Abstract
The coronavirus disease 2019 (COVID-19) pandemic poses a great threat to public health. Individuals who are immunocompromised because of the progression of the primary disease or receiving immunosuppressive medications are prone to severe COVID-19 complications and poor outcomes. Abundant data have shown that many COVID-19 vaccines are safe and effective in large-scale populations; however, these clinical trials have excluded immunocompromised populations. Available evidence indicates that immunocompromised populations have a blunted immune response to other vaccines, raising concerns regarding the efficacy of COVID-19 vaccination in these populations. Thus, there is an urgent need to delineate the efficacy of COVID-19 vaccines in these vulnerable populations. Here, we review the characteristics of specific humoral and cellular responses to COVID-19 vaccination in immunocompromised populations, including HIV-infected patients and those receiving immunosuppressive treatment, especially solid organ transplant recipients and those undergoing anti-CD20 treatment. We also addressed the challenges that immunocompromised populations will face in the future pandemic and the need for basic and clinical translational studies to highlight the best vaccination strategies for these populations.
Keywords: Anti-CD20 treatment, Coronavirus disease 2019, Human immunodeficiency virus, Immunocompromised, Solid organ transplant recipient, Vaccine
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for coronavirus disease 2019 (COVID-19), has had a global impact since its discovery. As of August 25, 2022, >595 million confirmed cases of COVID-19 and 6.4 million deaths have been reported worldwide (https://covid19.who.int/). Historically, vaccines have been the most powerful weapon to fight infectious diseases. The early and timely submission of the virus sequence to the World Health Organization (WHO) by the Chinese government significantly promoted the development of the COVID-19 vaccine.[1] Abundant data have shown that the COVID-19 vaccines developed by different research platforms are safe and effective for large-scale populations.[2–6] However, immunocompromised populations who had a higher risk of COVID-19-related hospitalization, severe disease, or death[7] were excluded from large clinical trials. Thus, the efficacy of these COVID-19 vaccines in immunocompromised patients requires urgent clarification.
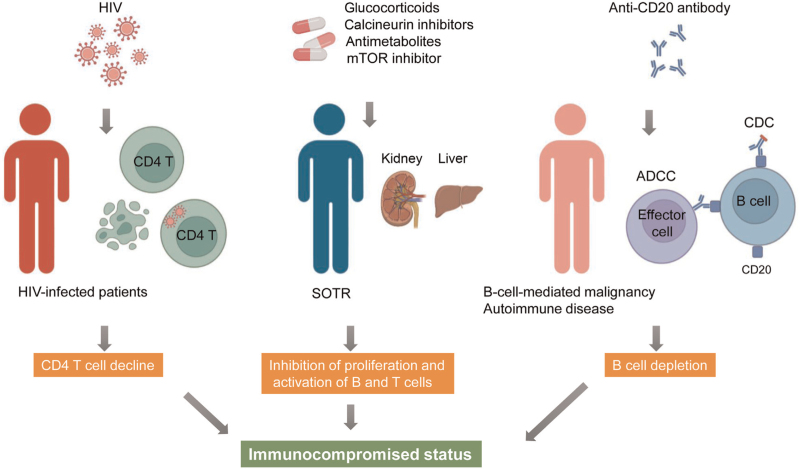
Immunocompromised conditions are usually observed in those with primary or secondary immunodeficiency caused by the progression of a disease or patients receiving immunosuppressive treatment. A lower vaccination response rate, such as that observed for the influenza vaccination, is commonly observed in immunocompromised populations.[8] Among the secondary immunodeficiencies, human immunodeficiency virus (HIV) infection represents the classical immunocompromised state caused by the disease progression. HIV mainly infects CD4+ T cells and leads to their death by apoptosis or pyroptosis.[9] Without antiretroviral therapy (ART), HIV destroys the immune system and leads to the progression of acquired immunodeficiency syndrome (AIDS). Among the patients with secondary immunodeficiency conditions caused by immunosuppressive medications, the immune response to the COVID-19 vaccine was reported to be the lowest in solid organ transplantation recipients (SOTRs) and those receiving anti-CD20 B cell-depleting treatment [Figure 1]. Thus, we mainly focused on these two populations receiving immunosuppressive treatments. Most of the SOTRs need to receive immunosuppressive drugs to prevent graft rejection, including glucocorticoids, calcineurin inhibitors (CNI), antimetabolites, and mammalian target of rapamycin inhibitors.[10] These immunosuppressive drugs may block the activation or proliferation of B and T lymphocytes, leading to a poor immune response to the COVID-19 vaccine. Anti-CD20 treatment is mainly used to treat B cell-mediated malignancies and various autoimmune disorders (eg, rheumatoid arthritis and multiple sclerosis).[11] However, treatment with anti-CD20 leads to non-specific and overall suppression of humoral immunity. Thus, the immune response to COVID-19 vaccination in patients receiving CD20 monoclonal antibodies was significantly impaired. Identifying the underlying risk factors for poor immune response to COVID-19 vaccination will aid in improving vaccination strategies for these vulnerable populations.
Figure 1.
Model for three immunocompromised conditions in this study. ADCC: Antibody-dependent cell-mediated cytotoxicity; CD: Cluster of differentiation; CDC: Complement-dependent cytotoxicity; HIV: Human immunodeficiency virus; mTOR: Mammalian target of rapamycin; SOTR: Solid organ transplantation recipient. This illustration was created using biorender.com.
In this review, we focus on the immunogenicity of different types of COVID-19 vaccines in immunocompromised populations, particularly HIV-infected individuals, SOTRs, and anti-CD20 recipients.
COVID-19 Vaccines
Types of COVID-19 vaccines
As of July 2022, the WHO has reported that there are 359 COVID-19 vaccine candidates being evaluated. Among them, 161 are in the clinical phase and 198 in the pre-clinical phase, including 39 candidates being or having been detected in phase III clinical trials and 11 candidates being or having been detected in phase IV clinical trials (https://www.who.int/teams/blueprint/covid-19/covid-19-vaccine-tracker-and-landscape).
COVID-19 vaccine coverage
Notably, 11 vaccines have been authorized by the WHO for emergency use [Table 1]. According to the production platforms, these vaccines can be mainly divided into the following four categories: (1) inactivated vaccines: inactivated whole SARS-CoV-2 virions were used to trigger an immune response with highly accepted safety; (2) recombinant protein vaccines: the simulated protein fragment or shell of the SARS-CoV-2 virion was used to trigger an immune response with the help of adjuvant; (3) virus vector vaccines: viral vectors that transfect the gene encoding SARS-CoV-2 proteins were used to trigger an immune response; (4) messenger ribonucleic acid (mRNA) vaccines: the genetically modified mRNA sequence of SARS-CoV-2 protein was used to trigger an immune response. Compared with other technologically traditional vaccines, mRNA vaccines can induce much stronger humoral responses and are more widely used worldwide.[12,13] Although inactivated-virus vaccines are mostly used in China to vaccinate residents, more than six domestic mRNA vaccines are being developed. For example, ArCoV produced by Abogen Biosciences is being examined in phase III clinical trials and might be used in COVID-19 vaccination programs.[14]
Table 1.
COVID-19 vaccines as emergency use issued by the WHO.
| Candidate vaccine | Vaccine platform description | Developer | Number of doses | Schedule | Route of administration | Storage temperature | Number of approved countries | Potential serious side effects |
| mRNA-1273 (Spikevax) | mRNA | Moderna | 2 | Day 0 + 28 | Intramuscular | 2–8°C for 30 days, −20°C for 6 months | 88 | Myocarditis and pericarditis |
| BNT162b2 (Comirnaty) | mRNA | Pfizer/BioNTech | 2 | Day 0 + 21 | Intramuscular | −70°C | 148 | – |
| COVOVAX (Novavax formulation) | Protein subunit | Serum Institute of India | 2 | Day 0 + 21 | Intramuscular | 2–8°C | 5 | – |
| Nuvaxovid | Protein subunit | Novavax | 2 | Day 0 + 21 | Intramuscular | 2–8°C | 39 | – |
| Ad5-nCov (Convidecia) | Replication-defective adenovirus type 5 vector | CanSino | 2 | Day 0 + 21 | Intramuscular | 2–8°C | 10 | Thrombotic thrombocytopenia syndrome |
| Ad26.COV2.S | Replication-incompetent human adenovirus type 26 vector | Janssen (Johnson & Johnson) | 1–2 | Day 0 or Day 0 + 56 | Intramuscular | 2–8°C | 113 | Thrombotic thrombocytopenia syndrome and Guillain-Barré syndrome |
| AZD1222 (Vaxzevria) | Replication-defective chimpanzee adenovirus vector | Oxford/AstraZeneca | 1–2 | Day 0 or Day 0 + 28 | Intramuscular | 2–8°C | 148 | Thrombotic thrombocytopenia syndrome and Guillain-Barré syndrome |
| Covishield | Replication-defective chimpanzee adenovirus vector | Serum Institute of India | 1–2 | Day 0 or Day 0 + 28 | Intramuscular | 2–8°C | 49 | Thrombotic thrombocytopenia syndrome and Guillain-Barré syndrome |
| Covaxin | Inactivated | Bharat Biotech | 2 | Day 0 + 14 | Intramuscular | 2–8°C | 14 | – |
| BBIBP-CorV (Covilo) | Inactivated | Sinopharm (Beijing) | 2 | Day 0 + 21 | Intramuscular | 2–8°C | 93 | – |
| CoronaVac | Inactivated | Sinovac | 2 | Day 0 + 14 | Intramuscular | 2–8°C | 56 | – |
COVID-19: Coronavirus disease 2019; mRNA: Messenger ribonucleic acid; WHO: World Health Organization. https://covid19.trackvaccines.org/agency/who/.
As of September 2022, approximately 67.7% of the global population is vaccinated with more than one dose of COVID-19 vaccine (https://covid19.who.int/). In China, inactivated-virus vaccines are primarily used; >90.5% of the population has completed primary vaccination, and 71.7% of the population has received a booster vaccination. In high-income countries, mRNA vaccines or adenovirus vector vaccines are primarily used; approximately 79.8% of the population receives more than one dose of the COVID-19 vaccine, 74.9% of the population completed primary vaccination, and 44.8% of the population received a booster vaccination (https://app.powerbi.com/). However, in low-income countries, only 53.2% of the population has received more than one dose of the COVID-19 vaccine, 37.5% of the population has completed primary vaccination, and 9.8% of the population has received a booster vaccination. An imbalance in accessing COVID-19 vaccines is particularly noteworthy in Africa. Owing to underdeveloped technology in Africa, the COVID-19 vaccines can rarely be domestically produced, and the population mainly relies on international support. Hence, Africa has the lowest vaccination rate worldwide, and this has been proven to be dangerous. Extreme vaccine inequities and low vaccination rates pose a great threat to immunocompromised patients.
Safety of COVID-19 vaccine
The safety of COVID-19 vaccines has been thoroughly and rigorously assessed in the general population. Serious side effects were infrequently reported after COVID-19 vaccination, such as thrombotic thrombocytopenia syndrome and Guillain-Barré Syndrome for several virus vector vaccines, and myocarditis and pericarditis for mRNA vaccine, mRNA-1273 [Table 1]. Generally, COVID-19 vaccinations in immunocompromised patients are safe. The side effects after COVID-19 vaccination are mild and temporary. Similar rate of side effects and no additional adverse effects of the COVID-19 vaccines have been reported in immunocompromised patients. Thus, we mainly focus on the immunogenicity of COVID-19 vaccines in the following sections.
Immunogenicity of the COVID-19 Vaccine in Specific Immunocompromised Populations
HIV-infected patients
HIV infection remains a global public health issue, with an estimated 38 million people living with HIV. In untreated patients, the CD4 T cell count declines continuously and eventually progresses to the AIDS stage, in which the immune system is severely weakened. Multiple large cohort studies have shown that HIV-infected patients, especially those with low CD4 T cell count and unsuppressed viremia, experience more severe COVID-19 and poorer clinical outcomes than those without HIV.[15–20] Thus, HIV-infected patients, particularly those with immunosuppression, should be prioritized to receive the COVID-19 vaccine to reduce the risk of COVID-19.
The immunogenicity of COVID-19 vaccines in HIV-infected individuals has been reported inconsistently. Some studies found poorer immunogenicity to the COVID-19 vaccine in HIV-infected individuals compared with healthy controls (HCs), but other studies showed comparable immunogenicity between these two groups. This discrepancy may be due to the different numbers of enrolled participants, the different methods used to monitor the humoral response, or the heterogeneity, including age, ethnicity, CD4 T cell count, etc., of the enrolled HIV-infected population. Many clinical trials are still ongoing to fully elucidate the immunogenicity of the COVID-19 vaccination in HIV-infected patients [Supplementary Table 1]. Here, we also summarized the humoral immune responses to the COVID-19 vaccine in HIV-infected patients in Table 2, and only studies that enrolled >50 HIV-infected patients were included. Accumulating evidence from large cohort studies has revealed worse immunogenicity of COVID-19 vaccines in HIV-infected patients, especially those with low CD4 T cell counts and unsuppressed viral loads. A study conducted in Brazil revealed that HIV-infected patients mount reduced immunogenicity compared with HCs after receiving two doses of inactivated CoronaVac vaccine, and HIV-infected patients with CD4 counts <500 cells/μL had lower immunogenicity than those patients with a CD4 count of at least 500 cells/μL.[21] In addition, 71% of HIV-infected patients were neutralizing antibody (NAb)-positive compared with 84% of HCs.[21] A study by Spinelli et al[22] reported that following COVID-19 mRNA vaccination, HIV-infected patients had a lower surrogate virus neutralization test response, particularly among those with lower CD4 T cell counts. Similarly, a study conducted in Switzerland also reported a lower titer of anti-receptor binding domain (RBD) antibody after SARS-CoV-2 mRNA vaccination in HIV-infected patients compared with that in healthy volunteers.[23] In addition, a recent study conducted by Madhi et al[24] showed that HIV-infected patients had attenuated immunoglobulin G (IgG) antibody against spike protein (anti-spike IgG) titers after receiving two doses of recombinant spike protein nanoparticle vaccine NVX-CoV2373 compared with their healthy counterparts, while the seroconversion rate was similar. Some real-world studies also found reduced RBD-IgG, Spike-IgG, or seropositivity in HIV-infected patients compared with HCs, and the antibody response is particularly poor in HIV-infected patients with CD4 T cell counts <200 cells/μL.[25,26] However, some studies reported similar immunogenicity induced by COVID-19 vaccination. For example, a study conducted by Madhi et al[27] evaluated the humoral response after receiving an adenovirus-vectored vaccine AZD1222 in HIV-infected patients, and they found similar RBD-IgG and NAb responses between HIV-infected patients and HIV-negative participants. In the era of ART, most HIV-infected patients have received ART treatment. However, there are still some patients who have not received ART and some studies have shown poor humoral response to the COVID-19 vaccine in HIV-infected patients with unsuppressed viremia.[22,28] Compared with the widely examined humoral response, antigen-specific T cell responses have only been detected in a few studies. Woldemeskel et al[29] evaluated cellular immunity using interferon gamma (IFN-γ) ELISpot assay in 12 HIV-infected patients following two doses of BNT162b2 mRNA vaccine and found no difference in cellular immunity between HIV-infected patients and HCs. Compared with HIV-negative individuals, no difference in cellular responses was observed in 54 HIV-infected patients receiving two doses of ChAdOx1 nCoV-19.[30] However, the immune status of the enrolled HIV-infected patients may have affected the cellular immune response. A case study revealed failed seroconvert and undetectable spike-specific T cells in one patient with advanced HIV infection (CD4 T-cell count: 20 cells/μL, viral load: 831,764 copies/mL).[28]
Table 2.
Summary of studies on humoral immune response to COVID-19 vaccination in HIV-infected patients compared with HCs.
| Reference | Journal | Vaccine | Numbers (PLWH vs. HC) | PLWH grouped based on CD4 T cell counts (cells/mL) | Detection time points after full course vaccination | Detected parameters | Findings |
| Liu et al[31] | Vaccines | CoronaVac | 55 vs. 21 | CD4 < 350 CD4 ≥ 350 |
5 weeks | Anti-RBD IgG | Similar between PLWH and HC, but lower in PLWH with CD4 counts <350 cells/μL |
| Ao et al[32] | Emerg Microbes Infect | CoronaVac or BBIBP-CorV | 139 vs. 120 | CD4 < 200 200 ≤ CD4 ≤ 500 CD4 > 500 |
40 days | Anti-RBD IgG Anti-spike IgG |
Lower in PLWH, especially in those with CD4 counts <200 cells/μL Lower titers in PLWH |
| Cai et al[33] | J Med Virol | CoronaVac or BBIBP-CorV | 143 vs. 50 | NA | 35.78 days | SARS-CoV-2 IgG NAb |
Lower in PLWH Similar |
| Netto et al[21] | Lancet HIV | CoronaVac | 215 vs. 296 | CD4 < 500 CD4 ≥ 500 |
6 weeks | Anti-spike IgG | Lower in PLWH, and lower seroconversion in those with CD4 counts <500 cells/μL |
| NAb | Lower in PLWH, especially in those with CD4 counts <500 cells/μL | ||||||
| Huang et al[34] | Viruses | CoronaVac or BBIBP-CorV | 94 vs. 51 | NA | NA | Anti-spike IgG NAb |
Lower in PLWH Similar |
| Zeng et al[35] | Vaccines | BBIBP-CorV CoronaVac |
65 vs. 65 67 vs. 65 |
CD4 < 350 CD4 ≥ 350 |
28 days | Anti-RBD IgG | Lower in PLWH, especially in those with CD4 counts <350 cells/μL |
| Bergman et al[36] | EBioMedicine | BNT162b2 | 90 vs. 90 | CD4 > 300 CD4 ≤ 300 |
14 days | Anti-RBD IgG | Similar |
| Jedicke et al[37] | HIV Med | BNT162b2 | 50 vs. 41 | NA | 35 days | Anti-spike IgG | Lower in PLWH |
| Levy et al[38] | Clin Microbiol Infect | BNT162b2 | 143 vs. 261 | NA | 18 days | Anti-RBD IgG | Lower in PLWH |
| NAb | Similar | ||||||
| Portillo et al[23] | Front Immunol | BNT162b2 or mRNA-1273 | 124 vs. 48 | NA | 30 days | Anti-RBD IgG | Lower in PLWH |
| Spinelli et al[22] | Clin Infect Dis | BNT162b2 or mRNA-1273 | 100 vs. 100 | NA | 35 days | SARS-CoV-2 IgG NAb |
Lower in PLWH Lower in PLWH |
| Antinori et al[39] | Clin Infect Dis | BNT162b2 or mRNA-1273 | 166 vs. 169 | CD4 < 200 200 ≤ CD4 ≤ 500 CD4 > 500 |
1 month | Anti-RBD IgG | Lower in PLWH, especially in those with CD4 counts <200 cells/μL |
| NAb | Lower in PLWH, especially in those with CD4 counts <200 cells/μL | ||||||
| Heftdal et al[40] | J Intern Med | BNT162b2 | 269 vs. 538 | NA | >1 week | Anti-RBD IgG | Lower in PLWH |
| NAb | Similar | ||||||
| Lombardi et al[41] | Lancet Reg Health Eur | mRNA-1273 | 62 vs. 8 | CD4 < 350 350 ≥ CD4 ≤ 500 CD4 > 500 |
28 days | Anti-spike IgG NAb |
Similar Similar |
| Tau et al[42] | Open Forum Infect Dis | BNT162b2 | 136 vs. 61 | CD4 < 300 CD4 ≥ 300 |
4.5 months | Anti-RBD IgG | Similar between PLWH and HC, but lower in PLWH with CD4 counts <300 cells/μL |
| Madhi et al[24] | Lancet HIV | NVX-CoV2373 | 58 vs. 1216 | NA | 14 days | Anti-spike IgG | Lower in PLWH |
| NAb | Lower in PLWH |
Anti-RBD IgG: IgG antibody against receptor-binding domain; Anti-spike IgG: IgG antibody against spike protein; CD4: Cluster of differentiation 4; COVID-19: Coronavirus disease 2019; HC: Healthy control; HIV: Human immunodeficiency virus; IgG: Immunoglobulin; NA: Not applicable; NAb: Neutralizing antibody; PLWH: People living with HIV; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Collectively, well-treated HIV-infected patients with immune status comparable to healthy individuals may exhibit a similar immune response to the COVID-19 vaccine compared with healthy individuals. However, some HIV-infected patient, especially those in immunosuppressed conditions with poor CD4 T cell counts and unsuppressed viremia, might mount an attenuated immune response to the COVID-19 vaccine.
SOTRs with immunosuppressive medication
SOTRs with kidney, liver, or lung transplants are at increased risk of severe SARS-CoV-2 infection or COVID-19-related mortality.[43–45] Immunosuppression in SOTRs is mainly due to the use of immunosuppressive medications aiming at preventing allograft rejection. The use of immunosuppressive drugs might block the cellular and humoral immune responses and result in a poor vaccine response, which has been observed in SOTRs receiving other vaccine (influenza, hepatitis B vaccine, etc.). For example, CNI (tacrolimus or cyclosporin) can block the activation of T cells, and mycophenolate mofetil (MMF) can inhibit the proliferation of T and B cells.
Although SOTRs were excluded from large COVID-19 vaccine clinical trials, vaccination of SOTRs has been suggested by professional societies. Larger cohort studies have consistently concluded that both antibody positivity and titers are extremely low in SOTRs after receiving the COVID-19 vaccine [Table 3].[46–60] Boyarsky et al[61] found that anti-spike IgG was only detectable in 54% (357/658) of SOTRs after two doses of SARS-CoV-2 mRNA vaccine. Bertrand et al[62] evaluated the anti-spike antibody in 225 kidney transplant recipients (KTRs) after two doses of the BNT162b2 vaccine, and only 8 KTRs (17.8%) developed SARS-CoV-2 antibodies. One study conducted by Rabinowich et al[55] evaluated 80 liver transplant recipients (LTRs) after two doses of BNT162b2 vaccination, of whom only 47.5% were positive for SARS-CoV-2 IgG antibodies, and the antibody titer was also reduced in LTRs. Havlin et al[63] reported that none of the lung transplant recipients developed SARS-CoV-2 IgG after the first and second doses of BNT162b2 vaccine. In addition, studies comparing the immunogenicity of COVID-19 vaccines in immunocompromised patients also identified SOTRs as the least likely to achieve seroconversion after COVID-19 vaccination.[36,64,65] Older age, shorter time from transplantation, high-dose of steroids, receiving triple immunosuppression regimen, regimens including MMF, and co-morbidities including diabetes and hypertension were identified as the risk factors for compromised humoral response.[47,50,51,55,57,66] In addition, impaired SARS-CoV-2-specific T cell responses have been reported in SOTRs.[57,67] Devresse et al[48] showed that KTRs with higher SARS-CoV-2 antibodies were more likely to mount a T-cell response. Interestingly, some SOTRs without an antibody response may exhibit a T-cell response.[48]
Table 3.
Summary of studies on humoral immune response to COVID-19 vaccination in SOTRs compared with HCs.
| Reference | Journal | Vaccines | Study population (n) | Detection time points after full course vaccination | Detected parameters (positivity) | Factors associated with worse humoral response |
| Benotmane et al[46] | Kidney Int | mRNA-1273 | KTR (205) | 1 month | SARS-CoV-2 IgG (48%) | Treatment with CNI, MMF, or steroids |
| Cucchiari et al[47] | Am J Transplant | mRNA-1273 | KTR (148) | 2 weeks | Anti-spike IgM/IgG (29.9%) | Diabetes, treatment with ATG during the last year |
| Devresse et al[48] | Transplantation | BNT162b2 | KTR (90) | 1 month | Anti-RBD IgG (64.4%) | NA |
| Eren Sadioglu et al[49] | Transpl Infect Dis | CoronaVac | KTR (118) | 1 month | Anti-SARS-CoV-2 IgG (18.8%) | Age and impaired renal function |
| Grupper et al[50] | Am J Transplant | BNT162b2 | KTR (136) | 16.5 days | Anti-SARS-CoV-2 IgG (37.5%) | Age, high-dose corticosteroids, and triple immunosuppression regimen including MMF |
| Guarino et al[51] | J Hepatol | BNT162b2 | LTR (365) | 4 weeks | Anti-spike IgG (74.8%) | Age, higher BMI, shorter time from transplantation, multiple immunosuppressive drugs, and antimetabolite therapy |
| Hallett et al[52] | J Heart Lung Transplant | BNT162b2 or mRNA-1273 | HTR (134) Lung transplant recipient (103) |
28 days | Anti-spike IgG (62% and 36%, respectively) | Antimetabolite regimen, shorter years (<6 years) from transplantation |
| Hod et al[53] | Transplantation | BNT162b2 | KTR (120) | 26.7 days | Anti-RBD IgG (43.4%) NAb (35%) |
MPA dose and hemoglobin level <13 g/dL |
| Midtvedt et al[54] | Transplantation | BNT162b2 | KTR (141) | 25–89 days | Anti-spike IgG (18%) | Age, treatment with MPA, especially in triple therapy |
| Rabinowich et al[55] | J Hepatol | BNT162b2 | LTR (80) | 14.8 days | Anti-spike IgG (47.5%) | Age, decreased renal function, and immunosuppression |
| Rozen-Zvi et al[56] | Clin Microbiol Infect | BNT162b2 | KTR (308) | 2–4 weeks | Anti-spike IgG (36.4%) | Age, lower eGFR, high MPA dose, and higher CNI blood level |
| Ruether et al[57] | Clin Gastroenterol Hepatol | BNT162b2 or mRNA-1273 or AZD1222 | LTR (141) | 29 days | Anti-spike IgG (63%) | Age, arterial hypertension, and immunosuppression other than CNI monotherapy |
| Shostak et al[58] | Lancet Respir Med | BNT162b2 | Lung transplant recipient (168) | 16 days | Anti-spike IgG (18%) | Treatment with mTOR inhibitor or antimetabolites |
| Strauss et al[59] | Liver Transpl | BNT162b2 or mRNA-1273 | LTR (161) | 30 days | Anti-RBD IgG (81%) | Treatment with antimetabolites, vaccination with BNT162b2 vaccine |
| Stumpf et al[60] | Lancet Reg Health Eur | BNT162b2 or mRNA-1273 | KTR (368) | 4–5 weeks | SARS-CoV-2 IgG/IgA (65%) | Immunosuppressive drug number, vaccination with BNT162b2 vaccine |
Anti-RBD IgG: IgG antibody against receptor-binding domain; Anti-spike IgG: IgG antibody against spike protein; ATG: Antithymocyte globulin; CNI: Calcineurin inhibitors; COVID-19: Coronavirus disease 2019; HC: Healthy control; HTR: Heart transplant recipient; Ig: Immunoglobulin; KTR: Kidney transplant recipient; LTR: Liver transplant recipient; MMF: Mycophenolate mofetil; MPA: Mycophenolic acid; mTOR: Mammalian target of rapamycin; NA: Not available; NAb: Neutralizing antibody; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; STOR: Solid organ transplantation recipient.
The extremely weak immune response to two doses of the SARS-CoV-2 vaccine urges the administration of a third dose of vaccine in SOTRs. Among those who were seronegative after a two-dose course of mRNA vaccination, the third dose induced a serological response in no more than 50% SOTRs.[68–71] In addition, Caillard et al[72] evaluated the fourth dose of mRNA in KTRs who did not respond adequately after three doses and found that 50% of patients reached the threshold of anti-spike IgG titers.
Overall, SOTRs exhibited extremely weak immune responses to the COVID-19 vaccine and should be vaccinated before transplantation whenever possible. In addition, humoral responses should be monitored especially in those who are more likely to show a suboptimal immune response, and additional boosters may be needed to potentiate immune responses in SOTRs.
Patients with anti-CD20 treatment
Multiple studies have indicated that patients receiving anti-CD20 treatment are at higher risk of severe COVID-19.[73,74] Anti-CD20 treatment is used to treat B-cell-mediated malignant tumors and autoimmune diseases but could lead to non-specific and overall suppression of humoral immunity in treated patients. Thus, the immune response to COVID-19 vaccination in patients receiving CD20 monoclonal antibodies was significantly impaired [Table 4]. A recent study compared the seroconversion rate and average antibody titer in 478 patients with autoimmune diseases (including rheumatoid arthritis and systemic lupus erythematosus) and 502 HCs after receiving two doses of the mRNA vaccine.[75] They found that patients with autoimmune diseases who commonly used rituximab or MMF had significantly lower seroconversion rates and average antibody titers than HCs.[75] These data imply that anti-CD20 treatment might lead to a suppressed humoral response. Boekel et al[76] performed an observational study to examine the immune response of patients with autoimmune diseases after receiving COVID-19 vaccines and found that significantly reduced anti-RBD antibody response was detected among patients treated with CD20 monoclonal antibodies. Apostolidis et al[77] found that, although no significant difference between antigen-specific CD4 and CD8 T cell responses was observed, the antibody response of patients with multiple sclerosis treated with CD20 monoclonal antibody after two doses of mRNA vaccine was significantly lower than that of HCs. Thus, anti-CD20 treatment suppressed the humoral response but not the cellular response to COVID-19 vaccines. In another study, however, Moor et al[78] reported that in patients receiving anti-CD20 treatment, 49% detected antibody response and 32% detected T-cell response after 1.79 months of vaccination with two doses of mRNA vaccine. The suppressive degree of anti-CD20 treatment on the humoral response to COVID-19 vaccines might be due to different doses and durations of anti-CD20 treatment in various conditions. A single-center observational study reported that after adjusting for relevant confounding factors, the seroconversion rate of patients with rheumatoid arthritis treated with 200 mg rituximab after receiving two doses of mRNA or virus vector COVID-19 vaccines was significantly higher than that of patients treated with 1000 mg rituximab.[79]
Table 4.
Summary of studies on humoral immune response to COVID-19 vaccination in anti-CD20-treated patients compared with HCs.
| Reference | Journal | Vaccine | Study population (n) | Detection time points after full course vaccination | Detected parameters (positivity) | Findings |
| Ferri et al[75] | J Autoimmun | BNT162b2 or mRNA-1273 | Autoimmune diseases (26) | 1–3 weeks | IgG-NAb (53.8%) | Rituximab treatment was associated with higher odds of vaccine non-response |
| Boekel et al[76] | Lancet Rheumatol | Unlimited | Autoimmune diseases (27) | 1–5 months | Anti-RBD IgG (43%) | Lower seroconversion rates and antibody titers in patients with anti-CD20 therapy |
| Apostolidis et al[77] | Nat Med | BNT162b2 or mRNA-1273 | Multiple sclerosis (20) | 25–30 days | Anti-spike (88.89%) Anti-RBD IgG (50%) |
Lower anti-spike and RBD antibody levels in patients with anti-CD20 therapy |
| Moor et al[78] | Lancet Rheumatol | BNT162b2 or mRNA-1273 | B cell-mediated malignancies and autoimmune disorders (96) | 1.79 months | Anti-spike IgG (49%) | Lower anti-spike antibody levels in patients with anti-CD20 therapy |
| van der Togt et al[79] | Rheumatology | Unlimited | Rheumatoid arthritis (196) | 2–6 weeks | Anti-SARS-CoV-2 IgG (28%) | The response rate was significantly lower for patients receiving 1000 mg rituximab compared with those receiving 200 mg rituximab |
Anti-RBD IgG: IgG antibody against receptor-binding domain; Anti-spike IgG: IgG antibody against spike protein; COVID-19: Coronavirus disease 2019; HC: Healthy control; Ig: Immunoglobulin; NAb: Neutralizing antibody; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
After COVID-19 vaccination, patients receiving anti-CD20 medication displayed severely impaired humoral responses, but potent T cell responses were preserved. In the absence of a humoral response, whether a vaccine-induced T-cell response exerts protective effects against SARS-CoV-2 infection is still unclear. Thus, the real-world effectiveness of the COVID-19 vaccine in patients receiving anti-CD20 therapy still needs to be confirmed.
Perspective and Challenges
Studies have demonstrated prolonged viral shedding and evolution in SARS-CoV-2-infected immunocompromised populations and suboptimal immune responses to COVID-19 vaccines in immunocompromised populations compared with the general populations.[80,81] However, as shown in immunocompromised populations, the COVID-19 vaccine is still helpful for decreasing the disease severity and hospitalization in these immunocompromised individuals.[82]
In most clinical studies conducted on immunocompromised populations, antibody levels or seroconversion rates are used as surrogate endpoints for vaccine efficacy. However, data are still needed to fully elucidate whether COVID-19 vaccination is protective against SARS-CoV-2 infection, hospitalization, severe diseases, and death in real-world settings. As shown in the general population, a booster dose can partially restore omicron cross-neutralization by neutralizing antibodies.[83] Thus, booster vaccination might be needed for immunocompromised populations, which should be fully evaluated. As neutralizing antibodies wane over time, longitudinal studies are needed to determine the dynamics of vaccine-induced antibodies in immunocompromised populations, which might shed light on when to deliver a booster vaccination.
In addition to humoral immunity, the T-cell immune response is important for protection against viral infections. A recent study conducted in rhesus macaques showed that cellular immunity contributes to protection against viral infection in the context of sub-protective antibody titers.[84] In immunocompromised populations, especially those receiving anti-CD20 treatment, SARS-CoV-2 specific-T cell activity could be detected in some individuals without a detectable humoral response. Although the cellular immunity induced by COVID-19 vaccination retains the ability to recognize SARS-CoV-2, including omicron variant,[85] the real-word protective effects mediated by the cellular immune response in the absence of humoral response are still unclear.
As SARS-CoV-2 is a zoonotic RNA virus with a high mutation rate, the currently dominant omicron variant may result from the evolution in immunocompromised patients.[86] Thus, to prevent further emergence of more dangerous and contagious variants, primary COVID-19 vaccination, especially booster vaccination, is highly recommended. Unfortunately, many advanced HIV-infected patients, especially those in areas within low vaccine-coverage, such as sub-Saharan Africa, cannot get access to COVID-19 vaccines. Accelerating the pace of global vaccination and establishing herd immunity to end the COVID-19 pandemic are urgently needed. Furthermore, with the prevalence of the omicron variant, most authorized vaccines are limited in inducing mucosal immunity.[87] Thus, novel vaccines that broadly target various variants or intranasal vaccines that elicit mucosal immunity should be developed to better protect vulnerable immunocompromised populations.
In all, no additional adverse effects of COVID-19 vaccination were reported, and COVID-19 vaccination is safe in secondary immunocompromised patients.[21,24,27] However, more clinical translational studies are needed to determine the real-world efficacy of various types of COVID-19 vaccines in immunocompromised populations and how best to protect these patients. This will involve determining the most suitable vaccine type, dose, and delivery schedule for immunocompromised patients, and whether a heterologous vaccination schedule is better than homologous vaccination. In addition, as these immunocompromised populations remain at risk of SARS-CoV-2 infection even after vaccination, regular epidemic prevention and control measures including masking and social distancing, should be implemented.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82101837), the Beijing Natural Science Foundation (No. 7222171), and the Emergency Key Program of Guangzhou Laboratory (No. EKPG21-30-4).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Song JW, Hu W, Shen L, Wang FS. Safety and immunogenicity of COVID-19 vaccination in immunocompromised patients. Chin Med J 2022;135:2656–2666. doi: 10.1097/CM9.0000000000002505
Jin-Wen Song and Wei Hu contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halperin SA, Ye L, MacKinnon-Cameron D, Smith B, Cahn PE, Ruiz-Palacios GM, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022; 399:237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021; 398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021; 326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021; 72:340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy 2014; 6:131–139. doi: 10.2217/imt.13.171. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Song JW, Huang HH, Fan X, Huang L, Deng JN, et al. NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1-infected patients. J Clin Invest 2021; 131:e138861.doi: 10.1172/JCI138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yilmaz EA, Özdemir Ö. Solid organ transplantations and COVID-19 disease. World J Transplant 2021; 11:503–511. doi: 10.5500/wjt.v11.i12.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du FH, Mills EA, Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 2017; 8:12.doi: 10.1007/s13317-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol 2022; 32:e2313.doi: 10.1002/rmv.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au WY, Cheung PPH. Effectiveness of heterologous and homologous covid-19 vaccine regimens: living systematic review with network meta-analysis. BMJ 2022; 377:e069989.doi: 10.1136/bmj-2022-069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen GL, Li XF, Dai XH, Li N, Cheng ML, Huang Z, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe 2022; 3:e193–e202. doi: 10.1016/S2666-5247(21)00280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosioni J, Blanco JL, Reyes-Urueña JM, Davies MA, Sued O, Marcos MA, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021; 8:e294–e305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021; 8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boffito M, Waters L. More evidence for worse COVID-19 outcomes in people with HIV. Lancet HIV 2021; 8:e661–e662. doi: 10.1016/S2352-3018(21)00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomah DK, Reyes-Urueña J, Díaz Y, Moreno S, Aceiton J, Bruguera A, et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. Lancet HIV 2021; 8:e701–e710. doi: 10.1016/S2352-3018(21)00240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinelli MA, Lynch KL, Yun C, Glidden DV, Peluso MJ, Henrich TJ, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV 2021; 8:e334–e341. doi: 10.1016/S2352-3018(21)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Sun J, Patel RC, Zhang J, Guo S, Zheng Q, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021; 8:e690–e700. doi: 10.1016/S2352-3018(21)00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netto LC, Ibrahim KY, Picone CM, Alves APPS, Aniceto EV, Santiago MR, et al. Safety and immunogenicity of CoronaVac in people living with HIV: a prospective cohort study. Lancet HIV 2022; 9:e323–e331. doi: 10.1016/S2352-3018(22)00033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinelli MA, Peluso MJ, Lynch KL, Yun C, Glidden DV, Henrich TJ, et al. Differences in post-mRNA vaccination SARS-CoV-2 IgG concentrations and surrogate virus neutralization test response by HIV status and type of vaccine: a matched case-control observational study. Clin Infect Dis 2022; 75:e916–e919. doi: 10.1093/cid/ciab1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portillo V, Fedeli C, Ustero Alonso P, Petignat I, Mereles Costa EC, Sulstarova A, et al. Impact on HIV-1 RNA levels and antibody responses following SARS-CoV-2 vaccination in HIV-infected individuals. Front Immunol 2021; 12:820126.doi: 10.3389/fimmu.2021.820126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi SA, Moodley D, Hanley S, Archary M, Hoosain Z, Lalloo U, et al. Immunogenicity and safety of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine in people living with and without HIV-1 infection: a randomised, controlled, phase 2A/2B trial. Lancet HIV 2022; 9:e309–e322. doi: 10.1016/S2352-3018(22)00041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noe S, Ochana N, Wiese C, Schabaz F, Von Krosigk A, Heldwein S, et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 2022; 50:617–623. doi: 10.1007/s15010-021-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haidar G, Agha M, Bilderback A, Lukanski A, Linstrum K, Troyan R, et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis 2022; 75:e630–e644. doi: 10.1093/cid/ciac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 8:e568–e580. doi: 10.1016/S2352-3018(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touizer E, Alrubayyi A, Rees-Spear C, Fisher-Pearson N, Griffith SA, Muir L, et al. Failure to seroconvert after two doses of BNT162b2 SARS-CoV-2 vaccine in a patient with uncontrolled HIV. Lancet HIV 2021; 8:e317–e318. doi: 10.1016/S2352-3018(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woldemeskel BA, Karaba AH, Garliss CC, Beck EJ, Wang KH, Laeyendecker O, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin Infect Dis 2022; 74:1268–1270. doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Han J, Li X, Chen D, Zhao X, Qiu Y, et al. COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines (Basel) 2021; 9:1458.doi: 10.3390/vaccines9121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ao L, Lu T, Cao Y, Chen Z, Wang Y, Li Z, et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect 2022; 11:1126–1134. doi: 10.1080/22221751.2022.2059401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai S, Liao G, Yu T, Gao Q, Zou L, Zhang H, et al. Immunogenicity and safety of an inactivated SARS-CoV-2 vaccine in people living with HIV: a cross-sectional study. J Med Virol 2022; 94:4224–4233. doi: 10.1002/jmv.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Yan Y, Su B, Xiao D, Yu M, Jin X, et al. Comparing immune responses to inactivated vaccines against SARS-CoV-2 between people living with HIV and HIV-negative individuals: a cross-sectional study in China. Viruses 2022; 14:277.doi: 10.3390/v14020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng G, Xu L, Feng S, Tang J, Wang X, Li G, et al. IgG antibody responses and immune persistence of two doses of BBIBP-CorV vaccine or CoronaVac vaccine in people living with HIV (PLWH) in Shenzhen, China. Vaccines (Basel) 2022; 10:880.doi: 10.3390/vaccines10060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine 2021; 74:103705.doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jedicke N, Stankov MV, Cossmann A, Dopfer-Jablonka A, Knuth C, Ahrenstorf G, et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med 2022; 23:558–563. doi: 10.1111/hiv.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy I, Wieder-Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–1855. doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–e563. doi: 10.1093/cid/ciac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heftdal LD, Knudsen AD, Hamm SR, Hansen CB, Møller DL, Pries-Heje M, et al. Humoral response to two doses of BNT162b2 vaccination in people with HIV. J Intern Med 2022; 291:513–518. doi: 10.1111/joim.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lombardi A, Butta GM, Donnici L, Bozzi G, Oggioni M, Bono P, et al. Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. Lancet Reg Health Eur 2022; 13:100287.doi: 10.1016/j.lanepe.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tau L, Turner D, Adler A, Marom R, Ahsanov S, Matus N, et al. SARS-CoV-2 humoral and cellular immune responses of patients with HIV after vaccination with BNT162b2 mRNA COVID-19 vaccine in the Tel-Aviv medical center. Open Forum Infect Dis 2022; 9:ofac089.doi: 10.1093/ofid/ofac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol 2020; 5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int 2020; 98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis 2021; 73:e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 2021; 99:1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant 2021; 21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devresse A, Saad Albichr I, Georgery H, Yombi JC, De Greef J, Belkhir L, et al. T-cell and antibody response after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation 2021; 105:e142–e143. doi: 10.1097/TP.0000000000003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eren Sadioğlu R, Demir E, Evren E, Aktar M, Şafak S, Artan AS, et al. Antibody response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis 2021; 23:e13740.doi: 10.1111/tid.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guarino M, Cossiga V, Esposito I, Furno A, Morisco F. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: the debate is open!. J Hepatol 2022; 76:237–239. doi: 10.1016/j.jhep.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallett AM, Greenberg RS, Boyarsky BJ, Shah PD, Ou MT, Teles AT, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant 2021; 40:1579–1588. doi: 10.1016/j.healun.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hod T, Ben-David A, Olmer L, Levy I, Ghinea R, Mor E, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS-CoV-2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation 2021; 105:e234–e243. doi: 10.1097/TP.0000000000003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Midtvedt K, Tran T, Parker K, Marti HP, Stenehjem AE, Goransson LG, et al. Low immunization rate in kidney transplant recipients also after dose 2 of the BNT162b2 vaccine: continue to keep your guard up!. Transplantation 2021; 105:e80–e81. doi: 10.1097/TP.0000000000003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozen-Zvi B, Yahav D, Agur T, Zingerman B, Ben-Zvi H, Atamna A, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect 2021; 27:e1171–e1173. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol 2022; 20:162–172.e69.doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shostak Y, Shafran N, Heching M, Rosengarten D, Shtraichman O, Shitenberg D, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med 2021; 9:e52–e53. doi: 10.1016/S2213-2600(21)00184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl 2021; 27:1852–1856. doi: 10.1002/lt.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 2021; 9:100178.doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Hanoy M, Laurent C, et al. Antibody and T Cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol 2021; 32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havlin J, Svorcova M, Dvorackova E, Lastovicka J, Lischke R, Kalina T, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant 2021; 40:754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahav G, Lustig Y, Lavee J, Ohad B, Magen H, Hod T, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine 2021; 41:101158.doi: 10.1016/j.eclinm.2021.101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376:e068632.doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Affeldt P, Koehler FC, Brensing KA, Adam V, Burian J, Butt L, et al. Immune responses to SARS-CoV-2 infection and vaccination in dialysis patients and kidney transplant recipients. Microorganisms 2021; 10:4.doi: 10.3390/microorganisms10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miele M, Busà R, Russelli G, Sorrentino MC, Di Bella M, Timoneri F, et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant 2021; 21:2919–2921. doi: 10.1111/ajt.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021; 326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charmetant X, Espi M, Benotmane I, Barateau V, Heibel F, Buron F, et al. Infection or a third dose of mRNA vaccine elicits neutralizing antibody responses against SARS-CoV-2 in kidney transplant recipients. Sci Transl Med 2022; 14:eabl6141.doi: 10.1126/scitranslmed.abl6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021; 174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med 2022; 175:455–456. doi: 10.7326/L21-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiavetti I, Ponzano M, Signori A, Bovis F, Carmisciano L, Sormani MP. Severe outcomes of COVID-19 among patients with multiple sclerosis under anti-CD-20 therapies: a systematic review and meta-analysis. Mult Scler Relat Disord 2022; 57:103358.doi: 10.1016/j.msard.2021.103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D’Abramo A, Vita S, Maffongelli G, Mariano A, Agrati C, Castilletti C, et al. Prolonged and severe SARS-CoV-2 infection in patients under B-cell-depleting drug successfully treated: a tailored approach. Int J Infect Dis 2021; 107:247–250. doi: 10.1016/j.ijid.2021.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferri C, Ursini F, Gragnani L, Raimondo V, Giuggioli D, Foti R, et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. J Autoimmun 2021; 125:102744.doi: 10.1016/j.jaut.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021; 3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moor MB, Suter-Riniker F, Horn MP, Aeberli D, Amsler J, Möller B, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021; 3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Togt CJT, Ten Cate DF, den Broeder N, Rahamat-Langendoen J, van den Bemt BJF, den Broeder AA. Humoral response to coronavirus disease-19 vaccines is dependent on dosage and timing of rituximab in patients with rheumatoid arthritis. Rheumatology (Oxford) 2022; 61:SI175–SI179. doi: 10.1093/rheumatology/keac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meiring S, Tempia S, Bhiman JN, Buys A, Kleynhans J, Makhasi M, et al. Prolonged shedding of SARS-CoV-2 at high viral loads amongst hospitalised immunocompromised persons living with HIV, South Africa. Clin Infect Dis 2022; 75:e144–e156. doi: 10.1093/cid/ciac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun J, Zheng Q, Madhira V, Olex AL, Anzalone AJ, Vinson A, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022; 182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS CoV-2 omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol 2022; 7:eabo2202.doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell 2022; 185:847–859.e11.doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cele S, Karim F, Lustig G, San JE, Hermanus T, Tegally H, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022; 30:154–162.e5.doi: 10.1016/j.chom.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lund FE, Randall TD. Scent of a vaccine. Science 2021; 373:397–399. doi: 10.1126/science.abg9857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.