ABSTRACT
Natural transformation enables bacteria to acquire DNA from the environment and contributes to genetic diversity, DNA repair, and nutritional requirements. DNA processing protein A (DprA) receives incoming single-stranded DNA and assists RecA loading for homology-directed natural chromosomal transformation and DNA strand annealing during plasmid transformation. The dprA gene occurs in the genomes of all known bacteria, irrespective of their natural transformation status. The DprA protein has been characterized by its molecular, cellular, biochemical, and biophysical properties in several bacteria. This review summarizes different aspects of DprA biology, collectively describing its biochemical properties, molecular interaction with DNA, and function interaction with bacterial RecA during natural transformation. Furthermore, the roles of DprA in natural transformation, bacterial virulence, and pilin variation are discussed.
KEYWORDS: DprA/SmfA, natural transformation, competence, extracellular DNA, DNA uptake sequence, RecA-DprA interaction, genetic competence
INTRODUCTION
Natural transformation (NT) involves the uptake of extracellular DNA (eDNA) from the environment and its integration into the bacterial genome (1, 2). eDNA constitutes a large gene pool outside the cell, either liberated from dead organisms or secreted by living cells through type IV and type II secretion systems (T2SSs) (1, 2). More than 80 bacterial species determined to have natural transformation potential express a conserved macromolecular protein complex and host-specific factors for the uptake and integration of eDNA into their genome (3–5) (Fig. 1). Bacterial species such as Deinococcus and Neisseria remain naturally competent during the growth phase (6, 7). However, in Bacillus subtilis and Streptococcus pneumoniae, the natural transformation system is not constitutively active; instead, its competence development, synthesis, and assembly of a macromolecular protein complex are transient and inducible, with very tight genetic regulation (8–10). The uptake of eDNA by the NT system is non-selective to the origin of eDNA, with some exceptions. For example, in Neisseria gonorrhoeae, uptake is restricted to its own DNA or an eDNA with a 10-nucleotide (nt) long (5′-GCCGTCTGAA-3′) DNA uptake sequence (DUS) (11). Similarly, Haemophilus influenzae can take up eDNA with a 9-nt long DNA sequence (AAGTGCGGT) with a 4-nt conserved GCGG motif in its core region (12).
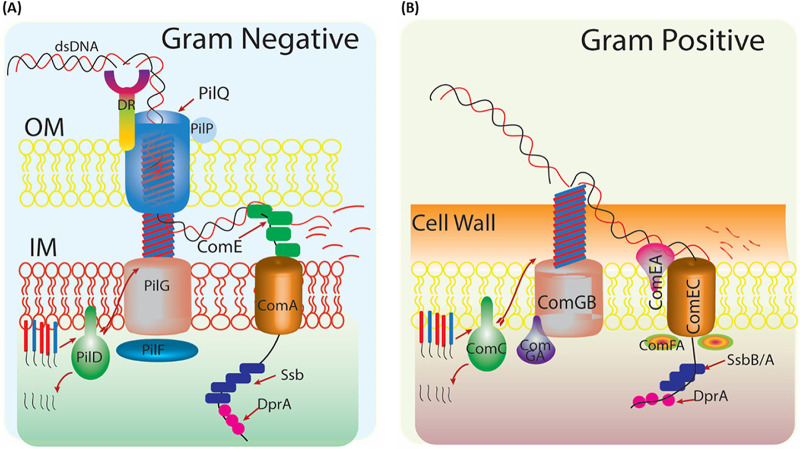
FIG 1.
Typical natural transformation machinery of Gram-negative and Gram-positive bacteria. (A) Schematic model of DNA uptake in Neisseria gonorrhoeae (Gram-negative). Cleavage of the minor pilin (PilE; red) and major pilin (PilV; blue) by pilin peptidase (PilD), assembled and recruited with the help of polytypic membrane protein (PilF) and traffic NTPase (PilG) to pilus fiber. DNA uptake sequence (DUS) in exogenous/incoming DNA recognized by its unidentified receptor, DR (DNA receptor). Secretin (PilQ) forms a channel with the help of pilot protein (PilP) through double-stranded DNA cross the outer membrane. In periplasm, DNA binding protein (ComE) is involved in uptake and delivery of single-stranded DNA (ssDNA) to cytoplasmic membrane protein (ComA). (B) Schematic model of DNA uptake in Bacillus subtilis (Gram-positive). The pseudopilus is composed of the major pseudopilin (ComGC; blue) and minor pseudopilin (ComGD, GE, and GC) and is processed by prepilin peptidase (ComC). The traffic NTPase (ComGA) and polytypic membrane protein (ComGB) are also involved in this process. Pseudopilus allows dsDNA to interact with a membrane-bound receptor (ComEA), which delivers DNA to cytoplasmic membrane protein (ComEC). An ATP binding protein (ComF) helps transport DNA across the membrane. Single-stranded DNA enters the cytoplasm, where SsbB/A and DprA protect it from nuclease action, and with the help of RecA, incoming DNA is integrated into the host genome.
The primary mechanism of natural transformation begins with the binding of a double-stranded eDNA to a long filament called type IV pilus (Tfp) or a transformation-specific pilus/pseudopilus through its nonspecific DNA binding activity (Fig. 1). The existence of Tfp has been reported in both Gram-negative and Gram-positive bacteria, including diverse phyla such as Deinococcus-Thermus (Thermus thermophilus, Deinococcus geothermalis), Firmicutes (Ruminococcus albus, Clostridium perfringens), and Cyanobacteria (Nostoc punctiforme, Microcystis aeruginosa, Synechocystis spp.) (13). The Tfp/pseudopilus uses the mechanical power to pull bound DNA through the outer membrane/peptidoglycan layer and delivers it to conserved translocase machinery located at the cytoplasmic membrane (2) (Fig. 1). Before entering into the cytoplasm, the incoming double-stranded DNA (dsDNA) is converted into single-stranded DNA (ssDNA) by a secretory nuclease (EndA) (2, 14). The conversion of dsDNA to ssDNA occurs at the outer membrane of Gram-negative bacteria and at the cell membrane of Gram-positive bacteria (Fig. 1A and B). The newly generated incoming ssDNA enters the cytoplasm with the help of Com transporter proteins. Due to the danger of degradation by nucleases, DNA processing protein A (DprA/SmfA/CilB) in different bacteria (here referred to as “DprA”) and RecA proteins together ensure the functional integrity of incoming DNA (15). At the final stage of integrating incoming DNA, DprA, along with accessory proteins, helps RecA to form nucleoprotein filament with incoming ssDNA and search for unique homologous sequences followed by the DNA strand exchange (DSE) reactions.
DprA was first identified in H. influenzae through mutagenesis, where the mutation did not affect plasmid DNA uptake, possibly due to illegitimate translocation of plasmid DNA, but abolished chromosomal natural transformation (16). A null dprA mutant of Campylobacter jejuni lost ~100-fold chromosome transformation efficiency (17), and the absence of dprA in Helicobacter pylori severely impaired natural transformation (18). In B. subtilis, lack of dprA/smfA affected plasmid DNA transformation rather than than chromosomal DNA transformation (19). DprA is a mediator for RecA loading and homology-directed RecA-mediated chromosomal transformation in chromosomal DNA transformation. The DprA and RecO proteins, along with single-strand annealing proteins, promote DNA strand annealing for the pairing and circularization of complementary strands coated by SsbA or SsbB during plasmid transformation or viral transfection (20, 21). RecO may compete with DprA for plasmid transformation (7, 22). In Neisseria meningitidis and N. gonorrhoeae, the dprA null mutant is deficient in genetic transformation (23). In S. pneumoniae, DprA plays a vital role in competence shutoff (24). The Escherichia coli dprA/smf gene could partially restore the transformation deficiency in a Haemophilus influenzae dprA mutant. Thus, it is apparent that DprA plays a vital role in natural transformation for most bacterial species.
DprA shows sequence conservation, and its occurrence in the bacterial genome is ubiquitous irrespective of a bacterium’s natural competence ability (4). In Gram-negative bacteria, the pervasive role of DprA in bacterial physiology and species-specific multifunction activity suggested its possible role in bacterial physiology in addition to natural transformation (25). To date, a large volume of research has been published on DprA, and much of it has demonstrated its involvement in molecular events linked to evolutionary consequences in bacteria. However, a comprehensive review describing its biochemical properties, functional interaction with RecA, and roles in natural transformation and bacterial virulence is lacking. This review provides an extensive yet judicious collection of recent findings on DprA and its functions in bacterial transformation, fitness, and virulence.
OCCURRENCE AND GENOMIC ORGANIZATION OF DprA
DprA homologs have been annotated in the genomes of >2,400 bacterial species, while the genomes of Archaeoglobus fulgidus, Rickettsia prowazekii, Chlamydia pneumoniae, Chlamydia trachomatis, Methanobacterium thermoautotrophicum, Methanococcus jannaschii, and Pyrococcus horikoshii lack dprA (26). Archaea such as Methanococcus voltae, Hermococcus kodakarensis, Methanothermobacter marburgensis, and a mutant of Pyrococcus furiosus have been classified as naturally competent, and type IV pilus-like structures facilitate this natural competence. However, among the 26 genome sequences available, Pyrococcus furiosus (Pfur) is the only archaean in which the dprA-like gene has a central Rossmann fold (RF) domain and shows ~33% identity with bacterial DprAs, suggesting that dprA-like genes exist outside Eubacteria. Furthermore, the absence of a dprA-like gene in natural competence-enabled archaea raises the possibility that these archaea possess DprA orthologues to fulfill the functions of DprA. Thus, the archaeal genome generally lacks dprA-like genes. Its occurrence in P. furiosus may have been due to horizontal gene transfer from ɛ-proteobacteria to fulfill a functional requirement other than natural transformation (27). Interestingly, sequence analyses of transformable bacteria do not form clusters but are distributed according to their taxonomic groups (28). A genome-wide sequence analysis suggested that DNA topoisomerases and DNA resolvase genes occasionally co-occur with dprA. For instance, in Neisseria, dprA is co-expressed with smg (putative RNA binding translational regulator) and topA (type IA topoisomerase) in an operon (6). In H. pylori strains, dprA is present with the ilvC, minD, and minE genes in upstream and some uncharacterized downstream open reading frames in an operon form (26). In H. influenzae, the dprA gene is co-transcribed in an operon with dprB (encodes RuvC) and an uncharacterized dprC (29). The Sxy/cAMP regulon regulates DNA uptake and processing genes, including the dprA gene of E. coli, H. influenzae, and Vibrio cholerae (30). The dprA gene of Riemerella anatipestifer ATCC 11845 presents as a distal gene of a putative operon comprised of RA0C_1074 (group II Hb), RA0C_1075 (RuvC), RA0C_1076 (uncharacterized), and RA0C_1077 (Lrp/AsnC) (31). The dprA gene in the radioresistant bacterium Deinococcus radiodurans exists in an operon with elongation factor P and an uncharacterized protein with two tetratricopeptide repeats. In conclusion, genome analysis has suggested that dprA gene occurs ubiquitously in bacteria and that archaea usually lack dprA-like genes. Furthermore, a causal relationship of dprA co-occurrence with DNA topoisomerases and DNA resolvase genes has been observed in some bacteria.
DOMAIN ORGANIZATION IN DprA PROTEIN
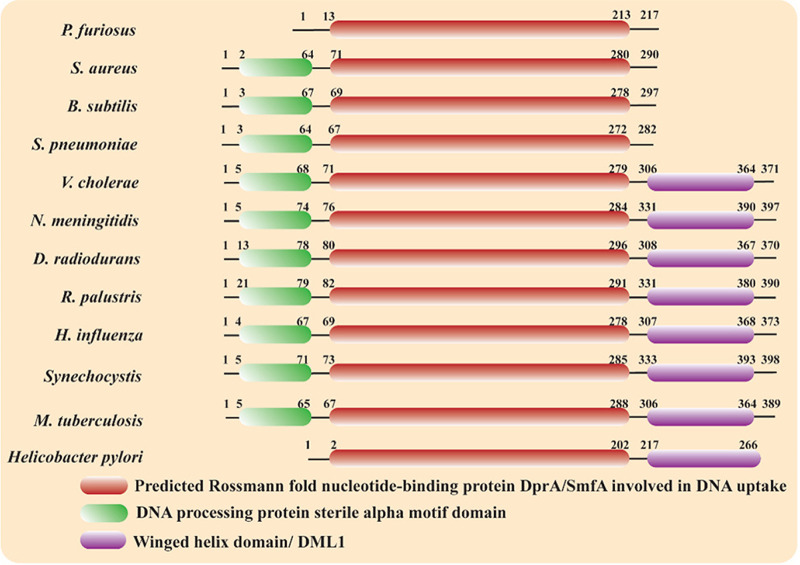
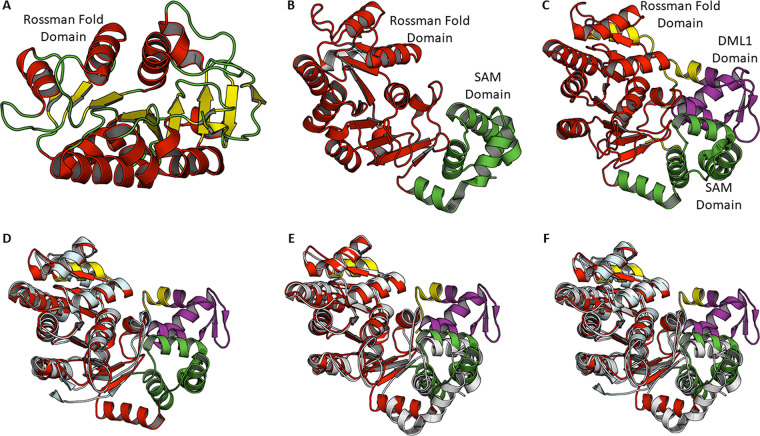
The DprA protein varies in size from 240 (C. jejuni) to 398 amino acids (Synechocystis and N. gonorrhoeae) (26). DprA consists of three domains: an N-terminal SAM domain, a central RF domain, and a C-terminal DML1 domain (Fig. 2). DprA proteins from different bacteria essentially have a central RF domain. The N-terminal SAM domain, which participates in protein-protein interaction, is common in most DprA except those of H. pylori and P. furiosus. DprA proteins from many other bacteria, including D. radiodurans, Mycobacterium tuberculosis, Rhodopseudomonas palustris, Neisseria meningitides, V. cholerae, H. influenzae, and Synechocytosis spp., show the presence of the C-terminal DML1 domain (Fig. 2). The crystal structures of DprA from three species, viz. H. pylori (DprAHp; PDB ID: 4LJK) (32), S. pneumoniae (DprASp; PDB ID: 3UQZ) (33) and R. palustris (DprARp; PDB ID: 3MAJ) (34) have been reported so far (Fig. 3). The crystal structure of DprAHp (H. pylori DprA) shows that the central RF domain consists of nine α-helices and nine β-strands. All nine strands form an extended structure. The helix flanks both sides of the β-strands to form a sandwiched structure (Fig. 3A). A similar architecture for the central RF is observed in the structures of DprASp and DprARp (Fig. 3B and C). Structural comparison of DprAHp with DprASp (S. pneumoniae DprA) or DprARp yields RMSD (root-mean-square deviation) values of 1.9 or 2.0 Å for aligned Ca, atoms, suggesting good similarity among structures (Fig. 3D to F). Only the central RF is visible in the DprAHp structure. DprASp structures show an N-terminal SAM domain consisting of five α-helices along with the central RF, while in the DprARp structure, all three domains are visible (Fig. 3).
FIG 2.
Domain architecture of DprA protein of selected bacteria. Central Rossmann fold (RF) is conserved and present in all DprA proteins, while the N-terminal SAM domain is common in all DprA except those of Helicobacter pylori and Pyrococcus furiosus. An additional C-terminal DML1 domain is present in the DprA of Deinococcus radiodurans, Mycobacterium tuberculosis, Rhodopseudomonas palustris, Neisseria meningitides, Vibrio cholera, Haemophilus influnzae, and Synechocytosis spp.
FIG 3.
Crystal structures of DprA. (A) Cartoon representation of DprA from H. pylori in which only the RF domain is observed. The central β-sheet is sandwitched between α-helices. (B) Crystal structures of DprA from S. pneumoniae showing RF domain (red) and N-terminal SAM domain (green). (C) Crystal structures of DprA from R. palustris show three domains: N-terminal SAM domain (green), central RF domain (red), and C-terminal DML1 domain (purple). Residues shown in yellow are linker regions. (D) Structural superposition of DprA from R. palustris and H. pylori (E) Structural superposition of DprA from R. palustris and S. pneumoniae (F) Structural superposition of DprA from R. palustris, S. pneumoniae and H. pylori.
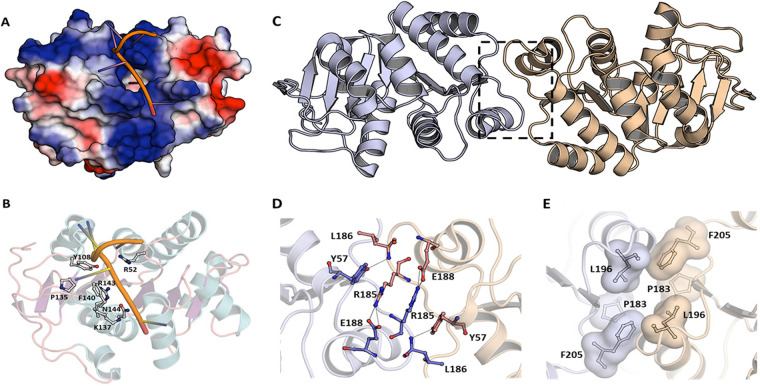
DprAs are DNA-binding proteins with a preference for ssDNA (28, 33, 35). Various parameters, including amino acid residues, domains, and the kinetics of their interactions with dsDNA and ssDNA, have been worked out in greater detail for DprABs, DprAHp, and DprASp (32, 33). Wang et al. (32) have obtained a DprAHp structure bound with 35-bp-long deoxythymine nucleotide (dT35) ssDNA. However, only seven dTs could be seen in the structure, as the electron density for the remaining dTs was not observed in the crystal structure. Both hydrophobic interactions and hydrogen bonds are involved in the binding of DNA to HpDprA. The DprA dimer interaction with ssDNA, DNA binding pocket residues, and the residues involved in DprA dimerization are shown in Fig. 4.
FIG 4.
Interaction of H. pylori DprA with ssDNA and DprA dimerization organization. (A) Electrostatic surface potential representation with bound ssDNA. (B) Residues involved in the DNA binding are shown in stick and ball model. Dimeric organization of H. pylori DprA in crystal structure. (C) Cartoon representation showing dimer interface. The two dimer subunits are shown in different colors. (D) Residues involved in hydrogen bond formation in H. pylori DprA dimerization. (E) Hydrophobic residues providing dimer interface. Residues are shown as sticks and their Van der Waals surface is also shown.
CELLULAR LOCALIZATION AND OLIGOMERIC PROPERTIES OF DprA
B. subtilis transformation-specific proteins (ComEA, ComEC, ComF, DprA, SsbB, RecA, CoiA, and pilin proteins) are either associated with the membrane or reside in the cytosol and relocalize to a single cell pole which is the preferred site for transforming eDNA uptake during transformation (19, 36). DprA bound with incoming ssDNA facilitates RecA filament formation for recombination during natural transformation (37). Time-lapse microscopy has shown that RecA forms a thread-like structure presumably on eDNA from the cell pole to nucleoid in B. subtilis competent cells when it is incubated with eDNA. The DprA of S. pneumoniae localizes at a single cell pole along with competence-specific σ factor (σX), ComW, ComD, ComE, and exogenous competence-stimulating heptadecapeptide (CSP) (38). The competence-dedicated alternative sigma factor σX assists DprA and ComD in their polar localization to facilitate competence shutoff (38).
The oligomeric nature of DprA was revealed by crystal structure and biochemical analysis of DprAHp (32). The DprAHp residues which form hydrophobic interaction in the dimeric form are Pro183 (P183), Leu196 (L196), and Phe205 (F205), while intermolecular hydrogen bonds form between Arg185 (R185) and Glu188 (E188) and between Tyr57 (Y57) and Leu186 (L186) (Fig. 4C to E). Full-length DprAHp forms an oligomer in the presence of glutaraldehyde, whereas DprAHp protein lacking C-terminal domain (RFHp) remains dimer, suggesting the crucial role of the C-terminal DML1Hp field in the formation of the higher-order oligomeric structure of DprAHp. Furthermore, the structural superimposition of the three DprA structures indicated that the dimeric form is essential for DprA biochemical functions and is a widely adopted quaternary structure (32).
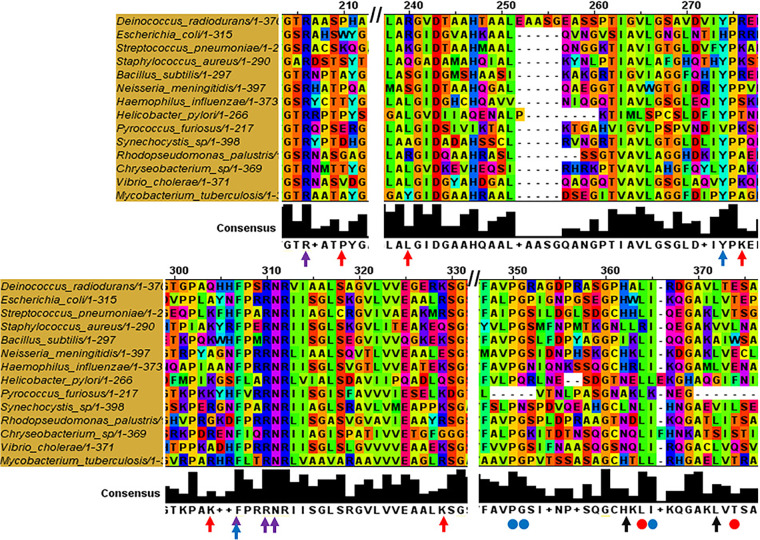
Unlike in DprAHp, the Pro248 (P248), Gly249 (G249), and Ile263 (I263) residues of DprASp form tail-to-tail dimers, and this type of dimerization is crucial for nucleoprotein complexes in vitro and genetic transformation (33). Interestingly, the DML1 domain at the C-terminal regions of DprARp and DprAEc has been shown not to affect the formation of RF-RF dimers by these DprAs, and dimerization seems to be similar as known for RF-RF dimers of DprASp. For DprASp, no variant(s) supporting N/N-terminal interaction have been isolated, suggesting a less crucial role of N/N-terminal interaction in DprASp dimerization. The residues involved in DprA dimerization and RecA interaction have considerable overlap. The DprA residues for RecA interaction appear to be evolutionarily less conserved. Nonetheless, the considerable overlap between DprA dimerization and RecA interaction interfaces has led to the hypothesis that RecA disrupts DprA dimerization, a prerequisite for DprA function, thereby giving RecA access to incoming ssDNA (33). Together, structural comparison of DprAHp, DprASp, DprARp, and the 3D models of E. coli DprA has revealed (i) the widespread association of a SAM and an RF domain in bacterial DprA, (ii) the evolutionary conservation of DprA dimerization potential and interface residues, and (iii) that the essential residues for self-dimerization of DprASp (P248, G249, and I263) are also identical in the DprARp, DprABs, and DprAEc proteins (Fig. 5).
FIG 5.
Sequence alignment of DprAs of different species highlighting conserved residues. Multiple sequence alignment was done using MUSCLE (Multiple Sequence Alignment-EMBL-EBI) and view using Jalview. Red arrow represents conserved lysine (K) of S. pneumoniae (K119, K144, K175, K202, and K225); purple and blue arrows represent conserved H. pylori DprA residues (R52, F-140, R-143, K137, and N-144) responsible for DNA binding; black arrows represent hydrophobic residues of H. pylori DprA (H264, and L273) crucial for dimerization. Red dot represents hydrophobic core dimerizing residues of H. pylori DprA (L196 and F205); blue dots represent the residues involved in self-dimerization of S. pneumoniae DprA (P248, G249, and I263).
DNA BINDING PROPERTIES OF DprA
DprA receives incoming DNA during natural transformation in its dimer conformation. The DprA of S. pneumoniae and its B. subtilis homolog (SmfA) bind cooperatively with ssDNA (28). DprA has shown higher affinity toward ssDNA versus dsDNA, except for D. radiodurans DprA (DprADr), which has equal affinity for ssDNA and dsDNA (39). The ability of DprADr to bind with dsDNA is an interesting phenotype and suggests a possible role of DprADr in DNA metabolism beyond natural transformation. Previously, RecA of D. radiodurans was shown to prefer dsDNA over ssDNA and was able to catalyze the inverse strand exchange reaction (40–43). The footing size of bacterial DprA is 30 to 40 bp on ssDNA, and therefore a minimum 50-bp length is essential for a stable nucleoprotein complex (6, 33). DprA has reversible nonspecific ssDNA binding activity and does not require DNA ends to bind. Unlike SSBEco, which destabilizes the helical structure of DNA, transmission electron microscopy analysis of DprA and ϕX ssDNA has revealed that DprASp and DprABs maintain or induce the DNA helical structure of DprA to form a tightly packed discrete complex with ϕX ssDNA (28). DprASp showed no affinity toward linear and relaxed dsDNA. However, it did show affinity toward negatively supercoiled ϕX DNA. This result was explained by the ability of the DprA protein to interact with ssDNA regions within ϕX DNA molecules and the apparent formation of a sleeve-like structure, which suggests that it can form a bridge or juxtapose two or more DNA molecules (28). Atomic force microscopy of DprABs revealed that depending on DprA concentrations, it forms discrete complexes without affecting the DNA helical structure (44).
The amino acid residues responsible for ssDNA binding are Arg52, Phe140, Arg143, and Asn144 for DprAHp (32) (Fig. 5, Fig. 4A and B). Multiple sequence alignment showed two motifs in DprA family proteins which are essential for ssDNA binding. The first “G-S/T/A-R”' motif is located in a loop (b3 to a3) region, and the corresponding residues are Gly50 to Arg52 in DprAHp; while the second “F/L/Y-X-X-R-N/D” motif is located in helix α6 and corresponds to residues Phe140 to Asn144 in DprAHp (32). The R123E mutant of DprA in R. anatipestifer cannot form nucleoprotein complexes and is deficient in transformation (31). Thus, RF plays an important role in DNA binding and other functions of DprA.
Together, DprA structure-function studies and biochemistry have suggested the following conclusions: (i) DprA binding with ssDNA is nonspecific; (ii) a dimer is required for optimal binding to ssDNA; (iii) the stable oligomeric complex of ssDNA-DprA dimer is observed at 1:1 stoichiometry (for DprAHp), and the ssDNA should be long enough (>50 nt) so that it can effectively be docked onto two specific binding pockets of DprA dimer (32); (iv) the C-terminal DML1-like domain contributes higher-order oligomer formation with the increased protein concentration because it possesses a weak secondary ssDNA binding site (45).
FUNCTIONAL INTERACTION OF DprA WITH OTHER GENOME INTEGRITY PROTEINS
RecA plays a vital role in natural transformation-mediated horizontal gene transfer, and DprA is a crucial protein associated with this process. During transformation, RecA loading onto incoming ssDNA is a multistep process (46–49). Various ssDNA binding proteins (SsbA, SsbB, and DprA) assist in this process (25, 44, 46, 50, 51). The interaction of DprA with RecA was initially recognized by FRET-based co-localization studies in B. subtilis (36). More recently, DprA interaction with RecA has been shown in many bacterial species, such as S. pneumoniae, R. palustris, and B. subtilis (24, 28, 33, 35, 44). DprA-RecA interactions have been shown by TAP-tag experiments, electron microscopy experiments in vitro, and Y2H (yeast two-hybrid) screening in vivo. RecA broadly interacts with two types of protein groups: (i) mediators which assist in RecA loading on ssDNA and (ii) modulators which are required for homology search and DNA strand exchange reaction. DprA belongs to the mediator group of RecA partners (46–49). RecAs of B. subtilis, D. radiodurans, and S. pneumoniae have higher activity with dATP. The RecA-dATP complex could assist DNA strand exchange without accessory factors such as DprA, SsbA, RecO, and SsbB (41, 52–54). On the other hand, the RecA·ATP complex cannot compete with a saturating concentration of SSB proteins. Therefore, the DprA protein helps the RecA.ATP to nucleate and polymerizes on the SsbA·ssDNA complex more efficiently than on protein-free ssDNA. The DprA of B. subtilis can facilitate active nucleoprotein filaments formation by RecA.ATP on ssDNA preincubated with SsbA, but not when bound with SsbB (46). The DprASp protein and its ortholog in B. subtilis may bind ssDNA cooperatively and have the ability to self-interact with RecA (24, 44). The DprASp-RecA-ssDNA filaments may catalyze the homology-dependent formation of joint molecules and limits the SSB barrier function during natural bacterial transformation (46).
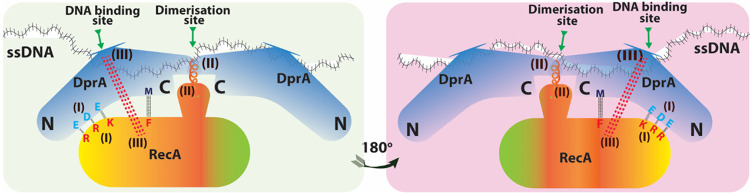
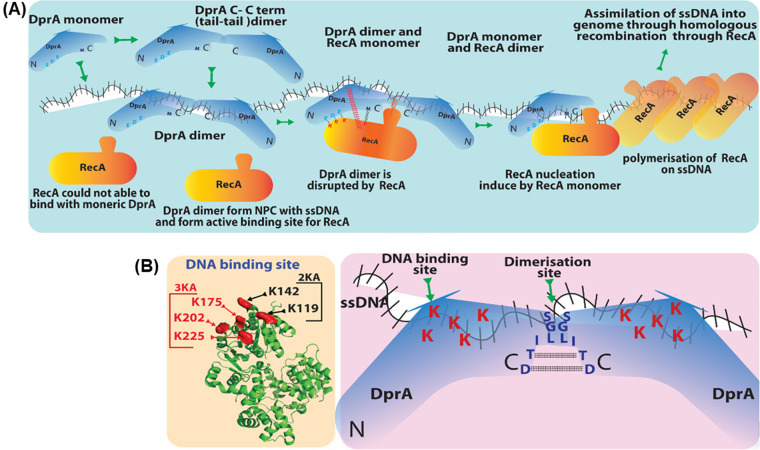
The acidic residues E235, D243, and E265 (EDE triad) in DprASp are crucial for DprASp-RecA interaction, and mutations in these residues lead to a loss of/weakened interaction with RecA (33). RecA residues necessary for DprA interaction have also been identified, and it has been shown that EDE-triad and methionine-238 residues of DprASp interact with R189/R226/K267 (RRK triad) and phenylalanine-230 residues at the N terminus of RecA, respectively (33) (Fig. 6). Additionally, the fact that the RecASp loop 2 region has a nonpolar motif (V212/G213/V214/M215/F216) suggests its role in RecA-DprA interaction, as the mutants of these residues decreased interaction activity. Because DprA and RecA can form self-oligomers, the heterogenic interaction of individual homopolymeric proteins is difficult to interpret. When RecA is present in an oligomeric form, the interacting region of this protein with DprA is largely inaccessible. Quevillon et al. (33) showed that DprASp binds with incoming ssDNA through its C/C dimer, followed by the nucleation of RecA on ssDNA. RecA interaction with DprASp destabilize the DprASp dimer interface, resulting in ssDNA transfer from DprA to RecA (Fig. 7A). The strong hydrophobic character of the loop 2 region of RecA might further facilitate the weakening of DprA homodimer binding to ssDNA, making DprA exit from ssDNA and concurrently loading RecA of DNA (33). Independently, Lisboa et al. (35) showed that a region in DprASp consisting of R115 and five basic residues (K119/K144/K175/K202/K225) surrounding it (Fig. 7B) was positioned at the opposite side of RecA interacting motif, close to its loop 2 region. Therefore, it is likely that the loop 2 region of RecA competes with the basic patch of DprA and paves the way to transfer ssDNA from the DprA dimer to RecA (35). Thus, the regulation of structural dynamics of RecA and DprA upon interaction for their functions would be an example of how two proteins with different oligomeric properties interact in heterogenic interaction for their functions.
FIG 6.
Residues important for RecA-DprA interaction. Three regions are responsible for DprAHp and RecA interaction. Region I represents DprAHp E235, D243, and E265 (EDE) triad interacting with R189, R226, and K267 (RRK) triad of RecAHp, respectively. Involvement of RecAHp F230 and DprAHp M238, which is present near EDE domain of DprAHp. Region II shows DprAHp dimerization domain with RecAHp N-terminal α-helix. Region III shows RecAHp polar loop 2 motifs interacting with DprAHp DNA binding site. Regions I and II are only accessible for the first RecA monomer binding to DprA and are entirely inaccessible for RecA oligomers. Region III has loop 2, responsible for the loading of RecA on ssDNA, as it competes with the basic patch of DprA to facilitate the transfer of ssDNA from DprA to RecA. Regions II and III are responsible for destabilization/disruption of the DprA dimer and transfer process.
FIG 7.
(A) DprA-mediated loading of RecA on ssDNA. DprASp dimer (C-C term) forms a nucleoprotein complex with ssDNA. DprASp dimer bound to ssDNA offers the best suitable platform for RecA nucleation. After ssDNA binding of DprASp dimer conformation change takes place, and sites of DprASp-RecA interaction open up (EDE triad of DprASp interacts with RRK triad of RecA). Once DprASp-RecA interaction is established, DprASp is converted to a monomer. This change allows RecA loading on ssDNA, followed by RecA nucleation, continued polymerization, and DprASp leaving the ssDNA. (B) Residues important for S. pneumoniae DprA protein DNA binding and dimerization. Five lysine (K119/K144/K175/K202/K225) residues on the basic face of DprASp are essential for DNA binding activity. DNA binding activity is completely abolished when lysine is mutated to alanine, whereas the partial patch mutation 3KA and 2KA produces a similar effect equal to that of R115A single mutant. This basic patch is localized opposite the RecA binding site.
Mismatch repair protein (MutS) may regulate recombination during B. subtilis horizontal gene transfer via natural chromosomal transformation. In B. subtilis, it has been shown that the natural chromosomal transformation frequency decreased exponentially with increased sequence divergence up to 15% in wild-type cells or cells lacking MutS2 or mismatch repair proteins (MutL, MutS, or both). However, if sequence divergence is more than 15%, the chromosomal transformation efficiency is ~100-fold higher in ΔmutS and ΔmutSL than in wild-type cells and ΔmutS2. Thus, transformation frequency decreased in a biphasic manner with increasing sequence difference of incoming DNA. The addition of MutS overcame the heterologous barrier between incoming DNA and dsDNA, possibly through the modulation of RecA filament on bidirectional ssDNA (55). RecA-mediated DSE in the presence of DprA, RecO, and SsbA proteins reconstituted in vitro led to the observation that DNA sequence divergence halts RecA strand exchange in a log-linear fashion and showed a minor priority for the 3′ end of duplex DNA (48). Natural chromosomal transformation (CT) in B. subtilis is governed by sequence divergence (SD) of up to 15%, and integration occurs through homology-directed CT.
In contrast, the interspecies boundaries prevail beyond 23% SD or more, and CT frequency decreases log-linearly. The interspecies CT is RecA-dependent, and the activity of the dprA, radA, recJ, recX, or recD2 genes is suggested to be crucial for interspecies CT, the generation of genetic diversity, and speciation (20). In addition to DprA’s native role in natural transformation to promote RecA-mediated bidirectional or reciprocal recombination, DprA may also promote RecA-catalyzed unidirectional gene conversion and produce pilin variation in Neisseria (see below, “DprA role in the regulation of bacterial virulence”).
DprA can protect ssDNA from nucleolytic degradation by ExoT (3′ to 5′ exonuclease), RecJ (5′ to 3′ exonuclease), and Mung Bean endonuclease in vitro and reverse the inhibitory effect of RecX and RecU on RecA-mediated DNA strand exchange (21, 56). The restriction-modification systems (RM systems) in bacteria discriminate self and non-self DNA and help maintain the genome integrity of an organism by reducing horizontal gene transfer in bacteria (29, 57). Dwivedi et al. (45) showed that DprAHp can protect dsDNA substrate from type II restriction endonucleases and stimulate methyltransferase activity in vitro. However, the involvement of type II RM systems in natural transformation by interacting with DprA is less likely in vivo because the incoming ssDNA during natural transformation was shown to be a poor substrate for type II restriction endonucleases (58).
The radiation-resistant bacterium D. radiodurans is naturally transformable, and various factors (PilQ, PilD, type IV pilins, PilB, PilT, ComEC-ComEA, and ComF) responsible for DNA uptake and translocation have been recently identified (7). Cells devoid of DprA have severely abolished transformation, and the absence of either recF, recO, or ddrB in a dprA– genetic background completely negates transformation (7). In vitro studies have suggested that DprADr has equal affinity binding for ssDNA and dsDNA and protects ssDNA from nucleolytic degradation. Furthermore, it has been suggested that the oligomerization and DNA binding properties of DprADr are essential to support RecA-catalyzed strand exchange reactions (SER) in vitro (39). Ithurbide et al. (7) did not observe a role of DprA in gamma radiation resistance. However, our current work on DprA’s role in gamma radiation resistance and DNA repair suggests that dprA plays an important role in gamma irradiation and showed faster DNA double-strand break repair kinetics and elevated rifampicin resistance mutation frequency (D.K.S., H.S.M., and Y.S.R., unpublished data).
The roles of DprA in replication and cell division are not apparent. However, indirect evidence has shown that lower DNA content in a dprA mutant of N. meningitides post-antibiotic treatment highlights impaired DNA replication in dprA mutant cells (6). Thus, different findings have suggested some direct or indirect functional interaction of DprA with many proteins involved in genome integrity and metabolism, which would be worth monitoring in the future.
DprA FUNCTIONS IN NATURAL TRANSFORMATION AND REGULATION OF COMPETENCE
DprA function is necessary for the natural transformation of chromosomal and plasmid DNA in R. anatipestifer, N. meningitidis, N. gonorrhoeae, D. radiodurans, and S. pneumoniae (6, 7, 15, 17, 23). However, in H. influenzae, DprA is needed for chromosomal DNA transformation but not for plasmid DNA transformation (16). The non-requirement of DprA for plasmid DNA transformation was an intriguing observation. Experimental evidence suggested that different topological forms of plasmid DNA may allow DNA translocation as intact dsDNA without degradation. This DNA uptake mode is called illegitimate transformation (59). Bacteria such as D. radiodurans and B. subtilis have redundant pathways for chromosomal DNA transformation, in which the RecO and DprA proteins are functionally redundant (7, 44, 60). The role of DprA in shutting off competence has been reported in S. pneumoniae (24).
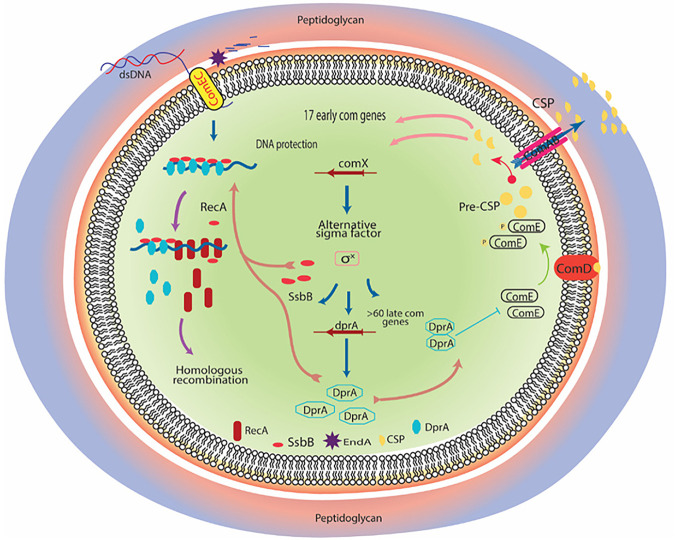
About ~80 bacterial species are capable of natural transformation (3). Among these, many naturally occurring species, such as Deinococcus and Neisseria, remain competent during their growth phase. However, B. subtilis and S. pneumoniae adopt a transient phase of competence to take up environmental DNA. Nutrition deprivation activates the competence of the B. subtilis bacterium and acquired DNA used for carbon sources during the stationary phase (60). DprA plays a more crucial role in regulating competence in S. pneumoniae (Fig. 8). The competence or X-state of S. pneumoniae is activated by competence-stimulating heptadecapeptide, encoded by the comC gene (9). CSP binding to ComDE (bacterial two-component system) can activate the classical mode of activation in which autophosphorylation of a sensor histidine kinase (ComD) and transphosphorylation of a response regulator (comE~P) activate the comAB, comCDE, and comX operon genes, which in turn activates a positive feedback loop of CSP (10, 38, 61) (Fig. 8). These changes lead to a sudden rise in CSP levels, rendering all cells X-state (competent). comX gene (master regulator of X-state) encodes competence-specific σ factor (σX) (Fig. 8). The genes which come under the control of σX are late com genes, including DNA uptake machinery genes, dprA, ssbB, and other recombination mediator proteins (62, 63). DprASp shuts down competence by suppressing comX expression (Fig. 8). DprAsp shifts the concentration of phosphorylated ComE toward unphosphorylated ComE. These changes lead to the suppression of comX promoter activity and, consequently, no activation of comX and comW genes (61). Together, these changes result in diminished or inhibited activation of σX and subsequent suppression of late competence genes, including dprA, which leads to shutoff of X- or competence state (Fig. 8). Johnston et al. (38) investigated the chronology of pneumococcal competence development and shutoff through spatiotemporal localization dynamics of the key regulators (DprA, σX, ComW, ComD, ComE, and exogenous CSP) at the single-cell level and suggested that two intertwined and transient transcription waves regulate S. pneumoniae competence. The stress-inducible phosphor-relay between ComD and ComE constitutes the first wave, while the second wave controls expression of an alternative sigma factor σX, regulating the expression of DprA which turns off competence through interaction with phosphorylated ComE. Experimental evidence has also suggested that σX physically conveys DprA next to ComD when all these proteins co-localize at one pole in competent cells. Through the polar DprA targeting function, σX mediates the timely shutoff of the pneumococcal competence cycle, preserving cell fitness. Thus, DprA-mediated regulation of competence is crucial for S. pneumoniae to switch swiftly between the competence and rapid growth phasse according to environmental conditions, nutritional state, and host factors (10, 38, 61). Moreover, in Acinetobacter baylyi, DprA-led shutoff of competence and regulation of natural transformation was shown to effectively reduce the cost of transformation if internalized DNA was utilized for the generation of the nucleotide pool and incorporated into DNA biosynthesis pathways. On the contrary, using internalized DNA for recombination and repair may have costly consequences because recombination with transforming DNA dramatically reduces transformant survival and induces DNA strand breaks (64).
FIG 8.
Regulation of competence in Streptococcus pneumoniae. Dynamicity of competence pathway in S. pneumoniae is regulated through competence-stimulating peptide (CSP). CSP is processed from pre-CSP (precursor form) and encoded by comC. ComAB is an ABC-type membrane transporter that converts pre-CSP into CSP and exports it; ComDE is a two-component signal transduction system (TCSs); and ComD (histidine kinase) binds with CSP to trigger its autophosphorylation (ComD~P) followed by transphosphorylation of its cognate response regulator molecule (ComE) by transfer of a phosphate group. ComE~P activates 17 early com genes, including comX, which encodes a competence-specific alternate sigma transcription factor (σX). dprA, ssbB, and more than 70 genes are regulated through σX alternate transcription factor. After internalization of DNA, elevated level of DprA sequester the ComE~P and consequently shuts off the competence pathway.
Transformation efficiency in S. pneumoniae is not dependent on the abundance of DNA; instead, it is entirely reliant on the level of cellular DprA. It has been estimated that approximately ~650 to ~2,250 DprA molecules per cell are required for maximum transformation efficiency, while the presence of fewer than 650 DprA molecules results in a rapid loss of transforming ssDNA. Interestingly, higher levels of DprA dimer (~4,000 molecules per cell) dictate competence shutoff in S. pneumoniae by either dephosphorylation or sequestering of ComE~P dimmers. In addition, a dprA null mutant of S. pneumoniae showed rapid cell lysis compared to the wild type due to a nonfunctioning shutoff pathway, indicating that the cell’s inability to shut off competence hurts cell viability and physiology (3).
DprACj (C. jejuni DprA) is involved in the natural transformation pathway of C. jejuni, affecting efficiency for both plasmid and chromosomal DNA. A dprA null mutant exhibited reduced transformation efficiency (2-log) for chromosomal DNA while altogether abolishing plasmid DNA electrotransformation (17, 65). This loss in transformation could be trans-complemented by wild-type DprA from H. pylori and H. influenza, suggesting the evolutionary conservation of DprA function across species.
DprA ROLE IN THE REGULATION OF BACTERIAL VIRULENCE
The absence of the dprA gene negatively impacts S. pneumoniae growth and virulence. This phenotype is due to DprA’s contribution to shutting off the competence state, but not to its role in DNA uptake and integration. A ΔdprA mutant of S. pneumoniae showed a growth defect when stimulated with CSP and had difficulty quitting the competence state. CSP-activated ComE positively regulates 24 early competence genes, including alternative sigma factor σX. The σX binds to the “com-box” in the promoter and regulates the expression of ~80 late competence genes. Among these genes, 16 are essential for competence and genetic transformation, and 14 late genes are necessary for bacterial fitness during bacteremia infection in the host (66, 67). Among these 14 late genes, cbpD, cibAB, and p1 promoter-regulated genes have roles in bacterial virulence by enhancing the release of pneumolysin and expression of lytA, radC, spd_0030, spd_0031, spd_0981, spd_1778, and spd_1828.
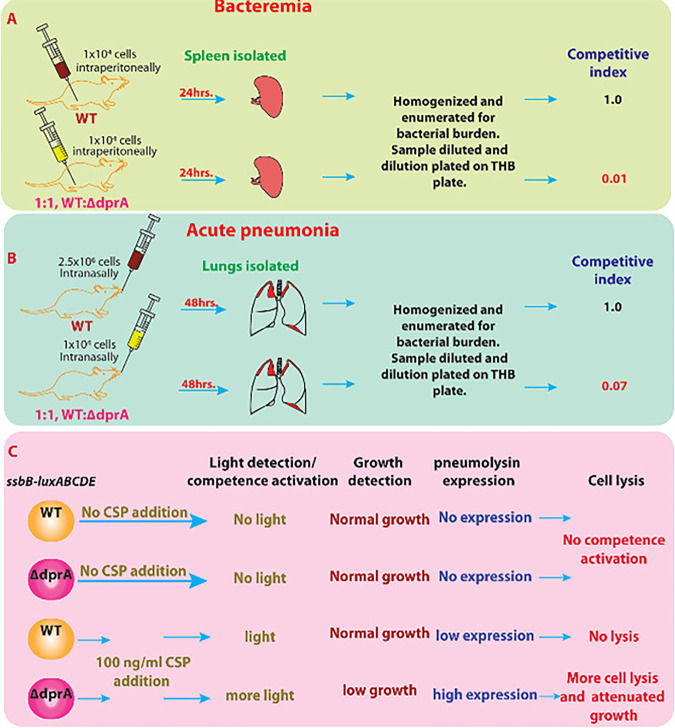
The importance of DprA in S. pneumoniae virulence during the infection cycle was tested in model in which the abilities of wild-type S. pneumoniae (D39) and a ΔdprA mutant to cause bacteremia and acute pneumoniae, respectively, were evaluated in a CD1 mouse model. dprA deletion severely affects the virulence and infectivity of S. pneumoniae and competitiveness to cause bacteremia, nasopharyngeal infection, and acute pneumonia infection (68) (Fig. 9A, B). Thus, DprA’s functional role in S. pneumoniae fitness during infection by regulating the competence state rather than by processing incoming DNA has been suggested. The inability of ΔdprA mutants to exit the competence state has contributed to attenuated virulence, perhaps by creating an imbalance in energy utilization by S. pneumoniae in host cells and excess energy use to maintain of competence (3) (Fig. 9C).
FIG 9.
DprA involvement in Streptococcus pneumoniae virulence and pathogenicity. Streptococcus pneumoniae D39 (WT) and ΔdprA mutant were used to evaluate bacteremia and acute pneumonia in CD1 mice. (A) For the bacteremia mouse model, 1 × 104 CFU bacteria were injected intraperitoneally. After 24 h, spleens were isolated from euthanized mice, homogenized dilutions were plated, and pneumonia burdens were calculated. (B) For the acute pneumonia mouse model, animals were anesthetized using isoflurane and intranasally administered 5 × 106 CFU pneumococci. After 48 h, mice were euthanized, lungs were removed, and bacterial burdens were calculated. The competitive index (CI) is the ratio of mutant versus wild type in the output sample divided by the input ratio of mutant versus wild type provided in the sample. A CI of >0.7 is defined as attenuated. (C) DprA is required to shut off competence and regulate ~100 “early” and “late” competence genes. Overexpression of early and late competence genes causes cellular stress and excess energy consumption (energy-deprived condition) in dprA mutant. Here, ssbB (late competence gene) is translation-fused with firefly luciferase gene to generate translation fusion construct SsbB-luxABCDE and measure the overexpression of late competence gene after competence activation through the addition of CSP1. A ΔdprA mutant of S. pneumoniae cannot shut off the competence pathway activated by CSP1 addition, leading to more energy consumption and causing unwanted cellular stress and energy deprivation. The ΔdprA mutant is susceptible to low CSP1 concentration for prolonged competence activation (more light), resulting in overexpression of allolytic factors LytA, CbpD, and CibAB. It also induces the fratricide immunity protein ComM. Combined action of low energy and excessive overexpression of allolytic enzyme, followed by the release of pneumolysin, potentially leads to bacterial death and attenuated virulence.
DprA ROLE IN BACTERIAL PILIN VARIATION
The pilus plays crucial roles in cellular adherence, bacterial aggregation, twitching motility, and natural DNA transformation in Neisseria (69–72). Pilin protein constitutes a major subunit of the Neisseria type IV pilus system (73–75). Variation in pilin antigenic determinants creates genetic diversity and plays vital roles in virulence, survival, cell adhesion, and escape from the host immune response in Neisseria and Streptococcus. Pilin variation is a convenient mechanism which allows an organism to introduce a high rate of recombinations without deleterious effects on genome function. Two mechanisms mediate pilus phase variation: a RecA-independent mechanism in which slippage and mispairing of the pilC gene strands lead to subunit variation in pilin, and RecA-dependent high-frequency gene conversion that promotes non-reciprocal DNA recombination events between multiple pilS loci and the pilE expression locus (72, 76–78). N. gonorrhoeae and related pathogens such as N. meningitidis use minor pilus protein (ComP) to take up DNA with DUS (DNA uptake sequence) and induce efficient DNA uptake to the periplasm (79–82). A novel function of DprA beyond transformation was shown for pilin protein variation in Neisseria (23). It was observed that dprA mutants show increased RecA-dependent pilin variation compared to isogenic parental strains (P < 0.05). However, this was restored to the parental level when DprA was complemented in trans. Using recA and a dprA double mutant further confirmed that DprA affects only RecA-dependent variation in pilus (23). Pilus variation in Neisseria invokes numerous recombination proteins (RecA, RecX, RecR, RecO, RecQ, RecG, and RuvABC) and mismatch repair proteins to offer gene conversion by non-reciprocal recombination process (83).
Pilin variation-related recombination events are unidirectional gene conversion, while natural transformation-led recombination is bidirectional or reciprocal. Although the role of DprA in bidirectional recombination is known, its role in unidirectional gene conversion was unclear. It is possible that DprA promotes RecA-catalyzed unidirectional gene conversion and produces pilin variation in Neisseria (23). The mechanisms which can explain DprA’s role in unidirectional gene conversion owed to its ability to load RecA on ssDNA, influencing and increasing DSE activity (23). Besides this, it is also likely that DprA binds with small single-stranded fragments generated during double-strand breaks at the pilus locus, which eventually load RecA and may stimulate invasion of the pilS locus (84).
CONCLUDING REMARK
The competence state enables bacteria to take up extracellular DNA from the environment. The natural transformation process involves the uptake of DNA followed by its processing and integration into the bacterial genome. The role of DprA is indispensable in natural transformation. It facilitates RecA functions during the integration of incoming ssDNA into the genome. Structure-function studies have identified important residues for homotypic and heterotypic interactions of DprA and RecA. DprA can alleviate the inhibitory effects of SSB, RecX, and RecU on RecA-mediated DNA strand exchange by directly antagonizing the inhibitory impact of RecX. dprA has been reported to occur in most bacterial species. However, many bacteria do not encode the other components of natural transformation machinery. This observation suggests a strong possibility of DprA having roles in other molecular events in bacterial physiology, possibly beyond natural transformation, such as regulation of virulence, pilin variation, and genome integrity maintenance. The molecular mechanisms underlying DprA’s role in the regulation of RecA function have been studied in a few bacteria, but are lacking in most bacteria which have DprA with complete competence machinery or DprA alone. A DprAsp-mediated shift in the concentration of phosphorylated ComE and its cascade leads to competence shutoff and energy conservation in S. pneumoniae, leading to speculation on this protein’s role as a stress-response regulator of DNA metabolism. The wide distribution of DprA in most bacteria, irrespective of their natural transformation ability, highlights possibly novel roles of DprA beyond natural transformation in bacterial physiology that would be worth exploring in the future.
ACKNOWLEDGMENT
The authors have no conflicts of interest regarding the manuscript’s content.
Contributor Information
Yogendra S. Rajpurohit, Email: ysraj@barc.gov.in.
Tina M. Henkin, Ohio State University
REFERENCES
- 1.Lorenz MG, Wackernagel W. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev 58:563–602. 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen I, Dubnau D. 2004. DNA uptake during bacterial transformation. Nat Rev Microbiol 2:241–249. 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 3.Johnston C, Mortier-Barriere I, Khemici V, Polard P. 2018. Fine-tuning cellular levels of DprA ensures transformant fitness in the human pathogen Streptococcus pneumoniae. Mol Microbiol 109:663–675. 10.1111/mmi.14068. [DOI] [PubMed] [Google Scholar]
- 4.Johnston C, Martin B, Fichant G, Polard P, Claverys J-P. 2014. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat Rev Microbiol 12:181–196. 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- 5.Brito IL. 2021. Examining horizontal gene transfer in microbial communities. Nat Rev Microbiol 19:442–453. 10.1038/s41579-021-00534-7. [DOI] [PubMed] [Google Scholar]
- 6.Hovland E, Beyene GT, Frye SA, Homberset H, Balasingham SV, Gómez-Muñoz M, Derrick JP, Tønjum T, Ambur OH. 2017. DprA from Neisseria meningitidis: properties and role in natural competence for transformation. Microbiology (Reading) 163:1016–1029. 10.1099/mic.0.000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ithurbide S, Coste G, Lisboa J, Eugénie N, Bentchikou E, Bouthier de la Tour C, Liger D, Confalonieri F, Sommer S, Quevillon-Cheruel S, Servant P. 2020. Natural transformation in Deinococcus radiodurans: a genetic analysis reveals the major roles of DprA, DdrB, RecA, RecF, and RecO proteins. Front Microbiol 11:1253. 10.3389/fmicb.2020.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claverys J-P, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in Gram-positive bacteria. Annu Rev Microbiol 60:451–475. 10.1146/annurev.micro.60.080805.142139. [DOI] [PubMed] [Google Scholar]
- 9.Pestova E, Håvarstein L, Morrison D. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol 21:853–862. 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Morrison DA. 1999. Identification of a new regulator inStreptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol 181:5004–5016. 10.1128/JB.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer-Smith R, Roberts S, Gurung N, Snyder LAS. 2016. DNA uptake sequences in Neisseria gonorrhoeae as intrinsic transcriptional terminators and markers of horizontal gene transfer. Microb Genom 2:e000069. 10.1099/mgen.0.000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith HO, Tomb JF, Dougherty BA, Fleischmann RD, Venter JC. 1995. Frequency and distribution of DNA uptake signal sequences in the Haemophilus influenzae Rd genome. Science 269:538–540. 10.1126/science.7542802. [DOI] [PubMed] [Google Scholar]
- 13.Pelicic V. 2008. Type IV pili: e pluribus unum? Mol Microbiol 68:827–837. 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 14.Parge HE, Forest KT, Hickey MJ, Christensen DA, Getzoff ED, Tainer JA. 1995. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 378:32–38. 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 15.Bergé M, Mortier-Barrière I, Martin B, Claverys J-P. 2003. Transformation of Streptococcus pneumoniae relies on DprA- and RecA-dependent protection of incoming DNA single strands. Mol Microbiol 50:527–536. 10.1046/j.1365-2958.2003.03702.x. [DOI] [PubMed] [Google Scholar]
- 16.Karudapuram S, Zhao X, Barcak GJ. 1995. DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J Bacteriol 177:3235–3240. 10.1128/jb.177.11.3235-3240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata T, Ando T, Israel DA, Wassenaar TM, Blaser MJ. 2005. Role of dprA in transformation of Campylobacter jejuni. FEMS Microbiol Lett 252:161–168. 10.1016/j.femsle.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Smeets LC, Bijlsma JJ, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. 2000. The dprA gene is required for natural transformation of Helicobacter pylori. FEMS Immunol Med Microbiol 27:99–102. 10.1111/j.1574-695X.2000.tb01418.x. [DOI] [PubMed] [Google Scholar]
- 19.Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. 2005. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell 122p:59–71. 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano E, Ramos C, Alonso JC, Ayora S. 2021. Recombination proteins differently control the acquisition of homeologous DNA during Bacillus subtilis natural chromosomal transformation. Environ Microbiol 23:512–524. 10.1111/1462-2920.15342. [DOI] [PubMed] [Google Scholar]
- 21.Serrano E, Carrasco B, Gilmore JL, Takeyasu K, Alonso JC. 2018. RecA regulation by RecU and DprA during Bacillus subtilis natural plasmid transformation. Front Microbiol 9:1514. 10.3389/fmicb.2018.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manfredi C, Suzuki Y, Yadav T, Takeyasu K, Alonso JC. 2010. RecO-mediated DNA homology search and annealing is facilitated by SsbA. Nucleic Acids Res 38:6920–6929. 10.1093/nar/gkq533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffin PM, Barber DA. 2016. DprA is required for natural transformation and affects pilin variation in Neisseria gonorrhoeae. Microbiology (Reading) 162:1620–1628. 10.1099/mic.0.000343. [DOI] [PubMed] [Google Scholar]
- 24.Mirouze N, Bergé MA, Soulet A-L, Mortier-Barrière I, Quentin Y, Fichant G, Granadel C, Noirot-Gros M-F, Noirot P, Polard P, Martin B, Claverys J-P. 2013. Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc Natl Acad Sci USA 110:E1035–E1044. 10.1073/pnas.1219868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav T, Carrasco B, Myers AR, George NP, Keck JL, Alonso JC. 2012. Genetic recombination in Bacillus subtilis: a division of labor between two single-strand DNA-binding proteins. Nucleic Acids Res 40:5546–5559. 10.1093/nar/gks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando T, Israel DA, Kusugami K, Blaser MJ. 1999. HP0333, a member of the dprA family, is involved in natural transformation in Helicobacter pylori. J Bacteriol 181:5572–5580. 10.1128/JB.181.18.5572-5580.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonson AB, Servin JA, Skophammer RG, Herbold CW, Rivera MC, Lake JA. 2005. Decoding the genomic tree of life. Proc Natl Acad Sci USA 102 (Suppl 1):6608–6613. 10.1073/pnas.0501996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortier-Barrière I, Velten M, Dupaigne P, Mirouze N, Piétrement O, McGovern S, Fichant G, Martin B, Noirot P, Le Cam E, Polard P, Claverys J-P. 2007. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130:824–836. 10.1016/j.cell.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 29.Humbert O, Dorer MS, Salama NR. 2011. Characterization of Helicobacter pylori factors that control transformation frequency and integration length during inter-strain DNA recombination. Mol Microbiol 79:387–401. 10.1111/j.1365-2958.2010.07456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Cameron AD, Redfield RJ. 2009. Sxy induces a CRP-S regulon in Escherichia coli. J Bacteriol 191:5180–5195. 10.1128/JB.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Tian X, Liu M, Wang M, Biville F, Cheng A, Zhu D, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Huang J, Tian B, Yu Y, Liu Y, Zhang L, Pan L, Rehman MU, Chen X. 2019. DprA is essential for natural competence in Riemerella anatipestifer and has a conserved evolutionary mechanism. Front Genet 10:429. 10.3389/fgene.2019.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Ding J, Zhang Y, Hu Y, Wang D-C. 2014. Structural insights into the unique single-stranded DNA-binding mode of Helicobacter pylori DprA. Nucleic Acids Res 42:3478–3491. 10.1093/nar/gkt1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quevillon-Cheruel S, Campo N, Mirouze N, Mortier-Barrière I, Brooks MA, Boudes M, Durand D, Soulet A-L, Lisboa J, Noirot P, Martin B, van Tilbeurgh H, Noirot-Gros M-F, Claverys J-P, Polard P. 2012. Structure-function analysis of pneumococcal DprA protein reveals that dimerization is crucial for loading RecA recombinase onto DNA during transformation. Proc Natl Acad Sci USA 109:E2466–E2475. 10.1073/pnas.1205638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang C, Xu X, Cui H, Chin S, Savchenko A, Edwards A, Joachimiak A, Midwest Center for Structural Genomics (MCSG) . 2010. Crystal structure of putative DNA processing protein DprA from Rhodopseudomonas palustris CGA009. MCSG, Lemont, IL. 10.2210/pdb3maj/pdb. [DOI] [Google Scholar]
- 35.Lisboa J, Andreani J, Sanchez D, Boudes M, Collinet B, Liger D, van Tilbeurgh H, Guérois R, Quevillon-Cheruel S. 2014. Molecular determinants of the DprA− RecA interaction for nucleation on ssDNA. Nucleic Acids Res 42:7395–7408. 10.1093/nar/gku349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer N, Hahn J, Dubnau D. 2007. Multiple interactions among the competence proteins of Bacillus subtilis. Mol Microbiol 65:454–464. 10.1111/j.1365-2958.2007.05799.x. [DOI] [PubMed] [Google Scholar]
- 37.Tadesse S, Graumann P. 2007. DprA/Smf protein localizes at the DNA uptake machinery in competent Bacillus subtilis cells. BMC Microbiol 7:105. 10.1186/1471-2180-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston CH, Soulet A-L, Bergé M, Prudhomme M, De Lemos D, Polard P. 2020. The alternative sigma factor σX mediates competence shut-off at the cell pole in Streptococcus pneumoniae. Elife 9:e62907. 10.7554/eLife.62907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma DK, Misra HS, Soni I, Rajpurohit YS. 2022. Characterization of DNA processing protein A (DprA) of the radiation-resistant bacterium Deinococcus radiodurans. Microbiol Spectr 10:e0347022. 10.1128/spectrum.03470-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajan R, Bell CE. 2004. Crystal structure of RecA from Deinococcus radiodurans: insights into the structural basis of extreme radioresistance. J Mol Biol 344:951–963. 10.1016/j.jmb.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 41.Kim JI, Cox MM. 2002. The RecA proteins of Deinococcus radiodurans and Escherichia coli promote DNA strand exchange via inverse pathways. Proc Natl Acad Sci USA 99:7917–7921. 10.1073/pnas.122218499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajpurohit YS, Bihani SC, Waldor MK, Misra HS. 2016. Phosphorylation of Deinococcus radiodurans RecA regulates its activity and may contribute to radioresistance. J Biol Chem 291:16672–16685. 10.1074/jbc.M116.736389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma DK, Siddiqui MQ, Gadewal N, Choudhary RK, Varma AK, Misra HS, Rajpurohit YS. 2020. Phosphorylation of deinococcal RecA affects its structural and functional dynamics implicated for its roles in radioresistance of Deinococcus radiodurans. J Biomol Struct Dyn 38:114–123. 10.1080/07391102.2019.1568916. [DOI] [PubMed] [Google Scholar]
- 44.Yadav T, Carrasco B, Hejna J, Suzuki Y, Takeyasu K, Alonso JC. 2013. Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA. J Biol Chem 288:22437–22450. 10.1074/jbc.M113.478347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwivedi GR, Sharma E, Rao DN. 2013. Helicobacter pylori DprA alleviates restriction barrier for incoming DNA. Nucleic Acids Res 41:3274–3288. 10.1093/nar/gkt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav T, Carrasco B, Serrano E, Alonso JC. 2014. Roles of Bacillus subtilis DprA and SsbA in RecA-mediated genetic recombination. J Biol Chem 289:27640–27652. 10.1074/jbc.M114.577924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrasco B, Yadav T, Serrano E, Alonso JC. 2015. Bacillus subtilis RecO and SsbA are crucial for RecA-mediated recombinational DNA repair. Nucleic Acids Res 43:5984–5997. 10.1093/nar/gkv545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrasco B, Serrano E, Sánchez H, Wyman C, Alonso JC. 2016. Chromosomal transformation in Bacillus subtilis is a non-polar recombination reaction. Nucleic Acids Res 44:2754–2768. 10.1093/nar/gkv1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenhart JS, Brandes ER, Schroeder JW, Sorenson RJ, Showalter HD, Simmons LA. 2014. RecO and RecR are necessary for RecA loading in response to DNA damage and replication fork stress. J Bacteriol 196:2851–2860. 10.1128/JB.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kidane D, Ayora S, Sweasy JB, Graumann PL, Alonso JC. 2012. The cell pole: the site of cross talk between the DNA uptake and genetic recombination machinery. Crit Rev Biochem Mol Biol 47:531–555. 10.3109/10409238.2012.729562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attaiech L, Olivier A, Mortier-Barrière I, Soulet A-L, Granadel C, Martin B, Polard P, Claverys J-P. 2011. Role of the single-stranded DNA-binding protein SsbB in pneumococcal transformation: maintenance of a reservoir for genetic plasticity. PLoS Genet 7:e1002156. 10.1371/journal.pgen.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrasco B, Manfredi C, Ayora S, Alonso JC. 2008. Bacillus subtilis SsbA and dATP regulate RecA nucleation onto single-stranded DNA. DNA Repair (Amst) 7:990–996. 10.1016/j.dnarep.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Grove DE, Anne G, Hedayati MA, Bryant FR. 2012. Stimulation of the Streptococcus pneumoniae RecA protein-promoted three-strand exchange reaction by the competence-specific SsbB protein. Biochem Biophys Res Commun 424:40–44. 10.1016/j.bbrc.2012.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steffen SE, Katz FS, Bryant FR. 2002. Complete inhibition of Streptococcus pneumoniae RecA protein-catalyzed ATP hydrolysis by single-stranded DNA-binding protein (SSB protein): implications for the mechanism of SSB protein-stimulated DNA strand exchange. J Biol Chem 277:14493–14500. 10.1074/jbc.M112444200. [DOI] [PubMed] [Google Scholar]
- 55.Carrasco B, Serrano E, Martín-González A, Moreno-Herrero F, Alonso JC. 2019. Bacillus subtilis MutS modulates RecA-mediated DNA strand exchange between divergent DNA sequences. Front Microbiol 10:237. 10.3389/fmicb.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le S, Serrano E, Kawamura R, Carrasco B, Yan J, Alonso JC. 2017. Bacillus subtilis RecA with DprA-SsbA antagonizes RecX function during natural transformation. Nucleic Acids Res 45:8873–8885. 10.1093/nar/gkx583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arber W. 2014. Horizontal gene transfer among bacteria and its role in biological evolution. Life (Basel) 4:217–224. 10.3390/life4020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson GG, Murray NE. 1991. Restriction and modification systems. Annu Rev Genet 25:585–627. 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- 59.Pifer ML. 1986. Plasmid establishment in competent Haemophilus influenzae occurs by illegitimate transformation. J Bacteriol 168:683–687. 10.1128/jb.168.2.683-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidane D, Carrasco B, Manfredi C, Rothmaier K, Ayora S, Tadesse S, Alonso JC, Graumann PL. 2009. Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet 5:e1000630. 10.1371/journal.pgen.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sung CK, Morrison DA. 2005. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J Bacteriol 187:3052–3061. 10.1128/JB.187.9.3052-3061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dagkessamanskaia A, Moscoso M, Hénard V, Guiral S, Overweg K, Reuter M, Martin B, Wells J, Claverys J-P. 2004. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol Microbiol 51:1071–1086. 10.1111/j.1365-2958.2003.03892.x. [DOI] [PubMed] [Google Scholar]
- 63.Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, Walling J, Li H, Mintz M, Tsegaye G, Burr PC, Do Y, Ahn S, Gilbert J, Fleischmann RD, Morrison DA. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol 51:1051–1070. 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 64.Hülter N, Sørum V, Borch-Pedersen K, Liljegren MM, Utnes ALG, Primicerio R, Harms K, Johnsen PJ. 2017. Costs and benefits of natural transformation in Acinetobacter baylyi. BMC Microbiol 17:34. 10.1186/s12866-017-0953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wassenaar TM, Fry BN, van der Zeijst BA1M. 1993. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene 132:131–135. 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 66.Lin J, Lau GW. 2019. DprA-dependent exit from the competent state regulates multifaceted Streptococcus pneumoniae virulence. Infect Immun 87:e00349-19. 10.1128/IAI.00349-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu L, Lin J, Kuang Z, Vidal JE, Lau GW. 2015. Deletion analysis of Streptococcus pneumoniae late competence genes distinguishes virulence determinants that are dependent or independent of competence induction. Mol Microbiol 97:151–165. 10.1111/mmi.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Y, Chang D, Xu H, Zhang X, Pan L, Xu C, Huang B, Zhou H, Li J, Guo J, Liu C. 2017. The virulence of Streptococcus pneumoniae partially depends on dprA. Braz J Microbiol 48:225–231. 10.1016/j.bjm.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sparling PF. 1966. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol 92:1364–1371. 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudel T, van Putten JP, Gibbs CP, Haas R, Meyer TF. 1992. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol 6:3439–3450. 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 71.Wolfgang M, Lauer P, Park HS, Brossay L, Hébert J, Koomey M. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol 29:321–330. 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 72.Chen T, Bolland S, Chen I, Parker J, Pantelic M, Grunert F, Zimmermann W. 2001. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J Biol Chem 276:17413–17419. 10.1074/jbc.M010609200. [DOI] [PubMed] [Google Scholar]
- 73.Lauer P, Albertson NH, Koomey M. 1993. Conservation of genes encoding components of a type IV pilus assembly/two-step protein export pathway in Neisseria gonorrhoeae. Mol Microbiol 8:357–368. 10.1111/j.1365-2958.1993.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 74.Cohen MS, Cannon JG. 1999. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis 179:S375–S379. 10.1086/513847. [DOI] [PubMed] [Google Scholar]
- 75.Lo H, Tang CM, Exley RM. 2009. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis 9:418–427. 10.1016/S1473-3099(09)70132-X. [DOI] [PubMed] [Google Scholar]
- 76.Hagblom P, Segal E, Billyard E, So M. 1985. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature 315:156–158. 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 77.Jonsson A-B, Nyberg G, Normark S. 1991. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J 10:477–488. 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cahoon LA, Seifert HS. 2011. Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol Microbiol 81:1136–1143. 10.1111/j.1365-2958.2011.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elkins C, Thomas CE, Seifert HS, Sparling PF. 1991. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol 173:3911–3913. 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodman SD, Scocca JJ. 1988. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA 85:6982–6986. 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berry J-L, Cehovin A, McDowell MA, Lea SM, Pelicic V. 2013. Functional analysis of the interdependence between DNA uptake sequence and its cognate ComP receptor during natural transformation in Neisseria species. PLoS Genet 9:e1004014. 10.1371/journal.pgen.1004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambur OH, Frye SA, Tønjum T. 2007. New functional identity for the DNA uptake sequence in transformation and its presence in transcriptional terminators. J Bacteriol 189:2077–2085. 10.1128/JB.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Criss AK, Bonney KM, Chang RA, Duffin PM, LeCuyer BE, Seifert HS. 2010. Mismatch correction modulates mutation frequency and pilus phase and antigenic variation in Neisseria gonorrhoeae. J Bacteriol 192:316–325. 10.1128/JB.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rotman E, Seifert HS. 2014. The genetics of Neisseria species. Annu Rev Genet 48:405–431. 10.1146/annurev-genet-120213-092007. [DOI] [PubMed] [Google Scholar]