Graphical abstract
Keywords: Pea (Pisum sativum L.), Variety, Nutrient composition, Phenolic compounds, Antioxidant activities
Highlights
-
•
The nutrient content of the 10 pea varieties varies greatly.
-
•
UPLC-QTOF-MS and HPLC-QQQ-MS/MS were both adopted to analyze the phenolic compounds.
-
•
A total of 38 key compounds were identified, including 12 phenolic compounds.
-
•
TPC and protocatechuic acid showed positive correlation with antioxidant capacity.
-
•
Most different pea varieties could be distinguished from each other.
Abstract
In this study, ten pea (Pisum sativum L.) varieties were compared in their nutrient composition, phenolic compounds, antioxidant properties and their diversity were deciphered by multivariate analysis of correlation analysis and principal component analysis (PCA). The ten pea cultivars are rich in nutrients with different contents in lipid (0.57 to 3.52%), dietary fiber (11.34 to 16.13%), soluble sugar (17.53 to 23.99%), protein (19.75 to 26.48%) and starch (32.56 to 48.57%). Through the UPLC-QTOF-MS and HPLC-QQQ-MS/MS analysis, the ethanol extracts of ten peas mainly included 12 kinds of phenolic substances and showed good antioxidant activities on the 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC). The phenolic content and protocatechuic acid showed a positive correlation with antioxidant capacity. All results provide theoretical basis for the development and rational application of different varieties of peas and their related products.
1. Introduction
The peas (Pisum sativum L.) are the second largest grain legume, which are widely grown in Europe, North America and Asia due to their strong adaptability and high nutritional economic value (Robinson & Domoney, 2021). Peas contain abundant nutritional composition, including protein, dietary fiber, fatty acids, trace elements and phenolics etc, which received increasing attentions in recent years (Gao et al., 2022). However, the differences of gene specificity and the growing environment conditions are likely to result in significant differences in their nutritional composition (Gao et al., 2022; Robinson et al., 2021). For example, there are differences in starch content between two different pea phenotypes: smooth (smooth seed surface) pea and wrinkled pea (wrinkled seed surface). The wide variety of microclimates may lead to bean production with high variability in its chemical composition (Fan and Beta, 2017, Gao et al., 2022). Therefore, systematic analysis of nutritional differences among pea varieties will help us to rationally enrich and improve the nutritional value and product value of pea resources.
The nutritional composition of peas is rich and diverse, the most important components are protein, carbohydrate, fat and beneficial to human body trace elements. Firstly, as a sustainable source of dietary protein, the protein content is abundant in the peas which could provide considerable energy for animal and human (Gorissen et al., 2018). However, the protein composition of peas from different sources are different due to the complexity of protein genes encoding peas, and environmental factors (Robinson et al., 2021). In addition, peas are also an important source of dietary fiber, and different varieties of peas have significant differences in dietary fiber content. For example, the total content of dietary fiber in dried peas were range from 14 to 26 %, but the beans have 23–32 % total dietary fiber (Brummer, Kaviani, & Tosh, 2015). In addition, peas are also rich in iron, zinc, selenium, vitamins B and other trace elements related to human health, which can adequately meet the human body's micronutrient requirements (Robinson et al., 2021).
Apart to the regular nutrients above, previous researches also pointed that phenolics, such as quercetin, protocatechuic acid and resveratrol, were the most important antioxidant bioactive substances in legume crops (Amarowicz & Shahidi, 2017), and also showed various effects in protecting against the development of cancer (Duenas, Estrella, & Hernandez, 2004) as well as a variety of other diseases (Gonzalez et al., 2018, Zhao et al., 2020). Types and contents of phenolic substances in different bean species were different. For instance, navy, pinto, small and black beans showed a significant difference in total phenolic acids content levels of 8.34, 52.90, 10.30 and 63.80 mg GAE/g, respectively, which may be the underlying factors for their differences in physiological functions (Ampofo & Ngadi, 2020). At present, few studies have reported the identification of phenolics of different pea varieties and the correlation of their physiological functions, which limits the well-targeted and high-value application of pea products (Fahim, Attia, & Kamel, 2019).
Currently, ultra-high performance liquid chromatography coupled with quadrupole time-of-flight spectrometers (UPLC-QTOF-MS) and triple quadrupole mass spectrometry (HPLC-QQQ-MS/MS) methods are both reliable analytical approaches for quickly identification and quantify multiple target components in a variety of complex matrices (Gai et al., 2021, Deng et al., 2021). For they could significantly reduce the analysis time and give excellent sensitivity with extremely low quantitation limit (Gai et al., 2021, Romera et al., 2018). However, to the best of our knowledge, this is the first time using UPLC-QTOF-MS combined with HPLC-QQQ-MS/MS method for the systematic analysis of bioactive phenolics in peas natural resources.
In the present work, we evaluated the nutritional components (moisture, ash, lipid, protein, dietary fiber, starch, etc.) of ten different peas from China, and the phenolics were also qualitatively and quantitatively analysed by the UPLC-QTOF-MS and HPLC-QQQ-MS/MS. The antioxidant activities of phenolic compounds on DPPH, FRAP and ORAC were also explored, respectively. All results of this research could provide basic data support for the research and potential applications on the nutritional function of different varieties of pea.
2. Materials and methods
2.1. Raw materials
The ten varieties of peas were purchased from different areas in China. The detailed information as follows: ZW.6 (Zhong Wan No.6) and ZW.9 (Zhong Wan No.9) from Langfang City, Hebei Province; ZW.8 (Zhong Wan No.8), JSP (Jian She pea) and CSRP (Chang Shou Ren pea) from Suqian City, Jiangsu Province; DMP (Da Ma pea) and XMP (Xiao Ma pea) from Baiyin City, Gansu Province; HL604 (He Lan 604), ZSHP (Zhu Sha Hong pea) and HMP (Hei Mei pea) from Chongqing. All the peas were ground into fine powder size and stored at room temperature in sealed plastic bags prior for furtherly analysis.
2.2. Chemical reagents
The solution of Gallic acid, folin-phenol and rutin were purchased from Shanghai Yuanye Biotechnology Co., ltd. The phenol was provided by Aladdin Biotechnology Co., ltd. (Shanghai, China). The hydrochloric acid, sulfuric acid, petroleum ether (30 ∼ 60 ℃), copper sullipide, potassium sullipide, boric acid, sodium nitrite, sodium carbonate, aluminum chloride, and sodium hydroxide were purchased from Xilong Scientific Co., ltd. (Shanghai, China). Standards of the cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin (HPLC grade, percent purity was ≥ 99 %) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and solvents were of analytical grade.
2.3. Nutrient composition analysis
The content of moisture, ash, protein, fatty acid, lipid and amino acid composition were determined by the method previously described (Kan et al., 2017). Meanwhile, the content of dietary fiber, starch and amylopectin were detected by the total dietary fiber detection kit and megazyme amylose/amylopectin kit, respectively. The phenol–sulphuric acid method was also used for the soluble sugar content analysis (DuBois, Gilles, Hamilton, Rebers, & Smith, 1956).The atomic absorption spectrophotometer (Perkin-Elmer, Model 2380, USA) was used to determine the content of the mineral elements. In brief, 5 g of pea sample was mixed with 5 mL concentrated nitric acid in the digestion tube for 1.5 h of digestion, and then been cooled down and transferred into a 25 mL volumetric flask for elemental analysis.
2.4. Qualitative and quantitative analysis of phenolic compounds
2.4.1. The extraction of free phenolic compounds
The extraction of phenolic compounds in peas was according to the previously described method with some modification (Chandrasekara & Shahidi, 2010). In brief, peas powder (1.00 g, through a 60-mesh sieve) was mixed with methanol/water (10 mL, 70:30 v/v) solution and then placed in an ultrasonic bath for 25 min treatment at room temperature. Setting mixture aside for 10 min at −20 ℃. The supernatants were collected after centrifugation at 4500 rpm for 10 min, then concentrated by rotary evaporation and topped up to 25 mL, and analyzed for the extraction of free phenolic compounds after filtering through a 0.22 µm PTFE membrane filter. All samples were performed in triplicate.
2.4.2. UPLC-QTOF-MS analysis
The phenolic compounds of 10 varieties peas were determined by a Waters Acuity ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-QTOF-MS) system (Waters, Milford, MA, USA), which equipped with the Agilent Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 m) and the work settings as follows: the injection volume, 5 µL; column temperature, 35 ℃; flow rate, 0.2 mL/min; mobile phase A, ultrapure water containing 0.1 % (v/v) formic acid; mobile phase B, methanol solution containing 0.1 % (v/v) formic acid. The mass spectrometry performed by a quadrupole time of flight mass spectrometer (Agilent6538), equipped with an electrospray ion source (ESI), and full-scan negative ion mode scanning. The data were obtained in the mass range of 50–1700 m/z. The detector conditions were as follows: capillary voltage, 4.0 kV; sampling cone voltage, 60 V; extraction cone voltage, 40 V; the temperature of dry gas (N2), 350 ℃; the flow rate of the cone gas, 10.0 L/min; atomizing gas pressure, 40 psi; The voltage of cracking, 175 V; ramp collision energy, 20 eV.
2.4.3. HPLC-QQQ-MS/MS analysis
The HPLC system (Agilent 1200 infinity series) equipped with the Agilent Eclipse XDB-C18 chromatographic column (4.6 mm × 250 mm, 5 µm) was used for quantitative analysis of phenolic compounds in peas. The chromatographic conditions: the injection volume, 10 µL; column temperature, 35 ℃; flow rate, 0.3 mL/min; mobile phase A, ultrapure water containing 0.1 % (v/v) formic acid; mobile phase B, Acetonitrile solution. The mass spectrometry condition: capillary voltage, 4.0 kV; the injection cone voltage, 35 V; the temperature of the ion source, 150 ℃; the velocity of desolvation gas (N2), 900 L/h. The Acquisition B03.01 and Qualitative Analysis B07.00 were used for data acquisition and processing.
2.5. Analyses of total phenolic (TPC) and total flavonoid content (TFC)
The total content of phenolic compounds in peas were determined according to the colorimetric method described with some modifications (Kan et al., 2017). Firstly, 20 µL gallic acid standards (0.025–1.6 mg/mL) or samples were mixed with 20 µL Folin-Ciocalteu reagent (0.2 M) in a 96 well plate and reacted for 30 s at 37 ℃ in a temperature incubator (SANYO Electric Co., ltd., Osaka, Japan), and then 60 µL saturated sodium carbonate (Na2CO3, 0.1 g/mL) solution was added for reacting 15 min. The absorbance was measured at 764 nm using a ELX800 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). In addition, the total flavonoids content (TFC) was estimated using the colorimetric assay according to previous report with some modifications (Chang, Yang, Wen, & Chern, 2002). A volume of 5 µL rutin standard or sample solution was mixed with 10 µL sodium nitrite (0.05 g/mL) solution and 40 µL distilled water in 96 microporous plate for reacting 5 min at 37 ℃ and then 10 µL aluminum chloride (0.1 g/mL) was added to react for 6 min. Finally, 30 µL sodium hydroxide (0.1 M) solution was added and reacting for 15 min. The absorbance of the mixture was then measured at 510 nm using a ELX800 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). All the samples were analyzed in triplicate.
2.6. In vitro antioxidant activity
2.6.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity assay
DPPH assay was measured according to the modified method reported earlier (Fuhrman, Volkova, Suraski, & Aviram, 2001). In brief, the Trolox was dissolved in 70 % methanol solution and DPPH was dissolved in pure methanol solution. Then the 25 µL tested samples (1 mg/mL) or standard solution were fully mixed with 175 µL DPPH solution (150 µM) in 96-well plate. The absorbance value was measured at 517 nm after 30 min reaction in the dark at 25℃. Trolox was used as reference standard and the concentration of Trolox standard curve are 12.5, 100, 200, 400, 500 µmol/L. All the results were determined three times in parallel.
2.6.2. Ferric reducing antioxidant power (FRAP) assay
TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mM), acetate buffer (3.1 g C2H3NaO2·3H2O and 16 m C2H4O2) and FeCl3·6H2O solution (20 mM) were mixed at a ratio of 1:10:1 to prepare the FRAP reagent. The 180 µL FRAP solution, 5 µL distilled water and sample or FeSO4 standard solution with different concentrations (0.05, 0.1, 0.2, 0.4, 0.6, 0.8 mmol/L) were mixed gently in each detection hole of 96-well plate, the absorbance value was measured at 593 nm after incubating at 37 ℃ for 5 min. The determination results were expressed as mmol FeSO4/g, which was determined three times in parallel (Benzie & Strain, 1996).
2.6.3. Oxygen radical absorbance capacity (ORAC) assay
The ORAC test was conducted by using the Abcam kit. Briefly, 25 µL antioxidant standard solution or sample and 150 µL luciferin were added together into an all-black 96-well plate (special for fluorescence testing), and each test well was mixed thoroughly. After incubating at 37 ℃ for 30 min, 25 µL free radical initiator was added to each test well and mixed well. Under the condition of Ex/Em = 480/520 nm, the absorbance value was measured every 1 to 5 min for 120 min. All results were expressed as mmol Trolox/g and measured in parallel for three times.
2.6. Statistical analysis
All the results were carried out in triplicate and expressed as mean value ± standard deviations. The software adopts SPSS Statistics 22 (IBM software, USA) was used for the Statistical data analysis. Significant difference was defined at p < 0.05 by Tukey’s test. Pearson correlation was used to analyze the correlation between variables. Principle component analysis (PCA) was used to convert the original variables into linear combination to explain the relationship between variables.
3. Results and discussion
3.1. Analysis of nutritional composition of ten pea varieties
3.1.1. Macronutrients composition analysis
The results of macronutrients composition analysis are shown in Table 1, the moisture content of peas showed little difference (11.23–14.63 %), which were similar to that of kidney beans (8.98–12.01 %) previous reported (Kan et al., 2018). The ash content range of samples was about 2.48–3.41 %, and no significant difference was observed among the 10 samples, which were also as same as that of raw chickpea (3.13 %), lentil (2.57 %), and yellow pea (2.63 %) reported (Xu et al., 2019). As an important carbohydrate fractions, soluble sugar (20–24 %) and starch (32–48 %) occupied a large proportion in peas (Table 1). The significant differences in the soluble sugar content could be observed among HL604 (27.30 %) and ZSHP (17.53 %). In addition, the lipid content of ZW.6 (3.5 %) was the highest than other peas, while HMP had only 0.5 % content of lipid which was the lowest samples. All the lipid content of peas were lower than those of soybean (14.92–22.19 %) and black soybean (14.13–20.45 %) reported (Kan et al., 2018), which also was comply with its characteristics of high protein and low lipid. It is worth noting that the protein content of peas was abundant (mostly distributed between 21 and 26 %), and even reached the same basic level as the meat protein content (22 %) (Henchion, Hayes, Mullen, Fenelon, & Tiwari, 2017). Among these peas, CSRP had the highest protein content (26.48 %), while DMP (19.75 %) had the lowest. Previous studies have shown that pea protein has low allergenicity, high nutritional value and could effectively prevent kidney disease and reduce the risk of cardiovascular disease).
Table 1.
Macronutrients composition analysis of different varieties of peas (%).g
| Variety | Protein | Soluble Sugar | Starch | Moisture | Ash | Fat |
|---|---|---|---|---|---|---|
| JSP | 22.06 ± 0.38c | 23.99 ± 0.80b | 47.78 ± 0.29ab | 14.63 ± 0.12a | 2.53 ± 0.15e | 1.66 ± 0.11c |
| ZW.9 | 26.09 ± 0.43a | 23.98 ± 0.51b | 48.57 ± 0.22a | 13.96 ± 0.27b | 2.92 ± 0.07bc | 0.84 ± 0.07d |
| DMP | 19.75 ± 0.89d | 21.83 ± 0.26cd | 48.20 ± 0.15a | 11.23 ± 0.20e | 2.60 ± 0.05de | 2.23 ± 0.18b |
| XMP | 23.05 ± 0.46bc | 20.76 ± 0.10d | 42.72 ± 0.71d | 13.39 ± 0.13cd | 2.78 ± 0.01cd | 1.00 ± 0.05d |
| ZSHP | 22.25 ± 0.70c | 17.53 ± 0.84f | 46.96 ± 0.49bc | 13.68 ± 0.34bc | 2.71 ± 0.11cde | 1.29 ± 0.26d |
| HL604 | 22.27 ± 0.59c | 27.30 ± 0.19a | 45.83 ± 0.33c | 12.97 ± 0.26d | 3.41 ± 0.03a | 1.25 ± 0.18d |
| ZW.8 | 21.80 ± 0.16c | 24.02 ± 0.15b | 47.71 ± 0.41ab | 13.38 ± 0.32cd | 2.80 ± 0.04cd | 1.05 ± 0.24d |
| ZW.6 | 23.02 ± 1.37bc | 21.74 ± 0.60cd | 41.32 ± 0.89d | 13.09 ± 0.06d | 2.92 ± 0.01cd | 3.52 ± 0.26a |
| HMP | 24.25 ± 1.13b | 22.42 ± 0.35c | 45.97 ± 0.40bc | 11.45 ± 0.35e | 2.48 ± 0.08e | 0.57 ± 0.11e |
| CSRP | 26.48 ± 0.39e | 19.39 ± 0.54e | 32.56 ± 0.22e | 11.25 ± 0.10e | 3.06 ± 0.14b | 1.61 ± 0.31bc |
a-f Indicates that there are significant differences between different pea varieties with the same in (p < 0.05).
gData are presented as means ± SD (n = 3).
3.1.2. Principal component analysis
Due to the complexity of the nutrient content of different pea varieties, it is difficult to identify the variety of peas by a particular nutrient content. PCA is an effective mathematical method that reduces the dimensionality of multivariate data, while preserving most of the variance. To visualize and characterize the variance observed for macronutrients composition analyzed among peas, the multivariate analysis was carried by PCA. As shown in Fig. 1, the PCA scores plot of ten peas along with its macronutrient composition represented 63.8 % (PC 1) and 16.5 % (PC 2) of the total variances. The cumulative variance contribution of PC1 and PC2 was 80.3 % (>80 %). PCA results showed that ZW8 and DMP can be clearly distinguished from other varieties.
Fig. 1.
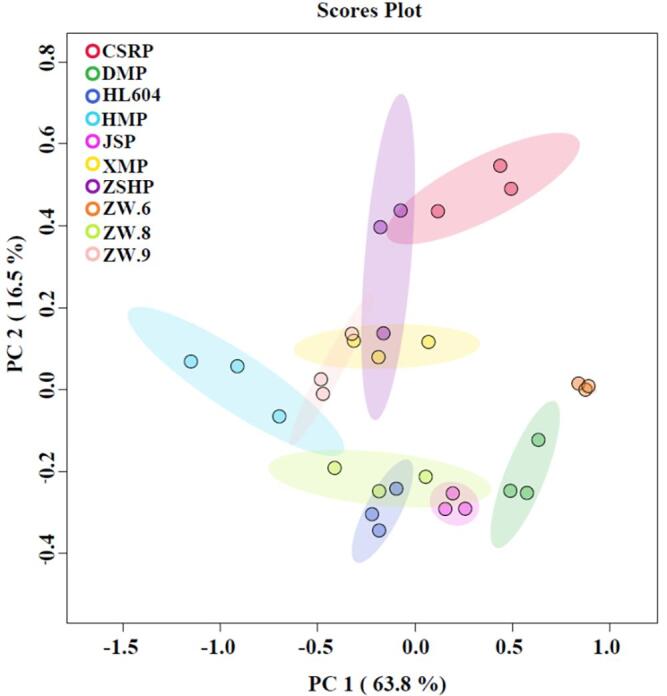
Principal component analysis between different varieties of peas based on macronutrients composition.
3.1.3. Amylose and dietary fiber content
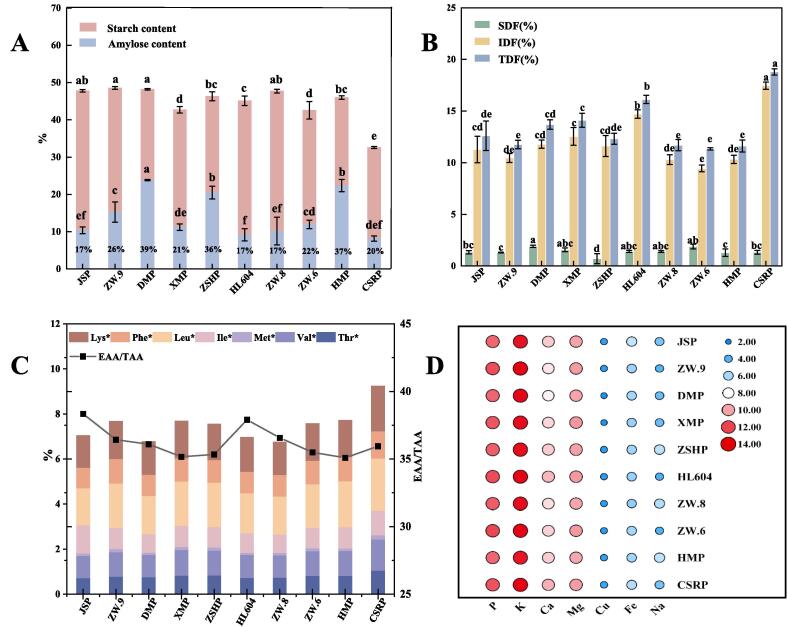
The results of Fig. 2A and Table S1 showed that starch was the highest nutrient component in peas. However, the amylose content in these peas presented a few difference, and the content value of DMP, ZSHP and HMP were 39.51 %, 39.51 % and 36.56 %, respectively, which were significantly higher than others (16.70–25.80 %) (p<0.05). Previous research had proved that the higher the amylose content in starch, the easier it would be convert into resistant starch (Faridah, Damaiyanti, Indrasti, Jayanegara, & Afandi, 2022), which could reduce the energy and blood sugar index of food effectively and increase the intake of human dietary fiber (Faridah et al., 2022). Moreover, Jiao et al. also found that high content of amylose in pea starch enable cellophane noodles excellent qualities, such as high elasticity, high shear strength and low breakage rate (Jiao et al., 2020). Consequently, DMP, ZSHP, HMP, ZW.6, ZW.9 are suitable raw material source of starch which used in food processing.
Fig. 2.
Nutrient composition analysis of different varieties of peas. (A) Starch and amylose. (B) Total dietary fiber (TDF), soluble dietary fiber (SDF) and insoluble dietary fiber (IDF); (C) Essential amino acids. Note: Thr = Threonin, Val = Valin, Met = Methionine, Ile = Isoleucine, Leu = Leucine, Phe = Phenylalanine, Lys = Lysine, EAA = Essential amino acid, TAA = Total amino acid; (D) Heat map of elements content.
The content of soluble dietary fiber (IDF), insoluble dietary fiber (SDF) and total dietary fiber content (TDF) of all peas were in range of 0.71–1.90 %, 9.45–17.46 % and 11.34–18.79 %, respectively (Fig. 2B and Table S1). Most varieties peas contained about 12 % of TDF, which was consistent with that of peas (11.7–14.81 %) reported previously (Stoughton-Ens, Hatcher, Wang, & Warkentin, 2010). CSRP had the highest content of TDF (18.79 %) including SDF (1.33 %) and IDF (17.46 %). While ZW.6 Peas had the lowest content of TDF (11.34 %). The conspicuous difference among these peas can be attribute to the environmental conditions and genetic characteristics of the species during the growing season as reported by the previous researches (Kan et al., 2018, Nikolopoulou et al., 2007).
3.1.4. Fatty acid and amino acid composition
The relative amount of unsaturated and saturated fatty acids is always used to evaluate the quality of oil products, for excessive intake of saturated fatty acids could cause some chronic diseases such as blood cholesterol, coronary heart disease and diabetes (Deron et al., 1998). The results of fatty acid composition in different peas are showed Table S2. The unsaturated fatty acid was about 90 %, and linoleic acid (C18:2n6c) was the main fatty acid (40 ∼ 47 %). The content of saturated fatty acid in peas was ranged from 5.54 to 8.12 % and the most component was stearic acid (C18:0) (4.42 ∼ 7.03 %). The results indicated that peas can be used as the safe food in our daily life due to its low content of saturated fatty acids.
In Fig. 2C and Table S3, the results of essential amino acid composition suggested that the ten kinds of peas contained same kinds of essential amino acids, and rich in Leucine (1.65 ∼ 2.31 %) and Lysine (1.42 ∼ 2.00 %), which were generally deficient in cereals (Gorissen et al., 2018). Besides, the JSP contained the highest proportion of essential amino acid (38.34 %), while HMP was the least (35.09 %). The 10 non-essential amino acids also have a similar composition (Table S3). The total amino acid content of peas was range from 18.37 % to 25.73 %, which was same as the chickpea grains reported, and the results were also consistent with the protein contents above (Nickhil et al., 2020).
3.1.5. Microelement content
The mineral elements in 10 peas were significant different (Fig. 2D and Table S4), the results indicated that CSRP and ZW.6 have the highest mineral element content among the ten pea species. It is worth noting that the content of Na and Fe contents are considerably different. The Na levels ranged from 8.20 to 55.97 mg/kg. In addition, Fe content in CSRP is the highest (101 mg/kg), while that in DMP is the lowest (45 mg/kg). These differences can be caused by the different field practices, cultivars, and soils (Pohl, Stelmach, Welna, & Szymczycha-Madeja, 2013). The results provide a certain reference for the establishment of fingerprints of different varieties pea.
In conclusion, nutritional content of different varieties peas presented significant differences. Pea protein in CSRP and DMP can be considered as an important source of human dietary protein. Meanwhile, DMP with the highest amylose content has potential for processing value.
3.2. UPLC-QTOF-MS analysis
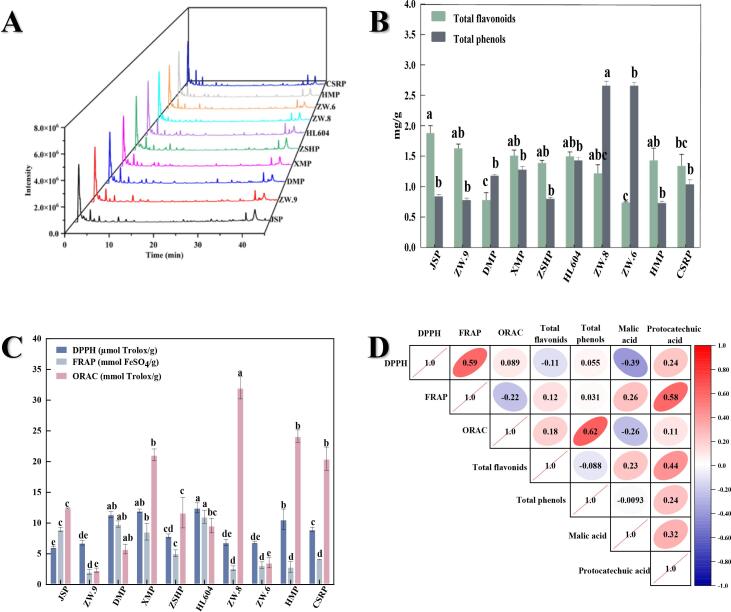
The phenolic compounds in peas are the important active substance, which was related with many health benefits. The crude phenolic compounds from peas were directly identified by UPLC-QTOF-MS and the identification of phenolic compounds was recognized by comparing retention time arrangement, peak spectra, mass-to-charge ratio, MS fragmentation, UV absorption spectrum, peak time of samples and standards, which were combined with information of database (Metlin) and related references (Peng, Li, Li, Deng, & Zhang, 2017). Taking the HL604 as an example, the total ion chromatogram of phenolic compounds and identification results from HL604 were shown in Fig. 3A and Table S6, and there were about 38 compounds were found through comparison with database (Metlin). The substances of each pea were not identical, which indicated that the germplasm resources have significant effects on the compound composition of the peas. In a word, 12 phenolic compounds were identified in the 10 pea cultivars, including pelargonidin 3-(6′'-p-coumarylglucoside)-5-(6′''-acetylglucoside), coumaric acid, protocatechuic acid, malic acid, quercetin di-pentoside, chinese wolfberry indican H, pyran galactose glucoside, (R)-Rutaretin 1′-(6′'-sinapoylglucoside), p-hydroxy benzoic acid, hexose gallic catechins, ganoderma lucidum acid H and pomelo peel. The types of phenolic compounds in ZW.8 were the most and the ZSHP were the least among all samples. The polysaccharide and the 2, 5- diketone gluconate were detected in all samples. besides, the HL604 and HMP had the highest oligosaccharide content, which are consistent with the above results of soluble sugar content (Table 1). The malic acid and 10 - methyl 17 alkanes acid were also found in the most varieties of peas. As shown in Table 2, the content of compounds in different varieties peas can be roughly estimated by the response value in the UPLC-QTOF-MS. The qualitative identification of phenolic substances in different pea varieties is summarized in the supplementary materials Table S6-S15.
Fig. 3.
(A) Total ion chromatogram of different varieties of pea; Total phenolic and flavonoid content (B) and antioxidant activity of different varieties of pea(C); (D) Correlation analysis between total phenols, total flavonoids, phenolic compounds and antioxidant activity.
Table 2.
Comparison of qualitative compound content in different varieties of pea.
| Name | DMP | HL604 | HMP | JSP | XMP | CSRP | ZW.8 | ZW.9 | ZW.6 | ZSHP |
|---|---|---|---|---|---|---|---|---|---|---|
| 3,6-dibromonaphthalene-2,7-diol | + | + | + | – | – | + | + | + | – | – |
| 1-Bromo-3-fluoropropan-2-ol | ++ | ++ | ++ | ++ | – | ++ | ++ | – | + | ++ |
| 1-Bromo-3-fluoropropane | ++ | ++ | ++ | ++ | ++ | ++ | ++ | – | ++ | – |
| 2,4,6-Trichlorophenol sodium salt | ++ | ++ | – | + | + | – | ++ | – | – | – |
| Benzaldehyde, 2-bromo- | – | – | – | – | – | – | ++ | – | + | – |
| Tetrasaccharide | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | ++ | ++ |
| Olisaccharide | +++ | ++++ | ++++ | ++ | ++ | +++ | +++ | ++ | +++ | +++ |
| Gluconic/galactonic acid | ++ | +++ | + | + | ++ | +++ | ++ | ++ | +++ | ++ |
| Trisaccharide | ++ | ++ | ++ | + | ++ | + | ++ | ++ | + | ++ |
| Itaconic acid | ++ | – | – | – | – | – | – | + | – | – |
| Malic acid | + | + | + | – | – | – | – | – | – | – |
| 2,5-diketogluconic acid | – | + | – | + | – | + | + | – | – | – |
| 3-Furoic acid | + | – | – | – | – | – | +++ | – | – | – |
| Protocatechuic acid | ++ | + | – | – | + | – | – | – | ||
| Trifluoro[(oxo-lambda ∼ 4 ∼ -sulfanylidene)amino]methane | ++ | ++ | ++ | ++ | +++ | – | + | + | + | – |
| 4-Hydroxybenzenesulfonic acid | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 2,4,6-Trinitrophenol--3-(1-methylazetidin-3-yl)pyridine (2/1) | + | – | – | – | – | – | + | – | – | – |
| Quercetin di-pentoside | ++ | +++ | – | ++ | – | + | + | – | – | – |
| 3-Amino-2,4,4,5,5-pentachlorocyclopent-2-en-1-one | + | ++ | + | – | – | – | – | – | – | – |
| 5-benzyl-2,4,6-trichloropyrimidine | ++ | + | + | + | ++ | + | + | ++ | – | – |
| Sodium thiocyanate | +++ | +++ | +++ | +++ | +++ | +++ | +++ | – | +++ | +++ |
| Methyl hydrogen sulfate | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ |
| 3,4,6-Trichloro-1-benzothiophene-2-carboxylic acid | ++ | ++ | – | – | – | – | ++ | – | ++ | – |
| 10-methyl-heptadecanoic acid | ++ | ++ | ++ | ++ | ++ | ++ | – | – | ++ | ++ |
| 4-bromo-2,5-difluorobenzoic acid | – | + | – | + | + | – | ++ | + | + | + |
| Galloc atechin hexoside | – | ++ | – | – | – | – | +++ | – | – | – |
| 3,4-Dichloro-5-[(3-iodoprop-2-yn-1-yl)oxy]furan-2(5H)-one | – | ++ | ++ | ++ | – | ++ | ++ | + | + | – |
| Soyasaponin Bb’ | – | ++ | – | – | + | + | – | – | – | – |
| Naringenin-4′-O-glucoside | – | + | – | – | – | – | + | – | – | – |
| Xanthine | – | – | + | – | – | + | – | – | – | – |
| 2-Nitrophenyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-beta-d-galactopyranoside | – | – | + | – | – | – | – | ++ | – | – |
| Acetamide, N-(4-chloro-2-(2-chlorobenzoyl)phenyl)-2-(cyclopentylamino)-N-methyl-, monohydrochloride | – | – | + | – | – | + | – | – | – | + |
| 1H,1H-Perfluoro-1-decanol | – | – | ++ | – | – | – | ++ | – | – | – |
| Bardoxolone methyl | – | – | + | – | – | – | + | – | + | – |
| Gymnodimine | – | – | + | – | – | – | – | – | ++ | – |
| Octadeca-11,13,15-trienoic acid Pinolenic Acid 1,4-Benzenediol, 2-dodecyl- |
– | – | ++ | ++ | ++ | – | – | ++ | – | – |
| 2-(1-Phenylhexyl)anthracene | – | – | +++ | – | ++ | – | +++ | +++ | +++ | +++ |
| Dipentyl 2-benzylbutanedioate | – | – | + | – | + | – | + | – | – | – |
| Ganoderic acid H | – | – | +++ | – | – | – | – | – | – | – |
| Didodecylbenzenesulphonic acid | – | – | ++ | ++ | – | – | – | – | ++ | – |
| 5-Bromo-2-(bromomethyl)-1,3-dinitrobenz | – | – | – | +++ | – | – | – | – | – | + |
| 4,6-Pyrimidinedicarboxamide | – | – | – | ++ | – | – | – | – | – | – |
| Aquayamycin | – | – | – | ++ | – | – | – | – | – | – |
| 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-3,5-bis(trifluoromethyl)-1H-pyrazole | – | – | – | ++ | – | + | – | – | + | – |
| (4-tert-Butylphenyl)(diphenyl)sulfanium pyrene-1-sulfonate | – | – | – | ++ | – | – | – | – | – | – |
| Saponin E | – | – | – | + | – | – | – | – | – | + |
| 4,4′-(1-Methyldecylidene)bisphenol | – | – | – | ++ | – | – | – | – | – | ++ |
| Pelargonidin 3-(6′'-p-coumarylglucoside)-5-(6′''-acetylglucoside) | – | – | – | – | ++ | ++ | ++ | – | + | – |
| Thielavin B | – | – | – | – | + | – | – | – | – | – |
|
– | – | – | – | ++ | – | +++ | – | – | – |
| Potassium 2,4,6-trichlorophenate | – | – | – | – | – | – | ++ | – | + | – |
|
– | – | – | – | – | – | – | – | ++ | +++ |
| (±)12,13-DiHOME | – | – | – | +++ | – | – | – | – | – | +++ |
-Data was not detected.
+, ++, +++ and ++++ The degree of substance content.
3.3. HPLC-QQQ-MS/MS analysis
Coumaric acid, protocatechuic acid, rosemary acid, pomelo peel and malic acid were the common five phenolic compounds in pea and which were selected for the qualitative analysis. Precursor ions, product ions and collision energy of quantitative substances are shown in Table S16. Contents were quantified with external standard method, and the concentration of standards were configured to 5–1000 ng/mL. The detection limit and quantification of the five phenolic compounds were as follows: coumaric acid (1.78, 5.92 ng/mL), protocatechuic acid (3.14, 10.46 ng/mL), rosemary acid (1.09, 3.62 ng/mL), pomelo peel (0.72, 2.40 ng/mL) and malic acid (25, 84 ng/mL).
Quantitative analysis results of phenolic compounds in different varieties of pea are shown in Table 3. The results indicated that the main ingredient of sample was malic acid, and the content range was from 17.25 to 56.62 µg/g. The JSP contained the highest amount of 56.62 µg/g, while the lowest was ZW.6 (17.25 μg/g). Meanwhile, there was no malic acid detected in the XMP and HMP. The protocatechuic acid content in all the samples were ranged from 2.63 to 16.64 µg/g, which have been reported to be a common phenolic widely found in legumes (Troszyńska, Estrella, López-Amóres, & Hernández, 2002). The highest content of coumaric acid was found in ZSHP (3.40 µg/g), and that of XMP was lowest (0.08 µg/g). The rosemary acid was detected in ZSHP and HMP, and both contents were 0.09 µg/g. In general, the contents of five phenolic compounds in ten pea varieties were obviously different, which is also one of the important factors leading to their different antioxidant activities and biological activities.
Table 3.
Quantitative analysis results of phenolic compounds in different varieties of pea (µg/g).h
| Variety | Coumaric acid | Malic acid | Protocatechuic acid | rosemary acid | pomelo peel |
|---|---|---|---|---|---|
| JSP | 1.15 ± 0.03d | 56.62 ± 0.09d | 10.87 ± 0.22d | ndi | ndi |
| ZW.9 | 0.52 ± 0.03c | 28.28 ± 0.37b | 2.94 ± 0.04a | ndi | ndi |
| DMP | nd | 33.95 ± 1.56b | 5.57 ± 0.15b | ndi | ndi |
| XMP | 0.08 ± 0.01a | nd | 11.51 ± 0.29d | ndi | ndi |
| ZSHP | 3.40 ± 0.07g | 42.66 ± 0.10c | 16.64 ± 0.60f | 0.09 ± 0.00a | ndi |
| HL604 | 0.61 ± 0.01c | 36.76 ± 2.34b | 14.62 ± 1.34e | ndi | 0.17 ± 0.03a |
| ZW.8 | 3.00 ± 0.05f | 27.48 ± 0.32b | 8.26 ± 0.06c | ndi | ndi |
| ZW.6 | 0.16 ± 0.04ab | 17.25 ± 0.17a | nd | ndi | ndi |
| HMP | 0.21 ± 0.04b | nd | 2.63 ± 0.05a | 0.09 ± 0.01a | ndi |
| CSRP | 1.34 ± 0.07e | 47.68 ± 0.39cd | 3.52 ± 0.08a | ndi | ndi |
a-f Indicates that there are significant differences between different pea varieties with the same in (p < 0.05).
h Data are presented as means ± SD (n = 3).
I nd, not detected or lower than limit of quantification.
3.4. TPC and TFC content
TPC and TFC are the most prominent antioxidant bioactive components of pea, which can be applied as antioxidants in food products (Zhao et al., 2021). TPC and TFC of different peas species are shown in Fig. 3B and Table S5. The TPC range of the samples was 0.66 ∼ 2.66 mg/g, which was similar to the kidney beans (2.07 ± 0.09 mg/g) previous reported (Zhu, Li, Deng, Li, & Zhang, 2020). The highest TPC was found in ZW.8 (2.66 mg /g), which was 2 ∼ 3 times higher than that of other peas. The TFC of peas at the range of 0.74 ∼ 1.88 mg /g, which was higher than Soybean (0.012 ± 0.001) reported (Zhu et al., 2020). The JSP contained the most flavonoids content (1.88 mg /g) among those peas. This result shows that peas are rich in flavonoids and phenolic compounds. It was also worth noting that the content and type of phenolic compounds vary with pea varieties, which was in accordance with previous reported that green lentils had the highest phenolic compounds among fourteen Canadian legume, while split red lentil had the lowest (Padhi, Liu, Hernandez, Tsao, & Ramdath, 2017).
3.5. Measurement of antioxidant activity
DPPH is a free radical with unpaired valence electrons in an atom on the nitrogen bridge, which has been widely used to reflect the free radical scavenging abilities of antioxidants (Eklund et al., 2005). There were significant differences among those samples, and the DPPH scavenging activities ranged from 6.06 to 12.49 µmol Trolox/g. The highest antioxidant activity of pea was the HL604 (12.49 µmol Trolox/g), the lowest value was found in the JSP (6.06 µmol Trolox/g) (Fig. 3C and Table S5). In addition, the FRAP value of samples ranged from 2.08 to 11.04 mmol Fe (II)/g and the value of HL604 reached to 11.04 mmol Fe (II)/g, which was consistent with DPPH scavenging activities. The lowest one was found in ZW.9 (2.08 Fe (II)/g). The ORAC value is also an effective method to determine the antioxidant activity of the extracts from peas, and the ORAC value ranged from 2.34 to 32.01 mmol Trolox/g. In the tested samples, the highest ORAC value was found in ZW.8 (32.01 mmol Trolox/g), which was nearly 15 times higher than the lowest (ZW.9, 2.34 mmol Trolox /g). Our results of antioxidant activity were higher than that of previous studies. Wojciech Rybiński et al. studied 30 grass pea (Lathyrus sativus) varieties and the results showed that the TEAC and FRAP were 0.015–0.037 mmol Trolox/g and 0.045–0.120 mmol Fe2+/g, respectively. (Rybiński, Karamać, Sulewska, Börner, & Amarowicz, 2018). Furthermore, Kan et al. reported that the DPPH scavenging activity of 26 kidney beans were from 1.07 to 7.48 μmol TE/g DW. (Kan et al., 2017).
According to above results, the phenolic compounds extracted from different peas showed significant differences in in vitro antioxidant activities, which might be due to the structure of antioxidants in peas is different and varied, and the mechanism of action for the removal of free radicals is different, so there is a certain selectivity for the removal of different free radicals. Previous studies also indicated that the extraction method has certain influence on the extraction of active substances from pea, and the difference in solubility of different phenols in methanol is also one of the reasons for the difference in antioxidant activity (Ren & Claire, 2021).
3.6. Correlation analysis
Correlation heat map between TPC, TFC content, phenolic compounds and antioxidant activity is shown in Fig. 3D (p<0.05). Since the content of rosemary acid and pomelo peel were below the limit of detection, the phenolic compounds of malic acid and protocatechuic acid were selected for correlation analysis with antioxidant activity. There is a positive correlation between TPC, TFC and oxygen radical absorption capacity of pea extracts. The results also showed that positive correlations were observed between all antioxidant capacity and protocatechuic acid. Therefore, we can find that due to different reactive oxygen species and differences in reaction mechanisms, selecting a single method for antioxidant capacity evaluation is not comprehensive.
4. Conclusion
In this work, ten varieties of peas from different parts of China were compared in their nutritional composition, main phenolic substances content and antioxidant capacities. CSRP had the highest protein content and JSP contained the highest proportion of essential amino acid, which could effectively supplement rich protein and amino acid food source. Furthermore, CSRP had the highest content of TDF and ZW.6 Peas showed the lowest content of TDF, which also provides a basis for us to develop low GI foods. PCA results showed that ZW8 and DMP can be clearly distinguished from other varieties. Besides, the content of soluble phenolic compounds in HL604 Peas was abundant, which mainly including malic acid, protocatechin, (p-coumaric acid and naringin. The in vitro antioxidant tests further proved that HL604 peas had the stronger radical scavenging capability in DPPH and FRAP. TPC and protocatechuic acid showed a positive correlation with antioxidant capacity. In summary, this research provided a theoretical basis for the development of novel legume-based foods with good processing and health characteristics.
CRediT authorship contribution statement
Shi-Kang Chen: Conceptualization, Investigation, Writing – original draft. Hai-Feng Lin: Investigation, Data curation. Xin Wang: Writing – review & editing. Yi Yuan: Writing – review & editing. Jun-Yi Yin: Conceptualization, Writing – review & editing, Supervision. Xiao-Xiao Song: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financed by the National Key R&D Program of China (2018YFE0108300) and Technological Innovation Guidance Science and Technology Project of Jiangxi Province (20192AEI91004), China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100599.
Contributor Information
Jun-Yi Yin, Email: yinjy@ncu.edu.cn.
Xiao-Xiao Song, Email: songxiaoxiao@ncu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Amarowicz R., Shahidi F. Antioxidant activity of broad bean seed extract and its phenolic composition. Journal of Functional Foods. 2017;38:656–662. doi: 10.1016/j.jff.2017.04.002. [DOI] [Google Scholar]
- Ampofo J.O., Ngadi M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrasonics Sonochemistry. 2020;64 doi: 10.1016/j.ultsonch.2020.104974. [DOI] [PubMed] [Google Scholar]
- Benzie I., Strain J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brummer Y., Kaviani M., Tosh S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Research International. 2015;67:117–125. doi: 10.1016/j.foodres.2014.11.009. [DOI] [Google Scholar]
- Chandrasekara A., Shahidi F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. Journal of Agricultural and Food Chemistry. 2010;58(11):6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- Chang C., Yang M., Wen H., Chern J. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. doi: 10.3168/jds.S0022-0302(02)74323-3. [DOI] [Google Scholar]
- Deng J., Xiang Z., Lin C., Zhu Y., Yang K., Liu T.…Zhu B. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT. 2021;151 doi: 10.1016/j.lwt.2021.112001. [DOI] [Google Scholar]
- Deron D., Fernstrom H., Campos H., Blanche P., Williams P., Krauss R. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. American Journal of Clinical Nutrition. 1998;67(5):828–836. doi: 10.1079/NRR19980009. [DOI] [PubMed] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Duenas M., Estrella I., Hernandez T. Occurrence of phenolic compounds in the seed coat and the cotyledon of peas (Pisum sativum L.) European Food Research and Technology. 2004;219(2):116–123. doi: 10.1007/s00217-004-0938-x. [DOI] [Google Scholar]
- Eklund P.C., Långvik O.K., Wärnå J.P., Salmi T.O., Willför S.M., Sjöholm R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Organic & Biomolecular Chemistry. 2005;3(18):3336–3347. doi: 10.1039/b506739a. [DOI] [PubMed] [Google Scholar]
- Fahim J.R., Attia E.Z., Kamel M.S. The phenolic profile of pea (Pisum sativum): A phytochemical and pharmacological overview. Phytochemistry Reviews. 2019;18(1):173–198. doi: 10.1007/s11101-018-9586-9. [DOI] [Google Scholar]
- Fan G., Beta T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. Journal of Food Composition and Analysis. 2017;62:217–222. doi: 10.1016/j.jfca.2017.07.001. [DOI] [Google Scholar]
- Faridah D.N., Damaiyanti S., Indrasti D., Jayanegara A., Afandi F.A. Effect of heat moisture treatment on resistant starch content among carbohydrate sources: A meta-analysis. International Journal of Food Science & Technology. 2022;57(4):1965–1974. doi: 10.1111/ijfs.15276. [DOI] [Google Scholar]
- Fuhrman B., Volkova N., Suraski A., Aviram M. White Wine with Red Wine-like Properties: Increased Extraction of Grape Skin Polyphenols Improves the Antioxidant Capacity of the Derived White Wine. Journal of Agricultural and Food Chemistry. 2001;49(7):3164–3168. doi: 10.1021/jf001378j. [DOI] [PubMed] [Google Scholar]
- Gai Q., Jiao J., Wang X., Fu Y., Lu Y., Liu J.…Xu X. Simultaneous quantification of eleven bioactive phenolic compounds in pigeon pea natural resources and in vitro cultures by ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-QqQ-MS/MS) Food Chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127602. [DOI] [PubMed] [Google Scholar]
- Gao L., Wu Y., Wan C., Wang P., Yang P., Gao X.…Gao J. Structural and physicochemical properties of pea starch affected by germination treatment. Food Hydrocolloids. 2022;124 doi: 10.1016/j.foodhyd.2021.107303. [DOI] [Google Scholar]
- Gonzalez A.L., Ciocci P.A., Fantinelli J.C., Schinella G.R., Mosca S.M., Rios J.L. Cardioprotection and natural polyphenols: An update of clinical and experimental studies. Food & Function. 2018;9(12):6129–6145. doi: 10.1039/c8fo01307a. [DOI] [PubMed] [Google Scholar]
- Gorissen S.H.M., Crombag J.J.R., Senden J.M.G., Waterval W.A.H., Bierau J., Verdijk L.B., van Loon L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchion M., Hayes M., Mullen A., Fenelon M., Tiwari B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods. 2017;6(7):53. doi: 10.3390/foods6070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao A., Yang Y., Li Y., Chen Y., Xu X., Jin Z. Structural properties of rice flour as affected by the addition of pea starch and its effects on textural properties of extruded rice noodles. International Journal of Food Properties. 2020;23(1):809–819. doi: 10.1080/10942912.2020.1761830. [DOI] [Google Scholar]
- Kan L., Nie S., Hu J., Wang S., Bai Z., Wang J.…Song K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chemistry. 2018;260:317–326. doi: 10.1016/j.foodchem.2018.03.148. [DOI] [PubMed] [Google Scholar]
- Kan L., Nie S., Hu J., Wang S., Cui S.W., Li Y.…Xie M. Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food and Chemical Toxicology. 2017;108:467–477. doi: 10.1016/j.fct.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Nickhil C., Debabandya M., Abhijit K., Saroj K.G., Manoj K.T., Yogesh S. Gaseous ozone treatment of chickpea grains, part I: Effect on protein, amino acid, fatty acid, mineral content, and microstructure. Food Chemistry. 2020;345 doi: 10.1016/j.foodchem.2020.128850. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou D., Grigorakis K., Stasini M., Alexis M.N., Iliadis K. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chemistry. 2007;103(3):847–852. doi: 10.1016/j.foodchem.2006.09.035. [DOI] [Google Scholar]
- Padhi E.M.T., Liu R., Hernandez M., Tsao R., Ramdath D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. Journal of Functional Foods. 2017;38:602–611. doi: 10.1016/j.jff.2016.11.006. [DOI] [Google Scholar]
- Peng H., Li W., Li H., Deng Z., Zhang B. Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr) Journal of Functional Foods. 2017;32:296–312. doi: 10.1016/j.jff.2017.03.003. [DOI] [Google Scholar]
- Pohl P., Stelmach E., Welna M., Szymczycha-Madeja A. Determination of the Elemental Composition of Coffee Using Instrumental Methods. Food Analytical Methods. 2013;6(2):598–613. doi: 10.1007/s12161-012-9467-6. [DOI] [Google Scholar]
- Ren Y., Claire T.Z.Y. A current review of structure, functional properties, andindustrial applications of pulse starches for value-addedutilization. Comprehensive Reviews in Food Science and Food Safety. 2021;20(3):3061–3092. doi: 10.1111/1541-4337.12735. [DOI] [PubMed] [Google Scholar]
- Robinson G.H.J., Domoney C. Perspectives on the genetic improvement of health- and nutrition-related traits in pea. Plant Physiology and Biochemistry. 2021;158:353–362. doi: 10.1016/j.plaphy.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera D., Mateo E.M., Mateo-Castro R., Gómez J.V., Gimeno-Adelantado J.V., Jiménez M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chemistry. 2018;267:140–148. doi: 10.1016/j.foodchem.2017.11.040. [DOI] [PubMed] [Google Scholar]
- Rybiński W., Karamać M., Sulewska K., Börner A., Amarowicz R. Antioxidant Potential of Grass Pea Seeds from European Countries. Foods. 2018;7(9):142. doi: 10.3390/foods7090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoughton-Ens M.D., Hatcher D.W., Wang N., Warkentin T.D. Influence of genotype and environment on the dietary fiber content of field pea (Pisum sativum L.) grown in Canada. Food Research International. 2010;43(2):547–552. doi: 10.1016/j.foodres.2009.07.011. [DOI] [Google Scholar]
- Troszyńska A., Estrella I., López-Amóres M.L., Hernández T. Antioxidant Activity of Pea (Pisum sativum L.) Seed Coat Acetone Extract. LWT - Food Science and Technology. 2002;35(2):158–164. doi: 10.1006/fstl.2001.0831. [DOI] [Google Scholar]
- Xu M., Jin Z., Simsek S., Hall C., Rao J., Chen B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chemistry. 2019;295:579–587. doi: 10.1016/j.foodchem.2019.05.167. [DOI] [PubMed] [Google Scholar]
- Zhao X., Sun L., Zhang X., Wang M., Liu H., Zhu Y. Nutritional components, volatile constituents and antioxidant activities of 6 chickpea species. Food Bioscience. 2021;41 doi: 10.1016/j.fbio.2021.100964. [DOI] [Google Scholar]
- Zhao D., Simon J.E., Wu Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Critical Reviews in Food Science and Nutrition. 2020;60(4):597–625. doi: 10.1080/10408398.2018.1546668. [DOI] [PubMed] [Google Scholar]
- Zhu L., Li W., Deng Z., Li H., Zhang B. The Composition and Antioxidant Activity of Bound Phenolics in Three Legumes, and Their Metabolism and Bioaccessibility of Gastrointestinal Tract. Foods. 2020;9(12):1816. doi: 10.3390/foods9121816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.