Abstract
Purpose:
We compared new cases detected per index case in familial hypercholesterolemia (FH) families with or without an identifiable monogenic etiology.
Methods:
We enrolled 52 FH probands with a pathogenic variant (FHg+) in LDLR, APOB, or PCSK9 and 73 probands without such a variant (FHg−). After direct contact by the study team, family members (FMs) of FHg+ probands could opt-in for genetic testing and FMs of FHg− probands were asked to provide a lipid profile. New cases were defined as presence of a pathogenic variant in FHg+ families and as low-density lipoprotein cholesterol ≥155 mg/dL in FHg− families.
Results:
Of 71 FHg+ probands seen by a genetic counselor, 52 consented and identified 253 FMs (111 consented and were tested, yielding 48 new cases). Of 101 FHg− probands who received counseling, 73 consented and identified 295 FMs (63 consented and were tested, yielding 17 new cases). New case detection per index case was significantly greater in FHg+ than in FHg− families (0.92 vs 0.23), a result of higher cascade testing uptake (43.9 vs 21.4%) and yield (43.2 vs 27.0%) in the former.
Conclusion:
New case detection rate was significantly higher in FH families with a monogenic etiology than in those without such an etiology owing to greater uptake and yield of cascade testing.
Keywords: Cascade testing, Familial hypercholesterolemia, Monogenic, Polygenic, Variant
Introduction
The Office of Public Health Genomics at the Centers for Disease Control and Prevention has labeled familial hypercholesterolemia (FH), Lynch syndrome, and hereditary breast and ovarian cancer as tier 1 conditions because these are relatively common genetic disorders associated with significant morbidity, and screening and treatment options are available to reduce adverse outcomes.1 Population screening for tier 1 conditions has been proposed,2-4 and new case detection could increase substantially by cascade testing of family members (FMs) of affected individuals,5 a compelling example of precision medicine at the population level. However, uptake of cascade testing for genetic disorders, particularly FH, is poor in the United States.6
FH is associated with significantly higher risk of early-onset coronary heart disease but remains vastly underdiagnosed.7-11 It is estimated that of approximately 1.3 million patients with FH in the United States, less than 10% have been identified despite established clinical scoring systems, such as the Dutch Lipid Clinic Network (DLCN) diagnostic criteria.7,8 A major reason for the low detection rate in the United States is the lack of a widely accepted screening strategy despite proposals for universal or targeted lipid screening.12,13
Although universal lipid screening remains a matter of debate,14 cascade testing (a form of targeted screening of FMs of affected individuals) is acknowledged to be the most cost-effective screening strategy for FH5,15-17 and is recommended by expert panels in the United States and Europe.17-20 A number of countries have assessed the efficacy of cascade testing for FH,21 but data for uptake and yield of cascade testing for FH in the United States are sparse, with initial reports indicative of low uptake.22,23 An added challenge is that at least half of patients with a clinical diagnosis of FH do not have a pathogenic/likely pathogenic (P/LP) variant in 1 of the 3 canonical genes for FH—LDLR, APOB, or PCSK924-26—and, in such cases, FMs have to be screened through a lipid profile. The basis of FH in these cases is unclear, although polygenic factors likely play a role.25,27
We therefore conducted a pragmatic clinical trial,28 the Cascade Testing for Familial Hypercholesterolemia study (NCT03640234), using direct contact of FMs of FH probands and compared new case detection rates in those with or without a P/LP variant. Efficiency of cascade testing was defined as new cases detected per index case (NCIC) for FH probands with (FHg+) or FH probands without (FHg−) an identifiable P/LP variant. Furthermore, we compared uptake and yield of cascade testing in the 2 groups to explain any observed differences in NCIC. In exploratory analyses of families without a P/LP variant, we assessed whether an elevated polygenic score (top quintile) for low-density lipoprotein cholesterol (LDL-C) or a DLCN score of ≥6 was associated with greater NCIC.
Materials and Methods
The study was approved by the Mayo Clinic Institutional Review Board.
Overall study design
The design of this pragmatic clinical trial has been previously described.29 Participants were enrolled at a single site (Mayo Clinic, Rochester, Minnesota) between November 2017 and December 2021. Those with a P/LP variant in any of the 3 canonical FH genes (FHg+) or with possible (DLCN score of 3-5), probable (DLCN score of 6-8), or definite (DLCN score of >8) FH based on the DLCN criteria but negative on genetic testing (FHg−) were invited to participate in the Cascade Testing for Familial Hypercholesterolemia trial at the time of genetic counseling (Figure 1). Participants were notified of their results in a face-to-face, phone, or telehealth appointment with a genetic counselor, and implications for FMs (blood relatives) were also discussed. Participants gave informed consent for the study team to contact relatives on their behalf and provided contact information for first-degree FMs. After contact, FMs of the proband could opt-in for genetic testing for FH or provide lipid profile data to the study team. A FM with the same P/LP variant as the proband or an LDL-C ≥155 mg/dL noted in the prior 36 months was considered a new case.
Figure 1. Overview of study workflow for probands and family members.
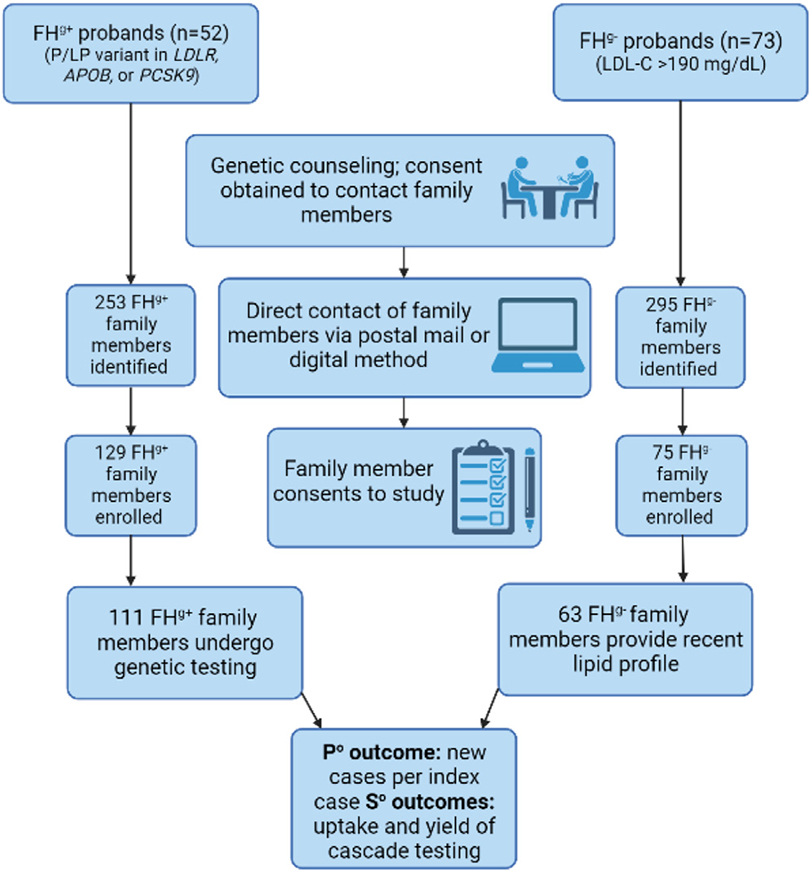
FHg+, familial hypercholesterolemia with a pathogenic variant; FHg−, familial hypercholesterolemia without a pathogenic variant; LDL-C, low-density lipoprotein cholesterol; P°, primary; P/LP, pathogenic/likely pathogenic; S°, secondary.
Participants
We aimed to enroll 50 FHg+ and 100 FHg− probands. Enrollment was stopped when we reached the target for FHg+ proband enrollment, 4 years after starting the trial. During this time, we enrolled 73 FHg− probands, falling short of our target owing to the COVID-19 pandemic. Of the 71 FHg+ probands with a P/LP variant in LDLR, APOB, or PCSK9 (FHg+), 52 consented to participate (Supplemental Figure 1). FHg+ probands were identified from (1) the Return of Actionable Variants Empirical (RAVE) study30 in which adults with hypercholesterolemia underwent sequencing of LDLR, APOB, and PCSK9, in a Central Laboratory Improvement Amendment-certified laboratory (n = 17), (2) the Familial Hypercholesterolemia Identification Registry, a registry of Mayo Clinic patients who met clinical criteria for FH (n = 23), and (3) the FH Clinic in the Department of Cardiovascular Medicine (n = 12).
FHg− probands were ascertained from the RAVE study on the basis of an LDL-C >190 mg/dl, an inclusion criterion of the study. As a part of the RAVE study, genetic testing, including a polygenic score for LDL-C, was performed (Supplemental Figure 2). Of 101 such probands in the RAVE study, 73 consented—36 FHg− participants had a DLCN score of ≥6 (probable/definite FH), and 37 participants had a DLCN score of 3 to 5 (possible FH). Demographic and clinical characteristics were obtained from the electronic health records of the consented probands. These characteristics included age, sex, race, ethnicity, education, and highest LDL-C level before study consent date.
Sequencing LDLR, APOB, and PCSK9 and genotyping of 12 single nucleotide variants associated with LDL-C
DNA from study participants was sent to a Central Laboratory Improvement Amendment-certified laboratory for targeted sequencing. The postcapture library DNA was subjected to sequence analysis on Illumina HiSeq platform (Illumina Inc., 1998, San Diego, CA) for 100 basepair paired end reads. Quality control metrics of the sequencing data and the process for identifying P/LP variants (including structural variants) have been described elsewhere.30 In FHg− individuals from the RAVE study, we calculated a polygenic score for LDL-C by combining 12 LDL-C–associated single nucleotide variants as previously described.27 The gene score used the weighted sum of the risk allele (ie, the LDL-C-raising allele), and the weights used were the corresponding per-allele (risk) beta coefficients reported in the literature. The score was divided into quintiles and disclosed to the FHg− proband.30 A list of P/LP variants found in the FHg+ probands is provided in Supplemental Table 1.
Disclosure of genetic test results to probands and contact of FMs
Participants received the results of the genetic testing in a face-to-face encounter, via phone, or telehealth appointment with a genetic counselor (Supplemental Figure 3). The counselor helped participants interpret and understand their results, highlighting the implications for FMs for both FHg+ and FHg− probands. Participants who consented were asked to provide a list of FMs for details on first-degree relatives and beyond (Supplemental Figure 4), along with their contact information and permission to contact the FMs (Figure 1). A letter or email was sent to FMs and stated that one of their relatives had been found to have elevated cholesterol levels, asking them to indicate their interest in participating in the study (Supplemental Material). FMs who indicated interest in the study were sent 2 copies of the informed consent form, one for their records and one to return to the study team, and a survey to gather demographic information by postal mail or email.
Cascade testing in FMs
For FMs who provided informed consent, we initiated cascade testing. A saliva collection kit (Invitae Corporation, 2010, San Francisco, CA) was used for DNA testing in FMs of FHg+ patients. Saliva collection kits were mailed to the homes of FHg+ FMs who then sent the sample directly to the laboratory. FMs of FHg− patients were asked to have a lipid profile drawn or to send the results of a lipid profile drawn in the preceding 36 months to the study team.
Disclosure of test results to FMs
Consented FHg+ FMs received genetic test results through postal mail or via a phone call by the study coordinator and were encouraged to see their primary care provider for further evaluation. FMs also received a letter that summarized their results as well as the key points related to hypercholesterolemia as a risk factor for coronary heart disease.
Outcomes
The primary outcome was NCIC, a measure of the efficiency of cascade testing, in the FHg+ and FHg− groups.21 NCIC was defined as the ratio of the number of newly diagnosed cases of FH to the number of probands.
NCIC can be conceptualized as the product of (FMs per proband) × uptake × yield. The latter two were secondary outcomes for the trial. Cascade testing uptake was defined as the proportion of FMs who consented and completed testing. Yield was defined as the proportion of FMs who met criteria as a new case among the FMs who consented and completed testing. In exploratory analyses, we compared NCIC in strata defined by the top quintile of polygenic score vs quintiles 1 to 4, and by a DLCN score of ≥6 vs a DLCN score of <6, in FHg− probands. New cases were defined as presence of a P/LP variant in the FHg+ group and LDL-C ≥155 mg/dL in adults (the threshold for defining an elevated LDL-C based on the DLCN criteria) in the FHg− group. For FMs on lipid lowering therapy, LDL-C levels were imputed using a multiplier on the basis of the type and dose of lipid lowering therapy.31
Power
We aimed to enroll 50 FHg+ and 100 FHg− probands, assuming an average of 3 FMs identified per proband; because of intrafamily correlation, this would mean a reduced effective number of 1.5 independent FMs per proband, which would lead to effective sample sizes of 75 and 150, respectively, for estimates and tests of group means and proportions.29 We were able to enroll 52 FHg+ probands and 73 FHg− probands out of a target of 100. We therefore performed a post hoc assessment of power (see Results section).
Statistical methods
Proband characteristics were compared using χ2 tests, t tests, Cochran Armitage test of trends, or Wilcoxon rank sum tests. The LDL-C levels in probands or FMs were imputed if they were on lipid lowering treatment at the time of testing.31 The primary outcome—NCIC—is the product of family size, participation rate, and positivity rate, all of which could potentially cluster in families. We used bootstrap analyses to account for the possibility of such clustering. CIs for NCIC, uptake, and yield were calculated using bootstrapping with 1000 iterations. Each bootstrap sample selected the corresponding number of probands (ie, 52 FHg+ and 73 FHg−) with simple random sampling with replacement and their corresponding FMs. Each parameter was calculated separately within each bootstrap sample and the 2.5th and 97.5th percentiles across all bootstrap samples defined the 95% CIs. Significance tests were based on calculating the bootstrap distribution for group differences and doubling the smaller of the proportion of these differences below or above zero as the observed 2-sided P value.
Results
Characteristics of the probands are shown in Table 1. Compared with FHg− probands, FHg+ probands were slightly younger and more often females. FHg+ probands had higher LDL-C levels (Table 1) and were more likely to be on lipid lowering therapy (single medication or combination of medications) at time of the highest LDL-C (Supplemental Table 2).
Table 1.
Demographic and clinical characteristics of probands who consented and completed testing
| Proband Type | |||
|---|---|---|---|
| Characteristic | FHg+ (n = 52) | FHg− (n = 73) | P value |
| Age at consent, y | 54.7 ± 16.4 | 61.3 ± 8.3 | .010a |
| Female sex | 35 (67.3%) | 36 (49.3%) | .045b |
| Non-Hispanic White | 49 (94.2%) | 71 (97.3%) | .394b |
| Years of education | |||
| 12-15 | 17 (37.0%) | 33 (47.1%) | .051c |
| 16 | 13 (28.3%) | 26 (37.1%) | |
| >16 | 16 (34.8%) | 11 (15.7%) | |
| Unknown | 6 | 3 | |
| Highest LDL-Cd, mg/dL | 264.5 (214.5-329.5) | 203.0 (193.0-225.0) | <.001e |
| Lipid lowering treatment at highest LDL-C | 14 (26.9%) | 4 (5.5%) | <.001b |
| Coronary heart disease at enrollmentf | 14 (26.9%) | 15 (20.7%) | .537 |
| Pathogenic variant | – | ||
| APOB | 13 (25.0%) | – | |
| LDLR | 37 (71.2%) | – | |
| PCSK9 | 2 (3.8%) | – | |
| LDL-C polygenic score quintileg | – | ||
| 1 | – | 2 (2.7%) | |
| 2 | – | 11 (15.1%) | |
| 3 | – | 14 (19.2%) | |
| 4 | – | 24 (32.9%) | |
| 5 | – | 22 (30.1%) | |
| DLCN scoreh | 5 (3-8) | 5 (4-6) | .762e |
| DLCN score ≥6h | 22 (42.3%) | 36 (49.3%) | .439b |
Values are n (%), mean ± SD, or median (IQR).
CHD, coronary heart disease; DLCN, Dutch Lipid Clinic Network; FHg+, familial hypercholesterolemia with a pathogenic variant; FHg−, familial hypercholesterolemia without a pathogenic variant; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
Two sample t test with unequal variance.
χ2 test.
Cochran Armitage test of trends.
Values were corrected for lipid lowering treatment use at the time of measurement.
Wilcoxon rank sum test.
CHD was defined as either a history of myocardial infarction, coronary revascularization, or coronary calcification >75th percentile for age and sex, >50% stenosis in a major coronary artery on angiography and at least moderate ischemia on stress imaging.
The quintiles were defined using cut-offs from the Return of Actionable Variants Empirical (RAVE) study.
The DLCN score presented does not include the 8 points for a pathogenic variant.
Of the 71 FHg+ probands approached, 52 enrolled in the study and identified 253 FHg+ FMs. Of the 129 FMs who consented to participate, 111 (86.0%) completed genetic testing, and 48 new cases (defined as presence of a P/LP variant) were detected (Supplemental Figure 1). Some FHg+ probands provided contact information for FMs beyond first-degree relatives, and they were contacted when a P/LP variant was confirmed in the first-degree relative (Supplemental Figure 4). Among FHg+ FMs, 11.7 % were aged <18 years and 14.4% were second- or third-degree relatives, whereas, all FHg− FMs were adult first-degree relatives (Supplemental Figure 4).
Of the 101 FHg− probands approached, 73 enrolled and identified 295 FHg− FMs. Of the 75 FMs who consented to participate, 63 (84.0%) provided a lipid profile, and 17 new cases (defined as LDL-C ≥155 mg/dL) were detected (Supplemental Figure 2).
Characteristics of the FMs are shown in Table 2. The age range was wider in FHg+ families (1-91 years) than in FHg− families (23-96 years) because children were only enrolled in FHg+ families. No children were provided by the FHg− probands for the study team to contact. In total, 10 FHg+ and 64 FHg− FMs responded that they were not interested in participating in the study via the study interest survey. FMs were enrolled from 25 states in the United States with approximately half of both FHg+ and FHg− FMs residing outside of Minnesota (Supplemental Table 3).
Table 2.
Demographic and clinical characteristics of family members who consented and completed testing
| Proband Type | ||
|---|---|---|
| Characteristic | FHg+ (n = 111) | FHg− (n = 63) |
| Age at consent, y | 47.4 ± 22.0 | 57.4 ± 17.1 |
| Unknown | 22 | 0 |
| Range, y | 1-91 | 23-96 |
| Age <18 y | 13 (11.7%) | 0 (0%) |
| Female sex | 60 (54.1%) | 35 (55.6%) |
| Non-Hispanic White | 107 (96.4%) | 60 (95.2%) |
| LDL-Ca, mg/dL | – | 133.0 (106.5-157.0) |
| LDL-C ≥155 mg/dLa | – | 17 (27.0%) |
| Lipid lowering treatment at provided LDL-C | – | 14 (22.2%) |
| Pathogenic variant | ||
| None | 63 (56.8%) | – |
| APOB | 19 (17.1%) | – |
| LDLR | 29 (26.1%) | – |
| PCSK9 | 0 (0.0%) | – |
| Degree of relationship | ||
| First | 95 (85.6%) | 63 (100.0%) |
| Second | 11 (9.9%) | 0 (0.0%) |
| Third | 5 (4.5%) | 0 (0.0%) |
Values are n (%), mean ± SD, or median (IQR).
FHg+, familial hypercholesterolemia with a pathogenic variant; FHg−, familial hypercholesterolemia without a pathogenic variant; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
Values were corrected for lipid lowering treatment use at the time of measurement. LDL-C measurements were only requested from the family members of FHg− probands.
Primary outcome
NCIC after cascade testing was significantly higher in FHg+ families than in FHg− families (0.92, 95% CI = 0.61-1.27 vs 0.23, 95% CI = 0.12-0.34; P < .001) (Table 3). Results were similar in a sensitivity analysis limited to only first-degree relatives (Supplemental Table 4). The higher NCIC in FHg+ families was a result of greater uptake and yield than in the FHg− families (see the following).
Table 3.
Participation rate, NCICa, uptake, and yield of cascade testing among family members by proband type
| Proband Type | |||
|---|---|---|---|
| Parameter With 95% CIsb | FHg+ | FHg− | P value |
| Number of probands | 52 | 73 | – |
| Number of family members identified | 253 | 295 | |
| Consented | 129 | 75 | – |
| Completed testing | 111 | 63 | – |
| Met criteria as a new case | 48 | 17 | |
| Mean number of family members per proband | 4.9 (3.9-5.9) | 4.0 (3.4-4.7) | .152 |
| Consented | 2.5 (1.9-3.1) | 1.0 (0.7-1.3) | <.001 |
| Completed testing | 2.1 (1.6-2.7) | 0.9 (0.6-1.1) | <.001 |
| Met criteria as a new case | 0.9 (0.6-1.3) | 0.2 (0.1-0.3) | <.001 |
| NCICc | 0.92 (0.61-1.27) | 0.23 (0.12-0.34) | <.001 |
| Uptake of cascade testingd | 43.9% (35.5-52.2) | 21.4% (15.8-26.7) | <.001 |
| Yield of cascade testinge | 43.2% (33.6-54.2) | 27.0% (16.7-38.5) | .032 |
FHg+, familial hypercholesterolemia with a pathogenic variant; FHg−, familial hypercholesterolemia without a pathogenic variant; LDL-C, low-density lipoprotein cholesterol; NCIC, new cases per index case.
New case defined as a family member who completed testing and had a pathogenic variant among relatives of FHg+ probands or had LDL-C ≥155 mg/dL among relatives of FHg− probands.
CIs were calculated using bootstrapping over 1000 iterations. Each bootstrap sample selected 52 FHg+ or 73 FHg− probands (simple random sampling with replacement) and their corresponding family members. Each parameter was calculated separately within each bootstrap sample and the 2.5th and 97.5th percentiles across all bootstrap samples defined the 95% CIs.
NCIC was calculated as the number of family members who met criteria as a new case divided by the total number of probands.
Uptake was defined as the proportion of family members who consented and completed testing among all identified family members.
Yield was defined as the proportion of family members who met criteria as a new case among family members who consented and completed testing.
Secondary outcomes
Uptake of cascade testing was significantly higher in FHg+ families than FHg− families. Of FHg+ FMs, 43.9% (95% CI = 35.5-52.2) consented and completed testing compared with 21.4% (95% CI = 15.8-26.7) of FHg− FMs. Similarly, the yield of cascade testing (proportion of new cases among FMs tested) was significantly higher in the FHg+ group (43.2%, 95% CI = 33.6-54.2) than in the FHg− group (27.0%, 95% CI = 16.7-38.5).
In FHg− families, there was a nonsignificant trend toward higher NCIC for probands with elevated polygenic score (quintile 5) compared with probands in quintiles 1 to 4 (0.32, 95% CI = 0.14-0.59 vs 0.19, 95% CI = 0.08-0.31; P = .28) (Table 4). We also observed a nonsignificant trend toward higher NCIC for FHg− probands with a DLCN score of ≥6 than the FHg− probands with a DLCN score of <6 (0.31, 95% CI = 0.14-0.50 vs 0.16, 95% CI = 0.05-0.29; P = .12) (Table 4). Polygenic score analyses were not performed in FHg+ families owing to FHg+ probands being recruited from other sources than the RAVE study.
Table 4.
Cascade testing metricsa in FHg− families by polygenic score quintile and DLCN score in probands
| FHg− Proband Polygenic Score Quintileb | FHg− Probandsc | |||||
|---|---|---|---|---|---|---|
| Parameter With 95% CIs | Q5 | Q1-Q4 | P value | DLCN ≥6 | DLCN <6 | P value |
| Number of probands | 22 | 51 | – | 36 | 37 | |
| Number of family members identified | 93 | 202 | 150 | 145 | ||
| Consented | 19 | 56 | – | 41 | 34 | – |
| Completed testing | 18 | 45 | – | 35 | 28 | – |
| Met criteria as a new case | 7 | 10 | 11 | 6 | ||
| Mean number of family members per proband | 4.2 (3.0-5.4) | 4.0 (3.3-4.7) | .702 | 4.2 (3.3-5.1) | 3.9 (3.1-4.7) | .722 |
| Consented | 0.9 (0.5-1.4) | 1.1 (0.7-1.5) | .444 | 1.1 (0.6-1.7) | 0.9 (0.6-1.3) | .488 |
| Completed testing | 0.8 (0.5-1.3) | 0.9 (0.5-1.2) | .848 | 1.0 (0.6-1.4) | 0.8 (0.5-1.1) | .440 |
| Met criteria as a new case | 0.3 (0.1-0.6) | 0.2 (0.1-0.3) | .280 | 0.3 (0.1-0.5) | 0.2 (0.1-0.3) | .116 |
| NCICd | 0.32 (0.14-0.591) | 0.19 (0.08-0.31) | .280 | 0.31 (0.14-0.50) | 0.16 (0.05-0.29) | .116 |
| Uptake of cascade testinge | 19.4% (10.5-30.8) | 22.3% (15.6-27.9) | .684 | 23.3% (14.7-33.1) | 19.3% (12.9-25.3) | .404 |
| Yield of cascade testingf | 38.9% (17.6-71.4) | 22.2% (11.6-32.6) | .176 | 31.4% (18.2-46.2) | 21.4% (7.3-39.2) | .364 |
FHg−, familial hypercholesterolemia without a pathogenic variant; LDL-C, low-density lipoprotein cholesterol; NCIC, new cases per index case.
New case is defined as a family member with an LDL-C ≥155 mg/dL.
CIs were calculated using bootstrapping over 1000 iterations. Each bootstrap sample selected 22 probands with polygenic score quintile 5 or 51 probands with polygenic score quintiles 1 to 4 (simple random sampling with replacement) and their corresponding family members. Each parameter was calculated separately within each bootstrap sample and the 2.5th and 97.5th percentiles across all bootstrap samples defined the 95% CIs.
CIs were calculated using bootstrapping over 1000 iterations. Each bootstrap sample selected 36 probands with DLCN score ≥6 or 37 probands with DLCN score <6 (simple random sampling with replacement) and their corresponding family members.
NCIC was calculated as the number of family members who met criteria as a new case divided by the total number of probands.
Uptake was defined as the proportion of family members who consented and completed testing among all identified family members.
Yield was defined as the proportion of family members who met criteria as a new case among family members who consented and completed testing.
Power (post hoc)
The FHg+ and FHg− families included an average of 4.9 and 4.0 FMs per proband, respectively, higher than the anticipated number of 3 in each arm. A comparison of the naive and bootstrap-based CIs for uptake and yield indicated that the effective percentage of independent observations exceeded prior assumptions, leading to greater power than originally estimated.29 The detectable group differences (with 80% power) in NCIC, uptake, and yield, were 0.48, 14.0%, and 21.0%, respectively, based on bootstrap standard errors.
Discussion
The main finding of this clinical trial was that new case detection after cascade testing for FH is significantly greater when a pathogenic variant is identified in the proband. This was because of higher uptake and yield of cascade testing in FH families with a pathogenic variant. Compared with prior reports,22,23 the uptake of cascade testing in both groups of FH families was much higher in our study, most likely a result of direct contact of FMs. To our knowledge, this is the first study in the United States to use direct contact to assess new case detection rate after cascade testing for FH.
The greater new case detection rate in the FHg+ group was because of a 2-fold higher uptake (43.9% vs 21.4%) and 1.6-fold higher yield (43.2% vs 27.0%) of cascade testing in FHg+ families than in FHg− families. The higher uptake may have been because of the greater effect on FHg+ FMs of learning about a monogenic condition in the proband. The lower yield in the FHg− group may be explained by probands having a polygenic etiology25,27 for FH, which could result in a lower proportion of FMs having elevated LDL-C than the approximately 50% predicted for those with monogenic FH.
We designed our pragmatic trial to replicate how a direct contact cascade testing program would be implemented in real-world practice. In individuals with a clinical diagnosis of FH, a pathogenic variant is not always identified.5 Therefore, case detection was based on genetic testing in FHg+ families and assessment of lipid profiles in the FHg− families. The latter group often have polygenic etiology,25,27 are high-risk nonetheless, and cascade testing is recommended.17-20 We noted a reasonable new case detection rate in such families, and our exploratory analyses motivate further investigation to assess whether the detection rate would be higher when the proband has a high polygenic score for LDL-C or a more severe clinical phenotype (eg, a DLCN score of ≥6).
Ajufo et al22 investigated the uptake of cascade testing in FH probands who received genetic testing vs those who received usual care, which included lipid profile testing. In the genetic testing group, only 13.1%, and in the usual care group, 8.8% of probands enrolled a FM for cascade testing.22 The NCIC was low (0.1) in both groups. In preliminary results from a study that returned FH results to 114 patients with 401 first-degree relatives, only 3.5% of FMs completed cascade testing.23 This is a much lower uptake of cascade testing than in our study (43.9% in FHg+ families and 21.4% in FHg− families).
The higher uptake and yield in our study is likely primarily owing to direct contact of FMs in contrast to probands contacting relatives themselves.22 In a systematic review of cascade testing for FH that examined NCIC in 10 studies (all from outside of the United States),21 direct contact of FMs and use of genetic testing were both associated with a higher NCIC. The greater NCIC observed in the FHg+ group suggests that readily available genetic testing increases efficiency of a cascade testing program for monogenic FH.
The current framework for cascade testing in the United States places the burden of contacting at-risk FMs on the proband using “Dear Family Member” letters.6,32 This passive approach is associated with low uptake of cascade testing for FH owing to multiple reasons, including complex family dynamics.5,33,34 Direct contact of FMs by health care providers appears to be more effective and could be implemented with the proband’s consent. 21,35-37 We did not encounter any issues related to direct contact, consistent with prior studies, indicating that direct contact is well accepted by FH probands and FMs.16,23,38
Despite direct contact, an enrollment gap for cascade testing persisted with only 51.0% of FHg+ and 25.4% of FHg− FMs participating. Further research is needed to better understand the reasons for this gap and to implement strategies to bridge it. Easier access to genetic/lipid testing and policies that facilitate direct contact of FMs by health care personnel could increase uptake of cascade testing and enable affected FMs of probands to seek timely treatment for FH.
Study limitations
A limitation of our study is that whereas genetic testing was available to FHg+ families, FHg− FMs were asked to provide lipid panel results or obtain new lipid profiles. This approach was necessitated by the pragmatic nature of our clinical trial but could have inflated the differences in cascade testing metrics between the 2 groups. Another factor that could have had a similar effect is that FHg+, but not FHg− probands, provided contact information for children and FMs beyond first-degree relatives. However, in sensitivity analyses limited to only first-degree relatives of the 2 proband groups, our inferences remained similar (Supplemental Table 4). Owing to logistical challenges, lipid level data could not be collected in FHg+ FMs. The method of direct contact used in this study may not be generalizable to all institutions. Most participants were non-Hispanic Whites, and additional studies are needed in diverse ancestry groups. The polygenic score for LDL-C available at the time of study initiation comprised 12 single nucleotide variants, and an expanded polygenic score has been recently reported.39 The COVID-19 pandemic affected our rate of FHg− proband enrollment. However, power to detect a difference in the main outcome was maintained given the higher-than-expected number of FMs per proband who enrolled in the study.
Conclusion
Our study demonstrates that new case detection after cascade testing for FH was higher in families with monogenic FH than in families without a monogenic etiology, a result of greater uptake and yield of cascade testing in the former. New case detection and uptake of cascade testing for both groups in our study was higher than what has been reported in near-contemporaneous studies in the United States,22,30 most likely a result of direct contact, for which we encountered no major barriers. Our findings provide evidence for adoption of cascade testing for FH in real-world clinical practice and suggest that direct contact of FMs and coordinated genetic/lipid testing can overcome the low uptake and yield of cascade testing for FH in the United States.23,36
Supplementary Material
Acknowledgments
This study was funded by a supplemental grant from the National Human Genome Research Institute (U01HG006379-S) as part of the Electronic Medical Records and Genomics (eMERGE) Network. I.J.K. is additionally funded by National Heart, Lung, and Blood Institute grants HL135879 and HL137010. Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: (NCT03640234).
Footnotes
Ethics Declaration
Probands were asked to complete a study consent form and provide contact information for first-degree family members and consent to the study team to contact such individuals. This study and the informed consent process were approved by the Mayo Institutional Review Board.
Conflict of Interest
The authors declare no conflict of interest.
Additional Information
The online version of this article (https://doi.org/10.1016/j.gim.2022.08.026) contains supplementary material, which is available to authorized users.
Data Availability
Data will be available upon request.
References
- 1.Green RF, Dotson WD, Bowen S, Kolor K, Khoury MJ. Genomics in public health: perspective from the Office of Public Health Genomics at the Centers for Disease Control and Prevention (CDC). Healthcare (Basel). 2015;3(3):830–837. 10.3390/healthcare3030830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury MJ, Feero WG, Chambers DA, et al. Correction: A collaborative translational research framework for evaluating and implementing the appropriate use of human genome sequencing to improve health. PLoS Med. 2018;15(8):e1002650. 10.1371/journal.pmed.1002650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray MF, Evans JP, Khoury MJ. DNA-based population screening: potential suitability and important knowledge gaps. JAMA. 2020;323(4):307–308. 10.1001/jama.2019.18640 [DOI] [PubMed] [Google Scholar]
- 4.Feero WG, Wicklund CA, Veenstra D. Precision medicine, genome sequencing, and improved population health. JAMA. 2018;319(19):1979–1980. 10.1001/jama.2018.2925 [DOI] [PubMed] [Google Scholar]
- 5.Knowles JW, Rader DJ, Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318(4):381–382. 10.1001/jama.2017.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: A scoping review of the literature. Health Aff (Millwood). 2018;37(5):801–808. 10.1377/hlthaff.2017.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidding SS, Champagne MA, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132(22):2167–2192. Published correction appears in Circulation. 2015;132(25):e397. 10.1161/CIR.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 8.Safarova MS, Kullo IJ. My approach to the patient with familial hypercholesterolemia. Mayo Clin Proc. 2016;91(6):770–786. 10.1016/j.mayocp.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Representatives of the Global Familial Hypercholesterolemia Community, Wilemon KA, Patel J, et al. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. 2020;5(2):217–229. Published correction appears in JAMA Cardiol. 2020;5(5):613. 10.1001/jamacardio.2019.5173 [DOI] [PubMed] [Google Scholar]
- 10.Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75(20):2553–2566. 10.1016/j.jacc.2020.03.057 [DOI] [PubMed] [Google Scholar]
- 11.de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133(11):1067–1072. 10.1161/CIRCULATIONAHA.115.018791 [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9(6)(suppl):S1–122.e1. Published correction appears in J Clin Lipidol. 2016;10(1):211. 10.1016/j.jacl.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Urbina EM, de Ferranti SD. Lipid screening in children and adolescents. JAMA. 2016;316(6):589–591. 10.1001/jama.2016.9671 [DOI] [PubMed] [Google Scholar]
- 15.Jackson CL, Huschka T, Borah B, et al. Cost-effectiveness of cascade genetic testing for familial hypercholesterolemia in the United States: a simulation analysis. Am J Prev Cardiol. 2021;8:100245. 10.1016/j.ajpc.2021.100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defesche JC, Lansberg PJ, Umans-Eckenhausen MA, Kastelein JJ. Advanced method for the identification of patients with inherited hypercholesterolemia. Semin Vasc Med. 2004;4(1):59–65. 10.1055/s-2004-822987 [DOI] [PubMed] [Google Scholar]
- 17.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–3490a. Published correction appears in Eur HeartJ. 2020;41(47):4517. 10.1093/eurheartj/eht273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. Published correction appears in Atherosclerosis. 2020;292:160-162. Published correction appears in Atherosclerosis. 2020;294:80-82. 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/ AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. Published correction appears in Circulation. 2019;139(25):e1182-e1186. 10.1161/CIR.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 20.Wierzbicki AS, Humphries SE, Minhas R, Guideline Development Group. Familial hypercholesterolaemia: summary of NICE guidance. BMJ. 2008;337:a1095. 10.1136/bmj.a1095 [DOI] [PubMed] [Google Scholar]
- 21.Lee C, Rivera-Valerio M, Bangash H, Prokop L, Kullo IJ. New case detection by cascade testing in familial hypercholesterolemia: A systematic review of the literature. Circ Genom Precis Med. 2019;12(11):e002723. 10.1161/CIRCGEN.119.002723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajufo E, deGoma EM, Raper A, Yu KD, Cuchel M, Rader DJ. A randomized controlled trial of genetic testing and cascade screening in familial hypercholesterolemia. Genet Med. 2021;23(9):1697–1704. 10.1038/s41436-021-01192-z [DOI] [PubMed] [Google Scholar]
- 23.Campbell-Salome G, Jones LK, Masnick MF, et al. Developing and optimizing innovative tools to address familial hypercholesterolemia underdiagnosis: identification methods, patient activation, and cascade testing for familial hypercholesterolemia. Circ Genom Precis Med. 2021;14(1):e003120. 10.1161/CIRCGEN.120.003120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578–2589. 10.1016/j.jacc.2016.03.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saadatagah S, Jose M, Dikilitas O, et al. Genetic basis of hypercholesterolemia in adults. NPJ Genom Med. 2021;6(1):28. Published correction appears in NPJ Genom Med. 2021;6(1):56. 10.1038/s41525-021-00190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72(6):662–680. 10.1016/j.jacc.2018.05.044 [DOI] [PubMed] [Google Scholar]
- 27.Talmud PJ, Shah S, Whittall R, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381(9874):1293–1301. 10.1016/S0140-6736(12)62127-8 [DOI] [PubMed] [Google Scholar]
- 28.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 29.Kullo IJ, Bailey KR. Design of a controlled trial of cascade screening for hypercholesterolemia: the (CASH) Study. J Pers Med. 2018;8(3):27. 10.3390/jpm8030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullo IJ, Olson J, Fan X, et al. The return of actionable variants empirical (RAVE) study, a Mayo Clinic genomic medicine implementation study: design and initial results. Mayo Clin Proc. 2018;93(11):1600–1610. 10.1016/j.mayocp.2018.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safarova MS, Liu H, Kullo IJ. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH Study. J Clin Lipidol. 2016;10(5):1230–1239. 10.1016/j.jacl.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadfield SG, Horara S, Starr BJ, et al. Family tracing to identify patients with familial hypercholesterolaemia: the second audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann Clin Biochem. 2009;46(Pt 1):24–32. 10.1258/acb.2008.008094 [DOI] [PubMed] [Google Scholar]
- 33.Kullo IJ. Familial hypercholesterolemia: a reportable disorder. Circulation. 2020;142(21):1999–2001. 10.1161/CIRCULATIONAHA.120.050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn J, Milo Rasouly H, Vasquez-Loarte T, et al. Do research participants share genomic screening results with family members? J Genet Couns. 2022;31(2):447–458. 10.1002/jgc4.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newson AJ, Humphries SE. Cascade testing in familial hypercholesterolaemia: how should family members be contacted? Eur J Hum Genet. 2005;13(4):401–408. 10.1038/sj.ejhg.5201360 [DOI] [PubMed] [Google Scholar]
- 36.Safarova MS, Kullo IJ. Lessening the burden of familial hypercholesterolemia using health information technology. Circ Res. 2018;122(1):26–27. 10.1161/CIRCRESAHA.117.312319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrikson NB, Wagner JK, Hampel H, et al. What guidance does HIPAA offer to providers considering familial risk notification and cascade genetic testing? J Law Biosci. 2020;7(1):lsaa071. 10.1093/jlb/lsaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwiter R, Brown E, Murray B, et al. Perspectives from individuals with familial hypercholesterolemia on direct contact in cascade screening. J Genet Couns. 2020;29(6):1142–1150. 10.1002/jgc4.1266 [DOI] [PubMed] [Google Scholar]
- 39.Graham SE, Clarke SL, Wu K-HH, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600 (7890):675–679. 10.1038/s41586-021-04064-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon request.


