Abstract
Introduction
Bovine immunodeficiency virus (BIV) is found worldwide in cattle under natural conditions. However, the effect of BIV infection on immune functions has not been fully characterised.
Material and Methods
Transcriptome analysis of BoMac cells after in vitro infection with BIV was performed using BLOPlus bovine microarrays. Genes identified as differentially expressed were subjected to functional analysis with the Ingenuity Pathway Analysis software (IPA).
Results
Out of 1,743 genes with altered expression, 1,315 were mapped as unique molecules. In total, 718 genes were identified as upregulated and 597 genes as downregulated. Differentially expressed genes were involved in 16 pathways related to immune response. The most enriched canonical pathway was leukocyte extravasation signalling. Interleukin-15 (IL-15) production was indicated as the most activated pathway and the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) signalling pathway was the most inhibited one. In addition, the study showed that the inflammatory response was decreased during BIV infection.
Conclusion
This is the first report to describe the microarray analysis of changes in gene expression upon BIV infection of bovine macrophages. Our data indicated how BIV influences the expression of genes and signalling pathways engaged in the immune response.
Keywords: BIV, bovine macrophage, microarray, gene expression
Introduction
Bovine immunodeficiency virus (BIV) belongs to the Lentivirus genus of the Retroviridae family and resembles human immunodeficiency virus (HIV) in its structural, genetic and antigenic features (13). Epidemiological evidence has shown that BIV appears to be widespread worldwide in bovids. The highest prevalence was noticed in Costa Rica (16) and the United States (28), while it was moderate in Japan (34), Pakistan (20) and Korea (6). Some reports also confirm BIV infections of cows in European countries: the Netherlands (17), Germany (22) and Poland (27) have been affected, but with lower seroprevalence. The most recent report on the occurrence of BIV in cattle comes from Mexico, showing a 28.94% prevalence (15).
The pathology of the course of BIV infection is not fully recognised. However, the cow from which the virus was isolated for the first time had evident persistent lymphocytosis, lymphadenopathy, central nervous
system lesions and emaciation (35). Subsequently, the virus was associated with clinical signs including weakness, emaciation and opportunistic bacterial infection (5). In calves experimentally infected with the FL112 strain of BIV, lymphadenopathy and non-suppurative meningoencephalitis have been reported (23). Although some studies showed a change in the CD4+/CD8+ T cell ratio (38, 40), the reduction of monocyte functions as phagocytic activity, chemotactic response, and delay in the antibody response against foreign antigens (25), immunodeficiency induced by BIV was never unambiguously confirmed (7). It is noteworthy that the Jembrana disease virus, which is referred to as bovine lentivirus type 2 because of its high similarity to BIV, is highly pathogenic and causes acute infections leading to death in cattle on the island of Bali (9). However, the significance of BIV infection in cattle has not been clearly established, and it is still unknown whether BIV induces a specific syndrome or whether it renders animals more susceptible to other infections.
Since BIV was predominantly detected in monocytes and macrophages following experimental and natural infection (14), they are suggested to be the virus’ target cells, and may be good candidates for the extended examination of the immune processes, for instance using a genome-wide approach. Assessing the gene transcript profiling of monocytes and macrophages, which are the first line of defence against viral infection, is therefore considered to be very important in the identification of genes and cellular pathways that may be involved in the pathogenesis of infectious diseases. Transcriptome profiling of HIV-infected monocytes and monocyte-derived macrophages, whether reported after an in vitro infection or in an ex vivo study, revealed dysregulation of several processes. These were activation of the MAPK and apoptotic signalling pathways (2), downregulation of the TLR signalling pathway (39) and induction of the STAT1 and STAT3 signalling pathway (1). To date, no similar data have been amassed for BIV infection.
Therefore, the aim of our study was to perform a genome-wide transcriptome analysis of bovine macrophages after infection with BIV. We investigated the host gene expression after the in vitro infection of a bovine macrophage (BoMac) cell line (30) with the FL112 strain of BIV (31) and compared it with the transcriptome of uninfected cells.
Material and Methods
BIV infection
The BIV virus stock was derived from the supernatant of foetal bovine lung (FBL) cells infected with BIV FL112. When the cells reached 70–80% of cytopathic effect, the BIV-containing supernatant was collected, centrifuged at 2,000 rpm for 15 min, aliquoted and stored at −70℃. The virus load was quantified by one-step RT-qPCR. Briefly, the BIV gag plasmid standard was constructed by the amplification and cloning of the 552 bp long gag gene fragment (primers BIVg723F/BIVg1274R) (Table 1) into the pDrive vector (PCR Cloning Kit; Qiagen, Hilden, Germany). In vitro transcription was performed using the MEGAscript Kit (Applied Biosystems, Foster City, CA, USA) with 1 μg of the linearised pDrive/gag BIV plasmid. The obtained standard BIV gag RNA was digested with TURBO DNase (Invitrogen, Waltham, MA, USA) to remove the DNA contamination, purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and evaluated spectrophotometrically using a Pearl Nanophotometer (Implen, Munich, Germany). Serial 10-fold dilutions from 106 to 100 copies/μL were prepared and used for the generation of the standard curve in a one-step RT-qPCR. To determine the number of BIV virions in the supernatant, viral RNA was isolated from 150 μL of aliquoted BIV supernatant using the NucleoSpin Viral RNA Kit (Macherey-Nagel, Düren, Germany) as described by the manufacturer. Then viral RNA which had previously been treated with DNase I, Amplification Grade (Invitrogen) was added to the RT-qPCR reaction (QuantiTect Probe RT-PCR Kit; Qiagen, Hilden, Germany) together with BIV gag-specific BIVg1074F/BIVg1202R primers and a BIVg1107P probe (Table 1).
Table. 1.
Primers and TaqMan probes used in this study for BIV and GAPDH gene amplification
| Primer/Probe | Sequence 5′ → 3′ | Position in target sequence | Product length (bp) | Application |
|---|---|---|---|---|
| BIVg723F | GAAGCAGACATCGAATCAGA | 723–742a | 552 | BIV standard |
| BIVg1274R | TCTTTTGTGGTTTCTGGAGC | 1255–1274 a | DNA | |
| BTG1F | TGATGCTGGTGCTGAGTATGTG | 4–25b | 182 | GAPDH standard |
| BTG2R | CCTTCAAGTGAGCCTGCAGCAA | 164–185 b | DNA | |
| BIVg1074F | ACAAGCCACCCTGATCTCAGTA | 1074–1095 a | 128 | RT-qPCR and |
| BIVg1202R | TCCTTGGGTTCCCTGATGAATGT | 1181–1202 a | qPCR- | |
| BIVg1107P | Cy5 - AACTTTCAGACAGTGGGTGCTGCAGG - BHQ3 | 1107–1132 a | - | BIV gag gene |
| BTG3F | CCACTGGGGTCTTCACTACCAT | 33–54 b | ||
| BTG4R | AAGTTAATTGCACCCGGGCTCT | 137–158 b | 126 | GAPDH qPCR- |
| BTG5P | JOE - CTGGAGAGGAGGGTGTAACAGGA - BHQ1 | 83–105 b | - |
The optimal conditions for in vitro infection were determined using different multiplicities of infection (MOI) (0.01, 0.1, 1 and 10) and durations of infection of BoMac cells with BIV (24, 48 and 72 h). The efficiency of the in vitro infection was measured by qPCR amplifying simultaneously the BIV gag gene and the bovine GAPDH gene. For qPCR the QuantiTect Multiplex PCR kit (Qiagen, Hilden, Germany) was used with the addition of 1 μg of template DNA isolated from BIV-infected BoMac cells and two sets of primers and probes designed to match the BIV gag gene (as above) and bovine GAPDH (BTG3F/BTG4R primers and a BTG5P probe) (Table 1). The highest efficiency of BIV infection was determined for MOI of 10 and 24 h.
BoMac cells were maintained in RPMI 1640 medium supplemented with 7% foetal calf serum, (Sigma-Aldrich, St. Louis, MO, USA) and 1× antibiotic antimycotic solution (Sigma-Aldrich). The cells were seeded into a P6 plate at 1.5 × 106 cells/well and infected the next day by incubating cells with the BIV-containing supernatant at MOI 10 for 18 h at 37°C with 5% CO2. Simultaneously, uninfected BoMac cultures were incubated in virus-free media under similar conditions. After incubation, cells were rinsed twice with fresh RPMI 1640 medium without serum and incubated for another 24 h.
Infections were repeated twice, in two independent replicates. Subsequently, the cells were washed with phosphate buffered saline, harvested separately from each well with a cell scraper and stored at −70°C as a dry pellet until total RNA extraction using the RNeasy MiniKit (Qiagen, Hilden, Germany), whereafter they were assigned to microarray and two-step RT-qPCR gene expression analysis.
Complementary DNA labelling, hybridisation and scanning protocol
A bovine long oligo microarray platform known as the BLOPlus microarray (Michigan State University, East Lansing, MI, USA) with 10,985 single spotted 70-mer probes was used for this experiment. The total RNA of BIV-infected and uninfected cells from both biological repeats was tested in duplicate. Total RNA was extracted both from infected and uninfected BoMac cells. After RNA quality and quantity assessment using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), 8 μg of RNA was reverse transcribed and the obtained complimentary DNA (cDNA) was purified and labelled overnight with the Superscript Indirect cDNA Labeling System (Invitrogen, Carlsbad, CA, USA). In four of the eight arrays, the control sample was labelled with Alexa 555 dye and compared to the infected sample labelled with Alexa 647 dye. The dyes assigned to the control and treated samples were swapped for the other four arrays. After labelling and volume reduction, the sample from the uninfected BoMac cell was combined with the sample from the BoMac/BIV cell and diluted in 120 μL of SlideHyb Glass Array Hybridization buffer 1 (Ambion, Naugatuck, CT, USA) and incubated at 70℃ for 5 min. Following this step, hybridisation was carried out using HybArray12 (Perkin Elmer, Waltham, MA, USA) through the following steps: O-ring conditioning at 75℃ for 2 min, probe introduction at 75℃, step down hybridisation at 42℃ for 6 h, 35℃ for 6h, and 30℃ for 6 h, followed by washing steps (five times in 2 × SSC/0.1% sodium dodecyl sulphate (SDS), 37℃; five times in 0.2 × SSC/0.1% SDS, 25℃; and five times in 0.2 × SSC, 25℃). Next the microarrays were spun to dryness and scanned using a ProScanArray Microarray Scanner (Perkin Elmer).
Microarray data analysis
Microarrays were processed using ProScanArray Express software (Perkin Elmer, Waltham, MA, USA) to generate spot analysis data. Normalisation was carried out by the LOWESS method and statistical analysis was performed with the R/Bioconductor packages. The median fluorescence intensity was used for data analysis. The obtained data was deposited in the NCBI Gene Expression Omnibus (GEO) with accession no. GSE218260. The genes chosen as differentially expressed (DEGs) were those with absolute fold-change >1.1. The DAVID Gene ID Conversion Tool (database for annotation, visualization, and integrated discovery, http://david.abcc.ncifcrf.gov/)was used to associate GenBank accession numbers retrieved from BLO array data files with official gene symbols. These official symbols of DEGs were submitted to the Ingenuity Pathway Analysis tool (IPA, http://www.ingenuity.com) (Qiagen, Redwood City, CA, USA) to reveal enrichment for genes engaged in the immune function.
Validation of differentially expressed genes using RT-qPCR. Sequences of oligomers from the BLOPlus microarray representing the genes chosen for RT-qPCR validation, namely DCTN6, ATR, LTA, IL-18, and HYAL2, were input to the BLAST tool to find additional sequence information for primer design, which was undertaken with the Primer3 tool (https://primer3.ut.ee). Two micrograms of the RNA of both BoMac and BoMac/BIV cells were digested separately with DNase I, Amplification Grade (Invitrogen, Carlsbad, CA, USA) as recommended by the manufacturer. Then 8 μL of each digestion reaction was combined with 1 μL of 50 μM oligo(dT)20 and 4 μL of 5 mM dNTPs mix and heated to 65℃ for 5 min. Then 5 × cDNA buffer, RNase inhibitor, dithiothreitol and dART reverse transcriptase were added, and reverse transcription was allowed to proceed according to the recommended protocol with the dART RT Kit (EURX, Gdańsk, Poland). Complementary DNA (cDNA) was quantified spectrophotometrically. Subsequently 50 ng of cDNA per qPCR reaction was used. We performed a qPCR reaction with primers specific for each gene using the QuantiTect SYBR Green Kit (Qiagen, Hilden, Germany) in a total reaction volume of 25 μL. The cycling conditions were as follows: 95°C for 15 min, and then 40 cycles of 95°C for 15 s, 50–54°C for 30 s depending on the primers and 72℃ for 30 s. The qPCR reaction was carried out in Rotor-Gene Q (Qiagen, Hilden, Germany) and the data were collected by the Rotor-Gene Assay Manager. For each primer set, standard curves made from serial dilutions of cDNA were used to estimate PCR reaction efficiency (E) using the formula E(%) = (10(−1/slope) − 1) × 100. Relative quantification of mRNA for each gene was conducted using E-methods with UCHL5 as the reference gene (26).
Results
Optimal infection conditions were determined by varying the MOI between 0.01, 0.1, 1 and 10 and the time after exposure of BoMac cells to BIV between 24 h, 48 h and 72 h. The qPCR results indicated that an MOI of 10 and 24 h were the optimal scheme for cell infection. Under these conditions both the virus titre (1.1 × 106 genomes/mL) and the copy number of proviral BIV DNA (15 copies/104 cells) were the highest, which indicates the peak of productive BIV infection in cultured BoMac cells.
To assess changes in the expression of host cellular genes in response to BIV infection, we analysed the transcriptome of BoMac cells by microarray analysis. This approach enabled us to show that 1,734 genes were differentially expressed upon BIV infection (FC >1.1 fold). The IPA core analysis was applied for all DEGs in order to identify the pathways and gene networks in which they were involved with the highest degree of significance. Out of 1,743 DEGs, 1,315 were mapped as unique molecules represented in the Ingenuity Knowledge Base (Table S1). In total 718 genes were identified as upregulated and 597 genes as downregulated. All 1,315 genes were used to identify overrepresented canonical pathways associated with the immune response including the following categories: cellular immune response, cytokine signalling, humoral immune response and pathogen-influenced signalling.
The IPA software indicated 16 pathways related to immune response (Table S2). The most enriched canonical pathway was leukocyte extravasation signalling with the following DEGs involved in this pathway: ABL1, ACTG2, CD44, CLDN3, CXCL12, GNAI3, ICAM3, MAPK10, MMP17, MMP23B, MMP9, NCF2, NCF4, PIK3C2B, PRKCG, PRKCZ, PTK2, PXN, RAP1B, VAV1, VAV3, VCAM1, WAS, and WIPF1 (Fig. 1.).
Fig. 1.
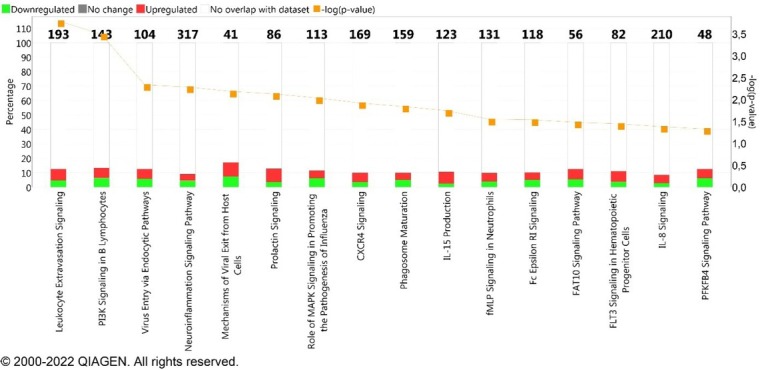
Top canonical pathways enriched in differentially expressed genes (DEGs). The value of −log(p) >1.3 reflects a significant association between the canonical pathway and the involved genes. The percentage indicates the number of DEGs overlapping with molecules associated to canonical pathways. The colours represent up- (red) and downregulated (green) genes. The numbers on top of the bars represent the total number of molecules associated to each pathway
Additionally, all customised canonical pathways were ordered according to the z-score value, which predicts pathway activation (value above 0) or inhibition (value below 0) (Fig. 2). In general, several interleukin and chemokine signalling pathways showed a shift of the activation state according to the z-score value. Interleukin (IL)-15 production was indicated as the most activated pathway (z-score 1.941) and the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) signalling pathway (z-score -0.447) was the most inhibited one (Fig. 2). The following molecules identified as DEGs were engaged in the IL-15 production pathway: ABL1, CLK2, CSK, DDR1, IGF1R, LCK, MET, MUSK, NTRK1, PRKCZ, PTK2, RYK and YES1. The molecules ATF2, ATF4, CREB1, CREB3L1, HK3 and PRKACA were involved in the PFKFB4 signalling pathway.
Fig. 2.
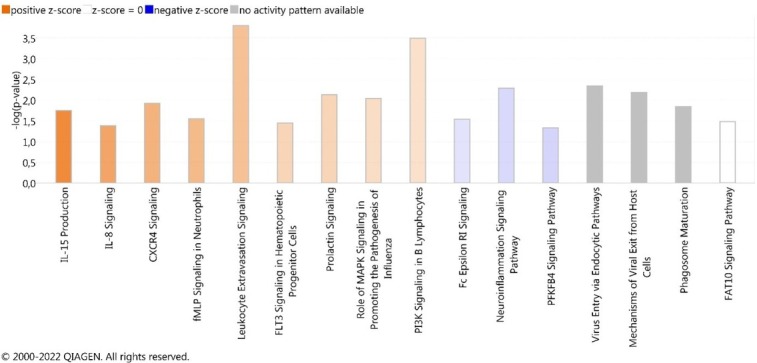
Most prominent canonical pathways ordered according to z-score value and their predicted activation state with regard to −log (p-value) greater than 1.3. The bars represent the p-values of overlap (−log) of differentially expressed genes in the dataset with known pathway-associated molecules. Pathways in orange are those predicted to be activated and pathways in blue are those predicted to be inhibited. The higher the intensity of the colours, the higher the absolute z-score
To further estimate the possible role of BIV infection in addition to canonical pathways, downstream effects analysis was conducted. The analysis makes it possible to predict the effect of molecular changes in gene expression on biological processes and disease. As a result, one of the highly implicated categories was inflammatory response, which according to the z-score value was predicted to be decreased. Next, the analysis showed 12 functions assigned to the inflammatory response with a number of genes that belong to a particular category (Table 2, Table S3). All of the 12 functions were predicted to be inhibited, and two of them, inflammation of joints and inflammatory response, were identified as statistically significant (z-score <−2).
Table 2.
Disease and biofunctions identified by IPA ordered with respect to activation z-score
| Diseases or functions annotation | P-value | Activation z-score | Number of molecules |
|---|---|---|---|
| Inflammation of joints | 4.61E−09 | −3.228* | 143 |
| Inflammatory response | 1.71E−06 | −2.003* | 103 |
| Activation of leukocytes | 6.71E−07 | −1.664 | 88 |
| Inflammation of respiratory system component | 6.93E−06 | −1.518 | 76 |
| Inflammation of body cavity | 4.07E−06 | −1.467 | 139 |
| Rheumatoid arthritis | 2.18E−07 | −1.387 | 102 |
| Inflammation of lungs | 3.60E−06 | −1.379 | 54 |
| Allergic pulmonary eosinophilia | 2.95E−06 | −1.342 | 12 |
| Inflammation of organs | 1.03E−07 | −1.088 | 187 |
| Inflammation of absolute anatomical region | 6.76E−06 | −1.049 | 153 |
| Immune response of tumour cell lines | 1.06E−05 | −0,788 | 29 |
| Dermatitis | 9.31E−06 | −0.363 | 68 |
Number of molecules – the number of analysis-ready genes that belonged to a disease or function category
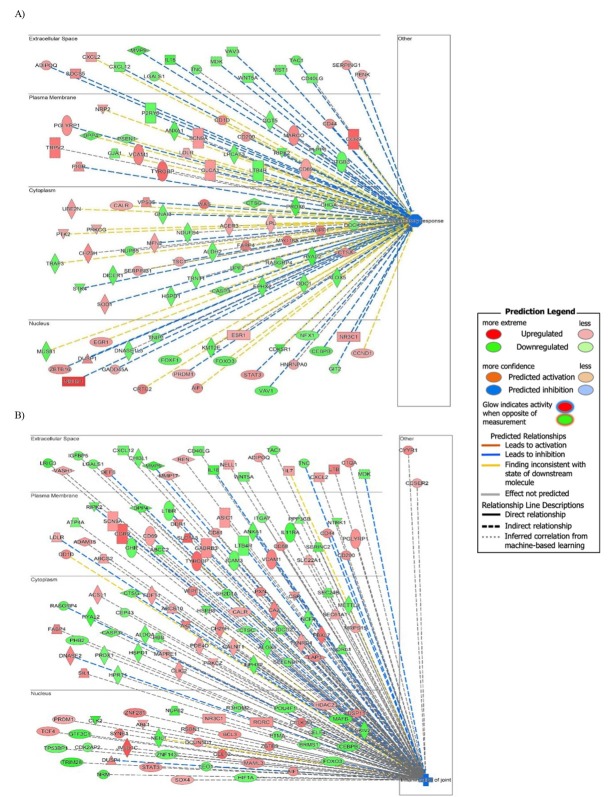
The representation of differentially expressed genes and their effect on selected biological functions is presented in Fig. 3. Both functions, i.e. inflammation of joints (A) and inflammatory response (B), included many genes which caused strong inhibition as predicted by IPA and was shown by the dark blue colour of the nodes connecting the analysed genes.
Fig. 3.
The inflammation of joints (A) and inflammatory response (B) pathways in BIV infected BoMac cells. The shapes of the nodes reflect the functional class of each gene product: transcriptional regulator (horizontal ellipse), transmembrane receptor (vertical ellipse), enzyme (rhombus), cytokine/growth factor (square), kinase (triangle), and complex/group/other (circle). Other indicators are explained in the prediction legend
Another IPA prediction concerns the regulator effects and displays a relationship between the regulator and disease or function and shows how regulators cause downstream effects on biology. In this prediction, a consistency score is calculated for each regulator effect network, based on findings from the literature. Higher scores are awarded to networks that are consistent with the predicted state of the regulator, the observed direction of expression of the target in the dataset and the expected impact on the downstream disease/ function. The IPA analysis identified the five most pronounced regulatory effects (Table 3) and what was interesting was that the highest consistency score was noted for inflammation of joints and the inflammatory response. Therefore, as previously in disease and functions analysis, the inflammation process was confirmed to be affected by BIV infection.
Table 3.
The top-scoring regulatory networks identified using IPA software
| Consistency score | Node total | Regulator total | Regulators | Target total | Target molecules in dataset | Diseases & functions |
|---|---|---|---|---|---|---|
| 1.291 |
20 |
4 |
ELF4, INSIG1, INSR, mir-148 |
15 |
ACSL1, C1QA, CA2, CD68, CXCL2, DUSP1, FABP4, FDFT1, GABRB3, HSPD1, LDLR, LGALS1, MMP9, PRDM1, VCAM1
|
Inflammation of joints |
| −7.211 |
15 |
1 |
INSR |
13 |
ALDH2, CLCA1, DUSP1,EGR1, FABP4,GADD45A, HSPD1, LGALS1, MFN2, MMP9, PRDM1, SERPINB1, VCAM1
|
Inflammatory response |
| −7.500 |
6 |
1 |
RABGEF1 |
4 |
CTSH, GBA, HEXB, LDLR |
Metabolism of protein |
| −7.506 |
5 |
1 |
ALDH1A2 |
3 |
ALDH1A3, CCN2, CD44 |
Neonatal death |
| −8.660 | 5 | 1 | DDX3X | 3 | CCND1, ODC1, RPS5 | Metabolism of protein |
* – statistically significant
To validate the gene expression profiles identified by microarray analysis, five differentially expressed genes were selected for RT-qPCR examination (Table 4). Consistently with the microarray results, the expression pattern revealed by RT-qPCR showed upregulation of ATR, LTA and DCTN6 and downregulation of the IL-18 and HYAL2 genes.
Table 4.
Comparison of gene expression changes observed in microarray analysis and RT-qPCR
| Gene | Reference sequence ID | Primer sequence (5′–3′) | FC | |
|---|---|---|---|---|
| Microarray | RT-qPCR | |||
| UCHL5 * | 174481* NM_ | F: ACAAAGACAACTTGCTGAGGAACCC | N/A | N/A |
| R: GGCAACCTCTGACTGAATAGCACTT | ||||
| ATR | XM_002685057.2 | F: AATGCACGTGTCCTTCGATA | 2.45 | 1.05 |
| R: TGAAAAGGCCAAGACTCATGT | ||||
| LTA | NM_001013401.2 | F: CCCTCAGAGCCTCGCTTT | 2.00 | 1.19 |
| R: GCGAGACATCAGAAGAAAGAGC | ||||
| IL-18 | NM_001562 | F: AGACAGGTTGATTTCCCTGGT | −1.50 | −1.15 |
| R: CCTGGAATCAGATCACTTTGG | ||||
| HYAL2 | XM_024982390 | F: GCGACCAGAGGGGGAACTC | −1.50 | −1.03 |
| R: TAGCACTGGCAGCGAAAGTGCA | ||||
| F: TGGTGGAGACTGCCTGCG | ||||
| DCTN6 | XM_024986251 | R: CCGACTAAAGAGGTTCTAGCAC | 3.00 | 1.12 |
* − Primers designed by Brym et al. (3)
Discussion
In the current study we optimised the in vitro model for BIV infection of BoMac cells with BIV and analysed the transcriptome of BoMac cells by microarray. We focused our attention on bovine macrophages since these cells play a crucial role in the immune system and many reports showed a harmful effect of BIV on monocyte/macrophage functions (7, 25). Furthermore, the host–virus interaction in the context of immune responses to BIV infection on the transcriptional level is poorly known. In our study we used the FL112 strain of BIV because this isolate exhibits different replication characteristics from the original R29 strain, which was attenuated during its long adaptation to in vitro cell cultures (10). Furthermore, FL112 was isolated from infected calves showing lymphadenopathy and an increased number of haemal lymph nodes (23).
Macrophages initiate and regulate wide-ranging immunological responses to many viral infections, including lentiviruses. On the other hand, there is evidence showing that the same virus type, a lentivirus HIV-1, leads to a loss of ability to trigger innate immune activation by macrophages (33). Moreover, by disrupting the innate response, HIV-1 avoids the antiviral host response and uses macrophages as a virus reservoir (8). This illustrates the diversity of the immune response following viral infection, which is regulated, among other interactions, by those between host and pathogen genes. Therefore, transcriptome analysis of host cells following viral infection is a convenient model for investigating host genes and molecular mechanisms involved in the antiviral response.
In this study, microarray data from BoMac cells infected with BLV and IPA analysis showed that the pathway most significantly enriched and changed by BIV infection is the canonical pathway related to leukocyte extravasation signalling (Fig. 1). Taking into consideration the respective values of the z-score, this pathway was significantly activated. Leukocyte extravasation is the process by which leukocytes migrate from blood to tissue during inflammation (21). It has been suggested that the ability of HIV-1 to activate leukocyte adhesion is the route by which the virus may exit the bloodstream and gain access to body tissues, leading to the establishment of reservoirs in tissues (37). Proviral DNA of BIV was present mainly in lymphoid and neural tissues in BIV-infected calves, which could indicate the involvement of the leukocyte extravasation signalling pathway (40). Among 16 pathways related to immune response, the IL-15 production pathway was indicated as the most activated (z-score 1.941). Interleukin 15 belongs to the 4-α-helix bundle family of glycoprotein cytokines that include IL-2, IL-3, IL-4 and IL-21. The secretion of IL-15 is strongly correlated with increasing HIV-1 viraemia and is one of the inflammation markers (32). This interleukin could be involved in modulating the HIV reservoir size by promoting the persistence of cells with integrated provirus or by enhancing HIV reactivation (12). Moreover, IL-15 secretion is significantly correlated with the upregulation of memory CD4 T cells, which increases susceptibility to simian immunodeficiency virus IV infection (11).
Our analysis also showed that the most inhibited pathway was the PFKFB4 signalling pathway (z-score – 0.447) (Fig. 2). Generally, the PFKFB4 signalling pathway has been associated with key aspects of cancer cell survival (19). However, recently Carlberg (4) pointed out that PFKFB4 was associated with genes mediating energy metabolism and is under the control of vitamin D receptors (VDRs). In addition to controlling cellular metabolism, VDRs regulate processes important for immunity. Moreover, immune system-related genes are upregulated by vitamin D3 in monocytes and dendritic cells (24, 36). Even though the mechanism of the binding of the bioactive form of vitamin D to VDRs in immune cells such as dendritic cells is still unclear, the results of the study by Vanherwegen et al. (36) suggested that the availability of glucose and the presence of PFKFB4 could be important for the cellular response. Therefore, it can be assumed that inhibition of the PFKFB4 signalling pathway may entrain the inhibition of the immune response.
In conclusion, both the IL-15 production and PFKFB4 signalling pathways play important roles in regulating the inflammatory response. Our studies have shown that both are significantly dysregulated following BIV infection, which consequently facilitates the establishment of the infection. In addition, this study showed that one of the inflammatory cytokines, IL-18 (18), was downregulated, which also confirms the inhibition of the inflammatory response during BIV infection (Table 4).
Despite the continuation of research on BIV, it is not yet known whether immunodeficiency is associated with BIV infection (7). Some reports have shown that BIV could favour secondary infections in naturally infected cows by masking the role of BIV as the primary pathogen. In this context our findings indicating dysregulation of the inflammatory response at the transcriptome level in BIV-infected BoMac cells may be helpful in explaining some pathological signs noted in cattle naturally co-infected with BIV (5, 29). This is also especially relevant to cattle co-infections with bovine leukemia virus and BIV, which were reported in several studies (16, 34) and contributed to poor productivity, impairment of the immune system and recurrent infections.
In summary, the results of this study revealed new mechanisms of interaction between cellular pathways and genes associated with the immune response following infection with BIV.
Acknowledgements
The authors acknowledge Dr Marcel Hulst and the ‘EPIZONE’ network of excellence for their assistance with microarray analysis. The authors thank Dr Chris Venables for sharing FBL/FL112 BIV cells, Dr Yahia Chebloune for the BIV molecular clone and Dr Thomas Vahlenkamp for providing BoMac cells with kind permission of Dr Judith R. Stabel.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was supported by the Polish Ministry of Science and Higher Education (grant N N308 182438). The IPA programme was funded by 6/487232/SPUB/SN/2021.
Animal Rights Statement: None required.
Supplementary Material.
References
- 1.Appelberg K.S., Wallet M.A., Taylor J.P., Cash M.N., Sleasman J.W., Goodenow M.M.. HIV-1 infection primes macrophages through STAT signalling to promote enhanced inflammation and viral replication. AIDS Res Hum Retroviruses. 2017;33:690–702. doi: 10.1089/aid.2016.0273.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown J.N., Kohler J.J., Coberley C.R., Sleasman J.W., Goodenow M.M.. HIV-1 activates macrophages independent of Toll-Like Receptors. PLoS One. 2008;3:e3664. doi: 10.1371/journal.pone.0003664.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brym P., Ruść A., Kamiński S.. Evaluation of reference genes for qRT-PCR gene expression studies in whole blood samples from healthy and leukemia-virus infected cattle. Vet Immunol Immunopathol. 2013;153:302–307. doi: 10.1016/j.vetimm.2013.03.004.. [DOI] [PubMed] [Google Scholar]
- 4.Carlberg C. Vitamin D signaling in the context of innate immunity: focus on human monocytes. Front Immunol. 2019. p. 10. [DOI] [PMC free article] [PubMed]
- 5.Carpenter S., Miller L.D., Alexandersen S., Whetstone C.A., Van Der Maaten M.J., Viuff B., Wannemuehler Y., Miller J.M., Roth J.A.. Characterization of early pathogenic effects after experimental infection of calves with bovine immunodeficiency-like virus. J Virol. 1992;66:1074–1083. doi: 10.1128/jvi.66.2.1074-1083.1992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho K.O., Meas S., Park N.Y., Kim Y.H., Lim Y.K., Endoh D., Lee S.I., Pjasjo K., Sugimoto C., Onuma M.. Seroprevalence of bovine immunodeficiency virus in dairy and beef cattle herds in Korea. J Vet Med Sci. 1999;61:549–551. doi: 10.1292/jvms.61.549.. [DOI] [PubMed] [Google Scholar]
- 7.Corredor A., St-Louis M.C., Archambault D.. Molecular and biological aspects of the bovine immunodeficiency virus. Curr HIV Res. 2010;8:2–13. doi: 10.2174/157016210790416343.. [DOI] [PubMed] [Google Scholar]
- 8.Covino D.A., Kaczor-Urbanowicz K.E., Lu J., Chiantore M.V., Fiorucci G., Vescio M.F., Catapano L., Purificato C., Galluzz C.M., Amici R., Andreotti M., Gauzzi M.C., Pellegrini M., Fantuzzi L. Transcriptome profiling of human monocyte-derived macrophages upon CCL2 neutralization reveals an association between activation of innate immune pathways and restriction of HIV-1 gene expression. Front Immunol. 2020. p. 11. [DOI] [PMC free article] [PubMed]
- 9.Desport M., Lewis J.. Jembrana disease virus: host responses, viral dynamics and disease control. Curr HIV Res. 2010;8:53–65. doi: 10.2174/157016210790416370.. [DOI] [PubMed] [Google Scholar]
- 10.Desport M., McLachlan S. Desport M. Lentiviruses and Macrophages: Molecular and Cellular Interactions. Caister Academic Press; Poole: 2010. The Bovine Lentiviruses: Pathogenesis and Cell Tropism; pp. 307–327. edited by. [Google Scholar]
- 11.Eberly M.D., Kader M., Hassan W., Rogers K.A., Zhou J., Mueller Y.M., Mattapallil M.J., Piatak M., Lifson J.D., Katsikis P.D., Roederer M., Villinger F., Mattapallilet J.J.. Increased IL-15production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182:1439–1448. doi: 10.4049/jimmunol.182.3.1439.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavegnano C., Brehm J.H., Dupuy F.P., Talla A., Ribeiro S.P., Kulpa D.A., Cameron C., Santos S., Hurwitz S.J., Marconi V.C., Routy J.P., Sabbagh L., Schinazi R.F., Sékalyet R.P.. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLOS Pathog. 2017;13:e1006740. doi: 10.1371/journal.ppat.1006740.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonda M.A., Braun M.J., Carter S.G., Kost T.A., Bess J.W., Arthur L.O., Van Der Maaten M.J.. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. Nature. 1987;330:388–391. doi: 10.1038/330388a0.. [DOI] [PubMed] [Google Scholar]
- 14.Gonda M.A., Luther D.G., Fong S.E., Tobin G.J.. Bovine immunodeficiency virus: molecular biology and virus-host interactions. Virus Res. 1994;32:155–181. doi: 10.1016/0168-1702(94)90040-X.. [DOI] [PubMed] [Google Scholar]
- 15.González‐Fernández V.D., Tórtora Pérez J.L., García Flores M.M., Aguilar Setién J.Á., Ramírez Álvarez H.. First evidence of bovine immunodeficiency virus infection in Mexican cattle. Transbound Emerg Dis. 2020;67:1768–1775. doi: 10.1111/tbed.13530.. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo G., Flores M., Bonilla J.A.. Detection and isolation of bovine immunodeficiency-like virus (BIV) in dairy herds of Costa Rica. J Vet Med Ser B. 1995;42:155–161. doi: 10.1111/j.1439-0450.1995.tb00696.x.. [DOI] [PubMed] [Google Scholar]
- 17.Horzinek M., Keldermans L., Stuurman T., Black J., Herrewegh A., Sillekens P., Koolen M.. Bovine immunodeficiency virus, immunochemical characterization and serological survey. J Gen Virol. 1991;72:2923–2928. doi: 10.1099/0022-1317-72-12-2923.. [DOI] [PubMed] [Google Scholar]
- 18.Ihim S.A., Abubakar S.D., Zian Z., Sasaki T., Saffarioun M., Maleknia S., Azizi G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front Immunol. 2022. p. 13. [DOI] [PMC free article] [PubMed]
- 19.Kotowski K., Rosik J., Machaj F., Supplitt S., Wiczew D., Jabłońska K., Wiechec E., Ghavami S., Dzięgiel P.. Role of PFKFB3 and PFKFB4 in cancer: genetic basis, impact on disease development/progression, and potential as therapeutic targets. Cancers (Basel) 2021;13:909. doi: 10.3390/cancers13040909.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meas S., Ohashi K., Tum S., Chhin M., Te K., Miura K., Sugimoto C., Onuma M.. Seroprevalence of bovine immunodeficiency virus and bovine leukemia virus in draught animals in Cambodia. J Vet Med Sci. 2000;62:779–781. doi: 10.1292/jvms.62.779.. [DOI] [PubMed] [Google Scholar]
- 21.Muller W.A.. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7–22. doi: 10.1177/0300985812469883.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muluneh A.. Seroprevalence of bovine immunodeficiency-virus (BIV) antibodies in the cattle population in Germany. J Vet Med Ser B. 1994;41:679–684. doi: 10.1111/j.1439-0450.1994.tb00280.x.. [DOI] [PubMed] [Google Scholar]
- 23.Munro R., Lysons R., Venables C., Horigan M., Jeffrey M., Dawson M.. Lymphadenopathy and non-suppurative meningo-encephalitis in calves experimentally infected with bovine immunodeficiency-like virus (FL112) J Comp Pathol. 1998;119:121–134. doi: 10.1016/S0021-9975(98)80057-1.. [DOI] [PubMed] [Google Scholar]
- 24.Nelson C.D., Reinhard T.A., Thacker T.C., Beitz D.C., Lippolis J.D.. Modulation of the bovine innate immune response by production of 1α,25-dihydroxyvitamin D3 in bovine monocytes. J Dairy Sci. 2010;93:1041–1049. doi: 10.3168/jds.2009-2663.. [DOI] [PubMed] [Google Scholar]
- 25.Onuma M., Koomto E., Furuyama H., Yasutomi Y., Taniyama H., Iwai H., Kawakami Y.. Infection and dysfunction of monocytes induced by experimental inoculation of calves with bovine immunodeficiency like virus. J Acquir Immune Defic Syndr. 1992;5:1009–1015. [PubMed] [Google Scholar]
- 26.Pfaffl M.W.. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45e–45. doi: 10.1093/nar/29.9.e45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rola-Łuszczak M., Kozaczyńska B., Kuźmak J.. Serological survey for bovine immunodeficiency virus in dairy cattle from Poland. Pol J Vet Sci. 2011;14:579–583. doi: 10.2478/v10181-011-0086-8.. [DOI] [PubMed] [Google Scholar]
- 28.StCyr Coats K., Pruett S.B., Nash J.W., Cooper C.R.. Bovine immunodeficiency virus, incidence of infection in Mississippi dairy cattle. Vet Microbiol. 1994;42:181–189. doi: 10.1016/03781135(94)90017-5.. [DOI] [PubMed] [Google Scholar]
- 29.Snider T.G., Hoyt P.G., Coats K.S., Graves K.F., Cooper C.R., Storts R.W., Luther D.G., Jenny B.F.. Natural bovine lentiviral type 1 infection in Holstein dairy cattle. I. Clinical, serological, and pathological observations. Comp Immunol Microbiol Infect Dis. 2003;26:89–101. doi: 10.1016/S0147-9571(02)00021-8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stabel J.R., Stabel T.J.. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet Immunol Immunopathol. 1995;45:211–220. doi: 10.1016/0165-2427(94)05348-V.. [DOI] [PubMed] [Google Scholar]
- 31.Suarez D.L., Van Der Maaten M.J., Wood C., Whetstone C.A.. Isolation and characterization of new wild-type isolates of bovine lentivirus. J Virol. 1993;67:5051–5055. doi: 10.1128/jvi.67.8.5051-5055.1993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan S., Qiu J., Rupert A.W., Hu Z., Higgins J., Dewar R.L., Stevens R., Rehm C.A., Metcalf J.A., Sherma B.T., Baseler M.W., Clifford H., Imamichi T.. Interleukin-15 (IL-15) strongly correlates with increasing HIV-1 viremia and markers of inflammation. PLoS One. 2016;11:e0167091. doi: 10.1371/journal.pone.0167091.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang J., Chain B.M., Miller R.F., Webb B.L., Barclay W., Towers G.J., Katz D.R., Noursadeghi M.. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. AIDS. 2009;23:2255–2263. doi: 10.1097/QAD.0b013e328331a4ce.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usui T., Meas S., Konnai S., Ohashi K., Onuma M.. Seroprevalence of bovine immunodeficiency virus and bovine leukemia virus in dairy and beef cattle in Hokkaido. J Vet Med Sci. 2003;65:287–289. doi: 10.1292/jvms.65.287.. [DOI] [PubMed] [Google Scholar]
- 35.Van Der Maaten M.J., Boothe A.D., Seger C.L.. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972;49:1649–1657. doi: 10.1093/jnci/49.6.1649.. [DOI] [PubMed] [Google Scholar]
- 36.Vanherwegen A.S., Eelen G., Ferreira G.B., Ghesquière B., Cook D.P., Nikolic T., Roep B., Carmeliet P., Telang S., Mathieu C., Gysemans C.. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J Steroid Biochem Mol Biol. 2019;187:134–145. doi: 10.1016/j.jsbmb.2018.11.011.. [DOI] [PubMed] [Google Scholar]
- 37.Weeks B.S.. The role of HIV-1 activated leukocyte adhesion mechanisms and matrix metalloproteinase secretion in AIDS pathogenesis. Int J Mol Med. 1998;1:361–366. doi: 10.3892/ijmm.1.2.361.. [DOI] [PubMed] [Google Scholar]
- 38.Whetstone C.A., Suarez D.L., Miller J.M., Pesch B.A., Harp J.A.. Bovine lentivirus induces early transient B-cell proliferation in experimentally inoculated cattle and appears to be pantropic. J Virol. 1997;71:640–644. doi: 10.1128/jvi.71.1.640-644.1997.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J.Q., Sassé T.R., Wolkenstein G., Conceicao V., Saksena M.M., Soedjono M., Perera S.S., Wang B., Dwyer D.E., Saksena N.K.. Transcriptome analysis of primary monocytes shows global down-regulation of genetic networks in HIV viremic patients versus long-term non-progressors. Virology. 2013;435:308–319. doi: 10.1016/j.virol.2012.10.026.. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S., Wood C., Xue W., Krukenberg S.M., Chen Q., Minocha H.C.. Immune suppression in calves with bovine immunodeficiency virus. Clin Diagnostic Lab Immunol. 1997;4:232–235. doi: 10.1128/cdli.4.2.232-235.1997.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.