Key Points
Question
Do household income levels factor into 21-gene recurrence score (RS) and mortality among patients with estrogen receptor (ER)-positive breast cancer?
Findings
In this cohort study involving 119 478 women, low income was associated with higher RS and worse survival. On subgroup analysis, similar findings were observed among those with RS below 26, while there was no survival difference between income levels among others with scores 26 or higher.
Meaning
These results suggest that low household income is associated with more aggressive tumor biology and significantly worse mortality among those with RS below 26.
This cohort study of US women with estrogen receptor (ER)-positive breast cancer examines the association of income levels with 21-gene recurrence scores and survival outcomes.
Abstract
Importance
While low income has been associated with a higher incidence of triple-negative breast cancer, its association with 21-gene recurrence score (RS) among patients with estrogen receptor (ER)-positive breast cancer remains unclear.
Objective
To evaluate the association of household income with RS and overall survival (OS) among patients with ER-positive breast cancer.
Design, Setting, and Participants
This cohort study used data from the National Cancer Database. Eligible participants included women diagnosed between 2010 and 2018 with ER-positive, pT1-3N0-1aM0 breast cancer who received surgery followed by adjuvant endocrine therapy with or without chemotherapy. Data analysis was performed from July 2022 to September 2022.
Exposures
Low vs high neighborhood-level household income levels defined as below vs above the median household income of $50 353 based on each patient’s zip code.
Main Outcomes and Measures
RS (a score ranged from 0 to 100 based on gene expression signatures indicating the risk of distant metastasis, with RS of 25 or below indicating non–high risk and RS above 25 indicating high risk) and OS.
Results
Among 119 478 women (median [IQR] age, 60 [52-67] years; 4737 [4.0%] Asian and Pacific Islander, 9226 [7.7%] Black, 7245 [6.1%] Hispanic, 98 270 [82.2%] non-Hispanic White), 82 198 (68.8%) and 37 280 (31.2%) patients had high and low income, respectively. Logistic multivariable analysis (MVA) showed that, compared with high income, low income was associated with higher RS (adjusted odds ratio [aOR], 1.11; 95% CI, 1.06-1.16). Cox MVA showed that low income was also associated with worse OS (adjusted hazards ratio [aHR], 1.18; 95% CI, 1.11-1.25). Interaction term analysis showed a statistically significant interaction between income levels and RS (interaction P < .001). On subgroup analysis, significant findings were noted among those with RS below 26 (aHR, 1.21; 95% CI, 1.13-1.29), while there was no significant OS difference between income levels among others with RS of 26 or higher (aHR, 1.08; 95% CI, 0.96-1.22).
Conclusions and Relevance
Our study suggested that low household income was independently associated with higher 21-gene recurrence scores and significantly worse survival outcomes among those with scores below 26, but not 26 or higher. Further studies are warranted to investigate the association between socioeconomic determinants of health and intrinsic tumor biology among patients with breast cancer.
Introduction
Socioeconomic status (SES) is measured using income, education, and occupational status and is a strong predictor of health outcomes.1 Low SES is a risk factor for unfavorable breast cancer outcomes regardless of racial and ethnic background.2,3 Decreased access to preventative care and cancer screening, with consequent effects on comorbidities of patients at their time of diagnosis presenting stage, have been implicated in the disparities associated with low SES.3 Differences in cancer treatment account for only a small fraction low SES disparities.3
Despite extensive research in the roles of socioeconomic inequities and breast cancer disparities, there is a paucity of studies evaluating the association of socioeconomic status with intrinsic tumor biology. For example, 21-gene recurrence score (RS), a score based on gene expression signatures indicating the risk of distant metastasis, has been incorporated into routine clinical care in the US.4,5 Although low SES and income levels are associated with a higher incidence of triple-negative breast cancer,6,7,8 the association between low income and RS among patients with estrogen receptor (ER)-positive breast cancer, the most common subtype, remains unclear. We performed an observational cohort study to evaluate the association of income levels with RS and survival among patients with nonmetastatic ER-positive breast cancer.
Methods
This study was reviewed and approved by the institutional review board at the Roswell Park Comprehensive Cancer Center with an exemption for informed consent requirements because data were deidentified and publicly available. It followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The National Cancer Database (NCDB) is a nationwide, clinical oncology database sponsored jointly by the American Cancer Society and the American College of Surgeons Commission on Cancer (CoC).9 It collected over 34 million cancer cases from more than 1500 CoC-accredited facilities,9 and it represented 80% of newly diagnosed breast cancer in the US.10 It also has been used for studies evaluating RS as shown in our previous studies.11,12 The database was queried for female patients diagnosed between 2006 and 2018 with ER-positive, pT1-3N0-1aM0 breast cancer who received surgery followed by adjuvant endocrine therapy. Patients were excluded if they had unknown RS or income levels. Follow-up was conducted until 2021. Other variables included for analysis were facility type, race and ethnicity, age, medical insurance, income and education level, Charlson-Deyo Comorbidity Score, year of diagnosis, histology, tumor grade, T and N staging, RS, lymphovascular space invasion, surgery, surgical margin, radiation therapy, and chemotherapy. Race and ethnicity were included as variables because they are considered factors in income levels and socioeconomic status. All missing values for each variable were grouped together and coded as unknown. Education and income levels were determined based on the 2016 American Community Survey data spanning years 2012 through 2016. It measured the percentage of adults who did not graduate from high school and the median household income adjusted for 2016 inflation, respectively, in each patient’s zip code and equally proportioned education and income ranges among all US zip codes. High vs low neighborhood-level income and education were determined by the median values of $50 353 and 10.9%, respectively, with the latter being the percentage of adults living in the zip code area who did not graduate from high school. Baseline characteristics were compared between those with known vs unknown RS and income levels. Other clinically relevant variables, including systemic therapy agents and duration, Karnofsky performance status, and breast cancer-specific survival, were not captured in the NCDB.
Our primary end points were RS and overall survival (OS). RS was defined as a score ranged from 0 to 100 indicating the risk of distant metastasis based on the gene expression signatures using pretreatment tumor specimens,13 with RS of 25 or below indicating non–high risk and RS above 25 indicating high risk for both node-negative5 and node-positive4 breast cancer.
OS was defined as the time interval between diagnosis and the last follow-up or death from any cause. Baseline characteristics were compared between high-income vs low-income levels using Fisher exact test and Mann-Whitney U test as appropriate. Logistic multivariable analysis (MVA) was constructed based on baseline patient and tumor characteristics as listed previously to evaluate variables associated with RS above 25. Cox MVA was used to evaluate OS. Cumulative incidence plots for overall mortality were generated. Survival data among patients diagnosed in 2018 were unavailable in the NCDB, and these patients were excluded for OS analysis. Cox MVA models included all variables listed previously.
Interaction term analysis was performed to evaluate any heterogeneous association between income levels and RS. If the interaction term was statistically significant, subgroup analyses were performed to compare the magnitude of income differences associated with OS stratified by RS. In order to reduce selection bias and further investigate the subgroup analysis results, propensity score matching between income levels was performed. Matching was based on all variables listed previously using nearest neighbor method in a 1:1 ratio with no replacements and a caliper distance of 0.25 of the standard deviation of the logit of the propensity score.14 Propensity scores were estimated using logistic regression models. To ensure adequate matching, standardized means differences for all matched variables were evaluated and calculated to be less than 0.1, suggesting negligible differences between income levels.15 Survival outcomes were reported after propensity score matching. Sensitivity analysis was also performed among non-Hispanic White women with high education level and private medical insurance in order to evaluate the association of income levels and OS.
All P values were 2-sided, and P < .05 was considered statistically significant. All analyses were performed using R version 4.0.3 (R Project for Statistical Computing).
Results
A total of 119 478 women (median [interquartile range (IQR)] age, 60 [52-67] years; 4737 [4.0%] Asian and Pacific Islander, 9226 [7.7%] Black, 7245 [6.1%] Hispanic, 98 270 [82.2%] non-Hispanic White) met our criteria and were included for analysis (Table 1). Of these, 82 198 (68.8%) and 37 280 (31.2%) patients had high and low household income levels, respectively. Median (IQR) follow up was 66.2 (47.9-90.3) months. A total of 36 084 women did not meet our criteria for analysis. Most baseline characteristics were comparable between those with known vs unknown RS or income levels (eTable 1 in Supplement 1). However, most patients with unknown RS or income levels also had missing values for education levels.
Table 1. Baseline Characteristics Stratified by Income Levels.
| Characteristics | Income, No. (%) | P value | |
|---|---|---|---|
| High | Low | ||
| Age, y | |||
| <50 | 16 996 (20.7) | 6259 (16.8) | <.001 |
| ≥50 | 65 202 (79.3) | 31 021 (83.2) | |
| Facility | |||
| Nonacademic | 52 353 (63.7) | 26 061 (69.9) | <.001 |
| Academic | 27 611 (33.6) | 10 271 (27.6) | |
| Not available | 2234 (2.7) | 948 (2.5) | |
| Race | |||
| Asian and Pacific Islander | 3968 (4.8) | 769 (2.1) | <.001 |
| Black | 3767 (4.6) | 5459 (14.6) | |
| Hispanic | 4525 (5.5) | 2720 (7.3) | |
| Non-Hispanic White | 69 938 (85.1) | 28 332 (76.0) | |
| Insurance | |||
| None | 717 (0.9) | 643 (1.7) | <.001 |
| Private | 52 399 (63.7) | 18 882 (50.6) | |
| Government | 28 249 (34.4) | 17 388 (46.6) | |
| Not available | 833 (1.0) | 367 (1.0) | |
| Educationa | |||
| Above median | 66 698 (81.1) | 9497 (25.5) | <.001 |
| Below median | 15 500 (18.9) | 27 783 (74.5) | |
| CDS | |||
| 0 | 71 138 (86.5) | 30 193 (81.0) | <.001 |
| 1 | 9070 (11.0) | 5540 (14.9) | |
| ≥2 | 1990 (2.4) | 1547 (4.1) | |
| Year | |||
| 2006-2013 | 32 908 (40.0) | 15 160 (40.7) | .047 |
| 2014-2018 | 48 792 (59.4) | 21 914 (58.8) | |
| Histology | |||
| Ductal or lobular carcinoma | 70 504 (85.8) | 32 265 (86.5) | <.001 |
| Other | 11 694 (14.2) | 5015 (13.5) | |
| PR | |||
| Positive | 74 507 (90.6) | 33 661 (90.3) | .06 |
| Negative | 7691 (9.4) | 3619 (9.7) | |
| T staging | |||
| 1 | 61 780 (75.2) | 27 334 (73.3) | <.001 |
| 2 | 19 267 (23.4) | 9402 (25.2) | |
| 3 | 1151 (1.4) | 544 (1.5) | |
| N staging | |||
| 0 | 69 896 (85.0) | 31 571 (84.7) | .12 |
| 1a | 12 302 (15.0) | 5709 (15.3) | |
| Grade | |||
| 1 | 22 911 (27.9) | 10 373 (27.8) | <.001 |
| 2 | 44 093 (53.6) | 19 615 (52.6) | |
| 3 | 12 176 (14.8) | 5936 (15.9) | |
| Other | 44 (0.1) | 26 (0.1) | |
| Not available | 2974 (3.6) | 1330 (3.6) | |
| RS | |||
| 0-15 | 40 443 (49.2) | 18 217 (48.9) | <.001 |
| 16-25 | 30 242 (36.8) | 13 144 (35.3) | |
| >25 | 11 513 (14.0) | 5919 (15.9) | |
| LVSI | |||
| No | 63 119 (76.8) | 28 205 (75.7) | <.001 |
| Yes | 10 413 (12.7) | 4231 (11.3) | |
| Not available | 8666 (10.5) | 4844 (13.0) | |
| Chemotherapy | |||
| No | 64 907 (79.0) | 29 354 (78.7) | .38 |
| Yes | 17 291 (21.0) | 7926 (21.3) | |
| Radiation | |||
| No | 25 148 (30.6) | 12 030 (32.3) | <.001 |
| Yes | 56 132 (68.3) | 24 838 (66.6) | |
| Not available | 904 (1.1) | 403 (1.1) | |
| Surgery | |||
| Lumpectomy | 55 886 (68.0) | 24 900 (66.8) | <.001 |
| Mastectomy | 26 293 (32.0) | 12 368 (33.2) | |
| Other | 19 (<0.1) | 12 (<0.1) | |
| Margin | |||
| Negative | 79 426 (96.6) | 35 988 (96.5) | .37 |
| Positive | 2487 (3.0) | 1144 (3.1) | |
| Not available | 285 (0.3) | 148 (0.4) | |
| Vital status | |||
| Alive | 78 156 (95.1) | 34 505 (92.6) | <.001 |
| Dead | 4042 (4.9) | 2775 (7.4) | |
Abbreviations: CDS, Charlson-Deyo comorbidity score; LVSI, lymphovascular space invasion; PR, progesterone receptor; RS, 21-gene recurrence score.
Median education level was 10.9% of adults living in a zip code who did not graduate from high school.
Logistic MVA showed that, compared with high household income, low household income was associated with higher RS (adjusted odds ratio [aOR] 1.11, 95% CI, 1.06-1.16; P < .001) (Table 2). A total of 4042 (4.9%) and 2775 (7.4%) women with high-income and low-income levels died during the follow-up, respectively (Table 1). Cox MVA showed that low income was also associated with worse OS (adjusted hazards ratio [aHR], 1.18; 95% CI, 1.11-1.25; P < .001) (Table 3). Interaction term analysis showed a statistically significant interaction between income levels and RS (interaction P < .001).
Table 2. Multivariable Logistic Regression Analysis Results for High 21-Gene Recurrence Score.
| Characteristic | aOR (95% CI) | P value |
|---|---|---|
| Income | ||
| Above median | 1 [Reference] | [Reference] |
| Below median | 1.11 (1.06-1.16) | <.001 |
| Race | ||
| Non-Hispanic White | 1 [Reference] | [Reference] |
| Asian and Pacific Islander | 1.04 (0.94-1.14) | .45 |
| Black | 1.19 (1.12-1.27) | <.001 |
| Hispanic White | 0.92 (0.85-0.99) | .04 |
| Age, y | ||
| <50 | 1 [Reference] | [Reference] |
| ≥50 | 1.04 (0.99-1.10) | .15 |
| Facility | ||
| Nonacademic | 1 [Reference] | [Reference] |
| Academic | 0.96 (0.93-1.00) | .08 |
| Insurance | ||
| None | 1 [Reference] | [Reference] |
| Private | 1.08 (0.91-1.28) | .38 |
| Government | 1.03 (0.87-1.22) | .77 |
| Educationa | ||
| Above median | 1 [Reference] | [Reference] |
| Below median | 1.04 (0.99-1.08) | .12 |
| CDS | ||
| 0 | 1 [Reference] | [Reference] |
| 1 | 1.02 (0.96-1.07) | .56 |
| ≥2 | 1.09 (0.98-1.21) | .11 |
| Year | ||
| For every 1 y increase | 0.98 (0.97-0.99) | <.001 |
| Histology | ||
| Ductal or lobular carcinoma | 1 [Reference] | [Reference] |
| Other | 0.74 (0.70-0.78) | <.001 |
| PR | ||
| Positive | 1 [Reference] | [Reference] |
| Negative | 6.55 (6.25-6.87) | <.001 |
| T staging | ||
| 1 | 1 [Reference] | [Reference] |
| 2 | 1.31 (1.26-1.37) | <.001 |
| 3 | 0.8 (0.67-0.94) | .007 |
| N staging | ||
| 0 | 1 [Reference] | [Reference] |
| 1a | 0.8 (0.75-0.84) | <.001 |
| Grade | ||
| 1 | 1 [Reference] | [Reference] |
| 2 | 2.76 (2.60-2.94) | <.001 |
| 3 | 16.83 (15.79-17.94) | <.001 |
| Other | 18.91 (11.32-31.43) | <.001 |
| LVSI | ||
| No | 1 [Reference] | [Reference] |
| Yes | 1.23 (1.17-1.30) | <.001 |
Abbreviations: aOR, adjusted odds ratio; CDS, Charlson-Deyo comorbidity score; LVSI, lymphovascular space invasion; PR, progesterone receptor.
Median education level was 10.9% of adults living in a zip code who did not graduate from high school.
Table 3. Multivariable Cox Regression Analysis Results for Overall Survival.
| Characteristics | aHR (95% CI) | P value |
|---|---|---|
| Income | ||
| Above median | 1 [Reference] | [Reference] |
| Below median | 1.18 (1.11-1.25) | <.001 |
| Race | ||
| Non-Hispanic White | 1 [Reference] | [Reference] |
| Hispanic White | 0.8 (0.72-0.90) | <.001 |
| Black | 1.08 (1.00-1.18) | .05 |
| Asian and Pacific Islander | 0.66 (0.56-0.79) | <.001 |
| Age, y | ||
| <50 | 1 [Reference] | [Reference] |
| ≥50 | 1.96 (1.77-2.17) | <.001 |
| Facility | ||
| Nonacademic | 1 [Reference] | [Reference] |
| Academic | 0.79 (0.75-0.84) | <.001 |
| Insurance | ||
| None | 1 [Reference] | [Reference] |
| Private | 0.64 (0.51-0.81) | <.001 |
| Government | 1.49 (1.19-1.87) | <.001 |
| Educationa | ||
| Above median | 1 [Reference] | [Reference] |
| Below median | 1.06 (1.00-1.12) | .046 |
| CDS | ||
| 0 | 1 [Reference] | [Reference] |
| 1 | 1.58 (1.49-1.68) | <.001 |
| ≥2 | 2.69 (2.47-2.94) | <.001 |
| Year | ||
| For every 1 y increase | 1.01 (1.00-1.03) | .07 |
| Histology | ||
| Ductal or lobular carcinoma | 1 [Reference] | [Reference] |
| Other | 0.98 (0.91-1.05) | .53 |
| PR | ||
| Positive | 1 [Reference] | [Reference] |
| Negative | 1.1 (1.02-1.18) | .01 |
| RS | ||
| 0-25 | 1 [Reference] | [Reference] |
| >25 | 1.91 (1.77-2.05) | <.001 |
| T staging | ||
| 1 | 1 [Reference] | [Reference] |
| 2 | 1.52 (1.45-1.61) | <.001 |
| 3 | 2.34 (1.99-2.75) | <.001 |
| N staging | ||
| 0 | 1 [Reference] | [Reference] |
| 1a | 1.61 (1.51-1.71) | <.001 |
| Grade | ||
| 1 | 1 [Reference] | [Reference] |
| 2 | 1.13 (1.07-1.21) | <.001 |
| 3 | 1.49 (1.37-1.61) | <.001 |
| Other | 0.86 (0.36-2.07) | .74 |
| LVSI | ||
| No | 1 [Reference] | [Reference] |
| Yes | 1.14 (1.07-1.23) | <.001 |
| Chemotherapy | ||
| No | 1 [Reference] | [Reference] |
| Yes | 0.71 (0.66-0.76) | <.001 |
| Radiation | ||
| No | 1 [Reference] | [Reference] |
| Yes | 0.59 (0.55-0.64) | <.001 |
| Surgery | ||
| Lumpectomy | 1 [Reference] | [Reference] |
| Mastectomy | 0.71 (0.65-0.77) | <.001 |
| Other | 1.46 (0.55-3.90) | .45 |
| Margin | ||
| Negative | 1 [Reference] | [Reference] |
| Positive | 1.19 (1.05-1.36) | .007 |
Abbreviations: aHR, adjusted hazards ratio; CDS, Charlson-Deyo comorbidity score; LVSI, lymphovascular space invasion; PR, progesterone receptor; RS, 21-gene recurrence score.
Median education level was 10.9% of adults living in a zip code who did not graduate from high school.
On subgroup analysis, similar findings were noted among those with RS below 26, while there were no significant OS differences between income levels among others with RS 26 or higher (Figure 1). This finding was again observed among 20 898 and 3782 matched pairs for RS below 26 (HR, 1.36; 95% CI, 1.25-1.48; P < .001) and 26 or higher (HR, 1.11; 95% CI, 0.96-1.28; P = .16) (Figure 2; eTable 2 in Supplement 1).
Figure 1. Forest Plot of Cox Multivariable Analysis for the Association of Survival and Income Levels Stratified by 21-Gene Recurrence Score.
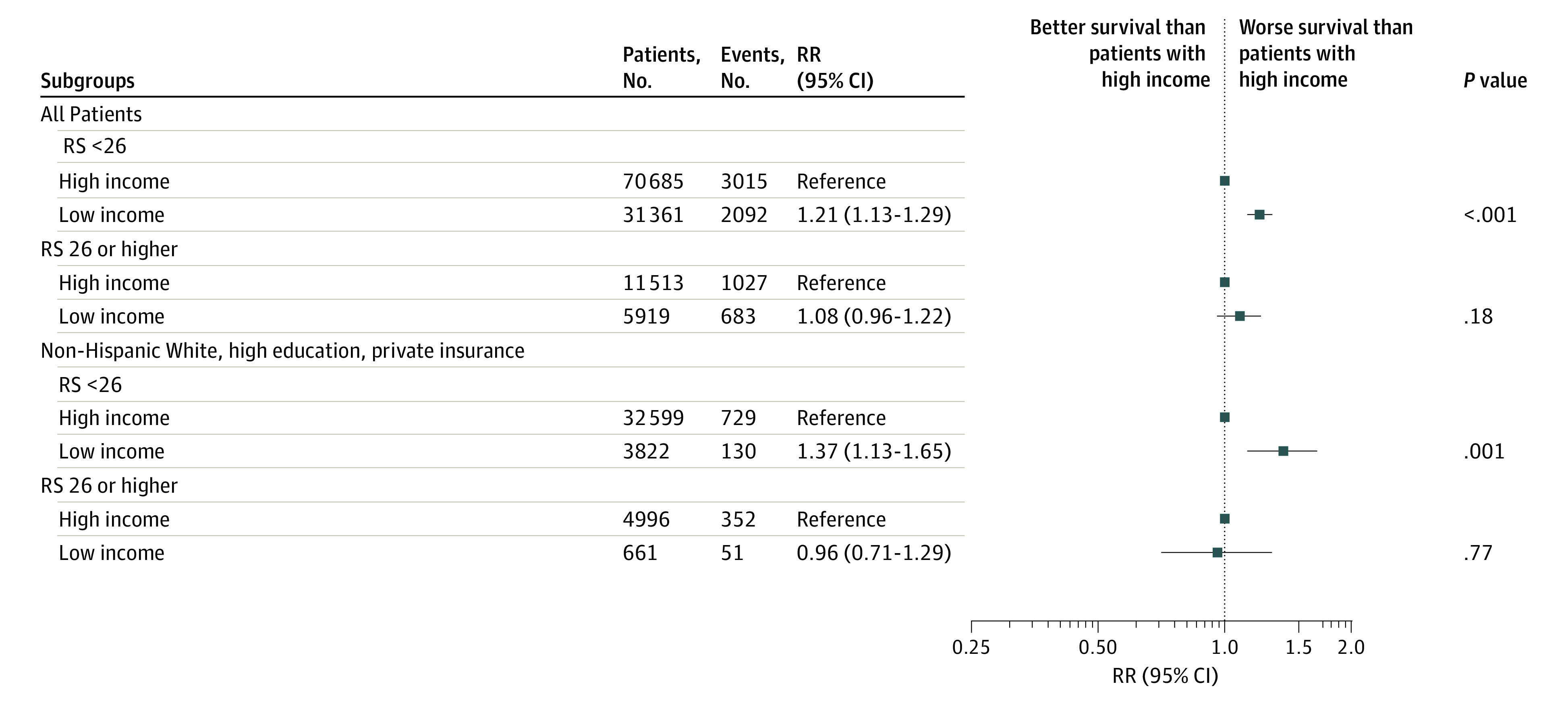
RR indicates risk ratio; RS, 21-gene recurrence score.
Figure 2. Cumulative Hazard of Overall Mortality Between High vs Low Income Stratified by 21-Gene Recurrence Score.
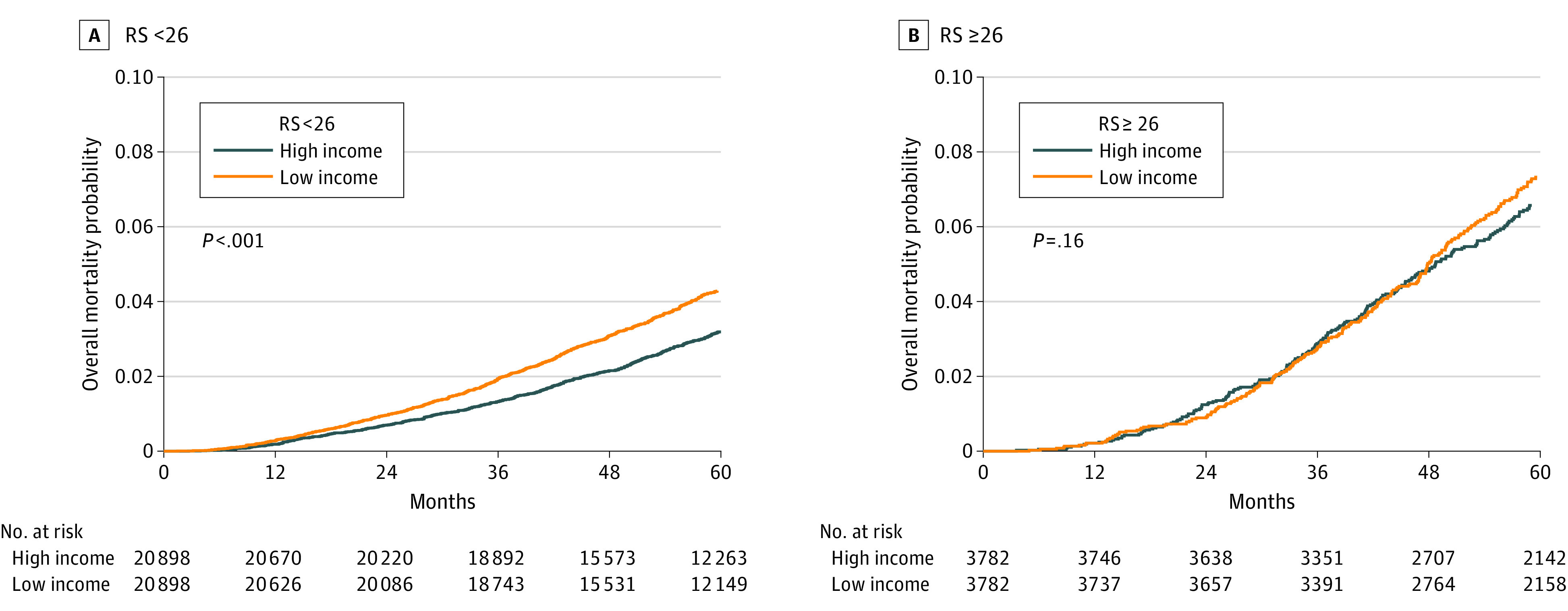
RS indicates 21-gene recurrence score.
On sensitivity analysis among non-Hispanic White women with high education and private medical insurance, logistic MVA similarly showed that, compared with high income, low income was associated with higher RS (aOR, 1.16; 95% CI, 1.05-1.28; P = .003). Cox MVA showed low income was associated with worse OS compared with high income (aHR, 1.24; 95% CI, 1.06-1.46; P = .007). The interaction term was statistically significant (interaction P = .03), with similar findings on subgroup analysis (Figure 1).
Discussion
To our knowledge, this study using a nationwide oncology database has been the largest to suggest that low household income is associated with higher RS and worse survival outcome among those with RS below 26. Breast cancer stage and therapy are highly correlated with education level, race, and health insurance in the US.2 This prompted our sensitivity analysis that only included non-Hispanic White women with high education and private medical insurance. Our findings remained consistent in this subgroup.
Our main finding on the association between low income and more aggressive tumor biology is consistent with other population-based studies suggesting a higher incidence of triple-negative breast cancer among patients with low SES.6,7,8 Similar findings were also noted among Hispanic women as well.16 However, these studies utilized census tract–level SES and the Yost index, instead of education and income levels separately. In our study, income levels, but not education, were associated with RS. A lack of association between education levels and breast cancer risk in our study was consistent with several population-based17 and prospective cohort18 studies.
Rationales for the association between income levels and intrinsic tumor properties as indicated by RS in our study remain unclear. Low income levels may lead to less cancer screening with consequent delay in diagnosis and worsening of tumor biology.3 Moreover, financial distress has been shown to result in increased psychological and emotional distress, poor quality of life, and depression.19 Such distress may lead to dysregulation in stress pathways,1,20 reduced tumor suppressor p53 function,21 and more aggressive tumor biology with distant metastasis.22 High RS also includes genes responsible for tumor proliferation and invasion.23 The limited efficacy of chemotherapy with high RS11,24 may explain the lack of significant association between income levels and survival outcomes among patients with RS of 26 or above in our study. Further studies are warranted to investigate the pathways connecting financial distress and pathways for tumorigenesis.
Limitations
This study had several limitations. Because of the retrospective nature of our study, some clinically relevant variables, such as performance status, tumor recurrence, breast cancer-specific survival, systemic therapy agents, and adherence to screening and treatments, were unavailable for analysis, which may have resulted in residual confounding despite matching and sensitivity analysis. In particular, most patients with unknown RS and/or income levels also had unknown education levels (eTable 1 in Supplement 1). Although education levels were not associated with high RS in our study (Table 2), the exclusion of such patients may have led to additional selection bias. Given the lack of patient-level income data, our findings may not be generalizable to individual patients from different socioeconomic backgrounds within the same zip code. In addition, given a small proportion of patients with high RS as shown in population-based studies,25,26 subgroup analysis in our study may be inadequately powered to detect OS differences among patients with high RS.
Conclusions
In our observational cohort study, low household income was independently associated with higher RS and worse survival outcomes among those with RS below 26, but not RS 26 or higher. Further studies are warranted to investigate the mechanism behind the association between socioeconomic determinants of health and intrinsic tumor biology among patients with breast cancer.
eTable 1. Baseline Patient, Tumor, and Treatment Characteristics Between Those With Unknown Versus Known 21-gene Recurrence Score and/or Income Levels
eTable 2. Baseline Patient, Tumor, and Treatment Characteristics After Matching
Data Sharing Statement
References
- 1.Williams DR, Mohammed SA, Shields AE. Understanding and effectively addressing breast cancer in African American women: Unpacking the social context. Cancer. 2016;122(14):2138-2149. doi: 10.1002/cncr.29935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496. doi: 10.1093/jnci/94.7.490 [DOI] [PubMed] [Google Scholar]
- 3.Silber JH, Rosenbaum PR, Ross RN, et al. Disparities in breast cancer survival by socioeconomic status despite Medicare and Medicaid insurance. Milbank Q. 2018;96(4):706-754. doi: 10.1111/1468-0009.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336-2347. doi: 10.1056/NEJMoa2108873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721-1728. doi: 10.1002/cncr.22618 [DOI] [PubMed] [Google Scholar]
- 7.Qin B, Babel RA, Plascak JJ, et al. Neighborhood social environmental factors and breast cancer subtypes among Black women. Cancer Epidemiol Biomarkers Prev. 2021;30(2):344-350. doi: 10.1158/1055-9965.EPI-20-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauscher GH, Campbell RT, Wiley EL, Hoskins K, Stolley MR, Warnecke RB. Mediation of racial and ethnic disparities in estrogen/progesterone receptor-negative breast cancer by socioeconomic position and reproductive factors. Am J Epidemiol. 2016;183(10):884-893. doi: 10.1093/aje/kwv226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallin K, Browner A, Palis B, et al. Incident cases captured in the National Cancer Database compared with those in U.S. population based central cancer registries in 2012-2014. Ann Surg Oncol. 2019;26(6):1604-1612. doi: 10.1245/s10434-019-07213-1 [DOI] [PubMed] [Google Scholar]
- 11.Ma SJ, Oladeru OT, Singh AK. Association of adjuvant chemotherapy with overall survival in patients with early-stage breast cancer and 21-gene recurrence scores of 26 or higher. JAMA Netw Open. 2020;3(5):e203876. doi: 10.1001/jamanetworkopen.2020.3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma SJ, Serra LM, Yu B, et al. Evaluation of risk stratification using gene expression assays in patients with breast cancer receiving neoadjuvant chemotherapy. Breast Cancer Res Treat. 2021;189(3):737-745. doi: 10.1007/s10549-021-06269-6 [DOI] [PubMed] [Google Scholar]
- 13.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530-536. doi: 10.1038/415530a [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haukoos JS, Lewis RJ. The Propensity Score. JAMA. 2015;314(15):1637-1638. doi: 10.1001/jama.2015.13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banegas MP, Tao L, Altekruse S, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat. 2014;144(3):625-634. doi: 10.1007/s10549-014-2882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conroy SM, Shariff-Marco S, Koo J, et al. Racial/ethnic differences in the impact of neighborhood social and built environment on breast cancer risk: the Neighborhoods and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2017;26(4):541-552. doi: 10.1158/1055-9965.EPI-16-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer JR, Boggs DA, Wise LA, Adams-Campbell LL, Rosenberg L. Individual and neighborhood socioeconomic status in relation to breast cancer incidence in African-American women. Am J Epidemiol. 2012;176(12):1141-1146. doi: 10.1093/aje/kws211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6(9):166. doi: 10.21037/atm.2018.03.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res. 2011;13(2):208. doi: 10.1186/bcr2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Z, Liu L, Zhang C, et al. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2012;109(18):7013-7018. doi: 10.1073/pnas.1203930109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang A, Le CP, Walker AK, et al. β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav Immun. 2016;57:106-115. doi: 10.1016/j.bbi.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 24.Ma SJ, Oladeru OT, Farrugia M, et al. Survival outcome of adjuvant chemotherapy and high 21-gene recurrence score in early-stage breast cancer. Breast J. 2021;27(1):27-34. doi: 10.1111/tbj.14130 [DOI] [PubMed] [Google Scholar]
- 25.Cheng R, Kong X, Wang X, Fang Y, Wang J. Oncotype DX breast recurrence score distribution and chemotherapy benefit among women of different age groups with HR-positive, HER2-negative, node-negative breast cancer in the SEER database. Front Oncol. 2020;10:1583. doi: 10.3389/fonc.2020.01583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, He ZY, Dong Y, Sun JY, Zhang WW, Wu SG. The distribution and outcomes of the 21-gene recurrence score in T1-T2N0 estrogen receptor-positive breast cancer with different histologic subtypes. Front Genet. 2018;9:638. doi: 10.3389/fgene.2018.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Patient, Tumor, and Treatment Characteristics Between Those With Unknown Versus Known 21-gene Recurrence Score and/or Income Levels
eTable 2. Baseline Patient, Tumor, and Treatment Characteristics After Matching
Data Sharing Statement


