Abstract
Background
Arterial occlusive events are an emerging problem in patients with chronic myeloid leukemia (CML) receiving tyrosine kinase inhibitor (TKI) therapy. Endothelial cell damage is thought to play an important role in the development of vascular events. Measurement of the peripheral vasodilator response by peripheral arterial tonometry (PAT) has reportedly been useful in the non-invasive assessment of endothelial dysfunction. To date, no studies have assessed endothelial function using PAT in patients with CML receiving TKIs.
Method
We measured the reactive hyperemia index (RHI) using PAT in young patients with CML (men aged ≤ 55 years and women aged ≤ 65 years) receiving TKIs.
Results
Thirty patients with CML were examined (mean age, 43.5 ± 9.8 years; men, 57%). The median RHI was 1.81. Among these patients, 16.7% and 83.3% were taking imatinib and second- or third-generation TKIs, respectively. There were no differences in the baseline characteristics between the low RHI (< 1.67, n = 10), borderline RHI (≥ 1.67 and < 2.10, n = 14), and normal RHI (≥ 2.10, n = 6) groups. Serum uric acid (UA) levels and the RHI were significantly negatively correlated (r = -0.40, p = 0.029).
Conclusion
One-third of young patients with CML receiving TKI therapy were classified as having a low RHI. The RHI was negatively correlated with serum UA level. Larger prospective studies are necessary to examine whether the RHI predicts cardiovascular events in such patients.
Keywords: Tyrosine kinase inhibitors, Chronic myeloid leukemia, Endothelial dysfunction, Uric acid
Introduction
Tyrosine kinase inhibitors (TKIs) have dramatically improved the prognosis of patients with chronic myeloid leukemia (CML). Imatinib, a first-generation BCR-ABL TKI, was approved for the treatment of CML in 2001. Currently second- (dasatinib, nilotinib, and bosutinib) and third-generation (ponatinib) TKIs are available for CML treatment and have showed rapid and deeper molecular responses (MRs) than imatinib. Four TKIs other than ponatinib are approved for first-line treatment and ponatinib is approved for patients with T315I mutations, which result in resistance to treatment with other TKIs [1–5]. Therefore, treatment-free remission following TKI cessation is an emerging goal for patients with CML for whom TKI use has yielded a deep and stable molecular response (patient who achieved MR4.5 and sustained 2 years). However, about half of patients relapsed within 2 years after cessation of TKI therapy in previous studies [5–7]. The management of various adverse events (AEs) has become a major issue in clinical practice. These include cardiovascular adverse events (CAEs), such as ischemic heart disease, cerebral infarction, and peripheral artery occlusive disease [8].
CAEs have been reported not only in patients with multiple risk factors for atherosclerosis, but also in young patients with CML who are considered to be at low risk [8]. In particular, nilotinib, ponatinib, and dasatinib are prone to result in CAEs. Incident rates among patients treated with nilotinib were 10.6% at 5 years and 24.8% at 10 years in one study [1]. The 5-year cumulative incidence rates of arterial occlusive events (including cardiovascular, cerebrovascular, and peripheral vascular events) in patients with CML treated with ponatinib and dasatinib were 31% [2] and 7%, respectively [5]. Although the pathophysiology of drug-induced CAEs remains unclear, several in vitro studies have revealed an effect of TKI on vascular endothelial cells (ECs) [9–11]. Hence, we hypothesized that various drug-induced effects on ECs may have roles to play in the development of CAEs in patients with CML treated with TKIs. However, an appropriate method and biomarker for the evaluation of EC functions has not been established.
Reactive hyperemia peripheral arterial tonometry (RH-PAT) using the EndoPAT 2000 system (Itamar Medical Inc., Caesarea, Israel) is a well-known non-invasive method for evaluation of the vascular endothelial function [12]. Furthermore, the reactive hyperemia (RH) response, as measured with the reactive hyperemia index (RHI), was an independent predictor of cardiovascular events beyond the traditional Framingham risk score during a 7-year follow-up study [13]. To date, no studies have investigated the clinical indicators of vascular endothelial function in patients with CML treated with TKI. This study aimed to evaluate the endothelial function using RH-PAT in patients with CML receiving TKI therapy and to assess the correlation between the RHI and multiple clinical factors.
Methods
Patient selection
This was a single-center, cross-sectional study. Consecutive patients with CML on TKI therapy who visited the hematology department of Juntendo University Hospital between January 2020 and July 2021 were considered eligible for this study. The inclusion criteria were men aged ≤ 55 years and women aged ≤ 65 years. We adopted these age thresholds for what is considered “premature atherosclerotic cardiovascular disease” as defined in the guidelines on cardiovascular disease [14]. Written informed consent was obtained from all patients. The Institutional Review Board of Juntendo University Hospital approved the study protocol, and all aspects of the study complied with the Declaration of Helsinki.
Data collection
Clinical data were collected from the patients’ medical records. We used blood sample data acquired on the day closest to the RH-PAT study within 30 days. We collected arterial blood pressure measurements at the time of the RH-PAT study.
The MR was assessed by quantitative reverse transcription polymerase chain reaction using peripheral blood on the International Scale (IS). We defined the major molecular response (MMR) (≤ 0.1% BCR-ABL on the IS) and deep MR (DMR) (≤ 0.01% BCR-ABL on the IS) according to the 2020 European LeukemiaNet recommendations [15, 16].
Endothelial function measurement
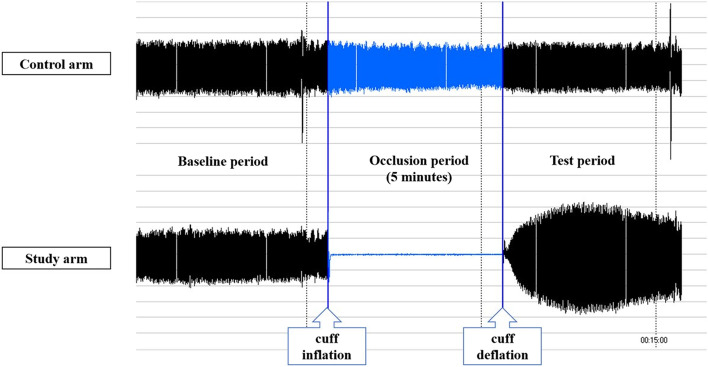
We used the EndoPAT 2000 system for RH-PAT to evaluate endothelial function. The detailed principles and measurement procedures were previously described [17]. RH-PAT was performed early in the morning with the patient on an empty stomach, in a quiet room with a steady room temperature. Patients were instructed to avoid their daily antihypertensive medication on the day of measurement. After 15 min of bed rest, the PAT probe was positioned on the second finger of each hand. After 5 min of baseline PAT in both hands, the blood pressure cuff was inflated on the test arm to 60 mmHg above the patient’s systolic pressure or at least 200 mmHg for 5 min, and PAT was performed for 5 min after cuff deflation. The increase in pulse amplitude in the hyperemic finger was recorded and analyzed, using an automated operator-independent algorithm, as the RHI (Fig. 1).
Fig. 1.
Representative reactive hyperemia peripheral arterial tonometry (RH-PAT) recording. Normal response is characterized by a significant increase in the signal amplitude in the test arm after cuff deflation compared with that at baseline
Statistical analysis
Data are presented as mean ± SD or median [interquartile range, IQR] values for continuous variables and as frequencies (%) for categorical variables. Qualitative data were compared using the chi-square or Fisher's exact probability test, and continuous variables were compared using the one-way analysis of variance (ANOVA). Pearson’s correlation coefficient or Spearman’s rank correlation coefficient was used to determine the relationship between clinical factors and the RHI. Stepwise multiple linear regression analysis was also performed to determine the independent determinants of the RHI. Multiple linear regression analysis included variables with a p-value < 0.1 in the correlation analysis. ANOVA p-value and coefficient of determination (R2) for regression analysis were shown. All analyses were conducted using SPSS Statistics 21.0 (IBM, Armonk, New York). Statistical significance was set at a two-tailed p-value of < 0.05.
Results
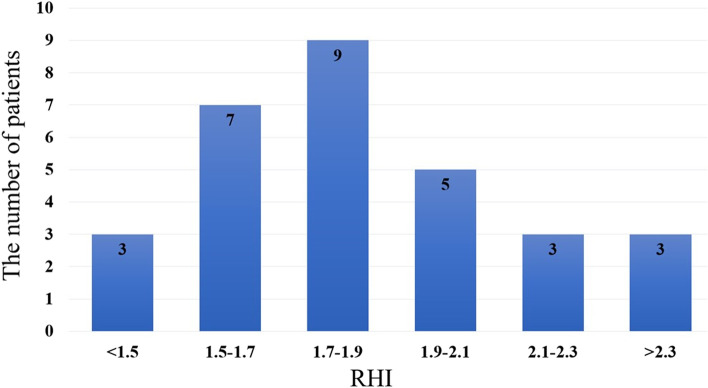
A total of 30 patients were included in this study. Table 1 shows the baseline characteristics of the patients. The mean age was 43.5 ± 9.8 years, 57.0% were men, 20.0% had hypertension, 53.3% had dyslipidemia, and 23.3% were current smokers. None of the patients were taking beta-blockers. The median duration of TKI therapy was 1466 [980–2256] days, and the median RHI was 1.81 [1.6–2.05]. In terms of TKI selection, 16.7% used imatinib, 40.0% used nilotinib, 13.3% used dasatinib, 16.7% used bosutinib, and 13.3% used ponatinib. In total, 18/30 (60.0%) achieved a DMR and 9/30 (30.0%) achieved an MMR. Figure 2 shows the distribution of the RHI in the study patients. Only six patients (20%) were within the normal range (RHI ≥ 2.10) [18].
Table 1.
Baseline characteristics
| N | RHI | ||||
|---|---|---|---|---|---|
| All |
Low RHI (< 1.67) |
Borderline RHI (≥ 1.67 and < 2.1) |
Normal RHI (≥ 2.1) |
P-value | |
| N = 30 | N = 10 | N = 14 | N = 6 | ||
| Age, yrs | 43.5 ± 9.8 | 42.6 ± 9.5 | 44.4 ± 11.6 | 43.0 ± 6.4 | 0.908 |
| Male/Female, n (%) | 17/13 (57/43) | 8/2 (80/20) | 6/8 (43/57) | 3/3 (50/50) | 0.181 |
| Height, cm | 166.3 ± 8.7 | 168.1 ± 9.0 | 163.6 ± 8.5 | 170.0 ± 7.8 | 0.252 |
| Weight, kg | 67.5 ± 13.6 | 69.9 ± 13.2 | 63.3 ± 12.4 | 73.2 ± 15.9 | 0.265 |
| BMI, kg/m2 | 24.3 ± 3.7 | 24.7 ± 4.0 | 23.5 ± 2.8 | 25.4 ± 4.9 | 0.535 |
| Systolic BP, mmHg | 122.6 ± 21.9 | 122.4 ± 17.1 | 128.1 ± 24.2 | 110.0 ± 21.0 | 0.245 |
| Diastolic BP, mmHg | 69.4 ± 15.6 | 74.0 ± 11.0 | 70.6 ± 15.5 | 58.7 ± 19.4 | 0.149 |
| Current smoker, n (%) | 7 (23.3) | 5 (50.0) | 2 (14.3) | 0 (0) | 0.151 |
| Former smoker, n (%) | 6 (20.0) | 1 (10.0) | 3 (21.4) | 2 (33.3) | 0.151 |
| Hypertension, n (%) | 6 (20.0) | 2 (20.0) | 3 (21.4) | 1 (16.7) | 0.971 |
| Dyslipidemia, n (%) | 16 (53.3) | 5 (50.0) | 8 (57.1) | 3 (50.0) | 0.926 |
| Diabetes mellitus, n (%) | 3 (10.0) | 2 (20.0) | 1 (7.1) | 0 (0) | 0.386 |
| MMR, n (%) | 9 (30.0%) | 2 (20.0%) | 4 (28.6%) | 3 (50.0%) | 0.683 |
| DMR, n (%) | 18 (60.0%) | 7 (70.0%) | 8 (57.1%) | 3 (50.0%) | 0.683 |
| Current medication | |||||
| TKI Imatinib | 5 (16.7) | 1 (10.0) | 2 (14.3) | 2 (33.3) | 0.151 |
| Nilotinib | 12 (40.0) | 3 (30.0) | 8 (57.1) | 1 (16.7) | |
| Dasatinib | 4 (13.3) | 1 (10.0) | 2 (14.3) | 1 (16.7) | |
| Bosutinib | 5 (16.7) | 2 (20.0) | 2 (14.3) | 1 (16.7) | |
| Ponatinib | 4 (13.3) | 3 (30.0) | 0 (0) | 1 (16.7) | |
| TKI duration, days | 1466 [980–2256] | 1643 [1213–2160] | 1352 [925–2128] | 1354 [1074–2370] | 0.830 |
| Statin, n (%) | 12 (40.0) | 3 (30.0) | 7 (50.0) | 2 (33.0) | 0.574 |
| ACE-I/ARB, n (%) | 5 (16.7) | 1 (10.0) | 3 (21.4) | 1 (16.7) | 0.760 |
| CCB, n (%) | 5 (16.7) | 1 (10.0) | 3 (21.4) | 1 (16.7) | 0.760 |
| Xanthine oxidase inhibitor, n (%) | 3 (10.0) | 2 (20.0) | 0 (0) | 1 (16.7) | 0.227 |
| Laboratory data | |||||
| WBC, × 103/µL | 6.5 ± 1.9 | 6.9 ± 1.9 | 6.7 ± 2.1 | 5.4 ± 1.5 | 0.277 |
| Hemoglobin, g/dL | 13.6 ± 1.7 | 14.6 ± 1.7 | 13.3 ± 1.9 | 12.8 ± 1.5 | 0.082 |
| Serum creatinine, mg/dL | 0.78 ± 0.25 | 0.84 ± 0.17 | 0.77 ± 0.32 | 0.72 ± 0.18 | 0.642 |
| Serum uric acid, mg/dL | 5.0 ± 1.2 | 5.7 ± 0.9 | 4.8 ± 1.2 | 4.5 ± 1.5 | 0.077 |
| Triglycerides, mg/dL | 83 [56–116] | 74 [56–163] | 72 [52–101] | 98 [78–169] | 0.243 |
| HDL-C, mg/dL | 61 ± 16 | 64 ± 20 | 62 ± 14 | 53 ± 15 | 0.450 |
| LDL-C, mg/dL | 110 ± 28 | 104 ± 29 | 122 ± 22 | 92 ± 32 | 0.068 |
| HbA1c, % | 5.6 ± 0.58 | 5.8 ± 0.86 | 5.5 ± 0.35 | 5.6 ± 0.28 | 0.362 |
BMI Body mass index, BP Blood pressure, MMR Major molecular response, DMR Deep molecular response, TKI Tyrosine kinase inhibitor, CCB Calcium channel blocker, ACE-I/ARB Angiotensin converting enzyme inhibitor/angiotensin II receptor blocker, WBC White blood cell, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol
Fig. 2.
Distribution of the reactive hyperemia index (RHI)
Patients were divided into three groups based on the RHI cutoff value proposed by Tanaka et al. [17]: low (< 1.67), borderline (≥ 1.67 and < 2.10), and normal (≥ 2.10) RHI groups. There were no differences in treatment responses among the three groups. Patients in the normal-RHI group (n = 6) tended to have lower hemoglobin levels, low-density lipoprotein (LDL) cholesterol levels, and serum uric acid (UA) levels than those in the low-RHI group (n = 10); however, these differences were not statistically significant. Selected TKIs in each group were similar between the normal and low-RHI groups (Table 1). There was also no difference between imatinib and newer generation TKIs in terms of the RHI (Fig. 3). In univariate analysis, the RHI was significantly correlated with diastolic blood pressure (r = -0.393, p = 0.032) and serum UA levels (r = -0.399, p = 0.029) (Table 2, Fig. 4). In the multiple linear regression model, serum UA levels were inversely associated with the RHI (p = 0.029) (Table 3).
Fig. 3.
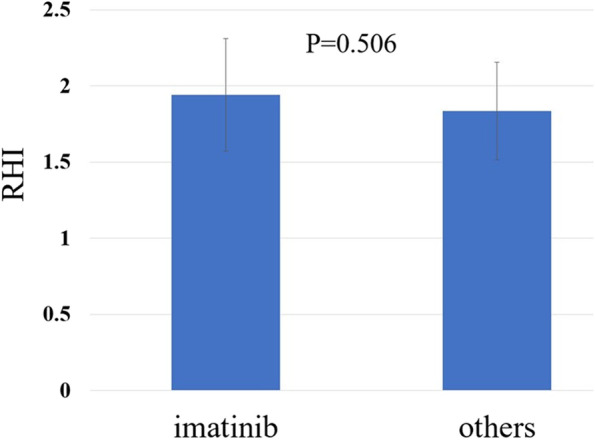
Comparison of the reactive hyperemia index (RHI) between patients receiving imatinib and those receiving second- or third-generation TKIs. No difference is seen between imatinib and newer TKIs in terms of the RHI
Table 2.
Univariate analysis of correlation between RHI and clinical indicators
| Variables | r | P-value |
|---|---|---|
| Age, yrs | 0.139 | 0.464 |
| Male sex | -0.222 | 0.239 |
| BMI, kg/m2 | 0.013 | 0.945 |
| Systolic BP, mmHg | -0.197 | 0.297 |
| Diastolic BP, mmHg | -0.393 | 0.032 |
| Current smoker | -0.36 | 0.051 |
| Hemoglobin, g/dL | -0.356 | 0.053 |
| Serum creatinine, mg/dL | -0.141 | 0.458 |
| Serum uric acid, mg/dL | -0.399 | 0.029 |
| Triglycerides, mg/dL | 0.070 | 0.713 |
| HDL-C, mg/dL | -0.163 | 0.390 |
| LDL-C, mg/dL | 0.025 | 0.894 |
| HbA1c, % | -0.118 | 0.534 |
BMI Body mass index, BP Blood pressure, TKI Tyrosine kinase inhibitor, CCB Calcium channel blocker, ACE-I/ARB Angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, WBC White blood cell, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol
Fig. 4.
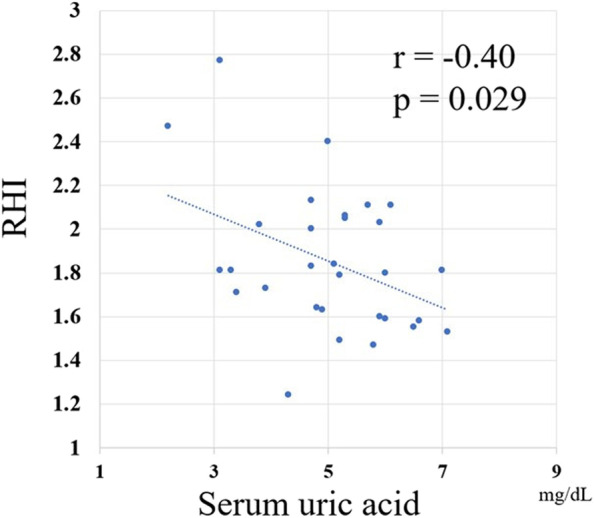
Correlation between the reactive hyperemia index (RHI) and serum uric acid (UA) levels. The RHI is significantly correlated with serum uric acid (UA) levels
Table 3.
Multiple linear regression analysis (dependent variable: RHI)
| Non-standardized coefficients (B) | Standardized coefficients (β) | P value | |
|---|---|---|---|
| Constant | 2.388 | < 0.0001 | |
| Serum uric acid, mg/dL | -0.107 | -0.399 | 0.029 |
Variables p < 0.1 in correlation analysis (diastolic blood pressure, smoker, hemoglobin, and uric acid) were included in the analysis. Overall R2 = 0.16, ANOVA p = 0.029
Discussion
To the best of our knowledge, this is the first study to evaluate vascular endothelial function using non-invasive RH-PAT in patients with CML receiving TKIs. Only 20% of the patients in this study exhibited normal RHI levels. Even in younger patients, who are generally considered to be at low risk, vascular endothelial function may have been impaired during TKI therapy. In univariate analysis, the RHI was significantly correlated with diastolic blood pressure and serum UA levels. Multiple regression analysis showed that serum UA levels were independently associated with the RHI. Because this is a cross-sectional study, it is unknown whether the RHI value can be a predictor of cardiovascular events in patients with CML; however, we believe that the findings from this pilot study will be useful for conducting prospective trials in the future.
TKI-associated arterial occlusive events in CML—the difference between first and newer generation TKIs
All TKIs approved for CML treatment share kinase inhibition activity against BCR-ABL; however, they differ in their kinase inhibition profiles (the so-called “off-target effect”), and some are vascular biology-related kinases [9]. Several reports have revealed the direct effects of TKIs on vascular ECs. For example, nilotinib upregulates pro-atherogenic adhesion-proteins (ICAM-1 and VCAM-1), which induces atherosclerosis on the cell surface [10]. In addition, Grover-Proakter et al. reported that nilotinib, dasatinib, and ponatinib induced more than 100 gene expression changes of human umbilical vein ECs compared to imatinib and bosutinib treated cells. Moreover, decreased tube formation and low cell viability were observed in human umbilical vein ECs treated with these three TKIs [19]. However, an appropriate method for evaluating EC function and damage during TKI therapy is lacking. Flow-mediated vasodilation (FMD) is a well-established method for assessing endothelial function and has been reported to be associated with cardiovascular events that develop during the treatment of multiple myeloma [20]. In addition, soluble ICAM-1 and endothelial function measured by FMD of the brachial artery are correlated with each other [21]. However, the assessment of brachial artery reactivity using ultrasound is technically challenging and involves a significant learning curve. It is recommended that at least 100 supervised scans and measurements are performed annually [22]. Conversely, PAT is a versatile, alternative method for non-invasive and reproducible assessment of endothelial function; it is also independent of the examiner's skills [12]. Therefore, we used RH-PAT to assess endothelial function in this study. FMD and RH-PAT are based on the same principle of the RH phenomenon and have been reported to significantly predict cardiovascular events with similar prognostic magnitudes; however, no statistically significant relationship was noted between them [23]. These findings suggest that these tests reflect different aspects of vascular function, and future research is required to investigate the differences in the predictability of cardiovascular events during TKI therapy.
In their review, Tanaka et al. [18] classified the RHI into three categories according to the risk of cardiovascular events: normal (≥ 2.10), borderline (≥ 1.67 and < 2.10), and abnormal (< 1.67). In our study, only six patients (20%) had an RHI within the normal range. Furthermore, to investigate the effects of newer generation TKIs (dasatinib, nilotinib, bosutinib, and ponatinib) on endothelial function, the RHI in patients treated with newer generation TKIs was compared with those treated with imatinib; however, the difference was not statistically significant. Given that the number of patients who received imatinib in our study was small, a study with a larger sample is necessary to compare the effects of imatinib with those of the newer generation TKIs on endothelial function.
UA and endothelial function
Several epidemiological studies have confirmed hyperuricemia as a risk factor for cardiovascular diseases [24, 25]. UA is the end product of purine metabolism and is catalyzed by xanthine oxidoreductase (XOR). Under conditions in which XOR activity is enhanced, UA and reactive oxygen species (ROS) are generated concomitantly. ROS may disrupt endothelial function through the reaction of superoxide (O2−) with nitric oxide (NO), leading to a decrease in NO bioavailability and increased production of peroxynitrite (ONOO−) [26]. Recent clinical studies have shown that FMD is more impaired in patients with hyperuricemia than in those without [27, 28]. Taher et al. showed that normal–increased UA levels were associated with endothelial dysfunction (RHI < 2.0) in women at low risk of cardiovascular diseases [29]. In addition, the serum UA level is associated with the RHI in patients with low-risk hypertension but not in patients with high-risk hypertension [30]. The authors of that report speculated that UA may be an important biomarker for investigating early signs of endothelial dysfunction in low-risk patients.
Early in the development of hematopoietic tumors and immediately after the start of chemotherapy, tumor cells rapidly disintegrate and produce excessive amounts of UA [31]. Tumor lysis syndrome (TLS) is a fulminant form of this pathology; however, the risk of TLS is low in patients with chronic-phase CML receiving TKI therapy [32]. All patients in this study had chronic-phase CML, and few had abnormally high serum UA levels. We found a significant inverse correlation between serum UA levels and the RHI, suggesting that UA affects vascular endothelial function even if it is within normal limits.
Limitations
This was a single-center, cross-sectional study based on a small number of patients, without any comparisons with healthy subjects. It remains unclear whether the RHI predicts cardiovascular events in patients with CML receiving TKIs. In addition, the differences between the effects of each TKI on endothelial function need to be clarified. To investigate these points, longitudinal studies with larger numbers of patients are required in the future.
Conclusions
The RHI, a clinical indicator of endothelial function, was negatively correlated with serum UA levels in young patients with CML receiving TKI therapy. One-third of young patients with CML receiving TKIs were classified as having a low RHI, although it was unclear whether the RHI predicted CAEs in these patients. Further research is required to investigate the relationship between drug-induced CAEs and the RHI in patients with CML.
Acknowledgements
Not applicable.
Abbreviations
- AE
Adverse event
- ANOVA
Analysis of variance
- CAE
Cardiovascular adverse event
- CML
Chronic myeloid leukemia
- DMR
Deep molecular response
- EC
Endothelial cells
- FMD
Flow-mediated vasodilation
- IS
International Scale
- MMR
Major molecular response
- MR
Molecular response
- PAT
Peripheral arterial tonometry
- RH
Reactive hyperemia
- RHI
Reactive hyperemia index
- ROS
Reactive oxygen species
- TKI
Tyrosine kinase inhibitor
- TLS
Tumor lysis syndrome
- UA
Uric acid
- XOR
Xanthine oxidoreductase
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by T.K., S.M., A.K., R.M., S.T., N.W., T.T., N.K., and T.M. The first draft of the manuscript was written by T.K. and S.M., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval was obtained from the ethics committee of Juntendo University (Approval number: H19-0191). The procedures used in this study adhered to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Tomoiku Takaku has received honoraria for lectures from Novartis, Pfizer, and Otsuka Pharmaceutical and research funding from Bristol Myers Squibb and Otsuka Pharmaceutical. Norio Komatsu was a board member of PharmaEssentia Japan, and received honoraria from Abbvie, Celgene, Otsuka Pharmaceutical Co., Ltd, Japan Tobacco Inc., Shire, Japan, Novartis, and Takeda Pharmaceutical Company Limited. None of the other authors have competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Boquimpani C, Pasquini R, Clark RE, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–453. doi: 10.1038/s41375-020-01111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, Dyagil I, Glushko N, Milojkovic D, le Coutre P, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231–237. doi: 10.1200/jco.2017.74.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre PD, Etienne G, Dorlhiac-Llacer PE, Clark RE, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–2340. doi: 10.1200/jco.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, Guerci-Bresler A, Legros L, Varet B, Gardembas M, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. doi: 10.1200/jco.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 7.Ross DM, Masszi T, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, Garcia-Gutierrez V, Gattermann N, le Coutre PD, Martino B, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–954. doi: 10.1007/s00432-018-2604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujioka I, Takaku T, Iriyama N, Tokuhira M, Kimura Y, Sato E, Ishikawa M, Nakazato T, Sugimoto KJ, Fujita H, et al. Features of vascular adverse events in Japanese patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a retrospective study of the CML Cooperative Study Group database. Ann Hematol. 2018;97:2081–2088. doi: 10.1007/s00277-018-3412-8. [DOI] [PubMed] [Google Scholar]
- 9.Haguet H, Douxfils J, Chatelain C, Graux C, Mullier F, Dogné JM. BCR-ABL tyrosine kinase inhibitors: which mechanism(s) may explain the risk of thrombosis? TH Open. 2018;2:e68–e88. doi: 10.1055/s-0038-1624566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadzijusufovic E, Albrecht-Schgoer K, Huber K, Hoermann G, Grebien F, Eisenwort G, Schgoer W, Herndlhofer S, Kaun C, Theurl M, et al. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia. 2017;31:2388–2397. doi: 10.1038/leu.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukegawa M, Wang X, Nishioka C, Pan B, Xu K, Ohkawara H, Hamasaki Y, Mita M, Nakamura K, Okamoto M, et al. The BCR/ABL tyrosine kinase inhibitor, nilotinib, stimulates expression of IL-1β in vascular endothelium in association with downregulation of miR-3p. Leuk Res. 2017;58:83–90. doi: 10.1016/j.leukres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/circulationaha.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/cir.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26:2172–2175. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 16.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, Clark RE, Cortes JE, Deininger MW, Guilhot F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060–1071. doi: 10.1161/hypertensionaha.118.11554. [DOI] [PubMed] [Google Scholar]
- 19.Gover-Proaktor A, Granot G, Pasmanik-Chor M, Pasvolsky O, Shapira S, Raz O, Raanani P, Leader A. Bosutinib, dasatinib, imatinib, nilotinib, and ponatinib differentially affect the vascular molecular pathways and functionality of human endothelial cells. Leuk Lymphoma. 2019;60:189–199. doi: 10.1080/10428194.2018.1466294. [DOI] [PubMed] [Google Scholar]
- 20.Kastritis E, Laina A, Georgiopoulos G, Gavriatopoulou M, Papanagnou ED, Eleutherakis-Papaiakovou E, Fotiou D, Kanellias N, Dialoupi I, Makris N, et al. Carfilzomib-induced endothelial dysfunction, recovery of proteasome activity, and prediction of cardiovascular complications: a prospective study. Leukemia. 2021;35:1418–1427. doi: 10.1038/s41375-021-01141-4. [DOI] [PubMed] [Google Scholar]
- 21.Witte DR, Broekmans WM, Kardinaal AF, Klöpping-Ketelaars IA, van Poppel G, Bots ML, Kluft C, Princen JM. Soluble intercellular adhesion molecule 1 and flow-mediated dilatation are related to the estimated risk of coronary heart disease independently from each other. Atherosclerosis. 2003;170:147–153. doi: 10.1016/s0021-9150(03)00253-3. [DOI] [PubMed] [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/hypertensionaha.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: the Framingham Study. Am J Epidemiol. 1985;121:11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 25.Gertler MM, Garn SM, Levine SA. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med. 1951;34:1421–1431. doi: 10.7326/0003-4819-34-6-1421. [DOI] [PubMed] [Google Scholar]
- 26.Maruhashi T, Hisatome I, Kihara Y, Higashi Y. Hyperuricemia and endothelial function: from molecular background to clinical perspectives. Atherosclerosis. 2018;278:226–231. doi: 10.1016/j.atherosclerosis.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, Rosano GM. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Tomiyama H, Higashi Y, Takase B, Node K, Sata M, Inoue T, Ishibashi Y, Ueda S, Shimada K, Yamashina A. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am J Hypertens. 2011;24:770–774. doi: 10.1038/ajh.2011.55. [DOI] [PubMed] [Google Scholar]
- 29.Taher R, Sara JD, Prasad M, Kolluri N, Toya T, Lerman LO, Lerman A. Elevated serum uric acid is associated with peripheral endothelial dysfunction in women. Atherosclerosis. 2019;290:37–43. doi: 10.1016/j.atherosclerosis.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Otani N, Toyoda S, Sakuma M, Hayashi K, Ouchi M, Fujita T, Anzai N, Tanaka A, Node K, Uemura N, et al. Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens Res. 2018;41:923–931. doi: 10.1038/s41440-018-0095-4. [DOI] [PubMed] [Google Scholar]
- 31.Firwana BM, Hasan R, Hasan N, Alahdab F, Alnahhas I, Hasan S, Varon J. Tumor lysis syndrome: a systematic review of case series and case reports. Postgrad Med. 2012;124:92–101. doi: 10.3810/pgm.2012.03.2540. [DOI] [PubMed] [Google Scholar]
- 32.Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.