Abstract
Background
Increasing copayments for higher-priced prescription medications has been suggested as a means to help finance drug coverage for elderly patients, but evaluations of the impact of such policies are rare. The objective of this study was to analyze the effect of reference-based pricing of angiotensin- converting enzyme (ACE) inhibitors on drug utilization, cost savings and potential substitution with other medication classes.
Methods
We analyzed 36 months of claims data from British Columbia for 2 years before and 1 year after implementation of reference-based pricing (in January 1997). The 119 074 patients were community-living Pharmacare beneficiaries 65 years of age or older who used ACE inhibitors during the study period. The main outcomes were changes over time in use of ACE inhibitors, use of antihypertensive drugs and expenditures for antihypertensive drugs, as well as predictors of medication switching related to reference-based pricing.
Results
We observed a sharp decline (29%) in the use of higher-priced cost-shared ACE inhibitors immediately after implementation of the policy (p < 0.001). After a transition period, the post-implementation utilization rate for all ACE inhibitors was 11% lower than projected from pre-implementation data. However, overall utilization of antihypertensives was unchanged (p = 0.40). The policy saved $6.7 million in pharmaceutical expenditures during its first 12 months. Patients with heart failure or diabetes mellitus who were taking a cost-shared ACE inhibitor were more likely to remain on the same medication after implementation of reference-based pricing (OR 1.12 [95% confidence interval, CI, 1.06–1.19] and 1.28 [95% CI 1.20–1.36] respectively). Patients with low-income status were more likely than those with high-income status to stop all antihypertensive therapy (OR 1.65 [95% CI 1.43–1.89]), which reflects a general trend toward discontinuation of therapy among these patients even before implementation of reference-based pricing.
Interpretation
Reference-based pricing in British Columbia achieved a sustained reduction in drug expenditures, and no changes in overall use of antihypertensive therapy were observed. Further research is needed on the overall health and economic effects of such policies.
Drugs are consuming an increasing share of Canada's health care dollar, and they now represent the second largest category of health expenditures after hospital services. In 2000, Canadian expenditures on prescription drugs had reached Can$11.4 billion, exceeding spending for physician services and representing a 10.3% increase from 1999.1 Spending for prescription drugs in the United States was US$78.9 billion dollars in 1997, which represented 8% of overall health care expenditures at the time, and these costs have been growing 2.5 to 3 times faster than total health care expenditures since then.2,3,4 People 65 years of age and older receive about 40% of all prescriptions.5
The increasing number of policies to contain drug expenditures in North America6 and Europe, including differential cost-sharing, have not been without controversy. A common form of differential cost sharing is reference-based pricing,7 whereby only the cost of a specific, less expensive “no-cost” drug within a therapeutic class is covered by drug benefit plans. For more costly drugs in the same therapeutic class, the patient must pay the difference between the reference price and the actual cost. Reference-based pricing is founded on the assumption that medication classes with therapeutic equivalence can be identified.
Critics of reference-based pricing argue that it may be impossible to identify therapeutically equivalent medications8 and that patients may switch to a less effective, low-cost treatment in critical circumstances, may reduce compliance or may stop therapy if they have to pay for their drugs. Such switching may be associated with more frequent visits to the physician, more medical procedures and more frequent hospital admissions.9,10,11,12,13,14,15,16,17 Access to specific medications may be disproportionately reduced among low-income or elderly patients.18 A recent time-series study found that a 25% cost-sharing policy for prescription drugs (up to an income- dependent deductible) in Quebec led to a 9.1% reduction in the use of essential drugs and increased by 14.2% the number of emergency department visits by elderly patients.19
Angiotensin-converting enzyme (ACE) inhibitors are effective antihypertensive medications that are also considered first-line agents for treatment of congestive heart failure in elderly patients.20,21,22,23,24,25,26 They also have benefits after recent myocardial infarction27,28 and for patients with diabetes or other chronic renal diseases.29,30 Although ACE inhibitors are commonly affected by differential cost-sharing policies, no data are available on the effects of such policies on utilization of these agents, particularly in subgroups of patients with heart failure, recent myocardial infarction or diabetes. It is essential that cost-containment policies not reduce adherence to antihypertensive drug therapy, which is already low.31
The British Columbia government introduced reference-based pricing for ACE inhibitors on Jan. 1, 1997. Costs for captopril, quinapril and ramipril were covered by the reference price of $27 per monthly supply. For other ACE inhibitors, patients paid any difference between the reference price ($27) and the actual drug cost.32
Physicians can request individual exemptions in cases of intolerance to the drug or treatment failure or if the patient is frail; 98% of such requests are approved.33 Prescriptions by cardiologists and pulmonary specialists are not affected by the policy. Patients with diabetes or asthma, as identified by their medication use, receive a general exemption from the policy.
We studied the impact of reference-based pricing on utilization of ACE inhibitors by elderly patients in British Columbia, expenditures for these drugs and switching of medications.
Methods
For pharmaceutical benefits within British Columbia's publicly funded health care system, the substance, the dose and the number of pills dispensed are entered into a computer network by trained pharmacists. Underreporting and misclassification appear minimal.34 Although previous reports indicate reasonable levels of accuracy and completeness of diagnostic coding in British Columbia's health care system,35 misclassification of diagnoses (according to the International Classification of Diseases, 9th revision [ICD-9]) is probably similar to that occurring in other research using administrative databases.36,37,38,39 We assembled patient characteristics and individual drug and health care utilization data by linking claims databases through personal health numbers.
The study population encompassed patients enrolled in Pharmacare Plan A, the province-wide pharmaceutical benefits program for all community-living residents 65 years of age and older (approximately 479 000 people in 1995 and 509 000 in 1998).40 We identified all patients for whom at least one ACE inhibitor was dispensed between January 1995 and June 1998 (n = 119 074). Patients who immigrated to British Columbia or who turned 65 years during the observation period were included and contributed information starting on the day of immigration or their 65th birthday. For studying the effects of reference-based pricing on use of and costs for all antihypertensives, we restricted the study population to people who had been using any ACE inhibitor before implementation of reference-based pricing (n = 59 623) (during the period from Oct. 1, 1995, to Mar. 31, 1996).
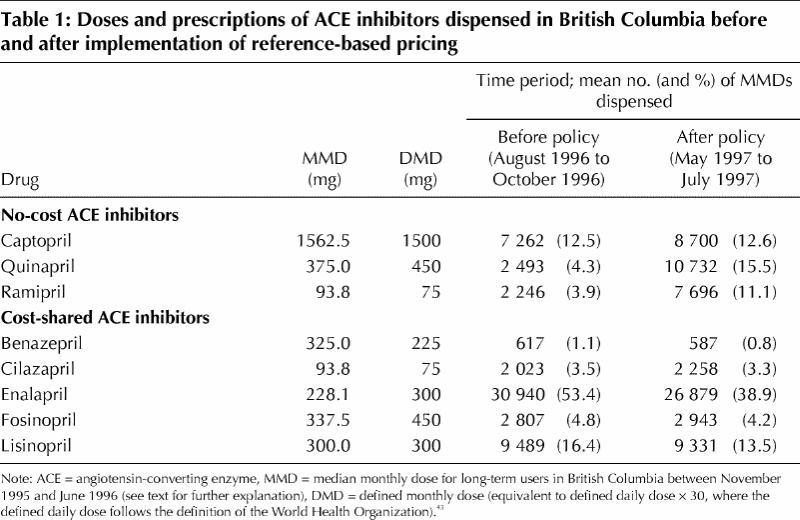
For each of the 8 ACE inhibitors, we determined the median monthly dose (MMD, in milligrams) dispensed during the 8-month period from Nov. 1, 1995, to June 30, 1996, for patients who filled at least one prescription during a period of 120 days before and a period of 120 days after the 8-month period. The MMD was a standard measure of dose across all available ACE inhibitors (Table 1).41,42 The MMDs were similar to defined monthly doses43 (Table 1).
Table 1
We plotted trends over time with 95% confidence limits. We used generalized linear models for repeated measures to estimate sudden changes in trends or levels of ACE inhibitor use after the introduction of reference-based pricing. Regression models included a constant term, a term for linear time trend before reference-based pricing, and binary indicators for a 5-month transition period from Dec. 1, 1996 (immediately after the policy announcement) to Apr. 30, 1997, and for the period after Apr. 30, 1997, to distinguish trends during the transition phase from any longer-term effects. We also included 2 linear time trends for those periods to measure changes in slope after the policy change.17,44,45 Because of the large number of observations, we assumed normal distributions of monthly rates. A Durbin–Watson test indicated autocorrelation of the data.46 Therefore, we assumed autocorrelated covariance structures in the regression models. We determined the statistical significance of regression coefficients with 2-sided t-tests, and we present slope estimates and percent changes between predicted and observed trends in utilization of medication. Two-sided p values for changes in level are reported only for the point of interruption of the trend lines.
For the analysis of predictors of switching medications, we constructed a pair of dispensings for each patient, one before and one after the policy change. The first dispensing of the pair was defined as the first dispensing of an ACE inhibitor after Sept. 1, 1996, but before Dec. 31, 1996; the second dispensing was defined as the last dispensing of an ACE inhibitor after Jan. 1, 1997, but before June 30, 1997. Patients who stayed on a high-priced cost-shared ACE inhibitor after implementation of reference-based pricing were subclassified as those with a policy exemption and those making a copayment. Some patients switched from a cost-shared drug to a no-cost drug. Among patients without a second dispensing of an ACE inhibitor, as defined above, we identified those who switched from a cost-shared ACE inhibitor to another antihypertensive and those who stopped all antihypertensive therapy for at least 6 months but were still eligible for pharmaceutical benefits and had not been admitted to hospital.
The characteristics of patients in each of these 5 utilization groups were compared with those of all remaining patients in 5 multiple logistic regression models. Thus, 4 utilization groups (excluding the group of interest) were collapsed to form the comparison group for each analysis. Multiplicative interaction terms were tested and rejected at an alpha level of 10%. Odds ratios and 95% confidence intervals are reported.
Potential predictors of switching were assessed for the 6 months before the policy announcement. These potential predictors were patient's age and sex; patient's income status (household-adjusted annual income based on individual premium- subsidy codes recorded in the Medical Services Plan database: high = greater than $19 000, moderate = $11 001 to $19 000, low = up to $11 000); cost of the drug (excluding dispensing fees) per MMD; diagnosis of acute or old myocardial infarction (ICD 410 or 412 respectively), diabetes mellitus (ICD 250), heart failure (ICD 428 or 402) or chronic renal failure (ICD 582–586) coded during at least 2 ambulatory visits or 1 hospital admission during the 6-month period; and prescribing physician's sex and graduation year. We also defined several comorbidity measures, including the number of different 3-digit ICD-9 codes, the chronic disease score (a health status measure based on a weighted sum of dispensings of specific classes of prescription medications47,48), and the number of physician visits, elective admissions to hospital and emergency admissions during the 6-month period.
Results
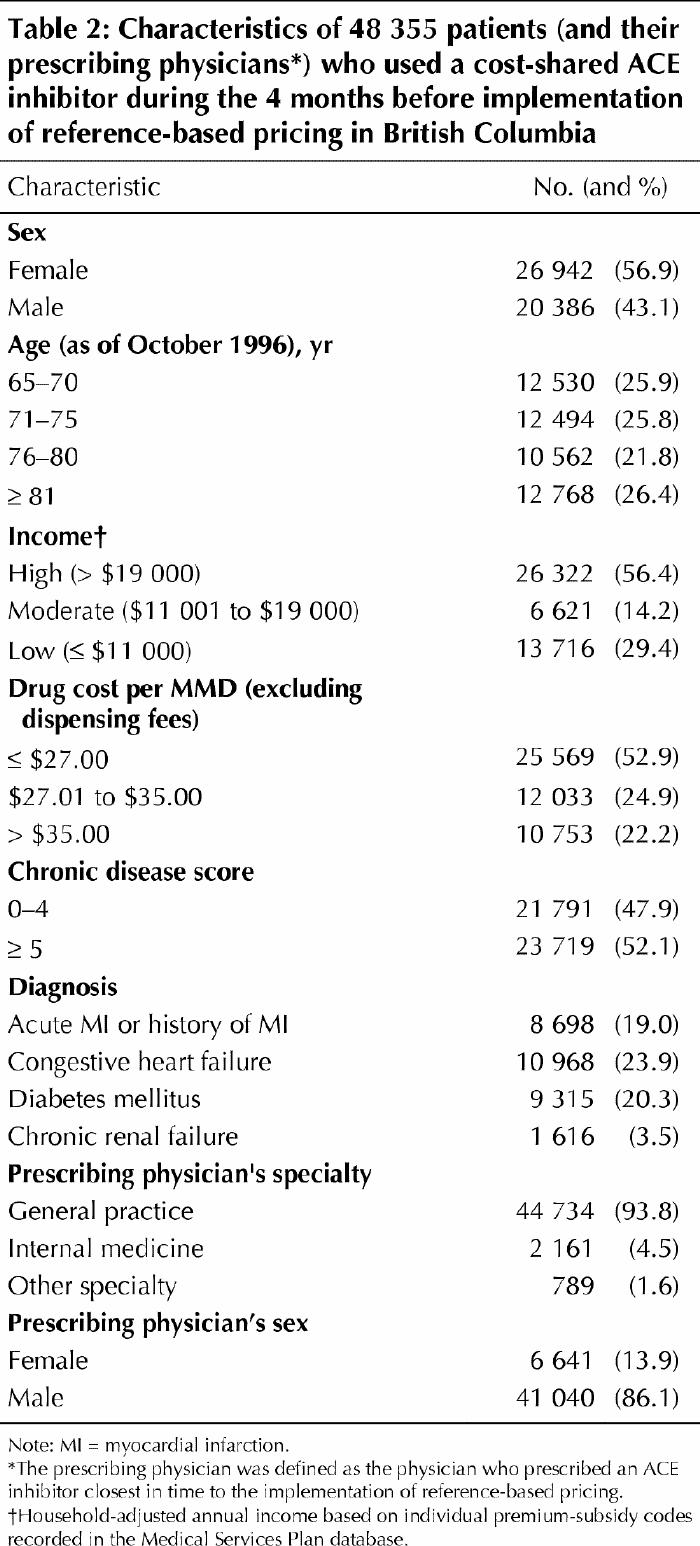
The characteristics of the 119 074 patients using ACE inhibitors were stable during the 42-month observation period. Throughout the period, women represented about 57% of the population, with little month-to-month variation. In the study, 56.4% of the patients had high incomes, 14.2% had moderate incomes and 29.4% were in the lowest income category (Table 2). The mean age was 73.3 (standard deviation 8.3) years, with a decreasing trend of 0.5 years of age per calendar year.
Table 2
In January 1995, 1230 MMDs of cost-shared ACE inhibitors were dispensed per 10 000 senior citizens (Fig. 1). The rate of dispensing of cost-shared ACE inhibitors increased by 16 MMDs (1.3%) per month (p < 0.001) before the reference-based pricing policy become effective in January 1997. After a sharp drop of 462 MMDs (29% of the predicted value) following the implementation of the policy (standard error [SE] 2.7%, p < 0.001), the utilization rate stabilized at about 1150 MMDs (38% below the rate projected for June 1997 on the basis of pre-implementation data).
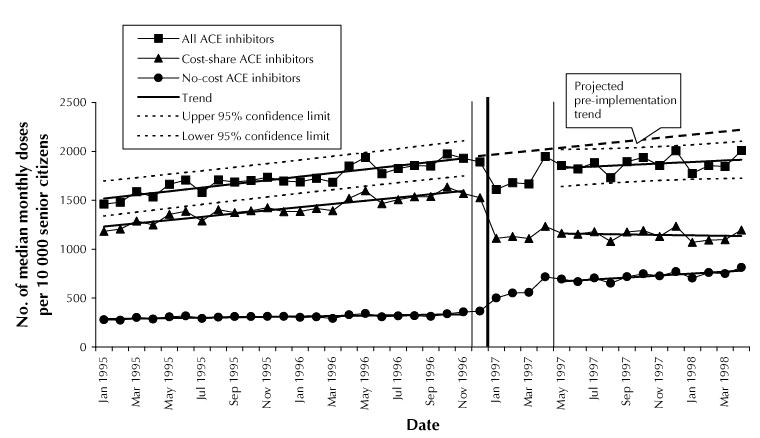
Fig. 1: Changes in utilization of angiotensin-converting enzyme (ACE) inhibitors in British Columbia senior citizens (residents at least 65 years of age). The thick vertical line marks the introduction of reference-based pricing; the 2 thinner flanking lines encompass the 5-month transition period. Utilization rates are adjusted for the length of individual months.
Utilization of no-cost ACE inhibitors during the pre-implementation period was 290 MMDs per 10 000 senior citizens, with a slight upward trend of 2 MMDs (0.7%) per month (p = 0.012). During the 5-month transition period, utilization increased by 70 MMDs (24.1%) per month (p < 0.001), reaching 625 MMDs in April 1997 (Fig. 1). This represents almost a 100% increase over the utilization predicted for April 1997 (350 MMDs) on the basis of the November 1996 rate (SE 9.8%, p < 0.001). The median prescription duration for patients who switched to no-cost ACE inhibitors decreased from 70 to 40 days. After May 1997 the rate of increase for no-cost ACE inhibitors stabilized at 8 MMDs per month (p = 0.007) (Fig. 1).
The overall utilization of ACE inhibitors dropped from 1930 MMDs per 10 000 senior citizens in November 1996 to a mean of 1710 MMDs during the first 3 months after implementation of the policy (Fig. 1), which corresponds to an 11.4% decrease (SE 3%, p = 0.02). Utilization then rose to 1825 MMDs in June 1997. This was still 11.0% below the value predicted for June 1997 (2050 MMDs) on the basis of the pre-implementation trend (1.3% increase per month). The rate of increase after implementation, 8 MMDs (0.5%) per month, was slightly but not significantly lower (p = 0.14) than the pre-implementation trend (Fig. 1).
In the cohort of 59 623 patients who used any ACE inhibitor between Oct. 1, 1995, and Mar. 31, 1996, we observed a nonsignificant 10% decrease in utilization of antihypertensive medications in January 1997 (p = 0.15 compared with the value predicted for January 1997 on the basis of November 1996 data; Fig. 2). In June 1997, utilization was 7% below the projected level. The post- implementation trend in antihypertensive use increased marginally faster than the pre-implementation trend (+0.12 and +0.07 defined monthly dose per month, p = 0.54). There was no change in use of antihypertensives (p = 0.40).
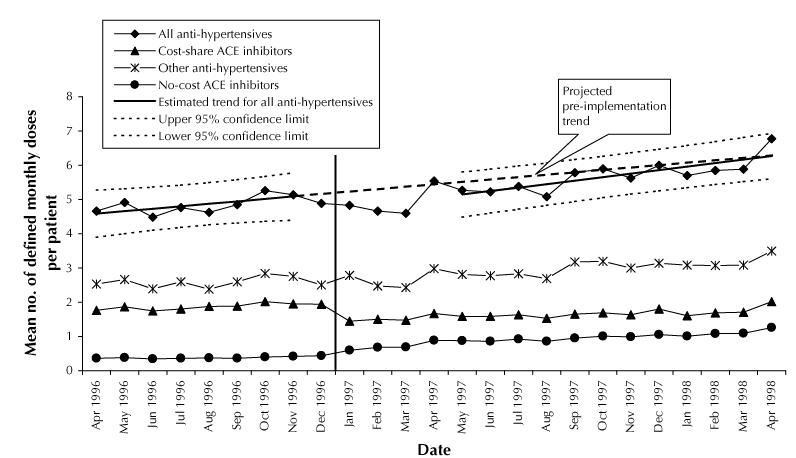
Fig. 2: Changes in utilization of antihypertensive medications for a cohort of 59 623 patients who used any ACE inhibitor from Oct. 1, 1995, to Mar. 31, 1996. The analysis adjusted for dropouts because of death, admission to long-term care or emigration during follow-up. The vertical line marks the introduction of reference-based pricing. Utilization rates are adjusted for the length of individual months.
Of the 48 355 patients who were receiving a cost-shared ACE inhibitor before implementation of reference-based pricing, 75% stayed on the same cost-shared drug after implementation (and 41% of these had an exemption), 18% switched to a no-cost ACE inhibitor, 4% switched to another class of antihypertensive medication, and 3% stopped all antihypertensive drug treatment. The associations of age, sex, income status and health care utilization with these switching patterns are presented in Table 3.
Table 3
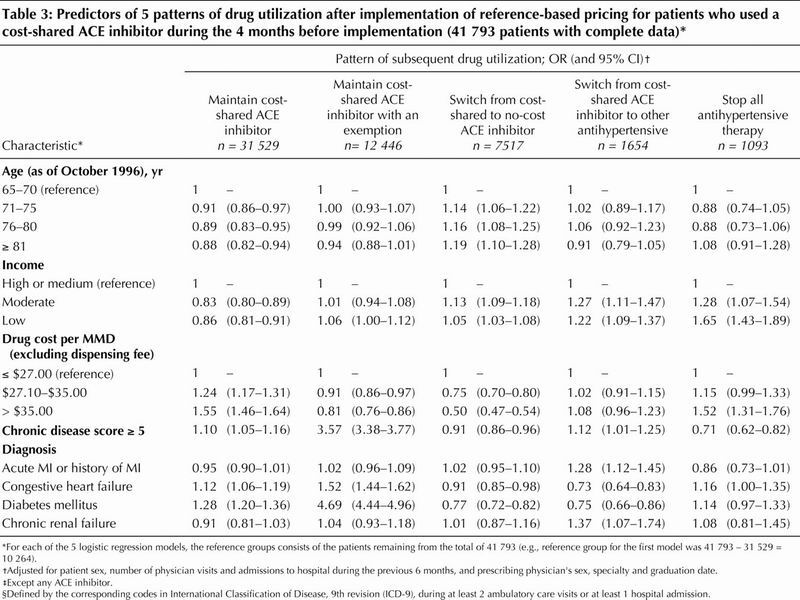
Among the 41 793 (86.4%) of these patients with complete covariate information, those with a high chronic disease score, congestive heart failure or diabetes were more likely to remain on their cost-shared ACE inhibitor than to make any of the possible switches (Table 3). Within these groups, patients with an exemption were even more likely to stay on their cost-shared ACE inhibitor (Table 3). Older patients were more likely to switch to a no-cost ACE inhibitor (after adjustment for health status). Patients with low-income status were less likely than those with high- income status to stay on a cost-shared ACE inhibitor. Patients who were receiving more expensive ACE inhibitors (drug cost more than $35/month) were more likely to stop all antihypertensive medications (with adjustment for income status).
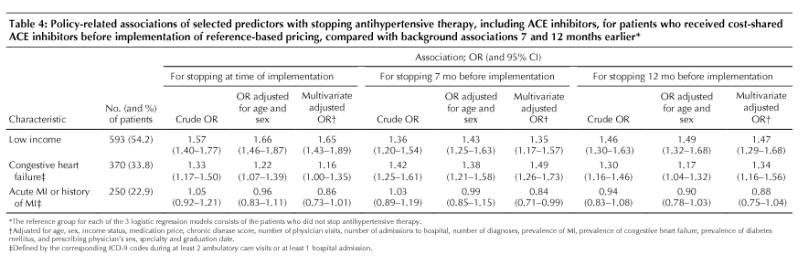
Patients with low-income status were more likely than those with high-income status to stop all antihypertensive therapy (Table 4), but these patients were also more likely to have discontinued therapy 7 and 12 months before implementation of the policy (Table 4).
Table 4
Before implementation of the policy, utilization of ACE inhibitors was dominated by more expensive enalapril preparations (53.5% of total consumption; Table 1). The policy did not appear to produce a systematic change in drug prices per MMD across substances (mean change –$0.15 [SD $0.19] per MMD). In the cohort of 59 623 patients who used ACE inhibitors between Oct. 1, 1995, and Mar. 31, 1996, there was a temporal decrease in mean monthly expenditures for antihypertensive medications, from $46 per patient in November 1996 to $38 in the first 3 months after implementation of the policy (Fig. 3). In June 1997, mean monthly expenditure per patient was $9.40 (19%) less than the expenditure projected from the pre-implementation trend; the rate of increase ($0.46 per patient per month) was also slightly lower than the projected trend.
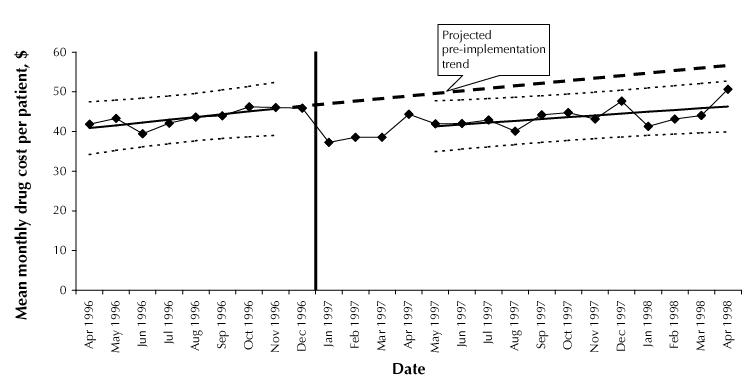
Fig. 3: Drug cost (excluding dispensing fees) for antihypertensive medications for a cohort of 59 623 patients who used any ACE inhibitor from Oct. 1, 1995, to Mar. 31, 1996. The analysis adjusted for dropouts because of death, admission to long-term care or emigration during follow-up. The vertical line marks the introduction of reference-based pricing.
On the basis of the difference between observed expenditures and expenditures projected from pre-implementation trends, we estimate that the cost savings to Pharmacare for all prescription antihypertensive medications was $6.7 million in the first year. This does not count potential savings related to patients who received their first prescription of a no-cost ACE inhibitor after implementation of the policy, patients who might have started a more expensive ACE inhibitor if there had been no reference-based pricing.
Interpretation
The heated controversy surrounding differential cost-sharing rests on weak empirical evidence.49,50,51,52 British Columbia's decision to introduce reference-based pricing for ACE inhibitors provided a quasi-experiment to study the effects of differential cost-sharing in a homogeneous and stable health care system.
ACE inhibitors were heavily marketed in British Columbia in 1995 and 1996, and their market share among elderly patients increased, partially because of concerns about the safety of calcium-channel blockers.53 With the introduction of reference-based pricing, the sizable increases in costs to patients for ACE inhibitors not listed as reference drugs caused an immediate and sustained reduction in their use, with an almost equal but delayed increase in the use of no-cost ACE inhibitors. The 29% reduction in the use of cost-shared ACE inhibitors among all elderly patients was comparable to the effects of Medicaid drug reimbursement caps on psychotropic agents in New Hampshire (15% to 49% reduction)42 and withdrawal of payments for unsubstantiated, nonscientific drug therapy in New Jersey (22% reduction).54 The slow increase in the use of no-cost ACE inhibitors resulted in a small and transient reduction in total dispensing of ACE inhibitors and antihypertensives. The transient reduction was caused in part by a reduction in prescription duration: we assume that after physicians switched patients to no-cost drugs, they scheduled visits with their patients sooner than usual to rule out intolerance or treatment failure.
The long-term reduction in use of ACE inhibitors was caused by a combination of dose reductions and the temporary or permanent discontinuation of therapy by 3% of patients who were receiving cost-shared ACE inhibitors. The hypothesis of substitution by other antihypertensives was rejected because the net trend in use of antihypertensives other than ACE inhibitors was unchanged by reference-based pricing.
We estimate that British Columbia saved about $6.7 million in antihypertensive medication costs during the first 12 months after implementing reference-based pricing. This estimate is conservative, because it does not account for patients who initiated ACE inhibitor treatment after implementation, among whom the proportion starting on no-cost ACE inhibitors was probably greater than before implementation. There are indications that the policy exemption system is working for most patients with such an exemption. Patients who were sicker and those with heart failure or diabetes were more likely to stay on their pre-implementation medications without having to pay any additional cost. The exemption did not depend on income status and did not generally give preference to older seniors, other than through health status. Some patients temporarily switched to no-cost ACE inhibitors but switched back to their usual medication after a testing phase, although we could not establish specific reasons for switching back (data not shown).
It was our objective here to focus on the most vulnerable patient groups. We found that patients with low-income status were the most likely to switch to no-cost ACE inhibitors, which raises ethical issues of differential income-related effects of the policy. In addition, low-income patients were more likely to stop all antihypertensive therapy. However, this group also had a greater rate of discontinuation of antihypertensive therapy 7 and 12 months before implementation of the policy. The 95% confidence limits before implementation spanned the effect estimate at the time of implementation, so the slight increase in the probability of discontinuation at the time of implementation was not significantly greater than the pre-implementation level. The generally low rates of adherence with antihypertensive therapy for low-income patients, independent of reference-based pricing, are well described.31,55,56
In general, patients were more likely to change to other groups of antihypertensives or to stop their therapy as medication price and, therefore, their share of the cost increased. However, 46% of patients maintained cost-shared ACE inhibitors despite higher out-of-pocket payments. Some patients' out-of-pocket payments were probably covered by additional insurance (e.g., public service pension plans or private-sector drug insurance policies.)
This study had several limitations. The administrative databases could not provide enough detailed information to distinguish the clinical appropriateness of stopping antihypertensive medication or reducing the dose in individual cases. The assessment of health status by proxies such as medication use, ICD-9 diagnoses and health care utilization is likely to adjust for much but not all of the potential confounding effect in the analysis of predictors of switching.
In conclusion, the implementation of reference-based pricing for ACE inhibitors in British Columbia achieved a sustained reduction in drug expenditures, despite the generous exemptions in the provincial benefits plan. Sicker patients and those with heart failure or diabetes were most likely to remain on their prior medication. However, the underlying concern about stopping all antihypertensive therapy in low-income patients, independent of reference-based pricing, remains to be solved. Further research is necessary to examine the impacts on health, health care utilization and total costs, specifically in the subgroups of patients switching treatments.
Acknowledgments
The study was supported by grants RO3 HSO9855 and RO1 HS10881 from the US Agency for Healthcare Research and Quality, Department of Health and Human Services, Rockville, Md.; the Drug Information Association, Fort Washington, Pa.; Pharmacare, British Columbia Ministry of Health, Victoria, BC; and the Harvard Pilgrim Health Care Foundation, Boston, Mass. Dr. Schneeweiss was supported by the Deutsche Forschungsgemeinschaft (schn 527/3 and schn 527/4); the Pharmacoepidemiology Teaching and Research Fund, Harvard School of Public Health; and the Takemi Associate Award, Harvard School of Public Health.
Footnotes
This article has been peer reviewed.
Contributors: Sebastian Schneeweiss developed the design and the statistical models, conducted the analyses and led the writing of the paper. Stephen Soumerai, Robert Glynn, Malcolm Maclure and Alexander Walker contributed to the design, the statistical analysis and the writing of the paper. Colin Dormuth was responsible for data manipulation and linkage; he also provided insights into the specifics of the databases and contributed to the design, the statistical analysis and the writing of the paper.
Competing interests: None declared for Sebastian Schneeweiss, Stephen Soumerai and Colin Dormuth. Robert Glynn has received research grants from Pfizer Inc. and Bristol–Myers Squibb for projects unrelated to the current study. Malcolm Maclure has received research funds from Pfizer Inc. for a project unrelated to the current study. Alexander Walker is employed by a division of United Health Group, a US health care provider with an interest in pricing policies.
Correspondence to: Dr. Sebastian Schneeweiss, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women's Hospital and Harvard Medical School, 221 Longwood Ave., Boston MA 02115; fax 617 232-8602; schneeweiss@post.harvard.edu
References
- 1.Canadian Institute for Health Information. Spending on drugs outpaces other health care spending, reports CIHI [press release]. Ottawa: The Institute; 2001 Mar 14. Available: www.cihi.ca/medrls/14mar2001.shtml (accessed 2001 Jan 9).
- 2.Iglehart JK. The American health care system. Expenditures. N Engl J Med 1999; 340:70-6. [DOI] [PubMed]
- 3.Levit K, Cowan C, Braden B, Stiller J, Sensenig A, Lazenby H. National health expenditures in 1997: more slow growth. Health Aff (Millwood) 1998; 17 (6):99-110. [DOI] [PubMed]
- 4.Kane NM. Pharmaceutical cost containment and innovation in the United States. Health Pol 1997;41(Suppl):S71-S89. [DOI] [PubMed]
- 5.Quinn K, Baker MJ, Evans B. A population-wide profile of prescription drug use in Saskatchewan, 1989. CMAJ 1992;146:2177-86. [PMC free article] [PubMed]
- 6.Huskamp HA, Rosenthal MB, Frank RG, Newhouse JP. The Medicare prescription drug benefit: How will the game be played? Health Aff (Millwood) 2000; 19(2):8-23. [DOI] [PubMed]
- 7.Schneeweiss S, Maclure M, Walker AM, Grootendorst P, Soumerai SB. On the evaluation of drug benefits policy changes with longitudinal claims data: the policy maker's versus the clinician's perspective. Health Pol 2001;55:97-109. [DOI] [PubMed]
- 8.Bourgault C, Elstein E, Le Lorier J, Suissa S. Reference-based pricing of prescription drugs: exploring the equivalence of angiotensin-converting-enzyme inhibitors. CMAJ 1999;161:255-60. Available: www.cma.ca/cmaj/vol-161/issue-3/0255.htm [PMC free article] [PubMed]
- 9.Canadian Cardiovascular Society. A position paper on drug-pricing strategies for prescription pharmaceuticals in Canada. Can JCardiol 1997;13:33-8. [PubMed]
- 10.McLaughlin PR. Reference-based pricing of prescription drugs. Can J Cardiol 1997; 13:31-2. [PubMed]
- 11.Boulet AP, Tessier G. Reference-based pricing in British Columbia: implications for cardiologists — an analysis. Can J Cardiol 1997;13:46-51. [PubMed]
- 12.British Columbia Pharmacy Association. Position statement, reference-based pricing. Richmond (BC): The Association; 1996.
- 13.McGregor M. Coverage of drug costs: reference-based pricing. Can J Cardiol 1998; 14:666-8. [PubMed]
- 14.Olley PM, McLaughlin PR. The Canadian Cardiovascular Society and reference-based drug pricing. Can J Cardiol 1998;14:669-70. [PubMed]
- 15.Roemer MI, Hopkins CE, Carr L, Gartside F. Copayments for ambulatory care: penny-wise and pound-foolish. Med Care 1975;13:457-66. [DOI] [PubMed]
- 16.Nelson AA, Reeder CE, Dickson WM. The effect of a Medicaid drug copayment program on the utilization and cost of prescription services. Med Care 1984; 22:724-36. [DOI] [PubMed]
- 17.Soumerai SB, Avorn J, Ross-Degnan D, Gortmaker S. Payment restrictions for prescription drugs in Medicaid: effects on therapy, cost, and equity. N Engl J Med 1987;317:550-6. [DOI] [PubMed]
- 18.Reeder CE, Nelson AA. The differential impact of copayment on drug use in a Medicaid population. Inquiry 1985;22:396-403. [PubMed]
- 19.Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 2001;285:421-9. [DOI] [PubMed]
- 20.Feldman RD, Campbell N, Larochelle P, Bolli P, Burgess ED, Carruthers SG, et al, for the Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension. 1999 Canadian recommendations for the management of hypertension. CMAJ 1999;161(12 Suppl):S1-S17. Available: www.cma.ca/cmaj/vol-161/issue-12/hypertension/hyper-e.htm [PMC free article] [PubMed]
- 21.Moser M, Abraham PA, Bennett WM, Brachfeld N, Goodman RP, McKenney JM, et al. The effects of benazepril, a new angiotensin-converting enzyme inhibitor, in mild to moderate hypertension: a multicenter study. Clin Pharmacol Ther 1991;49:322-9. [DOI] [PubMed]
- 22.Os I, Bratland B, Dalhof B, Gisholt K, Syvertsen JO, Tretli S. Lisinopril and nifedipine in essential hypertension: a Norwegian multicenter study on efficacy, tolerability and quality-of-life in 828 patients. J Hypertens Suppl 1991; 9 (6): S382-3. [PubMed]
- 23.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-35. [DOI] [PubMed]
- 24.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991;325:293-8. [DOI] [PubMed]
- 25.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fraction. N Engl J Med 1992;327:685-92. [DOI] [PubMed]
- 26.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-6. [PubMed]
- 27.Domanski MJ, Exner DV, Borkowf CB, Geller NL, Rosenberg Y, Pfeffer MA. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta-analysis of randomized clinical trials. JAm Coll Cardiol 1999;33:598-604. [DOI] [PubMed]
- 28.Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med 1995;46:455-6. [DOI] [PubMed]
- 29.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456-62. [DOI] [PubMed]
- 30.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 1996; 334:939-45. [DOI] [PubMed]
- 31.Feldman R, Bacher M, Campbell N, Drover A, Chockalingam A. Adherence to pharmacologic management of hypertension. Can J Public Health 1998; 89: I16-8. [DOI] [PMC free article] [PubMed]
- 32.Pharmacare. Antihypertensives: information on reference drug pricing in British Columbia. Victoria (BC): Ministry of Health; 1997.
- 33.Ensuring cost-effective drug strategies. In: Managing the cost of drug therapies and fostering appropriate drug use. Victoria (BC): Office of the Auditor General of British Columbia; 1998.
- 34.Anderson GM, Kerluke KJ, Pulcins IR, Hertzman C, Barer ML. Trends and determinants of prescription drug expenditures in the elderly: data from the British Columbia Pharmacare Program. Inquiry 1993;30:199-207. [PubMed]
- 35.Williams JI, Young W. Inventory of studies on the accuracy of Canadian health administrative databases [technical report]. Toronto: Institute for Clinical Evaluative Sciences; 1996.
- 36.Fowles JB, Lawthers AG, Weiner JP, Garnick DW, Petrie DS, Palmer RH. Agreement between physicians' office records and Medicare Part B claims data. Health Care Financ Rev 1995;16:189-99. [PMC free article] [PubMed]
- 37.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care 1994;32:81-90. [DOI] [PubMed]
- 38.Glynn RJ, Monane M, Gurwitz JH, Choodnovskiy I, Avorn J. Agreement between drug treatment data and a discharge diagnosis of diabetes mellitus in the elderly. Am J Epidemiol 1999;149:541-9. [DOI] [PubMed]
- 39.Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Flemming C, Baron JA. The accuracy of Medicare's hospital claims data: progress has been made but problems remain. Am J Public Health 1992;82:243-8. [DOI] [PMC free article] [PubMed]
- 40.BC Ministry of Management Services. BC stats [homepage]. Victoria: The Ministry. Available: http://www.bcstats.gov.bc.ca/index.htm (accessed 2002 Feb 6)
- 41.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin TJ, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991;325:1072-7. [DOI] [PubMed]
- 42.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of limiting Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994;331:650-5. [DOI] [PubMed]
- 43.Nordic Council on Medicines. Nordic drug index: defined daily doses. In: Nordic statistics on medicine. Part 2. Uppsala: The Council: 1985.
- 44.Gillings D, Makuc D, Siegel E. Analysis of interrupted time series mortality trends: an example to evaluate regionalized perinatal care. Am J Public Health 1981; 71:38-46. [DOI] [PMC free article] [PubMed]
- 45.Veney JE, Kaluzny AD. Trend analysis. In: Evaluation and decision making for health services. 2nd ed. Ann Arbor (MI): Health Administration Press; 1991.
- 46.Kennedy P. Autocorrelated disturbances. In: A guide to econometrics. 4th ed. Cambridge (MA): MIT Press; 1998. p. 123-5.
- 47.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992;45:197-203. [DOI] [PubMed]
- 48.Johnson RE, Hornbrook MC, Nichols GA. Replicating the chronic disease score (CDS) from automated pharmacy data. J Clin Epidemiol 1994;47:1191-9. [DOI] [PubMed]
- 49.Schneeweiss S, Schöffski O, Selke GW. What is Germany's experience on reference based drug pricing and the etiology of adverse health outcomes or substitution? Health Pol 1998;44:253-60. [DOI] [PubMed]
- 50.Holbrook A, O'Brien B, Grootendorst P. Reference-based pricing (RBP) of prescription drugs [letter]. Can J Cardiol 1997;13:689-90. [PubMed]
- 51.Weiss N, Heckbert SR. Thrombotic vascular events after change of statin [letter]. Lancet 1999;353:844. [DOI] [PubMed]
- 52.Soumerai SB, Ross-Degnan D, Fortess EE, Abelson J. A critical analysis of studies of state drug reimbursement policies. Research in need of discipline. Milbank Q 1993;71:217-52. [PubMed]
- 53.Maclure M, Dormuth C, Naumann T, McCormack J, Rangno R, Whiteside C, et al. Influences of educational interventions and adverse news about calcium-channel blockers on first-line prescribing of antihypertensive drugs to elderly people in British Columbia. Lancet 1998;352:943-8. [DOI] [PubMed]
- 54.Soumerai SB, Ross-Degnan D, Gortmaker S, Avorn J. Withdrawing payment for nonscientific drug therapy. JAMA 1990;263:831-9. [PubMed]
- 55.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. Compliance with antihypertensive therapy among elderly Medicaid enrollees: the role of age, gender, and race. Am J Public Health 1996;86:1805-8. [DOI] [PMC free article] [PubMed]
- 56.Caldwell JR, Theisen V, Kaunisto CA, Reddy PJ, Smythe PS, Smith DW. Psychosocial factors influence control of moderate and severe hypertension. Soc Sci Med 1983;17:773-82. [DOI] [PubMed]


