Abstract
As the most common sustained arrhythmia, AF is a complex clinical entity which remains a difficult condition to durably treat in the majority of patients. Over the past few decades, the management of AF has focused mainly on pulmonary vein triggers for its initiation and perpetuation. It is well known that the autonomic nervous system (ANS) has a significant role in the milieu predisposing to the triggers, perpetuators and substrate for AF. Neuromodulation of ANS – ganglionated plexus ablation, vein of Marshall ethanol infusion, transcutaneous tragal stimulation, renal nerve denervation, stellate ganglion block and baroreceptor stimulation – constitute an emerging therapeutic approach for AF. The purpose of this review is to summarise and critically appraise the currently available evidence for neuromodulation modalities in AF.
Keywords: Cardioneuroablation, atrial fibrillation, ganglionated plexus, autonomic nervous system
AF is a complex clinical entity which remains a difficult condition to durably treat in the majority of patients despite an improved understanding of its pathogenesis in the past two decades. AF is the most common sustained arrhythmia and is estimated to affect 37.5 million adults worldwide, with projections for its prevalence to continue to rise, corresponding with the increasing frequency of the risk factors related to its development.[1] AF diagnosis and treatment is associated with substantial financial cost, morbidity and mortality.[2–6] For these reasons, finding effective management strategies remains paramount.
Over the decades, the management of AF has attempted to evolve to match the increasing understanding of the triggers for its initiation and perpetuation, with a heightened role of catheter-based ablative strategies.[7–9] While traditional focus has been on pulmonary vein isolation (PVI), more recent advanced approaches, including isolation of the posterior wall of the left atrium, ablation of the vein of Marshall (VoM), superior vena cava isolation, left atrial appendage isolation, rotor mapping and ablation, non-PV trigger ablation, scar homogenisation and other strategies, have been explored to limit recurrence in those with persistent forms of AF but with only incremental additional effectiveness compared with PVI alone in clinical studies.[10–26] More recently, novel energy sources and mapping algorithms have also been explored for AF catheter ablation.[27–33] Even with more sophisticated approaches, superior long-term effective treatment for AF above what is achievable with PVI alone has been a challenge.[34–38]
However, could a key missing link lie outside the heart itself and be nestled within the nervous system? It is well understood that the autonomic nervous system (ANS) plays a dominant role in several arrhythmias, including AF, via a complex network of neural inputs, outputs and plexi, and is often an untargeted trigger in many management modalities.
Modulating, or in some instances eliminating, key neural connections to the heart have been studied as a means to reduce AF burden including vagal nerve stimulation and ablation of ganglionated plexi (GPs) located within or near the heart, but it is not common practice.[39–45] As the techniques for ablation of GPs are relatively straightforward and have more recently become better elucidated with emerging operator experience with cardioneuroablation in selected patients with vasovagal syncope, interest in GP ablation has grown among the electrophysiology community as an adjunctive approach to PVI for catheter ablation of AF patients.[46–49] However, there are many questions that need to be examined surrounding neuromodulation endpoints in catheter ablation procedures.[50] In this review, we explore the potential role of neuromodulation in the management of AF, including its principles, key technical considerations, shortcomings and potential future advances.
The Heart’s Little Brain: Regulator of Heart Function and Trigger of Arrhythmias
Through centuries of observation and experimentation, the intricate hierarchy of reflex arcs connecting higher centres in the medulla, hypothalamus, thalamus, amygdala cerebral cortex and thoracic ganglia with the heart have been elucidated. The extrinsic domain of the cardiac nervous system includes the autonomic ganglia and nerve fibres en route to the heart. A ganglion is a cluster of neuronal cell bodies outside the brain.[51–53] In the ANS, efferent axons from the ganglion to the effector organ are called postganglionic nerve fibres. While postganglionic nerve fibres of the sympathetic division extend from the sympathetic chain or paravertebral ganglia to the heart, ganglia of the parasympathetic division and their associated clusters of nerve cell bodies (GPs) – which are considered intrinsic – are distributed mainly within the epicardial area. From there the post-ganglionated intrinsic nerves extend towards specific atrial or ventricular regions around the sinoatrial node, the roots of caval and PVs, and near the atrioventricular node.
Location of Ganglionated Plexi Within the Heart
Several nests of ganglionated plexi have been identified within the heart via gross anatomical and histological studies which have defined the distribution of intrinsic cardiac ganglia in experimental and clinical studies:
the superior (anterior) right atrial GP (RSGP) located on the posterosuperior surface of the right atrium (RA) adjacent to the junction of the superior vena cava (SVC) and the RA;
the inferior (posterior) right atrial GP (RIGP) located adjacent to the interatrial groove;
the superior left atrial GP (LSGP) on the posterosuperior surface of the left atrium (LA) between the PVs;
the posterolateral (inferior) left atrial GP (LIGP) is identified on the posterolateral surface of the LA;
the posteromedial left atrial GP (PMLGP) on the posteromedial surface of the LA; and
the interatrial septal GP consisting of fusion and extensions of RIGP and PMLGP (Figures 1 and 2).[54,55]
Figure 1: The Schematic View of Ganglionated Plexi.
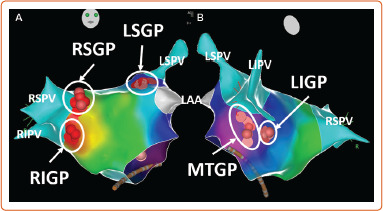
Pink and red dots show distribution of ablation points based on fragmented bipolar electrograms. LAA = left atrial appendage; LIGP = inferior left atrial ganglionated plexi; LIPV = left inferior pulmonary vein; LSGP = superior left atrial ganglionated plexi; LSPV = left superior pulmonary vein; MTGP = Marshall tract ganglionated plexi; RIGP = inferior right atrial ganglionated plexi; RIPV = right inferior pulmonary vein; RSGP = superior right atrial ganglionated plexi; RSPV = right superior pulmonary vein.
Figure 2: The Schematic View of Ganglionated Plexi.
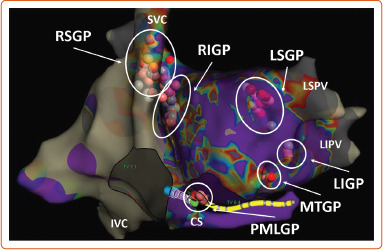
Pink and red dots show distribution of ablation points based on fragmented bipolar electrograms. CS = coronary sinus; IVC = inferior vena cava; LIGP = inferior left atrial ganglionated plexi; LSGP = superior left atrial ganglionated plexi; MTGP = Marshall tract ganglionated plexi; PMLGP = posteromedial left atrial ganglionated plexi; RIGP = inferior right atrial ganglionated plexi, RSGP = superior right atrial ganglionated plexi; SVC = superior vena cava.
However, it should be noted that a dense meshwork of neurons has been characterised by immunohistochemical methods as predominantly cholinergic, although adrenergic-, nitrergic- and peptidergic-positive fibres have also been identified within the GPs so it is too simplistic to assume that the GP behave as one unit. Furthermore, the specific neural elements responsible for the ablation outcomes remain unknown.[56]
The ligament of Marshall (LoM) is also considered part of the intrinsic cardiac ANS. Cholinergic nerve fibres originating in the LoM were found to innervate surrounding left atrial structures, including the PVs, left atrial appendage and coronary sinus.[51] The VoM is one of the structures contained within the LoM and permits access to it through the coronary sinus. However, the precise location and distribution and density of the GP may vary significantly among individuals, making it challenging to predict their precise location based on anatomy alone.[57] Table 1 shows the distribution of epicardial ganglia based on anatomical specimens and electrophysiological data.[55,57–62]
Table 1: Distribution of Ganglionated Plexi Based on Anatomical Specimens and Electrophysiological Data in Humans.
| Authors | GP Detection Method | Anatomical Area | ||||||
|---|---|---|---|---|---|---|---|---|
| SVC-RA Junction | IAS | LSPV-LAA | LIPV | IVC-CS | LoM | Other | ||
| Pachon et al. 2004,2020[59,60] | SA | GP 1 | GP 2 | GP 3 | * | GP 4 | N/D | * |
| Lellouche et al. 2007[62] | EGM | + | + | + | + | + | N/D | † |
| Armour 2008[55] | Heart sections | RSGP | RIGP | LSGP | LIGP | PMLGP | N/D | (-) |
| Nakagawa et al. 2009[58] | HFS | RSGP | RIGP | LSGP | LIGP | N/D | MTGP | (-) |
| Kim et al. 2018[57] | HFS | + | + | + | + | N/D | + | (-) |
| Aksu et al. 2020[61] | EGM | RSGP | RIGP | LSGP | LIGP | PMLGP | MTGP | (-) |
*Defined fragmented spectral potentials near the left inferior pulmonary vein insertion and the lateral wall of the right atrium and crista terminalis. However, these areas were not defined as GPs. †An A–H prolongation during ablation was seen in the anterior aspect of the mitral valve annulusi 6% of patients. CS = coronary sinus; EGM = electrogram; GP = ganglionated plexi; HFS = high-frequency stimulation; IAS = interatrial septum; LAA = left atrial appendage; LIGP = left inferior ganglionated plexi; LIPV = left inferior pulmonary vein; IVC-CS = inferior vena cava-coronary sinus junction; LoM = the ligament of Marshall; LSGP = superior left atrial GP; LSPV = left superior pulmonary vein; MTGP = Marshall tract ganglionated plexi; N/D = not defined; PMLGP = posteromedial left atrial GP; RA = right atrium; RIGP = inferior right atrial GP; RSGP = superior right atrial GP; SA = spectral analysis; SVC = superior vena cava.
Autonomic Tone, Ganglionated Plexi and Atrial Fibrillation
The role of the ANS as a trigger for the initiation and maintenance of AF is well established. First speculation of this association was highlighted by Coumel et al., who reported a small case series of 18 patients without structural heart disease who had recurrent paroxysms of AF/atrial flutter which appeared to be initiated by sinus rate slowing and atrial coupling attributed to vagal overactivity.[52] Derangements in sympathetic tone are also thought to play a central role in AF, possibly via cellular, structural and electrical changes which occur in the setting of states of heightened adrenergic tone, including hypertension, obstructive sleep apnoea and heart failure.[53,63]
Additional clues to the role of the ANS in the initiation and termination of AF have been demonstrated by circadian periodicity in which episodes of AF are more frequent in the early morning and evening.[64,65] Obstructive sleep apnoea (OSA) shares similar risk factors and may be a modifier for AF. Screening for OSA is currently recommended in patients undergoing attempts at rhythm control.[66] In a study of 101 patients, Mohammadieh et al. found that paroxysmal atrial fibrillation (PAF) patients with OSA had increased parasympathetic tone and relative reduction in sympathetic modulation during non-REM sleep when assessed by frequency-domain analysis of heart rate variability (HRV) suggesting vagal predominance as a contributor to AF in the subpopulation.[67] Another key clue of this role is the fact that therapies which blunt components of the autonomic nervous system suppresses AF in both animal and human studies.[68–74]
Endocardial Mapping of Ganglionated Plexi
From a clinical standpoint, the identification of the GPs and understanding of their anatomic clustering in the electrophysiology laboratory is an important step for targeting potential modulation. A study conducted by Po et al. demonstrated that application of high-frequency stimulation (HFS) of 20 Hz between 10–140 V at a 1–10 ms pulse width in anatomical regions where GP are known to be located resulted in a marked parasympathetic response, which was defined as an increase in the mean R-R interval by 50% during AF.[75] A similar method of GP identification using HFS, between 20–50 Hz, between 5–15 V and pulse width of 10 ms, was also shown to be able to precisely locate GP sites.[76] In a recently published randomised controlled trial (RCT), Kim et al. compared GP ablation without PVI against PVI in patients with paroxysmal AF.[77] To map GPs, two types of HFS techniques were used: synchronised HFS with 10 V, 80 ms duration, 40 Hz to detect the ectopy-triggering GPs and continuous HFS with 10 V, 20 Hz up to 10 seconds to detect the atrioventricular dissociating-GPs. In the GP ablation arm, only ectopy-triggering-GPs were targeted and ablated. The freedom from ≥30 seconds of atrial arrhythmia at 12-month follow-up was 50% (26 of 52) with GP ablation versus 64% (32 of 50) with PVI (p=0.09).
By using spectral analysis, Pachon et al. demonstrated that fibrillar potentials show fragmented and heterogeneous conduction properties and might result from incursions of neural and vascular structures and be used to detect autonomic innervation sites.[60] By using a classical band-pass filter setting of 30–500 Hz, Lellouche et al. analysed electrogram characteristics based on parasympathetic response during radiofrequency (RF) application and demonstrated that the best single predictor of parasympathetic response during RF application was the presence of at least four electrogram deflections at the ablation site.[62]
Stirrup et al. used 123I-metaiodobenzylguanidine (123I-mIBG) solid-state single-photon emission CT to map left atrial GPs, non-invasively.[78] All patients underwent cardiac CT as part of standard clinical care for delineation of LA and pulmonary venous anatomy prior to PVI. Following registration, the CT-derived LA segment was used as an anatomical constraint to define a region of search around the LA endocardium to facilitate identification of focal mIBG uptake adjacent to the atria. Focal increased mIBG activity within the search region was automatically overlaid on the CT-derived left atrial surface, generating a hybrid 3D image of left atrial innervation and anatomy. I-mIBG LA uptake areas were recorded and correlated with HFS. A total of 73 I-mIBG LA uptake areas were identified, of which 59 (81%) were HFS positive. The likelihood of this increased with reader confidence (92%).
More recently, our group demonstrated that RF catheter ablation guided by the identification of complex fractionated left atrial electrograms during sinus rhythm using 3D electroanatomical mapping correlated highly with the distribution of successful GP ablation sites without need for HFS, resulting in significant simplification of procedural workflow to add adjunctive GP ablation to PVI.[62,79–82] Briefly, after 3D mapping both atria, fragmented bipolar endocardial atrial electrograms are evaluated for the number of deflections at filter settings of 200–500 Hz by using the ablation catheter. The electrograms demonstrating greater or equal to 3–4 deflections in regions which are anatomically consistent with GP sites are tagged as ablation targets. Despite all these specialised techniques, it should be noted that the largest RCT to so far examine the role of adjunctive GP ablation to standard PVI used an anatomical approach for GP ablation.[83]
Neuromodulatory Strategies in the Management of Atrial Fibrillation
Several non-catheter neuromodulatory techniques have been explored in the management of AF both as alternative or adjunctive therapies to PVI with varying degrees of success. These include procedures targeting neural inputs within the heart and those in extracardiac structures (Figure 3). Targeting autonomic ganglionic plexi and alcohol injection in the VoM have yielded moderate results within cardiac structures. Techniques focused on neuromodulation extrinsic to the heart include transcutaneous vagal nerve stimulation, renal nerve denervation, stellate ganglion block and baroreflex receptor therapy.
Figure 3: Sites of Neuromodulation in the Management of Atrial Fibrillation.
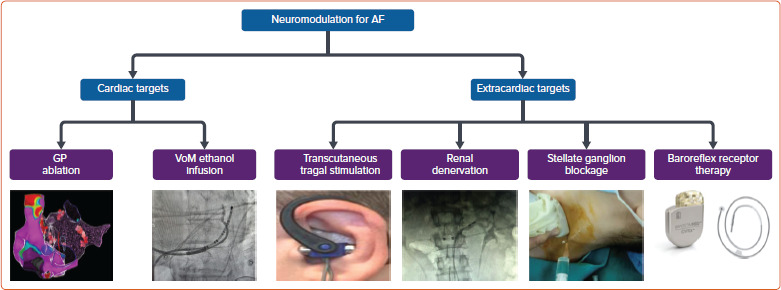
GP = ganglionated plexus. Source: Image for stellate ganglion blockage: Ma D et al.[122] Reproduced from Springer Nature under a Creative Commons CC BY licence.
Ganglionated Plexus Ablation
Several clinical trials have explored the use of GP ablation in the management of AF. While the results of these studies have been generally quite favourable, with decreased rates of AF recurrence when compared to PVI alone, it is worth noting that there is not a standardised method for performing these ablations.[75,83–86] Additionally, as a stand-alone treatment strategy for AF, GP ablation success rates have been lacklustre. Specifically, in one study examining the long-term impact of GP ablation during a 3-year follow-up period showed that isolated GP ablation was associated with significantly lower rates of freedom form atrial tachyarrhythmias (AT) without antiarrhythmic drug therapy when compared to circumferential PVI (34.3% versus 65.7%, p=0.008).[87] Several pooled analyses, including a recent RCT-only meta-analysis, have found that GP ablation as an additive strategy to standard PVI may be more beneficial in patients with paroxysmal rather than persistent AF.[88]
One of the factors that may limit the durability of GP ablation on freedom from AF may potentially be the phenomenon of nerve regeneration and reinnervation post-ablation. In one small canine study in which epicardial GP fat ablations were performed, features of restoration of vagal effects were noted within 4 weeks post-procedure and were suggestive of reinnervation.[89] Long-term recovery of autonomic tone has also been observed following cardioneuroablation for cardioinhibitory vasovagal syncope and may affect the durability of those therapeutic interventions as well.[90–92] However, given the additive benefit of combined GP ablation and PVI has been demonstrated to be long-term in comparison to PVI alone, the nerve regeneration hypothesis may not be universally true, or it may suggest that additional factors independent of nervous inputs may be involved in this population and warrants further investigation.
According to the published studies, an RF current should be applied in a point-by-point fashion in power-controlled mode with an open irrigated-tip catheter. RF energy should be limited to 30–35 W along the left atrial posterior wall and roof and to 40 W in the remaining areas for a duration of at least 30 seconds at each site.
Pulsed electric field (PEF) ablation is currently being investigated for human use for PVI and linear atrial ablation. Because every tissue has a different specific field threshold that induces necrosis, PEF-based irreversible electroporation induces selective myocyte necrosis without collateral damage to other tissues such as the oesophagus, the phrenic nerve, or the endothelial cells. However, in clinical cases and animal studies, transient bradycardia and even atrioventricular block have been observed with endocardial pulsed field applications in the LA. Wei et al. showed that nerves treated with irreversible electroporation were damaged after immediate direct injury with a full recovery after 2 weeks.[93] However, in an open-chest canine model, saline irrigated PEF with a changed setting (1,000 V, 100 ms) were directly delivered to visualised epicardial GP regions (LSGP, RSGP, LOM GP, oblique sinus GP and transverse sinus GP) using an anatomy-guided approach. Histological examination showed preserved function and structure of the atrial myocardium but also absence in acute structural change to nerves and GP. However, the local atrial effective refractory period (AERP) increased by 12–29% at different atrial sites following PEF applications suggesting perturbation of cardiac parasympathetic innervation in the acute model.[94] In a clinical comparison of 31 patients who underwent PVI using a lattice-tip catheter and PEF energy versus 13 patients who underwent PVI using RF energy, alteration in sinus node and AV node function was observed more frequently in the RF ablation group. Further, earlier recovery of GP function was noticed only in the PEF group, suggesting that RF ablation may be more advantageous in terms of long-term durable effectiveness for GP ablation.[95]
Vein of Marshall Ethanol Ablation
The VoM is one of the structures contained within the LoM and is embryologically derived from the left superior vena cava. Autonomic system inputs (parasympathetic and sympathetic) are part of the complexity of the LoM and play a role in the initiation and maintenance of AF.[51,96] Ablating these nervous inputs and abolishing vagal responses using ethanol infusions in the VoM has been shown to be successful in animal and human studies.[97]
The recently published VENUS trial showed that for individuals who had successful infusion of ethanol to the VoM at the time of AF ablation (PVI and non-PV sites), AF/AT recurrence rates 6–12 months post-procedure were significantly reduced with an (OR 0.57, 95% CI [0.37–0.90]), when compared to those who had PVI catheter ablation alone.[13,98] One of the proposed explanations for this technique reducing AF recurrence rates is that infusion of ethanol in the VoM leads to disruption of parasympathetic nerve connections that are key to triggering AF.[99] However, it has been noted that there is significant heterogeneity on the impact of VoM infusion on rhythm control depending on the lesion sets performed at the time of AF ablation and the procedural endpoint used.
A subsequent secondary analysis of the findings of the VENUS trial sought to explore this heterogenicity further.[100] The analysis showed that freedom from AF/AT was greatest if perimitral block was achieved (54.3% post VoM catheter ablation, 37% post catheter ablation alone, p=0.01) compared to when perimitral block was not achieved (34.0% post VoM catheter ablation, 37.0% post catheter ablation alone, p=0.583). Based on this finding, the authors concluded that using perimitral block as a therapeutic endpoint should be sought at the time of ablation.
Other than ablating the autonomic and the muscular fibres of the LoM, which can be arrhythmogenic per se, VoM ethanol infusion may affect the area around the left inferior pulmonary vein creating a low voltage zone.[101] This may facilitate acute and chronic block of the posterior mitral isthmus reducing the post-PVI perimitral flutters. As seen with GP ablation, the role of VoM ethanol infusion appears to be additive, reflecting the multifaceted nature of the pathophysiology of AF.
Transcutaneous Tragal Stimulation
Stimulation of the auricular branch of the vagal nerve via low-level transcutaneous tragal stimulation (LLTS), which is applied to the tragus of the ear, has been shown to be effective at suppressing AF by decreasing sympathetic tone and blunting inflammatory cytokines which may contribute to AF.[102–104]
In this non-invasive method of neuromodulation, flat metal clips are attached to the tragus and 20 Hz electrical stimuli are applied. In a recently sham-controlled double-blind trial, participants either attached ear clips to the tragus (intervention group) versus the earlobe (sham group) and performed LLTS for 1 hour daily over a 6-month period.[39] Participants in the active treatment group had an AF burden 85% lower than the sham group (p=0.01). Although this is a potentially exciting treatment prospect, responses to LLTS may be inconsistent within individual patients and predicting which patients will benefit most from this approach may be difficult.
One proposed novel approach to assist in patient selection and therapy adjustment is examining the burden of P-wave alternans (thought to be generated by the same mechanism as AF) at the time of initial LLTS. Using this method, people who had an increase in P-wave alternans after acute treatment had a lower AF burden at 6 months of chronic LLTS compared to those who did not have an acute increase.[40] Further studies are needed to define the role that this strategy will play as a stand-alone AF therapy or adjunct to other treatments including catheter-based therapies. Other considerations such as loss of efficacy due to physiological tolerance, as has been seen with chronic electrical peripheral nerve stimulation, will also need to be examined further.[105]
Renal Sympathetic Denervation
Another neuromodulation strategy in the management of AF to be recently examined is renal sympathetic denervation (RSD). The renal sympathetic nerves, which are located in the walls of the renal artery, interact closely with the central ANS (which in turn has inputs to the heart) and have been implicated in the development of resistant hypertension and AF. Based on this, it has been hypothesised that ablating these nervous connections could be related to the attenuation of afferent sympathetic input from the aorticorenal ganglion to the central nervous system, as well as attenuation of efferent signalling from the aorticorenal ganglion to the renal parenchyma, reducing renin–angiotensin–aldosterone system activation which alters sympathetic tone and leads to reductions in blood pressure and AF burden.[106]
The ERADICATE-AF trial was conducted to study this hypothesis further.[107] This RCT included 302 patients with uncontrolled hypertension and paroxysmal AF who underwent renal denervation in addition to PVI versus PVI alone. After a 12-month period of follow-up, freedom from AF and AT was noted to be significantly lower in the group undergoing both PVI and renal denervation when compared to the group undergoing PVI alone (72.1% versus 56.5%, p=0.006). This was accompanied by a significant reduction in systolic and diastolic blood pressures in the group undergoing both PVI and renal denervation.
It is worth noting that while these results in blood pressure reduction are much more impressive than those seen at the 6-month mark of the landmark SYMPLICITY HTN 3 trial which compared catheter-based renal denervation to a sham control (which showed no significant change to blood pressure), they align more with those of the recently published long-term follow-up.[108,109]
Important factors should be taken into consideration when examining the results of the ERADICATE-AF trial, however. First, there was no sham control arm – a limitation noted by the study authors – as well as some uncertainty about the specific mechanism of AF reduction. Renal sympathetic denervation decreased AF recurrence in ERADICATE-AF trial. However, the specific mechanism of AF reduction is not clear. One possibility is that denervation decreases hypertension, which decreases AF burden, or sympathetic denervation directly affects AF burden.
A systematic review and meta-analysis demonstrated a significant reduction of AF recurrence in the PVI + RSD group versus PVI alone (n=223 versus 228; pooled OR 0.63, 95% CI [0.50–0.80]; p<0.001, I2=0.0%) in select hypertensive patients.[110] As more data from clinical trials becomes available, RSD may be more favourably considered as a strategy to improve AF burden in selected patients with refractory hypertension for whom AF ablations are planned.
Stellate Ganglion Block
Blocking the stellate ganglion (SGB), a source of major sympathetic input to the heart has been shown to be beneficial in the management of drug-refractory ventricular arrhythmias.[111–113] However, the role of this technique has been less well studied in the management of AF.
In a small prospective study, 36 consecutive patients with paroxysmal AF were randomised to transcutaneous SGB using lidocaine or placebo prior to planned PVI. The ability to induce AF, AF duration as well as the atrial effective refractory period (AERP) were evaluated. There was a significant reduction in AF inducibility pre- and post-SGB (100% versus 54%, p<0.01), and a shortening of AF duration 5.5 (3.0–12.0 minutes) – 1.5 (0.0–5.8) minutes (p<0.01) before and after SGB.[114]
A subsequent RCT involving 200 patients showed significant reductions in AF episodes in the first 24 hours post-surgery in patients undergoing lobectomy who had right-sided SGB when compared to those who did not (3% versus 10%, p=0.045). To validate these findings further, other RCTs are further exploring the role of SGB in the prevention of postoperative AF, such as NCT05357690.
Given the infancy of this technique for AF management, many questions remain unanswered particularly surrounding the duration of its efficacy which may be only a few weeks when used for management of ventricular tachycardia.[115] Based on the limited data available and transient nature of its effect, restriction to periods of heightened AF risk such as that in the perioperative period for cardiothoracic surgeries appears to be valid.
Baroreflex Receptor Therapy
Baroreceptors in the carotid sinus and its accompanying reflex arcs play a dominant role in blood pressure homoeostasis through alterations in cardiac contractility, heart rate response and peripheral vascular resistance.[116] Abnormalities in the function of these baroreceptors have been implicated in patients with AF and heart failure.[117]
Animal models have demonstrated that low-level carotid baroreflex stimulation (LLCBS) was successful at suppressing AF and could reverse right atrial remodelling that occurred in the setting of a high right atrial pacing burden.[118,119] To date, there are limited human studies examining baroreflex receptor therapy (BRT) for the management of AF, however, its use in patients with heart failure has been explored and was found to be safe and effective in this population via the suppression of central sympathetic outflow.[120,121] The role of BRT in the management of AF has yet to be defined, with more data from human studies needed.
Conclusion
The autonomic nervous system is intimately involved in the pathophysiology of AF. Several methods to decrease AF burden via neuromodulation are being explored, some of which have demonstrated potential for clinical effectiveness as adjuncts, namely GP ablation, RSD and VoM ethanol infusion, to established catheter ablation PVI. The prospect of non-invasive techniques, such as transcutaneous tragal stimulation is exciting, but further research in this area is needed to guide clinical application.
Neuromodulation appears to offer at least incremental benefits to existing established management strategies for AF. Importantly, these methods remind us of the complex and multifaceted pathological processes that promote atrial AF and the need for a similar multifaceted approach to its management.
Clinical Perspective
The autonomic nervous system plays an important role in AF via a complex network of neural inputs, outputs and plexi.
Modulating, or in some instances eliminating, key neural connections to the heart may decrease AF burden.
Targeting autonomic ganglionated plexi and alcohol injection in the vein of Marshall in addition to pulmonary vein isolation may increase AF-free survival.
References
- 1.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–21. doi: 10.1177/1747493019897870. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Bailey KR et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–92. doi: 10.1016/J.JACC.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu BC et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. doi: 10.1161/circoutcomes.110.958165. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh A, Iglesias M, Khanna R, Beaulieu T. Healthcare utilization and costs associated with a diagnosis of incident atrial fibrillation. Heart Rhythm. 2022;3:577–86. doi: 10.1016/j.hroo.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lévy S, Steinbeck G, Santini L et al. Management of atrial fibrillation: two decades of progress — a scientific statement from the European Cardiac Arrhythmia Society. J Interv Card Electrophysiol. 2022;65:287–326. doi: 10.1007/S10840-022-01195-Z. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreidieh O, Varley AL, Romero J et al. Practice patterns of operators participating in the real-world experience of catheter ablation for treatment of symptomatic paroxysmal and persistent atrial fibrillation (REAL-AF) registry. J Interv Card Electrophysiol. 2022;65:429–40. doi: 10.1007/S10840-022-01205-0. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande R, AlKhadra Y, Singanallur P et al. Outcomes of catheter ablation versus antiarrhythmic therapy in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2022;65:771–802. doi: 10.1007/S10840-022-01365-Z. [DOI] [PubMed] [Google Scholar]
- 10.Oral H, Knight BP, Tada H et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–81. doi: 10.1161/HC0902.104712. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Shim J, Park J et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–61. doi: 10.1016/J.JACEP.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Yu HT, Kim TH et al. Electrical posterior box isolation in repeat ablation for atrial fibrillation: a prospective randomized clinical study. JACC Clin Electrophysiol. 2022;8:582–92. doi: 10.1016/J.JACEP.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Valderrábano M, Peterson LE, Swarup V et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA. 2020;324:1620–8. doi: 10.1001/JAMA.2020.16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrado A, Bonso A, Madalosso M et al. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21:1–5. doi: 10.1111/J.1540-8167.2009.01577.X. [DOI] [PubMed] [Google Scholar]
- 15.Arruda M, Mlcochova H, Prasad SK et al. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18:1261–6. doi: 10.1111/J.1540-8167.2007.00953.X. [DOI] [PubMed] [Google Scholar]
- 16.Schade A, Costello-Boerrigter L, Steinborn F et al. Voltage-guided ablation in persistent atrial fibrillation—favorable 1-year outcome and predictors. J Interv Card Electrophysiol. 2021;62:249–57. doi: 10.1007/s10840-020-00882-z. [DOI] [PubMed] [Google Scholar]
- 17.Jia H, Wang W, Yu B. Efficacy and safety of low voltage area ablation for atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 18.Aryana A, di Biase L, Pujara DK et al. Long-term durability of posterior wall isolation using the cryoballoon in patients with persistent atrial fibrillation: a multicenter analysis of repeat catheter ablations. J Interv Card Electrophysiol. 2021;62:161–9. doi: 10.1007/s10840-020-00887-8. [DOI] [PubMed] [Google Scholar]
- 19.Omuro T, Yoshiga Y, Ueyama T et al. An impact of superior vena cava isolation in non-paroxysmal atrial fibrillation patients with low voltage areas. J Arrhythm. 2021;37:965–74. doi: 10.1002/JOA3.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.di Biase L, Burkhardt JD, Mohanty P et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol. 2016;68:1929–40. doi: 10.1016/J.JACC.2016.07.770. [DOI] [PubMed] [Google Scholar]
- 21.Knecht S, Zeljkovic I, Badertscher P Role of empirical isolation of the superior vena cava in patients with recurrence of atrial fibrillation after pulmonary vein isolation – a multi-center analysis. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 22.Yorgun H, Şener YZ, Tanese N Long-term outcomes of left atrial appendage isolation using cryoballoon in persistent atrial fibrillation. EP Europace. 2022. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 23.Weng W, Birnie DH, Ramirez FD et al. Outcomes of a comprehensive strategy during repeat atrial fibrillation ablation. J Interv Card Electrophysiol. 2022;65:391–9. doi: 10.1007/S10840-022-01190-4. [DOI] [PubMed] [Google Scholar]
- 24.Ikenouchi T, Nitta J, Inaba O et al. Effect of isolation feasibility of non-pulmonary vein foci on efficacy of ablation for atrial fibrillation: comparison of the isolation and focal ablation methods. J Interv Card Electrophysiol. 2022;65:441–51. doi: 10.1007/S10840-022-01217-W. [DOI] [PubMed] [Google Scholar]
- 25.Narayan SM, Baykaner T, Clopton P et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2014;63:1761–8. doi: 10.1016/J.JACC.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohanty S, Mohanty P, Trivedi C et al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping: insights from a meta-analysis. Circ Arrhythm Electrophysiol. 2018;11:e005789. doi: 10.1161/CIRCEP.117.005789. [DOI] [PubMed] [Google Scholar]
- 27.Ekanem E, Reddy VY, Schmidt B et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace. 2022;24:1256–66. doi: 10.1093/europace/euac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haines DE, Kong MH, Ruppersberg P Electrographic flow mapping for atrial fibrillation: theoretical basis and preliminary observations. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 29.Skeete J, Sharma PS, Kenigsberg D et al. Wide area circumferential ablation for pulmonary vein isolation using radiofrequency versus laser balloon ablation. J Arrhythm. 2022;38:336–45. doi: 10.1002/JOA3.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suruga K, Suenari K, Nakano T et al. Comparison between cryoballoon and hot balloon ablation in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2022;64:281–90. doi: 10.1007/s10840-021-00978-0. [DOI] [PubMed] [Google Scholar]
- 31.Blockhaus C, Guelker JE, Feyen L Pulsed field ablation for pulmonary vein isolation: real-world experience and characterization of the antral lesion size compared with cryoballoon ablation. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 32.Higuchi K, Iwai S, Kato N The utility of combining continuous wavelet transform analysis and high-density voltage map in predicting the long-term outcomes after ablation of persistent atrial fibrillation. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 33.Bohnen M, Weber R, Minners J Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. EP Europace. 2022. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 34.Vanam S, Darden D, Munir MB et al. Characteristics and outcomes of recurrent atrial fibrillation after prior failed pulmonary vein isolation. J Interv Card Electrophysiol. 2022;64:715–22. doi: 10.1007/s10840-022-01160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma A, Jiang CY, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMOA1408288. [DOI] [PubMed] [Google Scholar]
- 36.Bisignani A, Cecchini F, Mugnai G et al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity-matched score comparison between different strategies. J Interv Card Electrophysiol. 2022;64:9–16. doi: 10.1007/s10840-021-00968-2. [DOI] [PubMed] [Google Scholar]
- 37.Spittler R, Bahlke F, Hoffmann BA et al. Durable pulmonary vein isolation but not complex substrate ablation determines the type of arrhythmia recurrence after persistent atrial fibrillation ablation. J Interv Card Electrophysiol. 2022;64:417–26. doi: 10.1007/s10840-021-01048-1. [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Li H, Yi S Comparing the efficacy of catheter ablation strategies for persistent atrial fibrillation: a Bayesian analysis of randomized controlled trials. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 39.Stavrakis S, Stoner JA, Humphrey MB et al. TREAT AF (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC Clin Electrophysiol. 2020;6:282–91. doi: 10.1016/J.JACEP.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulkarni K, Singh JP, Parks KA et al. Low-level tragus stimulation modulates atrial alternans and fibrillation burden in patients with paroxysmal atrial fibrillation. J Am Heart Assoc. 2021;10:e020865. doi: 10.1161/JAHA.120.020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi EK, Chen PS. Is the atrial neural plexis a therapeutic target in atrial fibrillation? Methodist Debakey CardioVasc J. 2015;11:82–6. doi: 10.14797/mdcj-11-2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pokushalov E, Turov A, Shugayev P et al. Catheter ablation of left atrial ganglionated plexi for atrial fibrillation. Asian Cardiovasc Thorac Ann. 2008;16:194–201. doi: 10.1177/021849230801600304. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto SI, Fujii M, Watanabe Y et al. Exploration of theoretical ganglionated plexi ablation technique in atrial fibrillation surgery. Ann Thorac Surg. 2014;98:1598–604. doi: 10.1016/j.athoracsur.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 44.Calò L, Rebecchi M, Sciarra L et al. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:22–31. doi: 10.1161/CIRCEP.111.964262. [DOI] [PubMed] [Google Scholar]
- 45.Kim MY, Aksu T. Ganglionated plexus ablation and pulmonary vein isolation: the future of AF ablation. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 46.Aksu T, Mutluer FO, Huang H. Cardioneuroablation for the treatment of vasovagal syncope and sinus bradycardia with atrial escape. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 47.Aksu T, Gopinathannair R, Gupta D, Pauza DH. Intrinsic cardiac autonomic nervous system: what do clinical electrophysiologists need to know about the “heart brain”? J Cardiovasc Electrophysiol. 2021;32:1737–47. doi: 10.1111/JCE.15058. [DOI] [PubMed] [Google Scholar]
- 48.Aksu T, Guler TE, Bozyel S, Yalin K. Selective vagal innervation principles of ganglionated plexi: step-by-step cardioneuroablation in a patient with vasovagal syncope. J Interv Card Electrophysiol. 2021;60:453–8. doi: 10.1007/s10840-020-00757-3. [DOI] [PubMed] [Google Scholar]
- 49.Aksu T, Padmanabhan D, Shenthar J et al. The benefit of cardioneuroablation to reduce syncope recurrence in vasovagal syncope patients: a case-control study. J Interv Card Electrophysiol. 2022;63:77–86. doi: 10.1007/s10840-020-00938-0. [DOI] [PubMed] [Google Scholar]
- 50.Linz D, Stavrakis S. Cardioneuroablation for vasovagal syncope: how to move beyond ‘learning by burning’? J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 51.Ulphani JS, Arora R, Cain JH et al. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293:H1629–35. doi: 10.1152/AJPHEART.00139.2007. [DOI] [PubMed] [Google Scholar]
- 52.Coumel P, Attuel P, Lavallée J et al. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645–56. [in French] [PubMed] [Google Scholar]
- 53.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 54.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–82. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 55.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93:165–76. doi: 10.1113/EXPPHYSIOL.2007.041178. [DOI] [PubMed] [Google Scholar]
- 56.Hanna P, Dacey MJ, Brennan J et al. Innervation and neuronal control of the mammalian sinoatrial node a comprehensive atlas. Circ Res. 2021;128:1279–96. doi: 10.1161/CIRCRESAHA.120.318458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim MY, Sikkel MB, Hunter RJ et al. A novel approach to mapping the atrial ganglionated plexus network by generating a distribution probability atlas. J Cardiovasc Electrophysiol. 2018;29:1624–34. doi: 10.1111/JCE.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagawa H, Scherlag BJ, Patterson E et al. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 59.Pachon MEI, Pachon-Mateos JC, Higuti C et al. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020;13:e007900. doi: 10.1161/CIRCEP.119.007900. [DOI] [PubMed] [Google Scholar]
- 60.Pachon M JC, Pachon MEI, Pachon MJC et al. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace. 2004;6:590–601. doi: 10.1016/J.EUPC.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Aksu T, Guler TE, Bozyel S et al. Usefulness of post-procedural heart rate response to predict syncope recurrence or positive head up tilt table testing after cardioneuroablation. Europace. 2020;22:1320–7. doi: 10.1093/EUROPACE/EUAA230. [DOI] [PubMed] [Google Scholar]
- 62.Lellouche N, Buch E, Celigoj A et al. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 2007;50:1324–31. doi: 10.1016/J.JACC.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 63.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8. doi: 10.1016/S0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 64.Bedford JP, Redfern O, Johnson A et al. Circadian variation in new-onset atrial fibrillation in patients in ICUs. J Crit Care. 2022;67:1–2. doi: 10.1016/J.JCRC.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita T, Murakawa Y, Sezaki K et al. Circadian variation of paroxysmal atrial fibrillation. Circulation. 1997;96:1537–41. doi: 10.1161/01.CIR.96.5.1537. [DOI] [PubMed] [Google Scholar]
- 66.Yeghiazarians Y, Jneid H, Tietjens JR et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144:e56–67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 67.Mohammadieh AM, Dissanayake HU, Sutherland K Does obstructive sleep apnoea modulate cardiac autonomic function in paroxysmal atrial fibrillation? J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PMC free article] [PubMed]
- 68.Richer LP, Vinet A, Kus T et al. Alpha-adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1175–80. doi: 10.1152/AJPREGU.00840.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leiria TLL, Glavinovic T, Armour JA et al. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton Neurosci. 2011;161:68–74. doi: 10.1016/J.AUTNEU.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Tan AY, Zhou S, Ogawa M et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–25. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan AY, Li H, Wachsmann-Hogiu S et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–43. doi: 10.1016/J.JACC.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 72.Morita N, Iida T, Nanao T et al. Effect of ganglionated plexi ablation by high-density mapping on long-term suppression of paroxysmal atrial fibrillation – the first clinical survey on ablation of the dorsal right plexus. Heart Rhythm. 2021;2:480–8. doi: 10.1016/j.hroo.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishida T, Takitsume A, Sugiura J et al. Catheter ablation of ganglionated plexi in patients with adenosine triphosphate-induced atrial fibrillation after pulmonary vein isolation. Heart Vessels. 2022;37:854–66. doi: 10.1007/S00380-021-01979-9. [DOI] [PubMed] [Google Scholar]
- 74.Călburean PA, Osório TG, Sieira J et al. High parasympathetic activity as reflected by deceleration capacity predicts atrial fibrillation recurrence after repeated catheter ablation procedure. J Interv Card Electrophysiol. 2021;60:21–9. doi: 10.1007/s10840-019-00687-9. [DOI] [PubMed] [Google Scholar]
- 75.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–9. doi: 10.1111/J.1540-8167.2009.01515.X. [DOI] [PubMed] [Google Scholar]
- 76.Lemery R, Birnie D, Tang ASL et al. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–96. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Kim MY, Coyle C, Tomlinson DR et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm. 2022;19:516–24. doi: 10.1016/j.hrthm.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stirrup J, Gregg S, Baavour R et al. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: technique and validation in patients with atrial fibrillation. J Nucl Cardiol. 2019;27:1939–50. doi: 10.1007/S12350-018-01535-5. [DOI] [PubMed] [Google Scholar]
- 79.Aksu T, Guler TE, Mutluer FO et al. Electroanatomic-mapping-guided cardioneuroablation versus combined approach for vasovagal syncope: a cross-sectional observational study. J Interv Card Electrophysiol. 2019;54:177–88. doi: 10.1007/S10840-018-0421-4. [DOI] [PubMed] [Google Scholar]
- 80.Aksu T, Guler TE, Bozyel S et al. Initial experience with fractionation mapping–guided ablation strategy in patients with long-standing persistent atrial fibrillation. J Interv Card Electrophysiol. 2021;61:405–13. doi: 10.1007/s10840-020-00834-7. [DOI] [PubMed] [Google Scholar]
- 81.Aksu T, Guler TE. Electroanatomical mapping-guided ablation during atrial fibrillation: a novel usage of fractionation mapping in a case with sinus bradycardia and paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2020;57:331–2. doi: 10.1007/s10840-019-00633-9. [DOI] [PubMed] [Google Scholar]
- 82.Rackley J, Nudy M, Gonzalez MD Pulmonary vein isolation with adjunctive left atrial ganglionic plexus ablation for treatment of atrial fibrillation: a meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. 2022. epub ahead of press. [DOI] [PubMed]
- 83.Katritsis DG, Pokushalov E, Romanov A et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25. doi: 10.1016/J.JACC.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 84.Scherlag BJ, Nakagawa H, Jackman WM et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13((Suppl 1)):37–42. doi: 10.1007/S10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 85.Scanavacca M, Pisani CF, Hachul D et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–85. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 86.Katritsis DG, Giazitzoglou E, Zografos T et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–8. doi: 10.1016/J.HRTHM.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 87.Mikhaylov E, Kanidieva A, Sviridova N et al. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: a 3-year follow-up study. Europace. 2011;13:362–70. doi: 10.1093/EUROPACE/EUQ416. [DOI] [PubMed] [Google Scholar]
- 88.Yan F, Zhao S, Wu W et al. Different effects of additional ganglion plexus ablation on catheter and surgical ablation for atrial fibrillation: a systemic review and meta-analysis. J Cardiovasc Electrophysiol. 2019;30:3039–49. doi: 10.1111/JCE.14258. [DOI] [PubMed] [Google Scholar]
- 89.Sakamoto SI, Schuessler RB, Lee AM et al. Vagal denervation and reinnervation after ablation of ganglionated plexi. J Thorac Cardiovasc Surg. 2010;139:444–52. doi: 10.1016/J.JTCVS.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukunaga M, Wichterle D, Peichl P et al. Differential effect of ganglionic plexi ablation in a patient with neurally mediated syncope and intermittent atrioventricular block. Europace. 2017;19:119–26. doi: 10.1093/europace/euw100. [DOI] [PubMed] [Google Scholar]
- 91.Zhao L, Jiang W, Zhou L et al. Atrial autonomic denervation for the treatment of long-standing symptomatic sinus bradycardia in non-elderly patients. J Interv Card Electrophysiol. 2015;43:151–9. doi: 10.1007/s10840-015-9981-8. [DOI] [PubMed] [Google Scholar]
- 92.Aksu T, Golcuk E, Yalin K et al. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. doi: 10.1111/pace.12756. [DOI] [PubMed] [Google Scholar]
- 93.Li W, Fan Q, Ji Z et al. The effects of irreversible electroporation (IRE) on nerves. PLoS One. 2011;6:e18831. doi: 10.1371/journal.pone.0018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Zyl M, Khabsa M, Tri JA et al. Open-chest pulsed electric field ablation of cardiac ganglionated plexi in acute canine models. J Innov Card Rhythm Manag. 2022;13:5061–9. doi: 10.19102/ICRM.2022.130704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stojadinović P, Wichterle D, Peichl P et al. Autonomic changes are more durable after radiofrequency than pulsed electric field pulmonary vein ablation. JACC Clin Electrophysiol. 2022;8:895–904. doi: 10.1016/J.JACEP.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 96.Kim DT, Lai AC, Hwang C et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–7. doi: 10.1016/S0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 97.Valderrábano M, Chen HR, Sidhu J et al. Retrograde ethanol infusion in the vein of Marshall regional left atrial ablation, vagal denervation, and feasibility in humans. Circ Arrhythm Electrophysiol. 2009;2:50–6. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valderrábano M, Peterson LE, Bunge R et al. Vein of Marshall ethanol infusion for persistent atrial fibrillation: VENUS and MARS clinical trial design. Am Heart J. 2019;215:52–61. doi: 10.1016/J.AHJ.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Báez-Escudero JL, Keida T, Dave AS et al. Ethanol infusion in the vein of Marshall leads to parasympathetic denervation of the human left atrium: implications for atrial fibrillation. J Am Coll Cardiol. 2014;63:1892–901. doi: 10.1016/J.JACC.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lador A, Peterson LE, Swarup V et al. Determinants of outcome impact of vein of Marshall ethanol infusion when added to catheter ablation of persistent atrial fibrillation: a secondary analysis of the VENUS randomized clinical trial. Heart Rhythm. 2021;18:1045–54. doi: 10.1016/J.HRTHM.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamakura T, André C, Duchateau J et al. Distribution of atrial low voltage induced by vein of Marshall ethanol infusion. J Cardiovasc Electrophysiol. 2022;33:1687–93. doi: 10.1111/JCE.15573. [DOI] [PubMed] [Google Scholar]
- 102.Clancy JA, Mary DA, Witte KK et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7:871–7. doi: 10.1016/J.BRS.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 103.Yu L, Huang B, Po SS et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc Interv. 2017;10:1511–20. doi: 10.1016/J.JCIN.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 104.Stavrakis S, Humphrey MB, Scherlag BJ et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–75. doi: 10.1016/J.JACC.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Günter C, Delbeke J, Ortiz-Catalan M. Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. J Neuroeng Rehabil. 2019;16:13. doi: 10.1186/S12984-018-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pokushalov E, Romanov A, Corbucci G et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70. doi: 10.1016/J.JACC.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 107.Steinberg JS, Shabanov V, Ponomarev D et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA. 2020;323:248–55. doi: 10.1001/JAMA.2019.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bhatt DL, Kandzari DE, O’Neill WW et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 109.Bhatt DL, Vaduganathan M, Kandzari DE et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised Symplicity HTN-3 Trial. Lancet. 2022;400:1405–16. doi: 10.1016/S0140-6736(22)01787-1. [DOI] [PubMed] [Google Scholar]
- 110.Kewcharoen J, Vutthikraivit W, Rattanawong P et al. Renal sympathetic denervation in addition to pulmonary vein isolation reduces the recurrence rate of atrial fibrillation: an updated meta-analysis of randomized control trials. J Interv Card Electrophysiol. 2021;60:459–67. doi: 10.1007/S10840-020-00748-4. [DOI] [PubMed] [Google Scholar]
- 111.Vaseghi M, Barwad P, Malavassi Corrales FJ et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017;69:3070–80. doi: 10.1016/J.JACC.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Ferrari GM, Dusi V, Spazzolini C et al. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–93. doi: 10.1161/CIRCULATIONAHA.115.015731. [DOI] [PubMed] [Google Scholar]
- 113.Tian Y, Wittwer ED, Kapa S et al. Effective use of percutaneous stellate ganglion blockade in patients with electrical storm. Circ Arrhythm Electrophysiol. 2019;12:e007118. doi: 10.1161/CIRCEP.118.007118. [DOI] [PubMed] [Google Scholar]
- 114.Leftheriotis D, Flevari P, Kossyvakis C et al. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm. 2016;13:2111–7. doi: 10.1016/j.hrthm.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 115.Hayase J, Patel J, Narayan SM, Krummen DE. Percutaneous stellate ganglion block suppressing VT and VF in a patient refractory to VT ablation. J Cardiovasc Electrophysiol. 2013;24:926–8. doi: 10.1111/JCE.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heusser K, Tank J, Engeli S et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–26. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 117.Gould PA, Yii M, Esler MD et al. Atrial fibrillation impairs cardiac sympathetic response to baroreceptor unloading in congestive heart failure. Eur Heart J. 2005;26:2562–7. doi: 10.1093/EURHEARTJ/EHI468. [DOI] [PubMed] [Google Scholar]
- 118.Linz D, Mahfoud F, Schotten U et al. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol. 2013;24:1028–33. doi: 10.1111/JCE.12171. [DOI] [PubMed] [Google Scholar]
- 119.Dai M, Bao M, Zhang Y et al. Low-level carotid baroreflex stimulation suppresses atrial fibrillation by inhibiting left stellate ganglion activity in an acute canine model. Heart Rhythm. 2016;13:2203–12. doi: 10.1016/J.HRTHM.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 120.Gronda E, Seravalle G, Brambilla G et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16:977–83. doi: 10.1002/EJHF.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zile MR, Lindenfeld JA, Weaver FA et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2020;76:1–13. doi: 10.1016/J.JACC.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 122.Ma D, Xue Y, Shi R. Stellate ganglion block as an intervention in refractory eosinophilic granulomatosis with polyangiitis: a case report. Allergy Asthma Clin Immunol. 2022;18:13. doi: 10.1186/s13223-002-00654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


