CASE
A 64-year-old male with a history of nonalcoholic steatohepatitis, alpha-1-antitrypsin deficiency, and an orthotopic liver transplant completed 5 months prior, presented to the hospital with a 1-day history of fever and fatigue. The patient had recently undergone evaluation for rash and pancytopenia and was ultimately diagnosed with graft-versus-host disease (GVHD) affecting his skin, gastrointestinal tract, and bone marrow. High-dose prednisone and twice-weekly etanercept were added to his prior immunosuppressant regimen of tacrolimus and mycophenolate. During a subsequent outpatient evaluation for bone marrow transplantation, he developed fever and fatigue again and was admitted to the hospital for further evaluation.
On presentation the patient was febrile to 38.2°C. His previous GVHD-related rash had resolved, and he denied any other localized symptoms. Initial laboratory studies were significant for a complete blood count showing pancytopenia, with a leukocyte count of 0.1 × 109/L (normal range, 3.4 × 109 to 9.6 × 109/L); electrolytes, renal function, and hepatic function testing were all within normal limits. Serum cytomegalovirus quantitative PCR (Roche Diagnostics, Indianapolis, IN) and Cryptococcus antigen (CrAg lateral flow immunoassay [LFA], IMMY Diagnostics, Norton, OK) testing were also performed, and results were negative. Chest X-ray did not show any focal consolidation or other acute findings. Bacterial blood cultures were obtained and grew methicillin-susceptible Staphylococcus aureus from the anaerobic bottle after 17 h of incubation. The patient was initially started on vancomycin and cefepime and later transitioned to cefazolin, 2 g every 8 h, once the susceptibility test results were available. However, at 86 h of incubation an aerobic blood culture bottle flagged positive and subculture onto sheep blood agar yielded Cryptococcus neoformans, which was identified by the BioFire Blood Culture Identification 2 (BCID2) panel (bioMérieux, Salt Lake City, UT) and confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics) using both the Bruker research-use-only and Mayo Clinic-developed spectrum library databases. Blood cultures obtained on each of the following 2 days also grew C. neoformans.
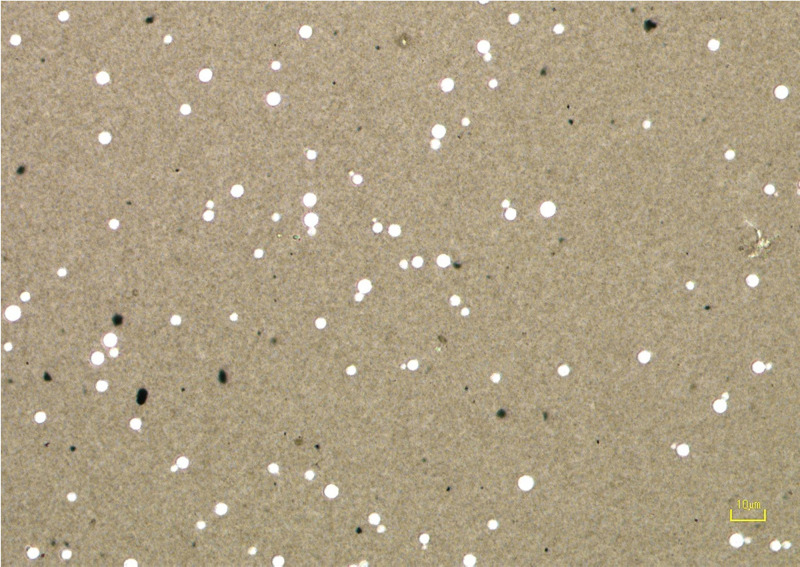
A repeat serum CrAg LFA performed 9 days later was also negative. To rule out postzone effect, the sample was serially diluted to 1:1,280; all dilutions remained negative on all samples tested. The C. neoformans isolate was subcultured on Sabouraud’s dextrose agar (SDA) at 30°C and 37°C. After 4 days, colonies were examined using India ink staining, which did not demonstrate the presence of a capsule (Fig. 1). Although the absence of capsule production in vitro is not definitive evidence of inhibited capsule production in vivo, together with the negative serum CrAg result, the findings were highly suggestive of infection with a capsule-deficient Cryptococcus isolate.
FIG 1.
India Ink stain of capsule-deficient Cryptococcus. In the presence of a capsule India ink displaces around the capsule, creating a clearing around the yeast cells. Lack of clearing around the cells is indicative of unencapsulated Cryptococcus.
The patient was initiated on liposomal amphotericin B, 4 mg/kg every 24 h, and flucytosine, 25 mg/kg every 6 h. Due to progressive thrombocytopenia, lumbar puncture was unable to be performed safely to collect cerebrospinal fluid (CSF) for further analysis (e.g., CrAg, fungal culture), although magnetic resonance imaging of the brain was not suggestive of cryptococcosis. The patient received combination antifungal therapy for 14 days, with clinical improvement and blood culture clearance. He was then transitioned to posaconazole, 300 mg daily, for both consolidation therapy and antifungal prophylaxis while neutropenic. Cefazolin was continued for a total of 4 weeks to treat his S. aureus bloodstream infection. He underwent matched, unrelated donor stem cell transplantation for GVHD after 3 weeks of antifungal therapy. His posttransplant course was complicated by a vancomycin-resistant Enterococcus faecium bloodstream infection and acute invasive pulmonary aspergillosis, although without evidence of relapsed cryptococcosis. As a result of his complicated posttransplant course, the patient passed away 33 days after his allogeneic stem cell transplant.
DISCUSSION
Cryptococcus species are facultative intracellular yeasts. These fungi are frequently encapsulated, and the capsule is primarily composed of the polysaccharides glucuronoxylomannan and glucuronoxylomannogalactan, which are major virulence factors. Historically, C. neoformans and Cryptococcus gattii represented the predominant pathogens, with multiple serotypes within each species. Recent phylogenetic studies, however, have led to a complete reorganization of the species complex, with C. neoformans containing the original serotype A, C. deneoformans encompassing serotype D, and at least five different species now recognized within the C. gattii complex (C. gattii, C. deuterogattii, C. tetragattii, C. decagattii, and C. bacillisporus) (1). C. neoformans is found worldwide in soil contaminated by bird droppings and decaying organic matter. C. gattii was primarily found in the tropical and subtropical areas but is now endemic in British Columbia, the Pacific Northwest, and California and is associated with several different tree species (1, 2).
Cryptococcus species are primarily opportunistic pathogens affecting immunocompromised individuals living with human immunodeficiency virus, cirrhosis, solid-organ transplantation, and hematologic malignancies or stem cell transplants, although infection in immunocompetent persons does occur more commonly with C. gattii (1, 3). The patient in this case was severely immunosuppressed due to a liver transplant, which was also complicated by GVHD. This is a rare complication of solid-organ transplantation, which most commonly affects liver or small-bowel transplant recipients and is associated with high mortality rates (4).
The most common clinical presentation for Cryptococcus species is pulmonary cryptococcosis, which can range from a solitary pulmonary nodule to severe pneumonitis. Cryptococcus has a predilection for dissemination, which commonly results in meningoencephalitis or fungemia. In immunocompromised patients or those with neurologic symptoms, it is recommended to obtain a lumbar puncture for evaluation, which, if intracranial pressure is elevated, also allows for therapeutic removal of CSF. Unfortunately, thrombocytopenia precluded this assessment in our patient (2).
Cryptococcus species are variably sized, narrow-necked budding yeasts with an average size of 6 μm (range, 2 to 10 μm) (1). Hematoxylin and eosin (H&E) stains do not stain Cryptococcus species well, but both the periodic acid-Schiff (PAS) stain and Grocott’s methenamine silver (GMS) stain can highlight the organisms in histopathology sections (1, 5). The Cryptococcus capsule is best visualized using the mucicarmine stain. The calcofluor white stain uses a fluorochrome that nonspecifically binds to chitin in fungal cell walls and can be used to visualize the organism in direct smears from body fluids and tissues, but it does not aid with visualization of the polysaccharide capsule (5). India ink staining is another rapid and inexpensive, but nonspecific, tool to detect encapsulated yeast cells. However, this stain is no longer routinely used due to its low sensitivity compared to that of CrAg tests (1, 5).
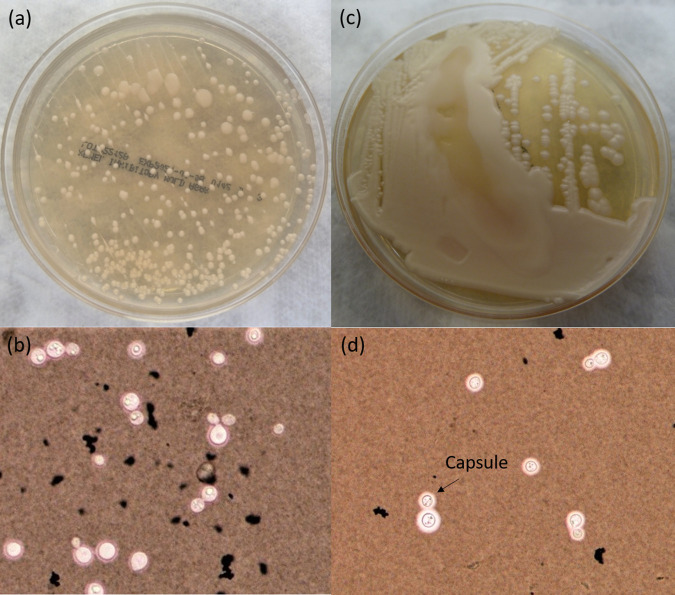
Cryptococcus spp. can be cultured on common mycology media such as Sabouraud’s dextrose agar (SDA), inhibitory mold agar (IMA), brain heart infusion (BHI) agar, and bird seed agar (Staib’s medium), but they also grow on blood agar plates used in bacteriology laboratories (5). Cryptococcus species are, however, sensitive to cycloheximide, and therefore, cycloheximide-containing media should be avoided (1). Colonies on SDA are characteristically white and creamy and typically mucoid due to capsule production. Interestingly, in this case, subculture of the original unencapsulated Cryptococcus isolate ultimately led to a mucoid appearance and capsule production after 10 days as demonstrated by positive India ink staining (Fig. 2). This is consistent with other case reports of unencapsulated Cryptococcus species with a negative antigen test (6). Fungal culture for Cryptococcus is considered the gold standard diagnostic method, although recovery of the organism from the specimen may take up to a week or longer depending on the initial organism burden. Following growth in culture, methods such as MALDI-TOF MS and Sanger sequencing, using targets such as the D1/D2 region of the large ribosomal subunit gene or the internal transcribed spacer region gene, can be utilized for identification and differentiation between the various Cryptococcus species (1, 5).
FIG 2.
Capsule modulation by Cryptococcus over time in vitro. (a) Nonmucoid cultures on Sabouraud’s dextrose agar (SDA) from original specimen 4 days postculturing; (b) India ink staining of culture indicating no visible capsule production; (c) mucoid Cryptococcus colonies following 10 days of subculture on SDA; (d) India ink staining of mucoid colonies, with clearing indicating the presence of a capsule.
Detection of CrAg can be achieved via latex agglutination (LA) or the more recently developed lateral flow immunoassay (LFA). Many laboratories have transitioned away from LA assays due to the requirement for reagent refrigeration, longer turnaround time (45 min), and lower sensitivity for non-HIV patients with cryptococcosis and for patients with C. gattii infections. In contrast, the CrAg LFA is a rapid (<15 min) and reliable method for detecting circulating antigen from either species in serum and CSF (7). The CrAg LFA provides a semiquantitative titer; however, it does not differentiate between Cryptococcus species (7). Although the CrAg LFA is highly sensitive and specific (i.e., >95%), false-negative results can occur due to high background from hemolyzed blood or postzone effect (7, 8). This immunologic phenomenon indicative of excess of antigen (postzone) in patient samples, as opposed to excess antibody (prozone) levels, ultimately results in the inadequate formation of antibody-antigen complexes and can lead to false-negative results by either agglutination or immunochromatographic methods. In cases in which the CrAg results are discrepant from results of other diagnostic testing, investigations may include performing additional specimen dilutions in an effort to dilute out the target analyte to reach an optimal antigen-antibody proportion, also known as the zone of equivalence (8). In this case, diluting the serum sample still did not result in a positive CrAg test. A false-negative CrAg test may also occur due to extremely low fungal burden or the lack of a capsule, as the CrAg tests detect the capsular glucuronoxylomannan antigen of Cryptococcus species.
Cryptococcus can modulate capsule formation based on host response, and thus, an early culture lacking a capsule (e.g., negative by India ink stain) suggests a capsule-deficient Cryptococcus, although this is not definitive. Lack of host immune pressures in vitro, as well as repeat subculturing, including at different temperatures (i.e., 30°C versus 37°C), can also affect capsule production. Thus, it is notoriously challenging to definitively identify a capsule-deficient Cryptococcus infection. False-positive CrAg LFA results are also possible and are reported to occur for patients with Trichosporon, Capnocytophaga, or Stomatococcus mucilaginosus infections (9). Additionally, low positive titers (i.e., ≤1:5) should be interpreted with caution for patients at low risk for cryptococcosis (9).
The mainstay antifungal treatment for severe forms of cryptococcosis is combination therapy with amphotericin B and flucytosine (2). In patients with fungemia, meningoencephalitis, or other forms of disseminated infection, induction treatment with combination amphotericin B and flucytosine for at least 2 weeks is recommended. However, a recent clinical trial showed that single high-dose infusion of liposomal amphotericin B followed by fluconazole and flucytosine was noninferior to a longer course of amphotericin B induction (10). In settings where such treatment is unavailable, alternatives include a longer course of amphotericin B monotherapy, high-dose fluconazole with flucytosine, or very-high-dose fluconazole. Induction therapy is followed by consolidation and maintenance therapy with fluconazole. Other triazoles, such as voriconazole and posaconazole, are expected to remain active, although clinical data regarding their efficacy are limited. Posaconazole was used as consolidation therapy in our patient due to concurrent need for antifungal prophylaxis targeting molds such as Aspergillus spp. and mucormycoses. Initial monotherapy with fluconazole is typically reserved for patients with mild or asymptomatic localized pulmonary cryptococcal infection.
While it has classically been assumed that capsule-deficient cryptococcal strains are less virulent, it remains unclear how capsule deficiency may affect clinical manifestations and outcomes in cryptococcosis; limited data suggest that these infections are similar to those with normal capsule production (11). Nonetheless, given the reliance on CrAg testing, the potential diagnostic delay due to capsule-deficient Cryptococcus presents a challenge that laboratory personnel and clinicians should be aware of and reinforces the importance of performing fungal culture for patients with suspected cryptococcosis.
SELF-ASSESSMENT QUESTIONS
-
1.
Which stains can best identify capsule production in Cryptococcus species?
-
a.
Hematoxylin and eosin (H&E)
-
b.
Fontana-Masson stain
-
c.
Mucicarmine
-
d.
Calcofluor white stain
-
a.
-
2.
False-negative CrAg results may occur due to:
-
a.
Short duration of cryptococcal fungemia
-
b.
Elevated rheumatoid factor
-
c.
Immunosuppression
-
d.
Postzone effect
-
a.
-
3.
What is the preferred initial treatment for fungemia with Cryptococcus species?
-
a.
Amphotericin B with flucytosine
-
b.
Itraconazole
-
c.
Fluconazole
-
d.
Posaconazole
-
a.
ANSWERS TO SELF-ASSESSMENT QUESTIONS
-
1.
Which stains can best identify capsule production in Cryptococcus species?
-
a.
Hematoxylin and eosin (H&E)
-
b.
Fontana-Masson stain
-
c.
Mucicarmine
-
d.
Calcofluor white stain
-
a.
Answer: c. The aluminum in mucicarmine stains forms a chelating complex with carmine giving it a positive charge which allows it to bind to low density acidic substrates such as the mucin present in the Cryptococcus capsule. Hematoxylin and eosin (H&E) does not allow for optimal visualization of Cryptococcus yeasts. Periodic acid-Schiff (PAS) and calcofluor white stains can help demonstrate narrow budding yeasts such as Cryptococcus but cannot differentiate the presence of a capsule. The Fontana-Masson stain is typically used to detect melanin-producing organisms. Although melanin production is a major virulence factor of neurotropic Cryptococcus, melanin is deposited in the cell walls, and therefore, Fontana-Masson does not stain the capsule.
-
2.
False-negative CrAg results may occur due to:
-
a.
Short duration of cryptococcal fungemia
-
b.
Elevated rheumatoid factor
-
c.
Immunosuppression
-
d.
Postzone effect
-
a.
Answer: d. The presence of excessive levels of antigen in the sample (postzone) can lead to inefficient complexing between the CrAg and both the soluble and adhered anti-CrAg antibodies on the lateral flow assay, leading to false-negative results. Other causes of false-negative CrAg results include low fungal organism burden and capsule-deficient Cryptococcus.
-
3.
What is the preferred initial treatment for fungemia with Cryptococcus species?
-
a.
Amphotericin B with flucytosine
-
b.
Itraconazole
-
c.
Fluconazole
-
d.
Posaconazole
-
a.
Answer: a. The combination amphotericin B and flucytosine is considered first-line therapy for severe cryptococcal infection, fungemia, and cryptococcal meningoencephalitis. High-dose fluconazole is used in later phases of treatment but can be an alternative initial treatment in combination with flucytosine if first-line therapy is unavailable. However, monotherapy with flucytosine should not be administered due to the possibility of rapid development of resistance. Posaconazole and itraconazole have anti-Cryptococcus activity but would not be routinely considered for initial treatment of severe or disseminated infection.
TAKE-HOME POINTS
-
•
Causes of false-negative cryptococcal antigen testing include low fungal burden, postzone effect, and the lack of or deficient capsule production. Fungal cultures should always be performed for patients with suspected cryptococcemia.
-
•
Cryptococcus species typically cause infection in immunocompromised patients, such as those with HIV and solid-organ transplant and/or stem cell transplant recipients, although infections in immunocompetent persons have been reported.
-
•
Cryptococcus is best visualized with periodic acid-Schiff (PAS) stain and Grocott’s methenamine silver (GMS) stain, although hematoxylin and eosin (H&E) and calcofluor white staining can be used.
-
•
Cryptococcemia and disseminated cryptococcal infections are typically treated with at least 2 weeks of combination amphotericin B and flucytosine followed by prolonged azole therapy.
Contributor Information
Elitza S. Theel, Email: theel.elitza@mayo.edu.
Carey-Ann D. Burnham, Pattern Bioscience
REFERENCES
- 1.Maziarz EK, Perfect JR. 2016. Cryptococcosis. Infect Dis Clin North Am 30:179–206. 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen M-H, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lui G, Lee N, Ip M, Choi KW, Tso YK, Lam E, Chau S, Lai R, Cockram CS. 2006. Cryptococcosis in apparently immunocompetent patients. QJM 99:143–151. 10.1093/qjmed/hcl014. [DOI] [PubMed] [Google Scholar]
- 4.Murali AR, Chandra S, Stewart Z, Blazar BR, Farooq U, Ince MN, Dunkelberg J. 2016. Graft versus host disease after liver transplantation in adults: a case series, review of literature, and an approach to management. Transplantation 100:2661–2670. 10.1097/TP.0000000000001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazzoni AF, Severo CB, Salles EF, Severo LC. 2009. Histopathology, serology and cultures in the diagnosis of cryptococcosis. Rev Inst Med Trop Sao Paulo 51:255–259. 10.1590/s0036-46652009000500004. [DOI] [PubMed] [Google Scholar]
- 6.Garber ST, Penar PL. 2012. Treatment of indolent, nonencapsulated cryptococcal meningitis associated with hydrocephalus. Clin Pract 2:e22. 10.4081/cp.2012.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis JN, Tenforde MW, Lechiile K, Milton T, Boose A, Leeme TB, Tawe L, Muthoga C, Rukasha I, Mulenga F, Rulaganyang I, Molefi M, Molloy SF, Ngidi J, Harrison TS, Govender NP, Mine M. 2020. Evaluation of a novel semiquantitative cryptococcal antigen lateral flow assay in patients with advanced HIV disease. J Clin Microbiol 58:e00441-20. 10.1128/JCM.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutakingirwa MK, Kiiza TK, Rhein J. 2020. “False negative” CSF cryptococcal antigen with clinical meningitis: case reports and review of literature. Med Mycol Case Rep 29:29–31. 10.1016/j.mmcr.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubbels M, Granger D, Theel ES. 2017. Low cryptococcus antigen titers as determined by lateral flow assay should be interpreted cautiously in patients without prior diagnosis of cryptococcal infection. J Clin Microbiol 55:2472–2479. 10.1128/JCM.00751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis JN, Lawrence DS, Meya DB, Kagimu E, Kasibante J, Mpoza E, Rutakingirwa MK, Ssebambulidde K, Tugume L, Rhein J, Boulware DR, Mwandumba HC, Moyo M, Mzinganjira H, Kanyama C, Hosseinipour MC, Chawinga C, Meintjes G, Schutz C, Comins K, Singh A, Muzoora C, Jjunju S, Nuwagira E, Mosepele M, Leeme T, Siamisang K, Ndhlovu CE, Hlupeni A, Mutata C, van Widenfelt E, Chen T, Wang D, Hope W, Boyer-Chammard T, Loyse A, Molloy SF, Youssouf N, Lortholary O, Lalloo DG, Jaffar S, Harrison TS, Ambition Study Group . 2022. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 386:1109–1120. 10.1056/NEJMoa2111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres HA, Prieto VG, Raad II, Kontoyiannis DP. 2005. Proven pulmonary cryptococcosis due to capsule-deficient Cryptococcus neoformans does not differ clinically from proven pulmonary cryptococcosis due to capsule-intact Cr. neoformans. Mycoses 48:21–24. 10.1111/j.1439-0507.2004.01068.x. [DOI] [PubMed] [Google Scholar]