Abstract
Tumor-associated macrophages (TAMs) are an essential proportion of tumor-infiltrating immune cells in the tumor microenvironment (TME) and have immunosuppressive functions. The high plasticity and corresponding phenotypic transformation of TAMs facilitate oncogenesis and progression, and suppress antineoplastic responses. Due to the uncontrolled proliferation of tumor cells, metabolism homeostasis is regulated, leading to a series of alterations in the metabolite profiles in the TME, which have a commensurate influence on immune cells. Metabolic reprogramming of the TME has a profound impact on the polarization and function of TAMs, and can alter their metabolic profiles. TAMs undergo a series of metabolic reprogramming processes, involving glucose, lipid, and amino acid metabolism, and other metabolic pathways, which terminally promote the development of the immunosuppressive phenotype. TAMs express a pro-tumor phenotype by increasing glycolysis, fatty acid oxidation, cholesterol efflux, and arginine, tryptophan, glutamate, and glutamine metabolism. Previous studies on the metabolism of TAMs demonstrated that metabolic reprogramming has intimate crosstalk with anti-tumor or pro-tumor phenotypes and is crucial for the function of TAMs themselves. Targeting metabolism-related pathways is emerging as a promising therapeutic modality because of the massive metabolic remodeling that occurs in malignant cells and TAMs. Evidence reveals that the efficacy of immune checkpoint inhibitors is improved when combined with therapeutic strategies targeting metabolism-related pathways. In-depth research on metabolic reprogramming and potential therapeutic targets provides more options for anti-tumor treatment and creates new directions for the development of new immunotherapy methods. In this review, we elucidate the metabolic reprogramming of TAMs and explore how they sustain immunosuppressive phenotypes to provide a perspective for potential metabolic therapies.
Keywords: Immune checkpoint inhibitors, Immunosuppression, Metabolism, Tumor-associated macrophages, Tumor microenvironment
Introduction
Peripheral blood and tissue resident tumor-associated macrophages (TAMs) constitute a tremendous segment of infiltrating myeloid cells in the tumor microenvironment (TME) of most malignant solid tumors. Importantly, TAMs display proangiogenic properties.[1–4] According to environmental perturbations, macrophages differentiate into two classes: anti-tumor M1-phenotype and pro-tumor M2-phenotype macrophages. The latter resemble TAMs. These two types of macrophages have their own metabolic profiles, which are adapted to their functions.
In terms of glucose metabolism, pro-inflammatory M1-like macrophages show an enhancement in glycolytic metabolism and the pentose phosphate pathway (PPP), whereas the tricarboxylic acid (TCA) cycle is impaired and mitochondrial oxidative phosphorylation (OXPHOS) is attenuated. However, anti-inflammatory M2-like macrophages elevate OXPHOS and diminish PPP. Both M1- and M2-like macrophages potentiate fatty acid synthesis. Furthermore, M1-like macrophages generate nitric oxide from L-arginine by expressing inducible nitric oxide synthase (iNOS), while anti-inflammatory M2-like macrophages harness arginase 1 (Arg-1) to metabolize L-arginine; glutamine metabolism is also increased in M2-like macrophages.[5] Therefore, the metabolic reprogramming that occurs in TAMs with tumor-promoting effects (similar to those of M2-like macrophages) has been extensively studied, and it has been discovered that TAMs metabolic reprogramming is intimately related to the properties of the tumor cells.
The rapid proliferation of tumor cells leads to altered metabolites in the TME, such as glucose starvation and lactate accumulation; metabolite level modifications, in turn, contribute to the metabolic reprogramming of TAMs, as metabolites can act as signaling molecules to hijack infiltrating immune cell phenotypes and functions, including highly plastic TAMs.[6–8] TAMs preferentially harness glycolysis for energy to contribute to the accumulation of lactate, enhance lipid intake and fatty acid oxidation (FAO) and upregulate Arg-1 and indoleamine 2,3-dioxygenase (IDO) expression, which results in the concentration changes of metabolites, such as ornithine and kynurenine, by affecting L-arginine and tryptophan metabolism. Concomitantly, TAMs also potentiate glutamate and glutamine metabolism. Metabolic reprogramming of glucose, lipid, and amino acid metabolism favors TAMs to maintain immunosuppressive phenotype and exert a pro-tumor function.
A series of metabolic reprogramming occurs in TAMs, and the efficacy of drugs targeting metabolic processes in reversing the immunosuppressive function of TAMs has been confirmed in mice and even in clinical studies. When metabolism targeted therapies are combined with immune checkpoint inhibitors (ICIs), efficacy is preferable, which provides more opportunities and options for future anti-tumor treatments. Nevertheless, the contradiction in metabolism between tumor cells and TAMs also presents challenges for clinical applications [Figure 1].
Figure 1.
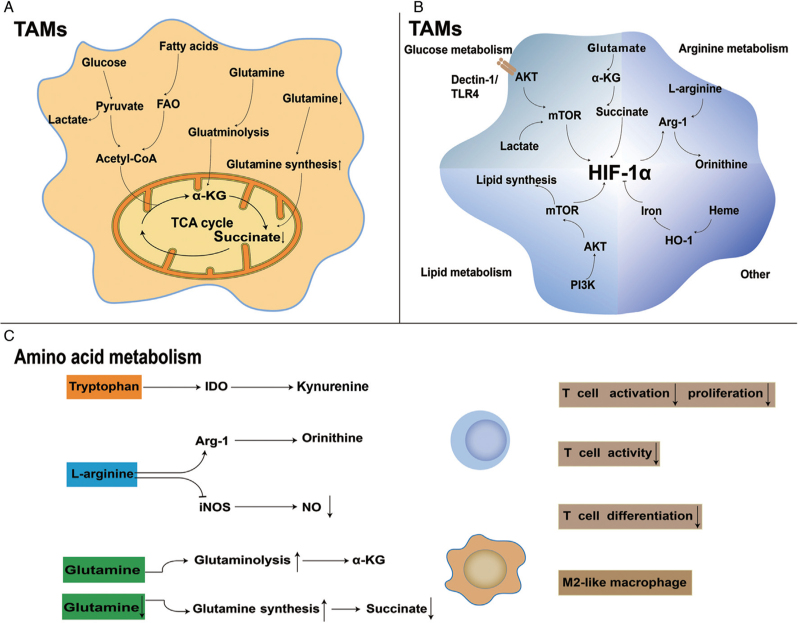
Metabolic reprogramming of tumor-associated macrophages (TAMs). (A) TAMs use glucose, lipids, and glutamine to enter the tricarboxylic acid cycle and produce ATP as energy source. (B) Relationship between metabolism and HIF-1α: mTOR is important for the transcription of HIF-1α. In addition, acidic and hypoxic tumor microenvironment (TME) can stimulate HIF-1α expression. Succinate, a glutamine metabolite, may partially respond to HIF-1α; whereas HIF-1α can affect the expression of Arg-1, affecting L-arginine metabolism. An excessive accumulation of intracellular iron inhibits HIF-1α activation. (C) Amino acid metabolism reprogramming in TAMs. TAMs upregulate IDO and Arg-1, increasing ornithine and kynurenine production and reducing the NO level, which have inhibitory effects on T cells. The α-ketoglutarate (α-KG) produced by glutaminolysis is beneficial for maintaining the M2-like phenotype. However, when intracellular concentrations of glutamine are low, the corresponding decrease in succinate also contributes to the immunosuppressive function of M2-like TAMs. Arg-1: Arginase 1; AKT: Protein kinase B; CoA: Coenzyme A; α-KG: α-ketoglutarate; FAO: Fatty acid beta-oxidation; HIF-1α: Hypoxia-inducible factor-1α; HO-1: Heme oxygenase-1; IDO: Indoleamine 2,3-dioxygenase; iNOS: Inducible nitric oxide synthase; mTOR: Mammalian target of rapamycin; NO: Nitric oxide; PI3K: Phosphatidylinositol-3-kinase; TCA: Tricarboxylic acid; TLR4: Toll-like receptor 4.
Glucose Metabolism
The “Warburg effect” occurs when tumor cells take up more glucose and make full use of aerobic glycolysis rather than OXPHOS to satisfy the demand for rapid proliferation. Increased aerobic glycolysis in tumor cells leads to glucose starvation and lactate accumulation, resulting in an acidic and hypoxic TME.[8] Subsequently, TAMs undergo a sequence of changes in glucose metabolism in favor of an immunosuppressive function, which further induces TME remodeling. Upon depletion of glucose in the TME, tumor cells take up large amounts of glucose to satisfy growth requirements. Nevertheless, studies have elucidated that TAMs are the major consumers of glucose in the TME.[9] However, compared to TAMs, tumor cells are more dependent on glucose to support their growth.[10] Glucose competition induces metabolic reprogramming of glycolysis and OXPHOS in TAMs.
Glycolysis
Glycolysis is the process by which glucose is metabolized into pyruvate in the cytoplasm under anaerobic conditions. Thereafter, pyruvate is broken down into lactate under the catalysis of lactate dehydrogenase (LDH). The formation of dysfunctional tumor vasculature and the consumption of oxygen by tumor cells develop a hypoxic TME, which upregulates glucose transporter 1 (GLUT-1) expression and improves glucose uptake in TAMs, thus counteracting glucose consumption by tumor cells.[11–13] TAMs exhibit elevated glycolysis following increased glucose uptake. For instance, in an in vitro treatment of macrophages grown in human melanoma cell-conditioned medium, TAMs showed an elevated expression of the genes encoding GLUT-1 and hexokinase 2 (HK2).[14,15] In addition, proteomic analyses demonstrated that glycolysis related enzymes, involving HK2, were up-regulated in myeloid-differentiated macrophages induced by extracts from patients with breast cancer and in TAMs isolated from patients suffering from pancreatic cancer, portending an improved glycolytic availability in these cells.[16,17] This suggests that there is increased glucose uptake and specific expression of glycolysis key enzymes, leading to elevated glycolysis and lactate accumulation in TAMs. Previously, lactate was considered merely as a by-product of this metabolic process, but new evidence has revealed that it also has numerous prominent physiological effects, such as the promotion of the TCA cycle.[18,19] In our study, we found that lactate in malignant pleural effusions affected macrophages function by regulating the synthesis of norepinephrine. Lactate accumulation may alter the epigenetic landscape of TAMs, so they have the characteristics of M2-like macrophages.[20–22]
The hypoxic TME can induce the expression of hypoxia-inducible factor (HIF)-1α, a momentous transcriptional factor that regulates the transcription of many genes involved in the glycolytic pathway or glucose transport in TAMs.[11,23,24] Two major pathways are significantly potentiated by HIF-1α transcription: Toll-like receptor/nuclear factor-κB (NF-κB) and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), which lead to an increase in glucose metabolism under oxygen-independent conditions.[25] Furthermore, HIF-1α promotes the transition of pyruvate to lactate via up-regulating the expression of these two enzymes: LDH (which catalyzes pyruvate to lactate) and pyruvate dehydrogenase kinase (which inactivates and restricts the entrance of pyruvate into the TCA cycle); further increasing lactate concentration.[13,25–27] Elevated lactate can motivate vascular endothelial growth factor (VEGF) expression and M2-like polarization of TAMs through HIF-1α mediation.[28] Taken together, enhanced glycolysis and elevated lactate concentrations produce immunosuppressive interactions in TAMs.
In the TME, tumor cells can also signal TAMs via lactate.[29] Lactate derived from tumor cells is transported into macrophages by monocarboxylic acid transporter (MCT) 1 and then generates pyruvate via LDH1, which has a competitive relationship with α-ketoglutarate (α-KG) to inhibit the expression of prolyl hydroxylase (PH), finally preventing proteasome degradation of HIF-1α, circumscribing ubiquitination, and triggering the glycolytic pathway.[30] Lactate derived from cancer cells potently induces Arg-1 in TAMs via stabilizing extracellular signal-regulated kinase 1/2 (ERK1/2), signal transducer and activator of transcription (STAT) 3 and HIF-1α, which stimulates tumor growth by suppressing T-cell responses.[15,28] With enhanced glycolysis in tumor cells and TAMs, the considerable glucose in the TME is reduced, which can lead to the suppression of T cell functions as well, thereby exacerbating the immunosuppressive capacity of the TME.[30–32] In the initial stages of tumorigenesis, TAMs preferentially utilize glycolysis for obtaining energy. However, with the accumulation of lactate in the TME and the gradual reduction of oxygen, OXPHOS predominates in later stages, meanwhile glucose uptake is reduced.[22,33,34]
Oxidative phosphorylation
TAMs can eventually differentiate into M2-like TAMs by increasing glycolysis and its metabolite lactate, culminating in exerting an immunosuppressive effect. Under these circumstances, tumor cells and infiltrating immune cells scramble the restrained glucose capacity. However, M2-like TAMs avoid this nutrient contention by preferentially utilizing OXPHOS.[35,36] OXPHOS is a common route that drives ATP synthesis by using the energy liberated during the decomposition of glucose, lipids, and amino acids. TAMs exhibit enhanced OXPHOS activity, thereby producing large amounts of ATP and completing the TCA cycle.[14,25] TAMs also exhibit high basal and maximal oxygen consumption rates and generate large quantities of mitochondrial ATP. In contrast, they have decreased PPP pathway expression, indicating that it may not be required for TAMs functions.[14,37] Despite increasing OXPHOS activity, TAMs express a glycolysis-dependent phenotype and are independent of OXPHOS and PPP.[37] Although glycolysis engenders less ATP per molecule of glucose than OXPHOS, it is fundamentally more significant for TAMs.
Lipid Metabolism
Lipids also ultimately generate ATP in the mitochondria via OXPHOS, which is critical for TAMs differentiation and function.[38] TAMs are loaded with a large quantity of lipid droplets, of which triglycerides (TGs), cholesterol, and phospholipids are the foremost components.[39–41] TAMs enhance lipid metabolism to induce the CD206+ major histocompatibility complex IIlow immunosuppression phenotype.[42]
Fatty acid
Lipid deposition in TAMs leads to activation of genes associated with fatty acid β-oxidation, including carnitine palmitoyl transferase-1A (CPT1A) (an FAO rate-limiting enzyme).[15,38] The source of fatty acids is the breakdown of TGs, which are the predominant lipids.[43] TGs can be metabolized via adipose triglyceride lipase to diacylglycerols (DGs), which are decomposed by hormone-sensitive lipase (HSL) to monoacylglycerols (MGs) and by monoacylglycerol lipase (MAGL/MGLL) to free fatty acids and glycerol.[43,44] MGLL deficiency is a pivotal proportion leading to lipid accumulation in TAMs (the accumulation of MGs, DGs, and TGs); thus, macrophages activate an M2-like phenotype.[39,45] Consistent with this, lipid accumulation in TAMs was completely inhibited in a mouse model of MGLL over-expression.[46]
TAMs express high levels of the scavenger receptor CD36, accumulate lipids, and use FAO as energy source. TAMs ultimately lead to colorectal cancer progression since they are programmed to promote the ectopic activation of abhydrolase domain containing 5 (ABHD5), a well-documented activator of lipolysis without which TAMs may not survive due to a lack of FAO and energy production.[47] High FAO levels accelerate mitochondrial OXPHOS, generate reactive oxygen species (ROS), phosphorylate Janus kinase (JAK) 1 and dephosphorylate Src homology 2 domain-containing phosphatase-1 (SHP1), resulting in the activation and transcription of STAT6 to regulate TAMs generation and function, which are necessary to reeducate TAMs.[38]
Receptor interacting protein kinase 3 (RIPK3) deletion enhances FAO through the ROS/caspase 1/peroxisome proliferator-activated receptors (PPAR) pathway and promotes M2 polarization of TAMs, whose immunosuppressive function can be prominently reeducated through up-regulating RIPK3 or inhibiting FAO.[48] The activation of RIPK3 balances the storage and degradation of lipids in tumor cells in a time-dependent manner.[15] In addition, the breakdown of PPAR-γ depends on caspase-1, and disrupted PPAR-γ can translocate to the mitochondria, thereby negatively regulating FAO and inducing lipid droplet accumulation and TAMs differentiation.[15,49] In our research, we found that, after interacting with tumor cells, TAMs regulated the expression of chemokine (C-C motif) ligand 20 (CCL20) through the lipid metabolism pathway; consequently, TAMs can exert their immunosuppressive effect. In summary, FAO is critical for TAMs survival and immunosuppressive phenotypes. Inhibition of fatty acid metabolism in TAMs has been proposed as a strategy to strengthen anti-tumor effects.[50]
Cholesterol
TAMs can utilize scavenger receptors including CD36 to take up lipids, which are decomposed into free cholesterol and fatty acids by lysosomal acid lipases in the lysosomes.[51] Solid neoplasms show high levels of free cholesterol and cholesteryl esters, although these are relatively absent in TAMs.[52] In our study, we observed that, in TAMs, increased cholesterol efflux led to a decrease in intracellular cholesterol content and inflammatory factors, resulting in immunosuppression. In addition, ovarian tumor cells promote the efflux of membrane cholesterols, which causes the formation of the M2-like TAMs and stimulates tumor progression.[53] This may be one of the reasons for the reduction in intracellular cholesterol levels in TAMs.
The cholesterol transporters ATP-binding cassette transporter A1/G1 (ABCA1/G1) mediate the reversal of cholesterol transport in macrophages.[54] The outward migration of TAMs membrane cholesterol enhances the IL-4 signaling pathway and inhibits interferon (IFN)-γ-induced gene expression, leading to pro-tumor effects.[55] The loss of intracellular cholesterol supports the conversion of macrophages into M2-like TAMs; thus, cholesterol transporters play a vital role in macrophage polarization.[56] Therefore, elevated cholesterol efflux is beneficial for maintaining the immunosuppressive phenotype of TAMs.
Phospholipids
Phosphatidylcholine and phosphatidylglycerol, the main cell surfactant components, are present at low levels in tumor tissues.[52] Phospholipids are the source of lipid second messengers that activate the PI3K/AKT/mTOR pathway, which is relevant to tumorigenesis and cancer progression and causes poor prognosis.[57] Arachidonic acid (AA), a widely studied phospholipid subgroup, is integral to the regulation of inflammation and cancer.[39] Free AA can be transformed into prostaglandins, oxygenated fatty acids, and leukotrienes via three major pathways, one of which is the cyclooxygenase (COX) pathway.[58] Phospholipid metabolism changes in TAMs after infiltration of the TME since COX1/2 are generally altered in diverse phases of macrophages development.[59] Prostaglandin E2 (PGE2) is a metabolite produced by AA under the mediation of COX2, which can be secreted into the TME to stimulate TAMs to produce chemokines beneficial to tumors, for which TAMs express a pro-tumor phenotype.[60,61]
Amino Acid Metabolism
In addition to the remodeling of glucose and lipid metabolism mentioned above, a large number of recent studies have revealed the reprogramming of amino acid metabolism in TAMs. TAMs upregulate the expression of Arg-1 and IDO and enhance glutamine synthesis and catabolism, which in turn leads to the accumulation of the corresponding metabolites. These changes favor polarization of TAMs into M2-like TAMs [Figure 2].
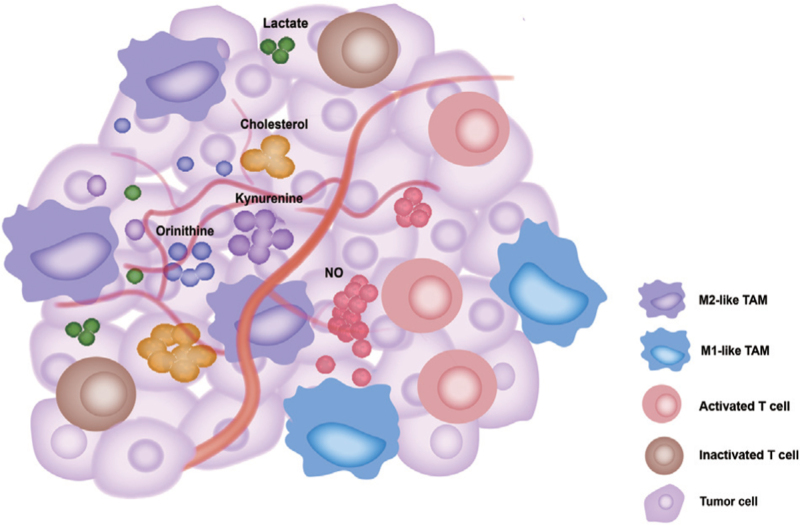
Figure 2.
M2-like TAMs undergo metabolic reprogramming, leading to changes in a series of metabolites in the TME to inhibit T cell activation. The accumulation of lactate (a metabolite from the anaerobic metabolism of glucose), kynurenine (a metabolite from tryptophan metabolism), and ornithine (a metabolite from arginine metabolism), and the efflux of cholesterol from TAMs inhibit infiltrating T cell functions at different degrees, further enhancing the immunosuppressive function of TME. In contrast, M1-like TAMs exert opposite functions, such as increasing NO production to inhibit tumor progression. NO: Nitric oxide; TAM: Tumor-associated macrophage; TME: Tumor microenvironment.
Arginine metabolism
As previously mentioned, TAMs maintain an immunosuppressive phenotype via upregulating the expression of Arg-1, resulting in a decrease in L-arginine and changes in the corresponding metabolites. The catabolism of L-arginine is mediated by Arg-1 and iNOS, which compete for the substrate L-arginine, generating a variety of metabolites that play diverse roles in tumors. It has been reported that TAMs can activate the transcription of Arg-1, which stimulates the breakdown of L-arginine into ornithine and urea. Interestingly, high levels of L-arginine in cells are essential for T cells survival and proliferation.[62,63] Depletion of L-arginine due to Arg-1 activation in TAMs promotes adjacent tumor cells and cause cancer progression.[64] Therefore, Arg-1 activity in TAMs can induce a pro-tumor phenotype, reducing T cells proliferation and cytokine production, which results in immunosuppression in the TME.[65] TAMs-derived ornithine favors the proliferation of tumor cells and can be converted by ornithine decarboxylase (ODC) into polyamines, including putrescine, spermidine, and spermine, thereby stimulating M2-related gene expression and stabilizing the tumor-promoting phenotype of TAMs.[66–69] Furthermore, ODC constrains M1-like TAMs activation through chromatin modification, leading to immunosuppression of TME.[67] TAMs preferentially take advantage of the Arg-1 pathway to metabolize L-arginine and form a competitive relationship with the iNOS pathway, which results in a deficit of NO.[70,71] Besides, NO can prevent M1-like phenotype to M2-like repolarization, because the inhibition of iNOS enables M1-like TAMs to repolarize into the M2-like phenotype upon exposure to IL-4 after LPS plus IFN-γ treatment. In the TME, a decline in NO levels increases the M2 phenotype and leads to immunosuppression.[72] Both the Arg-1 and iNOS pathways have been observed in TAMs, representing the M1/M2 phase in the ischemic tumor domain.[25] Co-expression of Arg-1 and iNOS at low arginine concentrations may be beneficial for the generation of ROS and reactive nitrogen species, and then suppresses the function of T cells in tumors. The remodeling of L-arginine metabolism in TAMs apparently favors the immunosuppressive phenotype.
Tryptophan metabolism
In malignant tumors, TAMs can deplete tryptophan in the local microenvironment through its uptake and catabolism, ultimately leading to immunosuppression.[73,74] Both TAMs and tumor cells can activate IDO to remodel the immunosuppressive environment through tryptophan consumption and the accumulation of tryptophan metabolites (kynurenine, 3-hydroxyanthranilate, and quinoline).[75,76] TAMs may strongly express IDO, which catabolizes tryptophan to kynurenine, an endogenous aryl hydrocarbon receptor (AHR) ligand.[77,78] Moreover, kynurenine can potently suppress the immune response of T cells; by mimicking AHR, kynurenine skews the conversion of naive T cell toward fork headbox p3 (FOXP3)+ regulatory T cell (Treg) and suppresses Th17 cell differentiation.[77,79] IDO+ TAMs inhibit T-cell viability, whereas pre-treatment of TAMs with IDO inhibitors reserves T-cell proliferation.[15,78,80] IDO activation can be induced by tumor necrosis factor-α, IFN-γ or prostaglandins, but TAMs squint toward the M2-like phenotype if IDO is over-expressed, while IDO silencing triggers an anti-tumor macrophage profile.[81] Therefore, TAMs can increase kynurenine by up-regulating IDO to consume tryptophan, which can produce immunosuppressive effects in the TME.
Glutamine and glutamate metabolism
The effects of glutamate metabolism in TAMs on their functional phenotypes have rarely been investigated.[5] In the TME, glutamine and glutamate have the same function; they provide energy to TAMs. In addition, glutamine powers tumor cells by being released into the TME.[69,82] Glutamatergic regulation of macrophages may be involved in the polarization of macrophages toward an immunosuppressive phenotype.[83] Moreover, glutamine deprivation has a substantial effect on M2 polarization.[84] Targeting glutamine metabolism promotes reprogramming and the pro-inflammatory phenotype of TAMs.[85] Glutamine synthetase (GS), an enzyme that synthesizes glutamine from glutamate, is probably maintaining the phenotype of M2-like TAMs. Inhibition of GS can reverse M2-like macrophages to an M1-like phenotype, manifested by lessened intracellular glutamine and incremental succinate.[86,87] TAMs in Lewis lung carcinoma (LLC) mouse models and patients with glioblastoma have been found to upregulate GS, which is induced in response to starvation and can elicit pro-tumorigenic TAMs polarization.[29,82] Macrophage-specific knockdown of GS reverses LLC-associated TAMs polarization to an anti-tumor phenotype and attenuates metastasis.[87] Not only is GS-mediated phenotypic transformation of macrophages significant, but glutamine catabolism also plays an essential role. It has been reported that α-KG from glutamine catabolism is critical for the alternative activation of macrophages (M2). Succinate is synthesized by c-aminobutyric acid and possibly promotes a partial reversal of the M2 phenotype to an M1-like phenotype.[28,88] A high α-KG/succinate proportion modulates M2 promotion, whereas a low proportion strengthens the pro-inflammatory phenotype of classically activated (M1) macrophages.[88] In summary, an increase in glutamine anabolism and catabolism is beneficial for inducing the transformation of TAMs to an M2-like phenotype.
Others
Iron metabolism
Iron is a potential mutagen that can cause tumor cells to behave more aggressively.[89,90] Tumor cells require excess iron at all times, and TAMs are key sources of iron. TAMs release iron into the TME to increase its availability.[91] Iron also influences the polarization of TAMs. Moreover, heme oxygenase 1-mediated activation of iron metabolism also contributes to TAMs polarization.[92] Intracellular iron deficiency may result in HIF activation, whereas high intracellular iron concentrations may induce an M1-like phenotype.[93,94] Hence, M2-polarized macrophages are set in an iron-export mode, while M1-polarized macrophages in an iron-retention mode.[95,96]
Nucleotide metabolism
Extracellular adenosine is a tumor metabolite that makes an impact on TAMs functions, phagocytosis and cytokine production. Adenosinergic signaling mediates various suppressive functions in infiltrating immune cells.[97,98] Myeloid cells devoid of adenosine receptor A2A prevent tumor progression and metastasis in a malignancy model.[99] In our study, A2A upregulated macrophages secretion of chemokine (C-X-C motif) ligand 5 (CXCL5) via the NF-κB pathway.
Reversing the Immunosuppression of TAMs Through Targeting Metabolism-Related Pathways
Due to TME remodeling, TAMs ultimately retain an immunosuppressive phenotype by reprogramming glucose, lipid, and amino acid metabolism. Targeting the metabolism-related pathways of TAMs presumably is conducive to the production of M1-like TAMs and thereby alters their immunosuppressive function. Simultaneously, the metabolism of tumor cells undergoes corresponding changes. Therefore, further studies are needed to determine whether treatments targeting metabolism-related pathways have anti-tumor effects.[100] Nonetheless, targeting metabolism-related pathways has been shown to be effective in suppressing tumors in mice and even in clinical trials, which works better in combination with ICIs, even in refractory tumors.[101,102]
Targeting glucose metabolism
At present, targeting the glucose metabolism-related pathways of TAMs is mainly focused on diminishing glycolysis to regulate the immunosuppressive effect of TAMs. 2-deoxyglucose (2-DG) can block the glycolytic pathway, thereby disrupting the polarization of M2-like TAMs. In addition to decreasing glycolysis, 2-DG impairs OXPHOS, resulting in the inhibition of ATP production, activation of JAK-STAT6, and failure of M2 polarization.[103,104] Meanwhile, in multiple in vitro and in vivo studies, 2-DG inhibited cancer cells survival, proliferation, and motility when combined with other targeted therapies; 2-DG has been used in clinical trials for tumor therapies, but it has exhibited strong toxicity.[105,106]
Although 2-DG has demonstrated toxicity in clinical trials, extensive malignancies have been treated with mTOR inhibitors, which can also inhibit glycolysis. In multiple mouse tumor models, it has been shown that hypoxia promotes the expression of DNA damage-inducible transcript 4 (DDT4, especially regulated in development and DNA damage response 1 [REDD1]), a well-known endogenous blocker of the mTOR complex 1 (mTORC1) in TAMs.[22] Thus, TAMs preferentially utilize oxidative metabolism while reducing glucose uptake under hypoxic conditions,[22] which has relationship with an enhanced angiogenic reaction and the formation of abnormal leaky blood vessels. It has been proved that mTORC1 inhibitors are paradoxically beneficial for tumor development due to glycolytic inhibition combined with activation of the neovascularization program.[22] Blockade of VEGFA expression in TAMs not only inhibits glycolysis, but also is detrimental to neo-angiogenesis, thereby reducing the infiltration of TAMs in the TME.[107,108]
Remarkably, therapeutic suppression of LDHs and/or MCTs predisposes TAMs toward an anti-tumor function and damages angiogenesis in malignancies. Several LDH and MCT inhibitors have entered phase I/II clinical trials, including AT-101 (a non-specific LDH inhibitor) and AZD3965 which can inhibit MCT1/2 expression.[109,110]
Besides, some drugs commonly used in the clinical treatment of non-tumor diseases have been shown to improve the inhibitory function by affecting the glucose metabolism of TAMs. Preclinical studies have demonstrated that the respiratory complex I inhibitor, metformin, can affect TAMs polarization by inhibiting M2-like reprogramming.[111–113] Acyclovir is an antibacterial and antiviral drug that polarizes macrophages to an M1-like anti-tumor phenotype by blocking the HIF-1 pathway and enhancing glucose uptake by pancreatic ductal adenocarcinoma.[114] These phenomena delineate that targeting the glucose metabolism pathway in TAMs is a promising direction.
Targeting lipid metabolism
The efficacy of targeting lipid metabolism has also been demonstrated in various mouse tumor models. Etomoxir is widely used as a CPT1 specific inhibitor.[115] CPT1 is upregulated by fatty acid uptake and oxidation.[46] Studies have indicated that etomoxir can inhibit the M2-like phenotype of TAMs and their precursor activity.[116]
Simvastatin can disrupt lipid rafts and is generally employed to decrease cholesterol level in clinical practice, which repolarizes TAMs and promotes the conversion of M2-like TAMs to the M1-like phenotype through cholesterol-related liver X receptor/ABCA1 modulation.[117] In addition, TAMs from mouse breast cancer models, especially M1-like phenotype, enhanced the expression of epithelial fatty acid binding protein (E-FABP), an intracellular lipid chaperone. Stimulation of TAMs with an E-FABP activator (EI-05) can significantly inhibit tumor growth by increasing lipid drop formation and IFN-β production.[118]
Targeting amino acid metabolism
Targeting pathways related to amino acid metabolism has shown remarkable efficacy. JHU083 is a precursor drug that extensively inhibits glutamine metabolism enzymes, targets glutamine metabolism, and reconstructs TAMs into an M1-like phenotype, strengthening anti-tumor therapies without influencing the total TAMs in tumors.[85] JHU083 also blocked glutamine metabolism in tumor cells, thereby inhibiting tumor growth in various mouse tumor models.[119]
TAMs up-regulate Arg-1 expression through the PI3K/AKT/mTOR pathway, resulting in an enhancement of the L-arginine metabolism, leading to immunosuppression. In mouse experiments, deletion of PI3Kγ and PIK3cg can inhibit the expression of Arg-1 and increase iNOS correspondingly, leading to an increase in intracellular L-arginine content, which ultimately results in immune activation and tumor suppression.[120,121]
Except for arginine metabolism, we have described above that TAMs strongly express IDO, thereby decomposing tryptophan and eventually producing inhibitory effects on T cells, resulting in an immunosuppressive function. For example, high IDO1 expression in sentinel lymph nodes was intimately related to tumor infiltrating lymphocyte reduction and poor prognosis in patients with melanoma.[122] Immunotherapy-treated IDO-knockout melanoma mice were found to live longer. IDO-inhibiting drugs hold promise as a new strategy for adjuvant therapy in IDO-expressing cancers [Table 1].[123,124]
Table 1.
Summary of clinical trials targeting metabolism-related pathway.
| Targeted metabolism | Molecular target | Agent | Metabolic changes | Effects on TAMs | Identifier | Stage | Conditions |
| Glucose metabolism | BRAF | Phenformin | Upregulates GLUT-1 or GLUT-3; inhibits OXPHOS | Reduces polarization and infiltration of M2-like TAMs | NCT03026517 | I | Melanoma |
| Hexokinase 2 | 2-deoxyglucose (2-DG) | Inhibits glucose uptake and glycolysis | Converts M2-like TAMs to M1-like TAMs | Completed (stopped due to toxicity) | I | Cancer in general | |
| HIF-1α | EZN-2968 | Inhibits HIF-1α | None | NCT01120288 | I | Advanced solid tumors with liver metastasis | |
| Lipid metabolism | COX1/2 | Aspirin | Suppresses oxidative stress and ROS metabolism | Increases M1 marker expression while decreases that of M2 | NCT04188119NCT02659384 | II | Ovarian cancer, HNSCC, TNBC, solid adult tumor, multiple myeloma |
| HMGCR | Lipophilic statins (simvastatin and lovastatin) | Influences cholesterol metabolism and reduces lactate production | None | NCT03275376 NCT03324425 | II | Hepatocellular carcinoma, squamous cell carcinoma, NSCLC, breast cancer | |
| SREBP-2 | Fatostatin | Inhibits the production of fatty acids and cholesterol synthesis; causes mitotic arrest | None | None | None | Prostate, pancreatic, and endometrial cancers | |
| Amino acid metabolism | mTOR | Ridaforolimus | Influences amino acid, glucose, nucleotide, fatty acid, and lipid metabolisms | Promotes M2 differentiation to cytotoxic M1 phenotype | NCT00086125NCT00122343NCT00538239 | III/Done | Hematological malignancies, metastatic endometrial cancer, sarcoma |
| IDO1 | Epacadostat | Inhibits tryptophan metabolism; reduces kynurenine | Reeducates M2-like TAMs to M1-like | NCT03832673 | II | Muscle-invasive urothelial cancer of the bladder | |
| Arginase-1 | L-norvaline andCB-1158 | Increases arginine metabolism via iNOS, increasing NO | Reeducates M2-like TAMs to M1-like | NCT02903914NCT03314935 | I/II | Advanced solid tumors | |
| GLS | CB-839 | Inhibits glutamine metabolism | Activates proinflammatory TAMs | NCT03875313NCT02861300 | I/II | Colorectal cancer, NSCLC, renal cell carcinoma, melanoma | |
| Adenosine metabolism | CD73 | Oleclumab | Reduces adenosine production | Enhances M1 macrophages predominance | NCT04262375 | II | Renal, pancreatic, head and neck cancer, and NSCLC with DNA methylation |
| Nucleotide biosynthesis | DHFRGARFT | MethotrexatePemetrexed | Impairs nucleotide biosynthesis | None | NCT00808639 | II | Breast cancer |
COX1/2: Cyclooxygenase 1/2; DHFR: Dihydrofolate reductase; GARFT: Glycinamide ribonucleotide formyltransferase; GLS: Glutaminase; GLUT: Glucose transporter; HIF-1α: Hypoxia-inducible factor-1α; HMGCR: 3-hydroxy-3-methyl glutaryl-coenzyme A reductase; HNSCC: Head and neck squamous cell carcinoma; IDO1: Indoleamine 2,3-dioxygenase 1; iNOS: Inducible nitric oxide synthase; mTOR: Mammalian target of rapamycin; NO: Nitric oxide; NSCLC: Non-small cell lung cancer; OXPHOS: Oxidative phosphorylation; ROS: Reactive oxygen species; SREBP-2: Sterol regulatory binding protein-2; TAMs: Tumor-associated macrophages; TNBC: Triple negative breast cancer.
Synergistic Application of Metabolism-Targeted Therapy and ICIs
Studies have found that the signaling level of programmed cell death ligand 1 (PD-L1) on TAMs affects the disease progression of melanoma and ovarian cancer, and that blockage of programmed cell death 1 (PD-1) and PD-L1 expression in TAMs can partially restore M1-like phenotype and function,[125,126] suggesting that combination of metabolism-related therapy and ICIs may address some bottlenecks in immunotherapy.
TAMs over-express COX2 and microsomal prostaglandin E synthase 1 (mPGES1), which promotes AA to activate PGE2, directly associated with PD-L1 expression.[127] Combination therapy with celecoxib (a selective COX2 inhibitor which boosts tumor cells apoptosis) and anti-PD-1 inhibits PD-L1 expression in B16-F10 melanoma and 4T1 breast cancer models.[128,129] Studies have found that 2,4-dinitrophenol (DNP) simultaneously restrained the expression of COX2 and PD-L1, inhibited the secretion of prostaglandins, blocked the oncogene c-Myc, and depressed the breast cancer (BC)-related protein bromodomain-containing protein 4 production and ERK1/2 phosphorylation in BC cells. DNP also exhibited strong anti-tumor effects in a triple-negative breast cancer (TNBC) mouse model.[130]
The co-expression of PD-L1 and lactate dehydrogenase A (LDHA), which are exceedingly expressed in TNBC cells and tissues, is related to adverse outcomes in TNBC. Both PD-L1 and LDHA functions are inhibited by miR-34a. Combining immunotherapy and metabolic therapy targeting PD-L1 and LDHA might be beneficial for the treatment of breast cancer (especially TNBC).[131] High levels of IDO1 exert an immunosuppressive effect, inhibiting the efficacy of anti-cytotoxic T lymphocyte associated antigen 4 and (PD1/L1) treatments, whereas the response to immunotherapy and chemotherapy is enhanced when IDO1 expression is suppressed.[132,133] A phase I/II clinical research indicated that the combination of nivolumab and IO102/IO103, an investigational vaccine targeting IDO and PD-L1, decreased tumor burden and increased progression-free survival.[134]
Conclusions and Perspectives
Reprogramming of cellular energy metabolism is an emerging hallmark of cancer.[135] In this review, we expound the metabolic reprogramming of TAMs connected with their immunosuppressive function. We elaborate on the glucose, lipid, and amino acid metabolism modifications needed for TAMs reprogramming. TAMs increase glycolysis and FAO, promote cholesterol efflux, up-regulate Arg-1 and IDO expression to elevate arginine and tryptophan metabolism, and enhance glutamine and glutamate metabolism, which ultimately favor TAMs to maintain an immunosuppressive phenotype.
Based on these metabolic changes, therapies targeting metabolism-related pathways have also been found to have favorable effects. In combination with PD-1/L1, inhibitors of COX2 and LDHA have also achieved encouraging results in refractory tumor models. Although immune checkpoint therapy has shown surprising efficacy, strategies targeting TAMs have gradually attracted attention in order to further address the tolerance phenomenon that occurs during treatment, but almost all of them are in the preclinical stage. Targeting TAMs reprogramming has shown potential for the therapeutic strategies of solid tumors.[136,137] This suggests that targeting metabolism-related pathways may provide new opportunities and options for future tumor immunotherapy. However, many questions remain unanswered: As the TME is a metabolically interrelated whole, how does targeting metabolism affect other immune cells? What about the serious adverse reactions associated with targeted metabolism, such as those associated with 2-DG? How can the TME metabolism balance be restored? With the continuous in-depth study of TAMs-related metabolism in the TME, we will open a new chapter in anti-tumor therapy.
Funding
This study was supported by grants from the National Key Research and Development Program of China (No. 2021YFE0110600) and the National Natural Science Foundation of China (Nos. 91942314, 82072578, 82002564, and 81872333).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang Y, Wang D, Yang L, Zhang Y. Metabolic reprogramming in the immunosuppression of tumor-associated macrophages. Chin Med J 2022;135:2405–2416. doi: 10.1097/CM9.0000000000002426
References
- 1.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol 2017; 10:58.doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res 2016; 2016:9720912.doi: 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 2019; 35:588–602. e10. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015; 21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. Biomark Res 2021; 9:1.doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Di Martino JS, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T, et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov 2018; 8:1006–1025. doi: 10.1158/2159-8290.CD-17-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitale I, Manic G, Galassi C, Galluzzi L. Stress responses in stromal cells and tumor homeostasis. Pharmacol Ther 2019; 200:55–68. doi: 10.1016/j.pharmthera.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab 2017; 25:1282–1293. e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021; 593:282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Wang J, Yadav DK, Bai X, Liang T. Glucose metabolism: the metabolic signature of tumor associated macrophage. Front Immunol 2021; 12:702580.doi: 10.3389/fimmu.2021.702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 2009; 114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1 α-Induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm 2017; 2017:9029327.doi: 10.1155/2017/9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 1996; 271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 14.Arts RJ, Plantinga TS, Tuit S, Ulas T, Heinhuis B, Tesselaar M, et al. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. Oncoimmunology 2016; 5:e1229725.doi: 10.1080/2162402X.2016.1229725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mojsilovic SS, Mojsilovic S, Villar VH, Santibanez JF. The Metabolic features of tumor-associated macrophages: opportunities for immunotherapy. Anal Cell Pathol 2021; 2021:5523055.doi: 10.1155/2021/5523055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penny HL, Sieow JL, Adriani G, Yeap WH, See Chi Ee P, San Luis B, et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology 2016; 5:e1191731.doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Chang C, Lu N, Wang X, Lu Q, Ren X, et al. Comprehensive proteomics analysis reveals metabolic reprogramming of tumor-associated macrophages stimulated by the tumor microenvironment. J Proteome Res 2017; 16:288–297. doi: 10.1021/acs.jproteome.6b00604. [DOI] [PubMed] [Google Scholar]
- 18.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell 2017; 171:358–371. e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017; 551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A 2017; 114:580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019; 574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab 2016; 24:701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Lu ZN, Song J, Sun TH, Sun G. UBE2C affects breast cancer proliferation through the AKT/mTOR signaling pathway. Chin Med J 2021; 134:2465–2474. doi: 10.1097/CM9.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley AM, Lynam-Lennon N, O’Neill H, O'Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol 2020; 17:298–313. doi: 10.1038/s41575-019-0247-2. [DOI] [PubMed] [Google Scholar]
- 25.Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol 2019; 10:1462.doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006; 3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 2015; 21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzone M, Menga A, Castegna A. Metabolism and TAM functions-it takes two to tango. FEBS J 2018; 285:700–716. doi: 10.1111/febs.14295. [DOI] [PubMed] [Google Scholar]
- 29.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem 2005; 280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 31.Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer 2020; 8:e001408.doi: 10.1136/jitc-2020-001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Yang Q, Li Z, Xu Z, Sun S, Wu Q, et al. Expression of monocarboxylate transporter 1 in immunosuppressive macrophages is associated with the poor prognosis in breast cancer. Front Oncol 2020; 10:574787.doi: 10.3389/fonc.2020.574787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol 2010; 184:1200–1209. doi: 10.4049/jimmunol.0902584. [DOI] [PubMed] [Google Scholar]
- 34.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4 (+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015; 162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed Cell Death Ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res 2018; 6:1260–1273. doi: 10.1158/2326-6066.CIR-17-0537. [DOI] [PubMed] [Google Scholar]
- 37.M de-Brito N, Duncan-Moretti J, C da-Costa H, Saldanha-Gama R, Paula-Neto HA, G Dorighello G, et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta Mol Cell Res 2020; 1867:118604.doi: 10.1016/j.bbamcr.2019.118604. [DOI] [PubMed] [Google Scholar]
- 38.Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res 2020; 80:1438–1450. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang Y, Miao H. Lipid metabolism in tumor-associated macrophages. Adv Exp Med Biol 2021; 1316:87–101. doi: 10.1007/978-981-33-6785-2_6. [DOI] [PubMed] [Google Scholar]
- 40.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010; 16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y, Zou T, Shen X, Nelson PJ, Li J, Wu C, et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020; 2:27–59. doi: 10.1002/mco2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med 2019; 11:e10698.doi: 10.15252/emmm.201910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun 2018; 38:27.doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ou J, Miao H, Ma Y, Guo F, Deng J, Wei X, et al. Loss of abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial-mesenchymal transition. Cell Rep 2014; 9:1798–1811. doi: 10.1016/j.celrep.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang W, Shi R, Kang X, Zhang X, Chen P, Zhang L, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun 2018; 9:2574.doi: 10.1038/s41467-018-04999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol 2021; 14:187.doi: 10.1186/s13045-021-01200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao H, Ou J, Peng Y, Zhang X, Chen Y, Hao L, et al. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat Commun 2016; 7:11716.doi: 10.1038/ncomms11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res 2020; 8:710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 49.Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen B, et al. Caspase-1 cleaves PPARγ for potentiating the pro-tumor action of TAMs. Nat Commun 2017; 8:766.doi: 10.1038/s41467-017-00523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res 2015; 3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017; 541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 52.Hoppstädter J, Dembek A, Höring M, Schymik HS, Dahlem C, Sultan A, et al. Dysregulation of cholesterol homeostasis in human lung cancer tissue and tumour-associated macrophages. EBioMedicine 2021; 72:103578.doi: 10.1016/j.ebiom.2021.103578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab 2019; 29:1376–1389. e4. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 2007; 117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer 2020; 1874:188394.doi: 10.1016/j.bbcan.2020.188394. [DOI] [PubMed] [Google Scholar]
- 56.Pradel LC, Mitchell AJ, Zarubica A, Dufort L, Chasson L, Naquet P, et al. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine-triggered polarization programs. Eur J Immunol 2009; 39:2270–2280. doi: 10.1002/eji.200838867. [DOI] [PubMed] [Google Scholar]
- 57.Tan AC. Targeting the PI3K/AKT/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac Cancer 2020; 11:511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colby JK, Jaoude J, Liu F, Shureiqi I. Oxygenated lipid signaling in tumor-associated macrophages-focus on colon cancer. Cancer Metastasis Rev 2018; 37:289–315. doi: 10.1007/s10555-018-9743-z. [DOI] [PubMed] [Google Scholar]
- 59.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 60.Shi SZ, Lee EJ, Lin YJ, Chen L, Zheng HY, He XQ, et al. Recruitment of monocytes and epigenetic silencing of intratumoral CYP7B1 primarily contribute to the accumulation of 27-hydroxycholesterol in breast cancer. Am J Cancer Res 2019; 9:2194–2208. [PMC free article] [PubMed] [Google Scholar]
- 61.Eruslanov E, Daurkin I, Vieweg J, Daaka Y, Kusmartsev S. Aberrant PGE2 metabolism in bladder tumor microenvironment promotes immunosuppressive phenotype of tumor-infiltrating myeloid cells. Int Immunopharmacol 2011; 11:848–855. doi: 10.1016/j.intimp.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kγ Drives pancreatic ductal adenocarcinoma progression. Cancer Discov 2016; 6:870–885. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016; 167:829–842. e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 2012; 32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 65.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015; 25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr 2007; 137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 67.Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A 2017; 114:E751–E760. doi: 10.1073/pnas.1614958114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van den Bossche J, Lamers WH, Koehler ES, Geuns JM, Alhonen L, Uimari A, et al. Pivotal advance: arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol 2012; 91:685–699. doi: 10.1189/jlb.0911453. [DOI] [PubMed] [Google Scholar]
- 69.Rabold K, Netea MG, Adema GJ, Netea-Maier RT. Cellular metabolism of tumor-associated macrophages - functional impact and consequences. FEBS Lett 2017; 591:3022–3041. doi: 10.1002/1873-3468.12771. [DOI] [PubMed] [Google Scholar]
- 70.Iniesta V, Gómez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J Exp Med 2001; 193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das P, Lahiri A, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog 2010; 6:e1000899.doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 2016; 17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999; 189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005; 22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol 2003; 81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 76.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res 2012; 72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 77.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 78.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010; 185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephens GL, Wang Q, Swerdlow B, Bhat G, Kolbeck R, Fung M. Kynurenine 3-monooxygenase mediates inhibition of Th17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur J Immunol 2013; 43:1727–1734. doi: 10.1002/eji.201242779. [DOI] [PubMed] [Google Scholar]
- 80.Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ, et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol 2012; 188:1117–1124. doi: 10.4049/jimmunol.1100164. [DOI] [PubMed] [Google Scholar]
- 81.Wang XF, Wang HS, Wang H, Zhang F, Wang KF, Guo Q, et al. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell Immunol 2014; 289:42–48. doi: 10.1016/j.cellimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Choi J, Stradmann-Bellinghausen B, Yakubov E, Savaskan NE, Régnier-Vigouroux A. Glioblastoma cells induce differential glutamatergic gene expressions in human tumor-associated microglia/macrophages and monocyte-derived macrophages. Cancer Biol Ther 2015; 16:1205–1213. doi: 10.1080/15384047.2015.1056406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanshiashvili L, Tsitsilashvili E, Dabrundashvili N, Kalandadze I, Mikeladze D. Metabotropic glutamate receptor 5 may be involved in macrophage plasticity. Biol Res 2017; 50:4.doi: 10.1186/s40659-017-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015; 42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest 2020; 130:3865–3884. doi: 10.1172/JCI131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mills EL, Kelly B, Logan A, Costa A, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016; 167:457–470. e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palmieri EM, Menga A, Martín-Pérez R, Quinto A, Riera-Domingo C, De Tullio G, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep 2017; 20:1654–1666. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 2017; 18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 89.Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M. Iron in the tumor microenvironment-connecting the dots. Front Oncol 2018; 8:549.doi: 10.3389/fonc.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thanan R, Oikawa S, Yongvanit P, Hiraku Y, Ma N, Pinlaor S, et al. Inflammation-induced protein carbonylation contributes to poor prognosis for cholangiocarcinoma. Free Radic Biol Med 2012; 52:1465–1472. doi: 10.1016/j.freeradbiomed.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Mertens C, Mora J, Ören B, Grein S, Winslow S, Scholich K, et al. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology 2018; 7:e1408751.doi: 10.1080/2162402X.2017.1408751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int Rev Immunol 2015; 34:82–100. doi: 10.3109/08830185.2014.969421. [DOI] [PubMed] [Google Scholar]
- 93.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011; 121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol 2011; 32:241–247. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Recalcati S, Minotti G, Cairo G. Iron regulatory proteins: from molecular mechanisms to drug development. Antioxid Redox Signal 2010; 13:1593–1616. doi: 10.1089/ars.2009.2983. [DOI] [PubMed] [Google Scholar]
- 96.Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev 2012; 11:883–889. doi: 10.1016/j.autrev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Vijayan D, Young A, Teng M, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 2017; 17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 98.Kepp O, Loos F, Liu P, Kroemer G. Extracellular nucleosides and nucleotides as immunomodulators. Immunol Rev 2017; 280:83–92. doi: 10.1111/imr.12571. [DOI] [PubMed] [Google Scholar]
- 99.Mantovani A, Marchesi F, Jaillon S, Garlanda C, Allavena P. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol 2021; 18:566–578. doi: 10.1038/s41423-020-00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab 2019; 30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 101.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med 2018; 10:eaan3311.doi: 10.1126/scitranslmed.aan3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab 2018; 28:463–475. e4. doi: 10.1016/j.cmet.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jian SL, Chen WW, Su YC, Su YW, Chuang TH, Hsu SC, et al. Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis 2017; 8:e2779.doi: 10.1038/cddis.2017.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett 2014; 355:176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 106.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013; 496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A 2016; 113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A 2016; 113:4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat 2001; 66:239–248. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 110.Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol 2011; 6:1757–1760. doi: 10.1097/JTO.0b013e31822e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res 2017; 5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol 2014; 92:543–552. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 113.Chiang CF, Chao TT, Su YF, Hsu CC, Chien CY, Chiu KC, et al. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget 2017; 8:20706–20718. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bulle A, Dekervel J, Deschuttere L, Nittner D, Van Cutsem E, Verslype C, et al. Anti-cancer activity of acriflavine as metabolic inhibitor of OXPHOS in pancreas cancer xenografts. Onco Targets Ther 2020; 13:6907–6916. doi: 10.2147/OTT.S245134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim K, Desousa BR, et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab 2018; 28:490–503. e7. doi: 10.1016/j.cmet.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Q, Wang H, Mao C, Sun M, Dominah G, Chen L, et al. Fatty acid oxidation contributes to IL-1β secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Mol Immunol 2018; 94:27–35. doi: 10.1016/j.molimm.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J, et al. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 2019; 9:265–278. doi: 10.7150/thno.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao E, Singh P, Zhai X, Li Y, Zhu G, Zhang Y, et al. Inhibition of tumor growth by a newly-identified activator for epidermal fatty acid binding protein. Oncotarget 2015; 6:7815–7827. doi: 10.18632/oncotarget.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019; 366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016; 539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas AC, Mattila JT. “Of mice and men”: arginine metabolism in macrophages. Front Immunol 2014; 5:479.doi: 10.3389/fimmu.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chevolet I, Speeckaert R, Haspeslagh M, Neyns B, Krüse V, Schreuer M, et al. Peritumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control. Br J Dermatol 2014; 171:987–995. doi: 10.1111/bjd.13100. [DOI] [PubMed] [Google Scholar]
- 123.Zhang T, Tan XL, Xu Y, Wang ZZ, Xiao CH, Liu R. Expression and prognostic value of indoleamine 2,3-dioxygenase in pancreatic cancer. Chin Med J 2017; 130:710–716. doi: 10.4103/0366-6999.201613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang L, Li A, Lei Q, Zhang Y. Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. J Hematol Oncol 2019; 12:125.doi: 10.1186/s13045-019-0804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018; 128:805–815. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A 2017; 114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosas C, Sinning M, Ferreira A, Fuenzalida M, Lemus D. Celecoxib decreases growth and angiogenesis and promotes apoptosis in a tumor cell line resistant to chemotherapy. Biol Res 2014; 47:27.doi: 10.1186/0717-6287-47-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016; 5:e1074374.doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin S, Muhammad N, Sun Y, Tan Y, Yuan H, Song D, et al. Multispecific Platinum(IV) complex deters breast cancer via interposing inflammation and immunosuppression as an inhibitor of COX-2 and PD-L1. Angew Chem Int Ed Engl 2020; 59:23313–23321. doi: 10.1002/anie.202011273. [DOI] [PubMed] [Google Scholar]
- 131.Huang X, Xie X, Wang H, Xiao X, Yang L, Tian Z, et al. PDL1 And LDHA act as ceRNAs in triple negative breast cancer by regulating miR-34a. J Exp Clin Cancer Res 2017; 36:129.doi: 10.1186/s13046-017-0593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 2005; 11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 134.Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol 2022; 15:24.doi: 10.1186/s13045-022-01242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer 2020; 19:50.doi: 10.1186/s12943-020-01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Y, Jin H, Song Y, Huang T, Cao J, Tang Q, et al. Targeting tumor-associated macrophages: a potential treatment for solid tumors. J Cell Physiol 2021; 236:3445–3465. doi: 10.1002/jcp.30139. [DOI] [PubMed] [Google Scholar]
- 137.Gao J, Liang Y, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol 2022; 13:888713.doi: 10.3389/fimmu.2022.888713. [DOI] [PMC free article] [PubMed] [Google Scholar]