Abstract
Parkinson's disease (PD) is the second most frequent neurodegenerative brain disease (NBD) after Alzheimer's disease (AD). Statins are the most common lipid‐lowering agents used in the management of dyslipidemia and the prevention of primary and secondary cardiovascular diseases (CVD) events. In addition, there is a controversial point regarding the role of serum lipids in the pathogenesis of PD. In this bargain, as statins reduce serum cholesterol so they affect the PD neuropathology in bidirectional ways either protective or harmful. Statins are not used in the management of PD, but they are frequently used in the cardiovascular disorders commonly associated with PD in the elderly population. Therefore, the use of statins in that population may affect PD outcomes. Concerning the potential role of statins on PD neuropathology, there are conflicts and controversies either protective against the development of PD or harmful by increasing the risk for the development of PD. Therefore, this review aimed to clarify the precise role of statins in PD regarding the pros and cons from published studies. Many studies suggest a protective role of statins against PD risk through the modulation of inflammatory and lysosomal signaling pathways. Nevertheless, other observations suggest that statin therapy may increase PD risk by diverse mechanisms including reduction of CoQ10. In conclusion, there are strong controversies regarding the protective role of statins in PD neuropathology. Therefore, retrospective and prospective studies are necessary in this regard.
Keywords: neurodegenerative brain disease, Parkinson's disease, statins
Abbreviations
- AD
Alzheimer's disease
- ANS
autonomic nervous system
- ApoAI
apolipoprotein A‐I
- BBB
blood–brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CVD
cardiovascular diseases
- HDL
high‐density lipoprotein
- HMG‐CoA
hydroxyl‐methyl‐glutaryl coenzyme A reductase
- LDL
low‐density lipoprotein
- MPP
1‐methyl‐4‐phenylpyridinium
- PD
Parkinson's disease
- PPAR‐α
peroxisome proliferator‐activated receptor
- ROS
reactive oxygen species
- SN
substantia nigra
- SREBP
sterol regulatory element binding protein
- Tregs
regulatory T cells
- VLDL
very low‐density lipoprotein
1. INTRODUCTION
Parkinson's disease (PD) is the second most frequent neurodegenerative brain disease (NBD) after Alzheimer's disease (AD). 1 , 2 PD was primarily recognized in 1817 by Doctor James Parkinson who illustrates shaking palsy. 3 Of note, PD is developing due to dopaminergic neuron loss in the substantia nigra (SN) with subsequent dopamine deficiency in the caudate nucleus and putamen. 1 These changes promote the development of motor dysfunctions including rigidity, resting tremors and bradykinesia. 4 Besides, numerous non‐motor disorders are present including apathy, depression, anxiety, autonomic disorders, dementia, neuropsychiatric disorders, cognitive dysfunction, and sleep disturbances. 5 Remarkably, the incidence of PD in the general population is 0.3% and reaches 4% above the age of 80 years. 6 The mean age of PD onset is around 60 years; though, early‐onset PD may develop in the younger age group 20–50 years. 7 The annual incidence of PD is 8–18 per 100 000. 7 , 8 Males are more affected than females by PD with a ratio of 3:2. 9 PD may be genetic and non‐genetic due to exposure to pesticides, and manganese though most PD cases are sporadic. 10 , 11
The neuropathological characteristic of PD is the deposition of Lewy bodies from aggregated α‐synuclein (Figure 1). 12
FIGURE 1.
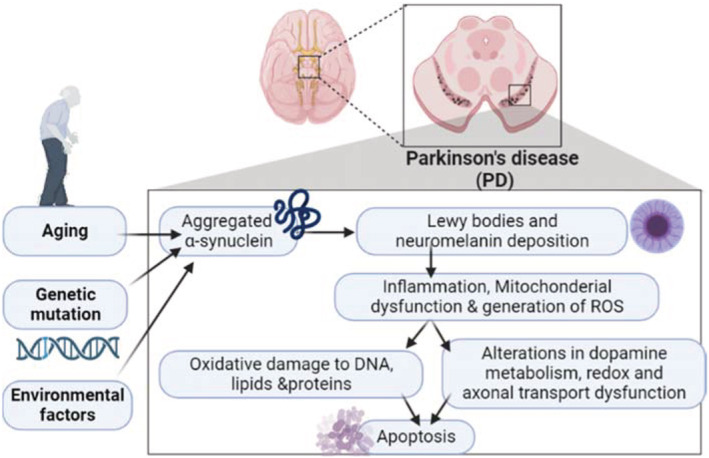
Pathophysiology of Parkinson's disease (PD): Aging, genetic mutations, and environmental factors promote the formation and aggregation of α‐synuclein with subsequent formation of Lewy bodies which lead to inflammation, mitochondrial dysfunction, and generation of reactive oxygen species (ROS). These changes lead to oxidative stress and DNA injury causing alteration of dopamine metabolism and axonal dysfunction with induction of apoptosis of dopaminergic neurons.
Concerning PD neuropathology, aggregation of α‐synuclein is not limited to the SN but extends throughout the entire brain such as the autonomic nervous system (ANS). 12 Deposition of α‐synuclein is progressive for many years before the onset of the symptomatic period. 12 Deposition of α‐synuclein is started at first in the ANS mostly in the dorsal motor nucleus of glossopharyngeal and vagus nerves and then extends to the other brain regions, this called stage I. 13 In stage II, α‐synucleinopathy extends more to brain stem areas including medulla oblongata, locus coeruleus, and pontine tegmentum. In stage III, the SN is mainly affected, and during stage IV α‐synucleinopathy there is intense degeneration of dopaminergic neurons in the SN and pathology of Lewy bodies extended to the temporal cortex. In the last V and VI stages, Lewy bodies are extremely deposited in the neocortex leading to the development of cognitive dysfunction. 13 These observations anticipated that PD neuropathology is not limited to SN degeneration. Remarkably, in the prodromal phase, non‐motor symptoms including anosmia, constipation, sleep disorders, and depression are developing before dopaminergic degeneration in the SN. After the development of motor symptoms, cognitive dysfunctions are propagated due to concern of the temporal cortex. 13 , 14 , 15 As well, PD is linked with the progression of a variety of inflammatory disorders, which are connected with the progression of PD neuropathology. 16
On the other side, statins are the most common lipid‐lowering agents used in the management of dyslipidemia, and primary and secondary prevention of cardiovascular diseases (CVD). 17 In general, statins are not used to treat PD. Statins are used because of CVD reasons. So, the benefit of statins needs to base on their benefit to prevent CVD disorders like stroke and ischemic heart diseases. 17
Statins had pleiotropic effects including stability of atherosclerotic plaques, endothelial protection, prevention of thrombotic events, and antioxidant, and anti‐inflammatory effects. 18 Therefore, statins can be used in a wide range of inflammatory disorders beyond cholesterol‐lowering effects. Statins act through competitive inhibition of hydroxyl‐methyl‐glutaryl coenzyme A (HMG‐CoA) reductase with subsequent inhibition of de novo cholesterol biosynthesis in the liver and other organs including the brain. 19 Reduction of cholesterol biosynthesis is associated with the upregulation of low‐density lipoprotein (LDL) receptors with the further reduction of LDL and circulating cholesterol. 20 In addition, statins increase the expression of protective high‐density lipoprotein (HDL) which reduces peripheral cholesterol concentration. 21 In addition, statins can reduce plasma triglyceride and very low‐density lipoprotein (VLDL) independent of lipoprotein A. 22
Prolonged use of statins is associated with the development of various adverse effects including muscle pain, increase risk of diabetes mellitus, and some cognitive dysfunction. 23 There are two types of statins either lipophilic like atorvastatin and simvastatin or hydrophilic like rosuvastatin. 24 Hydrophilic statins are less cross‐plasma membrane so they have less pleiotropic effects compared to lipophilic statins. 24 Of note, statins are also classified according to their nature, either natural like simvastatin and lovastatin, or synthetic like rosuvastatin. 24 Natural and synthetic statins have different pharmacokinetic properties but they have similar lipid‐lowering effects. Remarkably, statins lead to a dose‐dependent effect in reducing cholesterol and LDL. 25
Regarding the potential role of statins on PD neuropathology, there are conflicts and controversies either protective against the development of PD or harmful by increasing the risk for the development of PD. 26 , 27 Therefore, this review aimed to clarify the role of statins in PD from published studies regarding the pros and cons.
2. LIPID PROFILE IN PD
The brain contains the highest level of cholesterol than other organs and presents as an un‐esterified form. Cholesterol‐bound lipoprotein cannot cross the blood–brain barrier (BBB), so brain cholesterol is exclusively biosynthesized within the brain from glial and astrocyte cells and independent of plasma cholesterol. 28 Therefore, plasma lipid levels do not reflect brain cholesterol concentration, and PD‐related genes are engaged with brain cholesterol metabolism, suggesting a possible link between brain cholesterol and PD neuropathology. 28 Moreover, the reduction of apolipoprotein A‐I (ApoAI) is associated with PD severity and duration. 29 As well, the over‐expression of ApoE is linked with the advancement of PD neuropathology. 30 Higher levels of LDL and HDL in men but not in women reduce PD risk 31 suggesting a protective effect of lipoproteins against the development of PD.
It has been reported that serum lipids play a crucial role in the pathogenesis of NBDs including PD. 32 Increasing serum lipids and their metabolites are correlated with the induction of oxidative stress and aggregation of α‐synuclein in the dopaminergic neurons of the SN. 33 Brain cholesterol metabolism is highly disturbed in PD. 34 A case‐controlled study comprised 64 PD patients compared to 33 healthy control subjects illustrated that brain‐derived cholesterol metabolite (24‐OH‐cholesterol) was not changed compared with a significant reduction of peripherally derived cholesterol metabolite (27‐OH‐cholesterol) which reduced significantly. 34 However, Schönknecht et al. 35 observed that 24‐OH‐cholesterol was increased in the cerebrospinal fluid (CSF) of AD and PD. As well, CSF 24‐OH‐cholesterol is correlated with PD severity and duration. 36 Different studies revealed that high serum lipids and consumption of cholesterol are linked with a higher risk for the development of PD. 37 , 38 However, a meta‐analysis disclosed no association between high cholesterol levels and PD risk. 39
Notably, there are controversial findings concerning plasma lipids and the risk of PD. 31 , 37 However, hypercholesterolemia may increase the risk for the development of PD in young subjects. 37 A prospective study conducted by Hu et al. 37 found that involved 24 773 men and 26 153 women with hypercholesterolemia followed for more than 18 years illustrated that most patients develop PD. A meta‐analysis showed that hypercholesterolemia was associated with increased sporadic PD risk. 40
Moreover, expression of HMG‐CoA reductase is reduced in the fibroblast of PD patients with a subsequent decrease of brain cholesterol. 41 However, cholesterol metabolites are involved in the generation of Lewy bodies through induction aggregation of α‐synuclein. 42 Nevertheless, many studies revealed the protective effects of different plasma levels including cholesterol and triglyceride. 31 , 43 As well, serum lipids like cholesterol, triglyceride, LDL, and VLDL are reduced in PD patients compared with controls. 44 Notoriously, a case–control study observed that reduction of serum LDL was linked with the development of PD. 45 Similarly, a cohort study revealed that higher serum LDL and cholesterol level are associated with a lower risk for the development of PD. 31 Thus, higher cholesterol serum levels may attenuate the progression of PD. 46 Moreover, the highest intake of monosaturated fatty acids and cholesterol can decrease PD risk. 47
Of interest, the mutation in the genes linked with familial PD is connected with the propagation of dyslipidemia. 48 Therefore, there is a genetic connection between PD and hypercholesterolemia. 48 In contrast, higher levels of plasma lipids are associated with reduced risk for future PD. 43 These findings suggest a potential link between lipid metabolism and PD neuropathology, as lipid metabolism is affected by the genetic background of PD. Remarkably, Fabelo et al. 49 observed that marked alterations were found in the lipid raft of the frontal cortex in PD patients compared to the controls. In parallel, phosphatidylinositol and phosphatidylserine were also increased in PD patients. 49
These findings raised a controversial point regarding the role of serum lipids in the pathogenesis of PD. In this bargain, as statins reduce serum cholesterol, they affect the PD neuropathology in bidirectional ways either protective or harmful.
3. PROTECTIVE ROLE OF STATINS IN PD
Different studies revealed that statins have a neuroprotective effect against the development and progression of PD (Table 1). It has been reported that statins mainly the lipophilic ones which cross BBB negatively correlated with the incidence of PD independent of serum lipids. 50 Simvastatin is the mainly potent statin in crossing the BBB, and this particular statin drug negatively correlates with the incidence of PD and shows efficacy in animal models of PD. 50 However, PD mainly occurs in the aging population, who are more vulnerable to cholesterol or lipid‐related disorders, raising questions about whether this possible beneficial effect of statins in PD patients is cholesterol‐dependent or cholesterol‐independent. A clinical study performed by Wahner et al. 51 suggests the protective role of statins against the development of PD. A population‐based design that recruited 312 PD patients and 342 controls from California showed the strongest protective association between statin use and PD risk that was mainly observed in long‐term therapy >15 years. 51 Both gender and age were estimated in this study, though confounding CVD risk factors were not determined which may affect the causal relationship between PD and statins therapy. Of interest, age and sex not affect the clinical outcomes regarding use of statins in PD patients. 51 However, prolonged use of lipophilic statins can reduce PD risk mainly in women and the old age group. 52 A prospective study illustrated that PD risk was low with statins users, and continuation of statins therapy was associated with more reduction in PD risk. 52 Continuation of lipophilic statins was linked with a reduced risk of PD as compared with statin discontinuation, which was not modified by comorbidities or medications. There was no association between hydrophilic statins and the occurrence of PD. Among lipophilic statins, a significant association was observed for simvastatin and atorvastatin, particularly in women users. As for atorvastatin users, the beneficial effect was seen in the elderly subgroup. However, long‐term use of statins, either lipophilic or hydrophilic, was not significantly associated with PD in a dose/duration‐response relation. 52 Therefore, the continuation of lipophilic statin therapy was associated with a decreased incidence of PD as compared to discontinuation in statin users, especially in subgroups of women and the elderly. A long‐term follow‐up study is required to elucidate the potential valuable role of lipophilic statins in PD.
TABLE 1.
Beneficial effects of statins on PD.
| Study type | Findings | Ref. |
|---|---|---|
| Review | Lipophilic statins decrease the incidence of PD independent of serum lipids. | Roy and Pahan 50 |
| A population‐based design | Protective role of statins against the development of PD. | Wahneret al. 51 |
| Prospective study | Prolonged use of lipophilic statins reduces PD risk in women and the old age group. | Lee et al. 52 |
| Prospective study | Lipophilic statins are more effective than hydrophilic statins in the reduction of PD risk. | Gao et al. 53 |
| A longitudinal study | T2DM patients on statins develop less PD compared to statin non‐users. | Lin et al. 54 |
| A retrospective study | Prolonged use of statins for >4 years reduces the incidence of motor development. | Palermo et al. 55 |
| A systematic review and meta‐analysis | Protective effects of statins against the development of PD. | Bykov et al. 56 |
| A systematic review and meta‐analysis | Statin users mainly atorvastatin reduce PD risk. | Yan et al. 57 |
| An experimental study | Simvastatin attenuated LPS‐induced PD in rats. | Tan et al. 59 |
| Review | Statins attenuate the proinflammatory mediators and PD development. |
Bagheri et al. 60 |
| A systematic review and meta‐analysis | Statins use has a protective role against PD risk. | Wu et al. 62 |
Thus, lipophilic statins could be more effective in the reduction of PD risk as compared to hydrophilic statins. Likewise, a large‐scale prospective study revealed that statins users moderately decrease PD risk mainly in patients aged less than 60 years. 53 In this study, 38 192 men and 90 874 women on regular statins use were included and followed for 12 years revealed that the association between statins use and low PD risk was observed in participants younger than 60 years at baseline but not among those who were older 53 suggesting that regular use of statins was linked with a modest reduction in PD risk. A longitudinal study involved one million T2DM with or without statin therapy and followed for 7 years for the development of PD. In this study, T2DM patients on statins develop less PD compared to statin non‐users 54 suggesting a protective role of statins against the development of PD. The PD incidence rate was lower in statin users than in non‐users of statins. The crude hazard ratio of PD incidence in statin users was 0.65 in women and 0.60 in men compared with non‐users of statins. All statins except lovastatin exerted protective effects on PD incidence and had a significant dose‐dependent manner. 54
A retrospective study involved 181 participants, 104 were evaluated (42 patients on statins and 62 were not on statins) revealed that prolonged use of statins for >4 years reduces the incidence of motor development. 55 This study proposed that statins have a neuroprotective role against the development of motor symptoms in PD. A systematic review and meta‐analysis comprised 10 published studies confirmed the protective effects of statins against the development of PD. 56 Similarly, a systematic review and meta‐analysis comprised 17 published studies demonstrated that statin users mainly atorvastatin reduce PD risk. 57 These systematic review and meta‐analysis studies had some limitations including the results were obtained from observational studies, which are susceptible to various biases, selective, information, confounding, and follow‐up biases were unavailable in the cohort and case–control studies, the methods used to diagnose PD varied among the included studies, and most cases were diagnosed using medical records. Therefore, the included cases may have included some patients with secondary PD. Although the results from these studies suggest that statin drugs can reduce the risk of PD, consistent doses and courses of treatment have not yet been established. The numbers of subgroup analyses and analyses of different types of statins were small, and therefore, the results have a limited reference value. As well, researches on the application time, measurement, and types of statin drugs are still lacking, thus related researches are needed.
The protective effects of statins could be by different mechanisms including anti‐inflammatory and antioxidant effects. As well, statins through activation of sterol regulatory element binding protein (SREBP) attenuate the degeneration of dopaminergic neurons in the SN. 58 High cholesterol also stimulated the accumulation of α‐synuclein, and treatment with the cholesterol‐lowering drug lovastatin reduced 1‐methyl‐4‐phenylpyridinium (MPP)‐induced cell death by inhibiting the production of reactive oxygen species (ROS) but did not prevent lysosomal cholesterol increase nor affect α‐synuclein accumulation. Thus, there is a dual role of high cholesterol in PD, in which it acts both as a protector against lysosomal membrane permeabilization and as a stimulator of α‐synuclein accumulation. 58 In addition, statins prevent lysosomal dysfunction and aggregation of α‐synuclein. 59 An experimental study demonstrated that simvastatin attenuated LPS‐induced PD in a rat model by inhibiting inflammatory and oxidative stress‐induced degeneration of dopaminergic neurons in the SN. 59
As well, statins improve the regeneration of dopaminergic neurons and upregulate dopamine transporters. 58 , 59 Palermo et al. 55 revealed statins use for more than 4 years in PD patients reduce the risk of motor deterioration compared to PD patients' non‐used statins. Microglia are the primary innate immune system cells in the central nervous system (CNS). They are crucial for immunity, neurogenesis, synaptogenesis, neurotrophic support, phagocytosis of cellular debris, and maintaining CNS integrity and homeostasis. The CNS injury results in the activation of microglia known as microgliosis. 60 The activated microglia can release proinflammatory mediators leading to neuroinflammation which is associated with PD neuropathology. Evidence has indicated that statins have the potential to attenuate the proinflammatory mediators and subsequent PD by controlling the microglial activation and consequent reduction in neuroinflammatory mediators. 60 A recent mechanistic review demonstrated that statins attenuate the development of neuroinflammation and associated dopaminergic neuronal loss by inhibiting microglial activation and the release of proinflammatory cytokines. 60 An experimental study demonstrated that statins attenuate microglia‐induced neuronal injury by reducing the expression of inflammatory signaling pathways and glucose deprivation. 61 Up‐to‐date evidence from 15 observational studies revealed that statins use has a protective role against PD risk. 62 Following adjusting of confounding factors, the findings of this study showed that long‐duration statin use was linked with a decreased risk of PD. There was no significant decrease in the risk of PD in short‐term statin users. In addition, no significant difference in the reduction in the risk of PD was observed between men and women. 62 This study indicated that only long‐term statin therapy is effective against the development and progression of PD regardless of sex factor.
These findings suggest a protective role of statins against PD risk through the modulation of inflammatory and lysosomal signaling pathways as well as microglia activation.
4. HARMFUL ROLE OF STATINS IN PD
Numerous studies illustrated that statin therapy may be associated with a higher risk for the development of PD (Table 2), based on that cholesterol has a neuroprotective effect against the development of NBDs including PD. For example, Liu et al. 63 showed that statins use was linked with higher PD risk mainly in the initial period which was about 2.5 years. A retrospective case–control analysis involved identified 2322 incident PD cases having a minimum of 2.5 years of continuous enrollment before the earliest diagnosis of PD. A total of 2322 controls were then matched individually by age, gender, and a follow‐up window to explore the relationship of statin use with incident PD. This study revealed that the use of statin (especially lipophilics) was associated with a higher risk of PD, and the stronger association in initial use suggests a facilitating effect. 63 This duration of the study and associated confounding factors may restrict the final conclusions. Short duration of statins therapy <2 years may not be sufficient to affect PD neuropathology. 64 A previous study suggests that 2 years follow‐up is not sufficient to determine the effect of statins on the progression of PD. 64 However, the short‐term effects of statins may associate with an increased risk for the development of PD. 65 Of interest, the use of statins for less than 365 days was correlated with PD risk whereas the duration of more than 365 days was not correlated with PD risk. 65 Interestingly, long‐term statins therapy affects the cellular and molecular metabolic rate of dopaminergic neurons. 27 A recent study demonstrated that prolonged use of statins affects the availability of dopamine transporters in the putamen nucleus of PD patients compared to the controls. 27 This finding suggests that statins had detrimental effects on the expression of dopamine transporters in the SN, and may increase PD risk. Of note, 10‐year follow‐up of patients on statin therapy disclosed a greater risk for the development of PD compared to non‐statin users. 65 An updated clinical trial demonstrated that simvastatin has a potential role in the progression of PD. 66 A total of 216 patients progressed to the 80‐mg dose, the primary outcome analysis showed that the simvastatin group had an additional deterioration in motor symptoms while not taking medication at 24 months compared with the placebo group (p = .006). Thus, simvastatin was ineffective as a disease‐modifying therapy in patients with PD of moderate severity, providing no evidence to support proceeding to a phase 3 trial. 66 These observations may explain the duration‐dependent effect of statins in the progression of PD.
TABLE 2.
Detrimental effects of statins on PD.
| Study type | Findings | Ref. |
|---|---|---|
| A retrospective study | The use of statins (especially lipophilics) was associated with a higher risk of PD. | Liu et al. 63 |
| A prospective study | Short‐term effects of statins may associate with an increased risk for the development of PD. | Jeong et al. 65 |
| A clinical trial study | Simvastatin deteriorates motor symptoms in PD patients. | Stevens et al. 66 |
| An experimental study | Atorvastatin increases the severity of MPTP‐induced PD in mice | Kreisler et al. 67 |
| An experimental study | Statins may initially exacerbate motor dysfunction in PD via the reduction of expression of dopamine transporters. | Fukui et al. 71 |
| A prospective study | Lipophilic statins lead to a more detrimental effect on PD neuropathology. | Jeong et al. 27 |
| A case–control study | Lipophilic but not hydrophilic statins for more than 12 months were associated with a higher risk for the development of PD. | Kim et al. 74 |
| A prospective study | Hydrophilic statins are linked with the rapid progression of PD neuropathology. | Lewis et al. 75 |
The harmful effect of statins was suggested in a preclinical study, that atorvastatin increases the severity of 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced PD in mice. 67 A previous experimental study tested the capability of simvastatin, atorvastatin, fenofibrate, and bezafibrate (two synthetic peroxisome proliferator‐activated receptor‐alpha (PPAR‐α) agonists) to prevent dopaminergic cell death in the MPTP mouse model of PD illustrated that both of fenofibrate and bezafibrate via activation of peroxisome proliferator‐activated receptor (PPAR‐α) in the dopaminergic cell had neuroprotective effects in PD by inhibiting of inflammation, oxidative stress, and apoptosis. 67 However, simvastatin and atorvastatin were infective and produced negative impacts. 67
The underlying mechanism of the harmful effect of statins on PD neuropathology is through the reduction of CoQ10 which has an anti‐apoptotic effect and stabilizes the mitochondrial membrane. 68 Statin‐treated patients frequently feature decreased muscle coenzyme Q10 (CoQ10) contents, suggesting that statins might impair mitochondrial function. In cell cultures, statins diminish muscle oxygen consumption, promote mitochondrial permeability transient pore opening, and generate apoptotic proteins. 68 Animal models confirm the statin‐induced decrease in muscle CoQ10 but reveal no changes in mitochondrial enzyme activities. Human studies yield contradictory results, with decreased CoQ10, elevated lipids, decreased enzyme activities in muscle, and impaired maximal oxygen uptake in several but not all studies. Some patients are susceptible to statin‐induced myopathy due to variations in genes encoding proteins. 68
Reduction of dopamine transporters and/or endocytosis in the SN might be due to statins effects on brain cholesterol which is fundamental for functional dopamine transporters. 69 , 70 Therefore, statins may initially exacerbate motor dysfunction in PD via the reduction of expression of dopamine transporters. Further, statins may affect synaptic stability and may increase the risk of PD dementia. 71 Reduction in cellular cholesterol also resulted in increased basal autophagy and impairment of induction of autophagy by glucose deprivation. Together, these data indicate that a reduction in neuron‐derived cholesterol content, similar to that observed in the diabetic brain, creates a state of insulin and growth factor resistance that could contribute to CNS‐related complications of diabetes, including increased risk of neurodegenerative diseases, such as AD. 71 However, statins improve rather than reduce cognitive function in AD patients. 18 , 72 Notably, lipophilic statins lead to a more detrimental effect on PD neuropathology. 27 The detrimental effect of statins on PD neuropathology is through direct effects on the neurons rather than by reducing serum cholesterol. 27 As well, statins‐induced mevalonate metabolites promote PD neuropathology through the induction of apoptosis and microglial activation. 27 This study suggested that statins use is linked with the development of PD. However, this recent study had many limitations which affect the final conclusions. For example, biomarkers reflecting brain cholesterol levels, such as 24 S‐OH cholesterol and 27 S‐ OH cholesterol were not measured. As well, the use of statins can induce selection bias, in that statins are usually prescribed for patients with CVDs. In addition, duration of statin use prior to diagnosis of PD and dose of statins was not available in this study. 27
As well, an autoimmune response to autoantigens could be effective in the prevention of PD, and regulatory T cells (Tregs) inhibit this reaction. Thus, statins through induction expression of Tregs may attenuate the beneficial effect of autoimmune response with subsequent increased risk of PD. 73 A case–control study from the Korean population comprised 3026 PD patients compared to 12 104 healthy controls revealed that the use of lipophilic but not hydrophilic statins for more than 12 months was associated higher risk for the development of PD. 74 However, in this study, actual adherence of patients to statins therapy was not clarified. As well, blood cholesterol following statins therapy and its correlation with PD symptoms were not determined. Most of the cases were obtained from databases, so misdiagnosed PD cases cannot be excluded. Besides, the duration of statins therapy, dietary patterns, other medications, and confounding factors was not estimated. These factors may limit the final conclusion between statins use and PD risk.
Notoriously, hydrophilic statins are linked with the rapid progression of PD neuropathology, 75 and lipophilic statins did not affect the progression of PD according to clinical scores and imaging findings. 75 In addition, hydrophilic statins lead to the rapid progression of non‐motor symptoms of PD patients. 75 In this study, statins use was evaluated at baseline and subtyped to hydrophobicity (n = 125) with the determination of clinical and imaging scores following 18 months. 75 This study had a short duration of statins therapy that may affect the clinical outcome mainly for lipophilic statins which required long‐term effects. 27 Thus, statins class‐dependent effect on the pathogenesis of PD is reversed in this state. Genetic variation of HMG‐CoA reductase may affect statins effect on the PD neuropathology, and variability of haplotype 7 of HMG‐CoA reductase can decrease the neuroprotective effect of statins. 76 Variability in HMGCR affects statins response in patients with dyslipidemia. 76 These observations suggest that statins therapy for primary or secondary prevention of CVDs may increase PD risk by different mechanisms including reduction of CoQ10.
Taken together, according to the recent and updated studies, controversial points still present regarding the short‐ and long‐term effects of statins on PD neuropathology.
5. CONCLUSIONS
PD is the second most common NBD consequent to AD. Statins are the most common lipid‐lowering agents used in the management of dyslipidemia and the prevention of primary and secondary CVD events. Statins are not used in the management of PD, but they are frequently used in the cardiovascular disorders commonly associated with PD in the elderly population. Therefore, the use of statins in that population may affect PD outcomes. Statins have many pleiotropic effects like antioxidant and anti‐inflammatory effects. The possible effects of statins on PD neuropathology are conflicting either protective or harmful on PD neuropathology. As well, there is a controversial point regarding the role of serum lipids in the pathogenesis of PD. In this bargain, as statins reduce serum cholesterol, they may affect the PD neuropathology in bidirectional ways either protective or harmful. The protective role of statins against PD risk is through modulation of inflammatory, lysosomal signaling pathways, and microglia activation. However, long‐term statins therapy for primary or secondary prevention of CVDs may increase PD risk by different mechanisms including reduction of CoQ10. Taken together, according to the recent and updated studies, controversial points still present regarding the short‐ and long‐term effects of statins on PD neuropathology. Therefore, large‐scale prospective and retrospective studies are warranted in this regard to confirm the mechanistic role of statins in PD neuropathology.
AUTHOR CONTRIBUTIONS
Hayder M. Al‐kuraishy and Ali I. Al‐Gareeb conceptualized the manuscript, wrote, edited, and reviewed the main text and approved the final edition of the manuscript. Athanasios Alexiou, Marios Papadakis, Abdulrahman A. Alsayegh, Najlaa Hamed Almohmadi, Hebatallah M. Saad, and Gaber El‐Saber Batiha prepared the figures, wrote, corrected, amended, and approved the final edition of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENTS
No. Open Access funding enabled and organized by Projekt DEAL.
Al‐kuraishy HM, Al‐Gareeb AI, Alexiou A, et al. Pros and cons for statins use and risk of Parkinson's disease: An updated perspective. Pharmacol Res Perspect. 2023;11:e01063. doi: 10.1002/prp2.1063
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- 1. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):1‐21. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Kuraishy HM, Al‐Gareeb AI, Alsayegh AA, et al. A potential link between visceral obesity and risk of Alzheimer's disease. Neurochem Res. 2022;48:745‐766. doi: 10.1007/s11064-022-03817-4 [DOI] [PubMed] [Google Scholar]
- 3. Xiao‐dan W, Yong J. A 200‐year history of Parkinson's disease. Chin J Contemp Neurol Neurosurg. 2017;17(1):5. [Google Scholar]
- 4. Varalta V, Picelli A, Fonte C, et al. Relationship between cognitive performance and motor dysfunction in patients with Parkinson's disease: a pilot cross‐sectional study. Biomed Res Int. 2015;2015:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poewe W. Non‐motor symptoms in Parkinson's disease. Eur J Neurol. 2008;15:14‐20. [DOI] [PubMed] [Google Scholar]
- 6. Park J‐H, Kim D‐H, Kwon D‐Y, et al. Trends in the incidence and prevalence of Parkinson's disease in Korea: a nationwide, population‐based study. BMC Geriatr. 2019;19(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macleod AD, Henery R, Nwajiugo PC, Scott NW, Caslake R, Counsell CE. Age‐related selection bias in Parkinson's disease research: are we recruiting the right participants? Parkinsonism Relat Disord. 2018;55:128‐133. [DOI] [PubMed] [Google Scholar]
- 8. Ou Z, Pan J, Tang S, et al. Global trends in the incidence, prevalence, and years lived with disability of parkinson's disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9:776847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerri S, Mus L, Blandini F. Parkinson's disease in women and men: what's the difference? J Parkinsons Dis. 2019;9(3):501‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson's disease. The Lancet Neurology. 2020;19(2):170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JJ, Bandres‐Ciga S, Blauwendraat C, Gan‐Or Z, Consortium IPsDG . No genetic evidence for involvement of alcohol dehydrogenase genes in risk for Parkinson's disease. Neurobiol Aging. 2020;87(140):e19‐e140. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46:S30‐S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson's disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(s1):S71‐S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berg D, Borghammer P, Fereshtehnejad S‐M, et al. Prodromal Parkinson disease subtypes—key to understanding heterogeneity. Nat Rev Neurol. 2021;17(6):349‐361. [DOI] [PubMed] [Google Scholar]
- 15. Ferrazzoli D, Ortelli P, Madeo G, Giladi N, Petzinger GM, Frazzitta G. Basal ganglia and beyond: the interplay between motor and cognitive aspects in Parkinson's disease rehabilitation. Neurosci Biobehav Rev. 2018;90:294‐308. [DOI] [PubMed] [Google Scholar]
- 16. Koziorowski D, Tomasiuk R, Szlufik S, Friedman A. Inflammatory cytokines and NT‐proCNP in Parkinson's disease patients. Cytokine. 2012;60(3):762‐766. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Kuraishy HM, Al‐Gareeb AI, Hussien NR, Al‐Naimi MS, Rasheed HA. Statins an oft‐prescribed drug is implicated in peripheral neuropathy: the time to know more. J Pak Med Assoc. 2019;69(8):S108‐S112. [PubMed] [Google Scholar]
- 18. Alsubaie N, Al‐Kuraishy HM, Al‐Gareeb AI, et al. Statins use in Alzheimer disease: bane or boon from frantic search and narrative review. Brain Sci. 2022;12(10):1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Kuraishy HM, Al‐Gareeb AI. Acylation‐stimulating protein is a surrogate biomarker for acute myocardial infarction: role of statins. J Lab Physicians. 2017;9(3):163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Kuraishy HM, Hussian NR, Al‐Naimi MS, Al‐Gareeb AI. Statins role in vitiligo: a mini‐review. urk J Dermatol. 2020;14(1):1. [Google Scholar]
- 21. Al‐Kuraishy HM, Al‐Gareeb AI, Naji MT. Brain natriuretic peptide in patients with acute ischemic stroke: role of statins. Biomed Biotechnol Res J. 2020;4(3):239. [Google Scholar]
- 22. Kadhim SS, Al‐Windy SA, Al‐Nami MS, Al Kuraishy HM, Al Gareeb AI. Statins improve periodontal disease–induced inflammatory changes and associated lipid peroxidation in patients with dyslipidemia: two birds by one stone. J Int Oral Health. 2020;12(1):66. [Google Scholar]
- 23. Kadhim SS, Al‐Windy SA, Al‐Nami MS, Al‐kuraishy HM, Al‐Gareeb AI. Possible role of statins on the inflammatory biomarkers in patients with periodontal disease: a cross‐sectional study. Dent Hypotheses. 2019;10(3):70. [Google Scholar]
- 24. Al‐Kuraishy HM, Al‐Gareeb AI, Naji MT. Statin therapy associated with decreased neuronal injury measured by serum S100β levels in patients with acute ischemic stroke. Int J Crit Illn Inj Sci. 2021;11(4):246‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al‐Kuraishy HM, Hussien NR, Al‐Naimi MS, Al‐Gareeb AI, Lugnier C. Statins therapy improves acute ischemic stroke in patients with cardio‐metabolic disorders measured by lipoprotein‐associated phospholipase A2 (Lp‐PLA2): new focal point. Neurol India. 2021;69(6):1637‐1644. [DOI] [PubMed] [Google Scholar]
- 26. Becker C, Meier CR. Statins and the risk of Parkinson disease: an update on the controversy. Expert Opin Drug Saf. 2009;8(3):261‐271. [DOI] [PubMed] [Google Scholar]
- 27. Jeong SH, Lee HS, Chung SJ, et al. Effects of statins on dopamine loss and prognosis in Parkinson's disease. Brain. 2021;144(10):3191‐3200. [DOI] [PubMed] [Google Scholar]
- 28. Jin U, Park SJ, Park SM. Cholesterol metabolism in the brain and its association with Parkinson's disease. Exp Neurobiol. 2019;28(5):554‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiang JK, Wong YC, Siderowf A, et al. Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann Neurol. 2013;74(1):119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pang S, Li J, Zhang Y, Chen J. Meta‐analysis of the relationship between the APOE gene and the onset of Parkinson's disease dementia. Parkinson's Dis. 2018;2018:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rozani V, Gurevich T, Giladi N, et al. Higher serum cholesterol and decreased Parkinson's disease risk: a statin‐free cohort study. Mov Disord. 2018;33(8):1298‐1305. [DOI] [PubMed] [Google Scholar]
- 32. Xicoy H, Wieringa B, Martens GJ. The role of lipids in Parkinson's disease. Cell. 2019;8(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul R, Choudhury A, Borah A. Cholesterol–a putative endogenous contributor towards Parkinson's disease. Neurochem Int. 2015;90:125‐133. [DOI] [PubMed] [Google Scholar]
- 34. Huang X, Sterling NW, Du G, et al. Brain cholesterol metabolism and Parkinson's disease. Mov Disord. 2019;34(3):386‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schönknecht P, Lütjohann D, Pantel J, et al. Cerebrospinal fluid 24 S‐hydroxycholesterol is increased in patients with Alzheimer's disease compared to healthy controls. Neurosci Lett. 2002;324(1):83‐85. [DOI] [PubMed] [Google Scholar]
- 36. Meaney S, Lovgren‐Sandblom A, Brodin L, et al. Oxysterols and Parkinson's disease: evidence that levels of 24S‐hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett. 2013;555:102‐105. [DOI] [PubMed] [Google Scholar]
- 37. Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J. Total cholesterol and the risk of Parkinson disease. Neurology. 2008;70(21):1972‐1979. [DOI] [PubMed] [Google Scholar]
- 38. Miyake Y, Sasaki S, Tanaka K, et al. Dietary fat intake and risk of Parkinson's disease: a case‐control study in Japan. J Neurol Sci. 2010;288(1–2):117‐122. [DOI] [PubMed] [Google Scholar]
- 39. Gudala K, Bansal D, Muthyala H. Role of serum cholesterol in Parkinson's disease: a meta‐analysis of evidence. J Parkinsons Dis. 2013;3(3):363‐370. [DOI] [PubMed] [Google Scholar]
- 40. Klemann CJ, Martens GJ, Sharma M, et al. Integrated molecular landscape of Parkinson's disease. Npj Parkinson's Disease. 2017;3(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Musanti R, Parati E, Lamperti E, Ghiselli G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem Med Metab Biol. 1993;49(2):133‐142. [DOI] [PubMed] [Google Scholar]
- 42. Nakamura K, Mori F, Tanji K, et al. Isopentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathology. 2015;35(5):432‐440. [DOI] [PubMed] [Google Scholar]
- 43. Fang F, Zhan Y, Hammar N, et al. Lipids, apolipoproteins, and the risk of Parkinson disease: a prospective cohort study and a mendelian randomization analysis. Circ Res. 2019;125(6):643‐652. [DOI] [PubMed] [Google Scholar]
- 44. Guo X, Song W, Chen K, et al. The serum lipid profile of Parkinson's disease patients: a study from China. Int J Neurosci. 2015;125(11):838‐844. [DOI] [PubMed] [Google Scholar]
- 45. Huang X, Chen H, Miller WC, et al. Lower low‐density lipoprotein cholesterol levels are associated with Parkinson's disease. Mov Disord. 2007;22(3):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang X, Auinger P, Eberly S, et al. Serum cholesterol and the progression of Parkinson's disease: results from DATATOP. PLoS One. 2011;6(8):e22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan LC, Methawasin K, Tan E‐K, et al. Dietary cholesterol, fats and risk of Parkinson's disease in the Singapore Chinese health study. J Neurol Neurosurg Psychiatry. 2016;87(1):86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macías‐García D, Periñán MT, Muñoz‐Delgado L, et al. Serum lipid profile among sporadic and familial forms of Parkinson's disease. Npj Parkinson's Dis. 2021;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fabelo N, Martín V, Santpere G, et al. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson's disease and incidental Parkinson's disease. Mol Med. 2011;17(9):1107‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17(3):244‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Statin use and the risk of Parkinson disease. Neurology. 2008;70(16 Part 2):1418‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee Y‐C, Lin C‐H, Wu R‐M, et al. Discontinuation of statin therapy associates with Parkinson disease: a population‐based study. Neurology. 2013;81(5):410‐416. [DOI] [PubMed] [Google Scholar]
- 53. Gao X, Simon KC, Schwarzschild MA, Ascherio A. Prospective study of statin use and risk of Parkinson disease. Arch Neurol. 2012;69(3):380‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin KD, Yang CY, Lee MY, Ho SC, Liu CK, Shin SJ. Statin therapy prevents the onset of Parkinson disease in patients with diabetes. Ann Neurol. 2016;80(4):532‐540. [DOI] [PubMed] [Google Scholar]
- 55. Palermo G, Giannoni S, Giuntini M, et al. Statins in Parkinson's disease: influence on motor progression. J Parkinsons Dis. 2021;11:1‐12. [DOI] [PubMed] [Google Scholar]
- 56. Bykov K, Yoshida K, Weisskopf MG, Gagne JJ. Confounding of the association between statins and Parkinson disease: systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2017;26(3):294‐300. [DOI] [PubMed] [Google Scholar]
- 57. Yan J, Qiao L, Tian J, et al. Effect of statins on Parkinson's disease: a systematic review and meta‐analysis. Medicine. 2019;98(12):e14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eriksson I, Nath S, Bornefall P, Giraldo AMV, Öllinger K. Impact of high cholesterol in a Parkinson's disease model: prevention of lysosomal leakage versus stimulation of α‐synuclein aggregation. Eur J Cell Biol. 2017;96(2):99‐109. [DOI] [PubMed] [Google Scholar]
- 59. Tan W, Xue‐bin C, Tian Z, et al. Effects of simvastatin on the expression of inducible nitric oxide synthase and brain‐derived neurotrophic factor in a lipopolysaccharide‐induced rat model of Parkinson disease. Int J Neurosci. 2016;126(3):278‐286. [DOI] [PubMed] [Google Scholar]
- 60. Bagheri H, Ghasemi F, Barreto GE, Sathyapalan T, Jamialahmadi T, Sahebkar A. The effects of statins on microglial cells to protect against neurodegenerative disorders: a mechanistic review. Biofactors. 2020;46(3):309‐325. [DOI] [PubMed] [Google Scholar]
- 61. Lu D, Shen L, Mai H, et al. HMG‐CoA reductase inhibitors attenuate neuronal damage by suppressing oxygen glucose deprivation‐induced activated microglial cells. Neural Plast. 2019;2019:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu C‐C, Islam MM, Lee A‐J, et al. Association between statin use and risk of Parkinson's disease: evidence from 18 observational studies comprising 3.7 million individuals. J Pers Med. 2022;12(5):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu G, Sterling NW, Kong L, et al. Statins may facilitate Parkinson's disease: insight gained from a large, national claims database. Mov Disord. 2017;32(6):913‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolozin B, Wang SW, Li N‐C, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med. 2007;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jeong SM, Jang W, Shin DW. Association of statin use with Parkinson's disease: dose–response relationship. Mov Disord. 2019;34(7):1014‐1021. [DOI] [PubMed] [Google Scholar]
- 66. Stevens KN, Creanor S, Jeffery A, et al. Evaluation of simvastatin as a disease‐modifying treatment for patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2022;79:1232‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kreisler A, Gelé P, Wiart J‐F, Lhermitte M, Destée A, Bordet R. Lipid‐lowering drugs in the MPTP mouse model of Parkinson's disease: fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG‐CoA reductase inhibitors do not. Brain Res. 2007;1135:77‐84. [DOI] [PubMed] [Google Scholar]
- 68. Apostolopoulou M, Corsini A, Roden M. The role of mitochondria in statin‐induced myopathy. Eur J Clin Investig. 2015;45(7):745‐754. [DOI] [PubMed] [Google Scholar]
- 69. Jones KT, Zhen J, Reith ME. Importance of cholesterol in dopamine transporter function. J Neurochem. 2012;123(5):700‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schmitt M, Dehay B, Bezard E, Garcia‐Ladona FJ. Harnessing the trophic and modulatory potential of statins in a dopaminergic cell line. Synapse. 2016;70(3):71‐86. [DOI] [PubMed] [Google Scholar]
- 71. Fukui K, Ferris HA, Kahn CR. Effect of cholesterol reduction on receptor signaling in neurons. J Biol Chem. 2015;290(44):26383‐26392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Al‐kuraishy HM, Al‐Gareeb AI, Saad HM, Batiha GE‐S. Benzodiazepines in Alzheimer's disease: beneficial or detrimental effects. Inflammopharmacology. 2022;1‐10. doi: 10.1007/s10787-022-01099-4 [DOI] [PubMed] [Google Scholar]
- 73. Mascitelli L, Pezzetta F, Goldstein MR. Total cholesterol and the risk of Parkinson disease. Neurology. 2009;72(9):860‐861. [DOI] [PubMed] [Google Scholar]
- 74. Kim JH, Chang IB, Kim YH, Kwon MJ, Kim J‐H, Choi HG. Association between statin use and Parkinson's disease in Korean patients with hyperlipidemia. Parkinsonism Relat Disord. 2022;97:15‐24. [DOI] [PubMed] [Google Scholar]
- 75. Lewis MM, Albertson RM, Du G, Kong L, Foy A, Huang X. Parkinson's disease progression and statins: hydrophobicity matters. J Parkinsons Dis. 2022;12;1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pierzchlińska A, Droździk M, Białecka M. A possible role for HMG‐CoA reductase inhibitors and its association with HMGCR genetic variation in Parkinson's disease. Int J Mol Sci. 2021;22(22):12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.


