Abstract
Introduction
Ischemic stroke incidence appears to have decreased during the last decades, but most studies focus on the first-ever events and epidemiological data on recurrent stroke are scarce. The aim of our study was to investigate trends in incidence, risk factors, and medication in patients with first-ever and recurrent ischemic stroke between 2010 and 2019 in Sweden.
Methods
We included patients (≥18 years old) with ischemic stroke registered in the hospital-based Swedish Stroke Register (Riksstroke) 2010–2019. The coverage of Riksstroke was consistently high (about 90%) during this period. Data were stratified by first-ever and recurrent ischemic stroke in three different time periods (2010–2012, 2013–2016, and 2017–2019) and shown as crude and age-specific incidence rates per 100,000 person-years. Statistics Sweden provided census data on the Swedish population in different age groups.
Results
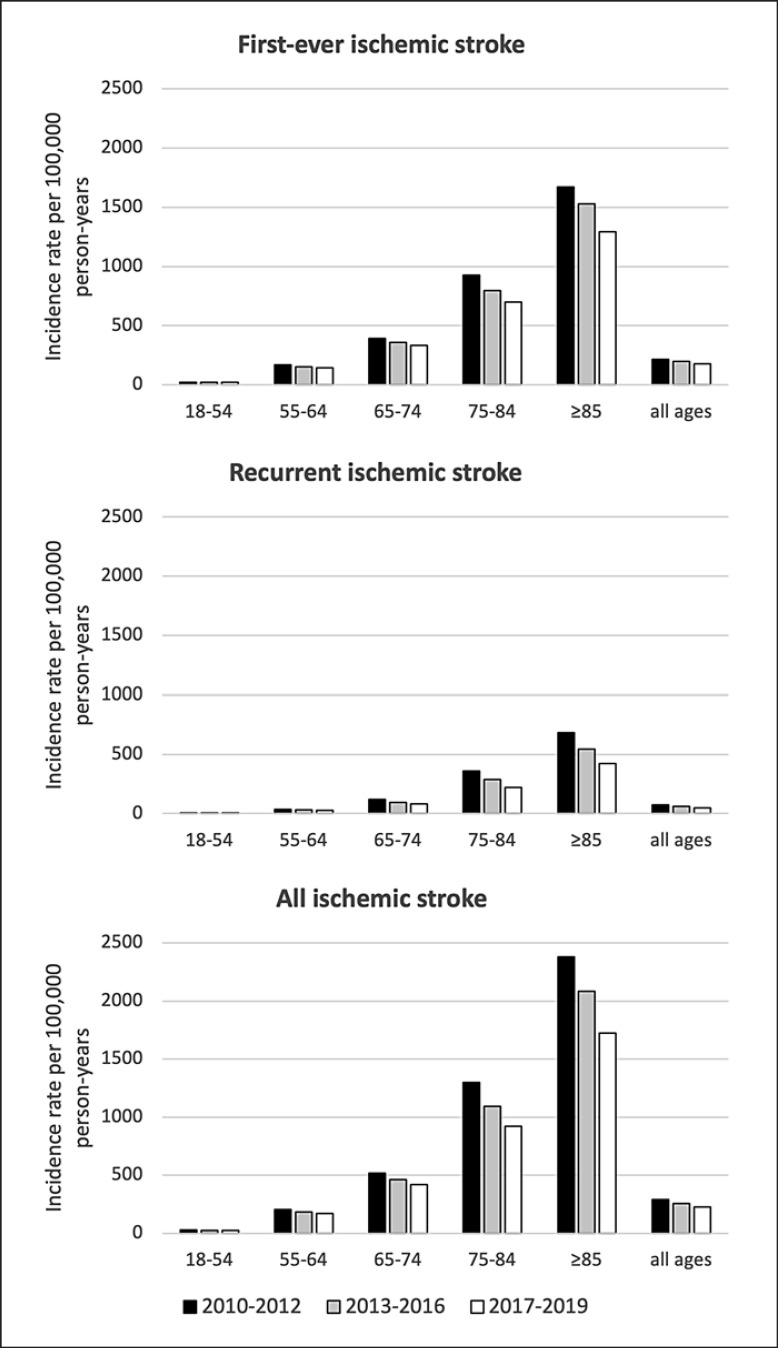
During the study period, 201,316 cases of ischemic stroke were registered in Riksstroke, including 153,865 (76.4%) cases of first-ever ischemic stroke and 46,248 (23.0%) cases of recurrent ischemic stroke (0.6% of cases unclassified). The crude incidence of first-ever ischemic stroke decreased by 17% from 216 (95% CI 214–218) to 179 (95% CI 177–181) between 2010–2012 and 2017–2019, whereas recurrent ischemic stroke decreased by 33% from 72 (95% CI 71–73) to 48 (95% CI 47–49). Between these time periods, diminishing ischemic stroke incidence was seen in all age groups with highest decline noted in those aged 75–84 years (928 [95% CI 914–943] to 698 [95% CI 686–709]; −25% in first-ever ischemic stroke and 361 [95% CI 351–370] to 219 [95% CI 213–226]; −39% in recurrent ischemic stroke) and ≥85 years (1,674 [95% CI 1,645–1,703] to 1,295 [95% CI 1,270–1,320]; −23% in first-ever ischemic stroke and 683 [95% CI 664–702] to 423 [95% CI 409–437]; −38% in recurrent ischemic stroke). Treatment with anticoagulants in patients with atrial fibrillation and lipid-lowering drugs increased considerably in patients with first-ever and recurrent ischemic stroke both at admission and discharge during the study period.
Conclusion
Whereas both first-ever and recurrent ischemic stroke rates declined in Sweden between 2010 and 2019, the proportional decline was almost double for recurrent ischemic stroke than for first-ever ischemic stroke and most pronounced in the elderly. Increased use of secondary preventive drugs, in particular anticoagulants in atrial fibrillation, appears to have contributed, but further studies on precise causes for the decline in recurrent ischemic stroke are needed.
Keywords: Epidemiology, Ischemic stroke, Recurrent stroke, Incidence, Sweden, Stroke registry
Introduction
Stroke remains the second leading cause of death and the third leading cause of death and disability combined worldwide [1]. Ischemic stroke is the most common subtype and accounts for about 65% of all stroke cases globally and up to 87% of all stroke cases in high-income countries [2, 3, 4]. Age-standardized stroke incidence and poststroke mortality have decreased in the last decades with more favorable trends in high-income countries [5, 6, 7, 8, 9]. Advances in primary and secondary prevention including better control of cardiovascular risk factors have been reported as likely reasons for the decline in stroke incidence [10, 11]. However, there is still a considerable gap between risk factor control according to guidelines and real-world stroke prevention [12].
Despite these trends, the burden of stroke is expected to increase because of global population growth and demographic changes with increasing life expectancy [8, 13, 14, 15, 16]. According to previous reports, the individual risk of stroke recurrence appears to have either been unchanged or slightly diminished in the last decades [17, 18, 19, 20]. However, epidemiological studies on ischemic stroke incidence usually focus on first-ever events, and epidemiological data on recurrent stroke are scarce, especially on a national level in high-income countries and low- and middle-income countries. The aim of this register-based, nationwide study was to analyze changes in ischemic stroke rates in Sweden over the last decade with focus on first-ever and recurrent ischemic stroke events including analyses based on age, risk factors, and anti-thrombotic medication.
Methods
Study Population
Data were obtained from the Swedish Stroke Register − Riksstroke, the Swedish quality register for stroke care. Riksstroke is a hospital-based stroke register, and all 72 Swedish acute care hospitals contribute to collecting and entering data. Between 2010 and 2019, the estimated coverage rate of Riksstroke was stable at about 90% of admitted acute stroke cases in Sweden without obvious differences in coverage between age groups or sex [2, 21]. Data on demography, history, risk factors, and medical treatment at admission and discharge of in-hospital care were collected and entered into Riksstroke by licensed health care workers. Patients aged ≥18 years, hospitalized between January 1, 2010, and December 31, 2019, with the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis of ischemic stroke (I63) were included.
Patients' Characteristics
Data on patient characteristics include sex, age, previous stroke, previous transient ischemic attack (TIA)/amaurosis fugax, cardiovascular risk factors (hypertension, diabetes mellitus, smoking, and atrial fibrillation [AF]), prestroke functional status, stroke severity, acute treatment, and medication at admission and discharge. The presence of hypertension was defined as treatment with blood pressure-lowering drugs at admission. AF was reported as present or absent and was not further specified as permanent or paroxysmal. Prestroke independency in activities of daily living (ADL) was defined as living in own home without home care service and being independent in dressing, toileting, and indoor mobility. Stroke severity was determined by the patients' level of consciousness at admission, using the Reaction Level Scale RLS-85 with categories of alert (RLS 1), drowsy (RLS 2–3), and comatose (RLS 4–8) [22]. Data on the National Institute of Health Stroke Score were missing in about 50% of patients and therefore not used in the analysis. Data on acute treatment include stroke unit care, iv thrombolysis, and thrombectomy. Data on medication include blood pressure-lowering drugs, antiplatelet drugs in patients without AF, anticoagulants in patients with AF, and lipid-lowering drugs. The analysis of medication at discharge excludes patients who died in hospital.
Patient characteristics data were stratified by first-ever, recurrent, and all ischemic stroke and in three different time periods (2010–2012, 2013–2016, and 2017–2019). Time periods including several years were chosen to reduce the effect of annual fluctuations and for comprehensive presentation. First-ever stroke was defined as a stroke in a patient with no record of a previous stroke at any time based on patient interview or available medical chart information. Recurrent stroke was defined as a stroke in a patient with a record of a previous stroke at any time. The Riksstroke data item “previous stroke” does not specify if it was ischemic or hemorrhagic nor the date of the previous event. Patients with both first-ever and recurrent ischemic stroke or more than one recurrent ischemic stroke under the study period were included with every ischemic stroke event recorded separately. Missing data were under 2% except for smoking (overall 10% missing) and the ADL parameters dressing, toileting, and mobility (overall between 2 and 3% missing).
Statistical Methods
Categorical variables were presented as frequencies with percentage and continuous data as medians. To analyze differences between groups, χ2 test was used for categorical variables. Incidence and relative incidence with associated confidence intervals were calculated through a Poisson regression model with number of stroke cases registered in Riksstroke as outcome, stratified for type of stroke, age, sex (in online suppl. Table 1a, b; for all online suppl. material, see www.karger.com/doi/10.1159/000527373), adjusted for time period, with logarithm of the size of the Swedish population ≥18 years as of December 31 each year (from Statistics Sweden) [23] as offset variable. For comparison of temporal trends, incidence rates were standardized to the European Standard Population as of 2013 [24]. Statistical analyses were performed using SPSS version 27.
Results
Characteristics of Patients with Ischemic Stroke
Between 2010 and 2019, 233,440 stroke cases were registered in Riksstroke. Of these, 201,316 (86.2%) cases were ischemic stroke and 29,263 (12.5%) cases were hemorrhagic strokes (1.2% of cases unclassified). Of all ischemic stroke cases, 153,865 (76.4%) were first-ever ischemic stroke and 46,248 (23.0%) were recurrent ischemic stroke (0.6% of cases unclassified). Over the studied time period, 184,162 individual patients had these 201,316 ischemic stroke events. Of these, 169,172 (91.9%) patients had a single ischemic stroke event and 14,990 (8.1%) patients had two or more ischemic stroke events (32,144 ischemic stroke events in total). Table 1 shows patient characteristics stratified by first-ever, recurrent, and all ischemic stroke in three different time periods (2010–2012, 2013–2016, and 2017–2019).
Table 1.
Patient characteristics including premorbid risk factors, stroke severity, acute treatment, premorbid status, and medication at admission and at discharge
| First-ever ischemic stroke |
Recurrent ischemic stroke |
All ischemic stroke |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010–2012 | 2013–2016 | 2017–2019 | 2010–2012 | 2013–2016 | 2017–2019 | 2010–2012 | 2013–2016 | 2017–2019 | ||
| Cases per time period, n | 48,969 | 61,508 | 43,388 | 16,248 | 18,316 | 11,684 | 65,753 | 80,348 | 55,215 | |
| Cases per year, n | 16,323 | 15,377 | 14,463 | 5,416 | 4,579 | 3,895 | 21,917 | 20,087 | 18,405 | |
| % of all ischemic stroke cases | 74.5 | 76.6 | 78.6 | 24.7 | 22.8 | 21.2 | − | − | − | |
| 18–54 years, % | 6.8 | 7.2 | 7.3 | 2.9 | 2.7 | 3.2 | 5.8 | 6.2 | 6.5 | |
| 55–64 years, % | 12.3 | 11.5 | 11.5 | 7.3 | 7.2 | 7.8 | 11.0 | 10.5 | 10.7 | |
| 65–74 years, % | 23.6 | 25.5 | 25.7 | 21.4 | 22.7 | 23.2 | 23.1 | 24.9 | 25.2 | |
| 75–84 years, % | 31.4 | 30.3 | 32.0 | 36.6 | 36.7 | 37.4 | 32.7 | 31.7 | 33.2 | |
| >85 years, % | 25.9 | 25.6 | 23.4 | 31.8 | 30.6 | 28.4 | 27.4 | 26.7 | 24.5 | |
| Nonmodifiable risk factors | ||||||||||
| Male sex, % | 50.8 | 51.5 | 52.9 | 52.2 | 54.8 | 56.5 | 51.1 | 52.3 | 53.6 | |
| Age, median (IQR), years | 77 (67–85) | 76 (68–85) | 76 (68–84) | 80 (72–86) | 80 (72–86) | 79 (72–85) | 78 (68–85) | 77 (69–85) | 77 (69–84) | |
| Age of men, median (IQR), years | 74 (65–82) | 73 (65–82) | 74 (65–81) | 78 (70–84) | 77 (70–84) | 77 (70–84) | 75 (66–83) | 75 (66–82) | 75 (66–82) | |
| Age of women, median (IQR), years 81 (71–87) | 80 (71–87) | 79 (71–86) | 83 (75–88) | 82 (75–88) | 81 (74–87) | 81 (72–87) | 81 (72–87) | 80 (71–87) | ||
| Modifiable risk factors, % | ||||||||||
| Hypertension | 57.7 | 59.7 | 60.8 | 72.6 | 74.7 | 76.7 | 61.3 | 62.9 | 64.1 | |
| Diabetes | 19.4 | 20.4 | 22.2 | 25.9 | 27.5 | 29.0 | 21.0 | 22.0 | 23.6 | |
| Smoking | 14.2 | 14.0 | 13.2 | 10.7 | 10.8 | 10.6 | 13.3 | 13.2 | 12.6 | |
| AF | 27.4 | 27.9 | 27.6 | 36.1 | 37.1 | 35.7 | 29.5 | 30.0 | 29.3 | |
| Previous TIA | 7.3 | 7.3 | 7.6 | 13.9 | 14.2 | 14.6 | 8.9 | 8.9 | 9.1 | |
| Number of RF >2 | 38.7 | 40.4 | 41.2 | 53.3 | 55.8 | 56.8 | 42.3 | 43.7 | 44.5 | |
| Premorbid status, % | ||||||||||
| Institutional Living | 6.8 | 7.2 | 6.5 | 15.9 | 15.6 | 13.4 | 9.1 | 9.1 | 8.0 | |
| ADL independency | 76.3 | 76.2 | 77.2 | 53.3 | 53.4 | 56.4 | 70.4 | 70.7 | 72.7 | |
| Stroke severity, % | ||||||||||
| Alert | 86.0 | 86.7 | 87.3 | 81.1 | 83.0 | 84.1 | 84.6 | 85.5 | 86.5 | |
| Drowsy | 10.1 | 9.3 | 8.9 | 13.0 | 11.6 | 11.1 | 10.8 | 9.8 | 9.3 | |
| Unconcious | 2.8 | 2.8 | 2.4 | 4.8 | 4.1 | 3.4 | 3.4 | 3.1 | 2.7 | |
| Acute treatment, % | ||||||||||
| SU direkt (including ICU) | 76.6 | 75.8 | 77.0 | 76.7 | 76.3 | 77.9 | 76.5 | 75.7 | 77.1 | |
| Iv trombolysis | 8.1 | 12.3 | 13.9 | 4.9 | 9.1 | 10.4 | 7.3 | 11.5 | 13.2 | |
| Trombectomy | 1.1 | 1.9 | 4.8 | 0.6 | 1.1 | 2.8 | 0.9 | 1.8 | 4.4 | |
| Medication at admission, % | ||||||||||
| BP-lowering drugs | 62.7 | 63.1 | 63.8 | 80.0 | 79.9 | 81.3 | 66.9 | 66.7 | 67.5 | |
| Antiplatelet drugs | 31.6 | 28.7 | 26.1 | 76.6 | 75.3 | 74.6 | 41.7 | 38.1 | 35.5 | |
| Anticoagulants | 18.4 | 27.7 | 39.6 | 25.6 | 38.6 | 53.8 | 20.5 | 30.8 | 43.3 | |
| Lipid-lowering drugs | 22.6 | 23.6 | 26.1 | 45.1 | 49.7 | 58.5 | 28.1 | 29.5 | 32.9 | |
| Medication at discharge, % | ||||||||||
| BP-lowering drugs | 76.3 | 77.1 | 77.3 | 83.5 | 82.7 | 83.3 | 77.9 | 78.1 | 78.5 | |
| Antiplatelet drugs | 89.8 | 89.6 | 90.5 | 88.9 | 88.2 | 87.8 | 89.4 | 88.9 | 89.9 | |
| Anticoagulants | 46.8 | 67.1 | 78.1 | 37.2 | 62.0 | 75.4 | 43.9 | 65.6 | 77.4 | |
| Lipid-lowering drugs | 64.1 | 71.8 | 79.8 | 61.8 | 69.8 | 79.0 | 63.4 | 71.1 | 79.6 | |
IQR, interquartile range; RF, risk factors; ADL, activities of daily living; SU, stroke unit; ICU, intensive care unit; Iv, intravenous; AF, atrial fibrillation; BP, blood pressure. Antiplatelet drugs in patients without AF, anticoagulants in patients with AF; deceased people excluded in medication at discharge.
On average, patients with recurrent ischemic stroke were older (median age 80 years, interquartile range [IQR] 72–86 years) and more likely to be male (54.3%) than patients with first-ever ischemic stroke (median age 77 years [IQR 67–85 years]; 51.7% male). Between time periods 2010–2012 and 2017–2019, median age decreased from 80 to 79 years in recurrent ischemic stroke and from 77 to 76 years in first-ever ischemic stroke. Proportions of male cases increased between these time periods with a higher increase in recurrent ischemic stroke (52.2% to 56.5% in recurrent ischemic stroke and 50.8% to 52.9% in first-ever ischemic stroke).
In all 3 time periods, patients with recurrent ischemic stroke had higher proportions of most risk factors compared with first-ever ischemic stroke (hypertension 74.5% vs. 59.4% [p < 0.001]; diabetes 27.3% vs. 20.6% [p < 0.001]; AF 36.4% vs. 27.6% [p < 0.001]; and previous TIA 14.2% vs. 7.4% [p < 0.001]). Only smoking was more common in first-ever ischemic stroke patients (13.9% vs. 10.7%; p < 0.001). Further, the proportion of patients with two or more risk factors was higher in recurrent ischemic stroke compared to first-ever ischemic stroke (55.2% vs. 40.1%; p < 0.001). Trends in risk factors between 2010–2012 and 2017–2019 were similar for first-ever and recurrent ischemic stroke with increasing proportions of hypertension and diabetes, slightly increasing proportions of previous TIA, stable proportions of AF, and slightly decreasing proportions of smoking (Table 1).
Before the index stroke, patients with recurrent ischemic stroke were more likely to live in an institutional setting (15.1% vs. 6.9%; p < 0.001) and less likely to be ADL-independent (54.1% vs. 76.5%; p < 0.001) compared to patients with first-ever ischemic stroke. Direct admission to a stroke unit was slightly more frequent for recurrent compared with first-ever ischemic stroke (76.8% vs. 76.4%; p < 0.001). The proportion of reperfusion treatment was higher in first-ever compared to recurrent ischemic stroke (iv thrombolysis 11.4% vs. 8.0%; p < 0.001 and thrombectomy 2.5% vs. 1.3%; p < 0.001). Between 2010–2012 and 2017–2019, reperfusion treatment increased in both first-ever ischemic stroke (iv thrombolysis 8.1% to 13.9%; p < 0.001 and thrombectomy 1.1% to 4.8%; p < 0.001) and recurrent ischemic stroke (iv thrombolysis 4.9% to 10.4%; p < 0.001 and thrombectomy 0.6% to 2.8%; p < 0.001).
Ischemic Stroke Incidence
The Swedish population aged ≥18 years increased between 2010 and 2019 from about 7.5 million to 8.14 million with the highest growth rate in the age groups of 65–74 years (+17%) and 75–84 years (+28%) [23]. Between the time periods 2010–2012 and 2017–2019, the absolute number of ischemic stroke cases decreased by 10,538 (16%). The number of first-ever ischemic stroke cases decreased by 5,581 (11.4%), and the number of recurrent ischemic stroke cases decreased by 4,564 (28.1%). The proportion of recurrent ischemic stroke of all ischemic stroke decreased from 2010–2012 to 2017–2019 from 24.7% to 21.2% (p < 0.001).
The crude incidence rate of first-ever ischemic stroke per 100,000 person-years decreased between 2010–2012 and 2017–2019 by 17% from 216 (95% CI 214–218) to 179 (95% CI 177–181), and the crude incidence rate of recurrent ischemic stroke decreased by 33% from 72 (95% CI 71–73) to 48 (95% CI 47–49). The crude incidence rate of all ischemic stroke decreased by 21% from 290 (95% CI 288–292) to 228 (95% CI 226–230). The age-standardized (adjusted to the European Standard Population 2013) incidence rates decreased in line with the crude rates between 2010–2012 and 2017–2019 (181 [95% CI 178–184] to 146 [95% CI 143–148]; −19% in first-ever ischemic stroke and 59 [95% CI 58–61] to 39 [95% CI 37–40]; −34% in recurrent ischemic stroke) (Table 2). Sex-specific incidence rates showed a decrease in ischemic stroke incidence in women and men in both first-ever ischemic stroke (210 [95% CI 208–213] to 169 [95% CI 166–171]; −20% in women and 222 [95% CI 219–224] to 190 [95% CI 187–192]; −14% in men) and recurrent ischemic stroke (68 [95% CI 66–69] to 42 [95% CI 41–43]; −38% in women and 76 [95% CI 74–77] to 55 [95% CI 53–56]; −28% in men) (online suppl. Table 1).
Table 2.
Age structure of study population, crude age-specific incidence rate and age-standardized incidence rate (ASR, European Standard Population 2013) per 100,000 person-years and relative incidence (Rl) between 2010–2012 and 2017–2019 of first-ever, recurrent and all ischemic stroke
| Age group | 2010–2012 |
2013–2016 |
2017–2019 |
Rl (95% Cl) | |||
|---|---|---|---|---|---|---|---|
| (years) | n/n at risk per year | rate (95% Cl) | n/n at risk per year | rate (95% Cl) | n/n at risk per year | rate (95% Cl) | |
| First-ever ischemic stroke | |||||||
| 18–54 | 1,107/4,606,835 | 24 (23–25) | 1,106/4,729,522 | 23 (23–24) | 1,062/4,869,818 | 22 (21–23) | 0.91 (0.86–0.95) |
| 55–64 | 2,002/1,172,398 | 171 (166–175) | 1,766/1,142,970 | 154 (151–158) | 1,665/1,167,951 | 143 (139–147) | 0.83 (0.80–0.87) |
| 65–74 | 3,859/978,573 | 394 (387–402) | 3,921/1,085,965 | 361 (355–367) | 3,716/1,110,099 | 335 (329–341) | 0.85 (0.83–0.87) |
| 75–84 | 5,128/552,322 | 928 (914–943) | 4,652/583,867 | 797 (785–808) | 4,633/664,172 | 698 (686–709) | 0.75 (0.73–0.77) |
| ≥85 | 4,227/252,504 | 1,674 (1,645–1,703) | 3,932/257,462 | 1,527 (1,503–1,551) | 3,386/261,470 | 1,295 (1,270–1,320) | 0.77 (0.75–0.79) |
|
| |||||||
| Total (≥ 18) |
16,323/7,562,632 | 216 (214–218) | 15,377/7,799,786 | 197 (196–199) | 14,463/8,073,510 | 179 (177–181) | 0.83 (0.82–0.84) |
| ASR (≥0) | 181 (178–184) | 163 (160–165) | 146 (143–148) | 0.81 | |||
|
| |||||||
| Recurrent ischemic stroke | |||||||
| 18–54 | 155/4,606,835 | 3 (3–4) | 126/4,729,522 | 3 (2–3) | 124/4,869,818 | 3 (2–3) | 0.76 (0.66–0.87) |
| 55–64 | 393/1,172,398 | 34 (32–35) | 331/1,142,970 | 29 (27–31) | 305/1,167,951 | 26 (24–28) | 0.78 (0.71–0.85) |
| 65–74 | 1,161/978,573 | 119 (115–123) | 1,041/1,085,965 | 96 (93–99) | 904/1,110,099 | 81 (78–84) | 0.69 (0.65–0.72) |
| 75–84 | 1,991/552,322 | 361 (351–370) | 1,682/583,867 | 288 (281–295) | 1,456/664,172 | 219 (213–226) | 0.61 (0.58–0.63) |
| ≥85 | 1,724/252,504 | 683 (664–702) | 1,400/257,462 | 544 (530–558) | 1,106/261,470 | 423 (409–437) | 0.62 (0.59–0.65) |
|
| |||||||
|
Total (≥18) ASR (≥0) |
5,416/7,562,632 |
72 (71−73) 59 (58–61) |
4,579/7,799,786 |
59 (58–60) 48 (47–49) |
3,895/8,073,510 |
48 (47−49) 39 (37–40) |
0.67 (0.66−0.69) 0.66 |
|
| |||||||
| All ischemic stroke | |||||||
| 18–54 | 1,266/4,606,835 | 27 (27–28) | 1,247/4,729,522 | 26 (26–27) | 1,189/4,869,818 | 24 (24–25) | 0.89 (0.85–0.93) |
| 55–64 | 2,415/1,172,398 | 206 (201–211) | 2,113/1,142,970 | 185 (181–189) | 1,976/1,167,951 | 169 (165–173) | 0.82 (0.79–0.85) |
| 65–74 | 5,055/978,573 | 517 (508–525) | 4,994/1,085,965 | 460 (453–466) | 4,631/1,110,099 | 417 (410–424) | 0.81 (0.79–0.83) |
| 75–84 | 7,168/552,322 | 1,298 (1,280–1,315) | 6,370/583,867 | 1,091 (1,078–1,104) | 6,107/664,172 | 920 (906–933) | 0.71 (0.69–0.72) |
| ≥85 | 6,013/252,504 | 2,381 (2,347–2,416) | 5,364/257,462 | 2,084 (2,056–2,111) | 4,502/261,470 | 1,722 (1,693–1,751) | 0.72 (0.71–0.74) |
|
| |||||||
|
Total (≥18) ASR (≥0) |
21,917/7,562,632 |
290 (288−292) 242 (239–246) |
20,087/7,799,786 |
258 (256−259) 212 (209–215) |
18,405/8,073,510 |
228 (226−230) 184 (181–187) |
0.79 (0.78−0.80) 0.76 |
Decreasing trends for ischemic stroke incidence between 2010–2012 and 2017–2019 were seen in all age groups with highest decline in those aged 75–84 years (−29%; 1,298 [95% CI 1,280–1,315] to 920 [95% CI 906–933]) and ≥85 years (−28%; 2,381 [95% CI 2,347–2,416] to 1,722 [95% CI 1,693–1,751]). The higher proportional decline in recurrent ischemic stroke compared to first-ever ischemic stroke was seen in all age groups (Fig. 1).
Fig. 1.
Trends in incidence rates of first-ever, recurrent, and all ischemic stroke. Incidence rates are given per 100,000 person-years for different age bands.
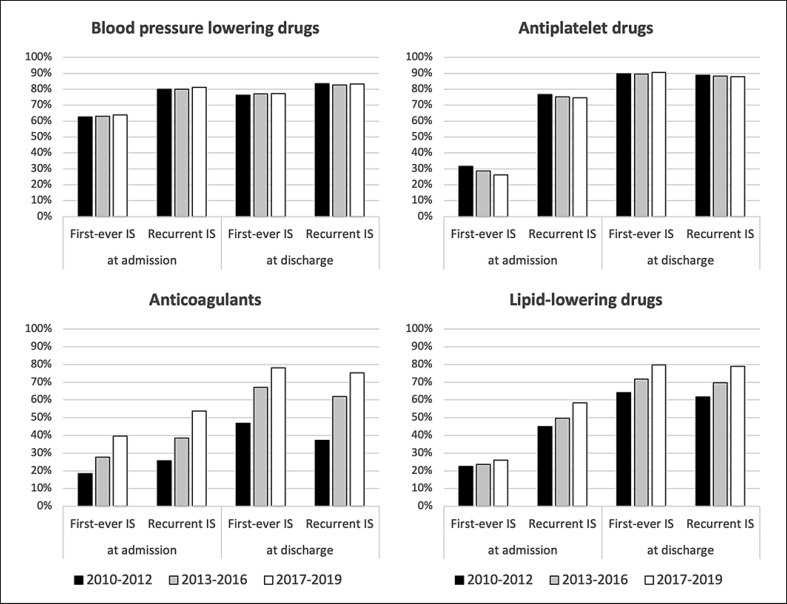
Medication at Admission and at Discharge
As shown in Table 1 and Figure 2, proportions of blood pressure-lowering and antiplatelet drugs were stable over the studied time period, both at admission and discharge. In contrast, increasing proportions were seen for anticoagulants and lipid-lowering drugs at admission and discharge between 2010–2012 and 2017–2019. In patients with first-ever ischemic stroke and AF, the proportion of anticoagulants at discharge increased by 31% (from 46.8% to 78.1%; p < 0.001) between 2010–2012 and 2017–2019. The highest proportional increases in both anticoagulants and lipid-lowering drugs at admission and discharge were seen in the older age groups ≥75 years. Medication at admission and discharge, stratified by age groups, is shown in online supplemental Table 2.
Fig. 2.
Trends in medication at admission and discharge stratified by first-ever and recurrent ischemic stroke (IS).
Discussion
Our study showed a decline in both first-ever and recurrent ischemic stroke incidence in Sweden between 2010–2012 and 2017–2019. The proportional decline was markedly higher in recurrent ischemic stroke (33%) compared to first-ever ischemic stroke (17%). The higher proportional decline of recurrent ischemic stroke was found in all age groups with the highest decline in age groups over 75 years. Risk factor burden was higher in recurrent ischemic stroke patients but with similar trends over time for both first-ever and recurrent schemic stroke. Across the study period, treatment with anticoagulants in patients with AF increased from 46.8% to 78.1% and treatment with lipid-lowering drugs increased from 64.1% to 79.8%. In contrast, treatment with blood pressure lowering and antiplatelet drugs did not change but remained consistently high.
Our results, showing a decline in first-ever ischemic stroke incidence, confirm findings in other recent studies from Scandinavian countries. A study based on nationwide data from the Danish Patient's Registry showed an increase in first-ever ischemic stroke incidence between 1996 and 2002 followed by a decrease between 2002 and 2016 [9]. Rosengren et al. analyzed trends in first-ever ischemic stroke incidence between 1987 and 2010 in Sweden. Based on data from the Swedish National Inpatient Register they reported a decline in incidence from the mid-1990s in patients between 45–64 and 65–84 years with a higher proportional decrease in the older age group [6]. However, they showed an increase in first-ever ischemic stroke in the young (18–44 years). In contrast, our more recent data encompassing 2010–2019 showed a decrease in age group 18–54 years, although smaller than in older age groups.
Studies on the epidemiology of recurrent ischemic stroke are scarce, especially on a national level, and most studies on recurrent ischemic stroke focus on the individual risk for ischemic stroke recurrence in a cohort followed longitudinally within a certain time period (usually 1 year). A population-based cross-sectional study from France (Dijon) recently showed an increased incidence in recurrent stroke between 1987–1994 and 2009–2015, while trends in first-ever stroke were stable [15]. In contrast, a recent systematic review of about 26 studies between 1997 and 2019 on the individual risk of ischemic stroke recurrence showed unchanged recurrence rates for stroke under this period [20]. A recent study from the United Kingdom encompassing about 6,000 patients with first-ever stroke showed a decline in the individual risk of stroke recurrence between the late 90s and the early 00′s but stable trends afterwards [19]. Pennlert et al. [17] showed a decline in risk of stroke recurrence in Sweden between the time periods 1995–1998 and 2004–2008. Modig et al. [25] analyzed the incidence of first and recurrent stroke in the older population (60–104 years) in Sweden between 1994 and 2014 using data from the Swedish National Patient Register and the Swedish Causes of Death Register. They reported a decline in both first-ever and recurrent stroke but differences in proportional decline between first-ever and recurrent stroke in Modig's study are difficult to compare with our data because of differences in definition and calculation of recurrent stroke incidence.
In our study, proportions of vascular risk factors (except for smoking) and the burden of vascular risk factors (two or more) were higher in recurrent than in first-ever schemic stroke. This result is in line with previous studies [26, 27] and may be related to a higher median age of recurrent ischemic stroke patients. Trends in risk factors between the time periods 2010–2012 and 2017–2019 were similar for first-ever and recurrent ischemic stroke and can neither explain the decline in overall ischemic stroke incidence nor the higher proportional decline in recurrent ischemic stroke.
The increase in anticoagulants is likely explained by the introduction of NOAC in 2011. The increased use of anticoagulants and lipid-lowering drugs, especially in older ischemic stroke patients, could reflect an improvement in primary and secondary prevention and might partly explain the higher proportional decline in ischemic stroke incidence in the elderly seen in our study. Increased prior-to-stroke treatment with anticoagulants and statins/lipid-lowering drugs could be seen in Denmark between 2005 and 2018 [28] and 1996–2016 [9] and the use of anticoagulants in patients with ischemic stroke and previous AF increased from 29.3% till 61.3% between 2006–2009 and 2014–2017 in Dijon, France [29].
The strength of our study is the nationwide design, the large number of included patients (over 200,000) and the consistently high coverage in Riksstroke with about 90% of all hospitalized stroke patients in Sweden, compared to the Swedish National Patient Register. A recent study showed that the completeness of case ascertainment of Riksstroke was 82% when compared to a population-based methodology, especially missing patients with high early case fatality rates, mild stroke severity, and those living in nursing care facilities [30]. However, the coverage rate and selection biases were probably stable during the study period which should allow conclusions about trends over time in ischemic stroke incidence.
A limitation of our study is that we have no information about the subtype (ischemic or hemorrhagic) or the date of the previous stroke events in cases of ischemic stroke recurrence. Early recurrent ischemic stroke events which occurred under the hospital stay or within 28 days after a stroke event are not registered in Riksstroke which could lead to an underestimation of early recurrent ischemic stroke events. The accuracy of the Riksstroke item on first-ever or recurrent stroke has not been specifically validated in the present data set. However, a validation study from 2003 showed a 90% agreement between the Riksstroke item and medical records [31]. Furthermore, data on compliance of secondary preventive drug therapies were not available.
In conclusion, we observed a marked decline in both first-ever and recurrent ischemic stroke rates in Sweden between 2010 and 2019 with an almost doubled proportional decrease in recurrent ischemic stroke by 33% compared to first-ever ischemic stroke by 17%. The higher proportional decrease in recurrent ischemic stroke was observed in all age groups and was most pronounced in the elderly. Increased use of secondary preventive drugs, in particular anticoagulants in AF, appears to have contributed, but further studies on precise causes for the decline in recurrent ischemic stroke are needed.
Statement of Ethics
The Swedish Ethical Review Authority approved the study and waived individual informed consent (#2019-06581).
Conflict of Interest Statement
Dr. Ullberg has received honoraria for an expert group assignment for AstraZeneca. Dr. Norrving has received honoraria for DSMB work for the THALES trial (AstraZeneca). The other authors have no conflicts of interest to disclose.
Funding Sources
This project is funded by the Swedish Government (under the “Avtal om Läkarutbildning och Medicinsk Forskning, ALF”), Elsa Schmitz' Stiftelse, Rut och Erik Hardebos donationsfond, and Sparbanksstiftelsen Färs och Frosta.
Author Contributions
All authors contributed to the study conception. Analysis of data was performed by Conrad Drescher and Mats Pihlsgård. Interpretation of data was performed by all authors. Supervision was performed by Fredrik Buchwald, Teresa Ullberg, Bo Norrving, and Jesper Petersson. An original draft was written by Conrad Drescher, and all authors revised previous versions of the manuscript and approved the final manuscript.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
We would like to thank the staff at Riksstroke, in particular statistician Fredrik Jonsson, for preparing the data from Riksstroke used in the study and all patients and staff reporting to the register.
Funding Statement
This project is funded by the Swedish Government (under the “Avtal om Läkarutbildning och Medicinsk Forskning, ALF”), Elsa Schmitz' Stiftelse, Rut och Erik Hardebos donationsfond, and Sparbanksstiftelsen Färs och Frosta.
References
- 1.Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20((10)):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riksstrokes årsrapport för 2019 års data Accessed April 15, 2022. Available from: https://www.riksstroke.org/wp-content/uploads/2020/09/Riksstroke_Årsrapport-2019_slutversionWEB-1.pdf.
- 3.Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54((2)):171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a Report From the American Heart Association. Circulation. 2021 Feb 23;143((8)):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8((4)):355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty-four-year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013 Sep;44((9)):2388–2393. doi: 10.1161/STROKEAHA.113.001170. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen M, Roth GA, Nichols E, Alam T, Abate D, GBD 2016 Stroke Collaborators Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18((5)):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Scott CA, Rothwell PM, Oxford Vascular S. Trends in stroke incidence in high-income countries in the 21st century: population-based study and systematic review. Stroke. 2020 May;51((5)):1372–1380. doi: 10.1161/STROKEAHA.119.028484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yafasova A, Fosbol EL, Christiansen MN, Vinding NE, Andersson C, Kruuse C, et al. Time trends in incidence, comorbidity, and mortality of ischemic stroke in Denmark (1996-2016) Neurology. 2020 Oct 27;95((17)):e2343–e2353. doi: 10.1212/WNL.0000000000010647. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363((9425)):1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 11.Vangen-Lonne AM, Wilsgaard T, Johnsen SH, Lochen ML, Njolstad I, Mathiesen EB. Declining incidence of ischemic stroke: what is the impact of changing risk factors? The tromso study 1995 to 2012. Stroke. 2017 Mar;48((3)):544–550. doi: 10.1161/STROKEAHA.116.014377. [DOI] [PubMed] [Google Scholar]
- 12.Boehme C, Toell T, Mayer-Suess L, Domig L, Pechlaner R, Willeit K, et al. The dimension of preventable stroke in a large representative patient cohort. Neurology. 2019 Dec 3;93((23)):e2121–e2132. doi: 10.1212/WNL.0000000000008573. [DOI] [PubMed] [Google Scholar]
- 13.Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006 Jun;13((6)):581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrom B, Jonsson AC, Nerbrand C, Norrving B, Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke. 2008 Jan;39((1)):10–15. doi: 10.1161/STROKEAHA.107.491779. [DOI] [PubMed] [Google Scholar]
- 15.Bejot Y, Bailly H, Graber M, Garnier L, Laville A, Dubourget L, et al. Impact of the ageing population on the burden of stroke: the Dijon Stroke Registry. Neuroepidemiology. 2019;52((1–2)):78–85. doi: 10.1159/000492820. [DOI] [PubMed] [Google Scholar]
- 16.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020 Aug;51((8)):2418–2427. doi: 10.1161/STROKEAHA.120.029606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennlert J, Eriksson M, Carlberg B, Wiklund PG. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke. 2014 Jun;45((6)):1839–1841. doi: 10.1161/STROKEAHA.114.005060. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom L, Irewall AL, Soderstrom L, Ogren J, Laurell K, Mooe T. One-year incidence, time trends, and predictors of recurrent ischemic stroke in Sweden from 1998 to 2010: an observational study. Stroke. 2017 Aug;48((8)):2046–2051. doi: 10.1161/STROKEAHA.117.016815. [DOI] [PubMed] [Google Scholar]
- 19.Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. 2020 Aug;51((8)):2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolmos M, Christoffersen L, Kruuse C. Recurrent ischemic stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2021 Aug;30((8)):105935. doi: 10.1016/j.jstrokecerebrovasdis.2021.105935. [DOI] [PubMed] [Google Scholar]
- 21.Soderholm A, Stegmayr B, Glader EL, Asplund K, Riksstroke C. Validation of hospital performance measures of acute stroke care quality. Riksstroke, the Swedish stroke register. Neuroepidemiology. 2016;46((4)):229–234. doi: 10.1159/000444679. [DOI] [PubMed] [Google Scholar]
- 22.Starmark JE, Stålhammar D, Holmgren E, Rosander B. A comparison of the glasgow coma Scale and the reaction level Scale (RLS85) J Neurosurg. 1988 Nov;69((5)):699–706. doi: 10.3171/jns.1988.69.5.0699. [DOI] [PubMed] [Google Scholar]
- 23.Statistics Sweden Folkmängden efter ålder och kön. År 1860-2020 [data base online] Accessed April 15, 2022. Available from: https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101A/BefolkningR1860N/
- 24.European Commission Revision of the European Standard Population: report of Eurostat's task force, 2013. Available from: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF.pdf/e713fa79-1add-44e8-b23d-5e8fa09b3f8f?t=1414782757000 Accessed April 15, 2022.
- 25.Modig K, Talback M, Ziegler L, Ahlbom A. Temporal trends in incidence, recurrence and prevalence of stroke in an era of ageing populations, a longitudinal study of the total Swedish Population. BMC Geriatr. 2019 Feb 4;19((1)):31. doi: 10.1186/s12877-019-1050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerrgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Stroke recurrence: predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology. 1997 Apr;48((4)):891–895. doi: 10.1212/wnl.48.4.891. [DOI] [PubMed] [Google Scholar]
- 27.Moerch-Rasmussen A, Nacu A, Waje-Andreassen U, Thomassen L, Naess H. Recurrent ischemic stroke is associated with the burden of risk factors. Acta Neurol Scand. 2016 Apr;133((4)):289–294. doi: 10.1111/ane.12457. [DOI] [PubMed] [Google Scholar]
- 28.Skajaa N, Adelborg K, Horvath-Puho E, Rothman KJ, Henderson VW, Casper Thygesen L, et al. Nationwide trends in incidence and mortality of stroke among younger and older adults in Denmark. Neurology. 2021 Mar 30;96((13)):e1711–e1723. doi: 10.1212/WNL.0000000000011636. [DOI] [PubMed] [Google Scholar]
- 29.Gabet A, Guenancia C, Duloquin G, Olie V, Bejot Y. Ischemic stroke with atrial fibrillation: characteristics and time trends 2006 to 2017 in the Dijon Stroke Registry. Stroke. 2021 Jun;52((6)):2077–2085. doi: 10.1161/STROKEAHA.120.030812. [DOI] [PubMed] [Google Scholar]
- 30.Aked J, Delavaran H, Norrving B, Lindgren A. Completeness of case ascertainment in Swedish hospital-based stroke registers. Acta Neurol Scand. 2020 Feb;141((2)):148–155. doi: 10.1111/ane.13187. [DOI] [PubMed] [Google Scholar]
- 31.Glader EL. Umeå, Sweden: Department of Public health and Clinical Medicine, Umeå university; 2003. Stroke care in Sweden - hospital care and patient follow-up based on riks-stroke, the national quality register for stroke care [dissertation] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.