Abstract
Background:
Although various therapies have been developed to treat malalignment in osteoarthritic knees, the pattern of malalignment progression is still unclear. This study aimed to identify homogeneous subgroups with distinct trajectories of malalignment progression in subjects with symptomatic knee osteoarthritis (KOA) and to determine corresponding risk factors.
Methods:
Eight-year follow-up (from 2004 to 2012) data on 1252 participants with symptomatic KOA from the Osteoarthritis Initiative were included. Varus/valgus angle progression was characterized by group-based trajectory models. Time-varying covariates were introduced into the model to investigate how they affected trajectories. Multinomial logistic regression for trajectory group membership was applied to ascertain risk factors.
Results:
Five subgroups were identified. Participants in the varus worsening trajectory (n = 166) or valgus worsening trajectory (n = 118) proceeded to worsen malalignment over time. The neutral trajectory (n = 378), varus stable trajectory (n = 328), and valgus stable trajectory (n = 262) maintained close to the initial varus/valgus angle over 8 years. Higher baseline Kellgren and Lawrence grade (odds ratio [OR] = 4.35, P < 0.001 for varus; OR = 3.85, P < 0.001 for valgus) and “severe” baseline malalignment (OR = 13.57, P < 0.001 for varus; OR = 23.04, P < 0.001 for valgus) were risk factors for worsening trajectories. The cutoff point of the baseline varus/valgus angle to discriminate between stable or worsening trajectory was −4.5° for varus and 3.6° for valgus.
Conclusions:
This study identified the malalignment progression pattern — minor malalignment (−4.5° to +3.6°) tends to remain stable, while major baseline malalignment is likely to progress. This provides a reference for therapy to prevent malalignment from deteriorating and emphasizes the necessity of determining the trigger factors for malalignment onset.
Keywords: Malalignment, Knee osteoarthritis, Group-based trajectory modeling
Introduction
Malalignment may be a cause or result of knee osteoarthritis (KOA).[1] As a cause,theeffectofmalalignmenton KOA has been well established. Knee varus or valgus increases the risk of osteoarthritis incidence[2,3] and progression[1] in the corresponding load-adding compartment. In addition, malalignment acts as a mediator between osteoarthritis progression and known risk factors, including obesity,[4] weak quadriceps strength,[5] and stage of osteoarthritis.[6] Therefore, malalignment is seen as a valuable therapeutic target for KOA, and various surgical and non-surgical management strategies have been developed.
Regardless of the goal for treating malalignment — including correcting existing malalignment or preventing future progression — the mechanism of knee malalignment development and deterioration should be investigated first. Compared with genetic, developmental, or post-traumatic malalignment, KOA-induced malalignment (or malalignment progression) deserves more focus due to its critical role in the vicious cycle of KOA malalignment, appropriateness for prospective research, and huge potential for prophylactic management. However, no previous studies have investigated how malalignment proceeds in KOA patients or those who are at risk of malalignment deterioration. These questions are valuable for several reasons, including better understanding the vicious cycle of malalignment and KOA,[7] developing more effective non-surgical treatments stratified by different malalignment trajectories,[8] and selecting the appropriate surgical window for those with rapidly worsening varus/valgus knees.
Group-based trajectory modeling (GBTM) is a statistical method[9,10] for analyzing behavioral, biological, or physical trajectories, that is, for the evolution of an outcome over age or time. Using this method, time information from longitudinal data was sufficiently utilized, and subgroups with different trajectories were determined. Unlike standard statistical approaches that analyze individual variability against the background of the mean population trend, GBTM characterizes subgroups that follow distinctive trajectories, even though subgroups are sometimes not identifiable ex-ante based on individual characteristics.[11] This study aims to illustrate the different progression trajectories of malalignment in symptomatic KOA and determine risk factors for unfavorable trajectories.
Methods
Ethical approval
Ethical approval was obtained by the Osteoarthritis Initiative (OAI) project. The registry number was NCT00080171 (clinicaltrials.gov). All participants signed the informed consent during the OAI project.
Subjects
From the pool of OAI participants (https://nda.nih.gov/oai), 1252 subjects with both symptomatic KOA at enrollment and femur tibia angle (FTA) measurements from at least two different visits were included in this study. Symptomatic KOA was defined as knees that have both of the following: (1) Frequent knee symptoms in the past 12 months, defined as “pain, aching or stiffness in or around the knee on most days” for at least 1 month during the past 12 months. (2) Radiographic knee OA, defined as definite tibiofemoral osteophytes (Osteoarthritis Research Society International atlas grades I–III,[12] equivalent to Kellgren and Lawrence [KL] grade >2) on the fixed flexion radiograph.
For those subjects with two symptomatic knees, the knee with a higher KL grade (or the left knee was selected if the KL grades were equal) was selected for this study.
Subjects with alignment measurements at fewer than two different timepoints or presenting abnormally large alterations in a short time (>10° in 1 year) were excluded [Figure 1].
Figure 1.
Flowchart of screening-eligible subjects. FTA: Femur tibia angle; KOA: Knee osteoarthritis; M: Month.
Alignment measurement
Standard posteroanterior fixed-flexion knee radiographs were acquired at baseline, 12-, 24-, 36-, 48-, 72-, and 96-month visits. A newly developed software method was used to measure FTA.[13] This method mitigated some difficulties associated with previous FTA measurements and had higher intra-reader and inter-reader reproducibility and a stronger correlation with hip knee ankle angle.[13] The software method is based on a dimensionless Cartesian coordinate system, with a line tangent to the distal margin of the medial and lateral femoral condyles as the x-axis, and a line perpendicular to the x-axis and tangent to the medial margin of the medial femoral epicondyle as the y-axis. The abscissa of the most lateral point of the lateral femoral epicondyle was set as 1.00. The femur axis was defined as a line perpendicular to the x-axis, with abscissa = 0.50. The tibial axis was determined by two midpoints of the medial and lateral sides of the tibial shaft at 1 and 10 cm below the tibial plateau. Valgus FTA was set as positive, while varus FTA was set as negative. The hip-knee-ankle (HKA) (varus/valgus angle) was calculated by the equation varus/valgus angle = 1.01 FTA + 4.3.[14]
Time-varying covariate and baseline risk factors
Since previous studies on malalignment etiology and progression were lacking, we assumed that malalignment was related to compartment width decrease and individual activity level. Using the above coordinates, the medial compartment was located between x = 0.15 and x = 0.30, and the lateral compartment was located between x = 0.70 and x = 0.90.[14] The width at the midpoint of each compartment was chosen to represent each compartment. The ratio of lateral compartment width/medial compartment width was used to reflect compartment-unbalanced KOA progression.
Data about potential risk factors were collected, including side, sex, race, body mass index, age, The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, WOMAC total score, Physical Activity Scale for the Elderly (Pase score), 12-Item Short Form Survey (SF-12): physical scale, SF-12: mental scale, Center for Epidemiologic Studies Depression Score, KL grading, presence of bumps in hand joints, family history of knee replacement, knee injury history, knee surgery history, extensor strength, and flexor strength. Due to the limited sample size, only factors selected based upon clinical experience or having a significant odds ratio (OR) in univariable logistic regression were included in the multinomial analysis to investigate whether they predicted group membership.
Statistical analysis
Group-based trajectory models were built via the Stata Traj plug-in (https://www.andrew.cmu.edu/user/bjones/) in Stata (version 14, Stata Corp., College Station, TX, USA[15]), which uses a discrete mixture model to model longitudinal data. The time metric was follow-up months, and the outcome was malalignment (varus/valgus angle). Malalignment trajectories over 8 years were identified and modeled with a censored normal distribution. We iteratively compared three to seven trajectories and allowed for up to a fourth-order polynomial in each trajectory. The final number of trajectories was decided based on the Bayesian information criterion (BIC)[16] and parsimony to the extent possible.[17] Average posterior probabilities were used to test the model fitness, with a value above 70% indicating optimal fit.[9] Then, time-varying covariates were introduced into the model to determine how they affected the trajectories. After that, multinomial analysis of baseline covariates was applied to investigate whether those covariates predicted the trajectory group membership assigned by the highest probabilities. These procedures followed the standard three-step method.[18]
Results
The study cohort was composed of 1252 participants with symptomatic KOA and at least two FTA measurements at an 8-year follow-up. After converting FTA to the varus/ valgus angle, the median baseline varus angle of the 859 patients was −2.9°, while the median baseline valgus angle of the 393 patients was 2.0°. Detailed characteristics of the included subjects are presented in Table 1.
Table 1.
Basic characteristics of included subjects of symptomatic knee osteoarthritis.
| Characteristics | Values |
| n | 1252 |
| Side (right/left) | 526 (42.0)/726 (58.0) |
| Sex (male/female) | 556 (44.4)/696 (55.6) |
| Race (White or Caucasian/non-White) | 894 (71.4)/358 (28.6) |
| Mean BMI (kg/m2) | 30.11 ± 5.10 |
| Mean age (years) | 61.34 ± 9.08 |
| Median WOMAC pain score, (range) | 4 (0–20) |
| Median WOMAC total score, (range) | 22 (0–96) |
| Median SF-12: physical scale, (range) | 46.21 (14.81–67.76) |
| Median SF-12: mental scale, (range) | 55.51 (10.33–69.84) |
| Median CES-D score, (range) | 5 (0–55) |
| KL grading (Grade 2/3/4) | 597 (47.7)/465 (37.1)/190 (15.1) |
| Bumps in hand joint (No/Yes) | 860 (68.7)/386 (30.8) |
| Family history of knee replacement | 201 (16.1) |
| Knee injury history | 523 (41.8) |
| Knee surgery history | 372 (29.7) |
| Medial joint space narrowing | 584 (46.6)/424 |
| (Grade 0/1–2/3) | (33.9)/243 (19.4) |
| Lateral joint space narrowing | 937 (74.8)/219 |
| (Grade 0/1–2/3) | (17.5)/95 (7.6) |
| Mean extensor strength (N) | 327.58 ± 133.69 |
| Mean flexor strength (N) | 168.16 ± 69.73 |
| Baseline malalignment (varus/valgus), n | 859/393 |
Data was presented by mean ± SD, n or n (%). BMI: Body mass index; CES-D: Center for Epidemiologic Studies Depression; KL: Kellgren and Lawrence; SF-12: 12-Item Short Form Survey; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
A five-trajectory model produced higher BIC values than models with fewer trajectories. A five-trajectory model had almost the same trends as models with six or seven trajectories but was more parsimonious [Supplementary Table 1]. The average posterior probabilities were above 0.70, indicating a good fit. The five-trajectory model was recalculated, including time-varying covariates (Pase score and the ratio of lateral compartment width to medial), and the shape of the trajectory plot was similar to the original model without adjustment.
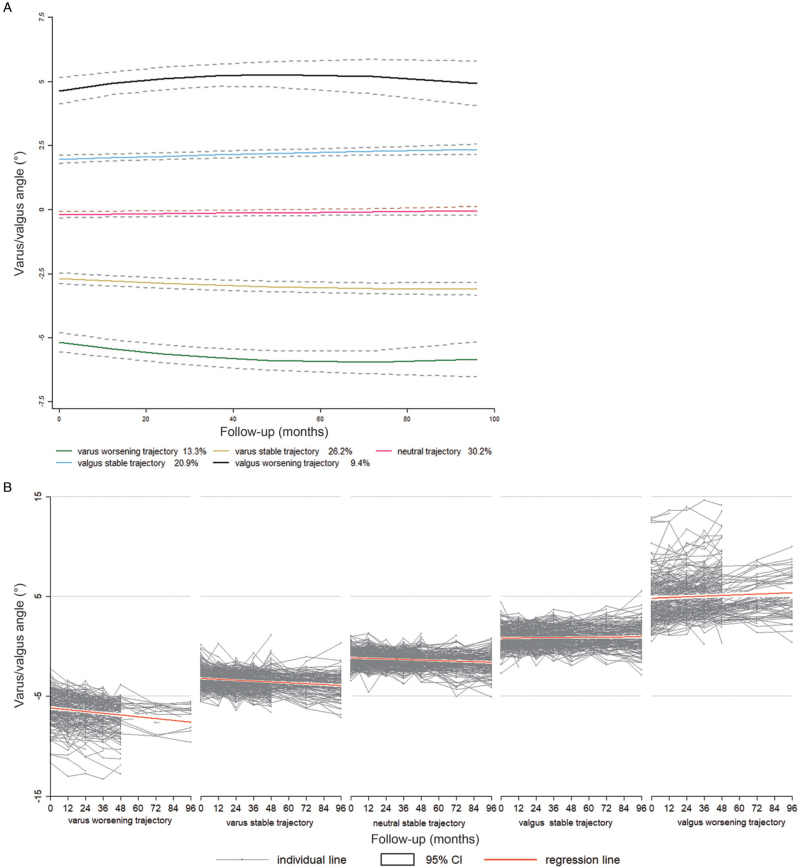
There were five distinct trajectories among the 1252 subjects. A total of 30.2% of subjects (n = 378) had a trajectory with almost neutral alignment at baseline and almost no deterioration afterward (neutral group). A total of 26.2% of subjects (n = 328) had a trajectory with slight varus malalignment but no obvious deterioration over 8 years (varus stable group). A total of 20.9% of subjects (n = 262) had a trajectory with slight valgus malalignment but no obvious deterioration over 8 years (stable valgus group). A total of 13.3% of subjects (n = 166) had a trajectory with obvious baseline varus malalignment and obvious sequential worsening (varus worsening group). A total of 9.4% of subjects (n = 118) had a trajectory with obvious valgus baseline malalignment and obvious sequential worsening (valgus worsening group) [Figure 2A].
Figure 2.
(A) Malalignment progression trajectory plot of Included subjects. (B) Individual malalignment changes among subjects in different trajectories. CI: Confidence interval.
Individual subject trajectories are presented in Figure 2B, with a regression line for each group to show the trends.
The estimated coefficients for time and time-varying covariates for each group are displayed in Table 2. In the varus worsening group, at a given time point, each unit increase in Pase score was associated with the worsening of varus, but the Pase score seemed to have no statistically significant effect in other groups. For joint width lateral (JWL)/joint width median (JWM), each unit increase was associated with a larger value in the varus/valgus angle in all five groups.
Table 2.
Parameter estimations for alignment trajectories (adjusted with time-varying covariates).
| Varus progression | Varus stable | Neutral | Valgus stable | Valgus progression | ||||||
| Variable | Parameter | P | Parameter | P | Parameter | P | Parameter | P | Parameter | P |
| Intercept | −4.888 | <0.001 | −2.300 | <0.001 | 1.668 | <0.001 | 4.861 | <0.001 | 6.776 | <0.001 |
| Month | −0.026 | <0.001 | −0.008 | <0.010 | 0.001 | 0.608 | 0.005 | 0.066 | 0.028 | <0.010 |
| Month2 | <0.001 | <0.050 | <0.001 | 0.098 | <0.001 | 0.900 | <0.001 | 0.638 | <0.001 | <0.050 |
| JWL/JWM | −0.054 | <0.001 | −0.281 | <0.010 | −1.844 | <0.001 | −2.903 | <0.001 | −2.224 | <0.001 |
| Pase score | −0.002 | <0.010 | <0.001 | 0.641 | <0.001 | 0.815 | <0.001 | 0.887 | 0.001 | 0.351 |
JWL: Joint space width of the lateral compartment; JWM: Joint space width of the medial compartment. Month2: The square of month, that is, the quadratic term in the multinomial model.
Table 3 presents the ORs of group membership by baseline predictors. For varus knees, KL grade >2 and more severe varus at baseline were associated with a higher risk of varus worsening group membership relative to the varus stable group (for KL grade >2, OR = 4.35, 95% confidence interval [CI] = [2.27, 8.33], P < 0.001; for more severe varus knees, OR = 13.57, 95% CI = [5.71, 32.24], P < 0.001). Similarly, KL grade >2 and more severe valgus at baseline were associated with a higher risk of valgus worsening group membership relative to the stable valgus group (for KL grade >2, OR = 3.85, 95% CI = [2.08, 7.14], P < 0.001; for more severe valgus knee, OR = 23.04, 95% CI = [6.86, 77.41], P < 0.001). In addition, the non-White race was a protective factor against valgus worsening (OR = 0.50, 95% CI = [0.26, 0.94], P < 0.03).
Table 3.
Baseline factors associated with trajectory group membership.
| Varus progression to varus stable | Valgus progression to valgus stable | |||
| Baseline variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Obesity | 0.90 (0.58, 1.38) | 0.63 | 0.77 (0.44,1.36) | 0.38 |
| Male | 1.51 (0.97, 2.35) | 0.06 | 0.80 (0.43, 1.49) | 0.49 |
| Older age | 0.87 (0.56, 1.36) | 0.56 | 0.82 (0.45, 1.49) | 0.53 |
| Non-Whites | 0.67 (0.39, 1.14) | 0.14 | 0.50 (0.26, 0.94) | 0.03 |
| With hand osteoarthritis | 1.28 (0.81, 2.04) | 0.28 | 1.14 (0.62, 2.11) | 0.66 |
| With knee injury history | 1.22 (0.75, 1.99) | 0.42 | 1.11 (0.58, 2.10) | 0.74 |
| With knee surgery history | 1.06 (0.64, 1.76) | 0.80 | 0.69 (0.34,1.40) | 0.31 |
| Higher KL grade (3 or 4) | 4.35 (2.27, 8.33) | <0.001 | 3.85 (2.08, 7.14) | <0.001 |
| More varus/valgus (than median) | 13.57 (5.71, 32.24) | <0.001 | 23.04 (6.86, 77.41) | <0.001 |
KL: Kellgren and Lawrence; OR: Odds ratio.
Since the varus/valgus angle was a continuous variable, the cutoff point of the baseline varus/valgus angle between the stable and worsening trajectories could be determined by the receiver operating characteristic (ROC) curve. A varus angle of −4.5° had the maximum value of the Youden index in varus knees. Similarly, a valgus angle of 3.6° showed the maximum value of the Youden index in the valgus knee [Supplementary Figure 1].
Considering heterogeneity from the extra-articular deformity or traumatic KOA, we repeated the analysis after excluding the participants with a knee injury or surgery.
The main results and conclusion were almost the same [Supplementary Figure 2, and Table 2].
Discussion
Malalignment is a critical factor in KOA incidence and progression and is therefore targeted in various KOA therapies. However, the malalignment progression trajectory is still unclear, leading to some difficulty in selecting the appropriate therapies for patients. The study's main finding was that knees with neutral or slight malalignment tended to remain stable in alignment, while knees with malalignment past thresholds (−4.5° for varus and 3.6° for valgus) tended to suffer deterioration of malalignment. In addition, higher initial OA grade and compartment-unbalanced KOA progression were risk factors for malalignment worsening.
This pattern of progression might be explained by the compensation of soft tissues. When minor knee varus/valgus occurs, the medial/lateral collateral ligaments and other soft tissues are stretched slightly and become tensed, preventing further varus worsening.[19] However, when the elongation of the ligaments (or other soft tissue) surpasses the tensile limits,[20] the compensatory mechanism fails, which leads to continuous worsening.
This study provided additional evidence for the vicious cycle of KOA malalignment. In the valgus worsening trajectory, a decrease in JWL/JWM, which indicated that relatively more lateral progression had occurred, was associated with an increased valgus angle. In the varus worsening trajectory, an increase in JWL/JWM, which indicated that relatively more medial progression had occurred, was associated with a decrease in the varus angle. Notably, a decrease in the varus angle meant that the varus had worsened since the varus angle was negative. This result was consistent with Hunter et al's[21] study, which reported an association between the change in joint space narrowing and the change in alignment. We also found that a higher baseline KL grade was a risk factor for malalignment worsening. Similarly, Hunter et al's study[22] demonstrated that cartilage loss, meniscal degeneration and position, osteophytes, bone attrition, and ligament damage were associated with variance in malalignment. Previous studies focused only on concurrent OA progression and malalignment progression or conducted simple cross-sectional observations, providing no information on causative relationships. We analyzed the baseline K1 grade and sequential malalignment worsening and determined that a higher KL grade was a risk factor for malalignment worsening.
The trajectories of malalignment progression suggested a pattern of inertia, that is, mild malalignment remained stable over time, while malalignment past a specific threshold worsened. A pattern of inertia was proposed by Felson et al[23] in the structural progression of KOA to describe how knees that had been experiencing radiographic deterioration were likely to further worsen, and knees that had been stable would remain stable. Similar inertia patterns in KOA structural progression and malalignment progression strongly suggest the possibility of a close bidirectional relationship in the vicious cycle. Waller et al[24] proposed that the vicious KOA-malalignment cycle may overwhelm any attempt, regardless of direct cartilage repair or disease-modifying pharmacotherapy, or at least make it increasingly difficult, to cease progression once the disease is well established. Therefore, it is critical to detect trigger factors for malalignment progression and halt the vicious cycle at the initial stage. This study found that a varus of −4.5° and a valgus of 3.6° were the cutoff points for stable and worsening trajectories. However, this study could not determine how baseline varus/valgus had formed. It was inferred that some of the initial deformities were inborn, and some were triggered by injury (overt or covert); further studies focused on varus/ valgus etiology are required.
This study could provide a reference for malalignment therapy candidate selection. Malalignment therapy modalities range from simple and non-invasive to complex and invasive procedures, including neuromuscular training, gait modification, foot orthoses, knee braces, cartilage transplantation, high tibial osteotomy, and joint distraction surgery. However, ideal candidates for specific therapies remain to be determined.[8,25] Malalignment treatment strategies include preventing the initial disease or progression, unloading the related compartment, or correcting the malalignment. The identification of the malalignment trajectory in this study could provide some theoretical basis for these therapies, especially for preventing worsening, since the greater the degree of malalignment, the more rapid the cartilage degradation and functional impairment.[1,6]
There were some limitations to this study. First, trigger factors that initiated malalignment were not investigated because most varus or valgus deformities in this study had formed before enrolment, and newly developed malalignment was infrequent among the included subjects. Studies on malalignment onset are still required. Second, the varus/valgus angle was calculated from the FTA. Although FTA had been used in many studies to calculate the varus/valgus angle, there was still some inaccuracy in subjects with abnormalities in the lower limbs. Third, some subjects did not receive FTA measurements at 72 and 96 months due to withdrawal or arthroplasty, reducing the sample sizes of the varus worsening trajectory and valgus worsening trajectory between 48 and 96 months; therefore, the corresponding trends at this period might be less reliable. Fourth, a tiny minority of subjects suffered an extremely rapid worsening, which has important clinical significance but was not separately analyzed in the study. This study only examined general trajectories, and those specific cases should be researched in more focused studies. Fifth, a few participants with minor varus or valgus experienced an inversion of malalignment at the follow-up; the small varus or valgus detected might be natural or from measuring error. Sixth, the malalignment of one knee might also be affected by the other knee, and this interaction was not considered in this study.
Conclusion
Malalignments in varus knees over −4.5° and valgus knees over 3.6° tended to deteriorate, while neutral or slightly misaligned knees were likely to maintain stable alignment. Non-White race and higher KL grade were risk factors for malalignment worsening. The triggers for initial malalignment and effective treatment to prevent malalignment worsening need to be studied in the future to halt the vicious cycle of KOA malalignment.
Funding
This study was funded by the grants from the National Natural Science Foundation of China (No. 81974347), China Postdoctoral Science Foundation (No. 2021M702351), Post-Doctor Research Project, West China Hospital, Sichuan University (No. 2020HXBH081), Medical Science and Technology Project of Health Commission of Sichuan Provincial (No. 21PJ040), and Sichuan University Postdoctoral Interdisciplinary Innovation Fund.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li M, Nie Y, Zeng Y, Wu Y, Wu L, Liu Y, Shen B. Eight-year trajectories of malalignment progression in symptomatic knee osteoarthritis. Chin Med J 2022;135:2570–2576. doi: 10.1097/CM9.0000000000002044
Mingyang Li and Yong Nie contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Sharma L, Song J, Felson D, Cahue S, Shamiyeh E, Dunlop D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001; 286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the multicenter osteoarthritis study and the osteoarthritis initiative. Arthritis Rheum 2013; 65:355–362. doi: 10.1002/art.37726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis 2010; 69:1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum 2004; 50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med 2003; 138:613–619. doi: 10.7326/0003-4819-1388-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cerejo R, Dunlop DD, Cahue S, Channin D, Song J, Sharma L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum 2002; 46:2632–2636. doi: 10.1002/art.10530. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 2013; 21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foroughi N, Smith RM, Lange AK, Singh MAF, Vanwanseele B. Progressive resistance training and dynamic alignment in osteoarthritis: a single-blind randomised controlled trial. Clin Biomech 2011; 26:71–77. doi: 10.1016/j.clinbiomech.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 10.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods 1999; 4:139. [DOI] [PubMed] [Google Scholar]
- 11.Nagin DS. Group-based trajectory modelling. 2010; Handbook of Quantitative Criminology New York, NY: Springer, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage 1995; 3:3–70. doi: 10.1016/j.joca.2006.11.009. [PubMed] [Google Scholar]
- 13.Iranpour-Boroujeni T, Li J, Lynch J, Nevitt M, Duryea J, Investigators O. A new method to measure anatomic knee alignment for large studies of OA: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2014; 22:1668–1674. doi: 10.1016/j.joca.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Neumann G, Hunter D, Nevitt M, Chibnik L, Kwoh K, Chen H, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage 2009; 17:761–765. doi: 10.1177/0049124113503141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res 2013; 42:608–613. doi: 10.1177/0049124113503141. [Google Scholar]
- 16.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001; 29:374–393. doi: 10.1177/0049124101029003005. [Google Scholar]
- 17.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 18.Van De Schoot R, Sijbrandij M, Winter SD, Depaoli S, Vermunt JK. The GRoLTS-checklist: guidelines for reporting on latent trajectory studies. Struct Equ Model 2017; 24:451–467. doi: 10.1080/10705511.2016.1247646. [Google Scholar]
- 19.Seering WP, Piziali RL, Nagel DA, Schurman DJ. The function of the primary ligaments of the knee in varus-valgus and axial rotation. J Biomech 1980; 13:785–794. doi: 10.1016/0021-9290(80)90240-7. [DOI] [PubMed] [Google Scholar]
- 20.Woo S, Debski R, Withrow J, Janaushek MA. Biomechanics of knee ligaments. Am J Sports Med 1985; 27:533–543. doi: 10.1177/03635465990270042301. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am 2009; 91:85–89. doi: 10.2106/JBJS.H.01409. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol 2005; 32:2192–2199. [PubMed] [Google Scholar]
- 23.Felson D, Niu J, Sack B, Aliabadi P, McCullough C, Nevitt M. Progression of osteoarthritis as a state of inertia. Ann Rheum Dis 2013; 72:924–929. doi: 10.1136/annrheumdis-2012-201575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011; 19:1823–1829. doi: 10.1007/s00167-011-1403-6. [DOI] [PubMed] [Google Scholar]
- 25.Kirkley A, Webster-Bogaert S, Litchfield R, Amendola A, MacDonald S, McCalden R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am 1999; 81:539–548. doi: 10.2106/00004623199904000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.