Abstract
Objectives
Fracture is a common injury after a traumatic event. The efficacy and safety of non-steroidal anti-inflammatory drugs (NSAIDs) to treat acute pain related to fractures is not well established.
Methods
Clinically relevant questions were determined regarding NSAID use in the setting of trauma-induced fractures with clearly defined patient populations, interventions, comparisons and appropriately selected outcomes (PICO). These questions centered around efficacy (pain control, reduction in opioid use) and safety (non-union, kidney injury). A systematic review including literature search and meta-analysis was performed, and the quality of evidence was graded per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. The working group reached consensus on the final evidence-based recommendations.
Results
A total of 19 studies were identified for analysis. Not all outcomes identified as critically important were reported in all studies, and the outcome of pain control was too heterogenous to perform a meta-analysis. Nine studies reported on non-union (three randomized control trials), six of which reported no association with NSAIDs. The overall incidence of non-union in patients receiving NSAIDs compared with patients not receiving NSAIDs was 2.99% and 2.19% (p=0.04), respectively. Of studies reporting on pain control and reduction of opioids, the use of NSAIDs reduced pain and the need for opioids after traumatic fracture. One study reported on the outcome of acute kidney injury and found no association with NSAID use.
Conclusions
In patients with traumatic fractures, NSAIDs appear to reduce post-trauma pain, reduce the need for opioids and have a small effect on non-union. We conditionally recommend the use of NSAIDs in patients suffering from traumatic fractures as the benefit appears to outweigh the small potential risks.
Keywords: pain, traumatic, fractures
WHAT IS ALREADY KNOWN ON THIS TOPIC
The use of non-steroidal anti-inflammatory drugs (NSAIDs) for analgesia after traumatic fracture is inconsistent and controversial due to uncertainty about the balance of harm (non-union) and benefit (analgesia and opioid reduction).
WHAT THIS STUDY ADDS
NSAIDs have a clear analgesic and opioid reduction effect with a minimal increase in non-union when prescribed to patients with acute traumatic fractures.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The benefits of NSAIDs in the treatment of acute traumatic fracture outweigh the potential harms.
Introduction
Opioids have traditionally been used for analgesia after trauma; however, misuse and abuse of opioids have reached epidemic proportions.1 In the last three decades, drug overdoses, largely due to opioids, have tripled.2 This public health crisis has led providers to consider alternative pharmaceuticals to control pain after trauma. Therefore, there is an upsurge in the interest of non-steroidal anti-inflammatories (NSAIDs) for acute pain control, particularly in trauma patients. However, the safety and efficacy of NSAIDs for acute pain control after fracture has not been well established, and debate remains about potential risks.
Traditionally, the most concerning adverse event related to NSAID use after orthopedic trauma is fracture non-union. Unfortunately, numerous attempts to study and quantify the relationship have led to conflicting results.3–23 Furthermore, alternative treatments to NSAIDs, including opioids, have demonstrated similar associations to non-union.18 A number of meta-analyses and systematic reviews have been performed in the non-trauma population with mixed results.24–26 It remains unclear whether increased pain medication (NSAIDs or opioids) directly increases rates of non-union or instead, the pain secondary to non-union increases the use of these medications. The risks and benefits of using NSAIDs for managing acute pain in patients with traumatic fractures remains unclear, and practices across North America vary greatly. The current Orthopedic Trauma Association (OTA) and American Academy of Orthopaedic Surgeons guidelines focus on acute musculoskeletal injuries and only explore fracture healing and do not discuss other potential harms such as acute kidney injury.27 28
We performed a systematic review and meta-analysis to develop evidenced-based recommendations to determine whether NSAIDs were safe and effective in patients with traumatic fractures, following the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology29 on behalf of Eastern Association for The Surgery of Trauma (EAST) and the OTA.
Objectives
A working group was formed under the EAST Guidelines Committee in collaboration with the Orthopedic Trauma Association to formulate an evidence-based guideline on NSAID use after orthopedic trauma.30 The GRADE methodology was used.31
Methods
Population, intervention and comparator (PIC) questions were generated a priori to the systematic literature review. Pertinent outcomes (O) were identified by the working group and then independently each member voted on outcomes using a scale of 1 to 9. Outcomes that received a rounded average score of 7 to 9 were deemed critical outcomes, those receiving a score of 4 to 6 were considered important but not critical and those receiving a score of 1 to 3 were considered of limited importance. Only critically important outcomes (7 to 9 rating) were considered in decision making for generating the final recommendations.
PICO 1: should NSAIDs be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that do not include NSAIDs to improve analgesia and reduce oral morphine equivalents (OMEs), without increases in non-union and acute kidney injury rates?
PICO 2: should ketorolac be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that do not include ketorolac to improve analgesia and reduce OMEs, without increasing non-union rates?
PICO 3: should selective NSAIDs (COX-2 inhibitors) be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that include non-selective NSAIDs to improve analgesia and reduce OMEs, without increasing non-union rates?
Acute kidney injury was deemed critical as an outcome for PICO 1 but not for PICO 2 and PICO 3 (score of 6).
COX-2 inhibitors included the coxib medications and meloxicam.
Identification of references
Our project was registered with the PROSPERO registry of systematic reviews and meta-analyses (CRD42020167575). Published literature was searched through MEDLINE (via Ovid), Embase (via Elsevier), Cochrane Central Register of Controlled Trials (via Wiley) and Web of Science (via Clarivate) databases by a professional librarian (SC) on March 18, 2020 and updated on February 25, 2021. The search used a combination of database-specific subject headings and keywords for the following concepts: NSAIDs, Opioids, Orthopedic Procedures, Fracture in various iterations and combinations. Results were limited to the English language. The full search strategy is available in online supplemental file 1.
tsaco-2022-001056supp001.pdf (414KB, pdf)
Studies that included adult (≥18 years old) trauma patients with any fracture were eligible for inclusion. Case reports, case series, commentaries, reviews, editorials and animal studies were excluded. For a study to be included in our final analysis, a clear comparison between patients receiving NSAIDs and control patients had to be present, as well as at least one of the critical outcomes reported. Studies in which NSAIDs were part of a multimodal approach to pain control compared with no multimodal pain control were excluded as the impact of NSAIDs was unable to be determined. Specifically, studies that did not report the specific impact of NSAIDs independent of other medications were not included.
Titles and abstracts were screened independently by two team members for inclusion in our meta-analysis. Conflicts were blindly adjudicated by a third member. Full-text review was also performed by two team members working independently, with conflicts adjudicated by a blinded third member. Included articles had their reference lists reviewed by two team members for identification of potential additional articles. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for our systematic review is depicted in figure 1.
Figure 1.
PRISMA flow diagram. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Data extraction and methodology
Data extraction from each included study was performed using a standardized data collection sheet and was performed in duplicate. Discrepancies were adjudicated by a third author. Data extracted included authors, journal, publication year, study design, number of patients, type of fracture(s), indication for NSAIDs (heterotopic ossification prevention vs pain control), type of NSAID(s) used, dose and duration (if available), number of patients in each experimental and control arms, as well as the critical outcomes previously listed. The definition of non-union in the literature is inconsistent and varied widely between studies. Due to the variability, the study definition was used and captured.
Meta-analysis was performed in Review Manager (RevMan V.5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) with random-effects modeling to generate forest plots. For dichotomous outcomes, the Mantel-Haenszel random effects model was used to calculate a pooled event rate and OR. For continuous outcomes, inverse variance was used to calculate a mean difference between interventions. Treatment effects were calculated with each study weight being proportional to the number of subjects it contributed to each outcome. Heterogeneity was calculated and quantified with I2. High heterogeneity was considered present for I2 values >75%, moderate for I2 values of 50% to 74% and low if I2 <50%.
Publication bias was evaluated using the Egger test, and the GRADE framework was applied to all quantified outcomes for assessment of bias, publication bias, inconsistency, imprecision and indirectness. Evidence profiles were created for each PICO using GRADEpro GDT software (GRADEpro Guideline Development Tool, McMaster University, 2015).
All committee members voted initially independently taking into consideration the quality of evidence, relationship of benefits and harms, perceived patient values and preferences, and resource utilization. Our PICO questions and analysis results (forest plots, GradePRO table, risk of bias assessment and summary of study types) were submitted to two external GRADE experts for blind review. No Institutional ethics was not necessary as we did not examine any individual patient data.
Results
Most studies were based in the USA, and 10 were prospective randomized control trials. Three included studies investigated the use of indomethacin for prevention of HO, as opposed to for pain control, and reported on outcomes identified as critically important, including non-union (table 1). Two studies12 18 used the same cohort of patients, and only Zura et al12 was used for quantitative synthesis.
Table 1.
Studies reporting on efficacy and safety of NSAIDs after orthopedic fracture in adult patients
| Author (ID) | State or country | Year | Study design | Study size | Patient population (types of fractures and surgery) | NSAIDs used | Duration (time or doses) | Indication for NSAIDs | Outcomes measured |
| Mehta23 | India | 1986 | Randomized control trial | 254 | Long bone fractures who underwent fixation | Dipyrone (500 mg) or ASA (500 mg) | One dose | Pain control | Pain |
| Hunter22 | USA | 2020 | Retrospective cohort | 502 | Malleolar ankle fractures who underwent fixation | ASA (325 mg) | Daily for 6 to 8 weeks | DVT prophylaxis | Non-union |
| Moed21 | USA | 1984 | Retrospective cohort | 35 | Acetabular fractures who underwent fixation | Indomethacin (25 mg) | TID for 6 weeks | Prevent HO | Non-union |
| Jeffcoach19 | USA | 2014 | Retrospective cohort | 193 | Femur, tibia and/or humeral fractures | 8 different NSAIDs | IQR 1.0 to 8.5 doses | Pain control | Non-union |
| Buchheit18 | USA | 2018 | Retrospective cohort | 309 330 | Bone fracture in year 2011 | Any NSAID, dose not reported | Acute (<30 days) Chronic (≥30 days) |
Not reported | Non-union |
| Adolphson17 | Sweden | 1993 | Randomized control trial | 33 | Colles’ fracture in postmenopausal women | Piroxicam (20 mg) | Daily for 8 weeks | Reduction in osteopenia | Pain |
| Weisz16 | USA | 2021 | Randomized control trial | 99 | Fracture of ribs, face, extremities or pelvis | IV Ibuprofen (800 mg) | Eight doses over 2 days | Pain | Pain, OMEs |
| Sagi15 | USA | 2014 | Randomized control trial | 98 | Acetabular fractures who underwent fixation | Indomethacin (75 mg) | Daily for 3 days, 1 week or 6 weeks | Prevent HO | Non-union |
| McDonald14 | USA | 2019 | Randomized control trial | 128 | Ankle fractures who underwent operative treatment | Ketorolac 30 mg IV intra-op+20 tabs Ketorolac 10 mg | 7 days | Pain | Pain, OMEs, non-union |
| Burd13 | USA | 2003 | Randomized control trial | 112 | Acetabular fractures who underwent fixation | Indomethacin (25 mg) | Three times a day for 6 weeks | Prevent HO | Non-union |
| Zura12 | USA | 2016 | Retrospective cohort | 309 330 | Bone fracture in year 2011 | Any NSAID, dose not reported | Not reported | Not reported | Non-union |
| Ortiz10 | Mexico | 2010 | Randomized control trial | 49 | Ankle fracture and pain >5/10 | Ketorolac (10 mg) Diclofenac (70 mg) Etoricoxib (60 mg) |
Two doses | Pain | Pain |
| Bayouth9 | USA | 2013 | Retrospective cohort (matched) | 42 | Rib fractures | IV Ibuprofen | 600 to 800 mg every 6 hours | Pain | Pain, OMEs |
| Eftekharian8 | Iran | 2017 | Randomized clinical trial | 50 | Mandibular fracture | Ketorolac | Single postoperative dose | Pain | Pain |
| Xu7 | USA | 2016 | Randomized clinical trial | 63 | Femoral or tibiofibular fractures | Ketorolac | In postoperative analgesia pump | Pain | Pain, OMEs |
| Tucker6 | USA | 2020 | Retrospective cohort | 17 689 | Operatively treated long-bone fractures | NSAID No NSAID |
Up to 90 days | Not reported | Non-union |
| Aliuskevicius3 | Denmark | 2021 | Randomized control trial | 96 | Non-surgically treated Colles’ fractures | Ibuprofen | 3 days or 7 days | Pain control | Pain |
| Haines5 | USA | 2020 | Retrospective cohort | 190 057 | ≥65 year old who underwent hip or femur fixation for fracture | Any NSAID | Not reported | Not reported | OMEs, acute kidney injury |
| George4 | USA | 2020 | Retrospective cohort | 339 864 | Single long-bone fracture or commonly paired long-bone fractures | Any NSAID, dose not reported; COX-2 or non-selective | Not reported | Not reported | Non-union |
†Institution specific.
‡For both groups combined.
ASA, aspirin; DVT, deep vein thrombosis; HO, heterotopic ossification; NSAIDS, non-steroidal anti-inflammatory drugs; OME, oral morphine equivalents.
PICO 1
Should NSAIDs be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that do not include NSAIDs to improve analgesia and reduce OMEs, without increases in non-union and acute kidney injury rates?
Non-union
Qualitative synthesis
Nine studies4 6 12–15 18 21 22 investigated the impact of NSAIDs on non-union (table 2). In general, non-union was not objectively defined or defined as persistent fracture at variable time-points. Three studies13–15 were randomized control trials, two13 15 of which investigated the use of indomethacin for the prevention of HO and reported on non-union. Most studies (n=6) found no relationship between NSAIDs and non-union, and of the three studies6 13 15 demonstrating an effect, two13 15 were studies on the long-term (6 weeks) use of indomethacin for the prevention of HO.
Table 2.
PICO 1.1 – impact of NSAIDs on non-union
| Author | Non-union definition | Groups | N | Male, n (%) | Age, year, mean (SD) | Non-union, n (%) | Lost to follow-up, n (%) | Study conclusions |
| Hunter22 | Persistent fracture at 24 weeks | ASA No ASA |
152 354 |
66 (43) 163 (46) |
44 (NA) 42 (NA) |
3 (3) 2 (1) |
54 (34) 154 (41) |
ASA for 6 to 8 weeks postoperatively does not influence time to union |
| Moed21 | Not defined | Indomethacin No indomethacin |
16 19 |
NA NA |
32 (NA) 32 (NA) |
0 (0) 0 (0) |
NA NA |
No problems with fracture healing noted |
| Buchheit18 | Not defined | NSAID Acute NSAID Chronic NSAID No NSAID |
2525 2180 527 306 579 |
NA NA |
NA NA |
227 (8.3) 183 (8.4) 44 (7.7) 15 022 (4.9) |
0% at 12 months (only complete cases included) | Numerous medications are associated with non-union including antibiotics, anticoagulants, bisphosphonates, opioids and NSAIDs. Non-union depends on fracture location. |
| Sagi15 | Lack of bridging callus and visible fracture line | Indomethacin 3 days 1 week 6 weeks Placebo |
72 24 25 23 26 |
(70%) (76%) (80%) (67%) |
41 (NA) 34 (NA) 42 (NA) 46 (NA) |
18 (25) 6 (35) 4 (24) 8 (62) 4 (19) |
29% 32% 43% 19% |
Indomethacin is not indicated in the prevention of HO after acetabular fracture. One week on indomethacin may be safe to prevent HO without an increase in non-union. |
| McDonald14 | Clinical healing (walking) and radiographic healing (blinded review) | Ketorolac No ketorolac |
64 64 |
23 (36) 30 (57) |
48 (15) 46 (17) |
0 (0) 0 (0) |
One, unclear which group | Ketorolac reduced post-op pain and OMEs but unclear effect on healing due to study power. |
| Burd13 | Not defined | Indomethacin No indomethacin |
38 74 |
NA NA |
40 (NA) 38 (NA) |
11 (29) 5 (7) |
NA NA |
Avoid NSAIDs for analgesia or anti-inflammatory purposes during healing of fractures. |
| Zura12 | Coded non-union or code for electrical bone stimulation | NSAID No NSAID |
23 847 285 384 |
NA NA |
NA NA |
661 (2.8) 14 588 (5.1) |
NA NA |
No impact of NSAIDs on healing; fracture healing is a diverse process and non-union can result from many risk factors. |
| Tucker6 | Coded non-union | NSAID No NSAID |
15 119 2 570 |
NA NA |
NA NA |
1 179 (7.8) 392 (10.4) |
NA NA |
Exposure to NSAIDs at any point during the 90-day postoperative period was associated with fracture non-union (no opioid adjustment) |
| George4 | Non-union diagnosis code or a procedure to treat non-union | NSAID No NSAID |
25 001 279 720 |
11 229 (45) 103 003 (37) |
49 (17) 60 (19) |
456 (1.8) 4 118 (1.5) |
All had 1- year follow-up | Filling a prescription for a non-selective NSAID after fracture was not associated with an increased risk of non-union in the subsequent year. Both COX-2-inhibitor and opioid prescription fills after fracture were associated with a greater risk of non-union. |
McDonald et al14 randomized 128 patients undergoing fixation or ankle fractures to receive 30 mg intravenous ketorolac intraoperatively and 20 tablets of 10 mg ketorolac postoperatively or usual care. Additionally, both groups were prescribed 81 mg of ASA for deep vein thrombosis prevention. At 12 weeks, there was no difference in experimental or control groups in non-union (17% vs 23% respectively, p=0.43). Buchheit et al18 and Zura et al12 used patient-level health claims data in North Carolina and evaluated 309 330 fractures and the impact of numerous medications, including NSAIDs, on fracture healing. Acute use of NSAIDs (<30 days) was not associated with non-union in any of the fractures studied (18 bones), OR 0.98 (95% CI 0.89 to 1.07). Patients chronically using NSAIDs (prior to fracture) did have increased non-union OR 1.22 (95%CI 1.15 to 1.43). Causality was not established. Other medications investigated did have an association with non-union including acute opioid use (OR 1.47, 95% 1.40 to 1.54) and anticonvulsants (OR 1.2, 95% CI (1.16 to 1.29)).
Moed et al21 investigated indomethacin for HO. In their retrospective cohort study, the authors compared patients with acetabular fractures who received 6 weeks of indomethacin compared with patients who did not receive indomethacin. No patients in either group experienced non-union. Hunter et al22 performed a retrospective chart review comparing patients receiving ASA for DVT prevention to patients who did not receive ASA. ASA was prescribed for 6 to 8 weeks after ankle fracture. Compared with patients without ASA use, there was no difference in non-union (3.1% vs 1%, p=0.21). Finally, George et al4 used claims data in Texas over 15 years to determine the association of NSAIDs (selective and non-selective) and opioids on non-union after any traumatic fracture. Any NSAID use had a higher incidence of non-union (1.2% vs 0.8%); however, this difference disappeared when only non-selective NSAIDs were considered (OR 1.08 95% CI 0.96 to 1.20). Notably, opioid prescription was associated with non-union as well (1.3% v 0.5%, OR 1.53 (95%CI 1.43 to 1.64)).
Three studies concluded there was an association between NSAID use and non-union. Tucker et al6 in a retrospective study of an insurance database found any use of postfixation NSAIDs (at any direction <90 days) was associated with non-union for subtrochanteric femur fractures (OR1.5, 95% 1.24 to 1.84), tibial shaft fracture (OR 1.42, 95% CI 1.19 to 1.69) and humeral shaft fracture (OR 1.2, 95% CI 1.0 to 1.46) as was tobacco use, peripheral vascular disease, obesity and infection. Studies from Burd et al13 and Sagi et al15 randomized patients with acetabular fractures to receive indomethacin for up to 6 weeks to prevent HO. Unlike Burd et al, Sagi included patients randomized to 3 days and 1 week as well as the longer 6 week duration. Both studies demonstrated a high proportion of patients experiencing non-union at 6 weeks (15% to 29%). Indomethacin for 3 to 7 days had no impact on non-union rates compared with placebo. Sagi et al had significant loss to follow-up but loss to follow-up was not reported by Burd et al.
Several studies investigated other factors that may be associated with non-union including tobacco use, obesity and other medications such as opioids, all of which demonstrated an association.12 18
Quantitative synthesis
Eight studies4 6 12 14 15 18 21 22 were included in the primary analysis (figure 2). A total of 51 687 patients received NSAIDs, while 581 702 did not. The non-union rate was 2.99% in the NSAID group compared with 2.18% in the no NSAID group (OR 1.45, 95% CI 1.04 to 2.01). Heterogeneity was very high (I2=93%) and was statistically significant (p<0.03). The high statistical heterogeneity may be due to differences in the fracture type, NSAID type and duration of use, or an unmeasured confounder.
Figure 2.
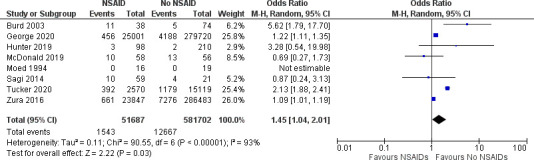
Odds of non-union in patients with traumatic fractures receiving NSAIDs compared with patients not receiving NSAIDS. NSAIDs, non-steroidal anti-inflammatories.
Reduction of morphine equivalents
Qualitative synthesis
Five studies5 7 9 14 16 examined the impact of NSAIDs on morphine equivalents (table 3). Weisz et al16 randomized fracture patients to receiving ibuprofen or placebo and measured OMEs at 48 hours. Patients receiving Ibuprofen had significantly lower OMEs compared with patients receiving placebo (76 mg vs 97 mg, p=0.017).
Table 3.
PICO 1.2 impact of NSAIDs on morphine equivalents
| Author | Outcome definition | Groups | N | Male, n (%) | Age, y, mean (SD) | OME/MME | Study conclusions |
| Weisz16 | OME at 48 hours | Ibuprofen Placebo |
54 44 |
38 (72) 34 (77) |
42 (16) 41 (16) |
23 (18) 27 (16) |
Ibuprofen significantly reduces opioids after traumatic fractures |
| McDonald14 | OME at 7 days | Ketorolac No ketorolac |
64 64 |
23 (36%) 30 (57%) |
48 (15) 46 (17) |
105 (89) 145 (104) |
Decreased use of opioid medications |
| Xu7 | OME at 48 hours | Ketorolac No ketrolac |
31 32 |
16(51) 17 (53) |
50 (11) 49 (12) |
130 (48 hours) 194 (48 hours) |
Significantly less opioid use in ketorolac group |
| Bayouth9 | OME at 7 days | Ibuprofen No Ibuprofen |
21 21 |
13 (62) 17 (81) |
52 (14) 53 (16) |
83 (54) 170 (115) |
Significantly less opioid use in ibuprofen group |
| Haines5 | OME during hospital day | NSAID No NSAID |
21 367 168 690 |
NR NR |
83 84 |
12 (13) 11 (13) |
Significantly less opioid use in the NSAID group |
MME, Morphine Milligram Equivalents; NSAIDS, non-steroidal anti-inflammatory drugs; OME, oral morphine equivalent.
Xu et al7 randomized patients undergoing orthopedic surgery from lower limb fractures to receiving a ketorolac pump or a non-ketorolac pain pump. Sufentanel dose at 48 hours was significantly less (194 vs 130 OME, p<0.05 (actual p value not reported)) for those patients receiving ketorolac. Bayouth et al9 performed a retrospective chart review of patients with rib fractures and compared patients receiving ibuprofen to patients receiving routine care. At 7 days, OMEs were significantly less in the ibuprofen group (83 mg vs 170 mg, p=0.004).
McDonald et al14 randomized 128 patients undergoing fixation or ankle fractures to receive 30 mg intravenous ketorolac intraoperatively and 20 tablets of 10 mg ketorolac postoperatively or usual care. Significantly fewer opioids (40 OMEs fewer, p=0.037) were used in the ketorolac group. Haines et al5 retrospectively analyzed a billing database of older adult trauma patients with hip fractures. Patients receiving NSAIDs (any type) had similar opioid use (milligram morphine equivalence, OME) during their hospital stay (11.43 v 12.01 mg, p=0.05), and this was significantly reduced in the NSAID group on multivariate regression (−0.23, 95% CI −0.41 to −0.06).
Quantitative synthesis
Due to differences in reporting of OMEs only two studies9 14 could be included for a quantitative synthesis for OMEs at 7 days (figure 3). There is a significant reduction in OMEs at 7 days in patients receiving NSAIDs (−56 mg, 95% CI −88 to −24) with moderate heterogeneity (I2=48%, p=0.17).
Figure 3.

Oral morphine equivalents in patients with traumatic fractures receiving NSAIDs compared with patients not receiving NSAIDS. NSAIDs, non-steroidal anti-inflammatories.
Analgesia
Qualitative synthesis
Mehta et al23 randomized patients with uncomplicated long-bone fractures to receive dipyrone or aspirin or placebo. Mean pain relief scores at 6 hours were lowest in dipyrone (3.2) and aspirin (2), and pain relief was worst for placebo (1.3) with a significant difference (p<0.001), table 4.
Table 4.
PICO 1.3 impact of NSAIDs on pain
| Author | Outcome definition | Groups | N | Male, n (%) | Age, years, mean (SD) | Pain outcome | Study conclusions |
| Mehta23 | Pain relief score (mean) at 6 hours | Dipyrone ASA Placebo |
91 93 70 |
74 (81) 65 (70) 54 (77) |
31 (1) 30 (1) 31 (1) |
3 2 1.3 |
Clear analgesic effect of single dose of dipyrone compared with ASA and placebo; ASA had worse side effect profile (16% abdominal discomfort). |
| Adolphson17 | Pain (VAS) at 10d, 4, 8 and 12 weeks | Piroxicam Placebo |
14 19 |
0 (0) 0 (0) |
NA NA |
2.1 (at 10 days) 3.2 (at 10 days) |
Significantly less pain in the Piroxicam group. |
| Weisz16 | Mean pain intensity difference | Ibuprofen Placebo |
54 44 |
38 (72) 34 (77) |
42 (16) 41 (16) |
1.1 (8 hours) 2.5 (8 hours) |
Ibuprofen significantly reduces pain after traumatic fractures. |
| McDonald14 | Pain on each postoperative day | Ketorolac No ketorolac |
64 64 |
23 (36) 30 (57) |
48 (15) 46 (17) |
30 (7 days) 30 (7 days) |
Ketorolac reduced pain in first 3 days postoperatively. |
| Xu7 | Pain (VAS) at 48 hours | Ketorolac No ketorolac |
31 32 |
16(51) 17 (53) |
50 (11) 49 (12) |
2 (48 hours) 2 (48 hours) |
No difference in pain control between ketorolac and no ketorolac groups when used in a postoperative pump. |
| Eftekharian8 | Pain (VAS) at 4 hours | Ketorolac Placebo |
25 25 |
15 (6) 15 (6) |
30 (8) 26 (8) |
1.08 (4 hours) 1.04 (4 hours) |
Single-dose ketorolac had limited efficacy on postoperative pain. |
| Bayouth9 | Pain scores during first 7 days | Ibuprofen No Ibuprofen |
21 21 |
13 (62) 17 (81) |
52 (14) 53 (16) |
7 (mean highest) 8 (mean highest) |
Significantly less pain during the 7 days post-trauma in the ibuprofen group. |
| Aliuskevicius3 | Pain (VAS) during first 2 weeks | Ibuprofen 3 days Ibuprofen 7 days Placebo |
24 26 30 |
8 (33) 7 (26) 8 (27) |
61 (8) 63 (11) 63 (9) |
3 (day 4) 2.5 (day 4) 4 (day 4) |
Significantly less pain in the first 7 days post-trauma in the 3 and 7 days ibuprofen groups. |
ASA, aspirin; d, day; NSAID, non-steroidal anti-inflammatory drugs; VAS, visual analog score.
Adolphson et al17 randomized postmenopausal patients to piroxicam or placebo after suffering from a Colles’ fracture. Pain at 10 days on a 11-point visual analog scale was significantly lower in the piroxicam group (2.1) compared with placebo (3.1, p<0.05 (actual p value not reported)). Weisz et al16 randomized fracture patients to receiving ibuprofen or placebo and measured pain intensity difference between groups. The patients receiving ibuprofen had significantly less pain in the first 8 hours of treatment (2.5/10 in the first 8 hours and 1.1/10 for ibuprofen group, p=0.013). Xu et al7 randomized patients undergoing orthopedic surgery from lower limb fractures to receiving a ketorolac pump or a non-ketorolac pain pump. There was no difference in visual analog pain scores at any time point up to 48 hours. Eftekharian et al8 randomized patients with mandible fractures to receive a single dose of 30 mg ketorolac or placebo in the postanesthesia unit at the onset of pain. There was no difference in pain intensity up to 4 hours of time.
Bayouth et al9 performed a retrospective chart review of patients with rib fractures and compared patients receiving ibuprofen to patients receiving routine care. Mean highest daily pain scores during the first 7 days of hospitalization was significantly lower in patients receiving ibuprofen (7v. 8, p<0.04). Similarly, mean lowest pain score was also significantly lower in the treatment group (3 vs 5, p<0.001).
Aliuskevicius et al3 randomized patients with Colles’ fractures who did not undergo surgery to receive placebo, ibuprofen (3 days) or ibuprofen (7 days). The ibuprofen groups had significantly less pain in the first 7 days after fracture but no difference after 7 days.
Quantitative synthesis
Due to difference in outcome definition and variable timing of pain scores a meta-analysis was not possible.
Acute kidney injury
Only a single study reported on acute kidney injury. Haines et al5 retrospectively analyzed a billing database of older adult trauma patients with hip fractures. There were similar rates of renal failure at baseline between the two groups (NSAID: 14% compared with no NSAIDs: 19%). There was no difference in new onset renal failure between groups (12% in no NSAIDs compared with 6% in NSAID group).
PICO 1 recommendation
We conditionally recommend NSAIDs to be used in analgesic regiments for adult patients (≥18 years old) with traumatic fracture. Despite the low overall quality of evidence, most studies showed no association between NSAIDs on non-union. The larger, better controlled studies demonstrated no increased risk of non-union compared with other, commonly prescribed analgesics, including opioids. There was a clear impact on reduction of acute pain and use of opioid analgesia. Eight authors voted for a strong recommendation and 11 authors voted for conditional recommendation.
PICO 2
Should ketorolac be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that do not include ketorolac to improve analgesia and reduce OMEs, without increasing non-union rates?
Qualitative synthesis
Three studies compared ketorolac use to no ketorolac use in patients with traumatic fractures (table 5). McDonald et al14 randomized 128 patients undergoing fixation or ankle fractures to receive 30 mg intravenous ketorolac intraoperatively and 20 tablets of 10 mg ketorolac postoperatively. Additionally, both groups were prescribed 81 mg of ASA for deep vein thrombosis prevention. Opioid consumption was lower in patients randomized to receive ketorolac (21 OMEs compared with 29 OMEs, p=0.04). Like the reduction in OMEs, pain scores were lower on postoperative days 1 to 3 in the patients receiving ketorolac. Finally, there was no difference in the proportion of patients with complete union (83% in ketorolac group compared with 77% in control group) at 12 weeks.
Table 5.
PICO 2 – summary of all outcomes
| Author | Outcome definition | Groups | N | Male, n (%) | Age, year, mean (SD) | Outcome | Study conclusions |
| Non-union | |||||||
| McDonald14 | Clinical healing (walking) and radiographic healing (blinded review) | Ketorolac No ketorolac |
64 64 |
23 (36) 30 (57) |
48 (15) 46 (17) |
0 (0) 0 (0) |
Ketorolac reduced post-op pain and OMEs but unclear effect on healing due to study power. |
| Morphine equivalents | |||||||
| McDonald14 | OME at 7 days | Ketorolac No ketorolac |
64 64 |
23 (36) 30 (57) |
48 (15) 46 (17) |
105 (89) 145 (104) |
Decreased use of opioid medications. |
| Xu7 | OME at 48 hours | Ketorolac No ketrolac |
31 32 |
16(51) 17 (53) |
50 (11) 49 (12) |
130 (48 hours) 194 (48 hours) |
Significantly less opioid use in ketorolac group. |
| Pain | |||||||
| Xu7 | Pain (VAS) at 48 hours | Ketorolac No ketorolac |
31 32 |
16(51) 17 (53) |
50 (11) 49 (12) |
2 (48 hours) 2 (48 hours) |
No difference in pain control between ketorolac and no ketorolac groups. |
| McDonald14 | Pain on each postoperative day | Ketorolac No ketorolac |
64 64 |
23 (36) 30 (57) |
48 (15) 46 (17) |
30 (7 days) 30 (7 days) |
Ketorolac reduced pain in first 3 days postoperatively. |
| Eftekharian8 | Pain (VAS) at 4 hours | Ketorolac Placebo |
25 25 |
15 (60) 15 (60) |
30 (8) 26 (8) |
1 (4 hours) 1 (hours) |
Single dose ketorolac was effective in the management of mild to moderate acute postoperative pain. |
MME, morphine milligram equivalents; OME, oral morphine equivalent; VAS, visual analog scale.
Xu et al7 randomized 63 patients to receive a post-operative pump containing ketorolac+sufentanil or sufentanil alone. There was no significant difference in pain at 48 hours but significantly less sufentanil was used in the group randomized to receive ketorolac.
Eftekharian et al8 randomized patients with mandible fractures to receive a single dose of 30 mg ketorolac or placebo in the postanesthesia unit at the onset of pain. There was a significant reduction in analgesic requirements but no difference in pain intensity at any time points.
Quantitative synthesis
Quantitative synthesis was not possible for any of the outcomes due to either a lack of two or more studies and inconsistent outcome definitions with respect to the timing of pain and methods to measure pain.
PICO 2 recommendation
We conditionally recommend ketorolac be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture. Ketorolac is associated with reduced opioid use and improved postoperative analgesia and is not associated with non-union. Five authors voted for a strong recommendation and 14 authors voted for conditional recommendation.
PICO 3
Should selective NSAIDs (COX-2 inhibitors) be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture versus routine analgesic regimens that include non-selective NSAIDs to improve analgesia and reduce OMEs, without increasing non-union rates?
Non-union
Qualitative synthesis
Jeffcoach et al19 report on a retrospective cohort study in patients with femur, tibia and/or humerus fractures at an academic level I trauma center. Non-union was not defined. In patients who received an NSAID (12%), most received a non-selective NSAIDs (93% ketorolac or ibuprofen, median of two doses) and seven patients (4%) experienced non-union. Only nine patients received a selective NSAID and none experienced a non-union (table 6). George et al4 used private health insurance claims during a 15-year period in patients with a long-bone fracture and 1-year of follow-up data. In the 2411 patients receiving a COX-2 inhibitor, 69 (2.9%) experienced non-union compared with 387 (1.7%)—22 590 patients—receiving a non-selective NSAID. The incidence for non-union in this cohort was similar to the rate of non-union (1.5%) in patients not receiving NSAIDs.
Table 6.
PICO 3 (selective vs non-selective NSAIDs)
| Author | Outcome definition | Groups | N | Male, n (%) | Age, years, mean (SD) | Outcome, n (%) | Study conclusions |
| Non-union | |||||||
| Jeffcoach19 | Not defined | Selective NSAID Non-Selective NSAID |
8 185 |
NR NR |
NR NR |
0 (0) 7 (3.8) |
No statement on selective NSAID use. |
| George4 | Non-union diagnosis code or a procedure to treat non-union | Selective NSAID Non-Selective NSAID |
2 411 22 590 |
11 229 (45) 103 003 (37) |
49 (17) 60 (19) |
69 (2.9) 387 (1.7) |
Filling a prescription for a non-selective NSAID after fracture was not associated with an increased risk of non-union in the subsequent year. Both COX-2-inhibitor and opioid prescription fills after fracture were associated with a greater risk of non-union. |
| Pain | |||||||
| Ortiz10 | Pain (VAS) at 24 hours | Ketorolac (non-selective) Diclofenac (non-selective) Etoricoxib (Selectve) |
15 17 17 |
NA NA NA |
39 (14) 38 (19) 37 (10) |
25 (24 hours) 25 (24 hours) 25 (24 hours) |
All studied NSAIDs were equally effective analgesics. |
NSAID, non-steroidal anti-inflammatory drugs; VAS, visual analog scale.
Ortiz et al10 randomized patients with closed ankles fractures to receive ketorolac (non-selective), diclofenac (non-selective) or etoricoxib (selective). There was no difference in the visual analog pain scores for any of the NSAIDs.
Quantitative synthesis
Due to the lack of studies, a meta-analysis was unable to be performed.
PICO 3 recommendation
We are unable to make a recommendation on whether selective NSAIDs (COX-2 inhibitors) versus non-selective NSAIDs be used in analgesic regimens for adult patients (≥18 years old) with traumatic fracture, due to the small number of studies identified. Four authors voted for a strong recommendation, two for conditional recommendation and 13 for no recommendation.
Using these guidelines in clinical practice
NSAIDs have a long history of use for analgesia in diseases with correctable and temporary causes of pain.27 Traumatic fractures are extremely common and fall into this category, but concerns related to the impact of NSAIDs on bone healing have been raised in animal studies.29 We aimed to address this question in addition to assessing the benefits of NSAIDs with respect to acute pain control and need for opioids in trauma patients. Through our literature review, we conditionally recommend NSAIDs to reduce acute pain and opioid use due to traumatic fractures, figure 4. The absolute difference between non-union in patients who receive or who do not receive NSAIDs in our analysis is <1%. This very small difference is outweighed by the reduction in opioids and improved analgesia. Some studies on longer duration NSAIDs (>30 days) demonstrated an association with non-union, but it remains unclear whether these patients had painful non-healing fractures requiring additional analgesic or that the NSAIDs lead to painful non-healing. Causality is impossible to determine from the current literature. Similar reviews on NSAIDs after orthopedic surgery have demonstrated the safety of NSAIDs for shorter durations (<2 weeks) and have identified indomethacin as particularly harmful.32
Figure 4.
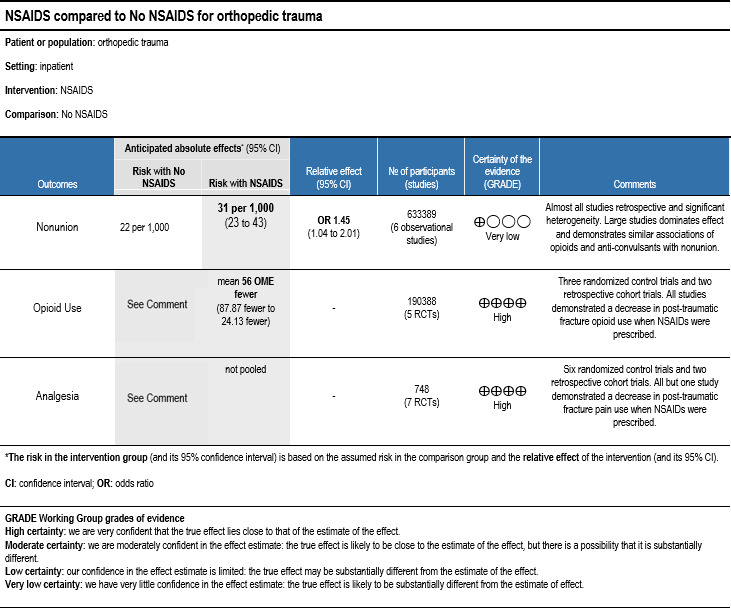
GRADEPro summary of findings.
There was limited evidence available for more narrow questions of specific NSAIDs such as ketorolac or classes of NSAIDs: selective or non-selective. Ketorolac has a clear impact on pain and opioid reduction after traumatic fracture but only a single study14 specifically commented on non-union (no difference). Again, the potential benefits of ketorolac seem to outweigh any risk of non-union. Similarly, few studies compared selective and non-selective NSAIDs, and therefore no recommendations could be made. Further study is needed specifically regarding classification of NSAIDs and duration of NSAID use after traumatic fracture. Additionally, dosing regimens for NSAID can be examined for deleterious effects such as non-union. The ceiling analgesic dose may often be lower than that prescribed in practice, and this should be used to prescribe the smallest dose to achieve the desired effect.33 34
There are number of limitations to our systematic review and meta-analysis. The first is the inherent bias in designs of included studies. A number of the included studies were retrospective. The largest contributors of patients were large retrospective cohort studies of administrative database that may suffer from coding errors, missing data and selection bias.5 12 18 Administrative studies also rely on prescription data and are unable to confirm compliance to NSAID regimen. Second, the definition of non-union was inconsistent between studies ranging from no definition in some studies to administrative coding or persistent fracture at 6 months in others. The third is the variability in the fracture type, presence of additional injuries (including additional fractures), indication for NSAID and type and duration of NSAID. Specifically, the analgesic ceiling dose of NSAID has been established as much lower than the dose in most studies.33 34 The higher dose given may contribute to the adverse events. Furthermore, some adverse events, such as gastrointestinal bleeding, were not identified as critically important. Finally, the heterogeneity of included studies does require consideration, particularly for the outcome of non-union (I2=94%). Our PICO questions were defined a priori and took a clinical lens on the safety and efficacy of NSAIDs for patients suffering from traumatic fracture(s) rather than specific fracture types, thus a subgroup analysis was not performed. Future work is needed to determine whether certain fracture locations or patterns warrant special consideration.
Conclusions
Based on the available evidence, non-steroidal anti-inflammatories have a clear effect on reducing post-traumatic fracture opioid use and improving pain control. These benefits outweigh the small risk of non-union.
Footnotes
Contributors: All authors contributed to the screening of articles, full-text review, data extraction and critical review of the article. PBM performed the meta-analysis. All authors voted on outcomes. PBM is responsible for the overall content and guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ERH reports research funding from The Patient-Centered Outcomes Research Institute, the Agency for Healthcare Research and Quality, the National Institutes of Health/National Heart, Lung, and Blood Institute, the Department of Defense/Army Medical Research Acquisition Activity and the Henry M. Jackson Foundation for the Advancement of Military Medicine. ERH is a volunteer past president of the Eastern Association for the Surgery of Trauma (EAST) and past chair of the EAST guidelines committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Stoicea N, Costa A, Periel L, Uribe A, Weaver T, Bergese SD. Current perspectives on the opioid crisis in the US healthcare system: a comprehensive literature review. Medicine (Baltimore) 2019;98:e15425. 10.1097/MD.0000000000015425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd RA, Seth P, David F, Scholl L. Morbidity and mortality weekly report. MMWR Surveillance Summaries 2021;70:293. 10.15585/mmwr.mm7008a4 [DOI] [Google Scholar]
- 3.Aliuskevicius M, Østgaard SE, Vestergaard P, Rasmussen S. The influence of ibuprofen on the healing of nonsurgically treated Colles’ fractures. Orthopedics 2021;44:105–10. 10.3928/01477447-20201216-04 [DOI] [PubMed] [Google Scholar]
- 4.George MD, Baker JF, Leonard CE, Mehta S, Miano TA, Hennessy S. Risk of nonunion with nonselective NSAIDs, COX-2 inhibitors, and opioids. J Bone Joint Surg Am 2020;102:1230–8. 10.2106/JBJS.19.01415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines KL, Fuller M, Vaughan JG, Krishnamoorthy V, Raghunathan K, Kasotakis G, Agarwal S, Ohnuma T. The impact of nonsteroidal anti-inflammatory drugs on older adult trauma patients with hip fractures. J Surg Res 2020;255:583–93. 10.1016/j.jss.2020.05.064 [DOI] [PubMed] [Google Scholar]
- 6.Tucker WA, Birt MC, Heddings AA, Horton GA. The effect of postoperative nonsteroidal anti-inflammatory drugs on nonunion rates in long bone fractures. Orthopedics 2020;43:221–7. 10.3928/01477447-20200428-06 [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Zhang N, Ni M, Wang B, Fang H. The comparative study on the combination of ketorolac tromethamine and sufentanil for postoperative analgesia in patients receiving traumatic lower limb surgery: a randomized controlled trial. Int J Clin Exp Med 2016;9:2969–76. [Google Scholar]
- 8.Eftekharian HR, Ilkhani Pak H. Effect of intravenous ketorolac on postoperative pain in mandibular fracture surgery; a randomized, double-blind, placebo-controlled trial. Bull Emerg Trauma 2017;5:13–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Bayouth L, Safcsak K, Cheatham ML, Smith CP, Birrer KL, Promes JT. Early intravenous ibuprofen decreases narcotic requirement and length of stay after traumatic rib fracture. Am Surg 2013;79:1207–12. 10.1177/000313481307901127 [DOI] [PubMed] [Google Scholar]
- 10.Ortiz MI, et al. Effectiveness of diclofenac, ketorolac and etoricoxib in the treatment of acute pain from ankle fracture. Proc West Pharmacol Soc 2014;53:46–8. [PubMed] [Google Scholar]
- 11.Zhang C, Zhou X. Effectiveness of various non-steroidal anti-inflammatory drugs in pain management of patients with vertebral fracture: a comparative clinical study. Trop J Pharm Res 2017;16:2275. 10.4314/tjpr.v16i9.32 [DOI] [Google Scholar]
- 12.Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg 2016;151:1–12:e162775. 10.1001/jamasurg.2016.2775 [DOI] [PubMed] [Google Scholar]
- 13.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003;85:700–5. 10.1302/0301-620x.85b5.13970 [DOI] [PubMed] [Google Scholar]
- 14.McDonald EL, Daniel JN, Rogero RG, Shakked RJ, Nicholson K, Pedowitz DI, Raikin SM, Bilolikar V, Winters BS. How does perioperative ketorolac affect opioid consumption and pain management after ankle fracture surgery? Clin Orthop Relat Res 2020;478:144–51. 10.1097/CORR.0000000000000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagi HC, Jordan CJ, Barei DP, Serrano-Riera R, Steverson B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma 2014;28:377–83. 10.1097/BOT.0000000000000049 [DOI] [PubMed] [Google Scholar]
- 16.Weisz RD, Fokin AA, Lerner V, Flynt A, Macias-Perez I, Pavliv L, Crawford M, Puente I. Intravenous ibuprofen reduces opioid consumption during the initial 48 hours after injury in orthopedic trauma patients. J Orthop Trauma 2020;34:341–7. 10.1097/BOT.0000000000001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adolphson P, Abbaszadegan H, Jonsson U, Dalén N, Sjöberg HE, Kalén S. No effects of piroxicam on osteopenia and recovery after Colles’ fracture. A randomized, double-blind, placebo-controlled, prospective trial. Arch Orthop Trauma Surg 1993;112:127–30. 10.1007/BF00449987 [DOI] [PubMed] [Google Scholar]
- 18.Buchheit T, Zura R, Wang Z, Mehta S, Della Rocca GJ, Steen RG. Opioid exposure is associated with nonunion risk in a traumatically injured population: an inception cohort study. Injury 2018;49:1266–71. 10.1016/j.injury.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 19.Jeffcoach DR, Sams VG, Lawson CM, Enderson BL, Smith ST, Kline H, Barlow PB, Wylie DR, Krumenacker LA, McMillen JC, et al. Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg 2014;76:779–83. 10.1097/TA.0b013e3182aafe0d [DOI] [PubMed] [Google Scholar]
- 20.Godoy Monzón D, Vazquez J, Jauregui JR, Iserson KV. Pain treatment in post-traumatic hip fracture in the elderly: regional block vs. systemic non-steroidal analgesics. Int J Emerg Med 2010;3:321–5. 10.1007/s12245-010-0234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moed BR, Karges DE. Prophylactic indomethacin for the prevention of heterotopic ossification after acetabular fracture surgery in high-risk patients. Journal of Orthopaedic Trauma 1994;8:34–9. 10.1097/00005131-199402000-00008 [DOI] [PubMed] [Google Scholar]
- 22.Hunter AM, Montgomery TP, Pitts CC, Moraes L, Anderson M, Wilson J, McGwin G, Shah A. Postoperative aspirin use and its effect on bone healing in the treatment of ankle fractures. Injury 2020;51:554–8. 10.1016/j.injury.2019.11.039 [DOI] [PubMed] [Google Scholar]
- 23.Mehta SD. A randomized double-blind placebo-controlled study of dipyrone and aspirin in post-operative orthopaedic patients. J Int Med Res 1986;14:63–6. 10.1177/030006058601400202 [DOI] [PubMed] [Google Scholar]
- 24.Marquez-Lara A, Hutchinson ID, Nuñez F, Smith TL, Miller AN. Nonsteroidal anti-inflammatory drugs and bone-healing: a systematic review of research quality. JBJS Rev 2016;4:1–14:e4. 10.2106/JBJS.RVW.O.00055 [DOI] [PubMed] [Google Scholar]
- 25.Borgeat A, Ofner C, Saporito A, Farshad M, Aguirre J. The effect of nonsteroidal anti-inflammatory drugs on bone healing in humans: a qualitative, systematic review. J Clin Anesth 2018;49:92–100. 10.1016/j.jclinane.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 26.Wheatley BM, Nappo KE, Christensen DL, Holman AM, Brooks DI, Potter BK. Effect of NSAIDs on bone healing rates: a meta-analysis. J Am Acad Orthop Surg 2019;27:e330–6. 10.5435/JAAOS-D-17-00727 [DOI] [PubMed] [Google Scholar]
- 27.Hsu JR, Mir H, Wally MK, Seymour RB, Orthopaedic Trauma Association Musculoskeletal Pain Task Force . Clinical practice guidelines for pain management in acute musculoskeletal injury. J Orthop Trauma 2019;33:e158–82. 10.1097/BOT.0000000000001430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AAOS . Pharmacologic, physical, and cognitive pain alleviation for musculoskeletal extremity/pelvis surgery. 2021
- 29.Guyatt GH, Gunn V, Yngve F-Y, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. Chin J Evid Based Med 2009;9:8–11. 10.1136/ebm.11.1.2-a [DOI] [Google Scholar]
- 30.Haut ER. Eastern association for the surgery of trauma (East) practice management guidelines and the perpetual quest for excellence. J Trauma Acute Care Surg 2020;89:1–10. 10.1097/TA.0000000000002709 [DOI] [PubMed] [Google Scholar]
- 31.Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P, Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee . The eastern association of the surgery of trauma approach to practice management Guideline development using grading of recommendations, assessment, development, and evaluation (grade) methodology. J Trauma Acute Care Surg 2012;73:S283–7. 10.1097/TA.0b013e31827013e9 [DOI] [PubMed] [Google Scholar]
- 32.Al Farii H, Farahdel L, Frazer A, Salimi A, Bernstein M. The effect of nsaids on postfracture bone healing: a meta-analysis of randomized controlled trials. OTA Int 2021;4. 10.1097/OI9.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motov S, Yasavolian M, Likourezos A, Pushkar I, Hossain R, Drapkin J, Cohen V, Filk N, Smith A, Huang F, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med 2017;70:177–84. 10.1016/j.annemergmed.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 34.Motov S, Masoudi A, Drapkin J, Sotomayor C, Kim S, Butt M, Likourezos A, Fassassi C, Hossain R, Brady J, et al. Comparison of oral ibuprofen at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med 2019;74:530–7. 10.1016/j.annemergmed.2019.05.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tsaco-2022-001056supp001.pdf (414KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article.



