Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) of EFSA was asked to deliver a scientific opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Bacillus subtilis CGMCC 13326 as a nutritional feed additive for all animal species. The additive is produced by a genetically modified production strain. Although the production strain harbours some genes coding for resistance to antimicrobials, viable cells and DNA of the production strain were not detected in the final product. Therefore, the use of B. subtilis CGMCC 13326 to produce vitamin B2 does not raise safety concerns. The use of riboflavin 80% produced by B. subtilis CGMCC 13326 in animal nutrition does not represent a safety concern for the target species, consumers and for the environment. In the absence of data, the FEEDAP Panel cannot conclude on the potential skin and eye irritation or potential toxicity by inhalation of the additive under assessment. Riboflavin is a known photosensitiser which may elicit skin and eye photoallergic reactions. The additive under assessment is effective in covering the animals' requirements of vitamin B2 when administered via feed.
Keywords: nutritional additive, vitamin B2, riboflavin, safety, Bacillus subtilis, genetically modified organism, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Kempex Holland BV 2 for authorisation of the product Vitamin B2 (riboflavin) produced by Bacillus subtilis CGMCC 13326, when used as a feed additive for all animal species (category: Nutritional additive; functional group: vitamins, pro‐vitamins and chemically well‐defined substances having a similar effect). During the assessment the applicant withdrew the request for use of the additive in water for drinking.
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 18 January 2021.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product Vitamin B2 (riboflavin) produced by a genetically modified Bacillus subtilis (CGMCC 13326), when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
The product Vitamin B2 produced by B. subtilis CGMCC 13326 has not been authorised in the EU.
The FEEDAP Panel has adopted several opinions on the use of vitamin B2 as a feed additive. Two opinions related to the safety and efficacy of vitamin B2 (80%) as riboflavin produced by B. subtilis KCCM‐10445 for all animal species (EFSA FEEDAP Panel, 2014, 2018a); an opinion on the safety and efficacy of vitamin B2 as riboflavin and riboflavin‐5′‐phosphate ester monosodium salt, produced by either B. subtilis DSM 17339 or B. subtilis DSM 23984 (EFSA FEEDAP Panel, 2016); another opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii (EFSA FEEDAP Panel, 2018b); another related to the safety and efficacy of vitamin B2 (riboflavin 5′‐phosphate ester monosodium salt) for all animal species when used in water for drinking (EFSA FEEDAP Panel, 2018c); and the last one related to the safety and efficacy of vitamin B2 produced by Eremothecium ashbyi CCTCCM 2019833 (EFSA FEEDAP Panel, 2021).
The ANS Panel issued an opinion on the re‐evaluation for riboflavin (E101(i)) and riboflavin‐5′‐phosphate (E 101(ii)) as part of the food additives re‐evaluation programme specified under Regulation (EU) No 257/20104 (EFSA ANS Panel, 2013).
Riboflavin is included in the European Pharmacopeia (PhEur), Monograph (MG) 10.0/0292 (PhEur, 2020).
2. Data and Methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 3 in support of the authorisation request for the use of Vitamin B2 (riboflavin) produced by a genetically modified strain of B. subtilis (CGMCC 13326) as a nutritional feed additive. 4 The dossier was received on 4/8/2020 and the general information and supporting documentation is available at https://open.efsa.europa.eu/questions/EFSA‐Q‐2020‐00637.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the active substance in animal feed. 5
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of the product containing Vitamin B2 produced using Bacillus subtilis CGMCC 13326 is in line with the principles laid down in Regulation (EC) No 429/2008 6 and the relevant guidance documents: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018d), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018e) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The assessment deals with the safety and efficacy of the product Vitamin B2 produced by B. subtilis CGMCC 13326 as a nutritional additive (functional group: vitamins, pro‐vitamins and chemically well‐defined substances having similar effect) in feed for all animal species.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The additive is produced by a genetically modified strain of B. subtilis which has been deposited ■■■■■ with the number CGMCC 13326. 7
A bioinformatic analysis of the whole genome sequence (WGS) of the production strain confirmed its identity as B. subtilis. 8 ■■■■■
■■■■■
The susceptibility of the production strain to the antibiotics recommended by the FEEDAP Guidance (EFSA FEEDAP Panel, 2018d) was tested by a broth microdilution method. 9 Three of the antibiotics tested showed a minimum inhibitory concentration (MIC) value above the FEEDAP cut‐off values, ■■■■■ Therefore, the production strain B. subtilis CGMCC 13326 is considered to be resistant to those antibiotics.
The WGS data of the production strain, including the two plasmid sequences, was interrogated for the presence of antimicrobial resistance (AMR) genes ■■■■■
In conclusion, the production strain harbours AMR genes coding for resistance to several antibiotics.
The WGS of the production strain was interrogated for the presence of virulence factors ■■■■■ The toxigenic potential of the production strain was assessed according to the Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018d). 10 ■■■■■ B. subtilis CGMCC 13326 is considered to be not toxigenic.
3.1.1.1. Information related to the genetically modified microorganism
Characterisation of the parental or recipient microorganism
■■■■■
Description of the genetic modification introduced in the parental strain
■■■■■: 11
■■■■■
■■■■■
■■■■■
■■■■■ 12
■■■■■
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
■■■■■. 15
3.1.3. Characterisation of the active substance
The product vitamin B2 produced by B. subtilis CGMCC 13326 contains riboflavin (International Union of Pure and Applied Chemistry (IUPAC) name: 7,8‐dimethyl‐10‐[(2S,3S,4R)‐2,3,4,5,‐tetrahydroxypentyl]benzo[g]pteridine‐2,4(3H,10H)‐dione, synonyms: vitamin B2, 7,8,‐dimethyl‐10‐(1’‐d‐ribityl)isoalloxazine; lactoflavin, 1‐deoxy‐1‐(7,8,dimethyl‐2,4‐dioxo‐3,4‐dihydrobenzo[g]pteridin‐10(2H)‐yl)‐d‐ribitol), which is identified by the CAS (Chemical Abstracts Service) number 83‐88‐5 and the EINECS (European Inventory of Existing Chemical Substances) number 201‐507‐1. The molecular formula of riboflavin is C17H20N4O6 and its molecular weight is 376.37 g/mol. The structural formula of riboflavin is shown in Figure 1.
Figure 1.
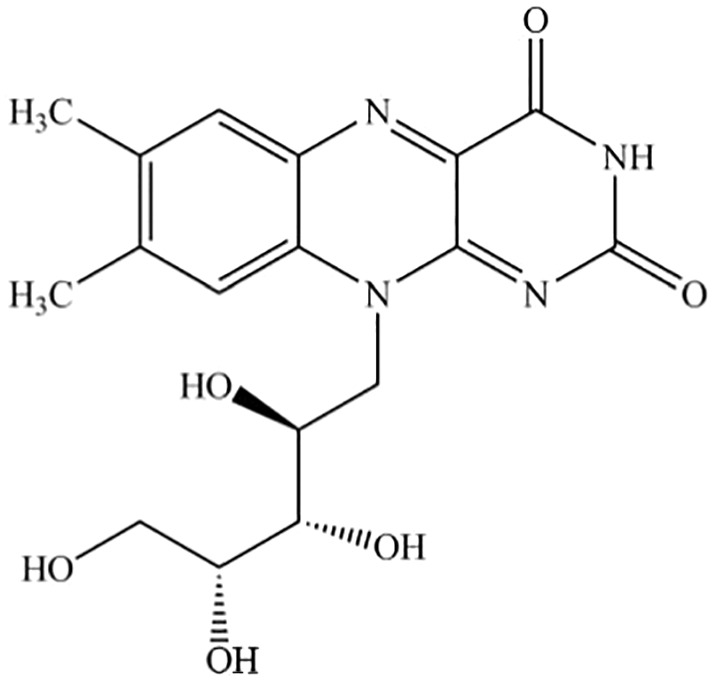
Structural formula of riboflavin
The active substance analysed in five batches resulted in a content of riboflavin of 100% on a dry matter (DM) basis in all the batches. 16 The presence of Bacillus cereus was tested in 3 batches of the active substance (25 g samples) and could not be detected. 17
3.1.4. Characterisation of the additive
The active substance is mixed with maize starch as a carrier. The specifications are for a product (formulated additive) containing ≥ 80% vitamin B2 in the form of riboflavin on a dry matter (DM) basis and ≤ 3% moisture and an unspecified amount of maize starch as a carrier.
Analytical data on batch‐to‐batch variation (five batches analysed) showed an average of 83.6% (range 82.7%–84.0%) vitamin B2 on an ‘as is’ basis, and an average 1.5% moisture (range 1.2%–1.8%). 18
3.1.4.1. Impurities
Three batches of the additive were analysed for impurities. Cadmium concentrations were below the limit of detection (LOD), 19 lead ranged 0.50–0.52 mg/kg, mercury ranged 0.094–0.098 mg/kg and arsenic ranged 0.050–0.055 mg/kg. Mycotoxins (aflatoxins, ochratoxin A, zearalenone, fumonisines [B1, B2, B3], deoxynivalenol and citrinin) were below the respective LOD except for ochratoxin A which ranged 11.1–12.2 μg/kg; and citrinin, that ranged 92–103 μg/kg. 20
Dioxins (polychlorinated dibenzofurans (PCDFs), polychlorinated dibenzo(p)dioxins (PCDDs)) and dioxin‐like polychlorinated biphenyls (DL‐PCBs) were measured in three batches of the final product and were below the corresponding limits of quantification (LOQs). 21 The levels of PCDD/F and the sum of PCDD/F and DL‐PCB (upper limit) were calculated to be 0.14 and 0.26 ng WHO‐TEQ/kg, respectively (in all three batches).
Lumiflavin (7,8,10‐trimethylbenzo[g]pteridine‐2,4(3H,10H)‐dione) is a toxic yellow photoderivative of riboflavin, produced by ultraviolet irradiation of riboflavin in alkaline solution. Impurities lumiflavin, 7,8‐dimethylbenzo[g]pteridine‐2,4(1H,3H)‐dione, 6.7‐dimethyl‐8‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]‐pteridine‐2,4(3H,8H)‐dione and 8‐(hydroxymethyl)‐7‐methyl‐10‐[(2S,3S,4R)‐2,3,4,5‐tetrahydroxypentyl]benzo[g] pteridine‐2,4(3H,8H)‐dione were analysed in three batches of the additive and showed compliance with the limits established in the PhEur (2020) monograph 10.0/0292. 22
The microbiological contamination of the final product was also evaluated. Enterobacteriaceae, Escherichia coli, yeasts and filamentous fungi were not detected in 1 g sample. Salmonella spp. was not detected in 25 g sample. 23
The presence of viable vegetative cells or spores of the production strain in the final product was tested in three batches of the riboflavin 98% fermentation product and three batches of the additive, respectively. ■■■■■ Therefore, it can be concluded that neither viable vegetative cells nor spores of the production strain were detected in the final product. 24 , 25
The presence of DNA from the production strain was tested in three batches of the final product and in triplicate ■■■■■. 26 ■■■■■. No DNA of the production strain was detected.
3.1.4.2. Physicochemical properties of the additive
The additive is an orange‐brown to yellow‐brown free‐flowing powder, having a bulk density of 500 kg/m3. 27 The additive is very slightly soluble in water (120 mg/L). 28
The dusting potential (Stauber–Heubach method) measured in three batches ranged 23–30 g/m3. 29 The particle size distribution of the additive (measured in three batches via laser diffraction analysis) showed that the percentage of particles < 10, < 50 and < 100 μm diameter ranged 32%–33%, 56%–58% and 68%–70%, respectively. 30
It is noted that the data available do not allow to exclude the presence of small/nano particles as foreseen in the Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles (EFSA SC, 2021). Therefore, the applicant was requested to provide information choosing any of the appraisal routes as indicated by the aforementioned guidance document. The applicant claimed that the safety of the additive can be adequately covered by the conventional risk assessment. 31
Based on the available information and considering the low supplemental level (up to 18 mg/kg feed, EFSA FEEDAP Panel, 2014) in commercial feed, it may be expected that potential riboflavin nanoparticles present in the additive would be at least partly dissolved in gastrointestinal tract. Any uptake of remaining nanoparticles from the gut would lead to dissolution of riboflavin in other body fluids and entry into known pathways of riboflavin metabolism, degradation, and elimination. Therefore, the Panel concluded that a conventional risk assessment will be sufficient in this case.
3.1.5. Stability and homogeneity
The shelf life of the additive (three batches) was tested when stored in sealed bags (protected from light) at room temperature for 12 months or at 40°C for 6 months. A loss of 1%–2% vitamin B2 was observed when stored at room temperature and no losses were observed when stored at 40°C. 32
The stability of the additive (three batches) was tested in a vitamin and mineral premixture for calves (without choline chloride) when supplemented at 0.5% and stored in sealed bags protected from light at room temperature for 6 months. 33 Only one batch showed a loss of 6%.
The stability of the additive (three batches) was tested in mash and pelleted compound feed for weaned pigs. 34 The basal diet consisted of barley, wheat and soybean meal, contained a background content of 2.5 mg vitamin B2/kg, and was supplemented with 20 mg additive/kg feed (corresponding to 16.7 mg vitamin B2/kg feed). Pelleting was performed at 70°C. After cooling, samples were stored at room temperature in sealed bags protected from light for 3 months. At the end of the storage period, the losses in mash feed ranged from 0 to 17%. As regards pelleted feed, only one batch showed a loss of 22% at the end of the storage period. The pelleting process caused no loss of vitamin B2.
The stability of the additive in water was tested at a concentration of 20 g/L when stored at room temperature (packaging not described) for 48 h. Losses ranged from 1% to 2% depending on the batch considered. 35
The pelleted compound feed described above was used to study the capacity of the additive to distribute homogeneously in feed. Ten subsamples were analysed for total vitamin B2 and the background vitamin B2 of the basal feed was subtracted, resulting in a coefficient of variation of 7%. 36
3.1.6. Conditions of use
The additive is aimed for all animal species and categories without any time limit or withdrawal period. It may be added directly in compound feed or complementary feed, or via premixtures. 4 No inclusion levels are proposed as they will depend on the dietary requirements of the different species.
3.2. Safety
3.2.1. Safety of the production organism
The strain B. subtilis CGMCC 13326 belongs to a species for which the qualified presumption of safety (QPS) approach to safety assessment applies (EFSA, 2007; EFSA BIOHAZ Panel, 2020). This approach requires the identity of the strain to be unequivocally established and evidence provided that the strain lacks toxigenic potential and does not show acquired resistance to antibiotics of human and veterinary importance and for genetically modified strains the safety of the genetic modification needs to be established.
The FEEDAP Panel notes that the identity of the production strain has been unambiguously established. Evidence was provided on the lack of toxigenic potential of the strain. However, the production strain is resistant to ■■■■■ and harbours several antimicrobial resistance genes. Nevertheless, viable cells (including vegetative cells and spores) and recombinant DNA of this production strain were not detected in the final product. Therefore, the use of B. subtilis CGMCC 13326 to produce vitamin B2 does not raise safety concerns.
3.2.2. Safety for the target species, consumers, and the environment
Safety concerns from the additive may derive either from riboflavin or from the residues of the fermentation process/production strain remaining in the final product. The active substance is produced by a genetically modified microorganism for which the recipient strain is considered by EFSA to qualify for the QPS approach to safety assessment and for which the genetic modification raises no toxicological concerns. The genetic modification introduced ■■■■■ resistance gene; however, this is not expected to have an impact on the toxicological profile of the production strain.
The nutrient requirements/recommendations of the target species for vitamin B2, the background levels of vitamin B2 in feed materials and the tolerance to overdoses of vitamin B2 were reviewed by the FEEDAP Panel in previous opinions (EFSA FEEDAP Panel, 2014, 2016, 2018b). The Panel concluded that the use levels based on the requirement/background levels would pose no safety concerns to the target species. The Panel is not aware of any more recent findings which would modify the above conclusion. The active substance used to formulate the additive is of high purity. The inclusion rate of riboflavin would usually not exceed 18 mg/kg complete feed. Moreover, the production strain is considered safe from the toxicological point of view. Therefore, it can be concluded that no safety concerns for the target animal would rise from the fermentation residues that may be present in the final additive and the product used to formulate it (i.e. maize starch). The FEEDAP Panel concludes that vitamin B2 produced by B. subtilis CGMCC 13326 is considered safe for the target species when used in feed to satisfy the nutritional requirements of the different target species.
The safety of riboflavin and consumer exposure to riboflavin were reviewed in previous opinions (EFSA NDA Panel, 2013; EFSA FEEDAP Panel, 2014, 2016). The FEEDAP Panel concluded that the supplementation of feed with riboflavin would not be of concern for the consumers. The Panel is not aware of any more recent findings which would modify the above conclusion.
The active substance riboflavin occurs in nature. Its use in animal nutrition is not expected to substantially increase the concentration in the environment. Considering that viable cells and recombinant DNA of the production strain B. subtilis CGMCC 13326 were not detected in the final product, a risk for the environment resulting from the use of the additive under assessment in animal nutrition is not foreseen.
The FEEDAP Panel concludes that the use of vitamin B2 produced by B. subtilis CGMCC 13326 is safe for the target species, for the consumer and for the environment.
3.2.3. Safety for the user
No data were provided on the effects of the additive on the respiratory system. The dusting potential (up to 30 g/m3) and the particle size distribution of the product (up to 70% of particles of < 100 μm diameter, see Section 3.1.4.2) indicate a risk of exposure by inhalation for people handling the additive.
No data were provided on the potential skin and eye irritation or dermal sensitisation of the additive under assessment. Riboflavin is a known photosensitiser which may elicit skin and eye photoallergic reactions.
In the absence of data, the FEEDAP Panel cannot conclude on the potential skin and eye irritation or potential toxicity by inhalation of the additive under assessment. Riboflavin is a known photosensitiser which may elicit skin and eye photoallergic reactions.
3.3. Efficacy
Data on requirement, allowances and recommendations for feed supplementation are easily accessible in the standard literature on animal nutrition. Dietary requirements are set for domestic animals except for ruminants, owing to microbial synthesis of riboflavin in the rumen (GfE, 1995, 2001, 2003; NRC, 1996, 2007, 2021).
Riboflavin (vitamin B2) has been used world‐wide in animal nutrition for decades. Owing to the long history of use and its established nutritional role in domestic animals, riboflavin when administered orally is regarded as effective in covering the animal's requirement for vitamin B2.
The FEEDAP Panel considers that vitamin B2 produced by B. subtilis CGMCC 13326 is an effective source in covering the animals' requirements when administered via feed.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation 37 and Good Manufacturing Practice.
4. Conclusions
The production strain harbours some genes for resistance to antimicrobials. However, viable cells and DNA of the production strain were not detected in the final product. Therefore, the use of B. subtilis CGMCC 13326 to produce vitamin B2 does not raise safety concerns.
The use of riboflavin 80% produced by B. subtilis CGMCC 13326 in animal nutrition does not represent a safety concern for the target species, consumers and for the environment.
In the absence of data, the FEEDAP Panel cannot conclude on the potential skin and eye irritation or potential toxicity by inhalation of the additive under assessment. Riboflavin is a known photosensitiser which may elicit skin and eye photoallergic reactions.
The additive under assessment is effective in covering the animals' requirements when administered via feed.
Abbreviations
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- AWT
Arbeitsgemeinschaft fűr Wirkstoffe in der Tierernährung e.V.
- CFU
colony forming unit
- CG
chemical group
- CV
coefficient of variation
- DL‐PCB
dioxin‐like polychlorinated biphenyl
- DM
dry matter
- EURL
European Union Reference Laboratory
- EINECS
European Inventory of Existing Chemical Substances
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- GfE
Gesellschaft fűr Ernährungsphysiologie
- HACCP
hazard analysis and critical control points
- IUPAC
International Union of Pure and Applied Chemistry
- LOD
limit of detection
- LOQ
limit of quantification
- MIC
minimum inhibitory concentration
- NRC
National Research Council
- OECD
Organisation for Economic Co‐operation and Development
- PCDD
polychlorinated dibenzo(p)dioxin
- PCDF
polychlorinated dibenzofuran
- PhEur
European Pharmacopoeia
- TEQ
toxic equivalents
- WHO
World Health Organization
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Anguita M, Brozzi R, Galobart J, Holzcknecht O, Pettenati E, Vettori MV and Tarrés‐Call J, 2023. Scientific Opinion on the safety and efficacy of a feed additive consisting of vitamin B2 (riboflavin) produced by Bacillus subtilis CGMCC 13326 for all animal species (Kempex Holland B.V.). EFSA Journal 2023;21(2):7874, 13 pp. 10.2903/j.efsa.2023.7874
Requestor European Commission
Question number EFSA‐Q‐2020‐00637
Panel members Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice Relevant information or parts of this scientific output have been blackened in accordance with the European Commission decision on the confidentiality requests formulated by the applicant and further confidentiality requests formulated by the applicant for which a decision by the European Commission is pending. The blackened text will be subject to review once the full decision on the confidentiality requests is adopted by the European Commission. The full output was shared with the European Commission, EU Member States and the applicant.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Matteo Innocenti, Martina Reitano, the WG on Microbiology and the Scientific Committee cross‐cutting WG on Nanotechnologies.>
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 1 February 2023
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Kempex Holland BV, Zeelandsedijk 15, 5408SL Volkel, The Netherlands.
FEED dossier reference: FAD‐2020‐0061.
Technical dossier/Supplementary information April 2022/FAD‐2020‐0061 Sin 050321 Add 290,421 Answers/reply to question 7.
The full report is available on the EURL website: https://joint‐research‐centre.ec.europa.eu/publications_en.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex 2.2.1.2a CONFID.
Technical dossier/Section II/Annex 2.2.1.2c COMFID.
Technical dossier/Section II/Annex 2.2.2.2.
Technical dossier/Section II/Annex 2.2.1.2f.
Technical dossier/Section II/Annex 2.2.1.2b.
Technical dossier/Supplementary information April 2022/FAD‐2020‐0061 Sin 210422/Annex 3 CONFID.
Technical dossier/Supplementary information April 2022/FAD‐2020‐0061 Sin 050321 Add 290,421 Answers.
Technical dossier/Section II/Annex 2.3.1a_CONFID.
Technical dossier/Section II/Annex 2.3.1b.
Technical dossier/Supplementary information April 2022/Annex 2. Method of analysis for riboflavin stated to be VDLUFA method III 13.9.1.
Technical dossier/Supplementary information April 2022/Annes 2.
Technical dossier/Section II/Annexes 2.1.3a and b. Analytical method for Vitamin B2: DIN EN 14152 (mod) and VDLUFA III 13.9.1.
Technical dossier/Section II/ Annex 2.1.3a. LOD in mg/kg for cadmium was 0.002.
Technical dossier/Section II/Annex 2.1.3a. LOD in μg/kg was 0.1 for aflatoxin, 17 for zearalenone, 25 for fumonisines (B1 to B3) and 134 for deoxynivalenol.
Technical dossier/Section II/Annexes 2.1.4.a1 to a3.
Technical dossier/supplementary information April 2022/Annex 1b.
Technical dossier/Section II/Annexes 2.1.3a and b; supplementary information April 2022/reply to question 5.
Technical dossier/Section II/Annex 2.1.4b CONFID.
Technical dossier/Supplementary information 210,422/Annex 4 b1 CONFID and Annexes 4c to 4 e.
Technical dossier/Section II/Annex 2.1.4c CONFID.
Technical dossier/Section II/Annex 2.5.2.
Technical dossier/Supplementary information April 2022/FAD‐2020‐0061_SIn_050321 Add 290,421 Answers
Technical dossier/Section II/Annexes 2.1.5.b1 to b3.
Technical dossier/Section II/Annexes 2.1.5a1 to a3.
Technical dossier/Supplementary information October 2022/ADME riboflavin
Technical dossier/Section II/Annex 2.1.3a.
Technical dossier/Section II/Annexes 2.4.1a and b.
Technical dossier/Section II/Annex 2.4.1b and c.
Technical dossier/Section II/Annex 2.1.3a; and supplementary information April 2022/Reply to question 6.
Technical dossier/Section II/Annex 2.4.1b.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- EFSA , 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(12):587, 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additive and Nutrient Sources added to food) , 2013. Scientific Opinion on the re‐evaluation for Riboflavin (E101(i)) and Riboflavin‐50‐phosphate (E 101(ii)). EFSA Journal 2013;11(10):3357, 49 pp. 10.2903/j.efsa.2013.3357 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernández Escámez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S, and Herman L 2020. Scientific Opinion on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA Journal 2020;18(2):5966, 56 pp. 10.2903/j.efsa.2020.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2014. Scientific Opinion on the safety and efficacy of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis for all animal species, based on a dossier submitted by VITAC EEIG. EFSA Journal 2014;12(1):3531, 2 pp. 10.2903/j.efsa.2014.3531 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016. Scientific Opinion on Safety and efficacy of vitamin B2 (riboflavin and riboflavin 5′‐phosphate ester monosodium salt) produced by Bacillus subtilis (DSM 17339 and DSM 23984) for all animal species based on a dossier submitted by DSM. EFSA Journal 2016;14(1):4349, 26 pp. 10.2903/j.efsa.2016.4349 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G , Aquilina, G , Azimonti, G , Bampidis, V , Bastos, ML , Bories, G , Chesson, A , Flachowsky, G , Gropp, J , Kolar, B , Kouba, M , López‐Alonso, M , López Puente, S , Mantovani, A , Mayo, B , Ramos, F , Saarela, M , Villa, RE , Wallace, RJ , Wester, P , Herman, L , Glandorf, B , Kärenlampi, S , Aguilera, J and Cocconcelli, PS , 2018a. Scientific Opinion on the safety of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM‐10445 for all animal species. EFSA Journal 2018;16(3):5223, 8 pp. 10.2903/j.efsa.2018.5223 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa L, Dierick N, Glandorf B, Herman L, Kärenlampi S, Leng L, Tebbe C, Aguilera J, Manini P, Tarres‐Call J and Wallace RJ, 2018b. Scientific Opinion on the safety and efficacy of vitamin B2 (riboflavin) produced by Ashbya gossypii for all animal species based on a dossier submitted by BASF SE. EFSA Journal 2018;16(7):5337, 19 pp. 10.2903/j.efsa.2018.5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V , Azimonti, G , Bastos, ML , Christensen, H , Dusemund, B , Kouba, M , Kos Durjava, M , López‐Alonso, M , López Puente, S , Marcon, F , Mayo, B , Pechová, A , Petkova, M , Ramos, F , Sanz, Y , Villa, RE , Woutersen, R , Costa, L , Dierick, N , Flachowsky, G , Mantovani, A , Wallace, RJ , Manini, P and Tarres‐Call, J , 2018c. Safety and efficacy of vitamin B2 (riboflavin 5′‐phosphate ester monosodium salt) for all animal species when used in water for drinking. EFSA Journal 2018;16(12):5531, 15 pp. 10.2903/j.efsa.2018.5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018d. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018e. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V , Azimonti, G , Bastos, ML , Christensen, H , Dusemund, B , Durjava, MF , Kouba, M , López‐Alonso, M , López Puente, S , Marcon, F , Mayo, B , Pechová, A , Petkova, M , Ramos, F , Sanz, Y , Villa, RE , Woutersen, R , Brantom, PG , Cocconcelli, PS , Glandorf, B , Herman, L , Maradona, MP , Saarela, M , Svensson, K , Tosti, L , Galobart, J , Manini, P , Pettenati, E , Pizzo, F , Tarrés‐Call, J and Anguita, M , 2021. Scientific Opinion on the safety and efficacy of the feed additive consisting of Vitamin B2/Riboflavin produced by Eremothecium ashbyi CCTCCM 2019833 for all animal species (Hubei Guangji Pharmaceutical Co., Ltd). EFSA Journal 2021;19(3):6462, 14 pp. 10.2903/j.efsa.2021.6462 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2013. Scientific Opinion on the substantiation of a health claim related to riboflavin (vitamin B2) and contribution to normal energy‐yielding metabolism pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal 2013; 11(10):3410, 10 pp. 10.2903/j.efsa.2013.3410 [DOI] [Google Scholar]
- EFSA Scientific Committee , More, S , Bampidis, V , Benford, D , Bragard, C , Halldorsson, T , Hernández‐Jerez, A , Bennekou, SH , Koutsoumanis, K , Lambré, C , Machera, K , Naegeli, H , Nielsen, S , Schlatter, J , Schrenk, D , Silano V, Turck, D , Younes, M , Castenmiller, J , Chaudhry, Q , Cubadda, F , Franz, R , Gott, D , Mast, J , Mortensen, A , Oomen, AG , Weigel, S , Barthelemy, E , Rincon, A , Tarazona, J and Schoonjans, R , 2021. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 2021;19(8):6769, 48 pp. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie) , 1995. Recommendations for the Supply of Energy and Nutrients to Beef Cattle. DLG‐Verlag, Frankfurt am Main, 85 pp. [in German]. [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie) , 2001. Recommendations for the Supply of Energy and Nutrients to Dairy Cows and Heifers. DLG‐Verlag, Frankfurt am Main, 136 pp. [in German]. [Google Scholar]
- GfE (Gesellschaft für Ernährungsphysiologie) , 2003. Recommendations for the Supply of Energy and Nutrients to Goats. DLG‐Verlag, Frankfurt am Main, 121 pp. [Google Scholar]
- Hosoya Y, Okamoto S, Muramatsu H and Ochi K, 1998. Acquisition of certain streptomycin‐resistant (str) mutations enhances antibiotic production in bacteria. Antimicrobial Agents and Chemotherapy, 42, 2041–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Academies of Sciences, Engineering, and Medicine) , 2021. Nutrient Requirements of Dairy Cattle. 8th Revised Edition. The National Academies Press, Washington, DC. 10.17226/25806 [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) , 1996. Nutrient Requirements of Beef Cattle. 7th Revised Edition. The National Academies Press, Washington DC, USA. 232 pp. [Google Scholar]
- NRC (National Research Council) , 2007. Nutrient Requirements of Small Ruminants. Sheep, Goats, Cervids, and New World Camelids. The National Academies Press, Washington DC, USA. 362 pp. [Google Scholar]
- PhEur (European Pharmacopeia) , 2020. Riboflavin, Monograph (MG) 0292. 10th Edition. Council of Europe (COE)—European Directorate for the Quality of Medicines, Strasbourg, France. [Google Scholar]


