Abstract
Background:
Reperfusion therapy is fundamental for ST-segment elevation myocardial infarction (STEMI). However, the details of contemporary practice and factors associated with reperfusion therapy in China are largely unknown. Therefore, this study aimed to explore reperfusion practice and its associated factors among hospitalized patients with STEMI in China.
Methods:
Patients with STEMI who were admitted to 159 tertiary hospitals from 30 provinces in China were included in the Improving Care for Cardiovascular Disease in China–Acute Coronary Syndrome project from November 2014 to December 2019. The associations of the characteristics of patients and hospitals with reperfusion were examined using hierarchical logistic regression. The associations between therapies and in-hospital major adverse cardiovascular events were examined with a mixed effects Cox regression model.
Results:
Among the 59,447 patients, 37,485 (63.1%) underwent reperfusion, including 4556 (7.7%) receiving fibrinolysis and 32,929 (55.4%) receiving primary percutaneous coronary intervention (PCI). The reperfusion rate varied across geographical regions (48.0%–73.5%). The overall rate increased from 60.0% to 69.7% from 2014 to 2019, mainly due to an increase in primary PCI within 12 h of symptom onset. Timely PCI, but not fibrinolysis alone, was associated with a decreased risk of in-hospital major adverse cardiovascular events compared with no reperfusion, with an adjusted hazard ratio (95% confidence interval) of 0.64 (0.54,0.76) for primary PCI at <12 h, 0.53 (0.37,0.74) for primary PCI at 12 to 24 h, 0.46 (0.25,0.82) for the pharmaco-invasive strategy, and 0.79 (0.54,1.15) for fibrinolysis alone.
Conclusions:
Nationwide quality improvement initiatives should be strengthened to increase the reperfusion rate and reduce inequality in China.
Trial registration:
Keywords: Acute coronary syndrome, Cardiovascular diseases, China, Fibrinolysis, Percutaneous coronary intervention, Quality improvement, Reperfusion, ST elevation myocardial infarction
Introduction
Ischemic heart disease (IHD) is one of the leading causes of mortality in China.[1] ST-segment elevation myocardial infarction (STEMI) is the most serious acute manifestation of IHD. A cornerstone of management for STEMI is early reperfusion in patients admitted within 12 h of symptom onset.[2–4] However, the reperfusion rate in patients with STEMI varies among countries. Evidence suggests that the reperfusion rate increased from 77.2% to 81.3% in Europe from 2006 to 2008,[5] and from 55.1% to 70.8% in the United States from 1990 to 2006.[6] However, in China, a nationwide multicenter survey carried out at 65 tertiary hospitals and 97 secondary hospitals (The China Patient-centered Evaluative Assessment of Cardiac Events Retrospective Study [PEACE]) showed that the adjusted reperfusion rate remained stagnant from 54.7% in 2001 to 55.2% in 2011.[7,8] Recently, our team reported an overall reperfusion rate of 57.4% from 2014 to 2018 at 150 tertiary hospitals and 42 secondary hospitals in the Improving Care for Cardiovascular Disease in China–Acute Coronary Syndrome (CCC-ACS) project, which was much lower than in Western countries.[9] The details of contemporary real-world reperfusion practice and its associated factors in hospitalized patients with STEMI in China are still unclear.[7,10,11]
Thus, based on the CCC-ACS project, we aimed to provide a detailed description of recent practice patterns in reperfusion for STEMI patients and associated factors to guide future quality improvement initiatives in China.
Methods
Ethical approval
Institutional review board approval was granted for this research with a waiver for informed consent by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. 2014018). This study is registered at www.ClinicalTrials.gov (No. NCT02306616), and the study complied with the Declaration of Helsinki.
Study design and patients
The CCC-ACS project was launched in 2014 as a collaborative initiative of the American Heart Association and the Chinese Society of Cardiology. Detailed information about the design and methodology of the CCC-ACS has been described previously.[12] In brief, participating hospitals were stratified and enrolled by geographical region and economic level. From Phase I (from November 2014 to June 2017) to Phase IV (from November 2018 to December 2019), 241 hospitals were enrolled, including 159 tertiary hospitals and 82 secondary hospitals [Supplementary Table 1]. The first 20 to 30 and 10 to 20 patients with ACS were consecutively reported to the database each month from tertiary and secondary hospitals, respectively. A standard web-based data collection platform (Oracle Clinical Remote Data Capture; Oracle Corporation, Redwood City, CA, USA) was used. Trained data abstractors at participating hospitals reported the required data from patients’ medical records. Third-party research associates performed regular quality audits to ensure the accuracy and completeness of the research data. The accuracy of medical record abstraction was 95.7%.
In this analysis, we used the data of 60,618 patients with STEMI from 159 tertiary hospitals in China between November 2014 and December 2019, based on the principal discharge diagnosis. Of these, 1171 patients (1.9%) were excluded because of missing data on reperfusion therapies. Finally, 59,447 patients were included in the analysis. The flowchart of participant recruitment is shown in Supplementary Figure 1.
Study variables
The key intervention variables were the use and type of reperfusion therapy, including no reperfusion, primary percutaneous coronary intervention (PCI) (including primary PCI at <12 h [within 12 h of symptom onset] and primary PCI at 12 to 24 h [12–24 h of symptom onset]), and fibrinolysis (including the pharmaco-invasive strategy and fibrinolysis alone) at the acute stage.[2] Timely PCI was defined as primary PCI and post-fibrinolysis PCI (pharmaco-invasive PCI and rescue PCI).[13]
Other study variables included demographic information (age, sex, medical insurance), risk factors (hypertension, diabetes mellitus, chronic heart failure, low-density lipoprotein cholesterol concentration of ≥70 mg/dL, and smoking), medical history (coronary heart disease, cerebrovascular disease, atrial fibrillation, renal failure, and bleeding), severe clinical conditions at admission (heart failure, cardiogenic shock, cardiac arrest, and Killip class), vital signs (estimated glomerular filtration rate [eGFR] of <60 mL·min−1·1.73 m−2, heart rate, systolic blood pressure), medications within 24 h of arrival (dual antiplatelet therapy [DAPT] status and angiotensin-converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB], β-blocker, and statin use), and hospital-level factors (geographic region and regional gross domestic product).
The outcomes were in-hospital major adverse cardiovascular events (MACEs) and a composite endpoint of all-cause death, reinfarction, stent thrombosis, and stroke during hospitalization, as documented in patients’ medical records.
Detailed definitions of other variables are provided in the Supplementary Methods. For each treatment, specialized inclusion and exclusion criteria were used and were counted as denominators [Supplementary Table 2].
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median (interquartile range [IQR]) and were compared using the Student's t-test, Wilcoxon's rank-sum test, analysis of variance, or the Kruskal-Wallis H test based on the data type and distribution. Categorical variables are described as n (percentage), and comparisons were made using the χ2 test or Fisher's exact test. The P values yielded by the multiple comparisons were corrected for multiplicity using the Bonferroni method.
To examine the association between patient characteristics, hospital characteristics, and reperfusion therapy, a hierarchical logistic regression analysis was performed using a random intercept of patients (level 1) clustered within hospital geographic regions (level 2). Candidate adjustment variables included patients’ individual characteristics (age, sex, medical insurance, time from symptom onset to admission, severe clinical conditions at admission [acute heart failure, cardiogenic shock, cardiac arrest], heart rate, systolic blood pressure, eGFR, diabetes mellitus, chronic heart failure, smoking, and history of disease [coronary heart disease, renal failure, and cerebrovascular disease]) and hospital characteristics (economic level of hospital location).
To evaluate the relationship between patterns of reperfusion therapy and in-hospital MACEs, two models were used after excluding patients who experienced MACEs within 1 day of admission, as follows: (i) a univariable Cox proportional hazards model and (ii) a mixed effects Cox regression model, clustering of patients within hospitals, while adjusting for age, sex, medical insurance, time from symptom onset to admission, severe clinical conditions at admission (acute heart failure, cardiogenic shock, cardiac arrest), vital signs (heart rate, systolic blood pressure, eGFR), diabetes mellitus, smoking, chronic heart failure, history of disease (coronary heart disease, renal failure, and cerebrovascular disease), transfer status, and medications in the first 24 h of arrival (DAPT, β-blockers, and ACEI/ARB).
We imputed the missing values of clinical variables using the sequential regression multiple imputation method implemented by IVEware software, version 0.2 (Survey Research Center, University of Michigan, Ann Arbor, MI, USA). Detailed information on the missing rate of each variable and the strategies used to manage missing data is presented in Supplementary Table 3. In addition, a sensitivity analysis was performed by further adjusting for the bleeding history using data from patients enrolled since July 2017 (information on bleeding history was only available after this date) to explored the factors associated with the use of reperfusion.
All statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) and R software (http://www.R-project.org). A two-sided P value of <0.05 was considered statistically significant.
Results
Patients’ characteristics according to the type of reperfusion
Among the 59,447 patients with STEMI, 37,485 patients (63.1%) underwent reperfusion with primary PCI (32,929 patients [55.4%]) or fibrinolysis (4556 patients [7.7%]). Compared with patients who underwent reperfusion, patients who did not undergo reperfusion were older and more likely to be female, had a more frequent medical history, and had an overall higher-risk profile. Regarding medications administered within the first 24 h of admission, patients were more likely to be administered β-blockers and less likely to be administered DAPT. Compared with patients who did not undergo reperfusion and patients who underwent fibrinolysis, patients who underwent primary PCI were more likely to be covered by urban insurance and to have a lower disease burden. Compared with patients who did not undergo reperfusion and patients who underwent primary PCI, patients who underwent fibrinolytic therapy were more likely to be transferred and covered by rural insurance (Table 1).
Table 1.
Characteristics of hospitalized patients with STEMI by practice of reperfusion.
| Characteristics | Total (n = 59,447) | No Reperfusion (n = 21,962) | Primary PCI (n = 32,929) | Fibrinolysis (n = 4556) |
| Age, years | 61.8 ± 12.6 | 63.5 ± 12.8‡ | 61.2 ± 12.5§ | 58.5 ± 11.5|| |
| Female | 12,937 (21.8) | 5572‡ (25.4) | 6593§ (20.0) | 772|| (16.9) |
| Medical insurance | ||||
| Urban insurance | 31,356 (52.7) | 10,337‡ (47.1) | 18,958§ (57.6) | 2061|| (45.2) |
| Rural insurance | 13,561 (22.8) | 6439‡ (29.3) | 5693§ (17.3) | 1429|| (31.4) |
| Self-paid | 8203 (13.8) | 2890‡ (13.2) | 4676§ (14.2) | 637‡§ (14.0) |
| Other | 6327 (10.6) | 2296‡ (10.5) | 3602‡ (10.9) | 429§ (9.4) |
| Risk factor | ||||
| Hypertension | 36,542 (61.5) | 13,515‡ (61.5) | 20,480‡ (62.2) | 2547§ (55.9) |
| Diabetes mellitus | 16,907 (28.4) | 6502‡ (29.6) | 9262§ (28.1) | 1143|| (25.1) |
| Chronic heart failure | 15,471 (26.0) | 6161‡ (28.1) | 8030§ (24.4) | 1280‡ (28.1) |
| Elevated LDL-C (≥ 70 mg/dL) | 51,324 (86.3) | 18,261‡ (83.1) | 29,221§ (88.7) | 3842‡ (84.3) |
| Smoking | 27,479 (46.2) | 8930‡ (40.7) | 16,124§ (49.0) | 2425|| (53.2) |
| History of disease | ||||
| Coronary heart disease | 4122 (6.9) | 1786‡ (8.1) | 2058§ (6.2) | 278§ (6.1) |
| Cerebrovascular disease | 4559 (7.7) | 2029‡ (9.2) | 2255§ (6.8) | 275|| (6.0) |
| Atrial fibrillation | 808 (1.4) | 340‡ (1.5) | 424§ (1.3) | 44§ (1.0) |
| Renal failure | 638 (1.1) | 327‡ (1.5) | 279§ (0.8) | 32§ (0.7) |
| Bleeding∗ | 93 (0.4) | 46‡ (0.7) | 38§ (0.3) | 9‡ (0.6) |
| Transferred-in | 29,663 (49.9) | 12,242‡ (55.7) | 14,069§ (42.7) | 3352|| (73.6) |
| Symptom-to-admission time†, h | 5.5 [2.5,15.5] | 17.6‡ [5.0,64.3] | 4.1§ [2.1,8.0] | 7.1|| [3.2,17.6] |
| Killip class | ||||
| I | 41,731 (70.2) | 13,854‡ (63.1) | 24,615§ (74.8) | 3262|| (71.6) |
| II–III | 14,808 (24.9) | 6838‡ (31.1) | 6899§ (21.0) | 1071|| (23.5) |
| IV | 2908 (4.9) | 1270‡ (5.8) | 1415§ (4.3) | 223§ (4.9) |
| Vital signs | ||||
| eGFR, mL·min−1·1.73 m−2) | 91.4 ± 38.2 | 89.0 ± 40.0‡ | 92.5 ± 37.3§ | 95.4 ± 35.6|| |
| Heart rate, beats/min | 78.2 ± 16.4 | 78.4 ± 16.9‡ | 78.3 ± 16.1‡ | 76.8 ± 15.8§ |
| Systolic blood pressure, mmHg | 127.2 ± 23.3 | 127.8 ± 23.1‡ | 127.2 ± 23.6§ | 124.4 ± 21.9|| |
| Medications in first 24 h | ||||
| DAPT | 56,518 (95.1) | 20,303‡ (92.4) | 31,830§ (96.7) | 4385§ (96.2) |
| ACEI or ARB | 27,786 (46.7) | 10,591‡ (48.2) | 15,056§ (45.7) | 2139‡§ (46.9) |
| β-blockers | 32,515 (54.7) | 12,388‡ (56.4) | 17,574§ (53.4) | 2553‡ (56.0) |
| Statins | 55,849 (93.9) | 20,427‡ (93.0) | 31,054§ (94.3) | 4368|| (95.9) |
| Hospital stays, days | 9.0 [7.0,12.0] | 10.0‡ [7.0,14.0] | 9.0§ [7.0,12.0] | 10.0‡|| [7.0,13.0] |
Data are presented as n (%), mean ± standard deviation or median [interquartile range].
Among 20,703 patients enrolled since July 2017.
Symptom-to-admission time was not available for 13,878 of 59,447 (23.3%) patients with STEMI.
Group differs significantly from type (in a row) where § or || is indicated.
ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; DAPT: Dual antiplatelet therapy; eGFR: Estimated glomerular filtration rate; LDL-C: Low-density lipoprotein cholesterol; PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
Geographical and temporal variations in the use of reperfusion
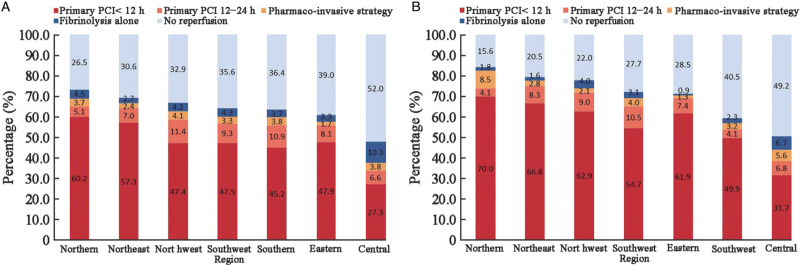
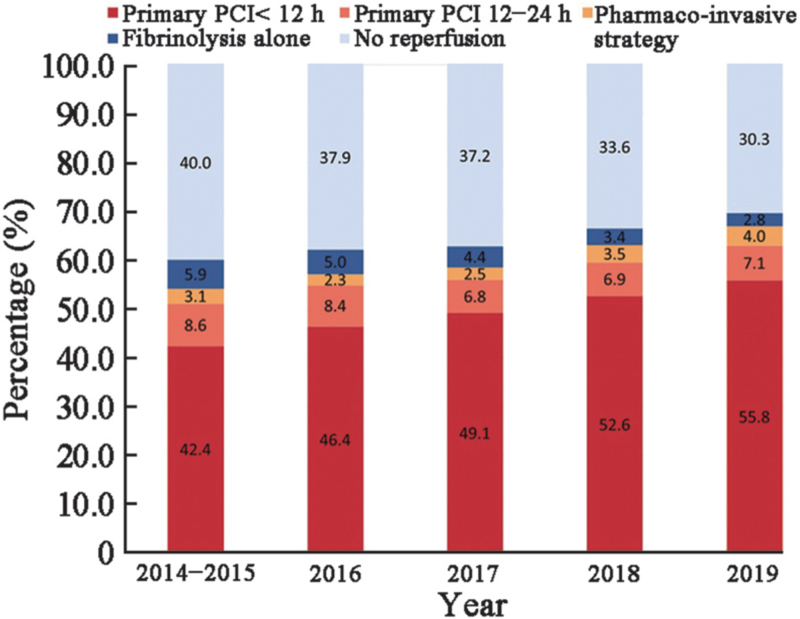
The rate of reperfusion varied across the seven geographical regions of China, ranging from 48.0% (4547/9482) to 73.5% (7393/10,052). In central China, more than half of hospitalized patients with STEMI (52.0%, 4935/9482) did not undergo any type of timely reperfusion therapy, and the lowest rate of primary PCI (33.9%, 3213/9482) was paralleled by the highest rate of fibrinolysis (14.1%, 1334/9482) (Figure 1). From 2014 to 2019, the rate of reperfusion continuously increased from 60.0% (11,310/18,834) to 69.7% (4664/6690); in particular, the rate of primary PCI increased from 51.0% (9622/18,834) to 62.9% (4207/6690). Meanwhile, the rate of fibrinolytic therapy decreased from 9.0% (1688/18,834) to 6.8% (457/6690). The rate of pharmaco-invasive therapy remained stagnant from 3.1% (577/18,834) to 4.0% (267/6690) over the course of the study (Figure 2). The fibrinolytic agent information was available for 2065 (45.3%) of the 4556 patients receiving fibrinolysis. Among those, 972 (47.1%) were treated with a non-specific fibrinolytic agent (including urokinase and streptokinase), 885 (42.8%) were treated with a specific thrombolytic agent (including reteplase, alteplase, and tenecteplase), and the remaining 208 (10.1%) were treated with other agents (types not available).
Figure 1.
Regional patterns of reperfusion therapy among hospitalized patients with STEMI in years (A) 2014–2019 and (B) 2019. PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
Figure 2.
Temporal trends in the pattern of reperfusion therapy among hospitalized patients with STEMI. Percentage of hospitalized patients with STEMI with different patterns of reperfusion therapy according to the year of admission. PCI: Percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
Procedural characteristics of STEMI patients who underwent primary PCI
Among the 32,929 patients with STEMI who underwent primary PCI, 28,280 patients (85.9%) underwent primary PCI at <12 h, while 4649 (14.1%) underwent primary PCI at 12 to 24 h. Compared with patients who underwent primary PCI at 12 to 24 h, patients who underwent primary PCI at <12 h had an overall lower-risk profile and were less likely to be transferred [Supplementary Table 4]. A large proportion of patients who underwent primary PCI did so via radial access (92.7%, 30,035/32,385). Thrombus aspiration was performed in 24.6% (8094/32,929) of patients. A total of 90.0% (29,619/32,926) of patients underwent stent implantation, among whom drug-eluting stents (DES) were used in 98.8% and bare-metal stents were used in 0.6% of patients. Of note, delays in door-to-balloon time were seen in the two primary PCI groups. A total of 72.8% (16,746/22,990) of patients in the primary PCI at <12 h group and 50.8% (1460/2874) of patients in the primary PCI at 12 to 24 h group met the door-to-balloon time goal of ≤90 min. The median door-to-balloon time was 60.0 min [IQR 33.0, 105.0] (Table 2).
Table 2.
Procedural characteristics of primary PCI in patients hospitalized with STEMI.
| Primary PCI | ||||
| Characteristics | Total (n = 32,929) | Primary PCI< 12 h (n = 28,280) | Primary PCI 12–24 h (n = 4649) | P value |
| Symptom-to-admission time∗, h | 4.0 (2.0, 8.0) | 3.9 (2.0, 6.7) | 14.9 (7.0, 24.3) | <0.001 |
| Multivessel CAD | 14,936 (45.4) | 12,739 (45.0) | 2197 (47.3) | 0.005 |
| Culprit vessel location | <0.001 | |||
| LM | 277 (0.8) | 225 (0.8) | 52 (1.1) | |
| LAD | 14,564 (44.2) | 12,444 (44.0) | 2120 (45.6) | |
| LCX | 3557 (10.8) | 3044 (10.8) | 513 (11.0) | |
| RCA | 13,313 (40.4) | 11,557 (40.9) | 1756 (37.8) | |
| Others | 717 (2.2) | 592 (2.1) | 125 (2.7) | |
| Uncertain | 501 (1.5) | 418 (1.5) | 83 (1.8) | |
| Number of narrow coronary arteries† | 0.006 | |||
| Nonobstructive CAD, < 50% | 381 (1.3) | 311 (1.2) | 70 (1.7) | |
| 1 | 15,955 (54.5) | 13,765 (54.9) | 2190 (52.5) | |
| 2 | 5890 (20.1) | 5010 (20.0) | 880 (21.1) | |
| ≥3 | 7040 (24.1) | 6005 (23.9) | 1035 (24.8) | |
| Door-to-balloon time‡, min | 60.0 (33.0, 105.0) | 58.0 (32.0, 98.0) | 90.0 (43.0, 240.0) | <0.001 |
| < 90min | 18,206 (70.4) | 16,746 (72.8) | 1460 (50.8) | <0.001 |
| Vascular access§ | 0.065 | |||
| Transradial access | 30,035 (92.7) | 25,842 (92.9) | 4193 (91.9) | |
| Transfemoral access | 2235 (6.9) | 1884 (6.8) | 351 (7.7) | |
| Others | 115 (0.4) | 97 (0.3) | 18 (0.4) | |
| Thrombus aspiration | 8094 (24.6) | 7157 (25.3) | 937 (20.2) | <0.001 |
| Implantation of stents|| | 0.596 | |||
| Yes | 29,619 (90.0) | 25,447 (90.0) | 4172 (89.7) | |
| No | 3307 (10.0) | 2830 (10.0) | 477 (10.3) | |
| Stent types ¶ | 0.001 | |||
| DES | 29,032 (98.8) | 24,968 (98.9) | 4064 (98.2) | |
| BMS | 190 (0.6) | 149 (0.6) | 41 (1.0) | |
| others | 161 (0.5) | 129 (0.5) | 32 (0.8) | |
| Number of implanted stents∗∗ | 1.0 (1.0, 2.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 2.0) | 0.004 |
| Other procedures during hospitalization | ||||
| IABP†† | 487 (3.9) | 409 (3.7) | 78 (5.4) | 0.002 |
| PTCA‡‡ | 613 (53.7) | 536 (53.5) | 77 (55.0) | <0.001 |
| CABG | 155 (0.5) | 120 (0.4) | 35 (0.8) | 0.002 |
Data are presented as n (%) or median (interquartile range).
Symptom-to-admission time was not available for 4153 of 32,929 (12.6%) patients.
Number of narrowed coronary arteries was not available for 3663 of 32,929 (11.1%) patients.
Door-to-balloon time was not available for 7065 of 32,929 (21.5%) patients.
Vascular access was not available for 544 of 32,929 (1.7%) patients.
Implantation of stents was not available for 3 of 32,929 (0%) patients.
Stent type was not available for 236 of 29,619 (0.8%) patients with stent implantations.
Total number of implanted stents was not available for 78 of 11,222 (0.7%) patients with stent implantations who were enrolled after July 2017.
IABP was based on 12,364 patients enrolled since July 2017.
PTCA was based on 1142 patients without stent insertion who were enrolled after July 2017.
P values are for comparisons among the two groups using the Student's t-test, Wilcoxon's rank-sum test, or χ2 test. BMS: Bare-metal stent; CAD: Coronary artery disease; CABG: Coronary artery bypass graft; DES: Drug-eluting stent; IABP: Intra-aortic balloon pump; LAD: Left anterior descending; LCX: Left circumflex; LM: Left main; PCI: Percutaneous coronary intervention; PTCA: Percutaneous transluminal coronary angioplasty; RCA: Right coronary artery; STEMI: ST-segment elevation myocardial infarction.
Factors associated with the use of reperfusion
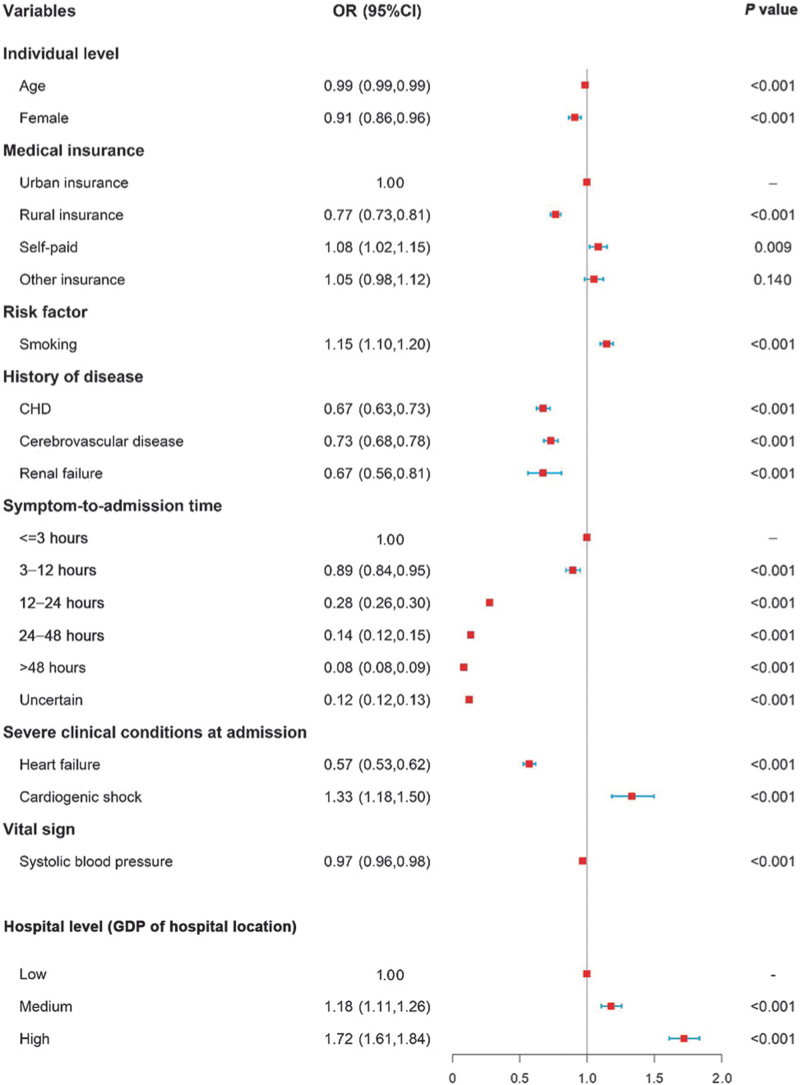
For individual-level variables, older, female, rural insurance, history of disease (including prior coronary heart disease, renal failure, and cerebrovascular disease), heart failure at admission, higher systolic blood pressure, and a longer time interval between symptom onset and admission were associated with a lower odds of reperfusion. Regarding hospital-level variables, hospitals in regions with middle and high levels of economic development had significantly higher odds of reperfusion than low economic regions. A dose-response relationship was noted between a shorter symptom-to-admission time, a higher regional gross domestic product, and a higher odds of reperfusion (Figure 3). We then conducted a sensitivity analysis to further adjust for the history of bleeding according to medical records. Similar results were obtained in the sensitivity analysis [Supplementary Figure 2].
Figure 3.
Association between patients’ characteristics and the use of reperfusion therapy. A hierarchical logistic regression model was clustered for patients within hospital geographic regions, adjusted for patients’ individual characteristics (age, sex, medical insurance, time from symptom onset to admission, severe clinical conditions at admission [acute heart failure, cardiogenic shock, cardiac arrest], heart rate, systolic blood pressure, eGFR, diabetes mellitus, chronic heart failure, smoking, history of disease [coronary heart disease, renal failure, and cerebrovascular disease]), and hospital characteristics (economic level of hospital location). CI: Confidence interval; CHD: Coronary heart disease; eGFR: Estimated glomerular filtration rate; GDP: Gross domestic product; OR: Odds ratio.
Practice of reperfusion and in-hospital outcomes
Compared with no reperfusion, all reperfusion strategies were associated with a lower risk of in-hospital MACEs according to the univariable Cox regression analysis. After full adjustment, the risk of MACEs was significantly lower with every type of timely PCI compared with no reperfusion (primary PCI <12 h group (hazard ratio [HR]: 0.64; 95% confidence interval [CI]: 0.54–0.76), primary PCI 12 to 24 h group (HR: 0.53; 95% CI: 0.37–0.74), and pharmaco-invasive strategy group (HR: 0.46; 95% CI: 0.25–0.82)), with the exception of fibrinolysis alone (HR: 0.79; 95% CI: 0.54–1.15) (Table 3).
Table 3.
Association between patterns of reperfusion therapy and in-hospital MACE.
| Therapy patterns | Case/Number∗ | Unadjusted HR (95% CI) | P value | Adjusted† HR (95% CI) | P value |
| No Reperfusion | 622/21,778 | 1.00 | – | 1.00 | – |
| Fibrinolysis alone | 59/2782 | 0.62 (0.45–0.85) | 0.003 | 0.79 (0.54–1.15) | 0.210 |
| Primary PCI | |||||
| < 12 h | 483/28,100 | 0.65 (0.57–0.74) | <0.001 | 0.64 (0.54–0.76) | <0.001 |
| 12–24 h | 72/4635 | 0.52 (0.39–0.69) | <0.001 | 0.53 (0.37–0.74) | <0.001 |
| Pharmaco-invasive strategy | 19/1745 | 0.39 (0.23–0.64) | <0.001 | 0.46 (0.25–0.82) | 0.009 |
The number of in-hospital MACEs was not available for 407 (0.7%) patients who experienced MACEs within 1 day of admission or had missing data regarding the detailed PCI strategies.
The adjusted model was clustered for patients within hospitals, and adjusted for age, sex, medical insurance, time from symptom onset to admission, severe clinical conditions at admission (acute heart failure, cardiogenic shock, cardiac arrest), vital signs (heart rate, systolic blood pressure, eGFR), diabetes mellitus, smoking, chronic heart failure, history of disease (coronary heart disease, renal failure, and cerebrovascular disease), transfer status, and medications administered in the first 24 h of arrival (DAPT, β-blockers, and ACEI/ARB). ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; CI: Confidence interval; DAPT: Dual antiplatelet therapy; eGFR: Estimated glomerular filtration rate; HR: Hazard ratio; MACE: Major adverse cardiovascular event; PCI: Percutaneous coronary intervention.
Discussion
This is the largest and most up-to-date real-world study exploring the practice of reperfusion in patients hospitalized with STEMI in China. We found that the rate of reperfusion (63.1%) among hospitalized patients with STEMI was far from optimal. Meanwhile, the use and pattern of reperfusion therapy varied across different geographical regions (48.0%–73.5%). There was a dramatic increase in the rate of reperfusion from 2014 to 2019 (from 60.0% to 69.7%), mainly due to the increase in the rate of primary PCI at <12 h (from 42.4% to 55.8%). Patient and hospital characteristics were both associated with reperfusion therapy. These findings identified areas that can be specially targeted to strengthen the use of early reperfusion and improve the quality of care for patients with STEMI in China.
Use of reperfusion among patients with STEMI
Among hospitalized patients with STEMI in China, the rate of early reperfusion (63.1%) from 2014 to 2019 was far from optimal. In fact, the rate was much lower than the rate in Sweden in 2014 (81.7%) and in the UK between 2004 and 2010 (76.8%).[14,15] The rate of primary PCI (55.4%) was also much lower than in Sweden in 2014 (78.0%).[14] Meanwhile, the rate of fibrinolytic therapy were extremely low (7.7%), and there was a huge gap between the extremely low rate of the pharmaco-invasive strategy and guideline recommendations.[2,3] This suggests that there may be an opportunity to emphasize the use of reperfusion therapy in China, including primary PCI and the pharmaco-invasive strategy.
Regarding the procedural characteristics of primary PCI, the four invasive procedures with Class I recommendations for primary PCI were widely adopted, including primary PCI within the first 90 min of arrival, stent treatment, and radial artery access.[2] Also, we identified a significant and rapid improvement in these invasive procedures in recent decades compared with the China Acute Myocardial Infarction (CAMI) registry in 2014[10] and the China PEACE study in 2011.[7]
Geographic variation in the use of reperfusion
There were large geographic variations in reperfusion practice. Hospitals in central China had significantly lower rates of reperfusion than other regions, which is consistent with a previous study.[16] Central China does not benefit from social health insurance programs and policy priorities as much as western regions and has become inefficient in medical service delivery compared with other regions.[17] Thus, specially targeted quality improvement efforts in central China will help to narrow regional care disparities for patients with STEMI.
Temporal trends in the type of reperfusion
We noticed a dramatic increase in the reperfusion rate from 2014 to 2019 in patients hospitalized with STEMI at tertiary hospitals in China, mainly due to the substantial and sustained increase in primary PCI at <12 h. The China PEACE registry, in which 40% of hospitals were tertiary hospitals, showed that the adjusted reperfusion rate remained stagnant from 54.7% in 2001 to 55.2% in 2011.[7] Subsequently, the CAMI report, in which 74.0% of hospitals were tertiary hospitals, showed that the reperfusion rate was 57.5% between 2013 and 2014.[10] To date, the overall reperfusion rate has increased, with a shift from fibrinolytic therapy to primary PCI at <12 h from 2014 to 2019. China started to build a social health insurance system in 2009,which focused on promoting public health services and equity and achieved near-universal health insurance coverage in 2011.[18] Meanwhile, a nationwide program (the China STEMI-PCI program) was established in 2011.[19] This program emphasized the development of chest pain centers, which have demonstrated improved in-hospital outcomes during the past decade in China.[20] Our findings are generally consistent with those of previous studies supporting the benefits of health reform on health resource allocation.[8,10]
A substantial proportion of reperfusion therapies are performed beyond recommended timelines.[10,21–23] We observed that almost 10% of patients underwent primary PCI at 12 to 24 h. Among the patients who underwent fibrinolysis, only 38.4% underwent the pharmaco-invasive strategy, as recommended in published guidelines.[2] The persistently marked underuse of guideline-recommended reperfusion, particularly the pharmaco-invasive strategy, calls for improvements in the selection of patients for early fibrinolysis and/or transfer PCI.
Factors associated with the use of reperfusion
Regarding the factors associated with the inefficient use of reperfusion, our study has shown that patient and hospital characteristics may be considered by care providers and may thus affect the delivery of guideline-recommended reperfusion strategies. As expected, patients who are elderly, female, with rural insurance and an overall high-risk profile are associated with a low odds of reperfusion therapy. A longer pre-hospital delay was also associated with a lower odds of reperfusion therapy, even in patients within 12 h of symptom onset, which is inconsistent with published guidelines.[2,3] Discrepancies in reperfusion therapy for STEMI might result from inadequate and inappropriate provider knowledge and concerning about potential patient arbitration and litigation due to the risk of treatment.[24–26] All patients enrolled in our study were from tertiary hospitals, and performance might be even worse at community hospitals and non-teaching hospitals. Quality improvement efforts require significant investment from hospitals and health care professionals.
Finally, we found that hospital-level factors and an unbalanced economy were associated with the use of reperfusion. Patients with STEMI who were hospitalized in low economic regions were generally less likely to undergo reperfusion therapy than patients hospitalized in other economic regions, which is consistent with previous studies.[16] A dose-response relationship was noted between higher economic development and reperfusion therapy. Our findings highlight the urgent need for more targeted policies in undeveloped regions to improve the overall use of reperfusion therapy in China.
The present study is subject to the limitations inherent in all observational studies. The major objective was to explore the current status of reperfusion practice and its associated factors among patients hospitalized with STEMI in China. Although we attempted to explore the potential associations between types of reperfusion therapies and number of in-hospital MACEs using a Cox regression model, the HR is unable to be used in the same way in observational studies as in randomized trials to explain the clinical benefits of the therapies. In addition, the present study only focused on in-hospital outcomes. Future studies with post-discharge data will help to examine the effect of reperfusion patterns on the long-term outcomes of patients with STEMI. Moreover, all participating hospitals were tertiary hospitals. Therefore, the findings cannot be generalized to all hospitals in China. Finally, clinical information was defined based on the information abstracted from in-patient records, and although the accuracy of medical record abstraction was 95.7% according to a third-party audit, the quality of documentation in the original medical records may have been affected by the quality of the data.
Conclusion
More than one-third of patients with STEMI do not undergo reperfusion therapy in China. The rate of reperfusion increased from 2014 to 2019, and the performance varied across the broad geography and unbalanced economy. Our findings indicate that timely reperfusion therapies, including primary PCI and pharmaco-invasive therapy, should be further strengthened, particularly in patients who are elderly, are female, have a long pre-hospital delay, and are from rural areas. This study emphasizes the urgent need for more targeted policies in Central China and in undeveloped regional hospitals to narrow the geographical and economic disparity and improve the overall use of reperfusion therapy.
Acknowledgements
We acknowledge all participating hospitals for their contributions to the project.
Conflicts of interest
The Improving Care for Cardiovascular Disease in China–Acute Coronary Syndrome (CCC-ACS) Project is a collaborative program of the American Heart Association and the Chinese Society of Cardiology. The American Heart Association has been funded by Pfizer and AstraZeneca for a quality improvement initiative through an independent grant for learning and change.
Supplementary Material
Footnotes
How to cite this article: Yang Y, Hao Y, Liu J, Yang N, Hu D, Sun Z, Zhao D, Liu J. Practice of reperfusion in patients with ST-segment elevation myocardial infarction in China: findings from the Improving Care for Cardiovascular Disease in China–Acute Coronary Syndrome project. Chin Med J 2022;135:2821–2828. doi: 10.1097/CM9.0000000000002257
Supplemental digital content is available for this article.
References
- 1.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019; 16:203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 4.Cardiology CSo. 2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction (in Chinese). Chin J Cardiovasc 2019; 47:766–783. doi: 10.3760/cma.j.issn.0253-3758.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Schiele F, Hochadel M, Tubaro M, Meneveau N, Wojakowski W, Gierlotka M, et al. Reperfusion strategy in Europe: temporal trends in performance measures for reperfusion therapy in ST-elevation myocardial infarction. Eur Heart J 2010; 31:2614–2624. doi: 10.1093/eurheartj/ehq305. [DOI] [PubMed] [Google Scholar]
- 6.Gibson CM, Pride YB, Frederick PD, Pollack CV, Jr, Canto JG, Tiefenbrunn AJ, et al. Trends in reperfusion strategies, door-to-needle and door-to-balloon times, and in-hospital mortality among patients with ST-segment elevation myocardial infarction enrolled in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J 2008; 156:1035–1044. doi: 10.1016/j.ahj.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015; 385:441–451. doi: 10.1016/S0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Murugiah K, Li J, Masoudi FA, Chan PS, Hu S, et al. Urban-rural comparisons in hospital admission, treatments, and outcomes for ST-segment-elevation myocardial infarction in China From 2001 to 2011: a retrospective analysis from the China PEACE study (Patient-Centered Evaluative Assessment of Cardiac Events). Circ Cardiovasc Qual Outcomes 2017; 10:e003905.doi: 10.1161/CIRCOUTCOMES.117.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao Y, Liu J, Liu J, Yang N, Smith SC, Jr, Huo Y, et al. Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation 2019; 139:1776–1785. doi: 10.1161/CIRCULATIONAHA.118.037655. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Yang Y, Wang C, Yang J, Li W, Zhang X, et al. Association of hospital-level differences in care with outcomes among patients with acute ST-segment elevation myocardial infarction in China. JAMA Netw Open 2020; 3:e2021677.doi: 10.1001/jamanetworkopen.2020.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao R, Patel A, Gao W, Hu D, Huang D, Kong L, et al. Prospective observational study of acute coronary syndromes in China: practice patterns and outcomes. Heart 2008; 94:554–560. doi: 10.1136/hrt.2007.119750. [DOI] [PubMed] [Google Scholar]
- 12.Hao Y, Liu J, Liu J, Smith SC, Jr, Huo Y, Fonarow GC, et al. Rationale and design of the Improving Care for Cardiovascular Disease in China (CCC) project: a national effort to prompt quality enhancement for acute coronary syndrome. Am Heart J 2016; 179:107–115. doi: 10.1016/j.ahj.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL. Timely PCI for STEMI--still the treatment of choice. N Engl J Med 2013; 368:1446–1447. doi: 10.1056/NEJMe1302670. [DOI] [PubMed] [Google Scholar]
- 14.Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J 2017; 38:3056–3065. doi: 10.1093/eurheartj/ehx515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, et al. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 2014; 383:1305–1312. doi: 10.1016/S0140-6736(13)62070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Q, Gao Y, Zheng X, Chen J, Masoudi FA, Lu Y, et al. Geographic variation in process and outcomes of care for patients with acute myocardial infarction in China from 2001 to 2015. JAMA Netw Open 2020; 3:e2021182.doi: 10.1001/jamanetworkopen.2020.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Hu X, Zhang X, Shang L, Yu M, Chen H. Equity and efficiency of medical service systems at the provincial level of China's mainland: a comparative study from 2009 to 2014. BMC Public Health 2018; 18:214.doi: 10.1186/s12889-018-5084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z. Launch of the health-care reform plan in China. Lancet 2009; 373:1322–1324. doi: 10.1016/S0140-6736(09)60753-4. [DOI] [PubMed] [Google Scholar]
- 19.Xinhua News Agency Launch of “Standardized Treatment Project for Acute Myocardial Infarction in China” (in Chinese) 2011 [Last accessed on September 22, 2020] Available at http://www.gov.cn/jrzg/2011-11/28/content_2005114.htm. [Google Scholar]
- 20.Fan F, Li Y, Zhang Y, Li J, Liu J, Hao Y, et al. chest pain center accreditation is associated with improved in-hospital outcomes of acute myocardial infarction patients in China: findings from the CCC-ACS project. J Am Heart Assoc 2019; 8:e013384.doi: 10.1161/JAHA.119.013384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017; 136:1908–1919. doi: 10.1161/CIRCULATIONAHA.117.030798. [DOI] [PubMed] [Google Scholar]
- 22.Aliprandi-Costa B, Morgan L, Snell LC, M DS, Kritharides L, French J, et al. ST-elevation acute myocardial infarction in Australia-temporal trends in patient management and outcomes 1999–2016. Heart Lung Circ 2019; 28:1000–1008. doi: 10.1016/j.hlc.2018.05.191. [DOI] [PubMed] [Google Scholar]
- 23.Rashid MK, Guron N, Bernick J, Wells GA, Blondeau M, Chong AY, et al. Safety and efficacy of a pharmacoinvasive strategy in ST-segment elevation myocardial infarction: a patient population study comparing a pharmacoinvasive strategy with a primary percutaneous coronary intervention strategy within a regional system. JACC Cardiovasc Interv 2016; 9:2014–2020. doi: 10.1016/j.jcin.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Du X, Gao R, Turnbull F, Wu Y, Rong Y, Lo S, et al. Hospital quality improvement initiative for patients with acute coronary syndromes in China: a cluster randomized, controlled trial. Circ Cardiovasc Qual Outcomes 2014; 7:217–226. doi: 10.1161/CIRCOUTCOMES.113.000526. [DOI] [PubMed] [Google Scholar]
- 25.Ranasinghe I, Rong Y, Du X, Wang Y, Gao R, Patel A, et al. System barriers to the evidence-based care of acute coronary syndrome patients in China: qualitative analysis. Circ Cardiovasc Qual Outcomes 2014; 7:209–216. doi: 10.1161/CIRCOUTCOMES.113.000527. [DOI] [PubMed] [Google Scholar]
- 26.Chinese doctors are under threat. Lancet 2010; 376:657.doi: 10.1016/S0140-6736(10)61315-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.