Abstract
Introduction
Cauda equina syndrome (CES) has significant medical, social, and legal consequences. Understanding the number of people presenting with CES and their demographic features is essential for planning healthcare services to ensure timely and appropriate management. We aimed to establish the incidence of CES in a single country and stratify incidence by age, gender, and socioeconomic status. As no consensus clinical definition of CES exists, we compared incidence using different diagnostic criteria.
Methods
All patients presenting with radiological compression of the cauda equina due to degenerative disc disease and clinical CES requiring emergency surgical decompression during a 1-year period were identified at all centres performing emergency spinal surgery across Scotland. Initial patient identification occurred during the emergency hospital admission, and case ascertainment was checked using ICD-10 diagnostic coding. Clinical information was reviewed, and incidence rates for all demographic and clinical groups were calculated.
Results
We identified 149 patients with CES in 1 year from a total population of 5.4 million, giving a crude incidence of 2.7 (95% CI: 2.3–3.2) per 100,000 per year. CES occurred more commonly in females and in the 30–49 years age range, with an incidence per year of 7.2 (95% CI: 4.7–10.6) per 100,000 females age 30–39. There was no association between CES and socioeconomic status. CES requiring catheterization had an incidence of 1.1 (95% CI: 0.8–1.5) per 100,000 adults per year. The use of ICD-10 codes alone to identify cases gave much higher incidence rates, but was inaccurate, with 55% (117/211) of patients with a new ICD-10 code for CES found not to have CES on clinical notes review.
Conclusion
CES occurred more commonly in females and in those between 30 and 49 years and had no association with socioeconomic status. The incidence of CES in Scotland is at least four times higher than previous European estimates of 0.3–0.6 per 100,000 population per year. Incidence varies with clinical diagnostic criteria. To enable comparison of rates of CES across populations, we recommend using standardized clinical and radiological criteria and standardization for population structure.
Keywords: Cauda equina syndrome, Incidence, Demographics, Spine
Introduction
Compression of the cauda equina can cause a neurological syndrome including back pain, leg pain, lower limb weakness or numbness, perianal or perineal sensory changes, or bladder, bowel, or sexual dysfunction [1]. There are no agreed clinical diagnostic criteria for cauda equina syndrome (CES) [1], and some clinical features, such as back pain and urinary dysfunction, are prevalent in the population [2, 3, 4]. This leads to a low rate of diagnosis of cauda equina compression on magnetic resonance imaging (MRI) when CES is suspected [5, 6, 7]. As CES can have debilitating medical, psychosocial, and legal consequences, a timely diagnosis is important [7, 8, 9, 10]. Lifestyle and population factors may affect rates of spinal degenerative disease [11], but healthcare service availability and medicolegal climates can also affect frequencies of diagnoses for poorly defined conditions such as CES [12, 13]. Establishing the incidence of CES according to defined criteria and across different population groups would aid healthcare service design to facilitate the best possible patient pathways and outcomes.
We aimed to establish the contemporary incidence of CES due to degenerative lumbar disc disease in Scotland along with any associations with age, sex, or socioeconomic status. We explored the effect of different diagnostic criteria and case ascertainment methods to facilitate comparison across studies and populations.
Materials and Methods
Patient Identification
We identified patients with a new diagnosis of CES in Scotland between June 1, 2018, and May 31, 2019. Patient identification was undertaken during the Understanding Cauda Equina Syndrome (UCES) study [14], registered at ISRCTN (ISRCTN16828522). This was a prospective observational study of CES. A favourable ethical opinion for the UCES study was given by the South East Scotland Research Ethics Committee in April 2018 (18/SS/0047). All hospitals in Scotland undertaking urgent spinal surgery participated, covering the whole population of Scotland.
Initial patient identification was through screening of acute referrals, admissions, and surgical cases by local clinicians. To ensure complete case ascertainment, all patients with a new ICD-10 code of G83.4 CES during the study dates were also identified from local hospital records, and all case notes were reviewed.
Public Health Scotland also confirmed there were 388 people with a new ICD-10 code G83.4 cauda equina syndrome (from the SMR01 General Acute Inpatient and Day Case Dataset) in Scotland during the study period (Freedom of Information Request Reference 2021-000872). Case notes for those admitted to a hospital in Scotland without spinal services were not reviewed as the data were provided anonymously. As there was no provision for emergency lumbar spine decompression in Scotland other than admission to one of the participating hospitals, none of those identified from the SMR01 dataset who were not also identified through the participating hospitals underwent surgical decompression for CES and met the inclusion criteria for the study.
Inclusion Criteria
Inclusion criteria were:
At least 18 years old;
Structural compression of the cauda equina due to a prolapsed intervertebral disc or a combination of disc prolapse and degenerative changes of the facet joints and/or posterior ligamentous complex;
Back and/or leg pain associated with any of: altered perianal or perineal sensation; bladder dysfunction; bowel dysfunction; sexual dysfunction; or bilateral sciatica;
offered surgical decompression for CES.
We excluded patients with nondegenerative causes of cauda equina compression (fracture, infection, tumour). All degenerative causes of canal compression or stenosis, including combinations of disc prolapse with facet joint arthritis or hypertrophic ligamentous changes were included. Patients with unilateral lower limb symptoms or neurological deficit (e.g., foot drop) were excluded unless they also met the inclusion criteria (e.g., foot drop plus urinary retention). All imaging was reviewed by two researchers in addition to the reporting radiologist to verify radiological cauda equina compression and the cause of compression.
Clinical Data Collection
Age, sex, and clinical details were extracted from the UCES study database [14]. The Scottish Index of Multiple Deprivation (SIMD) version 2, 2020 was used to divide participants into quintiles from quintile 1 (most deprived) to quintile 5 (least deprived) [15].
Population Statistics
Validated point population estimates for Scotland for June 30, 2018, by age and gender were sourced from National Records of Scotland [16].
Data Analysis
Population data were divided into 10 years age bands, a study inclusion age band (18 years or over), and a working age population (18–64 years). We included those aged 18–64 years in our definition of a working age population rather than using the Scottish government definition of working age of 16–64 years [16] as the UCES study included only those aged 18 years or over [14]. We included urinary retention requiring catheterization as a clinical diagnostic category for comparison to previous studies describing CES with retention [4]. We included those with a catheter inserted at any time point prior to operation. We also analysed a clinical category including those with either a catheter or a post-void residual volume of more than 200 mL as studies suggest that a post-void residual volume of more than 200 mL is a marker of urinary dysfunction when investigating those with CES [17]. Data analysis was carried out in R version 4.1.1 (2021). Incidence rates per 100,000 population per year and 95% confidence intervals (CIs) were calculated using the Poisson distribution and exact method as implemented in the epiR package using the method “inc.rate” and “exact.”
Results
One hundred and forty-nine patients with CES due to degenerative disc disease were identified in Scotland in the 1-year period. Identification methods and reasons for exclusion are shown in Figure 1. In the total Scottish population of 5.4 million, the crude incidence of CES was 2.7 (95% CI: 2.3–3.2) per 100,000 population per year. In the working age population of Scotland (18–64 years), the incidence of CES was 4.0 (95% CI: 3.3–4.7) per 100,000 population per year (see Table 1).
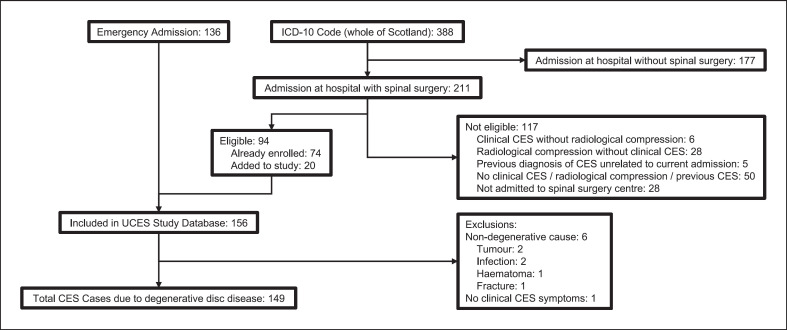
Fig. 1.
Flow diagram of patient identification. Initial patient identification was by the local clinical team during the emergency admission with CES. Case ascertainment was checked using diagnostic coding data for the study period for the ICD-10 code G83.4 Cauda Equina Syndrome. Additional eligible patients were added to the study database. ICD-10 coding for CES for the whole of Scotland was also sourced. Clinical notes were reviewed for all the 211 patients admitted at hospitals with spinal surgery but could not be reviewed for those admitted at hospitals without spinal surgery. As hospital sites with spinal surgery also have other specialties, it was possible that patients admitted at the hospital were not admitted to the spinal surgery centre. Notes were reviewed for the 28 patients admitted at the hospital site but not admitted to the spinal surgery centre. None had clinical CES and radiological compression of the cauda equina. Patients with causes of CES other than degenerative spine disease were excluded from this study but were initially included in the UCES database. Patients without clinical signs and symptoms of CES or without radiological cauda equina compression were excluded. One patient erroneously included in the UCES database did not have clinical CES and was not included in this study. Clinical CES was defined as back and or leg pain and any of: altered perianal or perineal sensation; bladder dysfunction; bowel dysfunction; sexual dysfunction; or bilateral sciatica. (CES: cauda equina syndrome; UCES: Understanding Cauda Equina Syndrome Study).
Table 1.
Incidence rates for CES in Scotland
| Total |
Female |
Male |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| population | patients (per year) |
incidence (95% CI) |
population | patients (per year) |
incidence (95% CI) |
population | patients (per year) |
incidence (95% CI) |
|
| Age groups | |||||||||
| 0–17 | 1,028,798 | − | − | 501,941 | − | − | 526,857 | − | − |
| 18–19 | 121,507 | 0 | 0 (0–3.0) | 59,268 | 0 | 0 (0–6.2) | 62,239 | 0 | 0 (0–5.9) |
| 20–29 | 732,964 | 13 | 1.8 (0.9–3.0) | 36,5145 | 10 | 2.7 (1.3–5.0) | 367,819 | 3 | 0.8 (0.2–2.4) |
| 30–39 | 709,255 | 47 | 6.6 (4.9–8.8) | 360,565 | 26 | 7.2 (4.7–10.6) | 348,690 | 21 | 6.0 (3.7–9.2) |
| 40–49 | 691,809 | 41 | 5.9 (4.3–8.0) | 355,673 | 23 | 6.5 (4.1–9.7) | 336,136 | 18 | 5.4 (3.2–8.5) |
| 50–59 | 791,347 | 21 | 2.7 (1.6–4.1) | 407,426 | 9 | 2.2 (1.0–4.2) | 383,921 | 12 | 3.1 (1.6–5.5) |
| 60–69 | 636,719 | 17 | 2.7 (1.6–4.3) | 328,462 | 8 | 2.4 (1.1–4.8) | 308,257 | 9 | 2.9 (1.3–5.5) |
| 70–79 | 462,067 | 7 | 1.5 (0.6–3.1) | 248,671 | 2 | 0.8 (0.1–2.9) | 213,396 | 5 | 2.3 (0.8–5.5) |
| 80+ | 263,634 | 3 | 1.1 (0.2–3.3) | 162,198 | 3 | 1.8 (0.4–5.4) | 101,436 | 0 | 0 (0.0–3.6) |
| Combined age groups Working age (18–64) | 3,383,188 | 134 | 4.0 (3.3–4.7) | 1,721,140 | 73 | 4.2 (3.3–5.3) | 1,662,048 | 61 | 3.6 (2.8–4.7) |
| Adults (18+) | 4,409,302 | 149 | 3.4 (2.9–4.0) | 2,287,408 | 81 | 3.6 (2.8–4.4) | 2,121,894 | 68 | 3.2 (2.5–4.1) |
| Whole population (0+) | 5,438,100 | 149 | 2.7 (2.3–3.2) | 2,789,349 | 81 | 2.9 (2.3–3.6) | 2,648,751 | 68 | 2.6 (2.0–3.3) |
Incidence is calculated per 100,000 population per year. 95% CIs are given in brackets. Incidence is not calculated for those under 18 years as children were not included in the study. The total population of Scotland, including children, is included in the whole population estimate. Working age is defined as 18–64 years and adults as 18 years and over. CES was defined as radiological compression of the cauda equina with back and/or leg pain associated with any of: altered perianal or perineal sensation, bladder dysfunction; bowel dysfunction; sexual dysfunction; or bilateral sciatica.
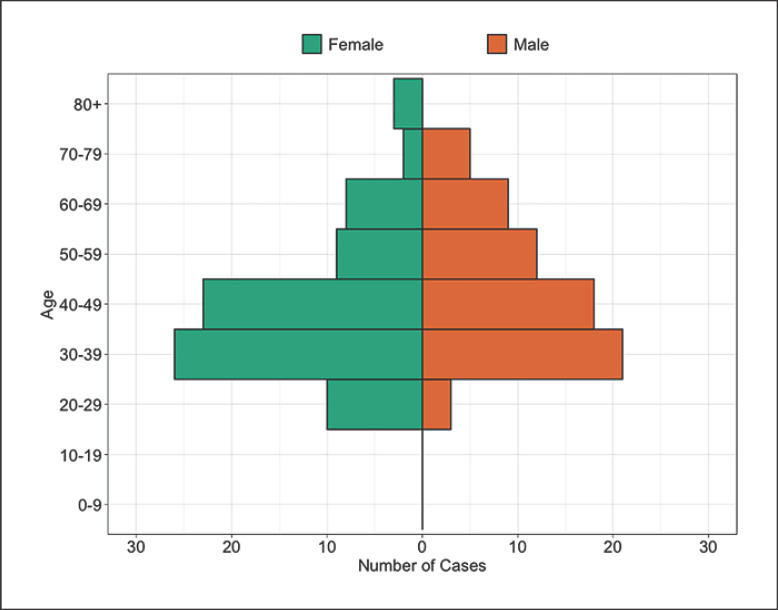
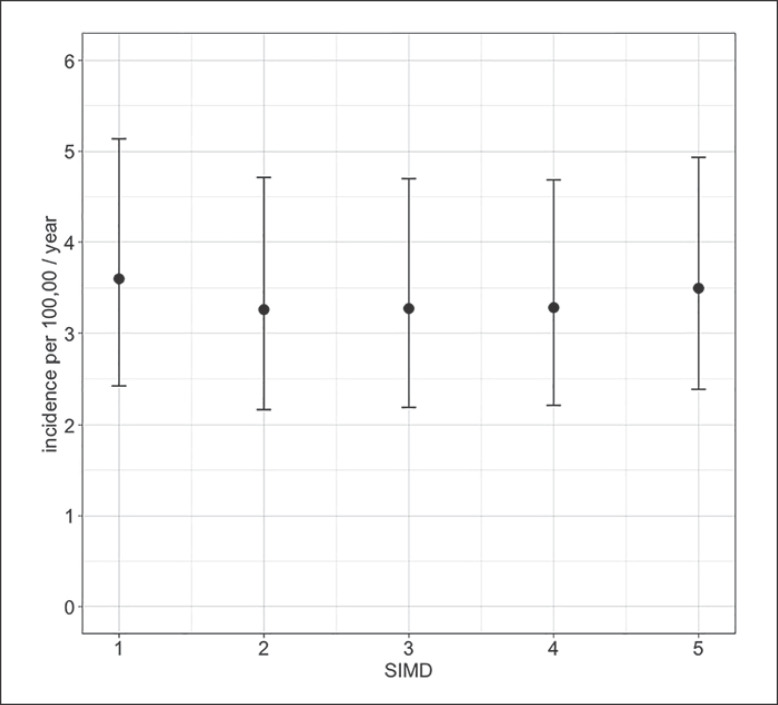
Eighty-one (54%) of patients with CES were female. The median age was 42 years with a range of 20–81 years. Age and gender distribution are shown in Figure 2. CES occurred more frequently between the ages of 30–49 years. After correction for the age and gender structure of the Scottish population, CES still occurred at higher rates in females and in the 30- to 49-year-old age groups (see Table 1). Females between 30 and 39 years had the highest incidence of CES at 7.2 (95% CI: 4.7–10.6) per 100,000 population per year. Incidence rates of CES were similar across the five SIMD categories with the least and most deprived groups equally affected (see Fig. 3).
Fig. 2.
Age and gender of patients with CES. All cases of CES in Scotland by 10 years age group and gender. All cases have clinical CES and radiological cauda equina compression. Raw numbers are presented with no correction for the underlying population structure. Orange bars to the right represent males. Green bars to the left represent females. CES, cauda equina syndrome.
Fig. 3.
Incidence of CES by socioeconomic status. Patients are divided into the quintiles of the Scottish Index of Multiple Deprivation (SIMD) where the most deprived fall into quintile one and the least deprived into quintile five. Incidence is presented as a point estimate per 100,000 population per year, with error bars representing 95% confidence intervals.
The effect of narrowing the clinical diagnostic criteria is shown in Table 2. Only 1 patient had bilateral radiculopathy without bladder, bowel, or sexual dysfunction or perianal or perineal sensory changes. A catheter was inserted at any time preoperatively for urinary retention in 50/149 (34%). Bladder scanning was carried out in 67/99 (68%) of those not catheterized. Six of these had no post-void residual volume documented, and 14/61 (23%) had a post-void residual volume of more than 200 mL, giving 64 patients with either a catheter inserted or a post-void residual volume of more than 200 mL. Of the 50 patients with a catheter inserted, 25 had documentation of whether they could feel the catheter. 14/25 (56%) could feel the catheter in the bladder and 11/25 (44%) could not feel the catheter. All patients with CES underwent emergency decompressive surgery. This was carried out within a day of admission in 141/149 (95%).
Table 2.
Incidence of CES using different diagnostic criteria
| Diagnostic criteria | Patients (per year) |
Incidence (95% CI) |
|---|---|---|
| Radiological compression of the cauda equina due to degenerative disc disease, identified from emergency admission or ICD-10 code, plus | ||
| Visceral symptoms or perianal sensory loss or bilateral radiculopathy | 149 | 3.4 (2.9–4.0) |
| Visceral symptoms or perianal sensory loss | 148 | 3.4 (2.8–3.9) |
| Urinary retention − catheterized or >200 mL post-void residual | 64 | 1.5 (1.1–1.9) |
| Urinary retention − catheterized | 50 | 1.1 (0.8–1.5) |
| New ICD-10 Code G83.4 CES applied during hospital admission between study dates plus | ||
| Admitted to any hospital in Scotland − no clinical review | 388 | 8.8 (7.9–9.7) |
| Admitted to hospital in Scotland with emergency spinal surgery − no clinical review | 211 | 4.8 (4.2–5.5) |
| Admitted to hospital in Scotland with emergency spinal surgery, has radiological compression of the cauda equina due to | 93 | 2.1 (1.7–2.6) |
| degenerative disc disease, plus visceral symptoms or saddle sensory loss or bilateral radiculopathy | ||
Incidence is calculated per 100,000 adults (over 18 years) population per year with 95% confidence intervals (CIs). The Scottish adult population was 4,409,302. Visceral symptoms include any new bladder, bowel, or sexual function disturbance. Perianal sensory loss includes reported unilateral or bilateral altered sensation or loss of sensation in the perianal or perineal regions or unilateral or bilateral loss of or altered light touch or pinprick sensation testing in the perianal or perineal regions. Post-void residual was measured using an ultrasound bladder scanner after voiding with the instruction to completely empty the bladder. Patients with unilateral radiculopathy only were not included in any of the categories. Catheterization includes those catheterized at any time point prior to decompression surgery.
The effect of patient identification method on the calculated incidence of CES is shown in Table 2. Six of the 136 patients initially identified from the emergency admission had causes of CES other than degenerative disease or were entered into the database but did not have clinical or radiological CES. Therefore, of the 149 final included patients, 130 (87%) were identified by local clinicians during their emergency admission (see Fig. 1).
Across Scotland, there were a total of 388 people with a new ICD-10 code for CES assigned during the study period (see Fig. 1). We reviewed case notes for the 211/388 (54%) admitted to a hospital with facilities for emergency spinal surgery. Those who were not admitted to a hospital with facilities for spinal surgery did not have a final clinical diagnosis of CES requiring surgical decompression as otherwise they would have been transferred to a hospital with facilities for emergency spinal surgery. Each patient was counted only once during the study period, even if admitted to multiple hospitals so those transferred to a hospital with spinal surgery from a hospital without spinal surgery were included in the numbers of those admitted at a hospital with spinal surgery facilities.
Of the 211 with a new ICD-10 code of CES who were admitted to a hospital with facilities for emergency spinal surgery, 118 (56%) did not have a final diagnosis of CES due to degenerative disease (see Fig. 1). One patient identified via ICD-10 coding had CES due to an infective cause, and the other 117 did not have clinical and radiological CES (see Fig. 1). If ICD-10 coding without case note review was used to identify those with CES in Scotland, 295/388 (76%) patients without clinical CES and radiological compression of the cauda equina due to degenerative disease would have been identified. Using ICD-10 coding followed by case notes review identified only 93 of the final 149 patients with CES.
Discussion
The crude incidence of CES in Scotland was 2.7 (95% CI: 2.3–3.2) per 100,000 population per year. CES occurred more frequently in those 30–49 years old, and in women, with an incidence of 7.2 (95% CI: 4.7–10.6) per 100,000 women aged 30–39 years per year. The incidence of CES was similar across socioeconomic groups. CES with urinary retention requiring catheterization preoperatively occurred in 1.1 (95% CI: 0.8–1.5) per 100,000 adult population per year.
Previous studies have reported incidence rates of CES ranging from 0.3 to 7.0 per 100,000 population per year [18]. Hurme et al. [19] investigated patients undergoing lumbar disc operations between 1975 and 1979 in Finland and found a rate of 0.48 per 100,000 population per year but did not define CES, so we cannot assess whether diagnostic criteria impacted on the lower incidence. Podnar [20] identified CES cases admitted to national rehabilitation centres in Slovenia and used a combination of clinical history, examination, neurophysiological findings, and radiological findings to make a diagnosis of CES with an incidence rate of 0.34 per 100,000 population per year. Only one patient in our cohort was admitted to a rehabilitation facility post-operatively, which may be due to different health service arrangements, earlier management, or less severe preoperative symptoms. Identifying acute presentations rather than rehabilitation admissions may explain our higher incidence.
Reito et al. [4] initially used ICD coding, and then notes review to identify those with CES presenting to an Emergency Department in Finland and found an incidence of 0.6 per 100,000 adult population per year. Patients were divided into CES-R (with retention) and CES-I (incomplete) [4]. Our rate of CES with urinary retention is higher (1.1 per 100,000 adults per year) than that found by Reito et al. [4] with CES-R (0.3 per 100,000 adults per year). Our higher incidence cannot therefore be due solely to a greater number of patients with less severe symptoms. Using the identification method used by Reito et al. [4], we found a higher overall incidence (2.1 per 100,000 adults per year, see Table 2), so our higher incidence cannot also be fully explained by study methodology.
All three of these studies identified few patients with CES (11 in 5 years [19]; 67 in 8 years [20]; 4 in 3 years [4]) in relatively small populations, which may lead to imprecise estimates with wide CIs. Conversely, Schoenfeld et al. identified an incidence of 7 per 100,000 population per year using ICD codes to identify cases in a database of serving American military personnel covering more than 13 million person-years [21]. A higher incidence was found in females and those over the age of 30 compared to under 30 years, which matches our findings of the highest rates in those aged 30–49 years [21]. Schoenfeld and Bader [21] found a higher incidence in those of more senior military rank, which may represent higher socioeconomic status. However, we did not identify any association with socioeconomic status, and it is possible that the association of seniority with age of 30–40 years may explain Schoenfeld et al.'s findings.
Using ICD codes alone significantly overestimated the incidence of CES in Scotland. We excluded over half (117/211; 55%) of those with an ICD code for CES. We purposely excluded those with CES due to fractures, infection, or tumours and those who did not undergo surgery. We did not identify any patients with CES who did not undergo surgery and there were very few cases due to causes other than degenerative disease (see Fig. 1), so it is unlikely the large number with the CES ICD-10 code is due to these cases. Although some identified by ICD coding had a large disc prolapse without any clinical symptoms of CES or were admitted as suspected CES but subsequently found not to have cauda equina compression, in many cases it was unclear from clinical notes review why the CES code had been applied. Coding may be more accurate in the USA, where income may be reliant on coding, unlike in the Scottish healthcare system, where coding is often performed by administrative staff and does not always have funding implications.
Along with differences in case identification methods, diagnostic criteria, and population structures, the higher incidence of CES in Scotland compared to other European countries, and lower incidence than in the USA could also reflect the healthcare system, imaging availability, and medicolegal environment. There are no specific data available for NHS Scotland, but CES accounted for £68 million (approximately $94 million) in NHS England medicolegal claims between 2013 and 2016, and the majority of claims were for delay or failure in diagnosis or treatment [9]. Since our study, the diagnosis and treatment of CES in NHS England have been highlighted as areas of clinical risk [22], with MRI access, particularly out of hours, described as inadequate [13, 23]. NHS provision and the medicolegal climate are similar in NHS Scotland and England. Increased litigation in spine surgery is associated with an increase in defensive medicine in the USA with increased length of stay, investigation, and hospital charges [24]. Spinal conditions associated with litigation may be less prevalent in countries with lower rates of litigation [12]. In Edinburgh, Scotland, the rate of cauda equina compression on MRI undertaken for suspected CES dropped from 22% in 2007 [5] to 7% in 2019 [25], suggesting either improved access to MRI or a decreasing threshold for imaging for suspected CES. The higher incidence of CES in Scotland compared to other European countries may reflect a lower threshold for diagnosing and operating on patients with CES. As clinical features in the previous studies of incidence are poorly described, this is difficult to confirm.
The strength of our study lies in complete population capture and complete clinical review. All hospitals undertaking emergency spinal surgery in Scotland provided prospective case identification and detailed clinical data. There are no other options for emergency spinal surgery in Scotland other than admission to the hospitals included in our study. No patients presenting would have been refused treatment, and it is uncommon for patients to seek healthcare outside of Scotland in an emergency situation. It is possible that some diagnoses of CES were missed during the study period, but it is more likely if symptoms persisted or progressed that diagnosis was delayed rather than not made at all. More precise incidence figures could be calculated using a larger population than the 5.4 million Scottish population or a longer study period. Due to the intensity of participant identification and clinical data entry at each site, the study was only run for one year. In the future, national clinical registries could be used to collect this data. Although the UCES study covered the whole of the UK, we did not have complete population coverage in the rest of the UK and therefore restricted this demographic analysis to the Scottish population.
Conclusion
In conclusion, the crude incidence of CES in 2018/2019 in the 5.4 million Scottish population was 2.7 (95% CI: 2.3–3.2) per 100,000 per year. The 95% CI for the incidence of CES was between 3.2 and 10.6 per 100,000 per year for those between 30 and 49 years old, but less than 5 per 100,000 per year outside this age range. The incidence of CES in Scotland is more than four times higher than previously reported European incidence rates. The incidence of CES depends on the diagnostic criteria used, identification methods, and the demographics of the underlying population, and these should be stated when describing the incidence of CES.
Statement of Ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. A favourable ethical opinion for the UCES study was given by the South East Scotland Research Ethics Committee in April 2018 (Reference 18/SS/0047). Written informed consent was given by those participating in the UCES study. Screening for eligibility and the use of anonymized data only did not require informed consent.
Conflict of Interest Statement
J. Woodfield, S. Lammy, A.A.B. Jamjoom, M.A.G. Fadelalla, P.C. Copley, M. Arora, S.A. Glasmacher, M. Abdelsadg, G. Scicluna, M.T.C. Poon, S. Pronin, A.H.C. Leung, S. Darwish, J. Brown, and N. Eames have no conflicts of interest to declare. I. Hoeritzauer declares honoraria for speaking about functional neurological disorders and has received payment for expert testimony on idiopathic urinary retention. P.F.X. Statham has received payment for expert testimony, acting for a number of both claimants and defenders in cases of CES, roughly in the proportion 2/3 defender, 1/3 claimant over about 20 years. A.K. Demetriades declares payment or honoraria for speaking for Integra, Stryker, and Safe Orthopaedics and declares unpaid leadership board roles for Global Neuro Foundation and European Association of Neurosurgical Societies.
Funding Sources
No specific funding was acquired for this study. NHS Lothian Department of Clinical Neurosciences Endowment fund provided funding for postage, stationary, and imaging transfer for the UCES Study. Castor EDC provided database access free of charge for the British Neurosurgical Trainee Research Collaborative. I. Hoeritzauer is funded by a NHS Research Scotland Fellowship. No funder or sponsor had any role in data analysis, writing of the manuscript, or the decision to submit for publication.
Author Contributions
J. Woodfield, I. Hoeritzauer, A.A.B. Jamjoom, S. Lammy, N. Eames, P.F.X. Statham contributed to study conception and design. J. Woodfield, S. Lammy, M. Fadelalla, P.C. Copley, M. Arora, A.H.C. Leung, G. Scicluna, M. Abdelsadg, S. Darwish, and A.A.B. Jamjoom identified and recruited participants, performed data entry, and analysis. S.A. Glasmacher, S. Pronin, and M.T.C. Poon contributed to data analysis. J. Woodfield, I. Hoeritzauer, A.A.B. Jamjoom, and S. Lammy drafted and revised the manuscript. M.T.C. Poon, P.C. Copley, A.K. Demetriades, J. Brown, N. Eames, and P.F.X. Statham revised the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
Anonymized individual participant data and a data dictionary will be available following proposal submission and review by the UCES steering committee via the chief investigator (julie.woodfield@ed.ac.uk) with a data access agreement.
Acknowledgments
We thank all patients who contributed to this study. We thank all the UCES collaborators across the UK, the UCES study steering committee, the British Neurosurgical Trainee Research Collaborative, the British Association of Spine Surgeons, the British Orthopaedic Trainees Association, and the Managed Service Network for Neurosurgery in Scotland for their support with the study.
Funding Statement
No specific funding was acquired for this study. NHS Lothian Department of Clinical Neurosciences Endowment fund provided funding for postage, stationary, and imaging transfer for the UCES Study. Castor EDC provided database access free of charge for the British Neurosurgical Trainee Research Collaborative. I. Hoeritzauer is funded by a NHS Research Scotland Fellowship. No funder or sponsor had any role in data analysis, writing of the manuscript, or the decision to submit for publication.
References
- 1.Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009 Nov;90((11)):1964–1968. doi: 10.1016/j.apmr.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Perry S, Shaw C, Assassa P, Dallosso H, Williams K, Brittain KR, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC incontinence study. J Public Health Med. 2000 Sep;22((3)):427–434. doi: 10.1093/pubmed/22.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010 Dec;24((6)):769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Reito A, Kyrola K, Pekkanen L, Paloneva J. Specific spinal pathologies in adult patients with an acute or subacute atraumatic low back pain in the emergency department. Int Orthop. 2018 May 29;42((12)):2843–2849. doi: 10.1007/s00264-018-3983-y. [DOI] [PubMed] [Google Scholar]
- 5.Bell DA, Collie D, Statham PF. Cauda equina syndrome: what is the correlation between clinical assessment and MRI scanning? Br J Neurosurg. 2007 Apr;21((2)):201–203. doi: 10.1080/02688690701317144. [DOI] [PubMed] [Google Scholar]
- 6.Domen PM, Hofman PA, Van Santbrink H, Weber WEJ. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009;16((3)):416–419. doi: 10.1111/j.1468-1331.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoeritzauer I, Pronin S, Carson A, Statham P, Demetriades AK, Stone J. The clinical features and outcome of scan-negative and scan-positive cases in suspected cauda equina syndrome: a retrospective study of 276 patients. J Neurol. 2018 Oct 8;265((12)):2916–2926. doi: 10.1007/s00415-018-9078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korse NS, Veldman AB, Peul WC, Vleggeert-Lankamp CLA. The long term outcome of micturition, defecation and sexual function after spinal surgery for cauda equina syndrome. PLoS One. 2017;12((4)):e0175987. doi: 10.1371/journal.pone.0175987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machin JT, Hardman J, Harrison W, Briggs TWR, Hutton M. Can spinal surgery in England be saved from litigation: a review of 978 clinical negligence claims against the NHS. Eur Spine J. 2018 Nov;27((11)):2693–2699. doi: 10.1007/s00586-018-5739-1. [DOI] [PubMed] [Google Scholar]
- 10.Hazelwood JE, Hoeritzauer I, Pronin S, Demetriades AK. An assessment of patient-reported long-term outcomes following surgery for cauda equina syndrome. Acta Neurochir. 2019 Sep;161((9)):1887–1894. doi: 10.1007/s00701-019-03973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steelman T, Lewandowski L, Helgeson M, Wilson K, Olsen C, Gwinn D. Population-based risk factors for the development of degenerative disk disease. Clin Spine Surg. 2018 Oct;31((8)):E409–12. doi: 10.1097/BSD.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 12.Schrader H, Sand T, Bovim G, Obelieniene D, Siurkiene D, Mickevičiene D, et al. Natural evolution of late whiplash syndrome outside the medicolegal context. Lancet. 1996 May 4;347((9010)):1207–1211. doi: 10.1016/s0140-6736(96)90733-3. [DOI] [PubMed] [Google Scholar]
- 13.Fountain DM, Davies SCL, Woodfield J, Kamel M, Majewska P, Edlmann E, et al. Evaluation of nationwide referral pathways, investigation and treatment of suspected cauda equina syndrome in the United Kingdom. Br J Neurosurg. 2019;33((6)):624–634. doi: 10.1080/02688697.2019.1648757. [DOI] [PubMed] [Google Scholar]
- 14.Woodfield J, Hoeritzauer I, Jamjoom AAB, Pronin S, Srikandarajah N, Poon M, et al. Understanding cauda equina syndrome: protocol for a UK multicentre prospective observational cohort study. BMJ Open. 2018;8((12)):e025230. doi: 10.1136/bmjopen-2018-025230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health Scotland Deprivation Guidance For Analysts. version 3.4. 2020. Jul, Available from: https://www.isdscotland.org/Products-and-Services/GPD-Support/Deprivation/_docs/PHS-Deprivation-Guidance-version-3-4.pdf (accessed Jul 20, 2021)
- 16.National Records of Scotland. Mid-Year Population Estimates Scotland: Methodology Guide 2021. Jun 25, Available from: https://www.nrscotland.gov.uk/files//statistics/population-estimates/mid-20/mid-year-pop-est-20-methodology.pdf (accessed Jul 20, 2021)
- 17.Katzouraki G, Zubairi AJ, Hershkovich O, Grevitt MP. A prospective study of the role of bladder scanning and post-void residual volume measurement in improving diagnostic accuracy of cauda equina syndrome. Bone Joint J. 2020 Jun;102-B((6)):677–682. doi: 10.1302/0301-620X.102B6.BJJ-2020-0195.R1. [DOI] [PubMed] [Google Scholar]
- 18.Hoeritzauer I, Wood M, Copley PC, Demetriades AK, Woodfield J. What is the incidence of cauda equina syndrome? A systematic review. J Neurosurg Spine. 2020 Feb 14;32((6)):832–841. doi: 10.3171/2019.12.SPINE19839. [DOI] [PubMed] [Google Scholar]
- 19.Hurme M, Alaranta H, Torma T, Einola S. Operated lumbar disc herniation: epidemiological aspects. Ann Chir Gynaecol. 1983;72((1)):33–36. [PubMed] [Google Scholar]
- 20.Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2007 Apr;35((4)):529–531. doi: 10.1002/mus.20696. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld AJ, Bader JO. Cauda equina syndrome: an analysis of incidence rates and risk factors among a closed North American military population. Clin Neurol Neurosurg. 2012 Sep;114((7)):947–950. doi: 10.1016/j.clineuro.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Healthcare Safety Investigation Branch Timely detection and treatment of cauda equina syndrome I2019/015. 2021. https://hsib-kqcco125-media.s3.amazonaws.com/assets/documents/HSIB_full_report_Timely_detection_and_treatment_of_cauda_equina_syndrome.pdf (accessed Aug 31, 2021)
- 23.Hutton M. Getting it Right First Time (GIRFT). Spinal Services. GIRFT Programme National Specialty Report. 2019. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2019/01/Spinal-Services-Report-Mar19-L1.pdf (accessed Sep 1, 2021)
- 24.Jackson KL, Rumley J, Griffith M, Linkous TR, Agochukwu U, DeVine J. Medical malpractice claims and mitigation strategies following spine surgery. Global Spine J. 2021 Jun;11((5)):782–791. doi: 10.1177/2192568220939524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodfield J, Brennan PM, Statham P, Stone J, Hoeritzauer I. Suspected cauda equina syndrome: no reduction in investigation, referral and treatment during the COVID-19 pandemic. annals. 2021 Jun;103((6)):432–437. doi: 10.1308/rcsann.2021.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data and a data dictionary will be available following proposal submission and review by the UCES steering committee via the chief investigator (julie.woodfield@ed.ac.uk) with a data access agreement.