Summary
Traumatic brain injury (TBI) has been recognized as an important risk factor for Alzheimer’s disease (AD). However, the molecular mechanisms by which TBI contributes to developing AD remain unclear. Here, we provide evidence that aberrant production of TDP-43 is a key factor in promoting AD neuropathology and synaptic and cognitive deterioration in mouse models of mild closed head injury (CHI). We observed that a single mild CHI is sufficient to exacerbate AD neuropathology and accelerate synaptic and cognitive deterioration in APP transgenic mice but repeated mild CHI are required to induce neuropathological changes and impairments in synaptic plasticity, spatial learning, and memory retention in wild-type animals. Importantly, these changes in animals exposed to a single or repeated mild CHI are alleviated by silencing of TDP-43 but reverted by rescue of the TDP-43 knockdown. Moreover, overexpression of TDP-43 in the hippocampus aggravates AD neuropathology and provokes cognitive impairment in APP transgenic mice, mimicking single mild CHI-induced changes. We further discovered that neuroinflammation triggered by TBI promotes NF-κB-mediated transcription and expression of TDP-43, which in turn stimulates tau phosphorylation and Aβ formation. Our findings suggest that excessive production of TDP-43 plays an important role in exacerbating AD neuropathology and in driving synaptic and cognitive declines following TBI.
Keywords: Traumatic brain injury, mild closed head injury, TDP-43, synaptic plasticity, neuroinflammation, neurodegeneration, tau phosphorylation
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease whose main hallmarks are extracellular accumulation and deposition of Aβ plaques and intraneuronal accumulation of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. AD is the most common cause of dementia in aging. Growing evidence suggests that traumatic brain injury (TBI) is an important risk factor for development of AD [18, 21, 22, 26-28, 35, 44, 54, 64]. Mild TBI, which includes concussions and subconcussive impacts, accounts for approximately 80% of all TBIs [24, 58, 68]. A single moderate to severe TBI or repetitive mild TBI may lead to AD-like neuropathology and neurocognitive decline later in life [1, 3, 5, 35, 47, 72, 73]. Accumulation of hyperphosphorylated tau and aggregation of TAR DNA-binding protein 43 (TDP-43) are two important neuropathological features in patients who have been exposed to TBI [5, 15, 30, 70, 71]. Some TBI patients also display Aβ pathology [5, 9, 25, 34, 35, 70]. Recent clinical studies have shown that TDP-43 inclusions are a distinct feature in AD, as more than 50% of AD patients display pathological TDP-43 inclusions [36, 37, 49, 55, 65, 74]. This suggests that there is a close link between TBI and AD [18, 20-22, 35, 70]. However, the molecular mechanisms by which TBI contributes to developing AD neuropathology are largely unclear.
Closed head injury (CHI) is blunt or non-penetrating head trauma. Clinically, the majority of mild TBI results from CHI. Previous studies from patients with TBI have shown that a single mild CHI may not induce chronic neuropathological changes and dementia [4, 41, 47, 52]. Animal studies using mouse models of mild CHI have also shown that repeated, but not single, mild CHI produces sustained neuroinflammation, neuropathology, and synaptic and cognitive impairments [14, 48, 51, 59, 63, 80]. However, a single mild CHI has been shown to exacerbate neuropathological changes and cognitive impairments in animal models of AD [43, 75]. This suggests that a single mild CHI may not lead to chronic neurodegenerative processes and dementia in healthy subjects, but it might be sufficient to trigger neuropathogenesis and accelerate or aggravate neuropathological changes among persons who are prone or susceptible to developing AD. Nevertheless, the molecular mechanisms responsible for single mild CHI-accelerated or repetitive mild CHI-induced AD neuropathology remain uncertain.
TDP-43 is a DNA and RNA binding protein shuttled between the cytoplasm and the nucleus, which regulates nuclear transcription, RNA splicing, and metabolism [39]. The role of TB-43 in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) has been extensively studied [16, 19, 38, 45, 56, 67]. However, less is known about TDP-43 in developing AD and in TBI-induced AD-like neuropathology. In the present study, we aimed to address these issues by examining: 1) whether single mild CHI exacerbates neuropathological changes and accelerates synaptic and cognitive declines in APP TG mice but not in WT animals; 2) whether neuropathological changes and synaptic and cognitive declines accelerated by a single mild CHI in APP TG mice or induced by repeated mild CHI in WT mice could be alleviated by knockdown of TDP-43; 3) whether overexpression of TDP-43 mimics the TBI-induced changes; 4) how TBI induces aberrant production of TDP-43; and 5) what TDP-43-associated molecular signaling mechanisms contribute to neuropathology in TBI. We provide evidence here that a single mild CHI is sufficient to accelerate neuropathological changes and synaptic and cognitive declines in APP TG mice but not in WT animals. Importantly, our results show that aberrant production of TDP-43 is a key factor in TBI-induced neuropathology and synaptic and cognitive impairments in mouse models of both single and repeated mild CHI. Knockdown of TDP-43 alleviates mild CHI-induced neuropathological changes in both amyloid-β precursor protein (APP) TG and WT mice, but the alleviation is reversed by rescue of the TDP-43 knockdown. Overexpression of human TDP-43 in the hippocampus exacerbates neuropathology and accelerates cognitive decline in APP TG mice, mimicking the single mild CHI-induced changes. Our results provide further evidence that TBI-triggered neuroinflammation promotes NF-κB-mediated transcription and expression of TDP-43, which in turn stimulates tau phosphorylation and Aβ formation by interacting with GSK3β and β-site amyloid precursor protein cleaving enzyme 1 (BACE1). Our study reveals a previously undefined role of TDP-43 in TBI-induced AD neuropathology and synaptic and cognitive declines.
Materials and Methods
Animals
C57BL/6J mice (Stock number: 000664) and 5xFAD APP transgenic (TG) mice (Stock number: 006554) were obtained from the Jackson Laboratory, as described previously [57]. P301S (PS19) tau TG mice (Stock number: 008169) were also obtained from the Jackson Laboratory, as described previously [76], for in vitro experiments. Both male and female mice at two to four months of ages were used in the present study and the animals were randomly assigned to groups. All animal studies were performed in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of University of Texas Health Science Center at San Antonio approved the care and use of the animals reported in this study.
Mild closed head injury
A mouse model of mild closed head injury (mild CHI) was used, as described previously [31, 80]. Briefly, single mild CHI was induced using an electromagnetic controlled stereotaxic impact device (Impact One™ Stereotaxic Impactor, Leica Biosystem, IL). Mice were placed in a stereotaxic frame after anesthesia with isoflurane. The skull was exposed by a midline skin incision. A 3 mm blunt metal impactor tip was positioned at 1.8 mm caudal to bregma and 2.0 mm left of midline. The injury was triggered by the electromagnetic device driving the tip to the exposed skull at a strike velocity of 3.0 m/s, depth 2.2 mm with a dwell time of 100 msec. After impact, the skin was sutured and the mice were allowed to recover from anesthesia on a warming pad and then returned to their home cages. For repeated mild CHI in WT mice, a second and third identical closed-skull TBI procedure was performed at days 2 and 3 after the first injury. For sham injuries, the same procedures and anesthesia were performed except that no hit was delivered.
Cell culture
Primary hippocampal neurons (astroglial cells ~ 5%) from postnatal P0 pups of APP-WT, APP-TG (5xFAD), PS19 tau-WT, and PS19 tau-TG mice were cultured as described previously [12, 31, 66, 78, 79]. Briefly, the hippocampi from pups were dissected out under microscope and triturated in serum-free culture medium after meninges were removed. Tissue was incubated in oxygenated trypsin for 10 minutes at 37°C and then mechanically triturated. Cells were spun down and resuspended in Neurobasal/B27 medium supplemented with 0.5 mM L-glutamine, penicillin/ streptomycin and 25 μM glutamate. Cells (1 × 106) were loaded into 6-well plates for immunoblot analysis. The medium were changed after 3 days of culturing and thereafter three times a week. The cultures were grown at 37°C in a humidified atmosphere of 5% CO2 until use.
HEK 293T cells were cultured for production and envelop of lentiviral vectors as described previously, and NG108-15 cells were cultured for assessing plasmid expression of TDP-43 [31, 66, 79].
Plasmid and lentiviral constructs
The FUGW lentiviral vectors (LV), as described previously [31, 66, 79], were used to insert a scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43 driven by a U6 promoter and the GFP reporter gene driven by an ubiquitin promoter. The followings are the primers for TDP-43 scramble control: gacgTTAATTAAAAAAAAA GGTGTCGTATTGTCGTAGTGT TCTCTTGAA ACACTACGACAATACGACACC AAACAAGGCTTTTCTCCAAGGGA 3’, and for TDP-43 shRNA: gacgTTAATTAAAAAAAAA GGTGTTTGTTGGACGTTGTAC TCTCTTGAA GTACAACGTCCAACAAACACC AAACAAGGCTTTTCTCCAAGGGA 3’. For shRNA-resistant TDP-43, we used the PCR-based mutagenesis method to generate shRNA-resistant cDNA constructs with four point mutations in the shRNA targeting sequence without altering the encoded amino acids as described previously [12]. The primers for the TDP-43 shRNA resistant construct are 5’GGAATCAGTGTACACATCTCCAATGCTGAACCTAAGCATAAT3’ and 5’GGAGATGTGTACACTGATTCCTTTAATGATCAAATCCTCTCCPCR3’. The PCR products were then digested and ligated into the BstB1-Pac1 sites in a modified FUGW2.1 construct with U6 promoter. The FUGW lentiviral vectors were also used for generate LV expressing scramble control or GSK3β-shRNA. The Oligos for scramble control or GSK3β-shRNA are 5’ gacgTTAATTAAAAAAAAA GATGAGGTCTACCTTAACCTG TCTCTTGAA CAGGTTAAGGTAGACCTCATC AAACAAGGCTTTTCTCCAAGGGA 3’ and 5’ gacgTTAATTAAAAAAAAA GCTCTGAATCGAAGGCTCTAT TCTCTTGAA ATAGAGCCTTCGATTCAGAGC AAACAAGGCTTTTCTCCAAGGGA 3’. pCMVΔ8.9 and VSVg vectors were used for viral envelope and production in 293T cells. The titer of the LV at least 1.0x1010 was used for in vivo injections, as described previously [66, 79]. The FUGW vectors were also used for generation of NF-kB p65 shRNA as described previously [79]. The oligos for scramble control and NF-kB p65 shRNA constructs are 5’GAATCATTACGCGAGACTGCA3’ and 5’AGGACCTATGAGACCTTCAAG3’.
Stereotaxic injection of LV and AAV
WT or 5xFAD mice at 2 months of age were anesthetized with ketamine/Xylazine (200/10 mg/kg) and placed in a stereotaxic frame. LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43, AAV9-CMV-hTDP-43.eGFP vectors (Vector Biolabs) or AAV9-CMV-eGFP were stereotaxically injected into the left side of the hippocampus in WT or APP TG mice at the coordinate: AP, −2.3, ML, 2, and DV, −2 (2.0 μl at 0.2 μl /min), as described previously [31, 66, 79]. Single or repeated mild CHI were induced 30 days following LV injections and all the assessments were carried out 30 days after mild CHI.
Hippocampal slice preparation
Hippocampal slices were prepared from mice as described previously [12, 13, 31, 66, 79, 80]. Briefly, after decapitation, brains were rapidly removed and placed in cold oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing: 125.0 NaCl, 2.5 KCl, 1.0 MgCl2, 25.0 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose, 3 pyruvic acid, and 1 ascorbic acid. Slices were cut at a thickness of 350-400 μm and transferred to a holding chamber in an incubator containing ACSF at 36 °C for 0.5 to 1 hour, and maintained in an incubator containing oxygenated ACSF at room temperature (~22-24 °C) for >1.5 h before recordings. Slices were then transferred to a recording chamber where they were continuously perfused with 95% O2, 5% CO2-saturated standard ACSF at ~32-34 °C.
Electrophysiological recordings
Field EPSP (fEPSP) recordings at hippocampal Schaffer-collateral synapses in response to stimuli at a frequency of 0.05 Hz were made using an Axoclamp 900A patch-clamp amplifier. Recording pipettes were pulled from borosilicate glass with a micropipette puller (Sutter Instrument) and filled with artificial ACSF (~4 MΩ). Long-term potentiation (LTP) at CA3-CA1 synapses was induced by a high-frequency stimulation (HFS) consisting of three trains of 100Hz stimulation (1 sec duration and a 20 sec inter-train interval), as described previously [12, 13, 66, 79, 80].
Reverse transcription and real-time PCR
Total RNA was prepared from harvested tissue with the RNeasy Mini Kit (Qiagen) and treated with RNase-free DNase (Qiagen) according to the manufacturer’s instructions. The RNA concentration was measured by spectrophotometer (DU 640; BECKMAN). RNA integrity was verified by electrophoresis in a 1% agarose gel. The iScript cDNA synthesis kit (BioRad) was used for the reverse transcription reaction. We used 1 μg total RNA, with 4 μl 5× iscript reaction mix and 1 μl iscript reverse transcriptase. The total volume was 20 μl. Samples were incubated for 5 min at 25 °C. All samples were then heated to 42 °C for 30 min, and reactions were stopped by heating to 85 °C for 5 min. Real-time RT-PCR specific primers for IL-1β, IL-6, TNFα, vimentin (Vim), and GAPDH were selected using Beacon Designer Software (BioRad) and synthesized by IDT (Coralville, IA). The primers used in the present study are listed in Supplemental Table 1. The PCR amplification of each product was further assessed using 10-fold dilutions of mouse brain cDNA library as a template and was found to be linear over five orders of magnitude and at greater than 95% efficiency. All the PCR products were verified by sequencing. The reactions were set up in duplicate in total volumes of 25 μl containing 12.5 μl 2× iQSYBR green Supermix (BioRad) and 5 μl template (1:10 dilution from RT product) with a final concentration of 400 nM of the primer. The PCR cycle was as follows: 95 °C/3 min, 45 cycles of 95 °C/30 sec, 58 °C/45 sec and 95 °C/1 min, and the melt-curve analysis was performed at the end of each experiment to verify that a single product per primer pair was amplified. Furthermore, the sizes of the amplified DNA fragments were verified by gel electrophoresis on a 3% agarose gel. The amplification and analysis were performed using an iCycler iQ Multicolor Real-Time PCR Detection System (BioRad). Samples were compared using the relative CT method. The fold increase or decrease were determined relative to naïve or sham controls after normalizing to a housekeeping gene using 2-ΔΔCT, where ΔCT is (gene of interest CT) - (GAPDH CT), and ΔΔCT is (ΔCT treated) - (ΔCT control), as described previously [13, 31, 66, 79, 80].
Western blots
Western blot assay was conducted to determine expression of APP, BACE1, ADAM10, neprilysin (NEP), Aβ42, TDP-43, acetylated tau (AC-Tau), p-tau (p-181), AT8 (p-tau-S202/T-205), tau-5 (total tau), p-GSK3β, PSD-95, PPARγ, p-NF-kB, glutamate receptor subunits, including GluA1, GluA2, GluN1, GluN2A, and GluN2B, and cytokines, including IL-1β, IL-6, and TNFα in hippocampal tissues or cultures from WT, APP TG (5xFAD) or tau TG (PS19). Tissues or cultures were extracted and immediately homogenized in RIPA lysis buffer and protease inhibitors, and incubated on ice for 30 min, then centrifuged for 10 min at 10,000 rpm at 4°C. Supernatants were fractionated on 4-15% SDS-PAGE gels (Bio-Rad) and transferred onto PVDF membranes (Bio-Rad). The antibodies used to detect the expression of proteins are listed in Supplemental Table 2. The membrane was incubated with specific antibodies at 4°C overnight. The blots were washed and incubated with a secondary antibody (goat anti-rabbit 1:2,000, Cell Signaling) at room temperature for 1 hr. Proteins were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences, UK). The densities of specific bands were quantified by densitometry using GE/Amersham Imager 680 UV. Band densities were normalized to the total amount of protein loaded in each well as determined by mouse anti β-actin (1:2,000, Santa Cruz), as described previously [13, 31, 66, 77, 79, 80].
ELISA
TBI-induced acceleration of Aβ42 formation in hippocampal tissues of APP TG mice that had received LV- Scr , LV-TDP-43-shRNA, or LV-Resc were detected using a colorimetric Aβ42 ELISA kit (AnaSpec, Inc.) according to the manufacture’s instruction [79]. TBI-induced IL-1β and TNFα in hippocampal tissues of WT were detected using ELISA kits (MilliporeSigma and MyBioSource) according to the instructions provided by the manufactures.
CHIP analysis
Chromatin Immunoprecipitation (CHIP) analysis was performed, as described previously [12, 66, 79], to determine the binding of NF-κBp65 at the promoters of the TDP-43 gene according to the manufacture’s instruction (MilliporeSigma). The primers for CHIP designed were based on the predicted potential NF-κB binding sites in the promoter region as followings: forward primer: 5’GCAAAGGCAGAAGACATTTAAG3’ and reverse primer: 5’ GGGCTAGGAGGGAAAGG3’ for the binding site 1; forward: 5’AAGCAATCACGGAGGTTGT’, and reverse: 5’ TGAGCTAACTGCCAAGGATG3’ for the binding site 2; and forward: 5’ TCCTGGAACTCACTCTGTAGAC3’ and reverse: 5’ CTGAGCTAACTGCCAAGGATG3’ for the binding site 3.
Immunohistochemistry
Immunofluorescence analysis was performed to assess TDP-43, Iba1, and GFAP in coronal brain sections. Animals were anesthetized with ketamine/xylazine (200/10 mg/kg) and subsequently transcardially perfused with PBS followed by 4% paraformaldehyde in phosphate buffer. The brains were quickly removed from the skulls and fixed in 4% paraformaldehyde overnight, and then transferred into the PBS containing 30% sucrose until sinking to the bottom of the small glass jars. Cryostat sectioning was made on a freezing Vibratome at 30 μm and a series five equally spaced (every 10 sections) sections were collected in 0.1M phosphate buffer. Free floating sections were immunostained using specific antibodies as listed in Supplemental Table 2 and followed by incubation with the corresponding fluorescent-labeled secondary antibody. 4′−6-Diamidino-2-phenylindole (DAPI), a fluorescent stain that binds strongly to DNA, was used to detect cell nuclei in the sections. The sections were then mounted on slides for immunofluorescence imaging using a Zeiss deconvolution microscope with the Slidebook software 6.0 (Intelligent Imaging Innovations.com, Denver, Colorado). The immunofluorescence intensity (in arbitrary densitometric units, ADU) of the channel detected in the region of interest as immunoreactivity of a specific antibody was imaged in each section and analyzed using SlideBook 6.0, as described previously [13, 31, 66, 77, 79, 80].
Novel object recognition test
The novel object recognition (NOR) test was performed to assess memory retention as described previously [66]. Briefly, animals were first allowed to acclimate to the testing environment (habituation). The test included two stages: training and testing. In the first stage of the test, the animal was confronted with two identical objects, placed in an open field, and in the second stage, the animal was exposed to two dissimilar objects placed in the same open field: one familiar object, used in the first phase, and the other novel object. Exploration of an object was defined as time spent with the head oriented towards and within two cm of the object. The time spent exploring each of the objects in stage two was detected using the EthoVision video tracking system (Noldus). The recognition index (RI) was calculated based on the following equation: RI =TN/TN+TF), where TN is the exploration time devoted to the novel object and TF is the exploration time for the familiar object, as described previously [2].
Morris water maze test
The classic Morris water maze (MWM) test was used to determine spatial learning and memory, as described previously [12, 13, 29, 31, 66, 79, 80]. Briefly, a circular water tank (diameter 120 cm and 75 cm in high) was filled with water and the water was made opaque with non-toxic white paint. A round platform (diameter 15 cm) was hidden 1 cm beneath the surface of the water at the center of a given quadrant of the water tank. WT or APP TG mice treated with sham or mild CHI received learning acquisition training in the Morris water maze for 7 days and each session was consisted of 4 trials. For each trial, the mouse was released from the wall of the tank and allowed to search, find, and stand on the platform for 10 seconds within the 60-second trial period. For each training session, the starting quadrant and sequence of the four quadrants from where the mouse was released into the water tank were randomly chosen so that it was different among the separate sessions for each animal and was different for individual animals. The mouse movement in the water pool was recorded by a video-camera and the task performances, including swimming paths, speed, and time spent in each quadrant were recorded using an EthoVision video tracking system (Noldus, version 14). A probe test was conducted 24 hours after the completion of the learning acquisition training. During the probe test, the platform was removed from the pool, and the task performances were recorded for 60 seconds.
Data analysis
Data are presented as mean ± S.E.M. Unless stated otherwise, one or two-way analysis of variance (ANOVA) followed by post-hoc tests were used for statistical comparison when appropriate. Differences were considered significant when P< 0.05.
Results
Single mild CHI accelerates neuropathology and cognitive decline in APP TG mice
To assess whether a single mild CHI exacerbates neuropathology and accelerates synaptic and cognitive impairments in APP TG mice, 5xFAD APP TG mice or age-matched wild type (WT) mice received a single mild CHI, as described previously [31, 80], at 2 months of age (Fig.1a). We observed that inflammatory markers, including vimentin (Vim) and proinflammatory cytokines (TNFα, IL-1β, and IL-6) in the hippocampus were significantly elevated in APP TG mice but not in WT mice 24 hrs following a single mild CHI (Fig. 1b). Importantly, a single mild CHI provoked accumulation and deposition of Aβ plaques in the brain of APP TG mice compared with sham, assessed 30 days after mild CHI (Fig. 1c). The single mild CHI-increased Aβ is likely associated with elevated expression of BACE1 in APP TG mice (Fig. 1d). Furthermore, a single mild CHI significantly increased expression of TDP-43, acetylated tau (AC-tau), and phosphorylated tau-T181 (p-tau181) in APP TG mice, but not in WT mice (Fig. 1d). It is worth noting that 5xFAD APP TG mice do not display tauopathy [57], suggesting that formation of AC-tau and p-tau in APP TG mice is triggered and caused by TBI. Of significance, a single mild CHI accelerated impairments in learning and memory as evidenced by results of the novel object recognition (NOR) and Morris water maze (MWM) tests in 3-month old APP TG mice (Fig. 1e&f). 5XFAD APP TG mice do not display learning and memory deficits before 5 months of age [13, 57]. This suggests that a single mild CHI accelerates neuropathological changes and cognitive decline in APP TG mice, but not in WT animals.
Figure 1.
Single mild closed head injury accelerates developing Alzheimer’s disease. a, Schematic illustration of the experimental protocol. WT and APP TG mice at two months of age received a single mild CHI, and all the assessments were made 30 days after TBI except for qPCR analysis. b, Expression of proinflammatory markers, vimentin (Vim) and cytokines (IL-1β, IL-6, TNFα), in the ipsilateral hippocampus is elevated by a single mild CHI in APP TG mice, but not in WT mice. The data are means ±SEM (ANOVA with Fisher's PLSD post-hoc test, n=3~5 animals/group). c, Single mild CHI facilitates formation of Aβ plaques in the brain of APP TG mice. Immunostaining analysis of 4G8 immunoreactivity. The data are means ±SEM (ANOVA with Bonferroni post-hoc test, n=5 animals/group). Scale bars: 400 μm. d, Single mild CHI accelerates neuropathological changes in TG mice. Immunoblot analysis of BACE1, TDP-43, AC-tau, and p-tau181 in the hippocampus of WT and TG mice that received a single mild CHI. The data are means ±SEM (ANOVA with Fisher's PLSD post-hoc test, n=3~4 animals/group). e, Single mild CHI expedites memory impairment in APP TG mice. The novel object recognition test was conducted in WT and TG mice 30 days after single mild CHI. The data are means ±SEM (ANOVA with Bonferroni post-hoc test, n=9~12 animals/group). f, Single mild CHI accelerates impairments in spatial learning and memory in APP TG mice. The Morris water maze test was conducted in WT and TG mice 30 days after single mild CHI. The data are means ±SEM. ***P<0.001 (ANOVA with repeated measures). The probe test was conducted 24 hours following 7-day learning acquisition training. The data are means ±SEM (ANOVA with Bonferroni post-hoc test, n=9~12 animals/group).
Silencing of TDP-43 prevents single mild CHI-promoted neuroinflammation, Aβ formation, and tau phosphorylation in APP TG mice
To determine whether TDP-43 is a common mechanism for single- and repeated mild CHI-induced neuropathology and synaptic and cognitive impairments, we designed and generated lentiviruses (LV) expressing scramble control (LV-Scr) or expressing TDP-43-shRNA using FUGW lentiviral vectors, as reported previously [66, 79]. To avoid non-specific off-target effects of shRNA knockdown, we also used a knockdown rescue strategy by shRNA silencing of endogenous TDP-43 while concurrently expressing an epitope-tagged shRNA-resistant TDP-43 (LV-Resc), as described previously [12]. As shown in Supplementary Fig. 1a, expression of TDP-43 was reduced in cells treated with LV-TDP-43-shRNA, but the decrease was revoked in cells treated with LV-Resc. Knockdown of TDP-43 was also confirmed by immunostaining analysis in the hippocampal sections from mice that were stereotaxically injected with LV-TDP-43-shRNA (Supplementary Fig. 1b).
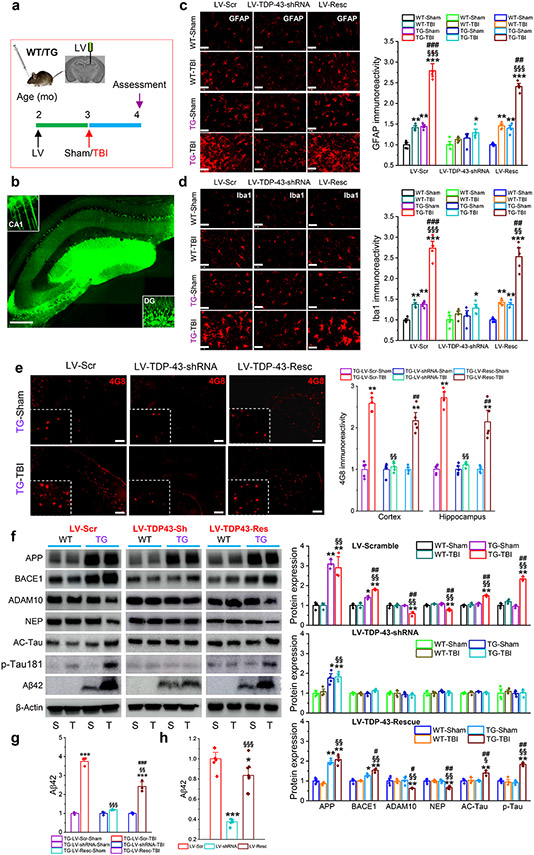
To determine the effects of knockdown of TDP-43 on single and repeated mild CHI-induced neuropathological changes, LV-Scr, LV-TDP-43-shRNA, or LV-Resc were injected into the hippocampus of WT or APP TG mice at 2 months of age, 30 days prior to a single or repeated mild CHI (Fig. 2a, b, Fig. 3a), as described previously [31, 66, 79]. Neuroinflammation, Aβ, and tau pathologies were assessed 30 days after a single mild CHI. As astrocytes and microglial cells are the main components in neuroinflammation, we first assessed immunoreactivity of GFAP and Iba1, specific markers of astrocytes and microglial cells, in the hippocampus of WT or APP TG mice that had received LV-Scr, LV-TDP-43-shRNA, or LV-Resc 30 days prior to a single mild CHI. A single mild CHI slightly increased reactivity of astrocytic and microglia in WT mice that received LV-Scr but robustly increased the reactivity in APP TG mice, supporting the idea that a single mild CHI intensifies neuroinflammatory responses in APP TG mice (Fig. 2c, d). However, the increased reactivity of astrocytes and microglia following single mild CHI was attenuated in both WT and APP TG mice that had received LV-TDP-43-shRNA. These results indicate that knockdown of TDP-43 prevents mild CHI-provoked neuroinflammation. This is further supported by results showing that silencing of TDP-43 reduced gliosis in WT mice that received repeated mild CHI (Fig. 3b, c). In addition, the TDP-43 knockdown-mediated decrease in reactivity of astrocytes and microglia was reversed by injection of LV-Resc in APP TG mice that received a single mild CHI or in WT mice that received repeated mild CHI (Fig. 2c, d and Fig. 3b, c).
Figure 2.
Silencing of TDP-43 alleviates single mild CHI-exacerbated neuropathology in APP TG mice. a, Schematic illustration of the experimental protocol. WT and APP TG mice at two months of age were stereotaxically injected with LV 30 days prior to a single mild CHI, and all the assessments were performed 30 days after a single mild CHI. b, A representative image showing expression of GFP in the hippocampus injected with LV. Scale bar: 200 μm. c, Immunoreactivity of GFAP (astrocytic marker) in the ipsilateral hippocampus of WT and APP TG mice that received LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The data are means ±SEM. *P<0.05, **P<0.01, ***P<0.001 compared with WT-Sham; §§§P<0.001 compared with WT-TBI; ##P<0.01, ###P<0.001 compared with TG-Sham (ANOVA with Bonferroni post-hoc test, n=5 animals/group). Scale bars: 40 μm. d, Immunoreactivity of Iba1 (microglial marker) in the ipsilateral hippocampus of WT and APP TG mice that received LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The data are means ±SEM. *P<0.05, **P<0.01, ***P<0.001 compared with WT-Sham; §§P<0.01, §§§P<0.001 compared with WT-TBI; ##P<0.01, ###P<0.001 compared with TG-Sham (ANOVA with Bonferroni post-hoc test, n=5 animals/group). Scale bars: 40 μm. e, Single mild CHI-aggravated formation of Aβ plaques is prevented by knockdown of TDP-43 in APP TG mice. The data are means ±SEM. **P<0.01 compared with TG-LV-Scr-Sham; §§P<0.01 compared with TG-LV-Scr-TBI; ##P<0.01 TG-LV-Resc-Sham (ANOVA with Fisher's PLSD post-hoc test, n=5 animals/group). f, Immunoblot analysis of hippocampal protein expressions in WT and APP TG mice that were injected with LV expressing scramble control, TDP-43-shRNA or shRNA-resistant TDP-43 prior to receive a single mild CHI. The data are means ±SEM. *P<0.05, **P<0.01 compared with WT-Sham; §P<0.05, §§P<0.001 compared with WT-TBI; #P<0.05, ##P<0.001 compared with TG-Sham (ANOVA with Bonferroni post-hoc test, n=3~4 animals/group). g, Quantification of Aβ42 displayed in f as it is not detectable in WT animals. The data are means ±SEM. ***P<0.001 compared with TG-LV-Scr-Sham; §§P<0.01, §§§P<0.001 TG-LV-Scr-TBI; ###P<0.01 TG-LV-Resc-Sham (ANOVA with Fisher's PLSD post-hoc test, n=3~4 animals/group). h, ELISA analysis of hippocampal Aβ42 in APP TG mice treated with LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The data are means ±SEM. *P<0.05, ***P<0.001 compared with LV-Scr, §§§P<0.001 LV-shRNA (ANOVA with Bonferroni post-hoc test, n=6~8 animals/group).
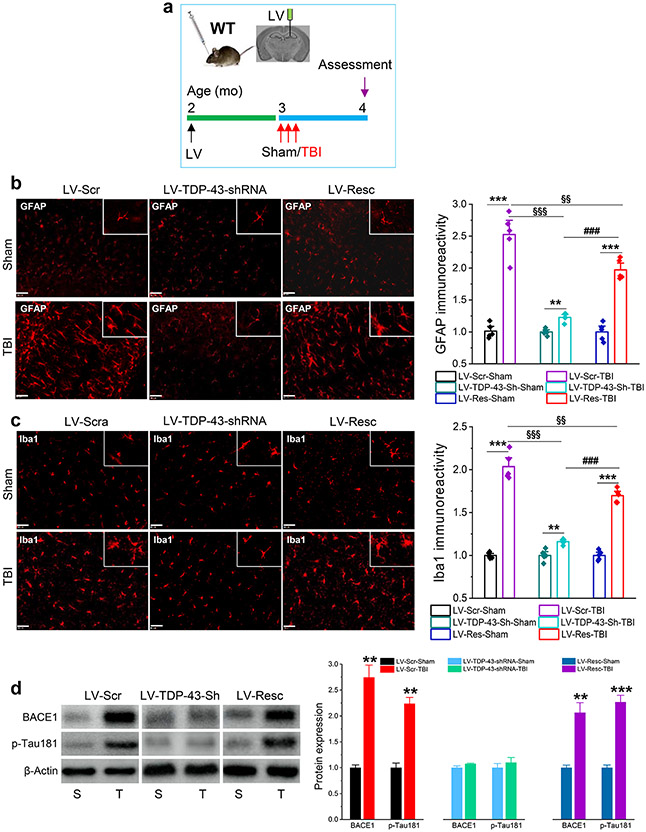
Figure 3.
Knockdown of TDP-43 reduces repeated mild CHI-induced neuroinflammation and neuropathology in WT animals. a, Schematic illustration of the experimental protocol. WT mice at two months of age were stereotaxically injected with LV 30 days prior to three repeated mild CHI (once a day for three days) and all the assessments were performed 30 days after mild CHI. b, Immunostaining analysis of immunoreactivity of GFAP in the ipsilateral hippocampus of WT mice that received LV expressing Scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The data are means ±SEM. **P<0.01, ***P<0.001 compared with LV-Scr-Sham; §§P<0.01, §§§P<0.001 compared with LV-Scr-TBI; ###P<0.001 compared with LV-shRNA-TBI (ANOVA with Bonferroni post-hoc test, n=5 animals/group). Scale bars: 200 and 40 μm. c, Immunoreactivity of Iba1 in the ipsilateral hippocampus of WT and APP TG mice that received LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The data are means ±SEM. **P<0.01, ***P<0.001 compared with LV-Scr-Sham; §§P<0.01, §§§P<0.001 compared with LV-Scr-TBI; ###P<0.001 compared with LV-shRNA-TBI (ANOVA with Bonferroni post-hoc test, n=5 animals/group). Scale bars: 200 and 40 μm. d, Immunoblot analysis of BACE1 and p-tau181 in the ipsilateral hippocampus in WT animals that received LV-Scramble, -TDP-43-shRNA or -Rescue. The data are means ±SEM. **P<0.01, ***P<0.001 compared with sham (ANOVA with Fisher's PLSD post-hoc test, n=6).
We next assessed whether knockdown of TDP-43 affects APP processing, Aβ formation, and tau phosphorylation in APP TG mice 30 days after exposure to a single mild CHI. We found that a single mild CHI robustly increased accumulation and deposition of Aβ plaques in the brain of 4-month-old APP TG mice that had received LV-Scr (Fig. 2e). However, the single mild CHI-induced increase in Aβ plaques in TG mice was averted by treatment with LV-TDP-43-shRNA, which prevents TBI-induced excessive production of TDP-43. In APP TG mice treated with LV-Resc, a single mild CHI still increased Aβ plaques. These results suggest that a single mild CHI accelerates or exacerbates accumulation of Aβ plaques in APP TG mice that receive LV-Scr, but the acceleration is prevented by silencing of TDP-43. Accelerated Aβ formation following a single mild CHI can likely be attributed to an increase in Aβ processing. We observed that expression of BACE1 was significantly elevated in APP TG mice that received LV-Scr but expression of ADAM10 and neprilysin (NEP) was downregulated. These changes were diminished by silencing TDP-43 in APP TG mice, although APP, which is transgenically overexpressed in APP TG mice, was not diminished (Fig. 2f). Moreover, increased Aβ42 following mild CHI was also suppressed by knockdown of TDP-43 in APP TG mice (Fig. 2f-h). In particular, a single mild CHI induced tau acetylation and phosphorylation in APP TG mice, but these changes were mitigated by TDP-43 shRNA (Fig. 2f). As expected, changes in β-amyloidosis and p-tau induced by single mild CHI reoccurred in APP TG mice that received LV-Resc. These results indicate that a single mild CHI facilitates APP processing and tau phosphorylation in APP TG mice.
To determine whether TDP-43 is also critical in repeated mild CHI-induced Aβ and tau neuropathology, WT mice were transfected with LV-Scr, LV-TDP-43-shRNA, or LV-Resc 30 days prior to three repeated mild CHI (Once a day for three days, Fig. 3a), as described previously [31, 80]. We observed that repeated mild CHI significantly increased expression of BACE1 and p-tau181 (Fig. 3d), but the increases were eliminated by silencing TDP-43. In mice treated with LV-Resc, expression of BACE1 and p-tau181 were increased again by repeated mild CHI. The data from both single and repeated mild CHI animal models suggest that TDP-43 plays an important role in TBI-induced Aβ formation and tau phosphorylation.
TBI-induced overproduction of TDP-43 disrupts synaptic integrity and impairs long-term synaptic plasticity
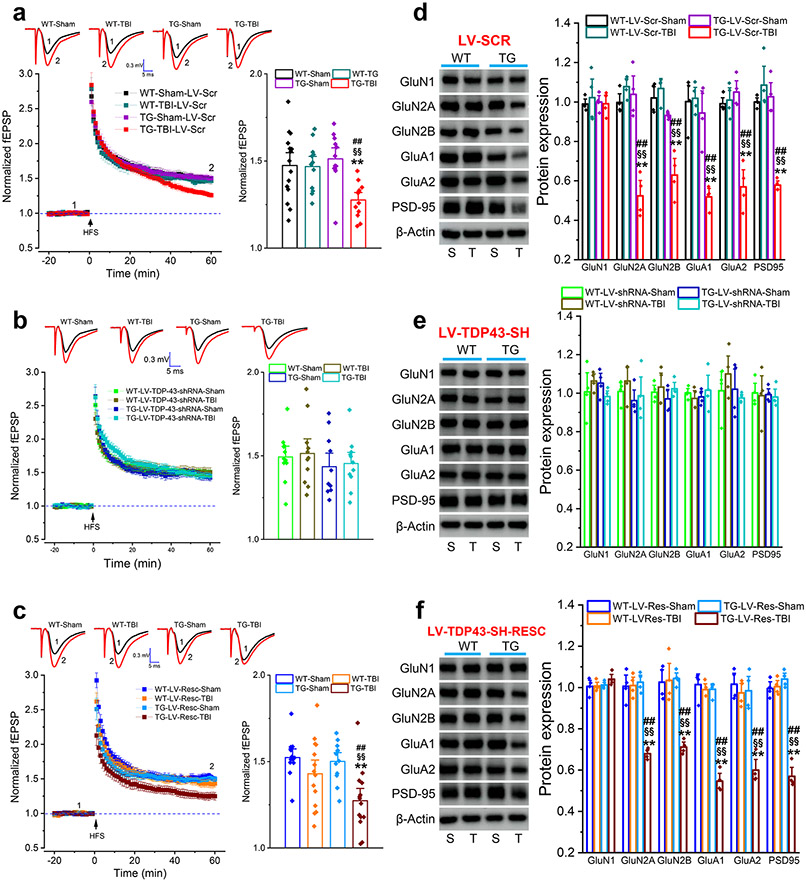
To determine whether TBI-induced aberrant production of TDP-43 contributes to disruption of synaptic integrity and plasticity, we assessed hippocampal long-term potentiation (LTP) in WT and APP TG mice 30 days after a single mild CHI. We found that a single mild CHI did not induce an impairment of LTP in WT mice that had received LV-Scr but significantly suppressed LTP in APP TG mice treated with LV-Scr (Fig. 4a). Single mild CHI-induced impairment of LTP in APP TG mice was prevented by knockdown of TDP-43 (Fig. 4b) but revoked by treatment with LV-Resc (Fig. 4c). Similarly, we observed that repeated mild CHI impaired LTP in WT mice that had received LV-Scr (Fig. 5a) and the impairment was prevented by silencing TDP-43 (Fig. 5b). As expected, repeated mild CHI impaired LTP in animals treated with LV-Resc (Fig. 5c).
Figure 4.
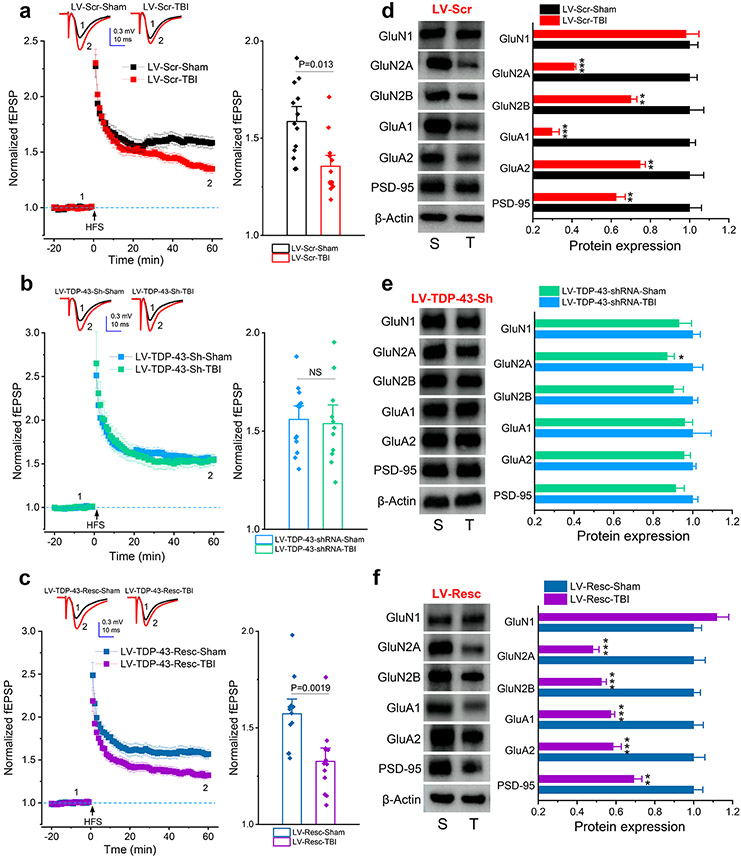
Single mild CHI-impaired long-term potentiation (LTP) and expression of synaptic proteins are prevented by knockdown of TDP-43 in APP TG mice. a~c, LTP at hippocampal CA3-CA1 synapses in WT and TG mice that received LV expressing scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. Recordings were made 30 days following single mild CHI. The data in bar graphs are means ±SEM averaged from 56 to 60 min following high-frequency stimulation (HFS). **P<0.01 compared with WT-Sham; §§P<0.01 compared with WT-TBI; ##P<0.01 compared with TG-Sham (ANOVA with Bonferroni post-hoc test, n=12~15 slices/5~6 animals/group). d, Expression of glutamate receptor subunits and PSD-95 in the hippocampus of WT and APP TG mice that received LV expressing scramble control. The analysis was performed 30 days after single mild CHI. The data are means ±SEM. *P<0.05, **P<0.01 compared with WT-LV-Scr-Sham, §P<0.05, §§P<0.01 compared with WT-LV-Scr-TBI; ##P<0.01 compared with TG-LV-Scr-Sham (ANOVA with Fisher's PLSD test post-hoc test, n=3~4 animals/group). e, Expression of glutamate receptor subunits and PSD-95 in the hippocampus of WT and APP TG mice that received LV expressing TDP-43-shRNA. The data are means ±SEM (n=3~4 animals/group). f, Expression of glutamate receptor subunits and PSD-95 in the hippocampus of WT and APP TG mice that received LV expressing shRNA-resistant TDP-43. The data are means ±SEM. *P<0.05, **P<0.01 compared with WT-LV-Resc-Sham, §P<0.05, §§P<0.01 compared with WT-LV-Resc-TBI; #P<0.05, ##P<0.01 compared with TG-LV-Resc-Sham (ANOVA with Fisher's PLSD post-hoc test, n=3~4 animals/group).
Figure 5.
Repeated mild CHI-impaired long-term potentiation (LTP) and expression of synaptic proteins are alleviated by silencing of TDP-43 in WT mice. (a~c) LTP at hippocampal CA3-CA1 synapses in WT mice that received LV expressing Scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. Recordings were made 30 days following three repeated mCHI. The data in bar graphs are means ±SEM averaged from 56 to 60 min following high-frequency stimulation (HFS). ANOVA with Bonferroni post-hoc test, n=12~15 slices/5~7 animals/group). (d~f) Expression of glutamate receptor subunits and PSD-95 in the hippocampus of WT mice that received LV expressing Scramble control, TDP-43-shRNA, or shRNA-resistant TDP-43. The analysis was performed 30 days after repeated mCHI. The data are means ±SEM. *P<0.05, **P<0.01, ***P<0.001 compared with Sham, (ANOVA with Fisher's PLSD test post-hoc test, n=6 animals/group).
To determine whether a single mild CHI reduces expression of synaptic proteins in APP TG mice, we assessed expression of PSD-95, NMDA receptor subunits GluN1, GluN2A and GluN2B, and AMPA receptor subunits GluA1 and GluA2. We found that a single mild CHI significantly downregulated expression of PSD-95 and glutamate receptor subunits except for GluN1 in the hippocampus of APP TG mice treated with LV-Scr (Fig. 4d). However, the expression of these important synaptic molecules in APP TG mice was prevented by silencing of TDP-43 (Fig. 4e) but expression was restored in mice treated with LV-Resc (Fig. 4f). Likewise, TDP-43 is also critical in repeated mild CHI-induced downregulation of synaptic proteins (Fig. 5d-f). These results provide evidence that a single mild CHI is sufficient to accelerate synaptic dysfunction in APP TG mice at 4 months of age and that limiting overproduction of TDP-43 following TBI is capable of maintaining the integrity and plasticity of synapses, which are important for cognitive function.
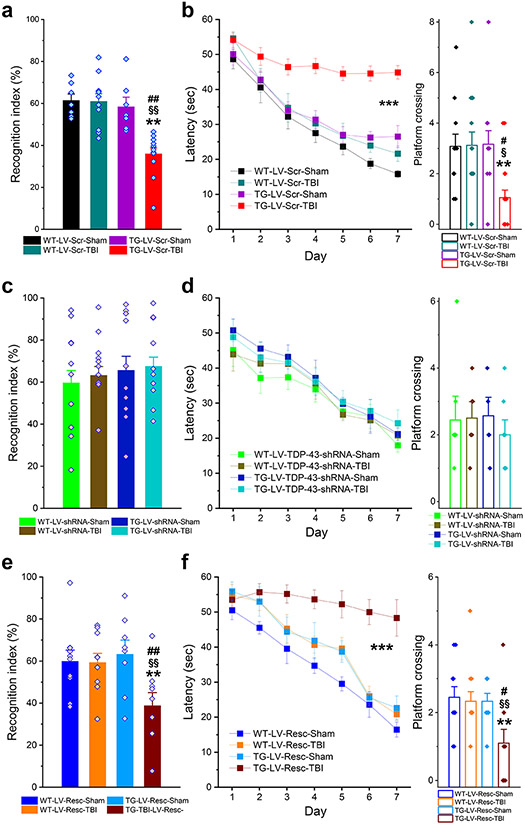
Silencing of TDP-43 prevents single and repeated mild CHI-impaired learning and memory
To determine whether TDP-43 contributes to single or repeated mild CHI-induced cognitive deficits, behavioral performance was assessed using the NOR and MWM tests in APP TG mice that received a single mild CHI or in WT mice that received repeated mild CHI. We found that mild CHI significantly impaired spatial learning and memory retention in both APP TG mice or WT mice that received LV-Scr (Figs. 6a, b and 7a, b). However, the impairments were prevented by knockdown of TDP-43 (Figs. 6c, d and 7c, d). As expected, TBI-induced impairments in learning and memory reoccurred in APP TG or WT mice that received LV-Resc (Figs. 6e, f and 7e, f). Our results indicate that mild CHI-impaired learning and memory are associated with overproduction of TDP-43.
Figure 6.
Silencing of TDP-43 prevents single mild CHI-accelerated cognitive decline in APP TG mice. a, Memory retention was assessed using the novel object recognition (NOR) test in WT and APP TG mice that received LV expressing scramble control. The test was conducted 30 days after single mild CHI. The data are means ±SEM. **P<0.01 compared with WT-LV-Scr-Sham, §§P<0.01 compared with WT-LV-Scr-TBI; ##P<0.01 compared with TG-LV-Scr-Sham (ANOVA with Bonferroni post-hoc test, n=8~11 animals/group). b, Spatial learning and memory retention were assessed using the Morris water maze (MWM) test. The data are means ±SEM (ANOVA with repeated measures). The probe test was conducted 24 hrs following 7 days of learning acquisition training. The data are means ±SEM. **P<0.01, compared with WT-LV-Scr-Sham; §P<0.05 compared with WT-LV-Scr-TBI; #P<0.05 compared with TG-LV-Scr-Sham. The data are means ±SEM. ***P<0.01 (ANOVA with Bonferroni post-hoc test). c, NOR test in WT and APP TG mice that received LV expressing TDP-43-shRNA. The data are means ±SEM (n=10 animals/group). d, MWM test in WT and APP TG mice that received LV expressing TDP-43-shRNA. e, NOR test in WT and APP TG mice that received LV expressing shRNA-resistant TDP-43. The data are means ±SEM. **P<0.01 compared with WT-LV-Resc-Sham, §§P<0.01 compared with WT-LV-Resc-TBI; ##P<0.01 compared with TG-LV-Resc-Sham (ANOVA with Bonferroni post-hoc test, n=8~10 animals/group). f, MWM test in WT and APP TG mice that received LV expressing shRNA-resistant TDP-43. The data are means ±SEM. ***P<0.01 (ANOVA with repeated measures). The probe test was conducted 24 hours after 7-day learning acquisition training. **P<0.01 compared with WT-LV-Resc-Sham, §§P<0.01 compared with WT-LV-Resc-TBI; #P<0.05 compared with TG-LV-Resc-Sham (ANOVA with Bonferroni post-hoc test).
Figure 7.
Knockdown of TDP-43 prevents repeated mild CHI-induced cognitive decline in WT mice. a, NOR test in animals that received LV expressing Scramble control. The test was conducted 30 days after three mild CHI. The data are means ±SEM (ANOVA with Bonferroni post-hoc test, n=11~14 animals/group). b, MWM test in animals that received expressing Scramble control. The data are means ±SEM (ANOVA with repeated measures). The probe test was conducted 24 hours after 7-day learning acquisition training. The data are means ±SEM (ANOVA with Bonferroni post-hoc test). c, NOR test in animals that received LV expressing TDP-43-shRNA. The data are means ±SEM (n=11~14 animals/group). d, MWM test in animals that received LV expressing TDP-43-shRNA. The data of the probe test are means ±SEM. e, NOR test in WT mice that received LV expressing shRNA-resistant TDP-43. The data are means ±SEM (ANOVA with Bonferroni post-hoc test, n=22~25 animals/group). f, MWM test in WT mice that received LV expressing shRNA-resistant TDP-43. The data are means ±SEM (ANOVA with repeated measures, n=11~12 animals/group). The probe test was conducted 24 hours after 7-day learning acquisition training. The data are means ±SEM (ANOVA with Bonferroni post-hoc test).
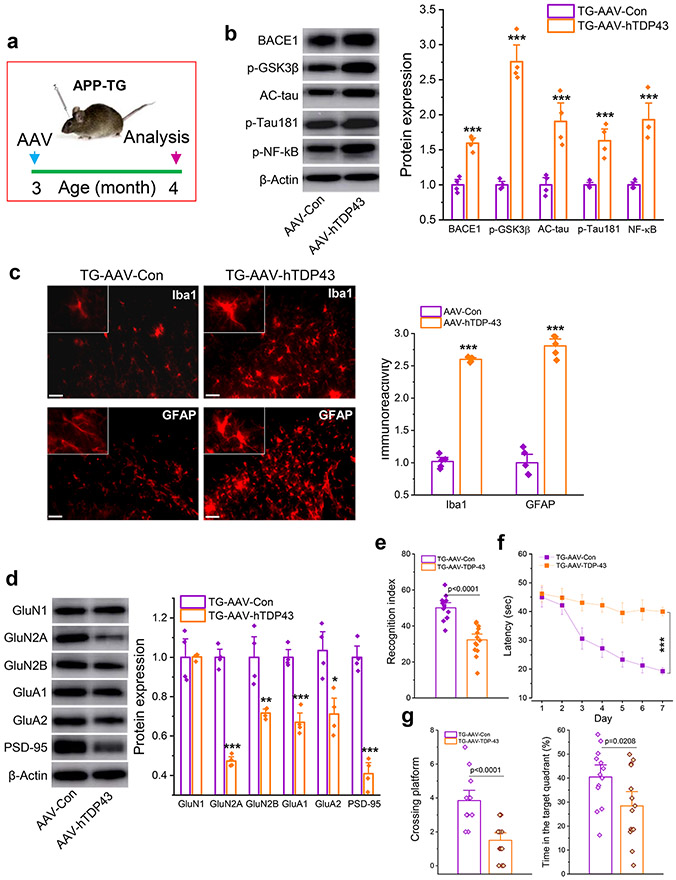
Overexpression of TDP-43 exacerbates neuropathology and promotes cognitive decline
To determine whether overexpression of TDP-43 and mild CHI produce similar changes in neuropathology and cognitive function, we used AAV vectors to overexpress human TDP-43 (AAV-hTDP43) in the hippocampus of APP TG mice (Fig, 8a and Supplementary Fig. 2). We found that AAV-mediated overexpression of human TDP-43 resulted in robust increases in expression of BACE1, phosphorylated glycogen synthase kinase-3β (p-GSK3β), AC-tau, p-tau, and phosphorylated NF-κB (p-NF-κB) in the hippocampus of APP TG mice (Fig. 8b). Overexpression of TDP-43 also provoked neuroinflammation as reactivity of microglia and astrocytes was significantly enhanced (Fig. 8c), suggesting that increased TDP-43 also escalates neuroinflammatory responses. Importantly, increased expression of TDP-43 downregulated expression of synaptic proteins, including PSD95 and glutamate receptor subunits, and accelerated deterioration in learning and memory assessed by NOR and MWM tests in APP TG mice (Fig. 8d-g). These data provide evidence that overexpression of TDP-43 induces AD neuropathology and cognitive deficits in APP TG mice, mimicking the changes induced by single mild CHI. We also observed that overexpression of TDP-43 caused impairments in learning and memory in WT mice (Supplementary Fig. 3).
Figure 8.
Overexpression of TDP-43 aggravates neuropathology and accelerates impairments of learning and memory in APP TG mice. a, Schematic illustration of the protocol for injection of AAV vectors. AAV9-CMV-hTDP-43.eGFP vectors or AAV9-CMV-eGFP control vectors were stereotaxically injected into the hippocampus of APP TG mice at three months of age and all the assessments were made 30 days after injection of AAV vectors. b, Immunoblot analysis of BACE1, p-GSK3β, AC-tau, p-tau181, and p-NF-kB. ***P<0.001 compared with TG-AAV-Con (ANOVA with Fisher's PLSD post-hoc test, n=4 animals/group). c, Immunoreactivity of microglia and astrocytes in the hippocampus of APP TG mice. ***P<0.001 compared with TG-AAV-Con (ANOVA with Bonferroni post post-hoc test, n=5 animals/group). d, Immunoblot analysis of glutamate receptor subunits and PSD-95 in the hippocampus of APP TG mice. *P<0.05, **P<0.01, ***P<0.001 compared with TG-AAV-Con (ANOVA with Fisher's PLSD post-hoc test, n=4 animals/group). e, NOR test in APP TG mice that received AAV-hTDP43. (ANOVA with Bonferroni post post-hoc test, n=13~14 animals/group). f, Learning acquisition in the MWM test. ***P<0.001 compared with TG-AAV-Con (ANOVA with repeated measures). g, The probe test that was performed 24 hrs following 7-day invisible training. (ANOVA with Bonferroni post post-hoc test, n=13~14 animals/group).
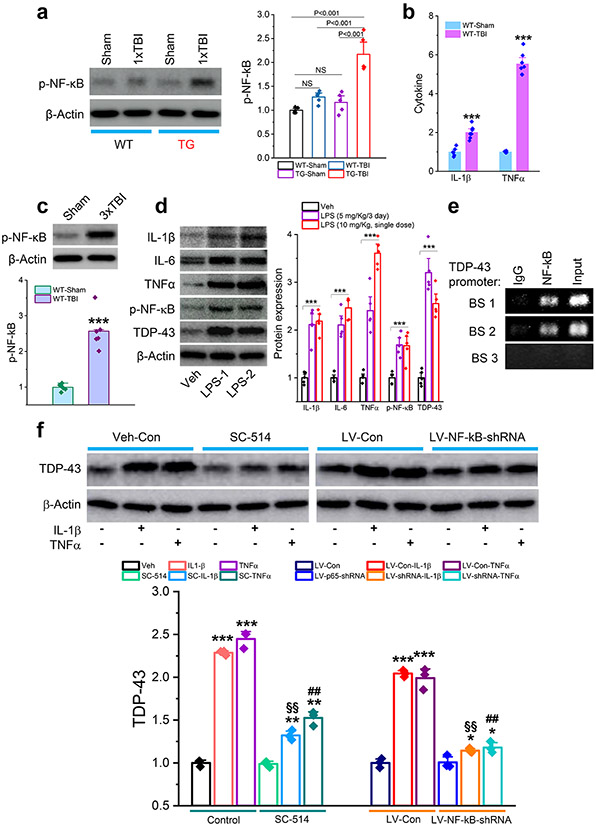
TBI-triggered neuroinflammation promotes NF-kB-mediated expression of TDP-43
To determine whether excessive production of TDP-43 following TBI is mediated via NF-kB signaling, we assessed p-NF-κB in WT and APP TG mice following single mild CHI. We found that a single mild CHI significantly increased p-NF-κB in APP TG mice but not in WT mice (Fig. 9a). The increased p-NF-κB in APP TG mice following single mild CHI can likely be attributed to elevated expression of cytokines and reactivity of astrocytes and microglia (Fig. 2c, d), as proinflammatory cytokines, including IL-1β and TNFα, were significantly elevated in the hippocampus of WT animals following repeated mild CHI (Fig. 9b). Correspondingly, p-NF-κB was increased in WT mice exposed to repeated mild CHI (Fig. 9c). These results suggest that TBI triggers inflammatory responses, which promote phosphorylation of NF-κB. To confirm this, we treated WT mice with lipopolysaccharide (LPS) to induce inflammation. We observed that either single or multiple doses of LPS significantly increased expression of cytokines (IL-1β, IL-6, and TNFα) and p-NF-κB in the hippocampus (Fig. 9d). Importantly, LPS treatment resulted in upregulation of TDP-43 expression, similar to that seen in TBI mice. These data indicate that TBI-induced expression of TDP-43 is likely mediated via inflammation-triggered NF-κB signaling. To test this prediction, we performed two sets of experiments. First, we conducted a chromatin immunoprecipitation (CHIP) analysis. We observed that the NF-κB p65 subunit displayed binding activity at the promoters of the TDP-43 gene (Fig. 9e), suggesting that NF-κB regulates transcription and expression of TDP-43. In the second experiment, we treated hippocampal neurons in vitro with IL-1β or TNFα in the absence or presence of SC-514, an NF-κB signaling pathway inhibitor. We found that IL-1β or TNFα elevated expression of TDP-43, but the elevation was attenuated by pharmacological inhibition of NF-κB (Fig. 9f). To further confirm that expression of TDP-43 is regulated through NF-κB signaling, we treated cultures with LV expressing p65-shRNA. While IL-1β or TNFα still significantly increased expression of TDP-43 in cultures treated with LV-control, the increase was diminished in cultures treated with LV-NF-κB-shRNA (Fig. 9f). These results provide evidence that TBI-triggered neuroinflammation promotes NF-κB-mediated aberrant expression of TDP-43.
Figure 9.
Neuroinflammation causes aberrant production of TDP-43 via NF-κB signaling. a, Expression of phosphorylated NF-κB (p-NF-κB) in the hippocampus of APP TG and WT mice that received a single mild CHI. The analysis was performed 30 days after the impact. The data are means ±SEM (ANOVA with Fisher's PLSD post-hoc test, n=5 animals/group). b, Cytokine formation in the hippocampus of WT mice that received repeated mild CHI. ELISA analysis IL-1β and TNFα was conducted 24 hours following the last impact. The data are means ±SEM. ***P<0.001 (ANOVA with Bonferroni post-hoc test, n=6~7 animals/group). c, Expression of p-NF-κB in the hippocampus of WT mice that received three mild CHI. The analysis was performed 30 days after repeated mild CHI. The data are means ±SEM (ANOVA with Fisher's PLSD post-hoc test, n=6 animals/group). d, Lipopolysaccharide (LPS) increases expression of cytokines, p-NF-κB, and TDP-43 in the hippocampus of WT mice. LPS was injected (i.p.) with repeated doses at 5 mg/kg (Once a day for three days, LPS-1) or a single dose at 10 mg/kg (LPS-2). Immunoblot analysis was performed 24 hours after injection. The data are means ±SEM. ***P<0.001 compared with the vehicle control (ANOVA with Fisher's PLSD post-hoc test, n=5 animals/group). e, Chromatin immunoprecipitation (CHIP) analysis of NF-κB p65 binding activity at the promoter of the TDP-43 gene. f, Proinflammatory cytokine-increased expression of TDP-43 is attenuated by pharmacological or genetic inhibition of NF-κB p65. Hippocampal neurons in cultures from control mice were treated with SC-541 (IKKβ inhibitor, 100 μM) or transfected with LV expressing NF-κB p65-shRNA for three days. IL-1β or TNFα (10 ng/ml)-induced expression of TDP-43 was assessed 24 hours after treatment with cytokines. The data are means ±SEM. *P<0.05, **P<0.01, ***P<0.001 compared with the vehicle control or LV-Con; §§P<0.01 compared with IL-1β; ##P<0.01 compared with TNFα (ANOVA with Fisher's PLSD test post-hoc test, n=3).
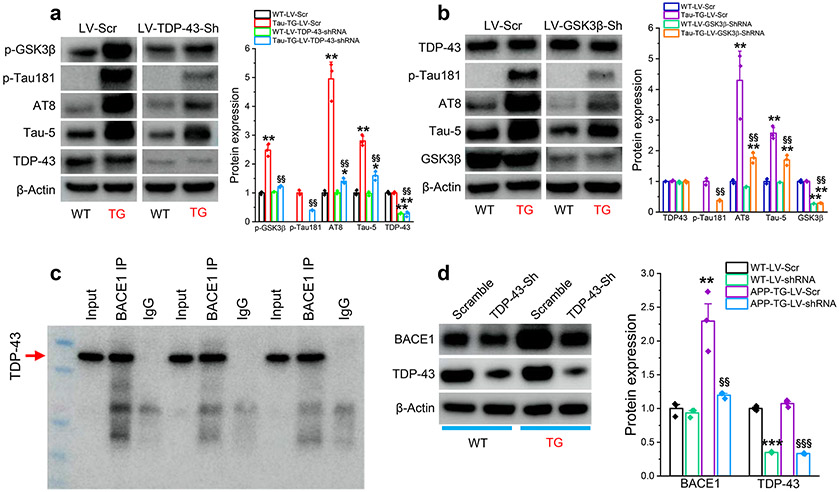
TDP-43 promotes tau phosphorylation and Aβ formation through interacting with GSK3β and BACE1
To assess whether TDP-43 is involved in processing tau phosphorylation and Aβ formation, we treated cultures of hippocampal neurons from PS19 tau TG mice with LV-TDP-43-shRNA or LV-Scr control. We observed that expression of TDP-43 was not significantly changed in hippocampal neurons cultured from PS19 tau TG mice. However, expression of p-GSK3β, p-tau181, AT8 (p-tau-S202/T205), and AT5 (total tau) were elevated in cultures treated with LV-Scr (Fig. 10a). The increased expression of these molecules was attenuated in cultures treated with LV-TDP-43-shRNA (Fig. 10a). GSK3β is the major kinase that phosphorylates tau, and it plays an important role in AD neuropathology [42, 46, 50, 69]. To confirm the role of GSK-3β in tau phosphorylation, we generated LV expressing GSK3β-shRNA. We found that knockdown of GSK-3β significantly reduced p-tau proteins in hippocampal neurons cultured from PS19 tau TG mice (Fig. 10b). However, silencing of GSK3β did not alter expression of TDP-43. These data suggest that GSK-3β is a downstream signaling molecule in TDP-43-promoted tau phosphorylation as knockdown of TDP-43 reduces p-GSK3β but silencing of GSK3β does not alter expression of TDP-43.
Figure 10.
TDP-43 stimulates tau phosphorylation and BACE1 expression. a, Knockdown of TDP-43 reduces phosphorylated GSK3β and prevents tau phosphorylation in cultured hippocampal neurons from tau TG mice (PS19). Hippocampal neurons in culture were treated with LV expressing TDP-43-shRNA or scramble control. The data are means ±SEM. *P<0.05, **P<0.01 compared with WT-LV-Scr; §§P<0.01 compared with Tau-TG-LV-Scr (ANOVA with Fisher's PLSD test post-hoc test, n=3). b, Silencing of GSK3β attenuates tau phosphorylation but does not affect TDP-43 expression in cultured hippocampal neurons from PS19 mice. Hippocampal neurons in culture were treated with LV expressing scramble control or GSK3β-shRNA. The data are means ±SEM. **P<0.01 compared with WT-LV-Scr; §§P<0.01 compared with Tau-TG-LV-Scr (ANOVA with Fisher's PLSD test post-hoc test, n=3). c, Immunoblot analysis of co-immunoprecipitation (co-IP) of BACE1 with TDP-43 in hippocampal tissues from WT mice (n=3 animals/group). d, Silencing of TDP-43 reduces expression of TDP-43 in hippocampal neurons cultured from 5xFAD mice. Hippocampal neurons in culture were treated with LV expressing TDP-43-shRNA or scramble control for three days. The data are means ±SEM. **P<0.01, ***P<0.001 compared with WT-LV-Scr; §§P<0.01 §§§P<0.001 compared with APP-TG-LV-Scr (ANOVA with Fisher's PLSD test post-hoc test, n=3).
Next, we asked whether TDP-43 interacts with BACE1, resulting in the increased Aβ formation seen in APP TG mice following mild CHI (cf. in Figs. 1c, d, 2e-h). To address this question, we first performed co-immunoprecipitation (IP)-Western blot analysis and found that interactions did occur between TDP-43 and BACE1 (Fig. 10c). We then assessed whether knockdown of TDP-43 reduced expression of BACE1 in vitro. Hippocampal neurons cultured from 5xFAD APP TG mice were treated with LV-TDP-43-shRNA or LV-Scr control. We found that silencing of TDP-43 led to a suppression of BACE1 expression (Fig. 10d), similar to that observed in vivo (Fig. 2f). In concert with our findings that knockdown of TDP-43 decreased total Aβ and Aβ42 in APP TG mice (Fig. 2e-h), these results suggest that TDP-43 promotes Aβ formation through interactions with BACE1.
Discussion
TBI has been recognized as an important risk factor of developing AD [18, 22, 27, 54]. However, the molecular mechanisms by which TBI contributes to development of AD remain unclear. In the present study, we provide evidence that aberrant production of TDP-43 is a key factor in TBI-induced neuropathology and synaptic and cognitive declines in mouse models of both single and repeated mild closed head injury. Our results show that a single mild CHI is sufficient to aggravate AD neuropathology and accelerate synaptic and cognitive deterioration in APP transgenic mice, while repeated mild CHI are needed to induce neuropathology and synaptic and cognitive impairments in WT animals. Neuropathology and synaptic and cognitive deficits induced by single mild CHI in APP TG mice or by repeated mild CHI in WT mice are alleviated by silencing TDP-43 in the hippocampus, and the alleviation is reversed by rescue of the TDP-43 knockdown. Overexpression of TDP-43 in the hippocampus induces AD neuropathology and cognitive impairments similar to the mild CHI-induced changes in APP TG mice. Our results provide further evidence that TBI-triggered neuroinflammation stimulates NF-kB-mediated transcription and expression of TDP-43, which in turn promotes tau phosphorylation and Aβ formation by provoking phosphorylation of GSK3β and expression of BACE1 (Fig. 11). Our study shows that aberrant production of TDP-43 exacerbates neuropathology and drives synaptic and cognitive declines following TBI.
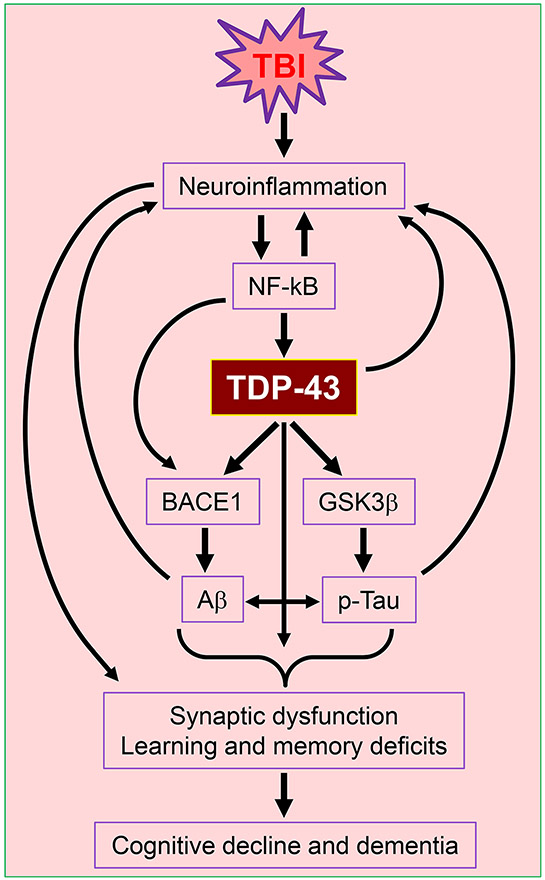
Figure 11.
Cartoon illustrating potential mechanisms of TBI-induced aberrant production of TDP-43 that causes neuropathology and synaptic and cognitive declines. TBI-triggered neuroinflammation promotes NF-κB-mediated transcription and expression of TDP-43, which in turn interacts with BACE1 and GSK3β, resulting in tau phosphorylation (p-tau) and Aβ formation. TDP-43 its own proteinopathy together with increased p-tau and Aβ drive synaptic dysfunction and learning and memory deterioration following TBI, which eventually lead to cognitive decline and dementia.
Previous studies have shown that repeated mild CHI, but not single mild CHI, induces neuroinflammation, neuropathology, and cognitive decline in mouse models of CHI [14, 48, 51, 59, 63]. Recent studies showed that a single mild CHI accelerates blood-brain barrier (BBB) leakage, neuropathology and synaptic and cognitive impairments in APP TG mice [43, 75]. These results suggest that a single mild CHI may not lead to chronic neurodegenerative processes and dementia in healthy subjects, but suggest that a single mild CHI likely accelerates or promotes neuropathological changes among persons who are susceptible to developing AD. We confirmed that a single mild CHI is sufficient to induce or accelerate neuropathological changes and synaptic and cognitive decline in APP TG mice, supporting the notion that TBI is a risk factor for AD.
TDP-43 is a highly conserved and ubiquitous RNA/DNA-binding protein involved in multiple cellular functions [19, 60], and deletion of TDP-43 is lethal for early embryonic development in mice [19, 40, 61]. Accumulated evidence indicates that aggregation of TDP-43 is a neurobiological feature in AD [5, 35-37, 47, 49, 55, 72, 74]. TDP-43 protein has been extensively studied due to its pathological inclusions in patients with ALS and FTLD and in animal models of these diseases [8, 10, 23, 45, 56, 67]. However, the role and mechanisms of aberrantly produced TDP-43 in the pathogenesis and neuropathology of AD and TBI are largely unknown. Our study provides evidence that excessive production of TDP-43 plays an important role in TBI-induced neuropathology and synaptic and cognitive declines, shown by the fact that preventing excessive production of TDP-43 following TBI reverses these changes. TDP-43 proteinopathy in ALS and FTLD is associated with TARDBP mutations, increased TDP-43 production and aggregation, and cytoplasmic mis-localization, as well as improper regulation of RNA metabolism and protein homeostasis [19, 23, 33, 45, 60]. Excessive production of TDP-43 following single mild CHI in APP TG mice or repeated mild CHI in WT animals contributes to neuropathology in TBI likely through some of these same mechanisms. In particular, overexpression of human TDP-43 in APP TG mice induces neuropathology and impairments in learning and memory, similar to the single mild CHI-induced changes in APP TG mice. We also discovered that silencing of TDP-43 results in reductions of p-GSK3β and p-tau. Knockdown of GSK3β in cultured neurons from tau TG mice does not alter the level of TDP-43, but it decreases p-tau, suggesting that GSK3β is a downstream signaling molecule of TDP-43 in phosphorylating tau. Acetylation of tau has been thought to be an early pathological event in tau phosphorylation and p-tau accumulation [17, 32, 53, 62]. Knockdown of either TDP-43 or GSK3β results in decreases in AC-tau and p-tau, suggesting that tau acetylation is upstream of tau phosphorylation. In addition, TDP-43 interacts with and increases expression of BACE1, which is likely via post-transcriptional regulation of gene expression and translation [6]. Thus, our study reveals a previously undefined mechanism by which TBI-induced overproduction of TDP-43 contributes to neuropathology and synaptic and cognitive impairments not only through TDP-43 proteinopathy itself, but also by promoting tau phosphorylation and Aβ formation through interactions with GSK3β and BACE1 (Fig. 11). It has been well acknowledged that pathogenesis of AD involves synergistic interactions between Aβ and tau [7, 11]. Based on the results from the present study, TDP-43 is also an important contributor to the pathogenesis of TBI-induced AD-like neurodegenerative disease by promoting tau phosphorylation and Aβ processing.
Overproduction of TDP-43 is a characteristic neurobiological feature in TBI-induced neuropathology [5, 35, 47, 72]. Pathological TDP-43 inclusions are also a distinct feature of AD [36, 37, 49, 55, 74]. However, little is known about how TDP-43 is overproduced following TBI. In the present study, we provide evidence that both TBI and proinflammatory factors (e.g., LPS and cytokines) increase expression of TDP-43, and the increase is attenuated by pharmacological or genetic inhibition of NF-kB signaling, suggesting that TBI-triggered neuroinflammation promotes TDP-43 expression by stimulating transcriptional activity of NF-κB. Our results suggest that excessive production of TDP-43 either resulting from a single mild CHI in APP TG mice or from repeated mild CHI in WT mice is an important mechanism common to both AD and TBI-induced AD-like neurodegenerative disease. Thus, strategies to limit excessive production of TDP-43 may provide a therapeutic approach for preventing development of TBI-induced AD neuropathology.
Supplementary Material
Acknowledgement
We thank Dr. Bryan W. Luikart of Dartmouth Medical School for providing FUGW lentiviral vectors and Dr. Zhao-qian Teng for participating in the initial work of this project. This work was supported by National Institutes of Health grants R01NS076815, R01MH113535, and R01AG058621 (to C.C.) and by startup funds from UT Health San Antonio, Joe R. & Teresa Lozano Long School of Medicine (to C.C.).
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
Data availability
Data supporting the findings of this manuscript are available in the main text and the supplementary Materials or from the corresponding authors upon request.
References
- 1.Al-Dahhak R, Khoury R, Qazi E, Grossberg GT (2018) Traumatic Brain Injury, Chronic Traumatic Encephalopathy, and Alzheimer Disease. Clin Geriatr Med 34:4, 617–635 Doi 10.1016/j.cger.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 2.Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:2, 93–110 Doi 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulsamy A, Corrigan F, Collins-Praino LE (2019) Cognitive and neuropsychiatric impairments vary as a function of injury severity at 12 months post-experimental diffuse traumatic brain injury: Implications for dementia development. Behav Brain Res 36566–76 Doi 10.1016/j.bbr.2019.02.045 [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Brody DL, Kochanek PM, Levin H, McKee A et al. (2016) Traumatic brain injuries. Nat Rev Dis Primers 216084 Doi 10.1038/nrdp.2016.84 [DOI] [PubMed] [Google Scholar]
- 5.Blennow K, Hardy J, Zetterberg H (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76:5, 886–899 Doi 10.1016/j.neuron.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 6.Blokhuis AM, Koppers M, Groen EJN, van den Heuvel DMA, Dini Modigliani S et al. (2016) Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways. Acta Neuropathol 132:2, 175–196 Doi 10.1007/s00401-016-1575-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71:4, 505–508 Doi 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- 8.Boeynaems S, Bogaert E, Van Damme P, Van Den Bosch L (2016) Inside out: the role of nucleocytoplasmic transport in ALS and FTLD. Acta Neuropathol 132:2, 159–173 Doi 10.1007/s00401-016-1586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breunig JJ, Guillot-Sestier MV, Town T (2013) Brain injury, neuroinflammation and Alzheimer's disease. Front Aging Neurosci 526 Doi 10.3389/fnagi.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budini M, Baralle FE, Buratti E (2014) Targeting TDP-43 in neurodegenerative diseases. Expert Opin Ther Targets 18:6, 617–632 Doi 10.1517/14728222.2014.896905 [DOI] [PubMed] [Google Scholar]
- 11.Busche MA, Hyman BT (2020) Synergy between amyloid-β and tau in Alzheimer's disease. Nat Neurosci 23:10, 1183–1193 Doi 10.1038/s41593-020-0687-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Zhang J, Fan N, Teng ZQ, Wu Y et al. (2013) Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 155:5, 1154–1165 Doi 10.1016/j.cell.2013.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Zhang J, Wu Y, Wang D, Feng G et al. (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep 2:5, 1329–1339 Doi 10.1016/j.celrep.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JS, Craft R, Yu GQ, Ho K, Wang X et al. (2014) Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice. PLoS One 9:12, e115765 Doi 10.1371/journal.pone.0115765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherry JD, Esnault CD, Baucom ZH, Tripodis Y, Huber BR et al. (2021) Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer's disease. Acta Neuropathol Commun 9:1, 86 Doi 10.1186/s40478-021-01189-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I et al. (2018) TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci 21:2, 228–239 Doi 10.1038/s41593-017-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP et al. (2011) The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun 2252 Doi 10.1038/ncomms1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dams-O'Connor K, Guetta G, Hahn-Ketter AE, Fedor A (2016) Traumatic brain injury as a risk factor for Alzheimer's disease: current knowledge and future directions. Neurodegener Dis Manag 6:5, 417–429 Doi 10.2217/nmt-2016-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer EMJ, Orie VK, Williams T, Baker MR, De Oliveira HM et al. (2020) TDP-43 proteinopathies: a new wave of neurodegenerative diseases. J Neurol Neurosurg Psychiatry 92:1, 86–95 Doi 10.1136/jnnp-2020-322983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delic V, Beck KD, Pang KCH, Citron BA (2020) Biological links between traumatic brain injury and Parkinson's disease. Acta Neuropathol Commun 8:1, 45 Doi 10.1186/s40478-020-00924-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djordjevic J, Sabbir MG, Albensi BC (2016) Traumatic Brain Injury as a Risk Factor for Alzheimer's Disease: Is Inflammatory Signaling a Key Player? Curr Alzheimer Res 13:7, 730–738 [DOI] [PubMed] [Google Scholar]
- 22.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74:7, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Wang L, Huntley ML, Perry G, Wang X (2018) Pathomechanisms of TDP-43 in neurodegeneration. J Neurochem Doi 10.1111/jnc.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner RC, Yaffe K (2015) Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 66:Pt B, 75–80 Doi 10.1016/j.mcn.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW (1993) Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett 160:2, 139–144 Doi 10.1016/0304-3940(93)90398-5 [DOI] [PubMed] [Google Scholar]
- 26.Gilbert M, Snyder C, Corcoran C, Norton MC, Lyketsos CG et al. (2014) The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer's disease: the Cache County Dementia Progression Study. Int Psychogeriatr 26:10, 1593–1601 Doi 10.1017/s1041610214000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L et al. (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54:6, 1316–1323 [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Sen N (2016) Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev Neurosci 27:1, 93–100 Doi 10.1515/revneuro-2015-0017 [DOI] [PubMed] [Google Scholar]
- 29.Hashem J, Hu M, Zhang J, Gao F, Chen C (2021) Inhibition of 2-Arachidonoylglycerol Metabolism Alleviates Neuropathology and Improves Cognitive Function in a Tau Mouse Model of Alzheimer's Disease. Mol Neurobiol 58:8, 4122–4133 Doi 10.1007/s12035-021-02400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heyburn L, Sajja V, Long JB (2019) The Role of TDP-43 in Military-Relevant TBI and Chronic Neurodegeneration. Front Neurol 10680 Doi 10.3389/fneur.2019.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M, Zhu D, Zhang J, Gao F, Hashem J et al. (2022) Enhancing endocannabinoid signalling in astrocytes promotes recovery from traumatic brain injury. Brain 145:1, 179–193 Doi 10.1093/brain/awab310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX et al. (2012) Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 135:Pt 3, 807–818 Doi 10.1093/brain/aws013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssens J, Van Broeckhoven C (2013) Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Hum Mol Genet 22:R1, R77–87 Doi 10.1093/hmg/ddt349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson VE, Stewart W, Arena JD, Smith DH (2017) Traumatic Brain Injury as a Trigger of Neurodegeneration. Adv Neurobiol 15383–400 Doi 10.1007/978-3-319-57193-5_15 [DOI] [PubMed] [Google Scholar]
- 35.Johnson VE, Stewart W, Smith DH (2010) Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat Rev Neurosci 11:5, 361–370 Doi 10.1038/nrn2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME et al. (2015) TDP-43 and pathological subtype of Alzheimer's disease impact clinical features. Ann Neurol Doi 10.1002/ana.24493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N et al. (2014) TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 127:6, 811–824 Doi 10.1007/s00401-014-1269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ et al. (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:5, 572–574 Doi 10.1038/ng.132 [DOI] [PubMed] [Google Scholar]
- 39.Klim JR, Pintacuda G, Nash LA, Guerra San Juan I, Eggan K (2021) Connecting TDP-43 Pathology with Neuropathy. Trends Neurosci 44:6, 424–440 Doi 10.1016/j.tins.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 40.Kraemer BC, Schuck T, Wheeler JM, Robinson LC, Trojanowski JQ et al. (2010) Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol 119:4, 409–419 Doi 10.1007/s00401-010-0659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A et al. (1999) Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 52:1, 78–84 Doi 10.1212/wnl.52.1.78 [DOI] [PubMed] [Google Scholar]
- 42.Lauretti E, Dincer O, Praticò D (2020) Glycogen synthase kinase-3 signaling in Alzheimer's disease. Biochim Biophys Acta Mol Cell Res 1867:5, 118664 Doi 10.1016/j.bbamcr.2020.118664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecca D, Bader M, Tweedie D, Hoffman AF, Jung YJ et al. (2019) (−)-Phenserine and the prevention of pre-programmed cell death and neuroinflammation in mild traumatic brain injury and Alzheimer's disease challenged mice. Neurobiol Dis 130104528 Doi 10.1016/j.nbd.2019.104528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Li Y, Li X, Zhang S, Zhao J et al. (2017) Head Injury as a Risk Factor for Dementia and Alzheimer's Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PLoS One 12:1, e0169650 Doi 10.1371/journal.pone.0169650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling SC, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79:3, 416–438 Doi 10.1016/j.neuron.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llorens-Martin M, Jurado J, Hernandez F, Avila J (2014) GSK-3beta, a pivotal kinase in Alzheimer disease. Front Mol Neurosci 746 Doi 10.3389/fnmol.2014.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LoBue C, Munro C, Schaffert J, Didehbani N, Hart J et al. (2019) Traumatic Brain Injury and Risk of Long-Term Brain Changes, Accumulation of Pathological Markers, and Developing Dementia: A Review. J Alzheimers Dis 70:3, 629–654 Doi 10.3233/jad-190028 [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Nguyen A, Villeda S, Zhang H, Ding Z et al. (2014) Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol 512 Doi 10.3389/fneur.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG et al. (2017) TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol 27:4, 472–479 Doi 10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina M, Garrido JJ, Wandosell FG (2011) Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front Mol Neurosci 424 Doi 10.3389/fnmol.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meehan WP 3rd, Zhang J, Mannix R, Whalen MJ (2012) Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71:4, 885–891 Doi 10.1227/NEU.0b013e318265a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM et al. (1999) Head trauma and risk of dementia and Alzheimer's disease: The Rotterdam Study. Neurology 53:9, 1959–1962 Doi 10.1212/wnl.53.9.1959 [DOI] [PubMed] [Google Scholar]
- 53.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V et al. (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67:6, 953–966 Doi 10.1016/j.neuron.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortimer JA, French LR, Hutton JT, Schuman LM (1985) Head injury as a risk factor for Alzheimer's disease. Neurology 35:2, 264–267 [DOI] [PubMed] [Google Scholar]
- 55.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA et al. (2018) TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun 6:1, 33 Doi 10.1186/s40478-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC et al. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:5796, 130–133 Doi 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- 57.Oakley H, Cole SL, Logan S, Maus E, Shao P et al. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci 26:40, 10129–10140 Doi 10.1523/jneurosci.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petraglia AL, Dashnaw ML, Turner RC, Bailes JE (2014) Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery 75 Suppl 4S34–49 Doi 10.1227/neu.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 59.Petraglia AL, Plog BA, Dayawansa S, Dashnaw ML, Czerniecka K et al. (2014) The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int 5184 Doi 10.4103/2152-7806.147566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel BK (2019) Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front Mol Neurosci 1225 Doi 10.3389/fnmol.2019.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sephton CF, Good SK, Atkin S, Dewey CM, Mayer P 3rd et al. (2010) TDP-43 is a developmentally regulated protein essential for early embryonic development. J Biol Chem 285:9, 6826–6834 Doi 10.1074/jbc.M109.061846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K et al. (2021) Reducing acetylated tau is neuroprotective in brain injury. Cell Doi 10.1016/j.cell.2021.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA et al. (2011) Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70:7, 551–567 Doi 10.1097/NEN.0b013e31821f891f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sivanandam TM, Thakur MK (2012) Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci Biobehav Rev 36:5, 1376–1381 Doi 10.1016/j.neubiorev.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 65.Smith DH, Johnson VE, Trojanowski JQ, Stewart W (2019) Chronic traumatic encephalopathy - confusion and controversies. Nat Rev Neurol 15:3, 179–183 Doi 10.1038/s41582-018-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song Y, Hu M, Zhang J, Teng ZQ, Chen C (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer's disease. EBioMedicine 39409–421 Doi 10.1016/j.ebiom.2018.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C et al. (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:5870, 1668–1672 Doi 10.1126/science.1154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sussman ES, Pendharkar AV, Ho AL, Ghajar J (2018) Mild traumatic brain injury and concussion: terminology and classification. Handb Clin Neurol 15821–24 Doi 10.1016/b978-0-444-63954-7.00003-3 [DOI] [PubMed] [Google Scholar]
- 69.Takashima A (2006) GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis 9:3 Suppl, 309–317 Doi 10.3233/jad-2006-9s335 [DOI] [PubMed] [Google Scholar]
- 70.Tsitsopoulos PP, Marklund N (2013) Amyloid-beta Peptides and Tau Protein as Biomarkers in Cerebrospinal and Interstitial Fluid Following Traumatic Brain Injury: A Review of Experimental and Clinical Studies. Front Neurol 479 Doi 10.3389/fneur.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner RC, Lucke-Wold BP, Robson MJ, Lee JM, Bailes JE (2016) Alzheimer's disease and chronic traumatic encephalopathy: Distinct but possibly overlapping disease entities. Brain Inj 30:11, 1279–1292 Doi 10.1080/02699052.2016.1193631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci 529 Doi 10.3389/fnagi.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Washington PM, Villapol S, Burns MP (2016) Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 275 Pt 3:0 3, 381–388 Doi 10.1016/j.expneurol.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson AC, Dugger BN, Dickson DW, Wang DS (2011) TDP-43 in aging and Alzheimer's disease - a review. Int J Clin Exp Pathol 4:2, 147–155 [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y, Wu H, Zeng J, Pluimer B, Dong S et al. (2021) Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol Commun 9:1, 74 Doi 10.1186/s40478-021-01178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N et al. (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:3, 337–351 Doi 10.1016/j.neuron.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Chen C (2018) Alleviation of Neuropathology by Inhibition of Monoacylglycerol Lipase in APP Transgenic Mice Lacking CB2 Receptors. Mol Neurobiol 55:6, 4802–4810 Doi 10.1007/s12035-017-0689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Chen C (2008) Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem 283:33, 22601–22611 Doi 10.1074/jbc.M800524200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Hu M, Teng Z, Tang YP, Chen C (2014) Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer's disease. J Neurosci 34:45, 14919–14933 Doi 10.1523/jneurosci.1165-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Teng Z, Song Y, Hu M, Chen C (2015) Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab 35:3, 443–453 Doi 10.1038/jcbfm.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this manuscript are available in the main text and the supplementary Materials or from the corresponding authors upon request.