Abstract
Mitochondria perform diverse functions in the cell and their roles during processes such as cell survival, differentiation and migration are increasingly being appreciated. Mitochondrial and actin cytoskeletal networks not only interact with each other, but this multifaceted interaction shapes their functional dynamics. The interrelation between mitochondria and the actin cytoskeleton extends far beyond the requirement of mitochondrial ATP generation to power actin dynamics, and impinges upon several major aspects of cellular physiology. Being situated at the hub of cell signaling pathways, mitochondrial function can alter the activity of actin regulatory proteins and therefore modulate the processes downstream of actin dynamics such as cellular migration. As we will discuss, this regulation is highly nuanced and operates at multiple levels allowing mitochondria to occupy a strategic position in the regulation of migration, as well as pathological events that rely on aberrant cell motility such as cancer metastasis. In this review, we summarize the crosstalk that exists between mitochondria and actin regulatory proteins, and further emphasize on how this interaction holds importance in cell migration in normal as well as dysregulated scenarios as in cancer.
Keywords: actin, actin-binding proteins, mitochondria, dynamics, cancer, cell migration
INTRODUCTION
Mitochondria are crucial organelles for energy production inside the cell, and therefore play critical role in energy-dependent biological processes (Noguchi & Kasahara, 2018; Xia et al., 2019). However, the functions of mitochondria extend far beyond their classical role as the powerhouse of the cell. These semi-autonomous organelles are important for thermogenesis, maintenance of cellular redox potential, calcium (Ca2+) homeostasis, reactive oxygen species (ROS) production, cell signaling, cellular apoptosis, amino acid biosynthesis, fatty acid oxidation (FAO) and macromolecular metabolism (Corbet & Feron, 2017; Ernster & Schatz, 1981; Vakifahmetoglu-Norberg, Ouchida, & Norberg, 2017). Mitochondria interact with cytoskeletal (actin cytoskeleton, microtubules, and intermediate filaments) components in cells, a feature that is conserved from lower eukaryotes (yeast) all the way up to higher vertebrates. Cytoskeletal interaction plays a key role in dynamic changes in morphology and cellular distribution of mitochondria, eventually modulating their function (Boldogh & Pon, 2006; Boldogh et al., 2001; Ligon & Steward, 2000; Loveless, Qadota, Benian, & Hardin, 2017; Moore & Holzbaur, 2018; Morris & Hollenbeck, 1995; Ordonez, Lee, & Feany, 2018; Stürmer, Baumann, & Walz, 1995). The overall goal of this comprehensive review is to discuss the bidirectional communication between mitochondrial network and specifically the actin cytoskeletal system. First, we provide a brief general introduction of mitochondrial dynamics and function. Next, we discuss how components and molecular regulators of actin cytoskeleton and mitochondria reciprocally regulate each other’s function. Finally, we analyze the connection between the actin cytoskeleton and mitochondria specifically during cell migration in pathophysiological contexts.
A PRIMER ON MITOCHONDRIAL DYNAMICS AND FUNCTION
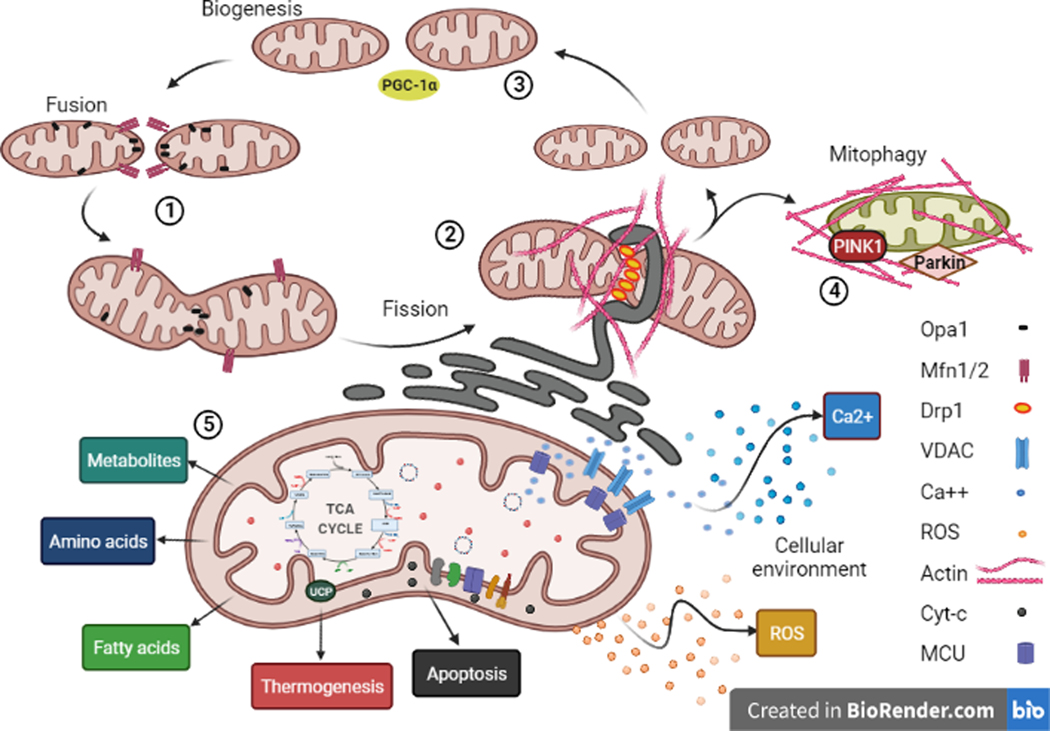
Mitochondria are highly dynamic organelles that undergo cycles of fission and fusion in order to maintain their function and cellular distribution. Mitochondrial fission takes place by membrane remodeling event initiated by the recruitment of the GTPase Dynamin-related protein 1 (Drp1) requiring the action of endoplasmic reticulum (ER)-associated filamentous actin (F-actin) network, and completed by the action of other dynamin-family proteins such as dynamin-2 (J. E. Lee, Westrate, Wu, Page, & Voeltz, 2016). During mitochondrial fusion, Outer Mitochondrial Membrane (OMM) is fused by the actions of mitofusins1 and 2 (Mfn1/2), followed by optic atrophy 1 (Opa1)-mediated fusion of the Inner Mitochondrial Membrane (IMM) (Tilokani, Nagashima, Paupe, & Prudent, 2018) (Fig 1). Impediment of mitochondrial network dynamics not only affect their function, but mitochondrial stress and dysregulation also triggers the intrinsic apoptotic cascade via cytochrome-c (cyt-c) release from the mitochondrial permeability transition pore (mPTP) (Kroemer, Galluzzi, & Brenner, 2007; Leung & Halestrap, 2008; Tait & Green, 2010). Given the crucial importance of mitochondria in cell survival, quality control of mitochondria is an important aspect of cellular function. In fact, damaged mitochondria undergo constant turnover through mitochondria-selective autophagy (a process known as mitophagy), requiring PINK1 (PTEN-associated kinase 1)-mediated mitochondrial recruitment of E3 ubiquitin ligase protein Parkin (Bingol & Sheng, 2016) (Fig 1).
Fig 1: Diverse mitochondrial functions in the cell:
Mitochondrial network undergoes constant steady-state dynamics and are involved in key cellular processes. The cellular gradients of mtROS and mtCa2+ are responsible for shaping the cytosolic environment.
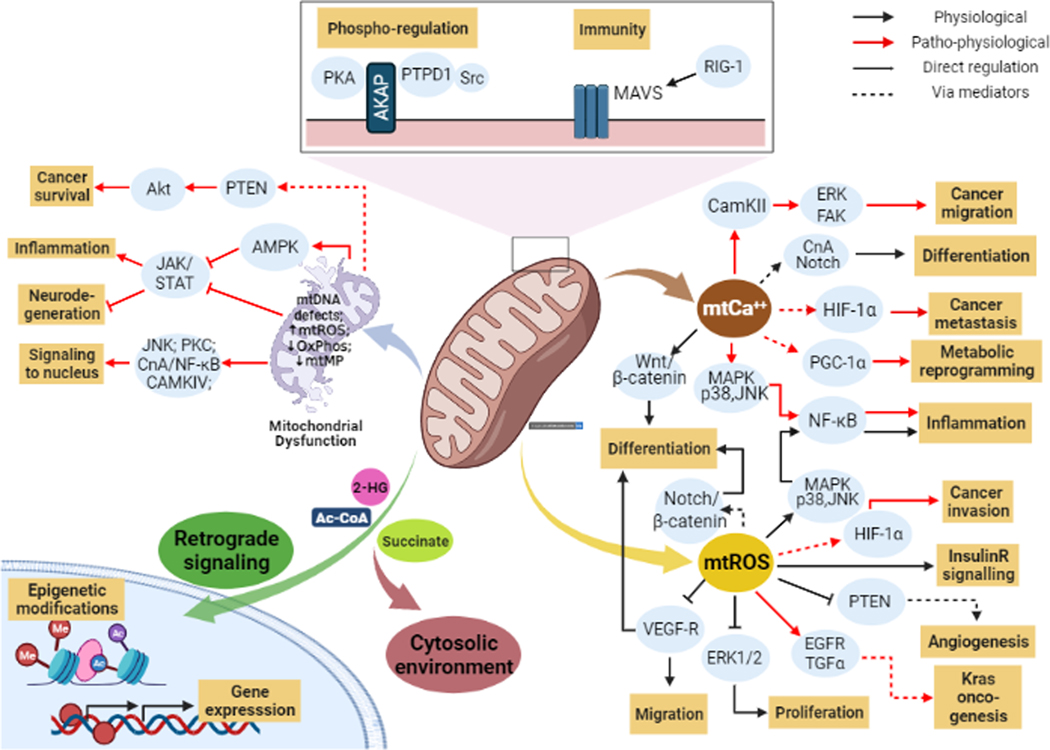
Mitochondrial function is highly diverse ranging from bioenergetics and biosynthesis (Fig 1) to regulation of important intracellular signaling pathways through signaling intermediate control (Fig 2). Therefore, mitochondrial activity impinges on virtually all biological processes including metabolism, cell migration/invasion, cell proliferation, differentiation, inflammatory response and neovascularization. The following section provides a brief summary of major aspects of mitochondrial functions.
Fig 2: Mitochondria act as a signaling hub:
Mitochondria perform various functions in the cell and coordinate diverse signaling pathways. These prominently involve fundamental pathways that control cell proliferation, differentiation, and migration as shown above.
Energy production.
Mitochondria are the major source of cellular ATP via the process of oxidative phosphorylation (OxPhos). Across the IMM, electron transport chain (ETC) complexes I-IV generate an electrochemical gradient (mitochondrial membrane potential, MMP) that drives ATP production by Complex V (Fernie, Carrari, & Sweetlove, 2004; Spinelli & Haigis, 2018).
Regulation of biosynthesis.
Several important biosynthetic pathways involve mitochondria at a central position in their regulation (Spinelli & Haigis, 2018). For example, mitochondria act as a hub for amino acid synthesis and heme metabolism (Cassago et al., 2012; Hancock, Liu, Alvord, & Phang, 2016; Sullivan et al., 2015). Mitochondria also provide the precursors for fatty acid synthesis and are indispensable for fatty acid oxidation (FAO) (Aon, Bhatt, & Cortassa, 2014; Mayr, 2015; Wanders, Ruiter, IJLst, Waterham, & Houten, 2010). In addition to providing the building blocks for cytosolic nucleotide synthesis, studies have also pointed that maintenance of nuclear genome integrity is also sensitive to mitochondrial nucleotide synthesis (M.-H. Lee, Wang, & Chang, 2014; Liya Wang, 2016).
Regulation of Cell Signaling.
Mitochondria receive, integrate and transmit many key signals in the intracellular milieu (Goldenthal & Marín-García, 2004; Zolotukhin et al., 2016) by several different mechanisms (illustrated in Fig 2). First, mitochondria are a major source for generating ROS (mtROS) which acts as a signaling intermediate to modulate a broad range of downstream signaling pathways and cellular activities (Zorov, Juhaszova, & Sollott, 2014). Examples of downstream actions of mtROS include: 1) ERK1/2-MAPK-mediated control of cell proliferation and growth (Weinberg et al., 2010), 2) PI3K/Akt-mediated regulation of angiogenesis (Connor et al., 2005; Sedding et al., 2013), 3) induction of Hypoxia-induced factor-1α (HIF-1α) signaling promoting cancer cell invasion (Hamanaka & Chandel, 2009; Shida et al., 2016), 4) modulation of JAK/STAT signaling and neurodegeneration (Monroe & Halvorsen, 2009), 5) AMP-kinase (AMPK)-dependent cellular energy sensing to control cell growth and metabolism (Choi et al., 2001; Hinchy et al., 2018; Jiang et al., 2019; J. Kim, Yang, Kim, Kim, & Ha, 2016; Mihaylova & Shaw, 2011; M. Quintero, Colombo, Godfrey, & Moncada, 2006; S. Wang, Song, & Zou, 2012; Wu, Viana, Thirumangalathu, & Loeken, 2012), and 6) regulation of Notch activation for Wnt-β-catenin signaling, a key pathway for cell fate and differentiation control (Hamanaka et al., 2013).
Second, mitochondria manage the levels of the ubiquitous second messenger Ca2+. Specifically, mitochondria associate with the ER as mitochondria-associated membranes (MAMs) that are required for Ca2+ homeostasis (van Vliet, Verfaillie, & Agostinis, 2014). Mitochondrial calcium (mtCa2+) control has been linked to the regulation of multiple signaling pathways including Akt/HIF-1α (Divolis, Mavroeidi, Mavrofrydi, & Papazafiri, 2016; Tosatto et al., 2016), calcineurin-mediated control of Notch signaling (Kasahara, Cipolat, Chen, Dorn, & Scorrano, 2013), CaMKII/ERK/FAK signaling (Sun et al., 2018) and p38-MAPK-dependent control of metabolic reprogramming via PGC-1 (Peroxisome proliferator-activated receptor-gamma coactivator-1alpha), a master inducer of mitochondrial biogenesis (Austin & St-Pierre, 2012; Gu et al., 2019).
Third, mitochondrial respiration can also regulate intracellular signaling. For example, the PI3K/Akt signaling pathway has been shown to be triggered by mitochondrial respiration (Pelicano et al., 2006). Mitochondrial stresses such as mitochondrial DNA (mtDNA) depletion and OxPhos inhibition can activate Ca2+-dependent kinases (PKC, JNK, MAPK and CaMKIV) and induce the NF-κB pathway (Biswas, Guha, & Avadhani, 2005). OxPhos dysfunction also affects the activity of JAK-STAT pathway through AMPK activation (D.-Y. Kim, Lim, Suk, & Lee, 2020). Furthermore, uncoupling proteins (UCP) that are localized on the IMM to regulate OxPhos activity (Brand & Esteves, 2005), have also been shown to be involved in regulating NF-κB/FAK/β-catenin signaling axis (Yu, Shi, Lin, Lu, & Zhao, 2020).
Fourth, mitochondrial metabolites (NAD+, acetyl-CoA, 2-hydroxyglutarate and succinate) post-translationally modify proteins to reciprocally regulate intracellular signaling (example: reciprocal regulation of AMPK-mTOR pathway and protein acetylation by acetyl-coA (Vancura et al., 2018), as well as contribute to mitochondria-to-nucleus retrograde signaling and epigenetic changes (Chandel, 2014; Chowdhury et al., 2011; M. Yang, Soga, & Pollard, 2013). We advise the reader to refer to several excellent articles that detail the role of mitochondrial metabolites and retrograde signaling in regulating cellular pathways (Castegna, Iacobazzi, & Infantino, 2015; Vyas, Zaganjor, & Haigis, 2016; D. Yang & Kim, 2019).
Fifth, mitochondria can also regulate cell signaling by acting as a physical platform to recruit other signaling molecules. For example, important protein kinases (e.g. PKA and Src) are recruited to OMM via AKAP scaffold proteins (Protein A‐kinase anchoring proteins), turning mitochondria into a potential structural platform to spatially regulate cell signaling (Livigni et al., 2006; Lucero, Suarez, & Chambers, 2019; Merrill & Strack, 2014).
BI-DIRECTIONAL COMMUNICATION BETWEEN MITOCHONDRION AND ACTIN CYTOSKELETON
Actin-dependent mitochondrial regulation
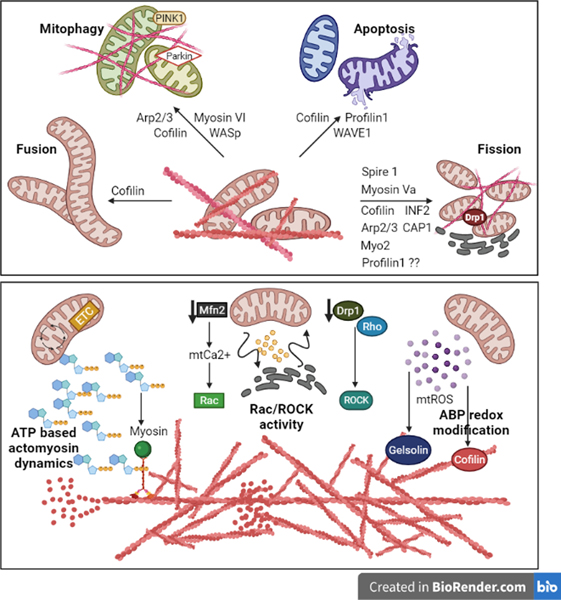
The existence of a connection between the actin cytoskeleton and mitochondria is known for several decades now. Electron microcopy observations of pig Leydig cells first showed that an actin envelope exists in direct contact with the mitochondria to immobilize them at a particular cytosolic location (Aguas, 1981). Experiments using ultra-purified mitochondrial fractions from mammalian cells further discovered the presence of actin in mitochondrial matrix and on the inner surface of the IMM (Etoh et al., 1990). Subsequent studies in yeast showed that defects in actin organization lead to defects in mitochondrial organization (Drubin, Jones, & Wertman, 1993). These findings are consistent with experimental evidence for actin-binding activity of mitochondria and the dependence on actin dynamics for mitochondrial motility (Boldogh et al., 2001; Lazzarino, Boldogh, Smith, Rosand, & Pon, 1994), as well as a direct correlation between mitochondrial function (ATP synthesis) and the functional state of the actin cytoskeleton (Stozharov, 1985). Although in higher eukaryotes, microtubule is the dominant player in directed movement of mitochondria, actin-mitochondria association is still important for short range transport, anchoring and cellular distribution of mitochondria (Kremneva, Kislin, Kang, & Khiroug, 2013; Saxton & Hollenbeck, 2012). Studies show that β-actin associates with mtDNA and its absence leads to impairment of mtDNA transcription, OxPhos activity and altered mitochondrial morphology (Reyes et al., 2011; Xie, Venit, Drou, & Percipalle, 2018). In vascular smooth muscle cells (VSMCs), actin organization and tension in the actin network alter mitochondrial network morphology and OxPhos activity (Bartolák-Suki et al., 2015). In human umbilical vascular endothelial cells, disruption of the actin network leads to decreased mtROS generation suggesting disrupted mitochondrial function (Ali, Pearlstein, Mathieu, & Schumacker, 2004). Collectively, these studies underscore the general importance of actin cytoskeleton in proper mitochondrial function. Since actin cytoskeletal organization is dynamically regulated by the actions of a wide range of actin-binding proteins (ABPs), we now review the involvement of individual ABPs in the regulation of mitochondrial function (Fig 3 provides a graphical summary of the main take home messages presented in this section). We break down the following section based on broad functional categories of major ABPs.
Fig 3: The crosstalk between mitochondrial function and ABP-mediated actin dynamics:
ABPs and the actin cytoskeleton can associate with the mitochondria to regulate their dynamics, biogenesis and mitophagy. Moreover, mitochondrial ATP, ROS and Ca2+ directly influence the activity of ABP-mediated actin dynamics.
Actin nucleation promoting factors (ANPFs):
The three well-studied actin nucleating factors are Arp2/3 complex, formin and SPIRE. The Arp2/3 complex is a conserved assembly of 7 proteins that nucleates new actin branches from existing parent filaments (Pollard, 2016). It can associate to the OMM and is required for mitochondrial motility and maintenance of morphology in yeast (Boldogh et al., 2001). Arp2/3 complex is also required for F-actin’s association to mitochondria and localized mitochondrial fission via Drp1, and abrogation of Arp2/3-mediated actin cycling impairs cellular balance of mitochondrial fission-fusion (S. Li et al., 2015; Moore, Wong, Simpson, & Holzbaur, 2016). Although experiments involving formin inhibitor SMIFH2 in the latter study suggested a possible co-operation between formin and Arp2/3 complex in maintenance of mitochondrial network equilibrium, off-target inhibitory effects of SMIFH2 on other ABPs (such as myosin) (Y. Nishimura, Shi, Zhang, et al., 2021) warrant a direct molecular validation for collaboration between Arp2/3 and formin in this process in future studies. Arp2/3 complex has also been recently implicated in the process of mitophagy (Kruppa et al., 2018). The formation of Arp2/3-nucleated F-actin cages is essential for the segregation of damaged mitochondria to prevent their re-fusion with the network, suggesting importance of Arp2/3 in the maintenance of a healthy mitochondrial network. Consistent with the critical role of Wiskott-Aldrich Syndrome protein (WASp) family of ABPs in signal-induced activation of Arp2/3 complex (Pollard, 2016), loss-of-function studies expectedly demonstrated the importance of WASp proteins in F-actin cage formation around damaged mitochondria and mitophagy (Kruppa et al., 2018), non-selective autophagy and OxPhos metabolic function of mitochondria (Danial et al., 2003; Rivers et al., 2020). Interestingly, the formation of a complex between WAVE1 (WASp-family verprolin homology protein 1) and mitochondrial apoptosis regulator Bad further indicated its involvement in modulating the mitochondrial apoptosis pathway. Therefore, WAVE1 might be a coordinator for mitochondrial metabolism and cell death signaling (J. Yang, Li, Wang, & Zhang, 2010).
Formins constitute a diverse family of proteins that can both nucleate and elongate actin filaments. Mammalian diaphanous 1 (mDia1- a major formin isoform) is involved in regulating short-range mitochondrial motility by anchoring mitochondria to the actin cytoskeleton (Minin et al., 2006) and mitochondrial morphology (D. Li, Dammer, Lucki, & Sewer, 2013; D. Li & Sewer, 2010). Since formins also interact with microtubules, long distance mitochondrial transport is also sensitive to perturbation of formins (Gaillard et al., 2011). Mass-spectrometric analysis has shown that mDia1 interacts with several components of the mitochondrial OxPhos complexes-I, -II, -III, -IV and –V, and influences mitochondrial biogenesis (Saleh, Subramaniam, Raychaudhuri, & Dhawan, 2019). Although mDia1 strongly associates with prohibitin 2 (Phb2 – an important regulator of mitochondrial bioenergetics) (Signorile, Sgaramella, Bellomo, & De Rasmo, 2019), the experimental evidence for mDia1’s involvement in mitochondrial OxPhos regulation is currently lacking in the literature. Functional association between formin and mitochondria has been most convincingly demonstrated in the context of mitochondrial fission, a process that requires bending of ER membrane around mitochondria and induction of a pre-constriction step to allow Drp1 recruitment (Friedman et al., 2011). ER-anchored inverted formin-2 (INF-2) promotes actin assembly around the mitochondria, a step critical for Drp1-mediated mitochondrial fission (Hatch, Ji, Merrill, Strack, & Higgs, 2016; Korobova, Ramabhadran, & Higgs, 2013). INF2-mediated actin polymerization also presumably drives mitochondrial fission in a Drp1-independent manner through promoting ER-to-mitochondria Ca2+ transfer, elevating mtCa2+ level and in turn resulting in IMM constriction (Chakrabarti et al., 2018). Recently, loss-of-function studies in keratinocytes also suggested INF2’s potential role as a mediator of mitochondria-involving intrinsic apoptotic pathway (Chen et al., 2019). However, whether INF2 mediates apoptosis directly or indirectly through its involvement in mitochondrial fission (a pre-requisite for mitochondria-driven apoptotic cascade) needs to be further scrutinized.
SPIRE belong to a third class of actin nucleating proteins that are operative at cell membranes and essential for vesicular trafficking inside the cells (Pylypenko et al., 2016). Spire-1C, a highly conserved isoform of Spire-1, localizes to the OMM and promotes actin filament assembly on the mitochondrial membrane independently of its formin-interaction. However, binding to INF2 formin appears to be required for Spire-1C-facilitated mitochondrial fission via ER-mediated mitochondrial constriction (Manor et al., 2015). Additionally, Spire-1C’s association with myosin-V at the OMM aids Drp1 recruitment (Araujo et al., 2019). Since mitochondrial motility is increased and decreased upon loss- and gain-of-functions of Spire-1C, respectively, Spire-1C likely plays a role in mitochondrial anchoring rather than stimulating motility. Given prior evidence for myosin-V in stalling vesicular trafficking in axons (Janssen et al., 2017), one can speculate that Spire-1C utilizes myosin-V’s interaction for its mitochondrial anchoring action.
Actin Elongating Factors (AEFs):
Formin (already discussed in the previous section) and Ena (enabled)/Vasodilator-stimulated phosphoprotein (VASP) proteins are the two major classes of AEFs that strongly influence the overall cytoskeletal architecture of cells. VASP knockdown affects cristae organization in the IMM and mitochondrial distribution in cells (Ying Wang et al., 2015). At the functional level, VASP function has been also linked to various metabolic regulations including mitochondrial fatty acid oxidation (Tateya et al., 2019, 2013; X. Wang, Pluznick, Settles, & Sansom, 2007). Whether these changes are due to VASP-dependent global cytoskeletal disturbances or other specific mechanisms remain unclear.
Actin depolymerizing factor (ADF):
Cofilin is a major F-actin depolymerizing and severing protein, targeted mutations of which results in altered mitochondrial OxPhos activity, mtROS generation, fatty acid oxidation as well as mitochondrial biogenesis in yeast (Kotiadis et al., 2012). Interestingly, cofilin-dependent changes in mitochondrial phenotypes were observed not only in mutants with altered actin cytoskeleton, but also in select mutants where mutations were introduced in residues with no role in actin regulation and involved post-transcriptional upregulation of mitochondrial function. The role of cofilin-dependent actin dynamics in regulating mitochondrial function was also established in mammalian contexts through demonstration of serum-response factor (SRF)-stimulated mitochondrial fusion and ATP production in neurons through cofilin inactivation (Beck et al., 2012). This is further supported by experimental observation of impaired Drp1 accumulation to the OMM and reduced mitochondrial fission in mouse embryonic fibroblasts (MEFs) upon cofilin downregulation (S. Li et al., 2015), a phenotype that is related to its actin depolymerizing function (Rehklau et al., 2017). Cofilin has also been shown to be important for mitophagy (G.-B. Li et al., 2018). Of further interest, post-translational modification (oxidation and dephosphorylation of select cysteine and serine residues) and mitochondrial translocation of cofilin is an early step in inducing mitochondrial swelling, cyt-c release and triggering mitochondria-mediated apoptosis in the setting of oxidative cellular injury that occurs independently of pro-apoptotic Bax activation (Chua et al., 2003; Klamt et al., 2009). However, there is also indication for cofilin-dependent regulation of mitochondria-initiated apoptosis through modulation of Bcl-2 proteins (Posadas, Pérez-Martínez, Guerra, Sánchez-Verdú, & Ceña, 2012). Overall, these observations suggest a possible role of cofilin as a stress-sensor during oxidative cellular injury where cofilin might be discharged from its role in actin dynamics and translocated to the mitochondria to mediate mitochondrial responses (Hoffmann, Rust, & Culmsee, 2019). Indeed, under stressed conditions, cofilin-mediated actin turnover is suppressed reducing ATP hydrolysis (Bernstein, Chen, Boyle, & Bamburg, 2006; Munsie, Desmond, & Truant, 2012).
G-actin-binding proteins:
Thymosin and profilin are the two major classes of G-actin binding proteins that determine the monomeric actin availability for polymerization. Thymosin-β4, a ubiquitously expressed actin monomer sequestering protein that inhibits actin polymerization, has been shown to stimulate mitochondrial anti-oxidant enzymes and downregulate ROS levels (Ho et al., 2008). Conversely, knocking down thymosin-β4 leads to increased mtROS production. Thymosin-β4 suppression leads to widespread mitochondrial defects including a decrease in mtDNA copy number, loss of mitochondrial membrane permeability, degeneration of cristae morphology and a metabolic shift to the glycolytic pathway (Tang & Su, 2011). Profilin, a nucleotide-exchange factor for actin that generally promotes actin polymerization utilizing its interaction with various poly-proline domain-bearing actin-assembly factors (e.g. formin and Ena/VASP), has also been shown to be important for maintenance of mitochondrial integrity and function (Wen, McKane, Stokasimov, & Rubenstein, 2011). Deletion of the Pfn1 (the major form of profilin) gene in hematopoietic stem cells also increases mtROS levels and alters mitochondrial membrane permeability (Zheng et al., 2014), phenotypes that are attributed to the loss of actin and poly-proline interactions of Pfn1.
Motor proteins:
The myosin superfamily of actin-based motor proteins in mammals is estimated to have 25–30 structurally and functionally distinct classes. Earlier studies in yeast showed that the Myo2 (belonging to myosin V class) motor links mitochondria to the actin-cytoskeleton and enables mitochondrial motility (Boldogh & Pon, 2006). Further, Myo2 also mediates proper inheritance of mitochondria into daughter cells during yeast cytokinesis (Eves, Jin, Brunner, & Weisman, 2012). Similar findings were extended related to Myo19 in human cells (O. A. Quintero et al., 2009; Rohn et al., 2014). Other forms of myosin (myosin II, V and VI) have also been functionally linked to mitochondrial anchoring (Kelleher et al., 2000; Pathak, Sepp, & Hollenbeck, 2010; Straub et al., 2020), and acto-myosin force generation and mitochondrial fission through concerted actions with other ABPs (e.g. formin, SPIRE) (Araujo et al., 2019; DuBoff, Götz, & Feany, 2012; Y. Nishimura, Shi, Li, Bershadsky, & Viasnoff, 2021; C. Yang & Svitkina, 2019). There is also experimental evidence for the role of myosins in mitochondrial quality control by mitophagy. For example, Myo19 degradation and decoupling of mitochondria from actin-based motility are crucial steps to prevent refusion of damaged mitochondria during mitophagy (López-Doménech et al., 2018). Myosin VI (interacts with Parkin) is involved in Parkin-mediated mitophagy upon CCCP-induced mitochondrial damage (Kruppa et al., 2018).
Other ABPs:
In addition to the ABPs listed above, several studies have linked the activities of actin cross-linking, severing and other polymerization-promoting proteins to the modulation of mitochondrial morphology and/or function. For example, actin cross-linking protein Filamin A interacts with the GTPase domain of Drp1 to accelerate its activity leading to mitochondrial hyperfission in hypoxic setting (A. Nishimura et al., 2018). F-actin bundling protein transgelin binds to p53 and induces mitochondria-mediated apoptosis pathway in a p53-dependent manner (Zhang, Yang, Zheng, & Chen, 2010). Transgelin knockdown alters the cellular distribution of mitochondrial network and elevates ROS levels (L. Yang et al., 2019). The actin-severing protein villin localizes to mitochondria and blocks apoptosis progression utilizing its actin-severing activity (Y Wang, George, Srinivasan, Patnaik, & Khurana, 2012). Suppression of villin-mediated actin dynamics leads to damaged mitochondrial morphology and renders cells sensitive to apoptosis (Roy et al., 2018). Cortactin (promotes actin polymerization) is one of the ABPs that is accumulated on mitochondrial surface along with F-actin when fission is blocked, and furthermore suppression of cortactin expression inhibits experimentally-induced mitochondrial fragmentation suggesting that cortactin’s action is important for maintenance of mitochondrial fission-fusion balance (S. Li et al., 2015). Finally, zyxin, a focal-adhesion associated protein that also promotes actin assembly, localizes to mitochondria and binds to mitochondrial anti-viral signaling protein (MAVS) on the OMM to regulate anti-viral innate immune responses (Kouwaki et al., 2017).
In summary, it is abundantly clear that the actin cytoskeleton and its regulatory proteins have an extensive involvement in regulating various aspects of mitochondrial physiology.
Mitochondrial Function Modulates Actin Dynamics
The previous section documents how actin cytoskeleton and its regulatory proteins are extensively involved in regulating various aspects of mitochondrial biology. In this section, we discuss our current understanding of how mitochondrial function influences actin cytoskeletal dynamics.
First, actin polymerization is an energy-demanding process, and actin dynamics is greatly impaired when mitochondrial ATP synthesis is blocked (Atkinson, Hosford, & Molitoris, 2004; Bernstein & Bamburg, 2003; Sablin et al., 2002) suggesting that the most evident way mitochondria influence actin cytoskeleton is through their role in supplying ATP. Mitochondrial ATP also fuels myosin light chain (MLC) of the myosin motors during vesicle trafficking and force generation across the cell (Bartolák-Suki et al., 2015; Verstreken et al., 2005). Second, proteomics analyses have shown that oxidative stress and OxPhos inhibition alters the expression levels of actin and many actin cytoskeleton-related proteins (Annunen-Rasila, Ohlmeier, Tuokko, Veijola, & Majamaa, 2007; Gielisch & Meierhofer, 2015). Third, proteins that regulate mitochondrial network dynamics can induce changes in F-actin organization. For example, Miro-1, a protein that tethers mitochondria to the cytoskeleton and enables organelle motility, leads to inhibition of myosin activity and defects in actin polymerization when silenced in expression (Morlino et al., 2014). Likewise, loss-of-function of mitochondrial fission protein Drp1 alters actin cytoskeleton through disrupting Ca2+ homeostasis (Ponte et al., 2020) and inhibition of the Rho/ROCK pathway (M. Yin et al., 2016). It is also possible that since in the absence of Drp1, mitochondria become incompetent to divide, they cannot be efficiently trafficked to the regions of active F-actin remodeling. Deletion of mitochondrial fusion protein Mfn2 also introduces changes in actin organization partly through affecting the activation status of Rac GTPase (W. Zhou et al., 2020). Fourth, ROS has well-established role in regulating actin dynamics (Moldovan, Irani, Moldovan, Finkel, & Goldschmidt-Clermont, 1999; Muliyil & Narasimha, 2014; Xu & Chisholm, 2014). Altered mitochondrial function and mtROS levels can impact actin cytoskeleton either by direct chemical modification of actin itself (affects polymerization ability of actin as well as the stability of actin filaments) or indirectly through altering the activities of select ABPs (Wilson & González-Billault, 2015). For example, cofilin and gelsolin activities are sensitive to cellular redox environment and mtROS levels (Bernstein et al., 2006; J.-S. Kim, Huang, & Bokoch, 2009; Minamide, Striegl, Boyle, Meberg, & Bamburg, 2000; Mittal, Siddiqui, Tran, Reddy, & Malik, 2014; Popova et al., 2010).
In summary, the crosstalk between mitochondria and actin is a highly coordinated process that shapes the dynamics of both the networks.
THE ACTIN -MITOCHONDRIA CROSSTALK IN CELL MIGRATION
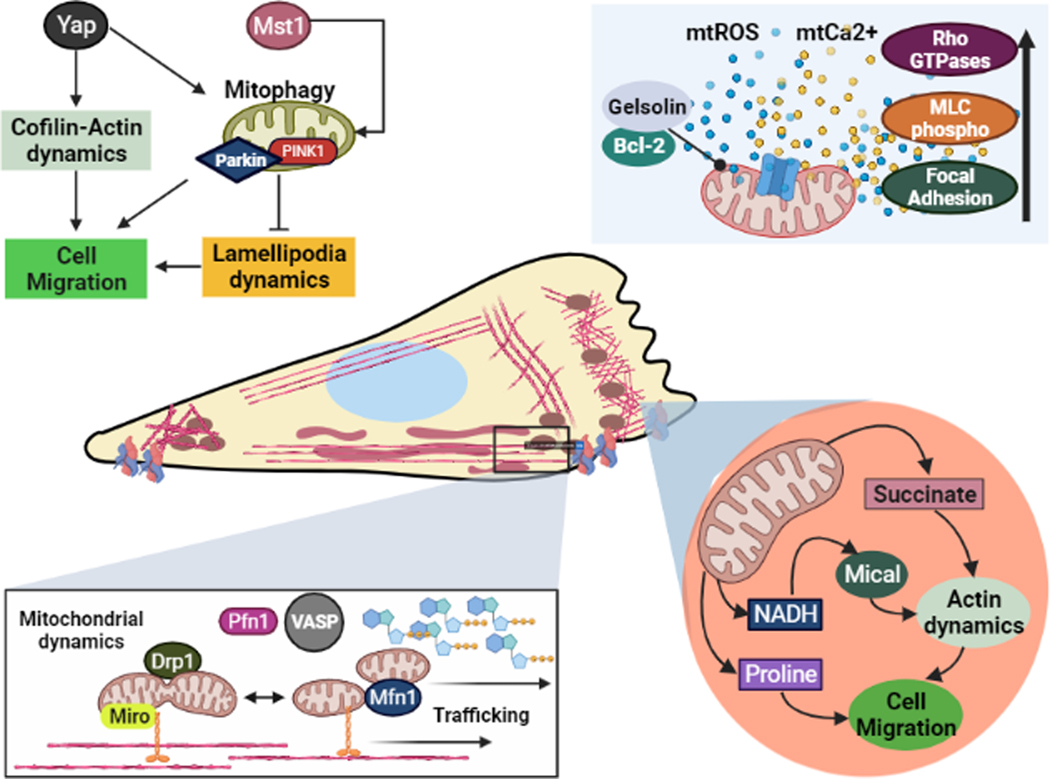
Actin-based cell migration is fundamental to development, wound healing, immune response and neovascularization. It is a tightly regulated and energetically expensive cellular process that involves sequential steps of membrane protrusion at the leading edge, formation of focal adhesions, forward translocation of the cell body, and finally, cell detachment from the rear end (Schaks, Giannone, & Rottner, 2019). In this section, we discuss how the interplay of mitochondria with structural and regulatory components of actin cytoskeleton regulates cell migration in physiological and pathological (specifically related to cancer) contexts (Fig 4 provides a graphical summary of the main take home messages presented in this section).
Fig. 4: Mitochondria-actin cytoskeleton crosstalk in cell migration:
During cell migration, the actin cytoskeleton and mitochondrial network interact closely to carry out lamellipodia-based motility. To sustain actin dynamics, mitochondria are strategically trafficked to the leading edge; where they modulate ABPs such as MICAL and Rho GTPases to directly affect actin-mediated cell migration.
Mitochondria, actin cytoskeleton and normal cell migration
Lamellipodial protrusion, the initiating step of cell migration, is driven by cycles of actin polymerization and depolymerization at the leading edge. Since this process requires ATP, mitochondrial OxPhos is critical for protrusive activities as experimentally supported by decreased F-actin polymerization and impairment in lamellipodia formation and cell migration when OxPhos is blocked (Zhao et al., 2013). Localized energy production at the sites of actin remodeling is facilitated by mitochondrial trafficking to the leading edge in response to local AMPK activation (Cunniff, McKenzie, Heintz, & Howe, 2016). Inhibition of mitochondrial trafficking to the leading edge by depletion of mitochondrial transport adaptor Miro leads to defects in mitochondrial coupling to actin cytoskeleton, actin polymerization, formation and turnover of adhesions, and cell migration (both single cell and collective) underscoring the importance of mitochondrial trafficking in cell migration (López-Doménech et al., 2018; Morlino et al., 2014; Schuler et al., 2017). Mitochondrial ATP synthesis is also involved in cell detachment from the substratum during migration. At the trailing edge, mitochondrial ATP generation powers MLC phosphorylation and is required for the relocation of cellular organelles during migration (Campello et al., 2006). Further, mitochondrial energy synthesis is closely linked to its fission-fusion dynamics. Migratory stimulation of VSMCs increases mitochondrial localization and Drp1 activity prior to cell migration (Li Wang et al., 2015). Drp1-mediated mitochondrial fragmentation is critical for the establishment of cell polarity, lamellipodia formation and efficient cell migration (H. J. Kim et al., 2015). Interestingly, the energy demand for cell migration can also dictate mitochondrial structural dynamics. For example, a microenvironment that requires less energy for cell migration (such as ameboid cell migration through less dense 3D extracellular matrix) promotes mitochondrial fragmentation lowering OxPhos resulting in higher AMPK activation. This can in turn elevate acto-myosin contractility (a key driver of ameboid cell migration) through stimulating myosin activity (via inhibition of myosin phosphatase) and actin remodeling (Crosas-Molist et al., 2021). Collectively, these observations suggest the existence of a complex sensory feedback loop between energy demand, mitochondrial dynamics and trafficking, and ATP-dependent cytoskeletal control during cell migration.
In addition to providing the energy demand for cell migration, mitochondria can also influence cell migration by acting as an important controller of secondary messengers, in particular Ca2+ and ROS. mtCa2+ homeostasis signals to the actin cytoskeleton to regulate cell migration. For example, in developing zebrafish embryos, silencing of mitochondrial calcium uniporter (MCU – responsible for mtCa2+ uptake) impairs F-actin remodeling leading to defects in cell migration (Prudent et al., 2013). Similar phenotypes were also observed in human cells where silencing of MCU led to changes in stiffness in actin cytoskeleton, loss of cell polarity, and reduced focal adhesion turnover resulting in impaired cell migration. At the molecular level, these phenotypes were correlated with reduced ER and cytosolic Ca2+ levels and suppression of Rho-GTPase activation and activity of calpain (a Ca2+-activated protein that is important for focal adhesion turnover) but were independent of intracellular energy levels (Prudent et al., 2016). Mitochondrial activity has been also linked to Ca2+ homeostasis and F-actin dynamics in in vivo wound healing models in C. elegans (Ponte et al., 2020; Xu & Chisholm, 2014). Wound-triggered Ca2+ influx also causes local production of mtROS which is an important signaling molecule. Depletion of mtROS blocks actin-based cell migration and wound healing through modulating Rho-GTPase activity, underscoring the importance of ROS as a downstream mediator of mitochondria-dependent control of cell migration (Xu & Chisholm, 2014). A recent mouse model study also corroborates the importance of mtROS signaling in cell migration during wound healing through an additional role of regulating α-smooth muscle actin (α-SMA) expression (S. Yang, Xu, Meng, & Lu, 2020).
Finally, mitochondrial metabolites also interplay with the actin cytoskeleton to control cell migration. For example, succinate produced from the TCA cycle can upregulate human mesenchymal stem cell migration through PKC activation (Ko et al., 2017). Succinate also promotes an increase in ATP production and mtROS generation to influence actin cytoskeleton. NADH is another mitochondrial metabolite that controls cell migration, (van Horssen et al., 2013; H. Yin et al., 2012). At the molecular level, intracellular NADH levels control the activity of MICAL (Microtubule-associated monooxygenase, calponin and LIM domain containing), a redox-sensitive protein that promotes actin cytoskeletal disassembly (Vanoni, Vitali, & Zucchini, 2013) and modulates cell migration (Y Wang et al., 2018). Thus, mitochondria-controlled intracellular NAD/NADH ratio is likely an important parameter to control the state of actin cytoskeleton and cell migration.
Mitochondria, actin cytoskeleton and cancer Cell Migration
Dysregulated migration promotes the dissemination of tumor cells and is a hallmark of malignant progression of cancer. We present several lines of key experimental evidence that support the role of actin-mitochondria crosstalk in cancer cell migration and metastasis.
First, transcriptomic analyses show evidence for dysregulated OxPhos and actin cytoskeleton signaling in metastatic circulating tumor cells (CTCs) (LeBleu et al., 2014). In fact, upregulation of PGC-1α-mediated mitochondrial metabolism confers increased invasive ability to CTCs. Second, mitochondrial translocation to the cortical actin cytoskeleton with promotion of cell motility has been shown to be a key mechanism of how tumor cells can readapt to therapeutic inhibition of oncogenic PI3K/Akt signaling (a pro-migratory pathway) regaining their invasive potential (Caino et al., 2015). Third, certain malignant cancer cells (breast and thyroid) are known to overexpress mitochondrial fission protein Drp1 and display more fragmented mitochondria than their non-metastatic counterparts (Ferreira-da-Silva et al., 2015; Zhao et al., 2013). Furthermore, silencing Drp1 or overexpression of Mfn1 (leads to elongated mitochondria) suppresses chemoattractant-induced recruitment of mitochondria in the lamellipodial region, formation of lamellipodia and breast cancer cell migration. Treatment with mitochondrial uncoupling agent or ATP synthesis inhibitor also induces a similar cell migration defect (Zhao et al., 2013). Contradicting these findings, migration of hepatocellular carcinoma cells (HCC) is in fact promoted by mitochondrial fission utilizing a Ca2+/CaMKII/FAK/ERK downstream signaling pathway (Sun et al., 2018). These apparently contradictory findings could either mean context-specific effect of mitochondrial dynamics on cell migration and/or an optimum range of mitochondrial fragmentation being most conducive for productive cell motility as excessive mitochondrial fragmentation could severely compromise its bioenergetic functionality thereby adversely affecting cell migration. Nonetheless, these observations support a causal relationship between mitochondrial dynamics/activity and tumor cell migration.
Fourth, as previously discussed in the context of normal cell migration, mtROS is a major player in regulating mitochondria-actin crosstalk in cancer scenarios. However, the effect of mtROS on cancer cell migration appears to be cell-line-specific. For example, downregulation of mtROS in certain cervical cancer cell lines (siHa and Ca-ski) led to formation of actin stress-fibers and circumferential F-actin ring suppressing their migration-promoting epithelial-to-mesenchymal transition ability (Shagieva et al., 2017). These findings are consistent with mtROS upregulation secondary to knockdown of actin-binding protein transgelin associated with increased migration and metastatic ability of breast cancer cells (L. Yang et al., 2019). However, in Hela cervical cancer cell line, suppression of mitochondrial complex-I (which also reduced mtROS production) led to enhanced cell adhesion and migration/invasion, a phenotype that was attributed to ROS-dependent alteration in the expression of certain ECM molecules (He et al., 2013). Although the reasons underlying this apparent discrepancy are unclear, it is possible that mitochondrial complex 1 inhibition could elicit pro-migratory response through unique metabolic reprograming.
Fifth, there is increasing appreciation of the role of mitophagy in controlling cancer cell migration and metastasis through regulation of actin cytoskeleton dynamics (Yigang Wang et al., 2020). Mitophagy has been suggested to directly modulate cell migration by altering F-actin and microtubule formation (H. Zhou et al., 2017). In pancreatic cancer cells, mammalian STE20-like kinase 1 (Mst1) of the Hippo signaling pathway upregulates mitophagy to promote cell migration and metastasis (Hu et al., 2020). In gastric cancer cells, knockdown of Hippo pathway effector Yes-associated protein (YAP) reduced mitophagy and F-actin level through excessive ROS production blunting lamellipodial dynamics and cell motility (Yan et al., 2018). Contrasting these pro-migratory effects of mitophagy, induction of Parkin-mediated autophagy inhibited the migratory ability of myeloma cells (Fan et al., 2020). Although mitophagy is generally viewed to improve the functionality of the mitochondrial network by eliminating dysfunctional mitochondria, in certain physiological setting mitophagy has been demonstrated to eliminate functional mitochondria as well (Twig & Shirihai, 2011), and this may explain study-specific differences with regard to the effect of perturbation of mitophagy on cancer cell migration
Clinical Significance of the Mitochondria-Actin/ABP Crosstalk
Metabolic changes as a consequence of altered mitochondrial function lie at the heart of progression of many diseases including cancer (Porporato, Filigheddu, Pedro, Kroemer, & Galluzzi, 2018). In the foregoing sections, we discussed how mitochondria and actin cytoskeleton regulate each other ultimately impacting cellular morphology, migration/invasion, and other aspects (e.g. cell survival, EMT) relevant for cancer progression. Oncogene-induced alteration in actin cytoskeletal structure is a hallmark of cancer cells. Although beyond the scope of this review, there is abundance of literature documenting dysregulated expressions of various ABPs causally linked to increased tumor aggressiveness in human cancer. However, ABPs have rarely been considered as therapeutic targets in cancer. With increasing evidence for structural and functional changes in mitochondria in cancer cells upon molecular perturbations of various ABPs (G. Li et al., 2015; Lin et al., 2019; C. Wang, Zhou, Vedantam, Li, & Field, 2008; Yao et al., 2013), targeting some of those ABPs that are important for regulating tumor progression, and amenable to pharmacological inhibition (Arp 2/3, formins and Pfn1 as a few examples) may offer dual benefits of at least partially achieving cytoskeletal and mitochondrial normalization. These types of proof-of-concepts should be explored in future studies. If successful, such strategies could pave the way for conceptually novel therapeutic directions in cancer. Furthermore, given the importance of mitophagy as a protective mechanism in the settings of cardiac ischemia and myocardial injury (Tan et al., 2020; J. Wang & Zhou, 2020; Yue Wang et al., 2021; H. Zhou, Ren, Toan, & Mui, 2021), and role of several ABPs in regulating mitophagy as we previously discussed, targeting actin-mitochondria crosstalk may have therapeutic potential in cardiovascular diseases.
CONCLUDING REMARK
The mitochondria exist as a complex and dynamic organelle network that bi-directionally communicates with actin cytoskeletal network to regulate many cellular functions including cell migration. This communication extends far beyond the classical paradigm of mitochondria supplying ATP to power actin polymerization during cell migration. While spatiotemporal controls of mitochondrial fission/fusion dynamics and their localization to the sites of actin remodeling through trafficking are important aspects for cell motility, the underlying molecular details of how motility-inducing signals couple to dynamic control of mitochondrial structure and localization are still not clear, and should be topics of interest for future studies. The interplay between the mitochondrial and actin cytoskeletal networks also holds importance in cancer cell metastasis. Given that the metabolic underpinnings of different cancer types are being increasingly appreciated, the plasticity that mitochondria bestow during cancer progression is set to become clearer with our better understanding of the actin-mitochondria crosstalk through future studies.
ACKNOWLEDGEMENTS
The authors wish to acknowledge funding from the National Cancer Center fellowship and Imaging Sciences in Translational Cardiovascular Training Program fellowship (T32-HL129964) (to Gau), DOD Grant #W81XWH-19–1-0768 (Roy) and NIH grants CA248873 (Roy) Yadav is a KVPY fellow (SX 1611105) funded by the Indian Institute of Science and an Infosys Foundation Scholar funded by the Infosys Foundation. Figures were created with BioRender.com.
ABBREVIATIONS
- ABP
Actin-binding proteins
- ADF
Actin depolymerizing factor
- AEF
Actin elongating factors
- AKAP
Protein A-kinase anchoring proteins
- AMPK
AMP-kinase
- ANPF
Actin nucleation promoting factors
- Arp2/3
Actin-related protein 2/3
- Cyt-c
Cytochrome-C
- Drp1
Dynamin-related protein1
- Ena/VASP
Enabled/vasodilator-activated phosphoprotein
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- FAO
Fatty acid oxidation
- IMM
Inner mitochondrial protein
- MAM
Mitochondrial Associated Protein
- mDia
Mammalian diaphanous
- Mfn11/2
Mitofusin 1 and 2
- MICAL
Microtubule-associated monooxygenase, calponin and LIM domain containing
- mtROS
Mitochondrial reactive oxygen species
- OMM
Outer mitochondrial membrane
- Opa1
Optic atrophy 1
- Pfn
Profilin
- ROS
Reactive oxygen species
- WASp
Wiskott-Aldrich syndrome protein
- WAVE1
WASp-family verprolin homology protein 1
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES:
- Aguas AP (1981). Direct contact between actin-like microfilaments and organelles in pig Leydig cells. Journal of Ultrastructure Research, 74(2), 175–182. 10.1016/s0022-5320(81)80075-5 [DOI] [PubMed] [Google Scholar]
- Ali MH, Pearlstein DP, Mathieu CE, & Schumacker PT (2004). Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. American Journal of Physiology. Lung Cellular and Molecular Physiology, 287(3), L486–96. 10.1152/ajplung.00389.2003 [DOI] [PubMed] [Google Scholar]
- Annunen-Rasila J, Ohlmeier S, Tuokko H, Veijola J, & Majamaa K. (2007). Proteome and cytoskeleton responses in osteosarcoma cells with reduced OXPHOS activity. Proteomics, 7(13), 2189–2200. 10.1002/pmic.200601031 [DOI] [PubMed] [Google Scholar]
- Aon MA, Bhatt N, & Cortassa SC (2014). Mitochondrial and cellular mechanisms for managing lipid excess. Frontiers in Physiology, 5, 282. 10.3389/fphys.2014.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JS, Silva-Junior RMP, Zhang T, Schiavon CR, Chu Q, Wu M, … Espreafico EM (2019). A novel role for Myosin-Va in mitochondrial fission. BioRxiv, 655803. 10.1101/655803 [DOI]
- Atkinson SJ, Hosford MA, & Molitoris BA (2004). Mechanism of actin polymerization in cellular ATP depletion. The Journal of Biological Chemistry, 279(7), 5194–5199. 10.1074/jbc.M306973200 [DOI] [PubMed] [Google Scholar]
- Austin S, & St-Pierre J. (2012). PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. Journal of Cell Science, 125(Pt 21), 4963–4971. 10.1242/jcs.113662 [DOI] [PubMed] [Google Scholar]
- Bartolák-Suki E, Imsirovic J, Parameswaran H, Wellman TJ, Martinez N, Allen PG, … Suki B. (2015). Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nature Materials, 14(10), 1049–1057. 10.1038/nmat4358 [DOI] [PubMed] [Google Scholar]
- Beck H, Flynn K, Lindenberg KS, Schwarz H, Bradke F, Di Giovanni S, & Knöll B. (2012). Serum Response Factor (SRF)-cofilin-actin signaling axis modulates mitochondrial dynamics. Proceedings of the National Academy of Sciences of the United States of America, 109(38), E2523–32. 10.1073/pnas.1208141109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, & Bamburg JR (2003). Actin-ATP hydrolysis is a major energy drain for neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(1), 1–6. 10.1523/JNEUROSCI.23-01-00002.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Chen H, Boyle JA, & Bamburg JR (2006). Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. American Journal of Physiology. Cell Physiology, 291(5), C828–39. 10.1152/ajpcell.00066.2006 [DOI] [PubMed] [Google Scholar]
- Bingol B, & Sheng M. (2016). Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radical Biology and Medicine, 100, 210–222. 10.1016/J.FREERADBIOMED.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Biswas G, Guha M, & Avadhani NG (2005). Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene, 354, 132–139. 10.1016/j.gene.2005.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh IR, & Pon LA (2006). Interactions of mitochondria with the actin cytoskeleton. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1763(5–6), 450–462. 10.1016/J.BBAMCR.2006.02.014 [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Yang H-C, Nowakowski WD, Karmon SL, Hays LG, Yates JR, & Pon LA (2001). Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proceedings of the National Academy of Sciences, 98(6), 3162 LP – 3167. 10.1073/pnas.051494698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, & Esteves TC (2005). Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metabolism, 2(2), 85–93. 10.1016/J.CMET.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Caino MC, Ghosh JC, Chae YC, Vaira V, Rivadeneira DB, Faversani A, … Altieri DC (2015). PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proceedings of the National Academy of Sciences, 112(28), 8638 LP – 8643. 10.1073/pnas.1500722112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, & Viola A. (2006). Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. The Journal of Experimental Medicine, 203(13), 2879–2886. 10.1084/jem.20061877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassago A, Ferreira APS, Ferreira IM, Fornezari C, Gomes ERM, Greene KS, … Ambrosio ALB (2012). Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proceedings of the National Academy of Sciences, 109(4), 1092–1097. 10.1073/PNAS.1112495109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegna A, Iacobazzi V, & Infantino V. (2015). The mitochondrial side of epigenetics. Physiological Genomics, 47(8), 299–307. 10.1152/physiolgenomics.00096.2014 [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Ji W-K, Stan RV, de Juan Sanz J, Ryan TA, & Higgs HN (2018). INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. The Journal of Cell Biology, 217(1), 251–268. 10.1083/jcb.201709111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS (2014). Mitochondria as signaling organelles. BMC Biology, 12(1), 34. 10.1186/1741-7007-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang C, Yu N, Si L, Zhu L, Zeng A, … Wang X. (2019). INF2 regulates oxidative stress-induced apoptosis in epidermal HaCaT cells by modulating the HIF1 signaling pathway. Biomedicine & Pharmacotherapy, 111, 151–161. 10.1016/J.BIOPHA.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, … Ha J. (2001). The regulation of AMP-activated protein kinase by H(2)O(2). Biochemical and Biophysical Research Communications, 287(1), 92–97. 10.1006/bbrc.2001.5544 [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Yeoh KK, Tian Y-M, Hillringhaus L, Bagg EA, Rose NR, … Kawamura A. (2011). The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Reports, 12(5), 463–469. 10.1038/embor.2011.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BT, Volbracht C, Tan KO, Li R, Yu VC, & Li P. (2003). Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nature Cell Biology, 5(12), 1083–1089. 10.1038/ncb1070 [DOI] [PubMed] [Google Scholar]
- Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, … Melendez JA (2005). Mitochondrial H2O2 Regulates the Angiogenic Phenotype via PTEN Oxidation. Journal of Biological Chemistry, 280(17), 16916–16924. 10.1074/JBC.M410690200 [DOI] [PubMed] [Google Scholar]
- Corbet C, & Feron O. (2017). Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochimica et Biophysica Acta. Reviews on Cancer, 1868(1), 7–15. 10.1016/j.bbcan.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Crosas-Molist E, Maiques O, Pandya P, Monger J, Graziani V, Malik S, … Sanz-Moreno V. (2021). AMPK is a Mechano-Metabolic Sensor Linking Mitochondrial Dynamics to Myosin II Dependent Cell Migration. SSRN Electronic Journal. 10.2139/ssrn.3845005 [DOI] [PMC free article] [PubMed]
- Cunniff B, McKenzie AJ, Heintz NH, & Howe AK (2016). AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Molecular Biology of the Cell, 27(17), 2662–2674. 10.1091/mbc.E16-05-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang C-Y, Krauss S, Ranger AM, … Korsmeyer SJ (2003). BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature, 424(6951), 952–956. 10.1038/nature01825 [DOI] [PubMed] [Google Scholar]
- Divolis G, Mavroeidi P, Mavrofrydi O, & Papazafiri P. (2016). Differential effects of calcium on PI3K-Akt and HIF-1α survival pathways. Cell Biology and Toxicology, 32(5), 437–449. 10.1007/s10565-016-9345-x [DOI] [PubMed] [Google Scholar]
- Drubin DG, Jones HD, & Wertman KF (1993). Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Molecular Biology of the Cell, 4(12), 1277–1294. 10.1091/mbc.4.12.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBoff B, Götz J, & Feany MB (2012). Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron, 75(4), 618–632. 10.1016/j.neuron.2012.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L, & Schatz G. (1981). Mitochondria: a historical review. The Journal of Cell Biology, 91(3 Pt 2), 227s–255s. 10.1083/jcb.91.3.227s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoh S, Matsui H, Tokuda M, Itano T, Nakamura M, & Hatase O. (1990). Purification and immunohistochemical study of actin in mitochondrial matrix. Biochemistry International, 20(3), 599–606. [PubMed] [Google Scholar]
- Eves PT, Jin Y, Brunner M, & Weisman LS (2012). Overlap of cargo binding sites on myosin V coordinates the inheritance of diverse cargoes. The Journal of Cell Biology, 198(1), 69–85. 10.1083/jcb.201201024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Price T, Huang W, Plue M, Warren J, Sundaramoorthy P, … Kang Y. (2020). PINK1-Dependent Mitophagy Regulates the Migration and Homing of Multiple Myeloma Cells via the MOB1B-Mediated Hippo-YAP/TAZ Pathway. Advanced Science, 7(5), 1900860. 10.1002/advs.201900860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, & Sweetlove LJ (2004). Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current Opinion in Plant Biology, 7(3), 254–261. 10.1016/j.pbi.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Ferreira-da-Silva A, Valacca C, Rios E, Pópulo H, Soares P, Sobrinho-Simões M, … Campello S. (2015). Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PloS One, 10(3), e0122308. 10.1371/journal.pone.0122308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, & Voeltz GK (2011). ER tubules mark sites of mitochondrial division. Science (New York, N.Y.), 334(6054), 358–362. 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J, Ramabhadran V, Neumanne E, Gurel P, Blanchoin L, Vantard M, & Higgs HN (2011). Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Molecular Biology of the Cell, 22(23), 4575–4587. 10.1091/mbc.E11-07-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielisch I, & Meierhofer D. (2015). Metabolome and proteome profiling of complex I deficiency induced by rotenone. Journal of Proteome Research, 14(1), 224–235. 10.1021/pr500894v [DOI] [PubMed] [Google Scholar]
- Goldenthal MJ, & Marín-García J. (2004). Mitochondrial signaling pathways: a receiver/integrator organelle. Molecular and Cellular Biochemistry, 262(1–2), 1–16. 10.1023/b:mcbi.0000038228.85494.3b [DOI] [PubMed] [Google Scholar]
- Gu L, Larson Casey JL, Andrabi SA, Lee JH, Meza-Perez S, Randall TD, & Carter AB (2019). Mitochondrial calcium uniporter regulates PGC-1α expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biology, 26, 101307. 10.1016/J.REDOX.2019.101307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, & Chandel NS (2009). Mitochondrial reactive oxygen species regulate hypoxic signaling. Current Opinion in Cell Biology, 21(6), 894–899. 10.1016/j.ceb.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, … Chandel NS (2013). Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Science Signaling, 6(261), ra8. 10.1126/scisignal.2003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CN, Liu W, Alvord WG, & Phang JM (2016). Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids, 48(3), 859–872. 10.1007/s00726-015-2134-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch AL, Ji W-K, Merrill RA, Strack S, & Higgs HN (2016). Actin filaments as dynamic reservoirs for Drp1 recruitment. Molecular Biology of the Cell, 27(20), 3109–3121. 10.1091/mbc.E16-03-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhou A, Lu H, Chen Y, Huang G, Yue X, … Wu Y. (2013). Suppression of mitochondrial complex I influences cell metastatic properties. PloS One, 8(4), e61677. 10.1371/journal.pone.0061677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, … Murphy MP (2018). Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. The Journal of Biological Chemistry, 293(44), 17208–17217. 10.1074/jbc.RA118.002579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JH-C, Tseng K-C, Ma W-H, Chen K-H, Lee OK-S, & Su Y. (2008). Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. The British Journal of Ophthalmology, 92(7), 992–997. 10.1136/bjo.2007.136747 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Rust MB, & Culmsee C. (2019). Actin(g) on mitochondria - a role for cofilin1 in neuronal cell death pathways. Biological Chemistry, 400(9), 1089–1097. 10.1515/hsz-2019-0120 [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang B, Wang L, Wang Z, Jian Z, & Deng L. (2020). Mammalian STE20‑like kinase 1 regulates pancreatic cancer cell survival and migration through Mfn2‑mediated mitophagy. Molecular Medicine Reports, 22(1), 398–404. 10.3892/mmr.2020.11098 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Janssen AFJ, Tas RP, van Bergeijk P, Oost R, Hoogenraad CC, & Kapitein LC (2017). Myosin-V Induces Cargo Immobilization and Clustering at the Axon Initial Segment. Frontiers in Cellular Neuroscience, 11, 260. 10.3389/fncel.2017.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Wang Y, Luo L, Shi F, Zou J, Lin H, … Luo Z. (2019). AMP-activated protein kinase regulates cancer cell growth and metabolism via nuclear and mitochondria events. Journal of Cellular and Molecular Medicine, 23(6), 3951–3961. 10.1111/jcmm.14279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, & Scorrano, L. (2013). Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science (New York, N.Y.), 342(6159), 734–737. 10.1126/science.1241359 [DOI] [PubMed] [Google Scholar]
- Kelleher JF, Mandell MA, Moulder G, Hill KL, L’Hernault SW, Barstead R, & Titus MA (2000). Myosin VI is required for asymmetric segregation of cellular components during C. elegans spermatogenesis. Current Biology, 10(23), 1489–1496. 10.1016/S0960-9822(00)00828-9 [DOI] [PubMed] [Google Scholar]
- Kim D-Y, Lim S-G, Suk K, & Lee W-H (2020). Mitochondrial dysfunction regulates the JAK-STAT pathway via LKB1-mediated AMPK activation ER-stress-independent manner. Biochemistry and Cell Biology = Biochimie et Biologie Cellulaire, 98(2), 137–144. 10.1139/bcb-2019-0088 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Shaker MR, Cho B, Cho HM, Kim H, Kim JY, & Sun W. (2015). Dynamin-related protein 1 controls the migration and neuronal differentiation of subventricular zone-derived neural progenitor cells. Scientific Reports, 5(1), 15962. 10.1038/srep15962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Huang TY, & Bokoch GM (2009). Reactive oxygen species regulate a slingshot-cofilin activation pathway. Molecular Biology of the Cell, 20(11), 2650–2660. 10.1091/mbc.e09-02-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yang G, Kim Y, Kim J, & Ha J. (2016). AMPK activators: mechanisms of action and physiological activities. Experimental & Molecular Medicine, 48(4), e224–e224. 10.1038/emm.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, … Shacter E. (2009). Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nature Cell Biology, 11(10), 1241–1246. 10.1038/ncb1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SH, Choi GE, Oh JY, Lee HJ, Kim JS, Chae CW, … Han HJ (2017). Succinate promotes stem cell migration through the GPR91-dependent regulation of DRP1-mediated mitochondrial fission. Scientific Reports, 7(1), 12582. 10.1038/s41598-017-12692-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, & Higgs HN (2013). An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science (New York, N.Y.), 339(6118), 464–467. 10.1126/science.1228360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiadis VN, Leadsham JE, Bastow EL, Gheeraert A, Whybrew JM, Bard M, … Gourlay CW (2012). Identification of new surfaces of cofilin that link mitochondrial function to the control of multi-drug resistance. Journal of Cell Science, 125(Pt 9), 2288–2299. 10.1242/jcs.099390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwaki T, Okamoto M, Tsukamoto H, Fukushima Y, Matsumoto M, Seya T, & Oshiumi H. (2017). Zyxin stabilizes RIG-I and MAVS interactions and promotes type I interferon response. Scientific Reports, 7(1), 11905. 10.1038/s41598-017-12224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremneva E, Kislin M, Kang X, & Khiroug L. (2013). Motility of astrocytic mitochondria is arrested by Ca2+-dependent interaction between mitochondria and actin filaments. Cell Calcium, 53(2), 85–93. 10.1016/J.CECA.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, & Brenner C. (2007). Mitochondrial membrane permeabilization in cell death. Physiological Reviews, 87(1), 99–163. 10.1152/physrev.00013.2006 [DOI] [PubMed] [Google Scholar]
- Kruppa AJ, Kishi-Itakura C, Masters TA, Rorbach JE, Grice GL, Kendrick-Jones J, … Buss F. (2018). Myosin VI-Dependent Actin Cages Encapsulate Parkin-Positive Damaged Mitochondria. Developmental Cell, 44(4), 484–499.e6. 10.1016/j.devcel.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino DA, Boldogh I, Smith MG, Rosand J, & Pon LA (1994). Yeast mitochondria contain ATP-sensitive, reversible actin-binding activity. Molecular Biology of the Cell, 5(7), 807–818. 10.1091/mbc.5.7.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, … Kalluri R. (2014). PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nature Cell Biology, 16(10), 1–15,992–1003. 10.1038/ncb3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Westrate LM, Wu H, Page C, & Voeltz GK (2016). Multiple dynamin family members collaborate to drive mitochondrial division. Nature, 540(7631), 139–143. 10.1038/nature20555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Wang L, & Chang Z-F (2014). The contribution of mitochondrial thymidylate synthesis in preventing the nuclear genome stress. Nucleic Acids Research, 42(8), 4972–4984. 10.1093/nar/gku152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AWC, & Halestrap AP (2008). Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochimica et Biophysica Acta, 1777(7–8), 946–952. 10.1016/j.bbabio.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Li D, Dammer EB, Lucki NC, & Sewer MB (2013). cAMP-stimulated phosphorylation of diaphanous 1 regulates protein stability and interaction with binding partners in adrenocortical cells. Molecular Biology of the Cell, 24(6), 848–857. 10.1091/mbc.E12-08-0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, & Sewer MB (2010). RhoA and DIAPH1 mediate adrenocorticotropin-stimulated cortisol biosynthesis by regulating mitochondrial trafficking. Endocrinology, 151(9), 4313–4323. 10.1210/en.2010-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-B, Zhang H-W, Fu R-Q, Hu X-Y, Liu L, Li Y-N, … Gao N. (2018). Mitochondrial fission and mitophagy depend on cofilin-mediated actin depolymerization activity at the mitochondrial fission site. Oncogene, 37(11), 1485–1502. 10.1038/s41388-017-0064-4 [DOI] [PubMed] [Google Scholar]
- Li G, Zhou J, Budhraja A, Hu X, Chen Y, Cheng Q, … Gao N. (2015). Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget, 6(3), 1834–1849. 10.18632/oncotarget.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Xu S, Roelofs BA, Boyman L, Lederer WJ, Sesaki H, & Karbowski M. (2015). Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. The Journal of Cell Biology, 208(1), 109–123. 10.1083/jcb.201404050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, & Steward O. (2000). Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. The Journal of Comparative Neurology, 427(3), 351–361. [DOI] [PubMed] [Google Scholar]
- Lin S, Huang C, Gunda V, Sun J, Chellappan SP, Li Z, … Yang S. (2019). Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments. Cell Reports, 28(11), 2824–2836.e8. 10.1016/j.celrep.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, … Feliciello A. (2006). Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Molecular Biology of the Cell, 17(1), 263–271. 10.1091/mbc.e05-09-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Doménech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, … Kittler JT (2018). Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. The EMBO Journal, 37(3), 321–336. 10.15252/embj.201696380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless T, Qadota H, Benian GM, & Hardin J. (2017). Caenorhabditis elegans SORB-1 localizes to integrin adhesion sites and is required for organization of sarcomeres and mitochondria in myocytes. Molecular Biology of the Cell, 28(25), 3621–3633. 10.1091/mbc.E16-06-0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero M, Suarez AE, & Chambers JW (2019). Phosphoregulation on mitochondria: Integration of cell and organelle responses. CNS Neuroscience & Therapeutics, 25(7), 837–858. 10.1111/cns.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Bartholomew S, Golani G, Christenson E, Kozlov M, Higgs H, … Lippincott-Schwartz J. (2015). A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. ELife, 4. 10.7554/eLife.08828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr JA (2015). Lipid metabolism in mitochondrial membranes. Journal of Inherited Metabolic Disease, 38(1), 137–144. 10.1007/s10545-014-9748-x [DOI] [PubMed] [Google Scholar]
- Merrill RA, & Strack S. (2014). Mitochondria: a kinase anchoring protein 1, a signaling platform for mitochondrial form and function. The International Journal of Biochemistry & Cell Biology, 48, 92–96. 10.1016/j.biocel.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, & Shaw RJ (2011). The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology, 13(9), 1016–1023. 10.1038/ncb2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, & Bamburg JR (2000). Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nature Cell Biology, 2(9), 628–636. 10.1038/35023579 [DOI] [PubMed] [Google Scholar]
- Minin AA, Kulik AV, Gyoeva FK, Li Y, Goshima G, & Gelfand VI (2006). Regulation of mitochondria distribution by RhoA and formins. Journal of Cell Science, 119(Pt 4), 659–670. 10.1242/jcs.02762 [DOI] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP, & Malik AB (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling, 20(7), 1126–1167. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan L, Irani K, Moldovan NI, Finkel T, & Goldschmidt-Clermont PJ (1999). The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxidants & Redox Signaling, 1(1), 29–43. 10.1089/ars.1999.1.1-29 [DOI] [PubMed] [Google Scholar]
- Monroe RK, & Halvorsen SW (2009). Environmental toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial disruption. Neurotoxicology, 30(4), 589–598. 10.1016/j.neuro.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Moore AS, & Holzbaur ELF (2018). Mitochondrial-cytoskeletal interactions: dynamic associations that facilitate network function and remodeling. Current Opinion in Physiology, 3, 94–100. 10.1016/j.cophys.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AS, Wong YC, Simpson CL, & Holzbaur ELF (2016). Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission–fusion balance within mitochondrial networks. Nature Communications, 7(1), 12886. 10.1038/ncomms12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlino G, Barreiro O, Baixauli F, Robles-Valero J, González-Granado JM, Villa-Bellosta R, … Sánchez-Madrid F. (2014). Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Molecular and Cellular Biology, 34(8), 1412–1426. 10.1128/MCB.01177-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, & Hollenbeck PJ (1995). Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. The Journal of Cell Biology, 131(5), 1315–1326. 10.1083/jcb.131.5.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muliyil S, & Narasimha M. (2014). Mitochondrial ROS regulates cytoskeletal and mitochondrial remodeling to tune cell and tissue dynamics in a model for wound healing. Developmental Cell, 28(3), 239–252. 10.1016/j.devcel.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Munsie LN, Desmond CR, & Truant R. (2012). Cofilin nuclear–cytoplasmic shuttling affects cofilin–actin rod formation during stress. Journal of Cell Science, 125(17), 3977–3988. 10.1242/jcs.097667 [DOI] [PubMed] [Google Scholar]
- Nishimura A, Shimauchi T, Tanaka T, Shimoda K, Toyama T, Kitajima N, … Nishida M. (2018). Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Science Signaling, 11(556). 10.1126/scisignal.aat5185 [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Shi S, Li Q, Bershadsky AD, & Viasnoff V. (2021). Crosstalk between myosin II and formin functions in the regulation of force generation and actomyosin dynamics in stress fibers. BioRxiv, 2021.08.15.456175. 10.1101/2021.08.15.456175 [DOI] [PubMed]
- Nishimura Y, Shi S, Zhang F, Liu R, Takagi Y, Bershadsky AD, … Sellers JR (2021). The formin inhibitor SMIFH2 inhibits members of the myosin superfamily. Journal of Cell Science, 134(8). 10.1242/jcs.253708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, & Kasahara A. (2018). Mitochondrial dynamics coordinate cell differentiation. Biochemical and Biophysical Research Communications, 500(1), 59–64. 10.1016/J.BBRC.2017.06.094 [DOI] [PubMed] [Google Scholar]
- Ordonez DG, Lee MK, & Feany MB (2018). α-synuclein Induces Mitochondrial Dysfunction through Spectrin and the Actin Cytoskeleton. Neuron, 97(1), 108–124.e6. 10.1016/j.neuron.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Sepp KJ, & Hollenbeck PJ (2010). Evidence That Myosin Activity Opposes Microtubule-Based Axonal Transport of Mitochondria. The Journal of Neuroscience, 30(26), 8984 LP – 8992. 10.1523/JNEUROSCI.1621-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Xu R-H, Du M, Feng L, Sasaki R, Carew JS, … Huang P. (2006). Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. The Journal of Cell Biology, 175(6), 913–923. 10.1083/jcb.200512100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD (2016). Actin and Actin-Binding Proteins. Cold Spring Harbor Perspectives in Biology, 8(8). 10.1101/cshperspect.a018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte S, Carvalho L, Gagliardi M, Campos I, Oliveira PJ, & Jacinto A. (2020). Drp1-mediated mitochondrial fission regulates calcium and F-actin dynamics during wound healing. Biology Open, 9(5). 10.1242/bio.048629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova EN, Pletjushkina OY, Dugina VB, Domnina LV, Ivanova OY, Izyumov DS, … Chernyak BV (2010). Scavenging of reactive oxygen species in mitochondria induces myofibroblast differentiation. Antioxidants & Redox Signaling, 13(9), 1297–1307. 10.1089/ars.2009.2949 [DOI] [PubMed] [Google Scholar]
- Porporato PE, Filigheddu N, Pedro JMB-S, Kroemer G, & Galluzzi L. (2018). Mitochondrial metabolism and cancer. Cell Research, 28(3), 265–280. 10.1038/cr.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas I, Pérez-Martínez FC, Guerra J, Sánchez-Verdú P, & Ceña V. (2012). Cofilin activation mediates Bax translocation to mitochondria during excitotoxic neuronal death. Journal of Neurochemistry, 120(4), 515–527. 10.1111/j.1471-4159.2011.07599.x [DOI] [PubMed] [Google Scholar]
- Prudent J, Popgeorgiev N, Bonneau B, Thibaut J, Gadet R, Lopez J, … Gillet G. (2013). Bcl-wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nature Communications, 4, 2330. 10.1038/ncomms3330 [DOI] [PubMed] [Google Scholar]
- Prudent J, Popgeorgiev N, Gadet R, Deygas M, Rimokh R, & Gillet G. (2016). Mitochondrial Ca(2+) uptake controls actin cytoskeleton dynamics during cell migration. Scientific Reports, 6, 36570. 10.1038/srep36570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylypenko O, Welz T, Tittel J, Kollmar M, Chardon F, Malherbe G, … Kerkhoff E. (2016). Coordinated recruitment of Spir actin nucleators and myosin V motors to Rab11 vesicle membranes. ELife, 5. 10.7554/eLife.17523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero M, Colombo SL, Godfrey A, & Moncada S. (2006). Mitochondria as signaling organelles in the vascular endothelium. Proceedings of the National Academy of Sciences of the United States of America, 103(14), 5379–5384. 10.1073/pnas.0601026103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, … Cheney RE (2009). Human Myo19 is a novel myosin that associates with mitochondria. Current Biology : CB, 19(23), 2008–2013. 10.1016/j.cub.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehklau K, Hoffmann L, Gurniak CB, Ott M, Witke W, Scorrano L, … Rust MB (2017). Cofilin1-dependent actin dynamics control DRP1-mediated mitochondrial fission. Cell Death & Disease, 8(10), e3063. 10.1038/cddis.2017.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, He J, Mao CC, Bailey LJ, Di Re M, Sembongi H, … Holt IJ (2011). Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Research, 39(12), 5098–5108. 10.1093/nar/gkr052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers E, Rai R, Lötscher J, Hollinshead M, Markelj G, Thaventhiran J, … Thrasher AJ (2020). Wiskott Aldrich syndrome protein regulates non-selective autophagy and mitochondrial homeostasis in human myeloid cells. ELife, 9. 10.7554/eLife.55547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn JL, Patel JV, Neumann B, Bulkescher J, Mchedlishvili N, McMullan RC, … Baum B. (2014). Myo19 ensures symmetric partitioning of mitochondria and coupling of mitochondrial segregation to cell division. Current Biology : CB, 24(21), 2598–2605. 10.1016/j.cub.2014.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Esmaeilniakooshkghazi A, Patnaik S, Wang Y, George SP, Ahrorov A, … Khurana S. (2018). Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation. Gastroenterology, 154(5), 1405–1420.e2. 10.1053/j.gastro.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablin EP, Dawson JF, VanLoock MS, Spudich JA, Egelman EH, & Fletterick RJ (2002). How does ATP hydrolysis control actin’s associations? Proceedings of the National Academy of Sciences of the United States of America, 99(17), 10945–10947. 10.1073/pnas.152329899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Subramaniam G, Raychaudhuri S, & Dhawan J. (2019). Cytoplasmic sequestration of the RhoA effector mDiaphanous1 by Prohibitin2 promotes muscle differentiation. Scientific Reports, 9(1), 8302. 10.1038/s41598-019-44749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton WM, & Hollenbeck PJ (2012). The axonal transport of mitochondria. Journal of Cell Science, 125(9), 2095–2104. 10.1242/jcs.053850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaks M, Giannone G, & Rottner K. (2019). Actin dynamics in cell migration. Essays in Biochemistry, 63(5), 483–495. 10.1042/EBC20190015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M-H, Lewandowska A, Caprio G. Di, Skillern W, Upadhyayula S, Kirchhausen T, … Cunniff B. (2017). Miro1-mediated mitochondrial positioning shapes intracellular energy gradients required for cell migration. Molecular Biology of the Cell, 28(16), 2159–2169. 10.1091/mbc.e16-10-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedding DG, Widmer-Teske R, Mueller A, Stieger P, Daniel J-M, Gündüz D, … Braun-Dullaeus R. (2013). Role of the phosphatase PTEN in early vascular remodeling. PloS One, 8(3), e55445. 10.1371/journal.pone.0055445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagieva G, Domnina L, Makarevich O, Chernyak B, Skulachev V, & Dugina V. (2017). Depletion of mitochondrial reactive oxygen species downregulates epithelial-to-mesenchymal transition in cervical cancer cells. Oncotarget, 8(3), 4901–4913. 10.18632/oncotarget.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida M, Kitajima Y, Nakamura J, Yanagihara K, Baba K, Wakiyama K, & Noshiro H. (2016). Impaired mitophagy activates mtROS/HIF-1α interplay and increases cancer aggressiveness in gastric cancer cells under hypoxia. International Journal of Oncology, 48(4), 1379–1390. 10.3892/ijo.2016.3359 [DOI] [PubMed] [Google Scholar]
- Signorile A, Sgaramella G, Bellomo F, & De Rasmo D. (2019). Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells, 8(1), 71. 10.3390/cells8010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli JB, & Haigis MC (2018). The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology, 20(7), 745–754. 10.1038/s41556-018-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stozharov A. (1985). Relationship between metabolic states of liver mitochondria and the G/F-actin ratio. Biomedica Biochimica Acta, 44(9), 1287–1294. [PubMed] [Google Scholar]
- Straub F, Welz T, Alberico H, Brandão RO, Huber A, Samol-Wolf A, … Kerkhoff E. (2020). The SPIRE1 actin nucleator coordinates actin/myosin functions in the regulation of mitochondrial motility. BioRxiv, 2020.06.19.161109. 10.1101/2020.06.19.161109 [DOI]
- Stürmer K, Baumann O, & Walz B. (1995). Actin-dependent light-induced translocation of mitochondria and ER cisternae in the photoreceptor cells of the locust Schistocerca gregaria. Journal of Cell Science, 108 (Pt 6, 2273–2283. [DOI] [PubMed] [Google Scholar]
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, & Vander Heiden MG (2015). Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell, 162(3), 552–563. 10.1016/J.CELL.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Cao H, Zhan L, Yin C, Wang G, Liang P, … Xing J. (2018). Mitochondrial fission promotes cell migration by Ca(2+) /CaMKII/ERK/FAK pathway in hepatocellular carcinoma. Liver International : Official Journal of the International Association for the Study of the Liver, 38(7), 1263–1272. 10.1111/liv.13660 [DOI] [PubMed] [Google Scholar]
- Tait SWG, & Green DR (2010). Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews Molecular Cell Biology, 11(9), 621–632. 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- Tan Y, Mui D, Toan S, Zhu P, Li R, & Zhou H. (2020). SERCA Overexpression Improves Mitochondrial Quality Control and Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. Molecular Therapy. Nucleic Acids, 22, 696–707. 10.1016/j.omtn.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tang M-C, & Su Y. (2011). Thymosin β₄ knockdown disrupts mitochondrial functions of SW480 human colon cancer cells. Cancer Science, 102(9), 1665–1672. 10.1111/j.1349-7006.2011.02002.x [DOI] [PubMed] [Google Scholar]
- Tateya S, Rizzo-De Leon N, Cheng AM, Dick BP, Lee WJ, Kim ML, … Kim F. (2019). The role of vasodilator-stimulated phosphoprotein (VASP) in the control of hepatic gluconeogenic gene expression. PloS One, 14(4), e0215601. 10.1371/journal.pone.0215601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateya S, Rizzo-De Leon N, Handa P, Cheng AM, Morgan-Stevenson V, Ogimoto K, … Kim F. (2013). VASP increases hepatic fatty acid oxidation by activating AMPK in mice. Diabetes, 62(6), 1913–1922. 10.2337/db12-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilokani L, Nagashima S, Paupe V, & Prudent J. (2018). Mitochondrial dynamics: overview of molecular mechanisms. Essays in Biochemistry, 62(3), 341–360. 10.1042/EBC20170104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, … Mammucari C. (2016). The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Molecular Medicine, 8(5), 569–585. 10.15252/emmm.201606255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, & Shirihai OS (2011). The interplay between mitochondrial dynamics and mitophagy. Antioxidants & Redox Signaling, 14(10), 1939–1951. 10.1089/ars.2010.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakifahmetoglu-Norberg H, Ouchida AT, & Norberg E. (2017). The role of mitochondria in metabolism and cell death. Biochemical and Biophysical Research Communications, 482(3), 426–431. 10.1016/J.BBRC.2016.11.088 [DOI] [PubMed] [Google Scholar]
- van Horssen R, Willemse M, Haeger A, Attanasio F, Güneri T, Schwab A, … Wieringa B. (2013). Intracellular NAD(H) levels control motility and invasion of glioma cells. Cellular and Molecular Life Sciences : CMLS, 70(12), 2175–2190. 10.1007/s00018-012-1249-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet AR, Verfaillie T, & Agostinis P. (2014). New functions of mitochondria associated membranes in cellular signaling. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1843(10), 2253–2262. 10.1016/J.BBAMCR.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Vancura A, Nagar S, Kaur P, Bu P, Bhagwat M, & Vancurova I. (2018). Reciprocal Regulation of AMPK/SNF1 and Protein Acetylation. International Journal of Molecular Sciences, 19(11), 3314. 10.3390/ijms19113314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni MA, Vitali T, & Zucchini D. (2013). MICAL, the flavoenzyme participating in cytoskeleton dynamics. International Journal of Molecular Sciences, 14(4), 6920–6959. 10.3390/ijms14046920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJT, Koh T-W, Zhou Y, & Bellen HJ (2005). Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron, 47(3), 365–378. 10.1016/j.neuron.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Vyas S, Zaganjor E, & Haigis MC (2016). Mitochondria and Cancer. Cell, 166(3), 555–566. 10.1016/J.CELL.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders RJA, Ruiter JPN, IJLst L, Waterham HR, & Houten SM (2010). The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. Journal of Inherited Metabolic Disease, 33(5), 479–494. 10.1007/s10545-010-9104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou G-L, Vedantam S, Li P, & Field J. (2008). Mitochondrial shuttling of CAP1 promotes actin- and cofilin-dependent apoptosis. Journal of Cell Science, 121(Pt 17), 2913–2920. 10.1242/jcs.023911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, & Zhou H. (2020). Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharmaceutica Sinica. B, 10(10), 1866–1879. 10.1016/j.apsb.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Li, Yu T, Lee H, O’Brien DK, Sesaki H, & Yoon Y. (2015). Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovascular Research, 106(2), 272–283. 10.1093/cvr/cvv005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Liya. (2016). Mitochondrial purine and pyrimidine metabolism and beyond. Nucleosides, Nucleotides & Nucleic Acids, 35(10–12), 578–594. 10.1080/15257770.2015.1125001 [DOI] [PubMed] [Google Scholar]
- Wang S, Song P, & Zou M-H (2012). AMP-activated protein kinase, stress responses and cardiovascular diseases. Clinical Science (London, England : 1979), 122(12), 555–573. 10.1042/CS20110625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pluznick JL, Settles DC, & Sansom SC (2007, December). Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. American Journal of Physiology. Renal Physiology, Vol. 293, pp. F1768–76. United States. 10.1152/ajprenal.00365.2007 [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng W, Zhang Y, Sun S, Zhao S, Chen Y, … Du J. (2018). MICAL2 promotes breast cancer cell migration by maintaining epidermal growth factor receptor (EGFR) stability and EGFR/P38 signalling activation. Acta Physiologica (Oxford, England), 222(2). 10.1111/apha.12920 [DOI] [PubMed] [Google Scholar]
- Wang Y, George SP, Srinivasan K, Patnaik S, & Khurana S. (2012). Actin reorganization as the molecular basis for the regulation of apoptosis in gastrointestinal epithelial cells. Cell Death and Differentiation, 19(9), 1514–1524. 10.1038/cdd.2012.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yigang, Liu H-H, Cao Y-T, Zhang L-L, Huang F, & Yi C. (2020). The Role of Mitochondrial Dynamics and Mitophagy in Carcinogenesis, Metastasis and Therapy. Frontiers in Cell and Developmental Biology, 8, 413. 10.3389/fcell.2020.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ying, Su K, Hu P-C, Gao F-F, Zhang J-W, & Wei L. (2015). The Ultrastructure of MCF-7 Breast Cancer Cells after Vasodilator-Stimulated Phosphoprotein Knockdown. Ultrastructural Pathology, 39(5), 318–323. 10.3109/01913123.2015.1027434 [DOI] [PubMed] [Google Scholar]
- Wang Yue, Jasper H, Toan S, Muid D, Chang X, & Zhou H. (2021). Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biology, 45, 102049. 10.1016/j.redox.2021.102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, … Chandel NS (2010). Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8788–8793. 10.1073/pnas.1003428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K-K, McKane M, Stokasimov E, & Rubenstein PA (2011). Mutant profilin suppresses mutant actin-dependent mitochondrial phenotype in Saccharomyces cerevisiae. The Journal of Biological Chemistry, 286(48), 41745–41757. 10.1074/jbc.M110.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, & González-Billault C. (2015). Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Frontiers in Cellular Neuroscience, 9, 381. 10.3389/fncel.2015.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Viana M, Thirumangalathu S, & Loeken MR (2012). AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia, 55(1), 245–254. 10.1007/s00125-011-2326-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Zhang Y, Jin K, Lu Z, Zeng Z, & Xiong W. (2019). Communication between mitochondria and other organelles: a brand-new perspective on mitochondria in cancer. Cell & Bioscience, 9(1), 27. 10.1186/s13578-019-0289-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Venit T, Drou N, & Percipalle P. (2018). In Mitochondria ?-Actin Regulates mtDNA Transcription and Is Required for Mitochondrial Quality Control. IScience, 3, 226–237. 10.1016/j.isci.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, & Chisholm AD (2014). C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Developmental Cell, 31(1), 48–60. 10.1016/j.devcel.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Qiu C, Sun W, Gu M, Xiao F, Zou J, & Zhang L. (2018). Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. Oncology Reports, 39(4), 1671–1681. 10.3892/or.2018.6252 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang C, & Svitkina TM (2019). Ultrastructure and dynamics of the actin-myosin II cytoskeleton during mitochondrial fission. Nature Cell Biology, 21(5), 603–613. 10.1038/s41556-019-0313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, & Kim J. (2019). Mitochondrial Retrograde Signalling and Metabolic Alterations in the Tumour Microenvironment. Cells, 8(3). 10.3390/cells8030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li J-H, Wang J, & Zhang C-Y (2010). Molecular modeling of BAD complex resided in a mitochondrion integrating glycolysis and apoptosis. Journal of Theoretical Biology, 266(2), 231–241. 10.1016/j.jtbi.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Yang L, Hong Q, Xu S, Kuang X, Di G, Liu G, … Yu S. (2019). Downregulation of transgelin 2 promotes breast cancer metastasis by activating the reactive oxygen species/nuclear factor‑κB signaling pathway. Mol Med Rep, 20(5), 4045–4258. 10.3892/mmr.2019.10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Soga T, & Pollard PJ (2013). Oncometabolites: linking altered metabolism with cancer. The Journal of Clinical Investigation, 123(9), 3652–3658. 10.1172/JCI67228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xu M, Meng G, & Lu Y. (2020). SIRT3 deficiency delays diabetic skin wound healing via oxidative stress and necroptosis enhancement. Journal of Cellular and Molecular Medicine, 24(8), 4415–4427. 10.1111/jcmm.15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Cai X, Liu C, Qin Y, Cheng H, Ji S, … Yu X. (2013). Profilin 1 potentiates apoptosis induced by staurosporine in cancer cells. Current Molecular Medicine, 13(3), 417–428. [PubMed] [Google Scholar]
- Yin H, van der Veer E, Frontini MJ, Thibert V, O’Neil C, Watson A, … Pickering JG (2012). Intrinsic directionality of migrating vascular smooth muscle cells is regulated by NAD(+) biosynthesis. Journal of Cell Science, 125(Pt 23), 5770–5780. 10.1242/jcs.110262 [DOI] [PubMed] [Google Scholar]
- Yin M, Lu Q, Liu X, Wang T, Liu Y, & Chen L. (2016). Silencing Drp1 inhibits glioma cells proliferation and invasion by RHOA/ ROCK1 pathway. Biochemical and Biophysical Research Communications, 478(2), 663–668. 10.1016/J.BBRC.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Yu J, Shi L, Lin W, Lu B, & Zhao Y. (2020). UCP2 promotes proliferation and chemoresistance through regulating the NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer. Biochemical Pharmacology, 172, 113745. 10.1016/J.BCP.2019.113745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-W, Yang Z-M, Zheng Y-C, & Chen Z-D (2010). Transgelin induces apoptosis of human prostate LNCaP cells through its interaction with p53. Asian Journal of Andrology, 12(2), 186–195. 10.1038/aja.2009.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, … Tu Y. (2013). Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene, 32(40), 4814–4824. 10.1038/onc.2012.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lu Z, Kocabas F, Böttcher RT, Costell M, Kang X, … Zhang CC (2014). Profilin 1 is essential for retention and metabolism of mouse hematopoietic stem cells in bone marrow. Blood, 123(7), 992–1001. 10.1182/blood-2013-04-498469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ren J, Toan S, & Mui D. (2021). Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Research Reviews, 66, 101250. 10.1016/j.arr.2020.101250 [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, … Chen Y. (2017). Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biology, 13, 498–507. 10.1016/j.redox.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hsu AY, Wang Y, Syahirah R, Wang T, Jeffries J, … Deng Q. (2020). Mitofusin 2 regulates neutrophil adhesive migration and the actin cytoskeleton. Journal of Cell Science, 133(17). 10.1242/jcs.248880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin PV, Belanova AA, Prazdnova EV, Mazanko MS, Batiushin MM, Chmyhalo VK, & Chistyakov VA (2016). Mitochondria as a Signaling Hub and Target for Phenoptosis Shutdown. Biochemistry. Biokhimiia, 81(4), 329–337. 10.1134/S0006297916040039 [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, & Sollott SJ (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews, 94(3), 909–950. 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.