Abstract
DNA methylation (DNAm) is a plausible mechanism underlying cardiometabolic abnormalities, but evidence is limited among youth. This analysis included 410 offspring of the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) birth cohort followed up to two time points in late childhood/adolescence. At Time 1, DNAm was quantified in blood leukocytes at long interspersed nuclear elements (LINE-1), H19, and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2), and at Time 2 in peroxisome proliferator-activated receptor alpha (PPAR-α). At each time point, cardiometabolic risk factors were assessed including lipid profiles, glucose, blood pressure, and anthropometry. Linear mixed effects models were used for LINE-1, H19, and 11β-HSD-2 to account for the repeated-measure outcomes. Linear regression models were conducted for the cross-sectional association between PPAR-α with the outcomes. DNAm at LINE-1 was associated with log glucose at site 1 [β = −0.029, p = 0.0006] and with log high-density lipoprotein cholesterol at site 3 [β = 0.063, p = 0.0072]. 11β-HSD-2 DNAm at site 4 was associated with log glucose (β = −0.018, p = 0.0018). DNAm at LINE-1 and 11β-HSD-2 was associated with few cardiometabolic risk factors among youth in a locus-specific manner. These findings underscore the potential for epigenetic biomarkers to increase our understanding of cardiometabolic risk earlier in life.
Keywords: cardiometabolic risk factors, population-based study, children and adolescents, Mexicans, biomarkers, epigenetics, DNA methylation
1. Introduction
Obesity is rising worldwide among children aged 5–19 years. In Latin America and the Caribbean region, prevalence rose over a 40-year period from 1.6% and 1.8% in 1975 to 10.4% and 13.4% in 2016 for girls and boys, respectively [1]. Obesity has been associated with increases in the risk and prevalence of cardiometabolic abnormalities among youth [2,3,4]. A cluster of cardiometabolic abnormalities, called metabolic syndrome [5,6], is considered a risk factor for cardiovascular disease (CVD) incidence, cardiovascular-related mortality, all-cause mortality [7,8], and other chronic diseases [9,10]. Rising prevalence of metabolic syndrome may be a driver of the CVD and type-2 diabetes epidemics [11]. Even though CVD outcomes are manifested in middle and late adulthood, cardiometabolic risk factors may become evident during childhood [12,13,14,15,16,17] and track into adulthood [4,18,19]. Identifying the determinants of cardiometabolic risk factors in youth could serve as a fundamental step for risk reduction and prevention [4,20].
Epigenetic modification is one potential underlying mechanism in obesity, cardiometabolic abnormalities, and CVD [21,22,23,24,25,26,27,28]. Previous research highlighted the importance of epigenetics as a potential biomarker for screening, diagnosis, prognosis, and individualized treatment regimens [23,24,29,30,31]. DNA methylation (DNAm), a commonly studied epigenetic modification, has been associated with CVD [21,22,23,24,25,26,27,32,33] and cardiometabolic risk factors, mainly in adults [21,28,34,35,36]. Existing evidence showed that DNAm during early development was associated with obesity and CVD risk later in life [37]; early embryogenesis is a particularly sensitive time period for epigenetic alteration by environmental factors that may contribute to disease risk [38]. However, adolescence is also a susceptible period for the impact of environmental stimuli on DNAm [39,40]. Furthermore, adolescence is characterized by changes in body composition and hormonal milieu [41]—the hallmarks for cardiometabolic abnormalities [42]. Despite the importance of this milestone, scare evidence is available investigating the potential of using DNAm as biomarkers for cardiometabolic health among children and adolescents.
The current study will address this gap in knowledge by examining the association of DNAm in blood leukocytes with cardiometabolic risk factors among Mexican children and adolescents enrolled in the Early Life Exposures in Mexico to ENvironmental Toxicants (ELEMENT) Cohort. Specifically, we quantified CpG site-specific DNAm at repetitive elements (long interspersed nuclear element-1, LINE-1), which comprises 15–17% of the human genome [43,44]. DNAm of LINE-1 is often used as a proxy measure for global DNAm [45], and it has been found to associate with CVD independent from well-established CVD risk factors in adults [46]. The other three genes are H19, 11β -hydroxysteroid dehydrogenase type 2 (11β-HSD-2), and peroxisome proliferator-activated receptor alpha (PPAR-α), which were selected based on their associations with components of cardiometabolic health. H19 is an imprinted gene with a role in regulating cell formation and proliferation, body weight, adipogenesis, and brown adipose tissue thermogenesis [47,48,49,50], and abnormal fat partitioning is a crucial underlying factor in impaired cardiometabolic health [51]. 11β-HSD-2 converts cortisol to an inactive metabolite called cortisone [52,53]. Previous studies have associated 11β-HSD-2 regulation with blood pressure [54,55,56], insulin sensitivity [57], and type 2 diabetes [58]. Blood pressure and glucose hemostasis are cornerstones in assessing cardiometabolic health; however, the latter is of great interest for Hispanic youth as insulin resistance was reported among normal-weight Mexican youth [59]. Lastly, PPAR-α controls multiple lipid metabolism pathways [60,61], and it was associated with serum triglycerides [62]. PPAR-α dysregulation is thought to play a role in dyslipidemia, diabetes, and obesity [63]. Based on functions of the genes and results from related studies, we hypothesized that altered DNAm of these regions would associate with cardiometabolic health measures (waist circumference, blood pressure, and serum glucose, high-density lipoprotein cholesterol, and triglycerides) in children and adolescents.
2. Results
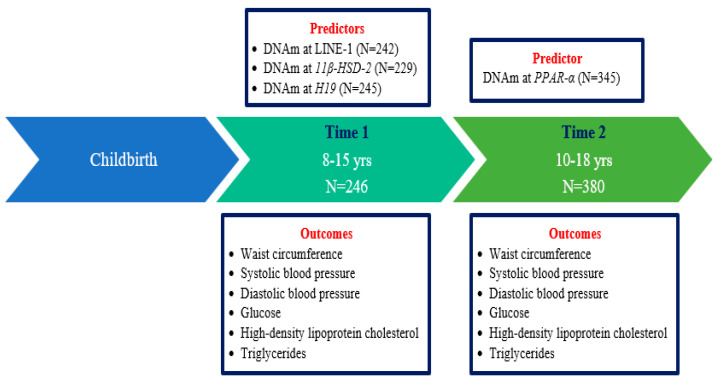
We assessed DNAm and cardiometabolic outcomes at one to two time points each in children from the ELEMENT cohort. The final sample sizes for LINE-1, 11β-HSD-2, and H19 were 242, 229, and 245 subjects, respectively, with DNAm at Time 1 and outcomes at Time 1 and/or Time 2. For PPAR-α, 345 subjects had DNAm and outcomes at Time 2 (Figure 1). Table 1 shows the demographic characteristics of the 410 children by time point. At Time 1, the mean (standard deviation (SD)) age of the sample was 10.34 (1.67) years and 53.25% were female. At Time 2, the mean age was 14.08 (2.03) years and 51.32% were female. Among cardiometabolic risk factors, only waist circumference and serum triglycerides values were greater at Time 2 than at Time 1. We examined the crude association between DNAm values across sites within each genomic region. We found that LINE-1, H19, and PPAR-α were moderately to strongly correlated with one another. 11β-HSD-2 sites were less correlated as we have observed in past studies with this same gene (Supplementary Tables S1–S4).
Figure 1.
Summary of the Main Predictors and Outcomes for this Study and Number of Participants with the Data from the Early Life Exposures in Mexico to ENvironmental Toxicants (ELEMENT) Cohort. Abbreviations: DNAm = DNA methylation; Long interspersed nuclear elements (LINE-1); 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2); Peroxisome proliferator-activated receptor alpha (PPAR-α).
Table 1.
Descriptive Statistics of Mother and Child Characteristics of the Early Life Exposures in the Mexico to ENvironmental Toxicants (ELEMENT) Analytical Sample.
| Time 1 n = 246 |
Time 2 n = 380 |
|
|---|---|---|
| Maternal Characteristics (At Time of Child’s Birth) | ||
| Years of education, % | ||
| <12 years | 121 (49.19) 1 | 196 (51.58) 2 |
| 12 years | 90 (36.59) 1 | 131 (34.47) 2 |
| >12 years | 34 (13.82) 1 | 52 (13.68) 2 |
| Age at childbirth, (years) | 26.86 (5.64) 1 | 26.47 (5.46) 2 |
| Parity, % | ||
| 1 | 90 (36.59) 1 | 144 (37.89) 2 |
| 2 | 89 (36.18) 1 | 135 (35.53) 2 |
| ≥3 | 66 (26.83) 1 | 100 (26.32) 2 |
| Marital status, % | ||
| Married | 175 (71.14) 1 | 274 (72.11) 2 |
| Other | 70 (28.46) 1 | 105 (27.63) 2 |
| Enrollment in calcium supplementation study, % | ||
| Not enrolled | 152 (61.79) 1 | 257 (67.63) 2 |
| Enrolled | 93 (37.80) 1 | 122 (32.11) 2 |
| Child Characteristics (At birth) | ||
| Female, % | 131 (53.25) | 195 (51.32) |
| Gestation age, (weeks) | 38.85 (1.49) 3 | 38.79 (1.61) 4 |
| Mode of delivery, % | ||
| Vaginal delivery | 140 (56.91) 5 | 220 (57.89) 6 |
| C-Section | 103 (41.87) 5 | 158 (41.58) 6 |
| Birth weight, (kg) | 3.15 (0.45) 7 | 3.15 (0.48) 6 |
| Breastfeeding duration, (months) | 8.15 (5.91) 1 | 8.09 (6.07) 2 |
| Child Characteristics (At follow-up visits) | ||
| Age, (years) | 10.34 (1.67) | 14.08 (2.03) |
| Body mass index Z-score for age | 0.85 (1.24) | 0.53 (1.26) 6 |
| Metabolic equivalents, (METs/week) | 31.38 (19.97) | 60.63 (38.76) |
| Total caloric intake, (kcal/day) | 2636.32 (839.83) | 2371.62 (936.82) |
| Pubertal onset, % | 103 (41.87) | 350 (92.11) 8 |
| Cardiometabolic risk factors (Outcomes) | ||
| Waist circumference, (cm) | 70.81 (10.71) | 79.14 (11.42) |
| Systolic blood pressure, (mmHg) | 102.74 (10.24) | 97.23 (9.62) |
| Diastolic blood pressure, (mmHg) | 65.58 (7.31) | 62.24 (6.71) |
| Fasting glucose, (mg/dL) | 86.98 (9.38) | 77.48 (7.05) 9 |
| High-density lipoprotein cholesterol, (mg/dL) | 58.76 (11.92) | 42.95 (8.87) 9 |
| Triglycerides, (mg/dL) | 87.89 (44.40) | 105.81 (57.47) 9 |
| DNAm (Predictors) | ||
| LINE-1 DNAm, % (averaged across 4 CpG sites) | 78.49 (2.31) 5 | N/A |
| 11β-HSD-2 DNAm, % (averaged across 5 CpG sites) a | −0.85 (1.34) | N/A |
| H19 DNAm, % (averaged across 4 CpG sites) | 58.31 (5.16) 1 | N/A |
| PPAR-α DNAm, % (averaged across 2 CpG sites) | N/A | 10.62 (2.09) 10 |
Means (SD) or count (percentages) are presented for continuous or categorical variables, respectively. Number of missing values 1 n = 245, 2 n = 379, 3 n = 242, 4 n = 377, 5 n = 243, 6 n = 378, 7 n = 244, 8 n = 373, 9 n = 342, 10 n = 358. a Negative values appear for 11β-HSD-2 because values are standardized to controls included on each plate to reduce the impact of pyrosequencing batch effects. Abbreviations: DNAm = DNA methylation; Long interspersed nuclear elements (LINE-1); 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2); Peroxisome proliferator-activated receptor alpha (PPAR-α).
2.1. Associations between the DNAm z-Score at LINE-1 and Repeated Measures of Cardiometabolic Risk Factors
In adjusted models, LINE-1 methylation levels were inversely associated with log serum fasting glucose at site 1 [β = −0.029, p = 0.0006]; for each one standard deviation increase in DNAm (i.e., +4%), there was an approximately 3% decrease in log fasting glucose. In addition, a positive association was detected between DNAm at site 3 and log serum fasting high-density lipoprotein cholesterol [β = 0.063, p = 0.0072], which means that for each one standard deviation increase in DNAm (i.e., +3%), there was an approximately 6% increase in log high-density lipoprotein cholesterol (Table 2). Sensitivity analysis (i.e., additionally adjusting for pubertal onset) did not attenuate the detected associations (Supplementary Table S5).
Table 2.
Associations between the DNAm z-score at LINE-1 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models (n = 242).
| LINE-1 z-Score at Site 1 | LINE-1 z-Score at Site 2 | LINE-1 z-Score at Site 3 | LINE-1 z-Score at Site 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | |
| Waist circumference (cm) (Total number of observations = 441, of which 43 (17.77%) subjects had one measurement and 199 (82.23%) subjects had two measurements) | ||||||||
| Model 1 | −0.5960 (1.0435) | 0.5684 | 1.1418 (1.4217) | 0.4227 | −0.4783 (1.1510) | 0.6781 | 0.2997 (0.9013) | 0.7398 |
| Model 2 | 0.5615 (1.0072) | 0.5777 | 0.9837 (1.3686) | 0.4730 | −1.7757 (1.1106) | 0.1111 | 0.3214 (0.8710) | 0.7124 |
| Systolic blood pressure (mmHg) (Total number of observations = 441, of which 43 (17.77%) subjects had one measurement and 199 (82.23%) subjects had two measurements) | ||||||||
| Model 1 | −0.4560 (0.8541) | 0.5939 | −0.1855 (1.1698) | 0.8741 | 0.1632 (0.9435) | 0.8628 | 0.9703 (0.7361) | 0.1887 |
| Model 2 | −0.9634 (0.8928) | 0.2817 | −0.00023 (1.2181) | 0.9999 | 0.4640 (0.9898) | 0.6397 | 0.8922 (0.7676) | 0.2464 |
| Diastolic blood pressure (mmHg) (Total number of observations = 441, of which 43 (17.77%) subjects had one measurement and 199 (82.23%) subjects had two measurements) | ||||||||
| Model 1 | −0.5185 (0.5769) | 0.3697 | −0.1316 (0.7927) | 0.8682 | 0.2271 (0.6379) | 0.7221 | 0.3619 (0.4966) | 0.4669 |
| Model 2 | −0.6759 (0.5947) | 0.2570 | −0.04549 (0.8136) | 0.9555 | 0.3404 (0.6613) | 0.6072 | 0.3674 (0.5094) | 0.4716 |
| Log-transformed fasting glucose (mg/dL) (Total number of observations = 438, of which 46 (19.01%) subjects had one measurement and 196 (80.99%) subjects had two measurements) | ||||||||
| Model 1 | −0.01570 (0.007838) | 0.0463 | 0.02427 (0.01086) | 0.0263 | −0.00357 (0.008708) | 0.6825 | −0.00361 (0.006726) | 0.5917 |
| Model 2 | −0.02864 (0.008211) | 0.0006 * | 0.02729 (0.01124) | 0.0160 | 0.01135 (0.009149) | 0.2162 | −0.00142 (0.007028) | 0.8402 |
| Log-transformed high-density lipoprotein cholesterol (mg/dL) (Total number of observations = 438, of which 46 (19.01%) subjects had one measurement and 196 (80.99%) subjects had two measurements) | ||||||||
| Model 1 | 0.02078 (0.01893) | 0.2733 | −0.02664 (0.02610) | 0.3083 | 0.01023 (0.02099) | 0.6265 | −0.01677 (0.01627) | 0.3039 |
| Model 2 | −0.01466 (0.02111) | 0.4881 | −0.02801 (0.02873) | 0.3306 | 0.06331 (0.02334) | 0.0072 * | −0.00571 (0.01822) | 0.7543 |
| Log-transformed triglycerides (mg/dL) (Total number of observations = 438, of which 46 (19.01%) subjects had one measurement and 196 (80.99%) subjects had two measurements | ||||||||
| Model 1 | −0.05170 (0.04055) | 0.2035 | −0.03424 (0.05541) | 0.5372 | 0.05445 (0.04481) | 0.2255 | −0.00392 (0.03498) | 0.9109 |
| Model 2 | −0.02698 (0.03945) | 0.4947 | −0.04343 (0.05383) | 0.4205 | 0.05072 (0.04378) | 0.2477 | 0.009633 (0.03392) | 0.7766 |
Long interspersed nuclear elements (LINE-1). Model 1 included LINE-1 z-scores at CpG sites 1, 2, 3, and 4 as fixed effects and a compound symmetry matrix structure to model the covariance structure of the repeated measurements for each outcome. Model 2 was additionally adjusted for the following fixed effects: age, sex, and duration of breastfeeding. * p < 0.008.
2.2. Associations between the DNAm z-Score at 11β-HSD-2 and Repeated Measures of Cardiometabolic Risk Factors
DNAm at site 4 showed an inverse association with log serum fasting glucose (mg/dL) [β = −0.018, p = 0.0018] (Table 3). In sensitivity analysis (i.e., additionally adjusting for pubertal onset), associations found with fasting glucose maintained similar magnitude and significance (Supplementary Table S6).
Table 3.
Associations between the DNAm z-score at 11β-HSD-2 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models (n = 229).
| 11β-HSD-2 z-Score at Site 1 | 11β-HSD-2 z-Score at Site 2 | 11β-HSD-2 z-Score at Site 3 | 11β-HSD-2 z-Score at Site 4 | 11β-HSD-2 z-Score at Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | Estimate (SE) | p-Value | |
| Waist circumference (cm) (Total number of observations = 415, of which 43 (18.78%) subjects had one measurement and 186 (81.22%) subjects had two measurements) | ||||||||||
| Model 1 | −0.3822 (1.0424) | 0.7142 | −0.08657 (0.7980) | 0.9137 | 0.2635 (0.9701) | 0.7862 | 0.5264 (0.7690) | 0.4943 | 0.2132 (0.7252) | 0.7690 |
| Model 2 | −1.1319 (1.0012) | 0.2595 | 0.2204 (0.7707) | 0.7751 | 0.6173 (0.9303) | 0.5076 | 0.5578 (0.7361) | 0.4493 | −0.1382 (0.6969) | 0.8430 |
| Systolic blood pressure (mmHg) (Total number of observations = 415, of which 43 (18.78%) subjects had one measurement and 186 (81.22%) subjects had two measurements) | ||||||||||
| Model 1 | −1.6096 (0.8326) | 0.0545 | −0.7568 (0.6372) | 0.2362 | 1.2770 (0.7754) | 0.1010 | 0.3766 (0.6161) | 0.5416 | −0.4901 (0.5780) | 0.3974 |
| Model 2 | −1.4026 (0.8695) | 0.1083 | −0.7320 (0.6688) | 0.2751 | 1.1599 (0.8074) | 0.1524 | 0.3305 (0.6404) | 0.6064 | −0.3520 (0.6029) | 0.5600 |
| Diastolic blood pressure (mmHg) (Total number of observations = 415, of which 43 (18.78%) subjects had one measurement and 186 (81.22%) subjects had two measurements) | ||||||||||
| Model 1 | −0.9251 (0.5519) | 0.0951 | −0.8601 (0.4222) | 0.0428 | 0.3540 (0.5143) | 0.4920 | 0.4535 (0.4092) | 0.2690 | −0.01360 (0.3827) | 0.9717 |
| Model 2 | −0.8686 (0.5624) | 0.1240 | −0.8775 (0.4322) | 0.0436 | 0.3201 (0.5221) | 0.5404 | 0.4519 (0.4148) | 0.2771 | 0.01427 (0.3888) | 0.9708 |
| Log-transformed fasting glucose(mg/dL) (Total number of observations = 412, of which 46 (20.09%) subjects had one measurement and 183 (79.91%) subjects had two measurements) | ||||||||||
| Model 1 | −0.00076 (0.007513) | 0.9193 | 0.001955 (0.005764) | 0.7348 | 0.006329 (0.006998) | 0.3668 | −0.01869 (0.005586) | 0.0010 * | 0.002692 (0.005216) | 0.6064 |
| Model 2 | 0.009223 (0.007893) | 0.2440 | −0.00184 (0.006079) | 0.7624 | 0.001102 (0.007320) | 0.8805 | −0.01837 (0.005817) | 0.0018 * | 0.007427 (0.005472) | 0.1762 |
| Log-transformed high-density lipoprotein cholesterol (mg/dL) (Total number of observations = 412, of which 46 (20.09%) subjects had one measurement and 183 (79.91%) subjects had two measurements) | ||||||||||
| Model 1 | 0.002550 (0.01874) | 0.8919 | −0.00550 (0.01438) | 0.7026 | −0.00829 (0.01745) | 0.6351 | −0.01132 (0.01390) | 0.4161 | 0.005434 (0.01303) | 0.6770 |
| Model 2 | 0.02693 (0.02073) | 0.1952 | −0.02151 (0.01596) | 0.1793 | −0.02199 (0.01925) | 0.2545 | −0.01714 (0.01524) | 0.2620 | 0.01880 (0.01442) | 0.1938 |
| Log-transformed triglycerides (mg/dL) (Total number of observations = 412, of which 46 (20.09%) subjects had one measurement and 183 (79.91%) subjects had measurements) | ||||||||||
| Model 1 | 0.02425 (0.04126) | 0.5572 | 0.03580 (0.03163) | 0.2588 | 0.004623 (0.03838) | 0.9042 | 0.01794 (0.03047) | 0.5566 | −0.00972 (0.02872) | 0.7354 |
| Model 2 | 0.01469 (0.04003) | 0.7140 | 0.03065 (0.03084) | 0.3212 | 0.01000 (0.03715) | 0.7880 | 0.01977 (0.02946) | 0.5029 | −0.01685 (0.02782) | 0.5453 |
11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2). Model 1 included 11β-HSD-2 z-scores for CpG sites 1, 2, 3, 4, and 5 as fixed effects and a compound symmetry matrix structure to model the covariance structure of the repeated measurements for each outcome. Model 2 was additionally adjusted for the following fixed effects: age, and sex. * p < 0.008.
2.3. Associations between the DNAm z-Score at H19 and Repeated Measures of Cardiometabolic Risk Factors
In the adjusted models, DNAm at none of the individual CpG sites was associated with the cardiometabolic outcomes (Supplementary Table S7). Results did not change in the two sensitivity analyses (Supplementary Tables S8 and S9).
2.4. Cross-Sectional Associations between the DNAm z-Score at PPAR-α and Cardiometabolic Risk Factors
In a cross-sectional analysis, DNAm was not associated with the cardiometabolic risk factors (Table 4). The sensitivity analysis (i.e., after adjusting for pubertal onset) showed the same result (Supplementary Table S10).
Table 4.
Cross-sectional Associations between DNAm z-scores at PPAR-α and Cardiometabolic Risk Factors using Linear Regression (n = 345).
| PPAR-α z-Score at Site 1 | PPAR-α z-Score at Site 2 | |||
|---|---|---|---|---|
| Estimate (SE) | p-Value | Estimate (SE) | p-Value | |
| Waist circumference (cm) (n = 345) | ||||
| Model 1 | 0.71915 (0.71474) | 0.3150 | −1.70941 (0.65445) | 0.0094 |
| Model 2 | 0.99917 (0.70529) | 0.1575 | −1.68127 (0.64618) | 0.0097 |
| Systolic blood pressure (mmHg) (n = 345) | ||||
| Model 1 | 0.58582 (0.60305) | 0.3320 | −1.02922 (0.55218) | 0.0632 |
| Model 2 | 0.49623 (0.57982) | 0.3927 | −0.66490 (0.53123) | 0.2116 |
| Diastolic blood pressure (mmHg) (n = 345) | ||||
| Model 1 | 0.58530 (0.42242) | 0.1668 | −0.57466 (0.38679) | 0.1383 |
| Model 2 | 0.58072 (0.40724) | 0.1548 | −0.34026 (0.37311) | 0.3624 |
| Log-transformed fasting glucose (mg/dL) (n = 310) | ||||
| Model 1 | 0.00598 (0.00614) | 0.3305 | 0.00016627 (0.00600) | 0.9779 |
| Model 2 | 0.00282 (0.00609) | 0.6443 | 0.00159 (0.00596) | 0.7900 |
| Log-transformed high-density lipoprotein cholesterol (mg/dL) (n = 310) | ||||
| Model 1 | −0.00813 (0.01303) | 0.5329 | 0.01206 (0.01273) | 0.3445 |
| Model 2 | −0.00419 (0.01309) | 0.7490 | 0.00857 (0.01280) | 0.5035 |
| Log-transformed triglycerides (mg/dL) (n = 310) | ||||
| Model 1 | 0.01232 (0.03058) | 0.6873 | 0.00118 (0.02989) | 0.9684 |
| Model 2 | 0.02086 (0.03057) | 0.4956 | −0.01116 (0.02989) | 0.7092 |
Peroxisome proliferator-activated receptor alpha (PPAR-α). Model 1 included PPAR-α z-scores for CpG sites 1 and 2. Model 2 was additionally adjusted for age, and sex.
As an explanatory analysis, we assessed the crude association between DNAm values and gene expression for PPAR-α. RNA-seq data were available for a small subset of subjects at the same time point (i.e., Time 3) (n = 65). Weak non-significant positive correlations were identified between mRNA and DNAm (site 1: Spearman’s correlation [rs] = 0.14, (p = 0.26); site 2: rs = 0.10, (p = 0.42); average of the two sites rs = 0.12, (p = 0.33)).
3. Discussion
In this study, the relationships between DNAm at LINE-1, 11β-HSD-2, H19, and PPAR-α with cardiometabolic risk factors were investigated among Mexican children and adolescents enrolled in a well-characterized birth cohort from Mexico City. Among cardiometabolic components, fasting glucose and high-density lipoprotein cholesterol were associated with DNAm of at least one genomic region. To the best of our knowledge, this is the first study investigating the potential of DNAm as a biomarker for cardiometabolic risk factors among Mexican youth using hypothesis-driven genomic regions.
The inverse and positive associations between LINE-1 DNAm and glucose and high-density lipoprotein cholesterol are in line with current evidence linking LINE-1 hypomethylation with genomic instability and CVD [46,64,65,66]. Furthermore, few studies conducted on adult populations showed inverse relationships between LINE-1 DNAm and impaired carbohydrate metabolism [67] and fasting glucose [62,68]. Scare and inconsistent evidence is available among pediatric populations with regard to cardiometabolic health and LINE-1 DNAm [69,70], where an inverse association detected with the waist circumference z-score [69] and null associations were reported with adiposity markers [70]. We acknowledge the complexity of crude comparisons across the studies because of the mismatch in the study endpoints and sample characteristics; therefore, future prospective studies are needed to strengthen the use of LINE-1 DNAm as a proxy for cardiometabolic health among youth.
We found that a one standard deviation increase in 11β-HSD-2 DNAm at site 4 (i.e., +2%) was associated with a decrease of 2% in fasting glucose. Our results could be explained in light of the limited studies that investigated the connection between 11β-HSD-2 and glucose metabolism in adult populations [57,58]. Müssig et al. reported inverse association between 1β-HSD2 activity and insulin sensitivity [57], and Jang and colleagues found higher 11β-HSD2 enzyme activity among subjects with type 2 diabetes [58]. It is worth noting that not only is 11β-HSD-2 expression regulated by other epigenetic modifications [71], age [72], and lifestyle factors [73], but a lack of association was also documented earlier between 11β-HSD2 enzyme activity and mRNA expression [58]. As our results showed the potential of 11β-HSD-2 DNAm as a cardiometabolic biomarker among youth, future studies are needed combining DNAm, gene expression, and enzyme activity assessment to strengthen the evidence for the role of 11β-HSD-2 in cardiometabolic risk.
The present study has multiple strengths, including the prospective assessment of the association between DNAm at four genomic regions and up to two repeated measures of cardiometabolic risk factors during a sensitive period of growth, development, and maturation. We used a robust statistical model to account for the longitudinal data structure and conducted site-specific analyses for examining the association between the DNAm of each region with cardiometabolic risk factors. Site-specific approaches may be better when the data are not as correlated or when some CpG sites are much more variable than others in order to capture the complexity of the data. Our data come from a well-characterized birth cohort, ELEMENT, which allowed for assessing whether any of the mother’s sociodemographic and reproductive characteristics would be potential confounding factors to account for. Furthermore, peripheral blood was used to quantify the DNAm because blood is an accessible tissue and commonly collected in clinical setting and epidemiological studies [31], which is a strength for investigating potential biomarkers for cardiometabolic risk factors among children.
With regard to the study limitations, the use of bisulfite treatment to measure DNAm does not distinguish between cytosine methylation (5mC) and cytosine hydroxymethylation (5hmC) [74], and 5hmC has its own distinct impact on gene regulation, which was not captured by our method. Therefore, the DNAm values might be confounded by hydroxymethylation because both 5hmC and 5mC are captured in the total DNAm percentage. Future studies should apply laboratory techniques that allow for distinguishing between 5hmC and 5mC. Additionally, our work has the limitation of including only DNAm without gene expression data for three of the four regions assessed. Because gene expression could be influenced by multiple factors, including other epigenetic modifications, physiological conditions, and lifestyle factors, we recommend future studies supplement the assessment of DNAm with gene expression and carefully take into account the other potential factors that influence gene expression. Such evidence will strengthen the use of DNAm as a clinical biomarker for cardiometabolic health if clinical validation studies confirm its utility.
We acknowledge the age heterogeneity as our analysis includes pre-teenagers and teenagers; given our small sample size, we did not explore the relationships stratified by age groups. Thus, future studies are needed to investigate the potential role of age in modifying the association between DNAm and cardiometabolic risk factors during pubertal transition. Additionally, the magnitude of detected associations was small, which might not be of clinical significance. However, small effect sizes are typically reported in epigenetic studies [62,65,67,68,75]. Because small effects may still have relevance for children’s health outcomes [75], further studies are needed to enhance our understanding of the cause-and-effect relationship between DNAm and cardiometabolic health by validating our results in independent large-scale population-based youth populations with objective assessment of lifestyle patterns known to influence DNAm. Such evidence will facilitate the progress toward increasing the reproducibility and strengthening the biological relevance of DNAm biomarkers. Additionally, despite our consideration for addressing multiple testing, we still acknowledge the possibility of reporting false positive results due to chance. Lastly, the possibility of residual confounding—such as smoking status and genetic variants—and reverse causation between DNAm and cardiometabolic outcomes cannot be ruled out.
4. Materials and Methods
4.1. Study Population
The analytical sample consisted of offspring who participated in two of three sequentially enrolled birth cohorts of the ELEMENT project in Mexico City, Mexico. A comprehensive description of the ELEMENT project and the eligibility and exclusion criteria are available elsewhere [76]. Briefly, the ELEMENT project included mother–child dyads recruited from maternity hospitals representing women from low- to middle-income population groups from 1997 to 2005 [77]. Mothers recruited for one of the birth cohorts were enrolled in a randomized controlled trial (RCT) that examined the role of daily calcium supplementation during pregnancy (1200 mg/day) in mitigating the effect of lead exposure on the neurobehavioral and physical developmental outcomes in offspring [76]. Offspring were followed at multiple time points in childhood and through adolescence; the aim of the follow-up visits was to follow as many children from the original birth cohort as possible, prioritizing younger ages at specific time points. The sample size for each follow-up visit was determined by the aims for the original grant-funded visit.
We utilized available data from two follow-up visits. In the first follow-up visit, herein called Time 1, we planned to follow 250 children aged between 8 and 15 years. We prioritized children according to availability of prenatal biological samples for offspring from the original birth cohorts [76]. The second follow up visit, Time 2, was conducted on average 2 years later (maximum time to follow-up was 4.6 years). We planned to follow >500 children from the original birth cohorts. We prioritized the 250 subjects from the Time 1 visit (of which a large majority (~90%) returned) and added additional ELEMENT children who were not included in the Time 1 visit. Based on a statistical power calculation and available funds, we selected a sub-sample of these for epigenetic analysis (all children at Time 1 and >350 at Time 2). Children were 10–18 years of age at Time 2.
The analytical sample for the genomic regions LINE-1, H19, and 11β-HSD-2 included children and adolescents who had DNAm data at Time 1 and had data for at least one of the six cardiometabolic risk factors (i.e., waist circumference, systolic and diastolic blood pressure, fasting glucose, triglycerides, high density lipoprotein cholesterol) at Time 1 and/or Time 2. DNAm at PPAR-α was measured only at Time 2; subjects with these data and at least one of the six cardiometabolic risk factors were included for the analytical sample for PPAR-α models. The National Institute of Public Health of Mexico and the University of Michigan institutional review boards approved the research protocols. Written informed consents were collected from mothers upon their enrollments and assent from adolescents.
4.2. Laboratory Measurements and Outcomes
4.2.1. DNA Methylation Analysis
The current study limits its focus to four genomic regions, which have previously been associated with cardiometabolic risk factors. Whole blood samples were collected via venipuncture into tubes containing ethylenediaminetetraacetic acid (EDTA) preservative (Paxgene and BD Vacutainer) by trained staff following standard protocols. High-molecular-weight DNA was extracted from blood leukocytes with the PAXgene Blood DNA kit (PreAnalytix, Switzerland) or the Flexigene kit (Qiagen). The extracted DNA samples were treated with sodium bisulfite using Epitect (Qiagen, Valencia, CA, USA) or EZ DNA Methylation kits (Zymo Research, Irvine, CA, USA) following the standard methods previously published [78]. The purpose of bisulfite treatment was to convert the un-methylated cytosines to uracil and to preserve the methylated cytosines. The bisulfite-treated DNA samples were amplified using HotStarTaq Master Mix (Qiagen), and primers designed to amplify each region of interest. Pyrosequencing was performed using either PyroMark Q96 MD (Qiagen) or PyroMark Q96 ID (Qiagen). Pyro Q-CpG Software calculated the percent methylation and performed internal quality control checks. At Time 1, DNAm was quantified for H19 (4 CpG sites in the imprinting control region), for LINE-1 (4 CpG sites in a conserved region across many LINE-1s), and for 11β-HSD-2 (5 CpG sites in the promoter region) and at Time 2 for PPAR-α (2 CpG sites in the promoter region) following the protocols published previously [79,80,81]. Information on these genomic regions and the primer sequences is presented in Supplementary Table S11 [77]. More than 10% of all samples and controls of human DNA with known percentages of DNAm (0%, 25%, 50%, 75%, and 100%) were run in duplicate and included in each pyrosequencing batch (96-well plate). The average of duplicate samples was used when applicable [82]. DNAm data from LINE-1, 11β-HSD-2, and H19 suggested a batch effect, and the methylation percentages were standardized to adjust for the batch effects as described previously [82]. We then standardized DNAm values for each region to have mean 0 standard deviation 1 based on the sample’s mean and standard deviation values to express the DNAm as a z-score, and these z-scores were used in statistical analysis.
Samples collected at Time 1 were not preserved for downstream RNA isolation. At Time 2, blood leukocytes preserved for RNA isolation were collected from all participants and archived. Of these, 72 were selected for next-generation sequencing of RNA (‘RNA-Seq’). Samples were prioritized for selection that had the highest quality and quantity of RNA and had complete datasets needed for previous questions of interest [83]. Of those, 65 were from participants included in this manuscript. The read count of PPAR-α from the RNA-seq was used to assess the relationship between DNAm and gene expression for PPAR-α. The RNA-seq protocol followed was previously described [83].
4.2.2. Cardiometabolic Risk Factors
Anthropometric Measures
Duplicate measurements were collected by trained research staff for body weight to the nearest 0.1 kg using a digital scale (BAME Model 420; Catálogo Médico) and InBody 230 (Biospace Co, Ltd, Seoul, Republic of Korea), height to the nearest 0.5 cm, and waist circumference to the nearest 0.1 cm using a non-stretchable measuring tape SECA (model 201, Hamburg, Germany) [84]. The average of the two measurements was used for the analysis [85]. These measurements were conducted at Time 1 and Time 2.
Blood Pressure Measurements
Duplicate readings of systolic and diastolic blood pressure were recorded in a seated position using a mercury sphygmomanometer (TXJ-10 MD 3000 model, Homecare, Nanjing, China), and the average of the two measurements was used for the analysis. These measurements were conducted at Time 1 and Time 2.
Fasting Biomarkers
At each follow-up visit (T1 and T2), trained research staff collected blood samples from children after an 8 h overnight fast. Fasting glucose and lipids were measured in serum at the Michigan Diabetes Research Center Chemistry Laboratory. Specifically, fasting glucose was assessed via automated chemiluminescence immunoassay (Immulite 1000; Siemens Medical Solutions). Triglycerides were quantified via an enzymatic colorimetric method using a Cobas Mira automated chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA). The level of high-density lipoprotein cholesterol was obtained by using direct high-density lipoprotein cholesterol (Roche Diagnostics) [85]. All serum markers were above the limit of detection (LOD).
4.3. Covariates
Based on prior knowledge of cardiovascular and metabolic health, covariates assessed for this research were classified as (1) maternal and child characteristics around the time of birth (sex, birth weight, gestation age, mode of delivery, duration of breastfeeding, and mothers’ age, marital status, parity, years of education, and enrollment in the calcium supplementation study during pregnancy) and (2) follow-up characteristics for the children, which were measured at the baseline visit for each exposure, e.g., child’s age, total caloric intake, physical activity measured as metabolic equivalents, and pubertal onset. In our statistical analysis section, we explained our rationale for selecting covariates in each adjusted model.
After childbirth, mothers reported household and demographic information, including their ages, marital status (married compared to any other status), parity status (1, 2, ≥3), and years of education (<12 yrs, 12 yrs, or >12 yrs), gestational age estimated by a registered nurse, and mode of delivery (vaginal, or C-section childbirth). The newborns were followed until 5 years of age, and information about self-reported breastfeeding duration was estimated [86]. Since cohort 3 was an RCT for daily calcium supplementation during the first trimester of pregnancy until 1-year postpartum and cohort 2 participants were not part of a trial, we created a binary indicator for mothers who received the calcium treatment (yes/no) with all mothers from cohort 2 falling into the ‘no’ category [76,87].
During each of two follow-up visits, total caloric intake was quantified using a semi-quantitative food frequency questionnaire (FFQ) that captured the intake over the previous week [84,88]. The FFQ was adapted from the Mexican National Health and Nutrition Survey, and FFQs were analyzed using food composition software developed by the National Institute of Public Health, Mexico [89]. A physical activity questionnaire was developed based on the Youth Activity Questionnaire (YAQ) and validated relative to 24 h physical activity recall among Mexican school-children aged 10 to 14 years in Mexico City [90]. For each self-reported physical activity, the corresponding metabolic equivalent was multiplied by the activity intensity [91]. The total metabolic equivalents per week were calculated by summing the metabolic equivalents for all activities. Puberty was assessed through Tanner staging for breast and pubic hair (for girls) or genitalia and pubic hair (for boys) [92,93] by trained physicians [94]. Consistent with previous ELEMENT publications in which pubertal onset was a covariate, we classified children as having pubertal onset when the Tanner Stage for either or both of pubic hair and genital development (boys) or pubic hair and breast development (girls) was greater than one [95,96,97].
4.4. Statistical Analysis
Outcomes were cardiometabolic risk factors: waist circumference, systolic blood pressure, diastolic blood pressure, glucose, high-density lipoprotein cholesterol, and triglycerides. Dependent variables of interest were DNAm z-scores at LINE-1, 11β-HSD-2, H19, and PPAR-α after standardizing the values based on the sample’s mean and standard deviation for each site. Outcomes and exposures were treated as continuous in our models. The demographic characteristics of the study participants were presented as the mean (SD) and counts (proportions) for continuous and categorical variables, respectively.
DNAm percentages were quantified at multiple loci (CpG sites) within the same genomic region (i.e., H19: 4 CpG sites, LINE-1: 4 CpG sites, 11β-HSD-2: 5 CpG sites, and PPAR-α:2 CpG sites). For each genomic region, the DNAm percentages at all CpG sites were included as repeated measures of the same variable in models of each outcome. To illustrate, the LINE-1 z-scores at CpG site 1, 2, 3, and 4 were included as four fixed effects in our models, and the same strategy was applied for other genes. This analytical approach was used in previous publications [98].
To examine the relationship between DNAm at Time 1 for LINE-1, 11β-HSD-2, and H19 and each cardiovascular risk factor outcome, separate linear mixed-effects models with a compound symmetry covariance structure were used to model the covariance structure of the repeated outcome assessed at Time 1 and 2. We used linear regression to assess the cross-sectional association between DNAm at PPAR-α and the outcomes because this gene was only measured at Time 2. For each exposure, the crude model included only DNAm z-scores at multiple CpG sites for a genomic region. Due to the biological plausibility for the sex and age difference in DNAm, we considered age and sex as mandatory covariates in any fully adjusted model. For the other covariates, we followed a parsimonious approach. Therefore, covariates were adjusted for only if they were potential confounders among our study population based on the significance of their statistical association with each gene of interest (i.e., p < 0.05). We investigated the confounding factors for each genomic region by examining the distribution of childbirth and follow-up characteristics across quartiles of average DNAm z-scores of all loci within the region using either analysis of variance or Kruskal-Wallis H tests for continuous covariates that were normally and non-normally distributed, respectively, and a chi-squared test for categorical covariates. Based on these investigations to select the confounding factors, only LINE-1 DNAm was associated with breastfeeding duration. Therefore, LINE-1 models included breastfeeding duration, in addition to age and sex (Supplementary Tables S12–S15).
Our mixed-effects models’ tables show information about the total sample size (i.e., number of unique subjects), total number of observations used in each model, and number of subjects with repeated measures for each outcome. Our linear regression models’ tables show information about the total sample size for each outcome. Collinearity was assessed in the linear regression models using variance inflation factors. We conducted sensitivity analyses. First, we adjusted for the pubertal onset at Time 1 for LINE-1, 11β-HSD-2, and H19 and at Time 2 for PPAR-α because puberty has been associated with DNAm [39]. We also repeated the analysis after excluding one outlier value in DNAm for H19. The SAS statistical software package, version 9.4, was used for analyses (SAS Corp, Cary, NC), and a p < 0.008 was considered a statistically significant association following correction for multiple testing of six outcomes (p < 0.008 or 0.05/6).
5. Conclusions
In conclusion, we observed associations between DNAm at specific CpG sites for LINE-1 and glucose and high-density lipoprotein cholesterol and for 11β-HSD-2 and glucose in a sample of Mexican youth. Our finding supplemented existing knowledge on the potential of epigenetics to identify the molecular mechanism underlying cardiometabolic abnormalities, and it could open the door for targeted interventions among youth. Nevertheless, our results merit further investigation to replicate, validate, and expand on the use of DNAm though carefully designed prospective studies in multiple independent pediatric populations. Moreover, since our study only focused on four genomic regions, we recommend future studies employ epigenome-wide approaches to identify all important genes for these outcomes in youth.
Acknowledgments
We gratefully acknowledge the mothers and children who participated in the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) study and American British Cowdray Medical Center (ABC) for providing facilities for this research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/epigenomes7010004/s1, Table S1: Spearman’s rank correlation coefficients between the DNAm z-scores at LINE-1 CpG sites; Table S2: Spearman’s rank correlation coefficients between the DNAm z-scores at 11β-HSD-2 CpG sites; Table S3: Spearman’s rank correlation coefficients between the DNAm z-scores at H19 CpG sites; Table S4: Spearman’s rank correlation coefficients between the DNAm z-scores at PPAR-α CpG sites; Table S5: Associations between the DNAm z-score at LINE- 1 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models Adjusted for Pubertal Onset (n = 242); Table S6: Associations between the DNAm z-score at 11β-HSD-2 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models Adjusted for Pubertal Onset (n = 229); Table S7: Associations between the DNAm z-score at H19 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models (n = 245); Table S8: Associations between the DNAm z-score at H19 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models after the Removal of Outlier DNAm Values (n = 244); Table S9: Associations between the DNAm z-score at H19 and Repeated Measures of Cardiometabolic Risk Factors using Mixed-effects Models Adjusted for Pubertal Onset (n = 245); Table S10: Cross-sectional Associations between the DNAm z-score at PPAR-α and Cardiometabolic Risk Factors using Linear Regression Adjusted for Pubertal Onset (n = 345); Table S11: Primer Sequences and Details of CpG Sites Assessed; Table S12: Average DNAm z-score at LINE-1 and Confounder Selection; Table S13: Average DNAm z-score at 11β-HSD-2 and Confounder Selection; Table S14: Average DNAm z-score at H19 and Confounder Selection; Table S15: Average DNAm z-score at PPAR-α and Confounder Selection.
Author Contributions
Data provision: J.M.G., D.C.D., K.E.P., A.C., M.M.T.-R. and L.A.T.-O.; conceptualization: A.A.A., J.M.G.,. K.E.P., H.M.K., E.A.R.-N. and A.B.; data analysis: A.A.A.; writing—original draft: A.A.A.; writing—review and editing: J.M.G. and K.E.P.; supervision: J.M.G. and K.E.P. All authors provided critically important intellectual contribution to the content and have read. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Boards of the University of Michigan and the National Institute of Public Health of Mexico.
Informed Consent Statement
Written informed consents were collected from mothers upon their enrollments in the ELEMENT project with assent from adolescents. No additional consent was required for publication.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to human subjects’ rights, but the data are available upon reasonable request to the ELEMENT PI, Karen Peterson (karenep@umich.edu) for review by the ELEMENT committee.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding Statement
Funding for the Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) was provided by the U.S. Environmental Protection Agency (US EPA) (RD83480019, RD83543601) and the National Institute for Environmental Health Sciences (NIEHS) (P20 ES018171, P01 ES02284401, and R35 ES031686). No additional financial support was received.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Di Cesare M., Soric M., Bovet P., Miranda J.J., Bhutta Z., Stevens G.A., Laxmaiah A., Kengne A.P., Bentham J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavares Giannini D., Caetano Kuschnir M.C., Szklo M. Metabolic syndrome in overweight and obese adolescents: A comparison of two different diagnostic criteria. Ann. Nutr. Metab. 2014;64:71–79. doi: 10.1159/000362568. [DOI] [PubMed] [Google Scholar]
- 3.Reinehr T., de Sousa G., Toschke A.M., Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 2007;92:1067–1072. doi: 10.1136/adc.2006.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flouris A.D., Bouziotas C., Christodoulos A.D., Koutedakis Y. Longitudinal preventive-screening cutoffs for metabolic syndrome in adolescents. Int. J. Obes. 2008;32:1506–1512. doi: 10.1038/ijo.2008.142. [DOI] [PubMed] [Google Scholar]
- 5.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Handelsman Y. Metabolic syndrome pathophysiology and clinical presentation. Toxicol. Pathol. 2009;37:18–20. doi: 10.1177/0192623308329288. [DOI] [PubMed] [Google Scholar]
- 7.Galassi A., Reynolds K., He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Gami A.S., Witt B.J., Howard D.E., Erwin P.J., Gami L.A., Somers V.K., Montori V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Ford E.S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 10.Stocks T., Bjorge T., Ulmer H., Manjer J., Haggstrom C., Nagel G., Engeland A., Johansen D., Hallmans G., Selmer R., et al. Metabolic risk score and cancer risk: Pooled analysis of seven cohorts. Int. J. Epidemiol. 2015;44:1353–1363. doi: 10.1093/ije/dyv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmet P., Magliano D., Matsuzawa Y., Alberti G., Shaw J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 12.Hong Y.M. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ. J. 2010;40:1–9. doi: 10.4070/kcj.2010.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenson G.S., Srinivasan S.R., Bao W., Newman W.P., 3rd, Tracy R.E., Wattigney W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 14.Sinha R., Fisch G., Teague B., Tamborlane W.V., Banyas B., Allen K., Savoye M., Rieger V., Taksali S., Barbetta G., et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 15.Marcovecchio M.L., Patricelli L., Zito M., Capanna R., Ciampani M., Chiarelli F., Mohn A. Ambulatory blood pressure monitoring in obese children: Role of insulin resistance. J. Hypertens. 2006;24:2431–2436. doi: 10.1097/HJH.0b013e328010918b. [DOI] [PubMed] [Google Scholar]
- 16.D’Adamo E., Impicciatore M., Capanna R., Loredana Marcovecchio M., Masuccio F.G., Chiarelli F., Mohn A.A. Liver steatosis in obese prepubertal children: A possible role of insulin resistance. Obesity (Silver Spring) 2008;16:677–683. doi: 10.1038/oby.2007.122. [DOI] [PubMed] [Google Scholar]
- 17.Giannini C., Diesse L., D’Adamo E., Chiavaroli V., de Giorgis T., Di Iorio C., Chiarelli F., Mohn A. Influence of the Mediterranean diet on carotid intima-media thickness in hypercholesterolaemic children: A 12-month intervention study. Nutr. Metab. Cardiovasc. Dis. 2014;24:75–82. doi: 10.1016/j.numecd.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Nicklas T.A., von Duvillard S.P., Berenson G.S. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: The Bogalusa Heart Study. Int. J. Sports Med. 2002;23((Suppl. 1)):S39–S43. doi: 10.1055/s-2002-28460. [DOI] [PubMed] [Google Scholar]
- 19.Morrison J.A., Friedman L.A., Wang P., Glueck C.J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Eloranta A.M., Schwab U., Venalainen T., Kiiskinen S., Lakka H.M., Laaksonen D.E., Lakka T.A., Lindi V. Dietary quality indices in relation to cardiometabolic risk among Finnish children aged 6–8 years—The PANIC study. Nutr. Metab. Cardiovasc. Dis. 2016;26:833–841. doi: 10.1016/j.numecd.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo A.G., Crujeiras A.B. Epigenetic biomarkers in metabolic syndrome and obesity. In: Sharma S., editor. Prognostic Epigenetics. Elsevier; Amsterdam, The Netherlands: 2019. pp. 269–287. [Google Scholar]
- 22.Zhang Y., Zeng C. Role of DNA methylation in cardiovascular diseases. Clin. Exp. Hypertens. 2016;38:261–267. doi: 10.3109/10641963.2015.1107087. [DOI] [PubMed] [Google Scholar]
- 23.Costantino S., Libby P., Kishore R., Tardif J.C., El-Osta A., Paneni F. Epigenetics and precision medicine in cardiovascular patients: From basic concepts to the clinical arena. Eur. Heart J. 2018;39:4150–4158. doi: 10.1093/eurheartj/ehx568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasher D., Greenway S.C., Singh R.B. The impact of epigenetics on cardiovascular disease. Biochem. Cell Biol. 2020;98:12–22. doi: 10.1139/bcb-2019-0045. [DOI] [PubMed] [Google Scholar]
- 25.Agha G., Mendelson M.M., Ward-Caviness C.K., Joehanes R., Huan T., Gondalia R., Salfati E., Brody J.A., Fiorito G., Bressler J. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease: A longitudinal study of 11 461 participants from population-based cohorts. Circulation. 2019;140:645–657. doi: 10.1161/CIRCULATIONAHA.118.039357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan L., Liu C., Hu J., Liu Y., Wang J., Chen G., Li Z., Chen H. Epigenetic mechanisms in coronary artery disease: The current state and prospects. Trends Cardiovasc. Med. 2018;28:311–319. doi: 10.1016/j.tcm.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 27.van der Harst P., de Windt L.J., Chambers J.C. Translational Perspective on Epigenetics in Cardiovascular Disease. J. Am. Coll. Cardiol. 2017;70:590–606. doi: 10.1016/j.jacc.2017.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samblas M., Milagro F.I., Martinez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14:421–444. doi: 10.1080/15592294.2019.1595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soler-Botija C., Galvez-Monton C., Bayes-Genis A. Epigenetic Biomarkers in Cardiovascular Diseases. Front. Genet. 2019;10:950. doi: 10.3389/fgene.2019.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Sun H., Tang W., Zhou L., Xie X., Qu Z., Chen M., Wang S., Yang T., Dai Y., et al. DNA methylation biomarkers in stool for early screening of colorectal cancer. J. Cancer. 2019;10:5264–5271. doi: 10.7150/jca.34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crujeiras A.B., Diaz-Lagares A. Epigenetic Biomarkers and Diagnostics. Elsevier; Amsterdam, The Netherlands: 2016. DNA methylation in obesity and associated diseases; pp. 313–329. [Google Scholar]
- 32.Kim M., Long T.I., Arakawa K., Wang R., Yu M.C., Laird P.W. DNA methylation as a biomarker for cardiovascular disease risk. PLoS ONE. 2010;5:e9692. doi: 10.1371/journal.pone.0009692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerman K., Sebastiani P., Jacques P., Liu S., DeMeo D., Ordovas J.M. DNA methylation modules associate with incident cardiovascular disease and cumulative risk factor exposure. Clin. Epigenetics. 2019;11:142. doi: 10.1186/s13148-019-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoun E., Issarapu P., di Gravio C., Shrestha S., Betts M., Saffari A., Sahariah S.A., Sankareswaran A., Arumalla M., Prentice A.M., et al. DNA methylation signatures associated with cardiometabolic risk factors in children from India and The Gambia: Results from the EMPHASIS study. Clin. Epigenetics. 2022;14:6. doi: 10.1186/s13148-021-01213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day S.E., Coletta R.L., Kim J.Y., Garcia L.A., Campbell L.E., Benjamin T.R., Roust L.R., De Filippis E.A., Mandarino L.J., Coletta D.K. Potential epigenetic biomarkers of obesity-related insulin resistance in human whole-blood. Epigenetics. 2017;12:254–263. doi: 10.1080/15592294.2017.1281501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costantino S., Mohammed S.A., Ambrosini S., Paneni F. Epigenetic processing in cardiometabolic disease. Atherosclerosis. 2019;281:150–158. doi: 10.1016/j.atherosclerosis.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Li E., Zhang Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014;6:a019133. doi: 10.1101/cshperspect.a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han L., Zhang H., Kaushal A., Rezwan F.I., Kadalayil L., Karmaus W., Henderson A.J., Relton C.L., Ring S., Arshad S.H., et al. Changes in DNA methylation from pre- to post-adolescence are associated with pubertal exposures. Clin. Epigenetics. 2019;11:176. doi: 10.1186/s13148-019-0780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y., Peterson K.E., Sanchez B.N., Dolinoy D.C., Mercado-Garcia A., Tellez-Rojo M.M., Goodrich J.M. Association of blood leukocyte DNA methylation at LINE-1 and growth-related candidate genes with pubertal onset and progression. Epigenetics. 2018;13:1222–1233. doi: 10.1080/15592294.2018.1556198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goran M.I., Gower B.A. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 41.Magge S.N., Goodman E., Armstrong S.C., Committee On N., Section On E., Section On O. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics. 2017;140:e20171603. doi: 10.1542/peds.2017-1603. [DOI] [PubMed] [Google Scholar]
- 42.Ardeljan D., Taylor M.S., Ting D.T., Burns K.H. The Human Long Interspersed Element-1 Retrotransposon: An Emerging Biomarker of Neoplasia. Clin. Chem. 2017;63:816–822. doi: 10.1373/clinchem.2016.257444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck C.R., Garcia-Perez J.L., Badge R.M., Moran J.V. LINE-1 elements in structural variation and disease. Annu. Rev. Genom. Hum. Genet. 2011;12:187–215. doi: 10.1146/annurev-genom-082509-141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sant K.E., Goodrich J.M. Methods for Analysis of DNA Methylation. In: McCullough S.D., Dolinoy D.C., editors. Toxicoepigenetics. Elsevier; Amsterdam, The Netherlands: 2019. pp. 347–377. [Google Scholar]
- 45.Muka T., Koromani F., Portilla E., O’Connor A., Bramer W.M., Troup J., Chowdhury R., Dehghan A., Franco O.H. The role of epigenetic modifications in cardiovascular disease: A systematic review. Int. J. Cardiol. 2016;212:174–183. doi: 10.1016/j.ijcard.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 46.Lai S., Du K., Shi Y., Li C., Wang G., Hu S., Jia X., Wang J., Chen S. Long Non-Coding RNAs in Brown Adipose Tissue. Diabetes Metab. Syndr. Obes. 2020;13:3193–3204. doi: 10.2147/DMSO.S264830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt E., Dhaouadi I., Gaziano I., Oliverio M., Klemm P., Awazawa M., Mitterer G., Fernandez-Rebollo E., Pradas-Juni M., Wagner W., et al. LincRNA H19 protects from dietary obesity by constraining expression of monoallelic genes in brown fat. Nat. Commun. 2018;9:3622. doi: 10.1038/s41467-018-05933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang R.C., Galati J.C., Burrows S., Beilin L.J., Li X., Pennell C.E., van Eekelen J., Mori T.A., Adams L.A., Craig J.M. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin. Epigenetics. 2012;4:21. doi: 10.1186/1868-7083-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowman A., Peterson K.E., Dolinoy D.C., Meeker J.D., Sanchez B.N., Mercado-Garcia A., Tellez-Rojo M.M., Goodrich J.M. Phthalate Exposures, DNA Methylation and Adiposity in Mexican Children Through Adolescence. Front. Public Health. 2019;7:162. doi: 10.3389/fpubh.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bray G.A., Heisel W.E., Afshin A., Jensen M.D., Dietz W.H., Long M., Kushner R.F., Daniels S.R., Wadden T.A., Tsai A.G., et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018;39:79–132. doi: 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel H., Dhangar K., Sonawane Y., Surana S., Karpoormath R., Thapliyal N., Shaikh M., Noolvi M., Jagtap R. In search of selective 11β-HSD type 1 inhibitors without nephrotoxicity: An approach to resolve the metabolic syndrome by virtual based screening. Arab. J. Chem. 2018;11:221–232. doi: 10.1016/j.arabjc.2015.08.003. [DOI] [Google Scholar]
- 52.Hintzpeter J., Stapelfeld C., Loerz C., Martin H.J., Maser E. Green tea and one of its constituents, Epigallocatechine-3-gallate, are potent inhibitors of human 11beta-hydroxysteroid dehydrogenase type 1. PLoS ONE. 2014;9:e84468. doi: 10.1371/journal.pone.0084468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friso S., Pizzolo F., Choi S.W., Guarini P., Castagna A., Ravagnani V., Carletto A., Pattini P., Corrocher R., Olivieri O. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199:323–327. doi: 10.1016/j.atherosclerosis.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 54.Drake A.J., McPherson R.C., Godfrey K.M., Cooper C., Lillycrop K.A., Hanson M.A., Meehan R.R., Seckl J.R., Reynolds R.M. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin. Endocrinol. 2012;77:808–815. doi: 10.1111/j.1365-2265.2012.04453.x. [DOI] [PubMed] [Google Scholar]
- 55.Krupp D., Shi L., Maser-Gluth C., Pietzarka M., Remer T. 11beta Hydroxysteroid dehydrogenase type 2 and dietary acid load are independently associated with blood pressure in healthy children and adolescents. Am. J. Clin. Nutr. 2013;97:612–620. doi: 10.3945/ajcn.112.047829. [DOI] [PubMed] [Google Scholar]
- 56.Mussig K., Remer T., Haupt A., Gallwitz B., Fritsche A., Haring H.U., Maser-Gluth C. 11beta-hydroxysteroid dehydrogenase 2 activity is elevated in severe obesity and negatively associated with insulin sensitivity. Obesity (Silver Spring) 2008;16:1256–1260. doi: 10.1038/oby.2008.218. [DOI] [PubMed] [Google Scholar]
- 57.Jang C., Obeyesekere V.R., Dilley R.J., Krozowski Z., Inder W.J., Alford F.P. Altered activity of 11beta-hydroxysteroid dehydrogenase types 1 and 2 in skeletal muscle confers metabolic protection in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2007;92:3314–3320. doi: 10.1210/jc.2006-2729. [DOI] [PubMed] [Google Scholar]
- 58.Barbosa-Cortes L., Villasis-Keever M.A., Del Prado-Manriquez M., Lopez-Alarcon M. Adiposity and Insulin Resistance in Children from a Rural Community in Mexico. Arch. Med. Res. 2015;46:214–220. doi: 10.1016/j.arcmed.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Bougarne N., Weyers B., Desmet S.J., Deckers J., Ray D.W., Staels B., De Bosscher K. Molecular Actions of PPARalpha in Lipid Metabolism and Inflammation. Endocr. Rev. 2018;39:760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 60.Burri L., Thoresen G.H., Berge R.K. The Role of PPARalpha Activation in Liver and Muscle. PPAR Res. 2010;2010:542359. doi: 10.1155/2010/542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellano-Castillo D., Moreno-Indias I., Sanchez-Alcoholado L., Ramos-Molina B., Alcaide-Torres J., Morcillo S., Ocana-Wilhelmi L., Tinahones F., Queipo-Ortuno M.I., Cardona F. Altered Adipose Tissue DNA Methylation Status in Metabolic Syndrome: Relationships Between Global DNA Methylation and Specific Methylation at Adipogenic, Lipid Metabolism and Inflammatory Candidate Genes and Metabolic Variables. J. Clin. Med. 2019;8:87. doi: 10.3390/jcm8010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Contreras A.V., Torres N., Tovar A.R. PPAR-alpha as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013;4:439–452. doi: 10.3945/an.113.003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guarrera S., Fiorito G., Onland-Moret N.C., Russo A., Agnoli C., Allione A., Di Gaetano C., Mattiello A., Ricceri F., Chiodini P., et al. Gene-specific DNA methylation profiles and LINE-1 hypomethylation are associated with myocardial infarction risk. Clin. Epigenetics. 2015;7:133. doi: 10.1186/s13148-015-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei L., Liu S., Su Z., Cheng R., Bai X., Li X. LINE-1 hypomethylation is associated with the risk of coronary heart disease in Chinese population. Arq. Bras. Cardiol. 2014;102:481–488. doi: 10.5935/abc.20140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baccarelli A., Wright R., Bollati V., Litonjua A., Zanobetti A., Tarantini L., Sparrow D., Vokonas P., Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.María Martín-Núñez G., Rubio-Martín E., Cabrera-Mulero R., Rojo-Martínez G., Olveira G., Valdés S., Soriguer F., Castano L., Morcillo S. Type 2 diabetes mellitus in relation to global LINE-1 DNA methylation in peripheral blood: A cohort study. Epigenetics. 2014;9:1322–1328. doi: 10.4161/15592294.2014.969617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turcot V., Tchernof A., Deshaies Y., Perusse L., Belisle A., Marceau S., Biron S., Lescelleur O., Biertho L., Vohl M.C. LINE-1 methylation in visceral adipose tissue of severely obese individuals is associated with metabolic syndrome status and related phenotypes. Clin. Epigenetics. 2012;4:10. doi: 10.1186/1868-7083-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perng W., Mora-Plazas M., Marin C., Rozek L.S., Baylin A., Villamor E. A prospective study of LINE-1DNA methylation and development of adiposity in school-age children. PLoS ONE. 2013;8:e62587. doi: 10.1371/journal.pone.0062587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunstan J., Bressler J.P., Moran T.H., Pollak J.S., Hirsch A.G., Bailey-Davis L., Glass T.A., Schwartz B.S. Associations of LEP, CRH, ICAM-1, and LINE-1 methylation, measured in saliva, with waist circumference, body mass index, and percent body fat in mid-childhood. Clin. Epigenetics. 2017;9:29. doi: 10.1186/s13148-017-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rezaei M., Andrieu T., Neuenschwander S., Bruggmann R., Mordasini D., Frey F.J., Vogt B., Frey B.M. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension. 2014;64:860–866. doi: 10.1161/HYPERTENSIONAHA.114.00002. [DOI] [PubMed] [Google Scholar]
- 71.Campino C., Martinez-Aguayo A., Baudrand R., Carvajal C.A., Aglony M., Garcia H., Padilla O., Kalergis A.M., Fardella C.E. Age-related changes in 11beta-hydroxysteroid dehydrogenase type 2 activity in normotensive subjects. Am. J. Hypertens. 2013;26:481–487. doi: 10.1093/ajh/hps080. [DOI] [PubMed] [Google Scholar]
- 72.Kargl C., Arshad M., Salman F., Schurman R.C., Del Corral P. 11beta-hydroxysteroid dehydrogenase type-II activity is affected by grapefruit juice and intense muscular work. Arch. Endocrinol. Metab. 2017;61:556–561. doi: 10.1590/2359-3997000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang Y., Pastor W.A., Shen Y., Tahiliani M., Liu D.R., Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONE. 2010;5:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breton C.V., Marsit C.J., Faustman E., Nadeau K., Goodrich J.M., Dolinoy D.C., Herbstman J., Holland N., LaSalle J.M., Schmidt R., et al. Small-Magnitude Effect Sizes in Epigenetic End Points are Important in Children’s Environmental Health Studies: The Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environ. Health Perspect. 2017;125:511–526. doi: 10.1289/EHP595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perng W., Tamayo-Ortiz M., Tang L., Sanchez B.N., Cantoral A., Meeker J.D., Dolinoy D.C., Roberts E.F., Martinez-Mier E.A., Lamadrid-Figueroa H., et al. Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open. 2019;9:e030427. doi: 10.1136/bmjopen-2019-030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y., Goodrich J.M., Dolinoy D.C., Sanchez B.N., Ruiz-Narvaez E.A., Banker M., Cantoral A., Mercado-Garcia A., Tellez-Rojo M.M., Peterson K.E. Accelerometer-measured Physical Activity, Reproductive Hormones, and DNA Methylation. Med. Sci. Sports Exerc. 2020;52:598–607. doi: 10.1249/MSS.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grunau C., Clark S.J., Rosenthal A. Bisulfite genomic sequencing: Systematic investigation of critical experimental parameters. Nucleic Acids Res. 2001;29:e65. doi: 10.1093/nar/29.13.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodrich J.M., Sanchez B.N., Dolinoy D.C., Zhang Z., Hernandez-Avila M., Hu H., Peterson K.E., Tellez-Rojo M.M. Quality control and statistical modeling for environmental epigenetics: A study on in utero lead exposure and DNA methylation at birth. Epigenetics. 2015;10:19–30. doi: 10.4161/15592294.2014.989077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Virani S., Dolinoy D.C., Halubai S., Jones T.R., Domino S.E., Rozek L.S., Nahar M.S., Padmanabhan V. Delivery type not associated with global methylation at birth. Clin. Epigenetics. 2012;4:8. doi: 10.1186/1868-7083-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoyo C., Murtha A.P., Schildkraut J.M., Jirtle R.L., Demark-Wahnefried W., Forman M.R., Iversen E.S., Kurtzberg J., Overcash F., Huang Z., et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6:928–936. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goodrich J.M., Dolinoy D.C., Sanchez B.N., Zhang Z., Meeker J.D., Mercado-Garcia A., Solano-Gonzalez M., Hu H., Tellez-Rojo M.M., Peterson K.E. Adolescent epigenetic profiles and environmental exposures from early life through peri-adolescence. Environ. Epigenet. 2016;2:dvw018. doi: 10.1093/eep/dvw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jansen E.C., Dolinoy D., Peterson K.E., O’Brien L.M., Chervin R.D., Cantoral A., Tellez-Rojo M.M., Solano-Gonzalez M., Goodrich J. Adolescent sleep timing and dietary patterns in relation to DNA methylation of core circadian genes: A pilot study of Mexican youth. Epigenetics. 2021;16:894–907. doi: 10.1080/15592294.2020.1827719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Betanzos-Robledo L., Rodriguez-Carmona Y., Contreras-Manzano A., Lamadrid-Figueroa H., Jansen E., Tellez-Rojo M.M., Perng W., Peterson K., Hebert J.R., Shivappa N., et al. Greater cumulative exposure to a pro-inflammatory diet is associated with higher metabolic syndrome score and blood pressure in young Mexican adults. Nutr. Res. 2020;81:81–89. doi: 10.1016/j.nutres.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perng W., Fernandez C., Peterson K.E., Zhang Z., Cantoral A., Sanchez B.N., Solano-Gonzalez M., Tellez-Rojo M.M., Baylin A. Dietary Patterns Exhibit Sex-Specific Associations with Adiposity and Metabolic Risk in a Cross-Sectional Study in Urban Mexican Adolescents. J. Nutr. 2017;147:1977–1985. doi: 10.3945/jn.117.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasper N., Peterson K.E., Zhang Z., Ferguson K.K., Sanchez B.N., Cantoral A., Meeker J.D., Tellez-Rojo M.M., Pawlowski C.M., Ettinger A.S. Association of Bisphenol A Exposure with Breastfeeding and Perceived Insufficient Milk Supply in Mexican Women. Matern. Child Health J. 2016;20:1713–1719. doi: 10.1007/s10995-016-1974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ettinger A.S., Lamadrid-Figueroa H., Mercado-Garcia A., Kordas K., Wood R.J., Peterson K.E., Hu H., Hernandez-Avila M., Tellez-Rojo M.M. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: A randomized controlled trial in Mexican women. Nutr. J. 2014;13:116. doi: 10.1186/1475-2891-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez-Ramirez S., Mundo-Rosas V., Jimenez-Aguilar A., Shamah-Levy T. Methodology for the analysis of dietary data from the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2009;51((Suppl. 4)):S523–S529. doi: 10.1590/S0036-36342009001000007. [DOI] [PubMed] [Google Scholar]
- 88.Ramírez Silva I., Barragán-Vázquez S., Rodríguez-Ramírez S., Rivera-Dommarco J.A., Mejía-Rodríguez F., Barquera-Cervera S., Tolentino-Mayo L., Flores-Aldana M., Villalpando-Hernández S., Ancira-Moreno M. Base de Alimentos de México (BAM): Compilación de la Composición de los Alimentos Frecuentemente Consumidos en el país, Version 18.1.1. 2021. [(accessed on 1 January 2023)]. Available online: https://insp.mx/informacion-relevante/bam-bienvenida.
- 89.Hernández B., Gortmaker S.L., Laird N.M., Colditz G.A., Parra-Cabrera S., Peterson K.E. Validez y reproducibilidad de un cuestionario de actividad e inactividad física para escolares de la ciudad de México. Salud Pública México. 2000;42:315–323. doi: 10.1590/S0036-36342000000400006. [DOI] [PubMed] [Google Scholar]
- 90.Ainsworth B.E., Haskell W.L., Whitt M.C., Irwin M.L., Swartz A.M., Strath S.J., O’Brien W.L., Bassett D.R., Jr., Schmitz K.H., Emplaincourt P.O., et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 91.Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chavarro J.E., Watkins D.J., Afeiche M.C., Zhang Z., Sanchez B.N., Cantonwine D., Mercado-Garcia A., Blank-Goldenberg C., Meeker J.D., Tellez-Rojo M.M., et al. Validity of Self-Assessed Sexual Maturation Against Physician Assessments and Hormone Levels. J. Pediatr. 2017;186:172–178.e173. doi: 10.1016/j.jpeds.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LaBarre J.L., Peterson K.E., Kachman M.T., Perng W., Tang L., Hao W., Zhou L., Karnovsky A., Cantoral A., Tellez-Rojo M.M., et al. Mitochondrial Nutrient Utilization Underlying the Association Between Metabolites and Insulin Resistance in Adolescents. J. Clin. Endocrinol. Metab. 2020;105:2442–2455. doi: 10.1210/clinem/dgaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aljahdali A.A., Peterson K.E., Cantoral A., Ruiz-Narvaez E., Tellez-Rojo M.M., Kim H.M., Hebert J.R., Wirth M.D., Torres-Olascoaga L.A., Shivappa N., et al. Diet Quality Scores and Cardiometabolic Risk Factors in Mexican Children and Adolescents: A Longitudinal Analysis. Nutrients. 2022;14:896. doi: 10.3390/nu14040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aljahdali A.A., Baylin A., Ruiz-Narvaez E.A., Kim H.M., Cantoral A., Tellez-Rojo M.M., Banker M., Peterson K.E. Sedentary patterns and cardiometabolic risk factors in Mexican children and adolescents: Analysis of longitudinal data. Int. J. Behav. Nutr. Phys. Act. 2022;19:143. doi: 10.1186/s12966-022-01375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Needham B.L., Smith J.A., Zhao W., Wang X., Mukherjee B., Kardia S.L., Shively C.A., Seeman T.E., Liu Y., Diez Roux A.V. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: The multi-ethnic study of atherosclerosis. Epigenetics. 2015;10:958–969. doi: 10.1080/15592294.2015.1085139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith J.A., Zhao W., Wang X., Ratliff S.M., Mukherjee B., Kardia S.L.R., Liu Y., Roux A.V.D., Needham B.L. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The Multi-Ethnic Study of Atherosclerosis. Epigenetics. 2017;12:662–673. doi: 10.1080/15592294.2017.1341026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to human subjects’ rights, but the data are available upon reasonable request to the ELEMENT PI, Karen Peterson (karenep@umich.edu) for review by the ELEMENT committee.