Infect Control. 2011;39:866–872. doi: 10.1016/j.ajic.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ACK, Cheng YC. Study of expiratory droplet dispersion and transport using a new Eulerian modeling approach. Atmos Environ. 2007;41:7473–7484. doi: 10.1016/j.atmosenv.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Shih T-P, Ko W-C, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rutte EA, Shattock AJ, Chitnis N, et al. Modelling the impact of Omicron and emerging variants on SARS-CoV-2 transmission and public health burden. Commun Med. 2022;2:1–7. doi: 10.1038/s43856-022-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yoo D, Ryu S, et al. Quantity, size distribution, and characteristics of cough-generated aerosol produced by patients with an upper respiratory tract infection. Aerosol Air Qual Res. 2019;19:840–853. doi: 10.4209/aaqr.2018.01.0031. [DOI] [Google Scholar]
- Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021 doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Basic routes of transmission of respiratory pathogens—a new proposal for transmission categorization based on respiratory spray, inhalation, and touch. Indoor Air. 2021;31:3–6. doi: 10.1111/ina.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shang Y, Yan Y, et al. Modelling of evaporation of cough droplets in inhomogeneous humidity fields using the multi-component Eulerian-Lagrangian approach. Build Environ. 2018;128:68–76. doi: 10.1016/j.buildenv.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Leong FY, Xu G, et al. Dispersion of evaporating cough droplets in tropical outdoor environment. Phys Fluids. 2020;32:113301. doi: 10.1063/5.0026360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J-X, Chen X. Can a toilet promote virus transmission? From a fluid dynamics perspective. Phys Fluids. 2020;32:065107. doi: 10.1063/5.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qian H, Hang J, et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Buildi Environ. 2021;196:107788. doi: 10.1016/j.buildenv.2021.107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chong KL, Ng CS, et al. Towards realistic simulations of human cough: effect of droplet emission duration and spread angle. Int J Multiphase Flow. 2022;147:103883. doi: 10.1016/j.ijmultiphaseflow.2021.103883. [DOI] [Google Scholar]
- Li W, Chong A, Lasternas B, et al. Quantifying the effectiveness of desk dividers in reducing droplet and airborne virus transmission. Indoor Air. 2022;32:e12950. doi: 10.1111/ina.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Reynolds JS, Szalajda JV, et al. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol Sci Technol. 2013;47:937–944. doi: 10.1080/02786826.2013.803019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linge K, Palmer J, Downey A, et al (2022) A tracer gas study of ventilation and air movement to inform potential SARS-CoV-2 airborne transmission. Anal Chem 10.26434/chemrxiv-2022-2rt0t
- Lipsitch M (2021) Seasonality of SARS-CoV-2: Will COVID-19 go away on its own in warmer weather? – Center for Communicable Disease Dynamics. https://ccdd.hsph.harvard.edu/will-covid-19-go-away-on-its-own-in-warmer-weather/. Accessed 3 Mar 2021
- Liu J, Duan Y. Saliva: a potential media for disease diagnostics and monitoring. Oral Oncol. 2012;48:569–577. doi: 10.1016/j.oraloncology.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Liu W, You X-Y. Transportation and risk analysis of influenza indoor and outdoor transportation and exposure risk analysis of influenza aerosol. Indoor Built Environ. 2012;21:614–621. doi: 10.1177/1420326X11426164. [DOI] [Google Scholar]
- Liu L, Wei J, Li Y, Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- Liu J, Xie W, Wang Y, et al. A comparative overview of COVID-19, MERS and SARS: review article. Int J Surg. 2020;81:1–8. doi: 10.1016/j.ijsu.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Allahyari M, Salinas JS, et al. Peering inside a cough or sneeze to explain enhanced airborne transmission under dry weather. Sci Rep. 2021;11:9826. doi: 10.1038/s41598-021-89078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li T, Deng Y, et al. Stability of SARS-CoV-2 on environmental surfaces and in human excreta. J Hosp Infect. 2021;107:105–107. doi: 10.1016/j.jhin.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Qian H, Zheng X, et al. Evaporation and dispersion of exhaled droplets in stratified environment. IOP Conf Ser: Mater Sci Eng. 2019;609:042059. doi: 10.1088/1757-899X/609/4/042059. [DOI] [Google Scholar]
- Liu K, Allahyari M, Salinas J, et al. Investigation of theoretical scaling laws using large eddy simulations for airborne spreading of viral contagion from sneezing and coughing. Phys Fluids. 2021;33:063318. doi: 10.1063/5.0054651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu L, Xu C, et al. Exploring the potentials of personalized ventilation in mitigating airborne infection risk for two closely ranged occupants with different risk assessment models. Energy Build. 2021 doi: 10.1016/j.enbuild.2021.111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yin D, Niu Y, et al. Effect of human thermal plume and ventilation interaction on bacteria-carrying particles diffusion in operating room microenvironment. Energy Build. 2022;254:111573. doi: 10.1016/j.enbuild.2021.111573. [DOI] [Google Scholar]
- Loh N-HW, Tan Y, Taculod J, et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anesth/j Can Anesth. 2020;67:893–894. doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordly K, Kober L, Jadidi M, et al. Understanding lifetime and dispersion of cough-emitted droplets in air. Indoor Built Environ. 2022 doi: 10.1177/1420326X221098753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Howarth AT, Adam N, Riffat SB. Modelling and measurement of airflow and aerosol particle distribution in a ventilated two-zone chamber. Build Environ. 1996;31:417–423. doi: 10.1016/0360-1323(96)00019-4. [DOI] [Google Scholar]
- Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China. Emerging Infect Dis J. 2020 doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Ou C, Hang J, et al. Role of pathogen-laden expiratory droplet dispersion and natural ventilation explaining a COVID-19 outbreak in a coach bus. Build Environ. 2022;220:109160. doi: 10.1016/j.buildenv.2022.109160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao N, An CK, Guo LY, et al. Transmission risk of infectious droplets in physical spreading process at different times: a review. Build Environ. 2020;185:107307. doi: 10.1016/j.buildenv.2020.107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson MJ, Yinda CK, Seifert SN, et al. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerging Infect Dis J—CDC. 2020 doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz JA, Finn A. Influenza and humidity – Why a bit more damp may be good for you! J Infect. 2015;71:S54–S58. doi: 10.1016/j.jinf.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Mittal R, Ni R, Seo J-H. The flow physics of COVID-19. J Fluid Mech. 2020 doi: 10.1017/jfm.2020.330. [DOI] [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L, Johnson GR, Ristovski ZD, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [Google Scholar]
- Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DH, Yinda KC, Gamble A, et al. Mechanistic theory predicts the effects of temperature and humidity on inactivation of SARS-CoV-2 and other enveloped viruses. Elife. 2021;10:e65902. doi: 10.7554/eLife.65902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi H, Shirzadi M, Tominaga Y, Mirzaei PA. CFD modeling of airborne pathogen transmission of COVID-19 in confined spaces under different ventilation strategies. Sustain Cities Soc. 2022 doi: 10.1016/j.scs.2021.103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Eng D, Nagano S, Tanaka Y (1992) Diffusion Characteristics Of Airborne Particles With Gravitational Settling In A Convection-Dominant Indoor Flow Field. 16
- Nardell EA. Catching droplet nuclei. Am J Respir Crit Care Med. 2004;169:553–554. doi: 10.1164/rccm.2401003. [DOI] [PubMed] [Google Scholar]
- Nayak R. Introduction to manikins. In: Nayak R, Padhye R, editors. Manikins for textile evaluation. Sawston: Woodhead Publishing; 2017. pp. 3–24. [Google Scholar]
- Netz RR, Eaton WA. Physics of virus transmission by speaking droplets. Proc Natl Acad Sci U S A. 2020;117:25209–25211. doi: 10.1073/pnas.2011889117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NNT, McCarthy C, Lantigua D, Camci-Unal G. Development of diagnostic tests for detection of SARS-CoV-2. Diagnostics. 2020;10:905. doi: 10.3390/diagnostics10110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2010;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Sakata S, Kaga A. A New methodology for studying dynamics of aerosol particles in sneeze and cough using a digital high-vision, high-speed video system and vector analyses. PLoS ONE. 2013;8:e80244. doi: 10.1371/journal.pone.0080244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Oshitani H, Kobayashi T, et al (2020) Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv. 10.1101/2020.02.28.20029272
- Oswin HP, Haddrell AE, Otero-Fernandez M, et al. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc Natl Acad Sci. 2022;119:e2200109119. doi: 10.1073/pnas.2200109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Pendar M-R, Páscoa JC. Numerical modeling of the distribution of virus carrying saliva droplets during sneeze and cough. Phys Fluids. 2020;32:083305. doi: 10.1063/5.0018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- Prather KA, Marr LC, Schooley RT, et al. Airborne transmission of SARS-CoV-2. Science. 2020;370:303–304. doi: 10.1126/science.abf0521. [DOI] [PubMed] [Google Scholar]
- Priyanka COP, Singh I, Patra G. Aerosol transmission of SARS-CoV-2: the unresolved paradox. Travel Med Infect Dis. 2020;37:101869. doi: 10.1016/j.tmaid.2020.101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psikuta A, Kuklane K, Bogdan A, et al. Opportunities and constraints of presently used thermal manikins for thermo-physiological simulation of the human body. Int J Biometeorol. 2016;60:435–446. doi: 10.1007/s00484-015-1041-7. [DOI] [PubMed] [Google Scholar]
- Quiñones JJ, Doosttalab A, Sokolowski S, et al. Prediction of respiratory droplets evolution for safer academic facilities planning amid COVID-19 and future pandemics: a numerical approach. J Build Eng. 2022;54:104593. doi: 10.1016/j.jobe.2022.104593. [DOI] [Google Scholar]
- Redrow J, Mao S, Celik I, et al. Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build Environ. 2011;46:2042–2051. doi: 10.1016/j.buildenv.2011.04.011. [DOI] [Google Scholar]
- Rezaei M, Netz RR. Airborne virus transmission via respiratory droplets: Effects of droplet evaporation and sedimentation. Curr Opin Colloid Interface Sci. 2021;55:101471. doi: 10.1016/j.cocis.2021.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S, Goldie S, Hill A, et al. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan AAR, Naser P, Naser J. Numerical study of when and who will get infected by coronavirus in passenger car. Environ Sci Pollut Res. 2022 doi: 10.1007/s11356-022-19824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman BE, Techet AH, Bush JWM, Bourouiba L. Visualization of sneeze ejecta: steps of fluid fragmentation leading to respiratory droplets. Exp Fluids. 2016 doi: 10.1007/s00348-015-2078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M, Gardner S, Wood S, et al. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J Infect Dis. 2020;221:372–378. doi: 10.1093/infdis/jiz582. [DOI] [PubMed] [Google Scholar]
- Sender R, Bar-On YM, Gleizer S, et al. The total number and mass of SARS-CoV-2 virions. PNAS. 2021 doi: 10.1073/pnas.2024815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Lim H, Park M, et al. Field study of the indoor environments for preventing the spread of the SARS-CoV-2 in Seoul. Indoor Air. 2022;32:e12959. doi: 10.1111/ina.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Jinadatha C, Lichtfouse E. Environmental chemistry is most relevant to study coronavirus pandemics. Environ Chem Lett. 2020;18:993–996. doi: 10.1007/s10311-020-01017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Okuyama K, Okazaki S, et al. Numerical simulation and experiment on the transport of fine particles in a ventilated room. Aerosol Sci Technol. 1996;25:242–255. doi: 10.1080/02786829608965394. [DOI] [Google Scholar]
- Smith SH, Somsen GA, van Rijn C, et al. Aerosol persistence in relation to possible transmission of SARS-CoV-2. Phys Fluids. 2020;32:107108. doi: 10.1063/5.0027844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither SJ, Eastaugh LS, Findlay JS, Lever MS. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerging Microbes Infect. 2020;9:1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Li J, Han J. How human thermal plume influences near-human transport of respiratory droplets and airborne particles: a review. Environ Chem Lett. 2021;19:1971–1982. doi: 10.1007/s10311-020-01178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JW, Liebner TJ, Craven BA, Settles GS. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6:S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JW, Nicolle AD, Klettner CA, et al. Airflow Dynamics of human jets: sneezing and breathing - potential sources of infectious aerosols. PLoS ONE. 2013;8:e59970. doi: 10.1371/journal.pone.0059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZF, Tu JY, Yeoh GH, Yuen RKK. Numerical studies of indoor airflow and particle dispersion by large Eddy simulation. Build Environ. 2007;42:3483–3492. doi: 10.1016/j.buildenv.2006.10.047. [DOI] [Google Scholar]
- Trancossi M, Carli C, Cannistraro G, et al. Could thermodynamics and heat and mass transfer research produce a fundamental step advance toward and significant reduction of SARS-COV-2 spread? Int J Heat Mass Transf. 2021;170:120983. doi: 10.1016/j.ijheatmasstransfer.2021.120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufnalska S, Lichtfouse E. Unanswered issues related to the COVID-19 pandemic. Environ Chem Lett. 2021;19:3523–3524. doi: 10.1007/s10311-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamatzi-Panagiotou A, Penchovsky R. Environmental factors influencing the transmission of the coronavirus 2019: a review. Environ Chem Lett. 2022;20:1603–1610. doi: 10.1007/s10311-022-01418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSciver M, Miller S, Hertzberg J. Particle image velocimetry of human cough. Aerosol Sci Technol. 2011;45:415–422. doi: 10.1080/02786826.2010.542785. [DOI] [Google Scholar]
- Vejerano EP, Marr LC. Physico-chemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen V, Aarnio M, Alava M, et al. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf Sci. 2020;130:104866. doi: 10.1016/j.ssci.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;189:1143–1144. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Han J. Will the COVID-19 pandemic end with the delta and omicron variants? Environ Chem Lett. 2022 doi: 10.1007/s10311-021-01369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wu H, Wan X-F. Transport and fate of human expiratory droplets—a modeling approach. Phys Fluids. 2020;32:083307. doi: 10.1063/5.0021280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cai K, Zhang R, et al. Novel one-step single-tube nested quantitative real-time PCR assay for highly sensitive detection of SARS-CoV-2. Anal Chem. 2020;92:9399–9404. doi: 10.1021/acs.analchem.0c01884. [DOI] [PubMed] [Google Scholar]
- Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li Y, Lung DC, et al. Aerosol transmission of SARS-CoV-2 due to the chimney effect in two high-rise housing drainage stacks. J Hazardous Mater. 2022;421:126799. doi: 10.1016/j.jhazmat.2021.126799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris A, Ali M, Khan AU, et al (2020) Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic options. Int J Clinical Virol;4:065–070. 10.29328/journal.ijcv.1001017
- Weber TP, Stilianakis NI. Inactivation of influenza a viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ. 2015;93:86–96. doi: 10.1016/j.buildenv.2015.06.018. [DOI] [Google Scholar]
- Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44:S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WF. On air-borne infection. Study II. Droplets and droplet nuclei. Am J Hyg. 1934;20:611–618. [Google Scholar]
- WHO (2020) Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed 7 Mar 2021
- WHO (2021) Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 6 Apr 2022
- WHO (2023) WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 30 Jan 2023
- World Health Organization . Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- Wu L, Liu X, Yao F, Chen Y. Numerical study of virus transmission through droplets from sneezing in a cafeteria. Phys Fluids. 2021;33:023311. doi: 10.1063/5.0040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao AT, Tong YX, Gao C, et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clinical Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Li Y, Chwang ATY, et al. How far droplets can move in indoor environments–revisiting the wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Xie X, Li Y, Sun H, Liu L. Exhaled droplets due to talking and coughing. J R Soc Interface. 2009 doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu W, Luo X, et al. Prediction and control of aerosol transmission of SARS-CoV-2 in ventilated context: from source to receptor. Sustain Cities Soc. 2022 doi: 10.1016/j.scs.2021.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Li X, Tu J. Thermal effect of human body on cough droplets evaporation and dispersion in an enclosed space. Build Environ. 2019;148:96–106. doi: 10.1016/j.buildenv.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Norvihoho LK, Zhou Z-F, et al. Investigation on the evaporation and dispersion of human respiratory droplets with COVID-19 virus. Int J Multiphase Flow. 2022;147:103904. doi: 10.1016/j.ijmultiphaseflow.2021.103904. [DOI] [Google Scholar]
- Zhang Z, Chen Q. Experimental measurements and numerical simulations of particle transport and distribution in ventilated rooms. Atmos Environ. 2006;40:3396–3408. doi: 10.1016/j.atmosenv.2006.01.014. [DOI] [Google Scholar]
- Zhang Z, Chen Q. Comparison of the Eulerian and Lagrangian methods for predicting particle transport in enclosed spaces. Atmos Environ. 2007;41:5236–5248. doi: 10.1016/j.atmosenv.2006.05.086. [DOI] [Google Scholar]
- Zhang H, Li D, Xie L, Xiao Y. Documentary research of human respiratory droplet characteristics. Procedia Eng. 2015;121:1365–1374. doi: 10.1016/j.proeng.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Chen W, Chan P, et al. Close contact behavior in indoor environment and transmission of respiratory infection. Indoor Air. 2020;30:645–661. doi: 10.1111/ina.12673. [DOI] [PubMed] [Google Scholar]
- Zhang M, Shrestha P, Liu X, et al. Computational fluid dynamics simulation of SARS-CoV-2 aerosol dispersion inside a grocery store. Build Environ. 2022;209:108652. doi: 10.1016/j.buildenv.2021.108652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Guan P. Modeling particle dispersion in personalized ventilated room. Build Environ. 2007;42:1099–1109. doi: 10.1016/j.buildenv.2005.11.009. [DOI] [Google Scholar]
- Zhao B, Zhang Y, Li X, et al. Comparison of indoor aerosol particle concentration and deposition in different ventilated rooms by numerical method. Build Environ. 2004;39:1–8. doi: 10.1016/j.buildenv.2003.08.002. [DOI] [Google Scholar]
- Zhao B, Yang C, Yang X, Liu S. Particle dispersion and deposition in ventilated rooms: testing and evaluation of different Eulerian and Lagrangian models. Build Environ. 2008;43:388–397. doi: 10.1016/j.buildenv.2007.01.005. [DOI] [Google Scholar]
- Zhao VXT, Wong TI, Zheng XT, et al. Colorimetric biosensors for point-of-care virus detections. Mater Sci Energy Technol. 2020;3:237–249. doi: 10.1016/j.mset.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Zhou C, Chan T, et al. Assessment of COVID-19 aerosol transmission in a university campus food environment using a numerical method. Geosci Front. 2022 doi: 10.1016/j.gsf.2022.101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Feng Y, Ma L. Impacts of human movement and ventilation mode on the indoor environment, droplet evaporation, and aerosol transmission risk at airport terminals. Build Environ. 2022;224:109527. doi: 10.1016/j.buildenv.2022.109527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Qi Y, Luzzatto-Fegiz P, et al (2020a) COVID-19: effects of environmental conditions on the propagation of respiratory droplets. Nano Lett 7 [DOI] [PubMed]
- Zhu S, Kato S, Yang J-H. Study on transport characteristics of saliva droplets produced by coughing in a calm indoor environment. Build Environ. 2006;41:1691–1702. doi: 10.1016/j.buildenv.2005.06.024. [DOI] [Google Scholar]
- Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan. China Global Health Res Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Kuehn TH, Verma H, et al. Association of airborne virus infectivity and survivability with its carrier particle size. Aerosol Sci Technol. 2013;47:373–382. doi: 10.1080/02786826.2012.754841. [DOI] [Google Scholar]