Abstract
Objective
To establish consensus definitions for necrotising otitis externa (NOE) to facilitate the diagnosis and exclusion of NOE in clinical practice and expedite future high-quality study of this neglected condition.
Design
The work comprised of a systematic review of the literature, five iterative rounds of consultation via a Delphi process and open discussion within the collaborative. An expert panel analysed the results to produce the final outputs which were shared with and endorsed by national specialty bodies.
Setting
Secondary care in the UK.
Participants
UK clinical specialists practising in infection, ear nose and throat (ENT) surgery or radiology.
Main outcome measures
Definitions and statements meeting the following criteria were accepted: (a) minimum of 70% of respondents in agreement or strong agreement with a definition/statement AND (b) <15% of respondents in disagreement or strong disagreement with a definition/statement.
Results
Seventy-four UK clinicians specialising in ENT, Infection and Radiology with a special interest in NOE took part in the work which was undertaken between 2019 and 2021. The minimum response rate for a Round was 76%. Consensus criteria for all proposed case definitions, outcome definitions and consensus statements were met in the fifth round.
Conclusions
This work distills the clinical opinion of a large group of multidisciplinary specialists from across the UK to create practical definitions and statements to support clinical practice and research for NOE. This is the first step in an iterative process. Further work will seek to validate and test these definitions and inform their evolution.
Keywords: MICROBIOLOGY, INFECTIOUS DISEASES, OTOLARYNGOLOGY, RADIOLOGY & IMAGING, Adult otolaryngology
Strengths and limitations of this study.
This Delphi process has engaged a large group of respondents—74 UK-based clinicians across the key three specialties expert in managing patients with necrotising otitis externa (ear nose and throat, infection and radiology).
The response rate to each of the Rounds is considered high for a Delphi study (>75%).
A broad recruitment strategy was employed, but we may have missed UK clinicians who are experts in this field.
We only recruited clinicians based in the UK.
Introduction
Necrotising otitis externa (NOE) is an under-recognised, poorly understood, severe infection of the external auditory canal (EAC) and lateral skull base. If detected late, this condition has a poor outcome with spread of infection to involve the cranial nerves, the base of skull and the central nervous system.1 Patients affected by NOE are generally frail and elderly with multiple comorbidities.2 3 This condition presents a challenge to ear, nose and throat (ENT) inpatient surgical units, which are generally ill equipped to manage complex, long-stay and commonly frail medical patients. The disease is associated with high mortality; one case-series reported overall survival of 38% at 5 years with disease-specific mortality of 14%.4 Early diagnosis and treatment may reduce the need for long-term antibiotic therapy and will reduce the risk of serious complications.
No established national or international guidelines exist for the diagnosis and management of NOE.5 Most published series are limited and of poor quality.2 3 Not surprisingly, the optimal strategy for diagnosis and management of NOE remains uncertain2 3 and there is considerable variability in how this condition is managed.6
Cohen and Friedman’s definition of NOE from 1987 is often cited7 and modified versions are used in some studies.2 However, publications often fail to explicitly state their criteria for defining a case of NOE, and for those that do, there is considerable variation in the definitions applied.2 To date there is no widely accepted case definition for NOE and none have been developed via consensus of multidisciplinary experts. The lack of an accepted definition has impeded progress in developing diagnostic and treatment algorithms.
Why is a consensus definition for NOE needed?
A diagnostic definition has two distinct uses. First and most importantly it provides the non-expert clinician with a clear set of criteria to facilitate diagnosis or the exclusion of NOE. Under recognition of NOE results in a delay in diagnosis increasing the risk of serious complications and poorer outcomes in an already frail population. Conversely, given that NOE is typically treated with prolonged courses of broad-spectrum antimicrobials, unnecessary treatment of individuals without NOE with extended regimens exposes frail patients to the serious risks associated with these agents8 as well as contributing more broadly to antimicrobial resistance.9–11 Accurate diagnostic processes for NOE are therefore important to optimise outcomes for patients with and without NOE. However, to date, no test with sufficient sensitivity and specificity to definitively diagnose or exclude NOE exists, and a poor evidence base is of little help to inform nuanced clinical decision-making.2 3
Second, a major limitation of the published literature on NOE is the lack of a consensus definition for NOE. As a result, publications likely reflect heterogenous populations and robust comparison across data sets is impossible. A consensus definition is needed to facilitate future high-quality study of the condition. For example, studies of new treatment regimens must include a robust case definition so findings can be critically appraised and applied to other patient cohorts.
What are the aims of the definitions/statements?
To be widely used and applied, consensus definitions and statements must be robust but also practical. For example, given that many sites in the UK do not have access to urgent MRI, inclusion of this as the sole modality in a diagnostic case definition would be problematic. At the start of the project, the following aims for consensus definitions/statements were therefore defined:
They should be implementable in all centres across the UK, from a small district general hospital to tertiary referral centres.
They should be highly specific (ie, describe a typical definite case of NOE and minimise the chances of misclassifying another condition), but not necessarily describe all potential presentations of NOE.
They are for guidance only and not prescriptive in terms of practice.
They should allow standardised description of cases to facilitate recruitment to clinical trials and comparison of cases across different cohorts.
They mark the start of an iterative process—as more, and better quality evidence becomes available these definitions/statements will be revisited and revised.
Methods
This project comprised of a systematic review of the literature, five iterative rounds of consultation via a Delphi process, with UK specialists, expert in managing NOE as well as open discussion within the collaborative. An expert panel analysed the results to produce the final guidance (figure 1). Consent from participants was implicit in their taking part and their support for publication.
Figure 1.
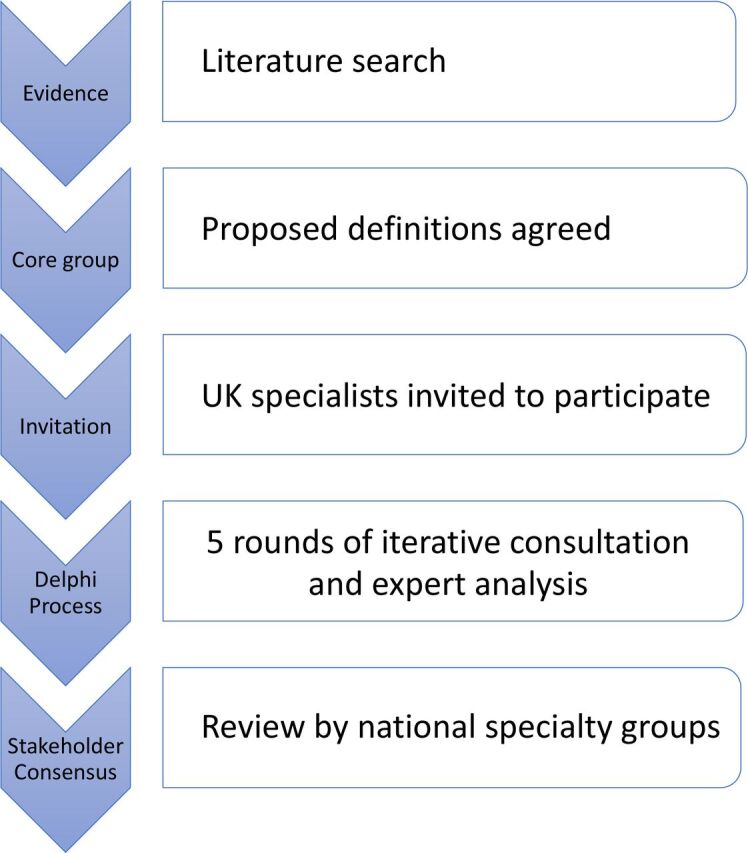
Overview of process to develop consensus case definitions and statements for necrotising otitis externa.
Systematic review
A systematic review of the literature for NOE was performed and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines12 (Takata et al, DOI.org/10.1111/coa.14041 ). The systematic review was registered on PROSPERO (PROSPERO ID: CRD42020128957). The search identified all English language clinical papers published on NOE. This revealed 422 publications, representing 16 528 patients. Sixty-four per cent of these publications were excluded from further analysis as they either included less than six patients and/or did not explicitly state the case definition applied. In the studies that did describe a case definition, the criterion used varied widely. Of note, no studies specifically addressing case definition were identified. The detailed results of this review has been published as a separate manuscript.
Delphi method
A Delphi method was used to reach consensus definitions for NOE, outcome definitions and key consensus statements. The Delphi method is a structured, flexible process of obtaining information from a group of experts by means of a series of questionnaires, each one refined based on feedback from respondents on a previous version.13 This iterative, multistage process is designed to transform opinion into group consensus, and is characterised by the following features: anonymity, allowing opinions to be expressed free from group pressure, iteration with controlled feedback from one round to the next, aggregation of group responses and expert input until consensus has been achieved.14–18 The method is ideally suited to amalgamate the opinions of a broad range of stakeholders, which was important given the lack of high-quality published evidence for NOE and the likely heterogenicity in practice across the UK.6
Participants
A core group of ENT, Infection and adiology senior consultant specialists with a special interest and expertise in NOE, set-up the UK NOE collaborative (MIA, ES, PP). This group, in consultation with national specialty organisations including the British Infection Association (BIA), ENT UK and the British Society for Otology (BSO) identified individuals with an interest in NOE, who were then invited to participate in the Delphi process by email. The same corresponding email address was used by the collaborative throughout the process and only one email address was used for each participant to ensure only one response was logged for each participant at each round. Questionnaires were set-up and analysed usingGoogle Forms or JISC. It was possible for the core group to identify if participants had replied, but individual responses were not reviewed in order toensureanonymity. All participants consented to publishing the results. The core group with other senior experts (PM-D (ENT consultant), MM (bone and joint infection surgeon), OMW (infection specialist)) facilitated the Delphi process and analysed the data.16
Definitions
After a literature review, the core group proposed definitions for definite, possible and complex NOE as well as definitions for outcomes including cure, non-response to treatment and relapse. They also proposed key consensus statements. These definitions and statements were shared with participants in a survey via email. Participants were asked to rate the extent to which they agreed with each definition/statement (strongly agree, agree, disagree and strongly disagree) on a Likert scale. The survey included the opportunity for individuals to comment after each definition/statement and at the end of the survey. Participants were encouraged to feedback on their reasons for disagreement or agreement with the proposed definitions/statements.
Following each round, results were shared with participants with explanations for proposed revisions to the definitions/statements from the expert group. The Delphi process comprised of five rounds, all of which were conducted by electronic survey apart from Round 3, which took the form of an in-person meeting.
Predefined consensus criteria
The following criteria were agreed for adoption of definitions/statements19:
Minimum of 70% of respondents in agreement or strong agreement with a definition/statement AND
<15% of respondents in disagreement or strong disagreement with a definition/statement.
Definitions/statements that met these criteria were accepted. Definitions that did not meet these criteria at each round were modified according to feedback and included in subsequent rounds. The Delphi process continued until consensus criteria were met for all definitions/statements.
Wider stakeholder review
The consensus case definitions/statements were shared with the British Infection Association (BIA), ENT UK, British Society Otology (BSO) and the British Society of Neuroradiologists (BSNR).
Patient and public involvement statement
There was no public/patient involvement in this study.
Results
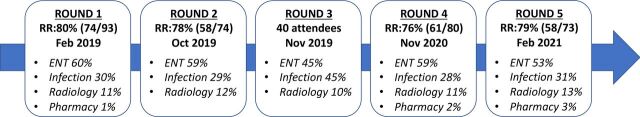
Email invitations explaining the objectives of the project and including the initial survey for Round 1 were sent to 93 identified specialists in the UK, of whom 74 responded (80%) (figure 2). Individuals who engaged with Round 1 were invited to participate in Round 2. Three individuals who had not participated in Rounds 1 and 2 attended and participated in the meeting for Round 3. Participants who had engaged in any of Rounds 1, 2 or 3 were invited to participate in Rounds 4 and 5 in addition to three individuals who had not been involved in the process prior to Round 4. The process took more than 2 years to complete, and some individuals were no longer contactable by initial email, meaning the number of possible respondents decreased for Round 5. The minimum response rate for a Round was 76%. The survey questions for each Round and raw data can be viewed in Supplementary Information which includes facilitator communiques with the collaborative (see online supplemental files 1–9). Consensus criteria for all case definitions, outcome definitions and consensus statements were met in Round 5. These are summarised in boxes 1–4. The final consensus definitions and statements were endorsed by the BIA, ENT UK, BSO and BSNR.
Figure 2.
Rounds in Delphi process showing response rate (RR) for each Round and specialty involvement. ENT = ear, nose and throat; RR = response rate.
Box 1. Consensus definitions for necrotising otitis externa (NOE).
Definitions of NOE
Definite NOE
NOE is diagnosed if ALL of the following are present:
· Otalgia and otorrhoea OR otalgia and a history of otorrhoea.
· Granulation OR inflammation of the external auditory canal.
· Histological exclusion of malignancy in cases where this is suspected.
-
· Radiological features consistent with NOE:
(i) CT imaging findings of bony erosion of the external auditory canal, together with soft tissue inflammation of the external auditory canal OR
(ii) MRI with changes consistent with NOE (eg, bone marrow oedema of the temporal bone with soft tissue inflammation of the external auditory canal).
Possible NOE
A severe infection of the external ear canal which does not show bony erosion of the external auditory canal on CT scan OR does not show changes consistent with NOE on MRI if this is performed (eg, bone marrow oedema of the temporal bone) AND which has ALL of the following characteristics:
· Otalgia and otorrhoea OR otalgia and a history of otorrhoea AND
· Granulation OR inflammation of the external auditory canal AND
-
· Any of the following features:
(i) Immunodeficiency.
(ii) Night pain.
(iii) Raised inflammatory markers (erythrocyte sedimentation rate/C reactive protein) in absence of other plausible cause.
(iv) Failure to respond to >2 weeks of topical anti-infectives and aural care.
Box 2. . Definition of complex disease.
Complex necrotising otitis externa (NOE)
Patients meeting the criteria for ‘definite’ NOE may be classified as ‘complex’ (or severe) if the following are present:
· Facial nerve or other lower cranial nerve palsy.
· Cerebral venous thrombosis on MRI or contrast enhanced CT.
· Extensive bone involvement as demonstrated by any of the following:
(i) CT showing bone erosion in other skull base locations in addition to the external ear canal wall (eg, around stylomastoid foramen, clivus, petrous apex, temporomandibular joint).
(ii) MRI showing bone marrow oedema extending to central skull-base.
(iii) CT or MRI showing extensive soft tissue oedema or inflammation or fluid collection below the skull base.
(iv) Intracranial spread of the disease (eg, dural thickening, extradural or subdural empyema, cerebral/cerebellar abscess).
Box 3. Consensus definitions for treatment outcomes.
Outcome definitions
Cure
A case of necrotising otitis externa (NOE) is considered treated and cured if a patient has no pain or otorrhoea for a minimum period of 3 months after completing antibiotic therapy.
Relapse
Relapse is recurrence of disease after the patient has been treated and cured ie, at least 3 months after stopping antibiotic therapy.
A relapsed case of NOE is a serious, invasive infection which occurs after the initial infection was considered to be treated and cured and is characterised by:
Recurrence of local disease
Recurrent otalgia OR recurrent otorrhoea
AND
Recurrent granulation OR inflammation
AND
Unchanged or progression of bony erosion of the external auditory canal on CT OR unchanged or progression of MRI changes such as bone marrow oedema of the temporal bone and soft tissue changes of the external auditory canal.
AND/OR
Development or recurrence of complex disease
Development or worsening of a lower cranial nerve palsy, base of skull osteomyelitis or development or worsening of other intracranial complication deemed a consequence of NOE and supported by radiological imaging.
Non-response to therapy
A case of NOE is defined as non-responsive to therapy if there is no improvement in otalgia or otorrhoea or inflammation or granulation tissue in the external auditory canal after 14 days of optimum analgesia, anti-infective therapy, aural care and optimisation of immune state.
Box 4. Consensus statements.
First-line imaging
CT scan is the initial imaging modality of choice for a suspected case of necrotising otitis externa (NOE).
Multidisciplinary approach
Once a diagnosis of definite NOE has been made, specialist review as part of a multidisciplinary team approach should be arranged.
Nomenclature
‘Necrotising Otitis Externa’ is the preferred name for this condition over ‘Malignant Otitis Externa’.
bmjopen-2022-061349supp001.pdf (12.3MB, pdf)
bmjopen-2022-061349supp002.pdf (32.3KB, pdf)
bmjopen-2022-061349supp003.pdf (133.3KB, pdf)
bmjopen-2022-061349supp004.pdf (1,005.8KB, pdf)
bmjopen-2022-061349supp005.pdf (70.3KB, pdf)
bmjopen-2022-061349supp006.pdf (58.2KB, pdf)
bmjopen-2022-061349supp007.pdf (18.3MB, pdf)
bmjopen-2022-061349supp008.pdf (344.3KB, pdf)
bmjopen-2022-061349supp009.pdf (11.3MB, pdf)
Discussion
This is the first published study which has sought to standardise diagnostic and outcome criteria for NOE, following consultation with experts working in the field from three specialties: ENT, Radiology and Infection. Consensus definitions/statements were obtained for all of the identified areas set out by the expert group at the start of the project.
The Delphi process is an ideal method for the development of diagnostic criteria in the absence of an available gold standard test or a robust evidence base,16 and has been used widely for this purpose.14 20–23 This method reduces bias, enhances transparency and allows the involvement of individuals from diverse clinical backgrounds and dispersed geographical locations. It also helps ensure that a single influential participant does not have a disproportionate influence on the process. One potential disadvantage of this method is the possible lack of individual responsibility and accountability, however in our work this was addressed in part by in-person discussions and encouragement of feedback from individuals at each round.
A major barrier to the agreement of these definitions/statements was the ongoing SARS-CoV-2 pandemic at the time the Delphi process was being conducted. This was a challenging time for all clinicians, especially Infection specialists, and as a result there were delays in engaging some key stakeholders. Similarly, due to widespread physical distancing we were unable to convene a planned in-person meeting to discuss the final results. However, the consistent response rate of ≥76% for all rounds in our study is noteworthy and should afford confidence in the final definitions/statements while acting as testament to the commitment of UK specialists to improve outcomes for this neglected condition. For context, response rates to Delphi surveys are usually low; one review reported that a response rate of 35–40% is typical during a first round consultation with 15–18 participants and that surveys with larger pools of participants tend to have lower response rates.24
Discussion at the in-person meeting confirmed it was not clinically appropriate to have a binary case definition for NOE given that currently available investigations cannot reliably distinguish patients with NOE from those without. For this reason, a decision was made to include a case definition for ‘possible’ NOE in the study outputs, to describe those patients without definitive evidence of NOE but for whom clinical suspicion is still high. This approach has been applied successfully in other infective conditions involving bone.25 26 Infection of the EAC is likely a continuum, with otitis externa and NOE extremes of the same disease process. Further work is needed to understand ‘possible’ NOE, the investigations that reliably distinguish these cases from definite NOE and the variables that determine the outcome of such cases.
The final consensus definitions for NOE adopted by the group include symptoms, signs and radiological changes as obligatory criteria. Specific radiological abnormalities are a relatively objective measure which can be standardised across sites and assessed in future work. While the ideal modality to diagnose NOE is debated,27–29 we chose to only include radiological changes on CT and MRI, given these modalities are most widely available in the UK.
Otalgia and the presence of granulation tissue or inflammation in the EAC were considered essential for diagnosis of a definite case in our definition. In contrast, only 78% and 76% of studies, respectively, were found to consider these features obligatory criteria in our systematic review (Takata et al DOI.org/10.1111/coa.14041). It is possible that our definition may be less sensitive and will wrongly exclude ‘true’ cases of NOE, without visible EAC changes or without pain. However, our definition is a starting point, which will evolve as data from a planned UK, multicentre observational study of NOE (Improving Outcomes in NOE (IONOE)) (NCT:04950985) and other studies emerge.
The role of the multidisciplinary team (MDT) working in the improvement of patient outcomes is well known.30–32 In the management of complex orthopaedic infections, time to diagnosis and clinical outcomes have both been shown to improve when MDTs function well.33 34 The benefits of an MDT approach are multifactorial; patients benefit from care, that is, co-ordinated, individualised and delivered by experts; clinicians benefit by having increased exposure to a larger number of cases which improves expertise; and the Unit benefits as the improvements in outcomes build morale.30 There are sparse data addressing the benefit of MDT working on outcomes for NOE. However, a UK study by Sharma et al, has shown that an MDT approach resulted in a shorter duration of therapy and lower mean hospital length of stay for patients with NOE.35 In our study there was strong support for an MDT model to manage NOE, but there was also concern that this would not be realistically achievable in the absence of dedicated local funding.
The term ‘malignant otitis externa’ (MOE) was first coined by Chandler in 1968 when reporting the first case series of severe temporal bone osteomyelitis, originating from the EAC, associated with Pseudomonas aeruginosa infection.36 Later the term ‘NOE’ was introduced.37 The terms MOE and NOE have since been used interchangeably to describe the condition. While the terms ‘necrotising’ and ‘malignant’ convey the aggressive and serious nature of the condition, they are both recognised to be misnomers in that they do not describe the pathophysiology of the condition. It was proposed and accepted that since malignancy is an important differential for this condition, it was preferable to use the term ‘necrotising otitis externa’.
This is the first published study which has sought to standardise diagnostic and outcome criteria for NOE, following consultation with experts. However, the results should be interpreted in the context of the limitations of the methods used. We tried to recruit broadly, but may have inadvertently missed some specialists. The data is collected from UK-based clinicians which may limit broader application of results. The decisions by the core group were led by the results of each round, which included comments by the participants, reducing any risk of bias.
Conclusion
This work distills the clinical opinion of a large group of multidisciplinary specialists from across the UK to create practical definitions and statements to support clinical practice and research for NOE. This is the first step in an iterative process. Further work will seek to validate and test these definitions and inform their evolution.
Supplementary Material
Acknowledgments
SHH is an NIHR Academic Clinical Lecturer in Infection and a Research Fellow at St Peter’s College, University of Oxford.
Footnotes
Twitter: @otolaryngolofox
SHH and MMK contributed equally.
Collaborators: Chris Aldren (NHS Frimley Health Foundation Trust); Victoria Alexander (St George’s University Hospitals NHS Trust and Epsom and St Helier University Hospitals NHS Trust); Fiona Andrewartha (Consultant in Infection, Nottinghamshire Healthcare NHS Foundation Trust); Helen Atkinson (Yorkshire and Humberside deanery); Manohar Bance (Consultant in ENT Surgery, Cambridge University Hospitals NHS Foundation Trust); Rupan Banga (Consultant in ENT Surgery, University Hospitals Birmingham NHS Foundation Trust); David Baring (NHS Lothian, Edinburgh); Tim Beale (University College London Hospitals NHS Foundation Trust); Alex Bennett (NHS Lothian, Edinburgh); Ian Bottrill (Oxford University Hospitals NHS Foundation Trust); F Kay Seymour (Barts Health NHS Trust); Philip Clamp (University Hospitals Bristol and Weston NHS Foundation Trust); Julia Colston (Kings College Hospital NHS Foundation Trust); Tumena Corrah (London North West University Healthcare NHS Trust); Lucy Dalton (University Hospitals Birmingham NHS Foundation Trust); Sudip Das (University Hospitals of Leicester NHS Trust); Eoghan deBarra (Beaumont Hospital, Royal College of Surgeons in Ireland Hospital Group, Dublin, Ireland); Jane Democratis (NHS Frimley Health Foundation Trust); Reena Dwivedi (Salford Royal NHS Foundation Trust); Chi Eziefula (Brighton and Sussex University Hospitals NHS Trust); Susannah Froude (University Hospital of Wales); Mark Gilchrist (Imperial College Healthcare NHS Trust); Laura Harrison (Oxford University Hospitals NHS Foundation Trust); Mary Hart (University Hospital of Wales); Carolyn Hemsley (Guys and St Thomas’ NHS Foundation Trust); Michael Hopkins (NHS Lothian, Edinburgh); Alex Howard (Liverpool University Hospitals NHS Foundation Trust); Harriet Hughes (Consultant in Infection, University Hospital of Wales); Arun Iyer (NHS Greater Glasgow and Clyde); Susan Jawad (University College London Hospitals NHS Foundation Trust); Gwennan Jones (University Hospital of Wales); Nicola Jones (Oxford University Hospitals NHS Foundation Trust); Gillian Jones (Brighton and Sussex University Hospitals NHS Trust); Hala Kanona (University College London Hospitals NHS Foundation Trust); Gerard Kelly (Leeds Teaching Hospitals NHS Trust); B Nirmal Kumar (Wrightington Wigan & Leigh NHS Foundation Trust); Steven Laird (Coventry and Warwickshire Partnership NHS Trust); Pankaj Lal (Liverpool University Hospitals NHS Foundation Trust); Martin Llewelyn (Brighton and Sussex University Hospitals NHS Trust); Simon K Lloyd (Manchester University Hospitals NHS Foundation Trust); Sarah Logan (University College London Hospitals NHS Foundation Trust); Sam Mackeith (Oxford University Hospitals NHS Foundation Trust); Philippa Matthews (Oxford University Hospitals NHS Foundation Trust); Martin McNally (Oxford University Hospitals NHS Foundation Trust); Nishchay Mehta (University College London Hospitals NHS Foundation Trust); Tamara Mitchell (Sheffield Teaching Hospitals NHS Foundation Trust); Hassan Mohammed (Newcastle Hospital NHS Foundation Trust); Peter Monksfield (University Hospitals Birmingham NHS Foundation Trust); Daniel Moualed (Great Western Hospital NHS Foundation Trust); Rupert Obholzer (Guys and St Thomas’ NHS Foundation Trust); John Phillips (Norfolk and Norwich University Hospitals NHS Foundation Trust); Peter Rea (University Hospitals of Leicester NHS Trust); Elisabeth Ridgway (Sheffield Teaching Hospitals NHS Foundation Trust); Philip Robinson (University Hospitals Bristol and Weston NHS Foundation Trust); Shakeel R. Saeed (The Royal National Throat, Nose and Ear Hospital and National Hospital for Neurology and Neurosurgery, London); Georgios Sakaglannis (University Hospitals of Leicester NHS Trust); Frances Sanderson (Imperial College Healthcare NHS Trust); Victoria Sinclair (Oxford University Hospitals NHS Foundation Trust); Avind Singh (London North West University Healthcare NHS Trust); Wendy Smith (Kettering General Hospital NHS Foundation Trust); Dominic StLeger (Manchester University Hospitals NHS Foundation Trust); David Summers (NHS Lothian, Edinburgh); Rebecca Sutherland (NHS Lothian, Edinburgh); Andrew Swift (Liverpool University Hospitals NHS Foundation Trust); Aaron Trinidade (Southend University Hospital NHS Trust); Matthew Trotter (University Hospital Coventry and Warwickshire NHS Trust); Michael Wareing (Barts Health NHS Trust); Glen Watson (Sheffield Teaching Hospitals NHS Foundation Trust); Martin Williams (University Hospitals Bristol and Weston NHS Foundation Trust); Mandy Williams (University Hospitals Bristol and Weston NHS Foundation Trust); Tom Wilson (Leeds and York Partnership NHS Foundation Trust); Ding Yang (University College London Hospitals NHS Foundation Trust); Phil Yates (Newcastle Hospital NHS Foundation Trust); Ahmed Youssef (Liverpool University Hospitals NHS Foundation Trust); Ivan Zammit (Newcastle Hospital NHS Foundation Trust).
Contributors: The conception and design of the work was done by SH, MMK, PM-D, ES, OMW, PP, MM and MIA. The data collection was done by SH, MMK, MP-S, MIA and the UK NOE Collaborative. The data analysis and interpretation was done by SH, MIA, MMK, ES, PM-D, OMW and PP. The first draft of the paper was done by SH and MIA. The article was critically reviewed and revised by SH, MMK, MP-S, PM-D, ES, OMW, PP, MM, MIA and the UK NOE Collaborative. MIA is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: on behalf of UK NOE Collaborative, Chris Aldren, Victoria Alexander, Fiona Andrewartha, Helen Atkinson, Manohar Bance, Rupan Banga, David Baring, Tim Beale, Alex Bennett, Ian Bottrill, F Kay Seymour, Philip Clamp, Julia Colston, Tumena Corrah, Lucy Dalton, Sudip Das, Eoghan deBarra, Jane Democratis, Reena Dwivedi, Chi Eziefula, Susannah Froude, Mark Gilchrist, Laura Harrison, Mary Hart, Carolyn Hemsley, Michael Hopkins, Alex Howard, Harriet Hughes, Arun Iyer, Susan Jawad, Gwennan Jones, Nicola Jones, Gillian Jones, Hala Kanona, Gerard Kelly, B Nirmal Kumar, Steven Laird, Pankaj Lal, Martin Llewelyn, Simon K Lloyd, Sarah Logan, Sam Mackeith, Philippa Matthews, Martin McNally, Nishchay Mehta, Tamara Mitchell, Hassan Mohammed, Peter Monksfield, Daniel Moualed, Rupert Obholzer, John Phillips, Peter Rea, Elisabeth Ridgway, Philip Robinson, Shakeel R. Saeed, Georgios Sakaglannis, Frances Sanderson, Victoria Sinclair, Avind Singh, Wendy Smith, Dominic StLeger, David Summers, Rebecca Sutherland, Andrew Swift, Aaron Trinidade, Matthew Trotter, Michael Wareing, Glen Watson, Martin Williams, Mandy Williams, Tom Wilson, Ding Yang, Phil Yates, Ahmed Youssef, and Ivan Zammit
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
We sought advice from the chair of the OUH Joint Research Office Study Classification Group who considered that this study did not require formal ethical approval. Participants gave informed consent to participate in the study before taking part.
References
- 1. Rubin Grandis J, Branstetter BF, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 2004;4:34–9. 10.1016/s1473-3099(03)00858-2 [DOI] [PubMed] [Google Scholar]
- 2. Mahdyoun P, Pulcini C, Gahide I, et al. Necrotizing otitis externa: a systematic review. Otol Neurotol 2013;34:620–9. 10.1097/MAO.0b013e3182804aee [DOI] [PubMed] [Google Scholar]
- 3. Byun YJ, Patel J, Nguyen SA, et al. Necrotizing otitis externa: a systematic review and analysis of changing trends. Otol Neurotol 2020;41:1004–11. 10.1097/MAO.0000000000002723 [DOI] [PubMed] [Google Scholar]
- 4. Stern Shavit S, Soudry E, Hamzany Y, et al. Malignant external otitis: factors predicting patient outcomes. Am J Otolaryngol 2016;37:425–30.:S0196-0709(16)30018-7. 10.1016/j.amjoto.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 5. Hasibi M, Ashtiani MK, Motassadi Zarandi M, et al. A treatment protocol for management of bacterial and fungal malignant external otitis: a large cohort in Tehran, Iran. Ann Otol Rhinol Laryngol 2017;126:561–7. 10.1177/0003489417710473 [DOI] [PubMed] [Google Scholar]
- 6. Chawdhary G, Pankhania M, Douglas S, et al. Current management of necrotising otitis externa in the UK: survey of 221 UK Otolaryngologists. Acta Otolaryngol 2017;137:818–22. 10.1080/00016489.2017.1295468 [DOI] [PubMed] [Google Scholar]
- 7. Cohen D, Friedman P. The diagnostic criteria of malignant external otitis. J Laryngol Otol 1987;101:216–21. 10.1017/s0022215100101562 [DOI] [PubMed] [Google Scholar]
- 8. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging 2010;27:193–209. 10.2165/11531490-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9. Bernstein JM, Holland NJ, Porter GC, et al. Resistance of Pseudomonas to ciprofloxacin: implications for the treatment of malignant otitis externa. J Laryngol Otol 2007;121:118–23. 10.1017/S0022215106002775 [DOI] [PubMed] [Google Scholar]
- 10. Rehman A, Patrick WM, Lamont IL. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J Med Microbiol 2019;68:1–10. 10.1099/jmm.0.000873 [DOI] [PubMed] [Google Scholar]
- 11. Wee I, Chin B, Syn N, et al. The association between fluoroquinolones and aortic dissection and aortic aneurysms: a systematic review and meta-analysis. Sci Rep 2021;11:11073. 10.1038/s41598-021-90692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Schulz KF, Simera I, et al. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217. 10.1371/journal.pmed.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitha A, Boulyana M, Hue V, et al. Consensus in diagnostic definitions for bone or joint infections in children by a Delphi method with European French-speaking experts. Acta Paediatr 2012;101:e350–6. 10.1111/j.1651-2227.2012.02716.x [DOI] [PubMed] [Google Scholar]
- 15. Windle PE. Delphi technique: assessing component needs. J Perianesth Nurs 2004;19:46–7. 10.1016/j.jopan.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 16. Eibling D, Fried M, Blitzer A, et al. Commentary on the role of expert opinion in developing evidence-based guidelines. Laryngoscope 2014;124:355–7. 10.1002/lary.24175 [DOI] [PubMed] [Google Scholar]
- 17. Beiderbeck D, Frevel N, von der Gracht HA, et al. Preparing, conducting, and analyzing delphi surveys: cross-disciplinary practices, new directions, and advancements. MethodsX 2021;8:101401. 10.1016/j.mex.2021.101401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhandari S, Hallowell MR. Identifying and controlling biases in expert-opinion research: guidelines for variations of Delphi, nominal group technique, and focus groups. J Manage Eng 2021;37. 10.1061/(ASCE)ME.1943-5479.0000909 [DOI] [Google Scholar]
- 19. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of delphi studies. J Clin Epidemiol 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 20. Olszewska E, Rutkowska J, Özgirgin N. Consensus-Based recommendations on the definition and classification of cholesteatoma. J Int Adv Otol 2015;11:81–7. 10.5152/iao.2015.1206 [DOI] [PubMed] [Google Scholar]
- 21. Weir N-JM, Pattison SH, Kearney P, et al. Criteria required for an acceptable point-of-care test for UTI detection: obtaining consensus using the Delphi technique. PLoS One 2018;13:e0198595. 10.1371/journal.pone.0198595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yung M, Tono T, Olszewska E, et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J Int Adv Otol 2017;13:1–8. 10.5152/iao.2017.3363 [DOI] [PubMed] [Google Scholar]
- 23. Rybak YE, Lai KSP, Ramasubbu R, et al. Treatment-Resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety 2021;38:456–67. 10.1002/da.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewin SR, Attoye T, Bansbach C, et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 2021;8:e42–50. 10.1016/S2352-3018(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury 2018;49:505–10. 10.1016/j.injury.2017.08.040 [DOI] [PubMed] [Google Scholar]
- 26. McNally M, Sousa R, Wouthuyzen-Bakker M, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021;103-B:18–25. 10.1302/0301-620X.103B1.BJJ-2020-1381.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morales RE, Eisenman DJ, Raghavan P. Imaging necrotizing otitis externa. Semin Roentgenol 2019;54:215–26. 10.1053/j.ro.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 28. van Kroonenburgh AMJL, van der Meer WL, Bothof RJP, et al. Advanced imaging techniques in skull base osteomyelitis due to malignant otitis externa. Curr Radiol Rep 2018;6:3. 10.1007/s40134-018-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehrotra P, Elbadawey MR, Zammit-Maempel I. Spectrum of radiological appearances of necrotising external otitis: a pictorial review. J Laryngol Otol 2011;125:1109–15. 10.1017/S0022215111001691 [DOI] [PubMed] [Google Scholar]
- 30. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: a review. Surg Neurol Int 2014;5(Suppl 7):S295–303. 10.4103/2152-7806.139612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ndoro S. Effective multidisciplinary working: the key to high-quality care. Br J Nurs 2014;23:724–7. 10.12968/bjon.2014.23.13.724 [DOI] [PubMed] [Google Scholar]
- 32. Prades J, Remue E, van Hoof E, et al. Is it worth reorganising cancer services on the basis of multidisciplinary teams (mdts)? A systematic review of the objectives and organisation of mdts and their impact on patient outcomes. Health Policy 2015;119:464–74. 10.1016/j.healthpol.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 33. Prendki V, Zeller V, Passeron D, et al. Outcome of patients over 80 years of age on prolonged suppressive antibiotic therapy for at least 6 months for prosthetic joint infection. Int J Infect Dis 2014;29:184–9. 10.1016/j.ijid.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 34. Ibrahim MS, Raja S, Khan MA, et al. A multidisciplinary team approach to two-stage revision for the infected hip replacement: a minimum five-year follow-up study. Bone Joint J 2014;96-B:1312–8. 10.1302/0301-620X.96B10.32875 [DOI] [PubMed] [Google Scholar]
- 35. Sharma S, Corrah T, Singh A. Management of necrotizing otitis externa: our experience with forty-three patients. J Int Adv Otol 2017;13:394–8. 10.5152/iao.2017.4399 [DOI] [PubMed] [Google Scholar]
- 36. Chandler JR. Malignant external otitis. Laryngoscope 1968;78:1257–94. 10.1288/00005537-196808000-00002 [DOI] [PubMed] [Google Scholar]
- 37. Kohut RI, Lindsay JR. Necrotizing (“ malignant ”) external otitis histopathologic processes. Ann Otol Rhinol Laryngol 1979;88(5 Pt 1):714–20. 10.1177/000348947908800520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061349supp001.pdf (12.3MB, pdf)
bmjopen-2022-061349supp002.pdf (32.3KB, pdf)
bmjopen-2022-061349supp003.pdf (133.3KB, pdf)
bmjopen-2022-061349supp004.pdf (1,005.8KB, pdf)
bmjopen-2022-061349supp005.pdf (70.3KB, pdf)
bmjopen-2022-061349supp006.pdf (58.2KB, pdf)
bmjopen-2022-061349supp007.pdf (18.3MB, pdf)
bmjopen-2022-061349supp008.pdf (344.3KB, pdf)
bmjopen-2022-061349supp009.pdf (11.3MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.