Highlights
-
•
The flavor substances changed significantly with different treatment methods.
-
•
36 VOCs were identified by GC-IMS, mainly including aldehydes and alcohols.
-
•
Roasting at 190 ± 10 °C for 70 s was the best heat treatment method.
Abbreviations: GC-MS, gas chromatograph-mass spectrometry; GC-IMS, gas chromatography-ion migration spectrometry; GC-O-MS, gas chromatograph-olfactometry-mass spectrometry; GMP, guanosine 5′-monophosphate; IMP, inosine 5′-monophosphate; AMP, adenosine 5′-monophosphate; FAAs, free amino acids; UPLC, ultra-performance liquid chromatography; ESI, electrospray ionization; SIM, selected-ion monitoring; HPLC, high-performance liquid chromatography; ICP-MS, Inductive Coupled Plasma Mass Spectrometer; RI, retention index; DT, drift time; LAV, laboratory analytical viewer; PCA, principal component analysis; TAV, taste active value; ND, not detected
Keywords: Coregonus peled, Taste extracts, Flavor compounds, Thermal treatments
Abstract
This study investigated the effects of different cooking methods on non-volatile flavor (free amino acids, 5′-nucleotides, and organic acids, etc.) of Coregonus peled meat. The volatile flavor characteristics were also analyzed by electric nose and gas chromatography-ion migration spectrometry (GC-IMS). The results indicated that the content of flavor substances in C. peled meat varied significantly. The electronic tongue results indicated that the richness and umami aftertaste of roasting were significantly greater. The content of sweet free amino acids, 5′-nucleotides, and organic acids was also higher in roasting group. Electronic nose principal component analysis can distinguish C. peled meat cooked (the first two components accounted for 98.50% and 0.97%, respectively). A total of 36 volatile flavor compounds were identified among different groups, including 16 aldehydes, 7 olefine aldehydes, 6 alcohols, 4 ketones, and 3 furans. In general, roasting was recommended and gave more flavor substances in C. peled meat.
Introduction
Coregonus peled, a salmonid, is a typical cold-water fish that is heavily farmed in China. It has recently become popular as a new type of ready-to-eat raw fish with a pleasant taste (Fan et al., 2021, Guo et al., 2019). Modern life often leaves little time for cooking fish at home, and cooking without good culinary skills can result in fish dishes with less desirable flavor (Deng et al., 2019). To improve storage stability and convenience for consumers, precooked products with nutritional value and pleasant sensory characteristics have emerged in the modern food processing industry (Wang et al., 2020).
Flavor is a critical sensory property that varies with different thermal treatments for aquatic products. Generally, two types of flavor substances have been identified in cooked fish products: non-volatile flavor compounds (free amino acids, 5′-nucleotides, organic acids, inorganic ions) and volatile flavor compounds (aldehydes, alcohols, ketones, esters) (Yang et al., 2022, Zhang et al., 2019). Both are produced and accumulated through lipid oxidation, enzymatic reaction, protein hydrolysis, microbial degradation, and Maillard reactions during thermal processing (Luo et al., 2022). Changes in physicochemical indicators can promote formation and metabolism of flavor components and textural properties (Liang et al., 2022).
Boiling, steaming, roasting, and frying are commonly used cooking methods for fish products. However, different heating times, temperatures, and cooking methods have different impacts on flavor-binding ability. Previous studies have shown that boiling, steaming, and sous-vide cooking produced little lipid hydrolysis or oxidation in European sea bass meat (Nieva-Echevarría et al., 2017). Similar results were found in cooked turbot meat using different cooking methods; turbot meat underwent a series of chemical reactions including the Maillard reaction and lipid oxidation, accumulating volatile compounds such as ketones, alcohols, acids, hydrocarbons, and aldehydes. Compared to steaming, frying and microwave heating significantly increased the number and proportion of characteristic compounds in cooked turbot, with more severe damage to fatty acids (Dong et al., 2018).
In recent years, the detection of food volatile flavor components has gained widespread attention in food industry. Gas chromatograph-mass spectrometry (GC–MS), gas chromatograph-olfactometry-mass spectrometry (GC-O-MS), two-dimensional gas chromatography, electronic nose, as well as gas chromatography -ion migration spectrometry (GC-IMS), etc. have been employed to analyze the flavor components in various types of foods (Wang, Chen, & Sun, 2020).
Compared with GC–MS, GC-IMS showed the great traits such as rapidness, high resolution and visualization through simple sample preparation, which has been employed in characterizing volatile flavor compounds of aquatic products prepared by different processing and storage conditions (Jin et al., 2021, Li et al., 2022).
Previous works have investigated the effect of super-chilling storage on shelf-life and quality indicators of C. peled muscle (Fan et al., 2021), flavor profile and microbial diversity of C. peled. caviar at different storage temperatures (Jiang et al., 2022). Wang et al (2020) studied the effects of different steaming conditions on quality characteristics of cooked C. peled, and found textural properties, cooking loss, color change, water holding capacity significantly correlated with the cooking condition. However, little information is available on the effects of different cooking methods on the flavor profiles of C. peled.
Herein, the objective of this study was to uncover the non-volatile flavor profiles (tastes, free amino acids, 5′-nucleotides, and organic acids, etc.) of C. peled cooked by different methods. Meantime, their volatile flavor profiles were also characterized by electric nose and GC-IMS methods. A whole information on flavor characteristics of C. peled cooked by different methods would be helpful for product development and quality control for precooked C. peled products in future.
Materials and methods
Materials and reagents
Fresh C. peled with a body weight of approximately 1–1.2 kg and a length of 38–42 cm (n = 30) was provided by Saihu Fishery Science and Technology Development Co., ltd. (Xinjiang Uygur Autonomous Region, China), who fished C. peled from Sailimu Lake, Xinjiang in September. C. peled were slaughtered and eviscerated, the scales and gills removed by the Saihu Fishery Science and Technology Development Co., ltd (Xinjiang Uygur Autonomous Region, China). After being washed with cold water, fish flesh was packed by vacuum and transported to the laboratory on ice by air transport.
Standards of guanosine 5′-monophosphate (GMP, purity ≥ 98 %), inosine 5′-monophosphate (IMP, purity ≥ 98 %), and adenosine 5′-monophosphate (AMP, purity ≥ 98 %) were purchased from Beijing Solarbio Science and Technology Co., ltd. (Beijing, China). 2, 4, 6-trimethylpridine (purity ≥ 98 %) was purchased from Shanghai Yuanye Biological Co., ltd. (Shanghai, China). Standard n-ketones (2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, and 2-nonanone, purity: 99 %) were bought from Sinopharm Chemical Reagent Co., ltd. (Beijing, China). All other reagents were analytical grade from premium suppliers.
Cooking treatments
Frozen C. peled was thawed at 4 °C for 12 h before the head, tail, and skin of the fish were removed. The dorsal muscle was removed and cut into 20 mm × 15 mm × 10 mm cuboids, and placed on ice in plastic wrap for further processing. The cuboids were randomly divided into six groups and subjected to different cooking methods, as described in previous studies (Wang et al., 2020, Zhao et al., 2021): (1) frying (A) at 190 ± 10 °C for 70 s; (2) roasting (B) at 190 ± 10 °C for 70 s; (3) steaming (C) at 100 °C for 4 min; (4) microwave heating (D) at 1700 W for 40 s; (5) sous-vide cooking (E) at 80 °C for 10 min; (6) air frying (F) at 180 °C for 10 min.
Preparation of taste extract
After cooling to room temperature, the treated samples were minced in a grinder (JYL-C010, Joyoung Co., Ltd., China). Approximately 100 g were mixed with 400 mL of ultrapure water and homogenized at 5000 rpm for 2 min with a homogenizer (T25, IKA Co., Germany). The supernatant (natural extract) was collected after centrifugation at 11,000 g for 20 min at 4 °C, and filtered to remove the lipid. This operation was repeated twice with the remaining precipitate. The supernatant was collected for later measurement. (Zhang et al., 2019).
Non-volatile taste compounds
Quantitation of electronic tongue measurement
The taste extracts were filtered through a 0.45-μm membrane and measured using an electronic tongue sensor system (TS-5000Z, Insent Inc., Japan). All sensors, including five lipid membrane sensors (bitterness, umami, saltiness, sourness, astringency) and three standard electrodes, were preconditioned in 0.01 M potassium chloride for 24 h. The test program referenced the method used by Pan et al. (2018), modifying it slightly. Each sample was measured six times.
Quantitation of free amino acids
Free amino acids(FAAs) was quantified using the method developed by Adeyeye (2009), with some modification. The natural extract (500 μL) was acid-hydrolyzed by adding 500 μL of 12 M HCl for 12 h. The hydrolysate (200 μL) was collected and mixed with 535 μL of 2 M NaOH to neutralize. The sample (10 μL) was mixed with 70 μL AccQ·Tag Ultra Borate Buffer and 20 μL AccQ·Tag Reagent. The reaction mixture was heated at 55 °C for 10 min, cooled, and loaded into the machine. The sample extracts were analyzed using a UPLC-Orbitrap-MS system (UPLC, Vanquish; MS, QE). The analytical conditions were: ultra-performance liquid chromatography(UPLC): column, Waters BEH C18 (50 mm × 2.1 mm, 1.7 μm); column temperature: 55 °C; flow rate: 0.5 mL/min; injection volume: 1 μL; solvent system: water (0.1 % formic acid), acetonitrile (0.1 % formic acid); gradient program: 95:5(v/v) at 0 min, 90:10 (v/v) at 5.5 min, 75:25 (v/v) at 7.5 min, 40:60 (v/v) at 8 min, 95:5 (v/v) at 8.5 min, 95:5 (v/v) at 13 min. HRMS data were recorded on a Q Exactive hybrid Q-Orbitrap mass spectrometer equipped with a heated electrospray ionization (ESI) source (Thermo Fisher Scientific) using selected-ion monitoring (SIM) acquisition methods. The ESI source parameters were: spray voltage: 3 kV; sheath gas pressure: 40 arb; aux gas pressure: 10 arb; sweep gas pressure: 0 arb; capillary temperature: 320 °C; aux gas heater temperature: 350 °C (Bao et al., 2018, Feng et al., 2016, Glauser et al., 2016, Marhabaie et al., 2014).
Quantitation of 5′-nucleotides analysis
The 5′-nucleotides(GMP, IMP, and AMP) were extracted and analyzed on a HPLC system (UltiMate 3000, Thermo Fisher Scientific Co., Ltd., Massachusetts, USA), according to the method modified from Wen et al. (2020). A chromatographic column (Acclaim PolarAdvantage II C18, 50 mm × 4.6 mm, 3 μm) with mobile phase A of methanol and mobile phase B of 20 mM KH2PO4-K2HPO4 buffer solution (v/v = 1:1, pH = 5.8). The sample was eluted at a flow rate of 1 mL/min with 0 % A and 100 % B for 0–6 min, 8 % A and 92 % B for 7–14 min, 35 % A and 65 % B for 15–20 min, and 0 % A and 100 % B for 21–23 min.
Quantitation of organic acids
The Succinic acid and lactic acid contents of the taste extracts were analyzed by high-performance liquid chromatography (HPLC, UltiMate 3000, Thermo Fisher Scientific Co., ltd., Massachusetts, USA) using a chromatographic column (Acclaim Polar Advantage II C18, 50 mm × 4.6 mm, 3 μm) with a mobile phase of Na2SO4 buffer solution (pH 2.5 modulated by mesylate) without gradient elution (Jing et al., 2022). The flow rate was 1 mL/min with 10-μL injection. The absorption was detected at 214 nm.
Quantitation of inorganic ion analysis
The contents of sodium (Na+), potassium (K+), phosphate (PO43-), and chlorine (Cl-) were determined using an inductive coupled plasma mass spectrometer (ICP-MS, Thermo Fisher Scientific Co., ltd., Massachusetts, USA) and the method used by Wen et al. (2020). The ICP-MS was equipped with a detector (DS5 defection stabilizer), a suppressor (AERS500), and an anion exchange column (AS14). The mobile phase was an Na2CO3/NaHCO3 mixed buffer (0.25 mol/L) with a 0.5 mL/min flow rate at room temperature to detect the 25-μL injection.
Volatile flavor analysis
Quantitation of electronic nose analysis
The volatile compounds of the six types of C. peled were determined using an electronic nose system (PEN3.0, AIRSENSE, Germany) equipped with ten types of sensors. Each cooked C. peled sample (1 g) was placed in a 10-ml glass injection bottle and equilibrated at room temperature for 15 min. The sensor cleaning time was 60 s; the auto-zero-time was 10 s; the sample preparation time was 5 s; the detection time was 60 s; the period between 54 s and 56 s, which exhibited a stable response curve, was used for data analysis (Ma et al., 2021).
Quantitation of gas chromatography-ion mobility spectrometry analysis
The volatile compounds in C. peled were determined by GC-IMS (FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH [G.A.S.], Dortmund, Germany) and the method used by Jin et al. (2021), with slight modification. Thermal C. peled sample (2 g) was placed in 20-mL headspace bottles and implanted (500 μL) using a high-temperature injector (85 °C) maintained at 60 °C for 20 min with an incubation speed of 500 rpm. An unbranched procedure was used. The samples were driven by high-purity nitrogen into a chromatographic column (MXT-5, 15 m,0.53 mm ID,1.0 μm df, Restek Corporation, USA) maintained at 60 ℃. The 99.99 % nitrogen gas was used as a vehicle at a programmed speed as follows: 2 mL/min for 2 min, 10 mL/min for 8 min, 100 mL/min for 10 min, and 150 mL/min for 5 min. The mixture gas was ionized in the IMS ionization cell. To prevent cross-pollution, the injector was compulsorily planed 30 s before each assay and 5 min after each assay. The n-ketones C4–C9 were used as foreign standards to estimate the retention index (RI) of each volatile chemical. Via collations of RI and the drift time (DT) through the instrumental database (FlavourSpec®, Germany), the volatile flavor substances were compared with standard chemicals in terms of DT and RI. The signal intensity denoted the height or the peak area.
Statistical analysis
The data were expressed as mean ± standard deviation (n ≥ 3), and a t-test was used for significance analysis (P < 0.05). Radar and bubble plots were plotted from a plug-in program using electric tongue device. A plug-in principal component analysis (PCA) and radar plots were acquired from electric nose system. The GC-IMS data and plot were obtained through GC × IMS Library Search, Laboratory Analytical Viewer (LAV), and gallery fingerprint plot for all volatile compounds identified between samples (Li et al., 2019).
Results and discussion
Non-volatile compounds of taste extracts
Electronic tongue measurement of taste extracts
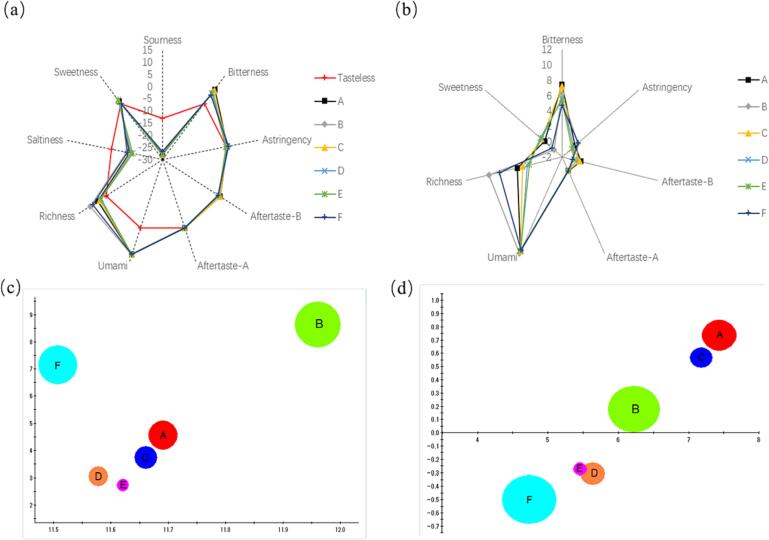
The effect of different cooking methods on the six basic tastes and three aftertastes of C. peled meat was investigated. As shown in Fig. 1a, the sourness and saltiness were lower than tasteless (reference solution) and the other taste indicators. Based on previous information, richness, bitterness, and sweetness were three significantly different taste indicators in the effective radar plot (Fig. 1b) with slight modification (without sourness and saltiness). The umami taste was another major taste indicator; the samples did not exhibit considerable differences. To determine the difference between major and minor taste indicators, a bubble plot (Fig. 1c) of umami, saltiness, and richness and a bubble plot (Fig. 1d) of bitterness, bitterness aftertaste, and astringent are presented. The umami value is large, but the difference is small. Between 11.5 and 12, the difference for richness (and umami aftertaste) is large: roasting (group B) is slightly larger than group F (air frying) and significantly larger than the other four cooking groups. The difference for bitter taste is also obvious; frying (A) and steaming (C) are significantly larger than other cooking groups. These results showed that taste profiles of C. peled meat cooked by different methods varied to some extent, and the specific taste compounds that induced taste changes in C. peled meat samples after cooking deserve further exploration.
Fig. 1.
Radar plots (a, b) and bubble plots (c, d) of electronic tongue measurement for C. peled meat cooked by different methods. The cooking methods A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively (a, b, c, d). Aftertaste A and B represent bitterness and umami, respectively (a, b).
Analysis of free amino acid content in taste extracts
The free amino acids (FAAs) produced by the protein in fish meat after heat treatment were important precursors for the characteristic flavor of C. peled meat. There were 21 FAAs detected in C. peled meat of the six cooking methods investigated. They showed some differences between them; 19 amino acids were common and two were uncommon (Table 1). Cystine was not detected in C. peled meat. The two uncommon amino acids were 4-hydroxy-l-proline and gamma-aminobutyric acid. The contents of histidine, alanine, and glycine were higher in each cooking group, indicating that these three amino acids were the main flavor substances in C. peled meat. Their contents were highest in roasting group(B) and lowest in steaming (C) (P < 0.05), indicating that roasting treatment is more conducive to release of flavor substances in C. peled meat.
Table 1.
Composition and contents of non-volatile taste compounds in C. peled meat cooked by different methods (mg/100 g).
| Component | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Lactic acid | 127.83 ± 3.02b | 167.70 ± 2.64a | 98.82 ± 1.40e | 114.71 ± 1.75c | 108.42 ± 1.01d | 126.91 ± 1.27b |
| Succinic acid | 1.46 ± 0.01b | 2.04 ± 0.07a | 0.81 ± 0.02d | 1.04 ± 0.03c | 0.85 ± 0.01d | 1.05 ± 0.01c |
| IMP | 78.72 ± 0.58b | 104.10 ± 1.46a | 76.41 ± 0.25c | 52.60 ± 0.05d | 48.42 ± 1.19e | 102.70 ± 0.08a |
| GMP | ND | ND | ND | 27.03 ± 0.07a | 27.07 ± 0.53a | ND |
| AMP | 0.96 ± 0.01b | 0.69 ± 0.02d | 0.88c | 0.27e | 0.93 ± 0.02b | 1.37a |
| Aspartic acid | 1.12 ± 0.01b | 1.34 ± 0.00a | 0.33 ± 0.03e | 0.54 ± 0.03d | 0.23 ± 0.03f | 1.04 ± 0.02c |
| Glutamic acid | 1.09 ± 0.02a | 0.93 ± 0.30a | 0.38 ± 0.06b | 1.01 ± 0.06a | 0.26 ± 0.03b | 1.24 ± 0.02a |
| Serine | 54.95 ± 0.54b | 82.69 ± 0.30a | 24.50 ± 1.99e | 26.76 ± 0.58d | 19.78 ± 0.28f | 48.81 ± 0.14c |
| Glycine | 284.20 ± 0.36b | 376.05 ± 2.59a | 127.11 ± 4.39e | 159.68 ± 1.31d | 96.18 ± 0.57f | 275.98 ± 0.57c |
| Threonine | 32.86 ± 0.27b | 46.76 ± 0.20a | 14.07 ± 1.26e | 16.37 ± 0.38d | 12.31 ± 0.15f | 28.87 ± 0.53c |
| Alanine | 154.26 ± 0.38b | 164.88 ± 0.02a | 60.96 ± 7.29e | 82.44 ± 0.41d | 47.57 ± 0.07f | 144.82 ± 0.38c |
| Histidine | 125.12 ± 0.10b | 167.04 ± 1.59a | 51.70 ± 2.03e | 63.54 ± 0.53d | 53.28 ± 0.56e | 105.18 ± 1.28c |
| Tyrosine | 9.44 ± 0.54b | 11.68 ± 0.06a | 4.06 ± 0.20e | 5.24 ± 0.07d | 3.70 ± 0.01e | 8.31 ± 0.03c |
| Leucine | 12.90 ± 0.03b | 13.80 ± 0.15a | 5.27 ± 0.54e | 6.88 ± 0.14d | 4.22 ± 0.02f | 12.01 ± 0.09c |
| Phenylalanine | 6.96 ± 0.06b | 9.18 ± 0.01a | 3.17 ± 0.23e | 4.15 ± 0.01d | 2.87 ± 0.02f | 6.33 ± 0.06c |
| Tryptophan | 2.60 ± 0.21b | 2.90 ± 0.09a | 1.08 ± 0.12e | 1.44 ± 0.00d | 0.97 ± 0.02e | 1.94 ± 0.01c |
| Isoleucine | 7.31 ± 0.02a | 7.55 ± 0.06a | 3.04 ± 0.28d | 3.84 ± 0.04c | 2.31 ± 0.02e | 6.73 ± 0.02b |
| Arginine | 8.17 ± 0.28b | 9.90 ± 0.07a | 2.71 ± 0.23e | 3.53 ± 0.03d | 0.88 ± 0.22f | 6.31 ± 0.50c |
| Proline | 24.05 ± 0.17b | 36.10 ± 0.20a | 9.63 ± 1.05e | 10.81 ± 0.29d | 7.10 ± 0.09f | 20.64 ± 0.03c |
| Lysine | 9.97 ± 0.18a | 8.00 ± 0.10b | 3.25 ± 0.23e | 4.12 ± 0.02d | 1.78 ± 0.16f | 7.01 ± 0.02c |
| Methionine | 7.70 ± 0.06b | 10.43 ± 0.19a | 3.13 ± 0.28e | 4.14 ± 0.11d | 2.25 ± 0.04f | 6.98 ± 0.07c |
| Valine | 11.89 ± 0.14b | 13.69 ± 0.17a | 4.63 ± 0.62e | 5.95 ± 0.21d | 3.68 ± 0.03f | 10.92 ± 0.02c |
| 4-Hydroxy-l-Proline | 1.83 ± 0.02a | 1.85 ± 0.06a | 0.68 ± 0.08d | 0.95 ± 0.03c | 0.47 ± 0.01e | 1.51 ± 0.01b |
| Gamma-Aminobutyric acid | 1.48 ± 0.00b | 1.78 ± 0.01a | 0.53 ± 0.04e | 0.65 ± 0.02d | 0.34 ± 0.00f | 1.22 ± 0.00c |
| Cystine | ND | ND | ND | ND | ND | ND |
| Asparagine | 4.00 ± 0.00a | 3.45 ± 0.06b | 1.65 ± 0.12e | 0.92 ± 0.04f | 1.93 ± 0.02d | 2.86 ± 0.02c |
| Glutamine | 37.75 ± 0.30a | 25.87 ± 0.20c | 14.15 ± 1.23f | 22.56 ± 0.67d | 16.78 ± 0.32e | 35.92 ± 0.61b |
| Na+ | 35.72 ± 3.20b | 62.81 ± 2.94a | 25.68 ± 2.37de | 30.41 ± 0.34 cd | 22.84 ± 0.37e | 34.81 ± 0.50bc |
| Cl- | 610.81 ± 13.60c | 903.68 ± 5.28a | 487.52 ± 35.40e | 584.68 ± 13.92 cd | 540.96 ± 12.51d | 681.72 ± 22.22b |
| K+ | 37.29 ± 0.95b | 61.27 ± 1.85a | 30.33 ± 4.30c | 37.97 ± 4.38b | 23.86 ± 0.82c | 41.80 ± 1.23b |
| PO43- | 598.83 ± 3.48c | 1029.27 ± 18.01a | 633.17 ± 29.10c | 732.81 ± 20.50b | 799.50 ± 16.04b | 1038.38 ± 49.70a |
Most FAAs can be categorized as umami, sweet, or bitter amino acids (Chen et al., 2022). Of the six cooked C. peled meat samples, the total sweet amino acids were the most prevalent, followed by bitter amino acids, and the umami amino acid contents were the lowest (Table 1). The bitter amino acid histidine, which has a low threshold, has a meaty and sweet flavor, and contributes more to the flavor of C. peled meat, which was strongly related to the type of heat treatment. The other bitter amino acids contributed little to the flavor due to their high thresholds. The sweet amino acids glycine and alanine had higher taste active value (TAV) with roasting treatment (group B), demonstrating that roasting treatment facilitates expression of sweet substances. The TAV of the umami glutamic acid was > 1, which contributed to the umani aroma and had a positive effect on heat-treated C. peled meat. The contents and contributions of flavor substances were significantly different in C. peled meat with different cooking methods. The C. peled meat in the roasting treatment (group B) exhibited a more pleasant flavor, while fewer positive taste substances were exhibited in steaming group of C. peled meat.
Different amino acids have different taste thresholds. Thus, the content of FAAs cannot be used to evaluate their contribution to the flavor of C. peled meat. Generally, TAV values are used to evaluate the contribution of amino acids to taste. TAV > 1 indicates that the substance contributes to the taste (Chen et al., 2014). As shown in Table 2, the TAV values of histidine and gamma-aminobutyric acid were > 1. Meantime, the TAV values of histidine and gamma-aminobutyric acid reached 2.59–8.36 and 1.66–8.63, respectively. After treatment with frying (group A), roasting (group B), and air frying (group F), the TAV values of glycine and alanine were > 1. The TAV values of glutamic acid were > 1 in all cooked C. peled meat samples except steaming. The TAV values of all amino acids except glutamic acid were significantly higher with roasting (group B) treatment than with other cooking treatments.
Table 2.
Taste thresholds and taste active values of non-volatile taste compounds in C. peled meat cooked by different methods.
| Component | Taste threshold mg/100 mL | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| Lactic acid | 67.55 | 1.89 ± 0.04b | 2.48 ± 0.04a | 1.46 ± 0.02e | 1.70 ± 0.03c | 1.60 ± 0.01d | 1.88 ± 0.02b |
| Succinic acid | 10.63 | 0.14b | 0.19 ± 0.01a | <0.10 | 0.1c | <0.10 | 0.1c |
| IMP | 23.53 | 3.35 ± 0.02b | 4.42 ± 0.06a | 3.25 ± 0.01c | 2.24d | 2.06 ± 0.05e | 4.36a |
| GMP | 8.14 | ND | ND | ND | 3.32 ± 0.01a | 3.33 ± 0.07a | ND |
| AMP | 86.80 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Aspartic acid | 53.24 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Glutamic acid | 16.18 | 2.34 ± 0.02a | 1.60 ± 0.01c | 0.88 ± 0.08f | 1.39 ± 0.04d | 1.04 ± 0.02e | 2.22 ± 0.04b |
| Serine | 262.75 | 0.21 ± 0.00b | 0.32 ± 0.01a | 0.10 ± 0.01d | 0.10d | 0.08 ± 0.01e | 0.19c |
| Glycine | 187.75 | 1.52 ± 0.01b | 2.00 ± 0.01a | 0.68 ± 0.02e | 0.86 ± 0.01d | 0.51 ± 0.00f | 1.47 ± 0.00c |
| Threonine | 416.85 | <0.10 | 0.11a | <0.10 | <0.10 | <0.10 | <0.10 |
| Alanine | 106.92 | 1.45 ± 0.01b | 1.54 ± 0.00a | 0.57 ± 0.07e | 0.77 ± 0.00d | 0.45 ± 0.01f | 1.36 ± 0.01c |
| Histidine | 20.00 | 6.25 ± 0.01b | 8.36 ± 0.08a | 2.59 ± 0.11e | 3.18 ± 0.03d | 2.66 ± 0.03e | 5.26 ± 0.06c |
| Tyrosine | 72.44 | 0.14 ± 0.01b | 0.16 ± 0.00a | <0.10 | <0.10 | <0.10 | 0.12 ± 0.01c |
| Leucine | 144.32 | <0.10 | 0.10 ± 0.01a | <0.10 | <0.10 | <0.10 | <0.10 |
| Phenylalanine | 743.40 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Tryptophan | 102.12 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Isoleucine | 131.20 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Arginine | 1306.50 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Proline | 287.75 | <0.10 | 0.13 ± 0.01a | <0.10 | <0.10 | <0.10 | <0.10 |
| Lysine | 1169.60 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Methionine | 74.60 | 0.10b | 0.14a | <0.10 | <0.10 | <0.10 | <0.10 |
| Valine | 351.45 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| 4-Hydroxy-l-Proline | 327.825 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Gamma-Aminobutyric acid | 0.2062 | 7.19 ± 0.02b | 8.63 ± 0.06a | 2.55 ± 0.20e | 3.16 ± 0.12d | 1.66 ± 0.01f | 5.91 ± 0.02c |
| Cystine | 24.22 | ND | ND | ND | ND | ND | ND |
| Asparagine | 660.60 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Glutamine | 730.75 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Na+ | 8.97 | 3.98 ± 0.36b | 7.00 ± 0.33a | 2.86 ± 0.26de | 3.39 ± 0.04 cd | 2.55 ± 0.04e | 3.88 ± 0.06bc |
| Cl- | 50.83 | 12.02 ± 0.27c | 17.78 ± 0.10a | 9.59 ± 0.70e | 11.50 ± 0.27 cd | 10.64 ± 0.25d | 13.41 ± 0.44b |
| K+ | 13.85 | 2.69 ± 0.07b | 4.42 ± 0.13a | 2.19 ± 0.31c | 2.74 ± 0.32b | 1.72 ± 0.06c | 3.02 ± 0.09b |
| PO43- | 142.46 | 4.20 ± 0.02c | 7.23 ± 0.13a | 4.44 ± 0.20c | 5.14 ± 0.14b | 5.61 ± 0.11b | 7.29 ± 0.35a |
Data are expressed as mean ± standard error. Different letters in the same row indicate significant differences between groups (P < 0.05). The cooking methods A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively. ND: not detected, Na+: sodium, K+: potassium, PO43-: phosphate, Cl-: chlorine, AMP: adenosine 5′-monophosphate, GMP: guanosine 5′ -monophosphate, IMP: inosine 5′-monophosphate.
Data are expressed as mean ± standard error. Different letters in the same row indicate significant differences between groups (P < 0.05). The cooking methods A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively.
Threshold data were from ChemTastes database (https://zenodo.org/record/5747393#.%20YwR3gu5BxPY).
Analysis of 5′-nucleotides in taste extracts
Table 1 and Table 2 also showed the taste profile of 5′-nucleotides (GMP, IMP, and AM) in taste extracts from cooked C. peled meat. The highest IMP content is found in 5′-nucleotide after different cooking treatments, compared to GMP and AMP. TAV of IMP are > 2. The contents of IMP in C. peled meat after roasting and air frying are the highest, reaching 4.42 and 4.36, respectively, indicating that umami taste was enhanced, consistent with the electronic tongue results (Fig. 1a,b). GMP was only detected in C. peled meat after microwave heating and sous-vide cooking, but not detected in the other four cooking groups. Umami plays an important role in food texture and can be obtained with the presence of glutamate and enhanced by addition of IMP and GMP (Chew et al., 2017). As an umami enhancer, IMP plays an important role in food taste (Rocha et al., 2020). AMP and GMP may interact with FAAs to enhance taste (Yamaguchi et al., 2013). The present results found that the effect of cooking method on 5′-nucleotides was significant. GMP is only present within C. peled meat after microwave heating and low temperature slow cooking. IMP and GMP were the taste-active compound in C. peled meat. A similar study about the effect of IMP and AMP on taste of Chinese mitten crab meat and shrimp meat was also reported by Chen & Zhang (2007).
Analysis of organic acids in taste extracts
In Table 1 and Table 2, both succinic acid and lactic acid were detected in C. peled meat after various cooking methods, and the content of lactic acid in C. peled meat after all cooking treatmenst exceeds its threshold value. The lactic acid content in C. peled meat after roasting is the highest, and has a TAV of 2.48, exhibiting an important contribution to taste. The present results showed that the effect of cooking methods on lactic acid was obvious, and it seemed that higher temperatures produce a higher lactic acid content. The content of succinic acid was well below the threshold, and did not contribute to taste. Zhang, Ma, & Dai (2019) found some organic acids like tartaric acid, malic acid, lactic acid, and succinic acid could be detected in cooked tuna samples, and tartaric acid and lactic acid were dominant organic acids. The present study investigated lactic acid, and succinic acid in C. peled meat after various cooking methods, and lactate is a potential taste factor of organic acids in cooked C. peled meat.
Analysis of inorganic ions in taste extracts
Some inorganic ions may enhance the flavor and taste of food products (Jiang, McPhedran, Hou, Chen, & Huang, 2023). In Table 1 and Table 2, inorganic ions (Na+, K+, PO43−, Cl−) were detected in C. peled meat after various cooking methods, are significantly higher in roasting group than in the other five cooking treatments; the values of these inorganic ions in C. peled meat after steaming and sous-vide cooking are significantly lower, probably because the two cooking treatments contained more water, resulting in a relatively low inorganic ion content in C. peled.
Volatile flavor compounds
Electronic nose analysis of cooked C. peled meat
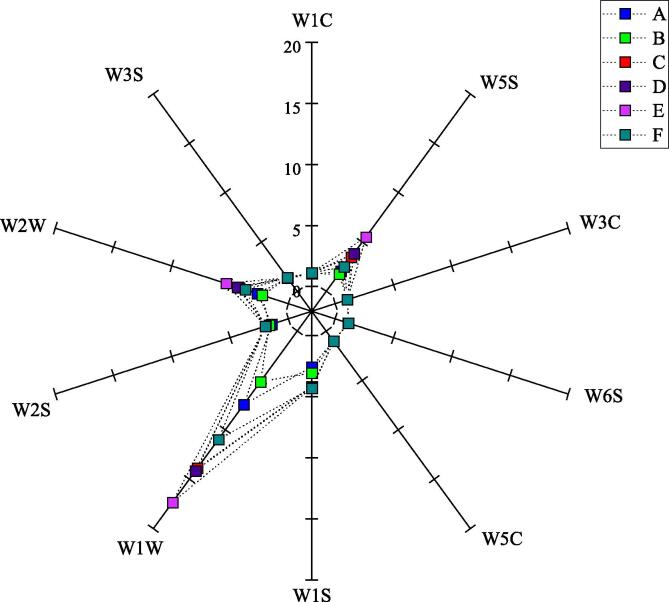
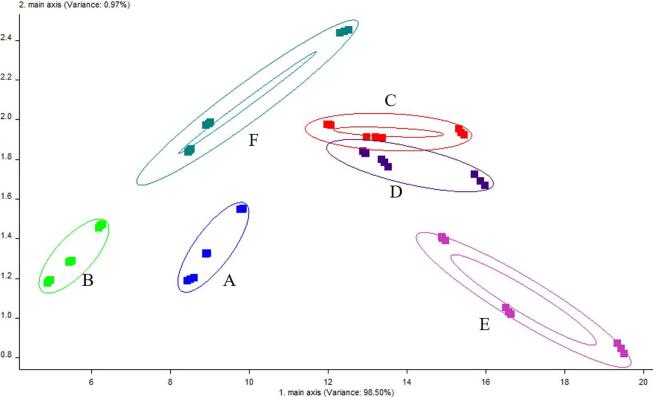
An electronic nose was used to analyze volatile flavor compounds in C. peled meat with different cooking treatments. The sensor signal intensities of volatile flavor compounds with different treatments are plotted in Fig. 2. C. peled meat samples produced almost no response in sensors W1C, W3C, W6S, W5C, and W3S, demonstrating that benzenes, ammonia, hydrides, short-chain alkanes, and long-chain alkanes were poorly represented and did not differ significantly among different cooking condition. For the W1S and W2S sensors, the response value for fish samples was not high, but there were differences between different cooked samples, indicating that different processing methods had an impact on the content of flavor substances in C. peled meat after cooking. C. peled meat samples had higher response values at W5S, W1W, and W2W; these three sensors can distinguish C. peled meat samples with different cooking treatments. Sous-vide cooking (group E) had the highest response value, followed by microwave heat (group D); roasting (group B) had the lowest value, indicating that the content of nitrogen oxides, inorganic sulfides, and organic sulfides was higher with sous-vide cooking treatment, and that roasting treatment was not conducive to formation of these substances. The PCA score plot result was displayed in Fig. 3, and PC1 and PC2 accounted for 98.50 % and 0.97 %, respectively. The cumulative contribution rate reached 99.47 %, indicating that the information contained in the first two principal components can better represent the overall data. Flavor substances produced in C. peled meat samples with different cooking treatments clustered together in one area with no obvious overlap, except for steaming (groups C) and microwave heating (group D), showing that the two cooked methods possessed certain similarity. These flavor characteristics of C. peled meat samples differ from each other, mainly caused by cooking state and conditions (Fedorov et al., 2021). Overall, electronic nose coupled with PCA can better distinguish flavor substances in C. peled meat after different cooking treatments.
Fig. 2.
Radar plot of electronic nose analysis of C. peled meat cooked by different methods. A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively. Note: W1C: aromatics; W5S: nitrogen oxides; W3C: ammonia and aromatic components; W6S: hydride; W5C: olefins and aromatic molecules; W1S: methane; W1W: sulfides; W2S: ethanol and some aromatics; W2W: organic sulfides: W3S: alkanes and aliphatics.
Fig. 3.
PCA score plot of electronic nose analysis of C. peled meat cooked by different methods. The groups A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively.
Gas chromatography-ion mobility spectrometry analysis of cooked C. peled meat
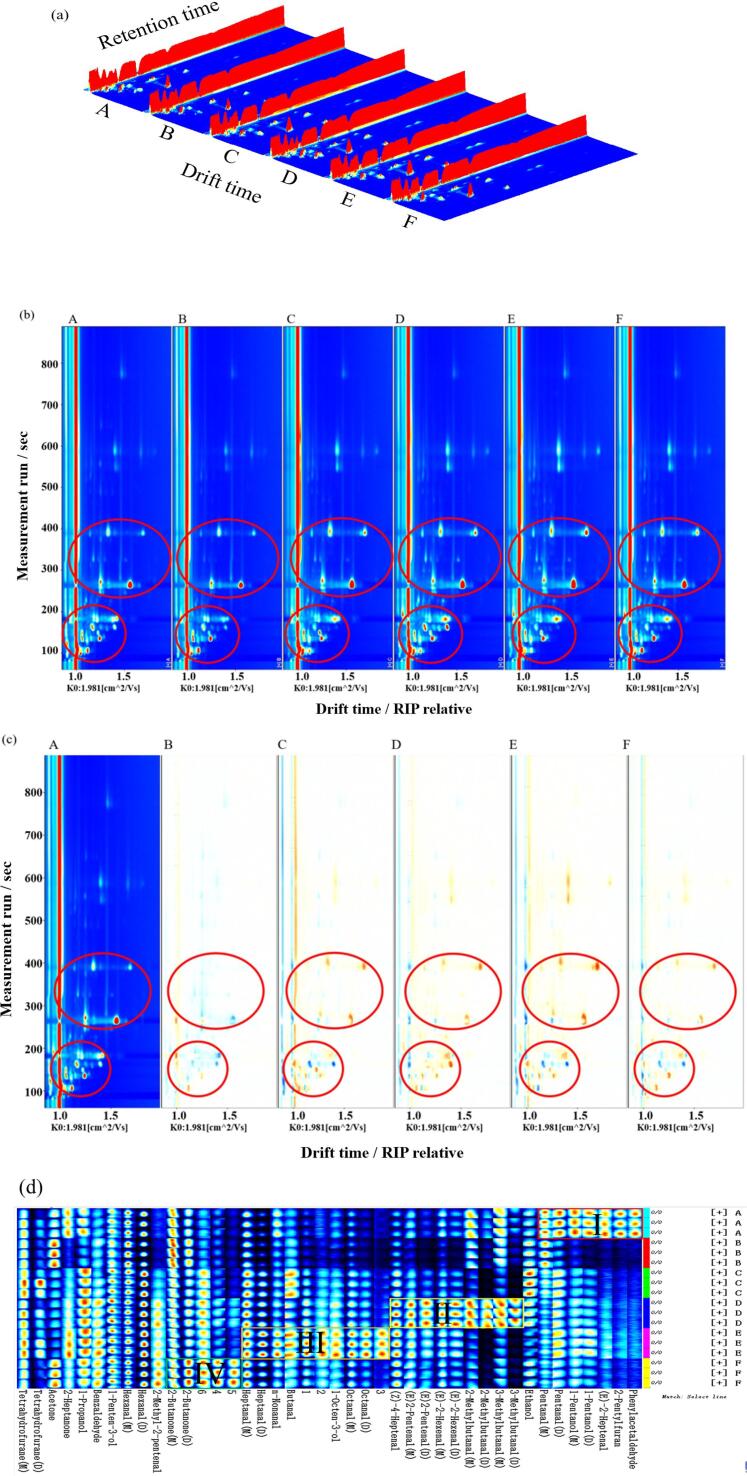
GC-IMS technology was used to identify the volatile organic compounds in the C. peled meat. Fig. 4(a) presents a three-dimensional graph. It is observed that the volatile organic compounds in C. peled meat cooked by different methods were generally similar, with subtle differences. In the three-dimensional graph, the difference is difficult to distinguish with the naked eye. Thus, the three-dimensional graph was converted to a two-dimensional graph (Fig. 4(b)) to see the difference between the types and contents of volatile flavor substances with different treatments more clearly (Cui et al., 2020). Fig. 4(c) uses frying sample (A) as the reference; the other five cooked samples are the deducted reference spectra. After deduction, the background is white. Red indicates a higher content of volatile flavor substances than the reference; blue indicates a lower content. The contents of volatile flavor substances increase or decrease in different treatment conditions (Jin et al., 2021, Zhao et al., 2022).
Fig. 4.
3D-topographic plots (a), 2D-topographic plots (b: vertical view; c: difference view), and gallery plot (fingerprint, d) of characteristic volatile organic compounds in C. peled meat cooked by different methods.
To distinguish the changes for all aroma substances, all peaks were used to draw fingerprints (Fig. 4(d)) to further analyze the volatile flavor substances in C. peled meat with different processing methods. Each row in the figure represents the volatile compounds in a C. peled meat sample; each column compares a volatile compound in different samples. The color intensity indicates the volatile compound content; brighter colors indicate higher content (Li et al., 2022). Each sample was measured in triplicate. A total of 42 signal peaks were detected in C. peled meat with different cooking methods. A total of 36 volatile organic compounds (monomers and dimers) were identified through comparison with the database in the instrumental software, including 16 aldehydes, 7 olefine aldehydes, 6 alcohols, 4 ketones, and 3 furans (Fig. 4(d) and Table 3).
Table 3.
Key volatile compounds in C. peled meat with different cooking methods detected by GC-IMS.
| Classification | Compound | Rt [sec] | Dt [a.u.] | RI | Odor description | Relative amount/% |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||||||
| aldehydes | n-Nonanal | 772.44 | 1.47252 | 1104.1 | fat, citrus, green | 1.57 ± 0.03a | 1.07 ± 0.03b | 1.06 ± 0.01b | 0.88 ± 0.06c | 1.50 ± 0.10a | 1.12 ± 0.03b |
| Octanal(M) | 590.886 | 1.40877 | 1013.7 | fat, soap, lemon, green | 3.61 ± 0.03d | 3.14 ± 0.16e | 5.03 ± 0.06b | 4.94 ± 0.08b | 6.46 ± 0.10a | 4.70 ± 0.11c | |
| Octanal(D) | 585.521 | 1.82116 | 1010.6 | fat, soap, lemon, green | 0.37 ± 0.02c | 0.28 ± 0.06d | 0.61 ± 0.02b | 0.62 ± 0.02b | 1.09 ± 0.06a | 0.59 ± 0.02b | |
| Benzaldehyde | 528.041 | 1.15726 | 980.7 | almond, burnt sugar | 0.42 ± 0.04b | 0.63 ± 0.08a | 0.42 ± 0.08b | 0.56 ± 0.05a | 0.59 ± 0.06a | 0.67 ± 0.04a | |
| Heptanal(M) | 391.701 | 1.33588 | 903.7 | fat, citrus, rancid | 6.26 ± 0.10e | 6.81 ± 0.12d | 8.19 ± 0.08a | 7.11 ± 0.06c | 7.88 ± 0.16b | 7.25 ± 0.04c | |
| Heptanal(D) | 394.965 | 1.69303 | 905.8 | fat, citrus, rancid | 2.48 ± 0.10e | 2.38 ± 0.06e | 5.57 ± 0.02b | 5.36 ± 0.04c | 7.72 ± 0.05a | 4.82 ± 0.16d | |
| Hexanal(M) | 263.769 | 1.26028 | 792.7 | grass, tallow, fat | 9.63 ± 0.17b | 10.88 ± 0.09a | 8.73 ± 0.17c | 7.22 ± 0.09e | 7.00 ± 0.25e | 7.71 ± 0.15d | |
| Hexanal(D) | 262.065 | 1.56495 | 790.8 | grass, tallow, fat | 21.22 ± 0.10c | 17.91 ± 0.25d | 24.85 ± 0.48a | 21.90 ± 0.06b | 24.85 ± 0.43a | 21.80 ± 0.04b | |
| Pentanal(M) | 184.23 | 1.18731 | 692.4 | almond, malt, pungent | 2.47 ± 0.06b | 2.86 ± 0.02a | 1.51 ± 0.01c | 1.04 ± 0.03e | 1.08 ± 0.00e | 1.21 ± 0.04d | |
| Pentanal(D) | 183.106 | 1.42591 | 690.7 | almond, malt, pungent | 3.53 ± 0.04a | 1.69 ± 0.06e | 2.58 ± 0.04b | 2.15 ± 0.01c | 2.63 ± 0.05b | 2.05 ± 0.07d | |
| 2-Methylbutanal(M) | 169.04 | 1.16656 | 660.3 | cocoa, almond | 2.13 ± 0.05a | 1.79 ± 0.03c | 0.70 ± 0.02e | 1.91 ± 0.02b | 0.68 ± 0.01e | 1.06 ± 0.03d | |
| 2-Methylbutanal(D) | 167.242 | 1.40448 | 656.1 | cocoa, almond | 1.14 ± 0.03b | 0.51 ± 0.01c | 0.10 ± 0.01e | 2.14 ± 0.11a | 0.11 ± 0.00e | 0.23 ± 0.02d | |
| 3-Methylbutanal(M) | 158.853 | 1.18228 | 636.1 | malt | 2.68 ± 0.07a | 2.52 ± 0.07b | 0.95 ± 0.04d | 2.44 ± 0.05b | 1.03 ± 0.02d | 1.42 ± 0.01c | |
| 3-Methylbutanal(D) | 159.452 | 1.40762 | 637.5 | malt | 1.32 ± 0.08b | 0.75 ± 0.02c | 0.15 ± 0.02e | 2.75 ± 0.14a | 0.16 ± 0.01e | 0.30 ± 0.01d | |
| Butanal | 138.744 | 1.29222 | 583.3 | pungent, green | 0.41 ± 0.01e | 0.55 ± 0.01c | 0.67 ± 0.02a | 0.37 ± 0.02f | 0.58 ± 0.01b | 0.48 ± 0.01d | |
| Phenylacetaldehyde | 652.164 | 1.26254 | 1047 | hawthorne, honey, sweet | 0.41 ± 0.04a | 0.12 ± 0.02b | 0.12 ± 0.02b | 0.13 ± 0.02b | 0.13 ± 0.01b | 0.12 ± 0.01b | |
| subtotal | 59.63 ± 0.56c | 53.89 ± 0.19e | 61.25 ± 0.67b | 61.52 ± 0.28b | 63.49 ± 0.58a | 55.52 ± 0.03d | |||||
| olefine aldehyde | (E)-2-Heptenal | 493.974 | 1.25547 | 963.5 | soap, fat, almond | 0.40 ± 0.03a | 0.13 ± 0.02d | 0.14 ± 0.01 cd | 0.17 ± 0.02bc | 0.20 ± 0.01b | 0.17 ± 0.02bc |
| (E)-2-Hexenal(M) | 327.215 | 1.17772 | 853.8 | apple, green | 1.18 ± 0.01b | 0.67 ± 0.03e | 0.89 ± 0.02d | 1.39 ± 0.03a | 1.05 ± 0.05c | 1.15 ± 0.01b | |
| (E)-2-Hexenal(D) | 326.363 | 1.51611 | 853.1 | apple, green | 0.21 ± 0.01c | 0.08 ± 0.02e | 0.18 ± 0.01d | 0.38 ± 0.01a | 0.25 ± 0.01b | 0.22 ± 0.03bc | |
| (E)2-Pentenal(M) | 228.052 | 1.10605 | 751.9 | strawberry, fruit, tomato | 0.85 ± 0.02b | 0.48 ± 0.02d | 0.74 ± 0.01c | 1.15 ± 0.02a | 0.87 ± 0.02b | 0.86 ± 0.04b | |
| (E)2-Pentenal(D) | 227.153 | 1.36108 | 750.8 | strawberry, fruit, tomato | 0.19 ± 0.01c | 0.09 ± 0.01d | 0.19 ± 0.02c | 0.49 ± 0.02a | 0.30 ± 0.03b | 0.27 ± 0.03b | |
| (Z)-4-Heptenal | 388.852 | 1.15067 | 901.8 | biscuit, cream | 0.94 ± 0.05e | 1.10 ± 0.01d | 1.46 ± 0.04c | 1.67 ± 0.01ab | 1.72 ± 0.04a | 1.62 ± 0.02b | |
| 2-Methyl-2-pentenal | 300.369 | 1.16082 | 829.5 | 0.12 ± 0.01d | 0.12 ± 0.02d | 0.15 ± 0.01c | 0.20 ± 0.01b | 0.17 ± 0.00c | 0.26 ± 0.01a | ||
| subtotal | 3.91 ± 0.05c | 2.67 ± 0.03d | 3.76 ± 0.05c | 5.47 ± 0.09a | 4.55 ± 0.11b | 4.56 ± 0.09b | |||||
| alcohol | 1-Octen-3-ol | 561.763 | 1.15598 | 996.7 | mushroom | 0.51 ± 0.01bc | 0.41 ± 0.07d | 0.49 ± 0.05c | 0.58 ± 0.02b | 0.73 ± 0.05a | 0.56 ± 0.02bc |
| 1-Penten-3-ol | 182.657 | 0.94698 | 690 | butter, pungent | 6.33 ± 0.14b | 6.08 ± 0.03c | 6.70 ± 0.07a | 6.64 ± 0.16a | 6.37 ± 0.08b | 6.75 ± 0.03a | |
| Ethanol | 99.226 | 1.05441 | 452.6 | sweet | 3.04 ± 0.06d | 4.19 ± 0.03b | 4.67 ± 0.10a | 3.32 ± 0.02c | 1.98 ± 0.03e | 3.13 ± 0.02d | |
| 1-Propanol | 129.12 | 1.11925 | 555.3 | alcohol, pungent | 1.13 ± 0.08bc | 0.80 ± 0.05d | 1.32 ± 0.08a | 1.20 ± 0.06abc | 1.25 ± 0.05ab | 1.10 ± 0.07c | |
| 1-Pentanol(M) | 245.925 | 1.25359 | 773 | balsamic | 1.32 ± 0.01a | 0.48 ± 0.01e | 0.59 ± 0.02d | 0.73 ± 0.01c | 0.76 ± 0.03b | 0.79 ± 0.01b | |
| 1-Pentanol(D) | 246.327 | 1.50933 | 773.4 | balsamic | 0.32 ± 0.01a | 0.06 ± 0.01e | 0.14 ± 0.01d | 0.16 ± 0.01c | 0.21 ± 0.01b | 0.15 ± 0.01 cd | |
| subtotal | 12.66 ± 0.18b | 12.01 ± 0.08c | 13.92 ± 0.23a | 12.64 ± 0.24b | 11.31 ± 0.08d | 12.48 ± 0.10b | |||||
| furfuran | 2-Pentylfuran | 557.164 | 1.25301 | 994.5 | green bean, butter | 0.48 ± 0.02a | 0.13 ± 0.01c | 0.13 ± 0.01c | 0.15 ± 0.01c | 0.19 ± 0.02b | 0.15 ± 0.02c |
| Tetrahydrofurane(M) | 151.961 | 1.06384 | 618.8 | 2.91 ± 0.35bc | 3.44 ± 0.05a | 3.10 ± 0.28ab | 2.44 ± 0.24d | 2.78 ± 0.17bcd | 2.57 ± 0.09 cd | ||
| Tetrahydrofurane(D) | 150.763 | 1.23049 | 615.7 | 1.14 ± 0.41b | 1.31 ± 0.08b | 2.20 ± 0.75a | 1.17 ± 0.42b | 1.78 ± 0.40ab | 0.92 ± 0.11b | ||
| subtotal | 4.53 ± 0.78ab | 4.87 ± 0.12ab | 5.42 ± 1.03a | 3.75 ± 0.65b | 4.75 ± 0.56ab | 3.63 ± 1.20b | |||||
| ketone | 2-Heptanone | 375.332 | 1.26028 | 892.7 | cream | 0.52 ± 0.03a | 0.24 ± 0.01c | 0.25 ± 0.01c | 0.31 ± 0.01b | 0.34 ± 0.02b | 0.33 ± 0.02b |
| 2-Butanone(M) | 133.983 | 1.06699 | 569.7 | fruit | 4.20 ± 0.13b | 5.51 ± 0.05a | 2.86 ± 0.03d | 2.88 ± 0.02d | 2.60 ± 0.02e | 3.27 ± 0.01c | |
| 2-Butanone(D) | 134.882 | 1.24516 | 572.3 | fruit | 6.40 ± 0.11c | 9.21 ± 0.22a | 5.71 ± 0.16e | 6.10 ± 0.07d | 5.60 ± 0.04e | 8.82 ± 0.07b | |
| Acetone | 104.919 | 1.12254 | 474.4 | Pungent,butter | 5.54 ± 0.22c | 9.15 ± 0.14a | 3.60 ± 0.16d | 3.36 ± 0.08d | 2.50 ± 0.09e | 6.80 ± 0.16b | |
| subtotal | 16.66 ± 0.27c | 24.11 ± 0.21a | 12.42 ± 0.27d | 12.65 ± 0.13d | 11.04 ± 0.13e | 19.22 ± 0.18b | |||||
The Ⅰ region represents the unique volatile flavor substances of frying treatment, including pentanol(M), pentanol(D), 1-propanolm, (E)-2-heptenal, 2-pentylfuran, and phenylacetaldehyde. The II region represents the unique flavor substances of microwave heating treatment, including (E)-2-pentenal(M), (E)-2-pentenal(D), (E)-2-hexenal(M), (E)-2- hexenal(D), 2-methylbutanal(M), 2-methylbutanal(D), 3-methylbutanal(M), and 3-methylbutanal(D). The III region represents the unique flavor substances of sous-vide cooking treatment, including heptanal(M), heptanal(D), n-nonanal, butanal, 1-octen-3-ol, octanal(M), octanal(D), and (Z)-4 -heptenal. The IV region represents the unique flavor substances of air frying treatment, including 2-butanone (M) and 2-butanone (D). Therefore, the characteristic volatile fingerprint of C. peled meat cooked by different methods are different from each other.
The volatile organic compound content in C. peled meat was further summarized and compared using the peak volume normalization method. It is observed in Table 3 that the content of aldehydes was the greatest in each sample, ranging from 53.89 % to 63.49 %. Aldehydes are formed mainly by oxidation and decomposition of fatty acids, and have a low threshold, indicating a greater impact on the overall flavor of fish samples (Yang et al., 2017). The content of hexanal (grass, fat) (monomer, dimer) was the highest. The content in groups steaming and sous-vide cooking was significantly higher than in the other cooking groups. High concentrations of aldehydes can produce an unpleasant spoilage smell (Ana et al., 2020). The content of total aldehydes in group sous-vide cooking was the highest (P < 0.05). The content in group roasting was the lowest, indicating that roasting was more conducive to the final product exhibiting a pleasing smell. Olefine aldehydes are derived mainly from the degradation of linoleic acid and linolenic acid, including (E)-2-heptenal, (E)-2-hexenal (monomer, dimer), (E) −2-pentenal (monomer, dimer), (Z)-4-heptenal, and 2-methyl-2-pentenal (Luo et al., 2022). The content of ketones was second only to that of aldehydes (11.04–24.11 %), including 2-heptanone (cream), 2-butanone (fruit) (monomer, dimer), and acetone (butter). The content in group roasting was significantly higher than in other groups. Studies have shown that ketones can reduce fish smell (Dong et al., 2018, Cui et al., 2020). Although the threshold of ketones was lower, it still made a positive contribution to the flavor of fish samples.
The content of alcohol substances ranked third (11.31– 13.92 %), including 1-octen-3-ol, 1-penten-3-ol, ethanol, n-propanol, and 1-pentanol (monomer, dimer). The content of 1-penten-3-ol (butter) and ethanol (sweet) was relatively high; the flavor threshold of alcohols is higher than that of aldehydes, which can impart buttery, sweet, and other odors to fish meat (Fratini et al., 2012). The content of furans was low, including 2-pentylfuran and tetrahydrofurane (monomer, dimer). There was no significant difference between cooking groups, indicating that different cooking treatments had little effect on furans in C. peled meat. Overall, the cookeing methods had a great influence on formation of certain volatile flavor compounds in C. peled meat. Sous-vide cooking treatment produced a higher content of aldehydes and more unpleasant odors. Roasting treatment facilitated formation of more positive odors in C. peled meat, probably because of combined effects of high temperature, protein denaturation, lipid oxidation, and Maillard reaction, etc. (Jin et al., 2021, Jing et al., 2022).
Data are expressed as mean ± standard error. Different letters in the same row indicate significant differences between groups (P < 0.05). The cooking methods A, B, C, D, E, and F stand for frying, roasting, steaming, microwave heating, sous-vide cooking, and air frying, respectively. M and D suffixed after the chemicals indicated monomer and dimer, respectively. Odor descriptions were searched from https://www.thegoodscentscompany.com/search2.html.
Conclusions
In summary, non-volatile (electric tongue, free amino acids, 5′-nucleotides, and organic acids, etc) and volatile flavor compounds (electric nose and GC-IMS) in C. peled meat cooked by different methods were detected and analyzed. In terms of non-volatile taste, the content of sweet free amino acids, 5′-nucleotides, and organic acids was higher in roasting samples. Sweet amino acids, umami glutamic acid, lactic acid, IMP, and inorganic ions were main taste active components in cooked C. peled meat. In terms of volatile odor, a total of 36 volatile flavor compounds were identified among different groups by GC-IMS technology, including 16 aldehydes, 7 olefine aldehydes, 6 alcohols, 4 ketones, and 3 furans. PCA of electronic nose detection results indicated that the flavor substances differed greatly from various cooking methods and could be well distinguished (the first two components accounted for 98.50 % and 0.97 %, respectively.). In general, roasting was the best method that gives more flavor substances in C. peled meat after cooking. These results might provide a reference for selection of thermal processing methods, and development of pre-cooked C. peled products in future.
Author contributions
Wengang Jin conducted the investigation, wrote the original draft, and performed plot analysis. Xinru Fan, Caiyan Jiang, Yang Liu, Kaiyue Zhu, and Xiaoqing Miao performed partial analysis, visualization, and the language check. Pengfei Jiang reviewed and edited the manuscript, supervised the work, and acquired funding.
CRediT authorship contribution statement
Wengang Jin: Investigation, Writing – original draft. Xinru Fan: Visualization, Data curation, Formal analysis. Caiyan Jiang: Visualization, Data curation, Formal analysis. Yang Liu: . Kaiyue Zhu: Visualization, Data curation, Formal analysis. Xiaoqing Miao: . Pengfei Jiang: Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Key Science and Technology Program of Liaoning Province (2020JH1/10200001), and China Scholarship Council (202008610071).
Data availability
The authors do not have permission to share data.
References
- Adeyeye E.I. Amino acid composition of three species of Nigerian fish: Clarias anguillaris, Oreochromis niloticus and Cynoglossus senegalensis. Food Chemistry. 2009;113(1):43–46. doi: 10.1016/j.foodchem.2008.07.007. [DOI] [Google Scholar]
- Ana B., María H.T., Iria S., Ortega M.J., Cristina G.G., Marina B.P. Types and distribution of bioactive polyunsaturated aldehydes in a gradient from mesotrophic to oligotrophic waters in the alboran ´ sea (western mediterranean) Marine Drugs. 2020;18(3):159–167. doi: 10.3390/md18030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y.L., Boeren S., Ertbjerg P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Science. 2018;135:102–108. doi: 10.1016/j.meatsci.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Chen G., Li J., Sun Z., Zhang S., Li G., Song C., et al. Rapid and sensitive ultrasonic-assisted derivatisation microextraction (UDME) technique for bitter taste- free amino acids (FAA) study by HPLC-FLD. Food Chemistry. 2014;143:97–105. doi: 10.1016/j.foodchem.2013.07.099. [DOI] [PubMed] [Google Scholar]
- Cui Z.K., Yan H., Manoli T., Mo H.Z., Li H.B., Zhang H. Changes in the volatile components of squid (illex argentinus) for different cooking methods via headspace–gas chromatography–ion mobility spectrometry. Food Science &. Nutrition. 2020;8(10):5748–5762. doi: 10.1002/fsn3.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.W., Li X., Zhao Y., Chen S.J., Yao H.Z., Wang H., et al. Effects of short-term low salinity stress on non-volatile flavor substances of muscle and hepatopancreas in Portunus trituberculatus. Journal of Food Composition and Analysis. 2022;109 doi: 10.1016/j.jfca.2022.104520. [DOI] [Google Scholar]
- Chew B.L., Fisk I.D., Fray R., Tucker G.A., Bodi Z., Ferguson A., et al. The effect of adenosine monophosphate deaminase overexpression on the accumulation of umami-related metabolites in tomatoes. Plant Cell Reports. 2017;36(1):81–87. doi: 10.1007/S00299-016-2058-z. [DOI] [PubMed] [Google Scholar]
- Deng X., Lei Y., Yu Y., Lu S., Zhang J. The discovery of proteins associated with freshness of coregonus peled muscle during refrigerated storage. Journal of Food Science. 2019;84(6):1266–1272. doi: 10.1111/1750-3841.14639. [DOI] [PubMed] [Google Scholar]
- Dong X.P., Li D.Y., Huang Y., Wu Q., Liu W.T., Qin L., et al. Nutritional value and flavor of turbot (Scophthalmus maximus) muscle as affected by cooking methods. International Journal of Food Properties. 2018;21(1):1972–1985. doi: 10.1080/10942912.2018.1494196. [DOI] [Google Scholar]
- Fratini G., Lois S., Pazos M., Parisi G., Medina I. Volatile profile of Atlantic shellfish species by HS-SPME GC/MS. Food Research International. 2012;48(2):856–865. doi: 10.1016/j.foodres.2012.06.033. [DOI] [Google Scholar]
- Fan X., Jin Z., Liu Y., Chen Y., Konno K., Zhu B., et al. Effects of super-chilling storage on shelf-life and quality indicators of Coregonus peled based on proteomics analysis. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110229. [DOI] [PubMed] [Google Scholar]
- Fedorov F.S., Yaqin A., Krasnikov D.V., Kondrashov V.A., Ovchinnikov G., Kostyukevich Y., et al. Detecting cooking state of grilled chicken by electronic nose and computer vision technique. Food Chemistry. 2021;345 doi: 10.1016/j.foodchem.2020.128747. [DOI] [PubMed] [Google Scholar]
- Feng P., Gao M., Burgher A., Zhou T.H., Pramuk K. A nine-country study of the protein content and amino acid composition of mature human milk. Food & Nutrition Research. 2016;60(1):31042. doi: 10.3402/fnr.v60.31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G., Grund B., Gassner A.-L., Menin L., Henry H., Bromirski M., et al. Validation of the mass-extraction-window for quantitative methods using liquid chromatography high resolution mass spectrometry. Analytical Chemistry. 2016;88(6):3264–3271. doi: 10.1021/acs.analchem.5b04689. [DOI] [PubMed] [Google Scholar]
- Guo X., Qiu H.H., Deng X.R., Mao X.Y., Guo X.B., Xu C.J., et al. Effect of chlorogenic acid on the physicochemical and functional properties of Coregonus Peled myofibrillar protein through hydroxyl radical oxidation. Molecules. 2019;24(17):3205. doi: 10.3390/molecules24173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Cai W., Shang S., Miao X., Dong X., Zhou D., et al. Comparative analysis of the flavor profile and microbial diversity of high white salmon (coregonus peled) caviar at different storage temperatures. LWT. 2022;169 doi: 10.1016/j.lwt.2022.114068. [DOI] [Google Scholar]
- Jiang X., McPhedran K.N., Hou X., Chen Y., Huang R. Assessment of the trace level metal ingredients that enhance the flavor and taste of traditionally crafted rice-based products. LWT. 2023 [Google Scholar]
- Jin W., Pei J., Chen X., Geng J., Chen D., Gao R., et al. Influence of frying methods on quality characteristics and volatile flavor compounds of giant salamander (Andrias davidianus) Meatballs. Journal of Food Quality. 2021;2021:1–10. doi: 10.1155/2021/8450072. [DOI] [Google Scholar]
- Jing B., Fan Y., Zhu L.L., Wang Y.Y., Hou H. Characteristic flavor of Antarctic krill (Euphausia superba) and white shrimp (Penaeus vannamei) induced by thermal treatment. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132074. [DOI] [PubMed] [Google Scholar]
- Li M., Yang R., Zhang H., Wang S., Chen D., Lin S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chemistry. 2019;290:32–39. doi: 10.1016/j.foodchem.2019.03.124. [DOI] [PubMed] [Google Scholar]
- Liang R., Lin S.Y., Chen D., Sun N. Differentiation of Penaeus vannamei from different thermal processing methods in physico-chemical, flavor and sensory characteristics. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132092. [DOI] [PubMed] [Google Scholar]
- Luo X.Y., Xiao S.T., Ruan Q.F., Gao Q., An Y.Q., Hu y., Xiong S.B. Differences in flavor characteristics of frozen surimi products reheated by microwave, water boiling, steaming, and frying. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131260. [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan L., Liu H.J., Liu H.Y., Zhou Y., Li M.N., et al. Analysis of the changes of volatile fl avor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Science and Human Wellness. 2022;12:173–182. doi: 10.1016/j.fshw.2022.07.035. [DOI] [Google Scholar]
- Ma M.M., Mu T.H., Zhou L. Identification of saprophytic microorganisms and analysis of changes in sensory, physicochemical, and nutritional characteristics of potato and wheat steamed bread during different storage periods. Food Chemistry. 2021;348(20) doi: 10.1016/j.foodchem.2020.128927. [DOI] [PubMed] [Google Scholar]
- Marhabaie M., Leeper T.C., Blackledge T.A. Protein composition correlates with the mechanical properties of spider (Argiope trifasciata) dragline silk. Biomacromolecules. 2014;15(1):20–29. doi: 10.1021/bm401110b. [DOI] [PubMed] [Google Scholar]
- Nieva-Echevarría B., Manzanos M.J., Goicoechea E., Guillén M.D. Changes provoked by boiling, steaming and sous-vide cooking in the lipid and volatile profile of European sea bass. Food Research International. 2017;99:630–640. doi: 10.1016/j.foodres.2017.06.043. [DOI] [PubMed] [Google Scholar]
- Pan J.F., Hui J., Shang M.J., Chang X., Lian H.L., Li H.W., et al. Physiochemical properties and tastes of gels from Japanese Spanish mackerel (Scomberomours niphonius) surimi by different washing processes. Journal of Texture Studies. 2018;49(6):578–585. doi: 10.1111/jtxs.12357. [DOI] [PubMed] [Google Scholar]
- Rocha R.A.R., Ribeiro M.N., Silva G.A., Rocha L.C.R., Pinheiro A.C.M., Nunes C.A., et al. Temporal profile of flavor enhancers MAG, MSG, GMP, and IMP, and their ability to enhance salty taste, in different reductions of sodium chloride. Journal of Food Science. 2020;85(5):1565–1575. doi: 10.1111/1750-3841.15121. [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Chen H.T., Sun B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS) Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Wang K.X., Lin X.Y., Zhao W., Fan X.R., Yu W.Y., Ma Z., et al. Low-temperature steaming improves eating quality of whitefish. Journal of Texture Studies. 2020;51(5):830–840. doi: 10.1111/jtxs.12540. [DOI] [PubMed] [Google Scholar]
- Wen X.Y., Chen A.H., Wu Y.P., Yang Y.Y., Xu Y.S., Xia W.S., et al. Comparative evaluation of proximate compositions and taste attributes of three Asian hard clams (Meretrix meretrix) with different shell colors. International Journal of Food Properties. 2020;23(1):400–411. doi: 10.1080/10942912.2020.1733015. [DOI] [Google Scholar]
- Yang Y., Zhang X., Wang Y., Pan D.D., Sun Y.Y., Cao J.X. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. European Journal of Lipid Science and Technology. 2017;119(10) doi: 10.1002/ejlt.201600512. 1600512. [DOI] [Google Scholar]
- Yang J., Mai R.J., Wu S.L., Dong H., Zhao W.H., Bai W.D. Characterization of flavor active volatile and non-volatile compounds in the Chinese dry-cured red drum (Sciaenops ocellatus) Journal of Aquatic Food Product Technology. 2022;31(2):200–213. doi: 10.1080/10498850.2021.2024635. [DOI] [Google Scholar]
- Yamaguchi H., Nakaya M., Kaneko G., Yoneda C., Mochizuki T., Fukami K., et al. Comparison in taste and extractive components of boiled dorsal muscle and broth from half-smooth golden puffer Lagocephalus spadiceus caught in Japan with those of the same fish imported. Fisheries Science. 2013;79(2):327–334. doi: 10.1007/s12562-012-0585-2. [DOI] [Google Scholar]
- Zhang N., Ayed C., Wang W., Liu Y. Sensory-guided analysis of key taste-active compounds in pufferfish (Takifugu obscurus) Journal of Agricultureal and Food Chemisty. 2019;67(50):13809–13816. doi: 10.1021/acs.jafc.8b06047. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma X., Dai Z. Comparison of nonvolatile and volatile compounds in raw, cooked, and canned yellowfin tuna (Thunnus albacores) Journal of Food Processing and Preservation. 2019;43(10):e14111. [Google Scholar]
- Zhao W.Y., Zhao M.Y., Wang K.X., Wei D.Y., Qin L., Dong X.P. Effect of frying methods on the eating quality of coregonus peled meat. Food Science. 2021;42(4):72–79. doi: 10.7506/spkx1002-6630-20190823-239. [DOI] [Google Scholar]
- Zhao T., Cao Z., Yu J., Weng X., Benjakul S., Guidi A., et al. Gas-phase ion migration spectrum analysis of the volatile flavors of large yellow croaker oil after different storage periods. Current Research in Food Science. 2022;5:813–822. doi: 10.1016/j.crfs.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Deng S., Liu Y., Huang F., Liu J., Han D., Zhang C., et al. Evaluation of volatile flavor compounds in bacon made by different pig breeds during storage time. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129765. [DOI] [PubMed] [Google Scholar]
- Zhang M.X., Wang X.C., Liu Y., Xu X.L., Zhou G.H. Isolation and identification of flavour peptides from Puffer fish (Takifugu obscurus) muscle using an electronic tongue and MALDI-TOF/TOF MS/MS. Food Chemistry. 2012;135(3):1463–1470. doi: 10.1016/j.foodchem.2012.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.