ABSTRACT
Testing of cellular therapy products for Mycoplasma is a regulatory requirement by the United States Food and Drug Administration (FDA) to ensure the sterility and safety of the product prior to release for patient infusion. The risk of Mycoplasma contamination in cell culture is high. Gold standard testing follows USP <63> which requires a 28-day agar and broth cultivation method that is impractical for short shelf-life biologics. Several commercial molecular platforms have been marketed for faster raw material and product release testing; however, little performance data are available in the literature. In this study, we performed a proof-of-principle analysis to evaluate the performance of five commercial molecular assays, including the MycoSEQ Mycoplasma detection kit (Life Technologies), the MycoTOOL Mycoplasma real-time detection kit (Roche), the VenorGEM qOneStep kit (Minerva Biolabs), the ATCC universal Mycoplasma detection kit, and the Biofire Mycoplasma assay (bioMérieux Industry) using 10 cultured Mollicutes spp., with each at four log-fold dilutions (1,000 CFU/mL to 1 CFU/mL) in biological duplicates with three replicates per condition (n = 6) to assess limit of detection (LOD) and repeatability. Additional testing was performed in the presence of tumor infiltrating lymphocytes (TILs). Based on LOD alone, the Biofire Mycoplasma assay was most sensitive followed by the MycoSEQ and MycoTOOL which were comparable. We showed that not all assays were capable of meeting the ≤10 CFU/mL LOD to replace culture-based methods according to European and Japanese pharmacopeia standards. No assay interference was observed when testing in the presence of TILs.
KEYWORDS: Mollicutes, cellular therapy, biologics, sterility, Mycoplasma, Acholeplasma, Spiroplasma
INTRODUCTION
Mollicutes (or interchangeably, Mycoplasma) are cell-wall-lacking prokaryotic organisms of medical and industrial microbiologic importance. Unlike other bacteria, Mollicutes cannot be observed on a Gram stain as they lack peptidoglycan needed to retain crystal violet. Additionally, they are exceedingly small compared with “typical” prokaryotes (0.1 to 0.2 μm in size). Culture requires the use of highly enriched media and extended incubation times (>5 days) because they are auxotrophic for many biosynthetic pathways. These traits, combined with the uncultivable nature of certain Mycoplasma spp., makes their detection a complex and costly endeavor.
In the United States, sterility testing for Mycoplasma in cellular and gene therapy products is a regulatory requirement to ensure the safety of biologics for human use as prescribed by the United States Food and Drug Administration (FDA) in the Code of Federal Regulations. The FDA accepts testing standards published in the United States Pharmacopeia (USP). Other international pharmacopeia standards, such as the European Pharmacopoeia (Ph. Eur.) and the Japanese Pharmacopeia (JP), may also be applicable depending on geographical product distribution. Gold standard testing for Mycoplasma is defined in USP <63> (1), Ph. Eur. chapter 2.6.7 (2), and JP XVIII (3). Briefly, gold standard testing requires the culturing of Mycoplasma using a combination of three methods, including broth culture, culture on permissive solid agar, and fluorescent antibody detection of noncultivable organisms grown on a cellular monolayer (Fig. 1). The long turnaround time (28 days) and large product volume (~15 mL) required for compendial testing are incompatible with biological products that have a short shelf life (48 to 72 h).
FIG 1.
Compendial testing requirements for Mycoplasma analysis on cell and gene therapy products. Primary product is subjected to analysis by (1) culture on cells (commonly Vero cells) with analysis via fluorescence microscopy for Mycoplasma contamination, (2) culture of primary product in broth medium with subsequent subculture onto permissive solid agar, and (3) culture directly onto solid agar incubated for 14 days prior to examination for distinctive Mycoplasma colonies. Figure created with BioRender.com.
The rapid emergence of cellular therapies over the last 2 decades has led to revolutionary treatments for a myriad of human diseases that had been previously thought untreatable (4). Historically, contamination of cell culture products with Mollicutes was common, with some reports estimating the presence of Mycoplasma in 15 to 30% of American Type Culture Collection (ATCC) cell lines and approximately 11% of all RNA sequencing (RNA-seq) data sets (Table 1) (5–7). Therefore, the detection of Mycoplasma in vaccines and cellular products remains a safety concern, as these organisms can go undetected easily in raw materials and cell culture due to the absence of obvious morphological/cytological changes. Molecular assays have become favorable alternatives to counter the limitations of the culture-based pharmacopeia standards. The European Pharmacopoeia and Japanese Pharmacopeia both recognize molecular testing for Mycoplasma as an acceptable test method if the limit of detection (LOD) is ≤10 CFU/mL compared with agar and broth culture and ≤100 CFU/mL compared with the indicator cell method (2, 3). In the United States, however, any non-USP <63> method is considered an alternative method that requires rigorous end-user validation to meet the equivalency specifications as outlined in USP <1223> (8), despite premarket and beta-testing studies that may be available by the vendor to the FDA in the form of a Drug Master File. And, unlike clinical in vitro diagnostic (IVD) assays, FDA 510(k) clearance is not an option for assays used for cGMP product release (9).
TABLE 1.
Mollicutes type strains utilized within this study
| Organism | USP <63> | Ph. Eur. 2.6.7. | JP XVIII | Reported cell culture contaminanta | Suitability for use |
|---|---|---|---|---|---|
| Acholeplasma laidlawii PG8 (ATCC 23206) | Xe | X | X | Yes | Vaccines and/or cell-derived materials/cultures for human and veterinary use when an antibiotic has been used during production |
| Mycoplasma arginini G230 (ATCC 23838) | X | X | Yes | Core challenge organism in QCMD proficiency testing surveys 2018–2021 | |
| Mycoplasma fermentans PG18 (ATCC 19989) | X | X | X | Yes | Vaccines or cell banks for human use |
| Mycoplasma gallisepticum PG31 (ATCC 19610) | X | X | No | When avian material has been used during production or when the vaccine or cell culture is intended for use in poultry | |
| Mycoplasma hominis PG21 (ATCC 23114) | Yes | Clinical relevance | |||
| Mycoplasma hyorhinis PG42 (ATCC 17981) | X | X | X | Yes | Nonavian veterinary vaccines or cell cultures |
| Mycoplasma orale CH19299 (ATCC 23714)b | X | X | X | Yes | Vaccines for human and veterinary use |
| Mycoplasma pneumoniae FH (ATCC 15531) | X | X | X | No | Vaccines or cell banks for human use |
| Mycoplasma pulmonis PG34 (ATCC 19612) | No | Core challenge organism in QCMD proficiency testing surveys 2018–2021 | |||
| Mycoplasma salivarium PG20 (ATCC 23064) | X | Yes | Core challenge organism in QCMD proficiency testing surveys 2018–2021 | ||
| Mycoplasma synoviae WVU 1853 (ATCC 25204)b | X | X | X | No | When avian material has been used during production or when the vaccine or cell bank is intended for use in poultry |
| Spiroplasma ixodetis Y32 (ATCC 33835)c | Xd | Xd | Xd | No | Use of or exposure to insect or plant material during production |
M. orale and M. synoviae failed to grow on culture and were excluded from further analysis.
S. ixodetis was cultured in lieu of S. citri due to the inability to purchase the latter organism. Both organisms are genetically similar and have compatible growth requirements.
S. citri ATCC 29747 is the listed reference organism in USP <63>, Ph. Eur. 2.6.7., and JP XVIII.
X indicates that the organism is listed as an indicator organism in the respective pharmacopeia document.
To date, several assays have been marketed for the detection of Mollicutes in cellular therapy products; however, little performance data are available in the literature. In this study, we conducted a proof-of-principle study to compare the performance of the following five commercially available assays marketed in the United States for Mycoplasma testing: the MycoSEQ Mycoplasma detection kit (Life Technologies), the MycoTOOL Mycoplasma real-time detection kit (Roche), the VenorGEM qOneStep kit (Minerva Biolabs), the ATCC universal Mycoplasma detection kit (American Type Culture Collection), and the Biofire Mycoplasma assay (bioMérieux Industry). Testing was performed using 10 Mollicutes type strains cultured as per the harmonized compendial methods to verify the inoculum concentration (Fig. 1). Testing was performed at log-fold dilutions and in replicates by two different analysts to assess for the limit of detection and repeatability of each platform. Additionally, three clinically relevant Mollicutes type strains were tested in the presence of tumor infiltrating lymphocytes (TILs) to evaluate whether this product matrix would lead to potential assay inhibition for the detection of low-level contaminants. This proof-of-principle study provides a comprehensive insight into the analytical performance of a wide variety of platforms for Mycoplasma product release testing from an end-user laboratory perspective.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains (Table 1) were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and stored as per manufacturer’s instructions prior to culture. Type strains were selected based on quality-control strains listed in international pharmacopeia standards (Table 1), reported cell culture contaminants (5–7), and organisms that have been frequently associated with human or avian origin. Isolates were initially cultured in Hayflick’s broth and Hayflick’s agar (Hardy Diagnostics, Santa Maria, CA). For Mycoplasma species, broth was inoculated at 10−1 to 10−3 (vol/vol) and incubated at 37°C until color change indicated metabolic growth (orange-yellow for glucose metabolizers and red-purple for arginine metabolizers). Agar was inoculated, wrapped in parafilm (Thermo Fisher Scientific, Waltham, MA), and incubated at 35 to 37°C with 5% CO2 until colonies were visible under a dissection scope (×20 magnification). Subsequent subcultures were expanded and passaged as described above with no more than 15 passages from initial inoculation as per USP <63> requirements. For strains that failed to grow on Hayflick’s broth or agar (M. hyorhinis, M. salivarium, M. synoviae, M. orale, M. pulmonis, and M. pneumoniae), SP4 medium and agar with arginine (Hardy Diagnostics, Santa Maria, CA) were utilized using the incubation conditions described above. For Spiroplasma ixodetis, cultures were incubated at 30°C (non-CO2) utilizing SP4 broth and agar with arginine until orange-yellow color change and colony growth were observed.
When ~30 mL of culture volume had been obtained, sterile glycerol (Sigma-Aldrich, St. Louis, MO) was added at a 10% (vol/vol) concentration, and stocks were aliquoted into 0.5-mL volumes. Stocks were frozen at −80°C for further study.
Stock concentration and limit of detection quantitation.
Frozen stocks of organisms were thawed on ice for determination of CFU per mL (CFU/mL), as well as color change units (CCU) as described previously (10, 11). Briefly, organism stocks were diluted, in duplicate, by 1:10 from 10−1 to 10−10 in either Hayflick or SP4 medium. For each dilution, 20 μL was cultured onto solid agar. Plates were sealed with parafilm wrap (Thermo Fisher Scientific) and incubated at 35 to 37°C with 5% CO2. Tubes used for serial dilution were incubated at 37°C without CO2 for 1 to 2 weeks. CCU were determined by the terminal dilution that indicated metabolic growth. Colonies were enumerated (between 30 and 300) for a single dilution. The average of two independent dilution sets was used to determine a final average CFU/mL. This CFU/mL was correlated with CCU as an internal control for organism titer within stock vials at –80°C.
Once the CFU/mL had been determined for each organism, stocks were diluted to 105 CFU/mL in growth medium (Hayflicks or SP4) for LOD studies. Further dilutions were performed using RPMI 1640 medium (Sigma-Aldrich) to minimize the presence of inhibitory substances (i.e., exogenous DNA and serum present in growth medium) prior to extraction. Final concentrations of 103, 102, 101, and 100 CFU/mL were completed for each strain, and all bacterial stocks were frozen in 300-μL aliquots at −80°C until the time of extraction. Dilutions were carried out in biological duplicate with three replicates per condition (n = 6) to account for repeatability studies. In addition, serial dilutions in SP4 or Hayflicks medium were performed, and 0.2 mL of each concentrate was cultured onto solid agar to ensure organism viability and to verify CFU/mL.
Due to reagent costs of the Biofire Mycoplasma assay, triplicate testing (rather than sextuplicate) was performed for each condition tested. Separate aliquots of thawed organism-dilution combinations were utilized for the Biofire Mycoplasma assay as extraction and amplification were performed in the pouch as per the manufacturer’s instructions. All other assays were tested using a single eluate per organism-dilution combination to minimize potential extraction variability. Concentrations ranging between 102, 101, 100, and 10−1 CFU/mL were tested for initial evaluation and assay comparison.
Determination of the LOD for each test platform and organism was defined as the terminal dilution in which all replicates were detected.
DNA extraction and PCR conditions.
DNA was extracted using the PrepSEQ express nucleic acid extraction kit (Applied Biosystems, San Francisco, CA). DNA was stored at −20°C prior to downstream testing.
All PCR assays were performed following manufacturer’s instructions. The ABI 7500 fast real-time PCR system was used for the MycoSEQ Mycoplasma detection kit (Thermo Fisher Scientific), VenorGEM qOneStep assay (Minerva Biolabs, Inc., Skillman, NJ), and the MycoTOOL Mycoplasma real-time PCR kit (Roche CustomBiotech, Basel, Switzerland). An analysis of the MycoSEQ Mycoplasma detection kit was performed using the AccuSEQ real-time detection software v.2.0 (Thermo Fisher Scientific). Run files for the VenorGEM qOneStep assay and the MycoTOOL Mycoplasma real-time PCR kit were uploaded to the Thermo Fisher connect platform and analyzed using the cloud-based relative quantitation analysis module with default analysis group settings for cycle threshold (CT) value determination. CT values were plotted to show dynamic ranges of each assay and analyte. Because the MycoTOOL kit was performed on an ABI 7500 fast real-time PCR system rather than a LightCycler (Roche), 18 cycles were added to the terminal CT value following technical advice provided by the manufacturer to account for touch-down PCR steps.
Gel electrophoresis for the ATCC universal Mycoplasma detection kit was performed using the E-Gel 1.2% with SYBR safe and E-gel simple runner system (Invitrogen, Thermo Fisher Scientific). Amplicon bands were visualized using the GelDoc Go imaging system and Image Touch Lab Software (Bio-Rad Laboratories) at 5-s exposure with UV light.
The Biofire Mycoplasma assay (bioMérieux Industry) was performed following the manufacturer’s instructions using a 0.2-mL sample volume. Testing was performed on the FilmArray 2.0 industry system intended for the pharma industry Mycoplasma panels. Note, this platform is different from the instrument and software available for clinical IVD assays, such as the respiratory, gastrointestinal, blood culture, and meningitis panels.
Tumor infiltrating lymphocyte generation and dilution experiments.
Tumor infiltrating lymphocytes (TILs) were generated as described elsewhere (12). TILs were diluted to a concentration of 105 cells/mL in RPMI and stored on ice. Concentrated stocks of M. hyorhinis, A. laidlawii, and M. pneumoniae were thawed on ice and diluted in RPMI to concentrations of 104, 103, and 102 CFU/mL. A total of 30 μL of each dilution was added to 270 μL of TILs for each organism/dilution combination. For controls at each organism/dilution combination, 30 μL of each dilution was added to 270 μL of RPMI. Negative controls consisted of media and TILs inoculated with 30 μL of RPMI. Testing was performed in duplicate across two different users to assess for repeatability. Samples were extracted using the PrepSEQ express nucleic acid extraction kit (Applied Biosystems). PCR assays and analysis were performed as described above.
RESULTS
The LOD for each assay/organism combination and reproducibility performance are provided in Table 2 and Table S1 in the supplemental material, respectively. Overall, a wide spectrum of LODs were observed. Based on LOD alone, the Biofire Mycoplasma assay was the most sensitive of the assays examined. MycoSEQ and MycoTOOL performed similarly followed by the ATCC universal Mycoplasma detection kit and the VenorGEM qOneStep. Overall, 80% of assays failed to detect Spiroplasma ixodetis despite genetic similarities and growth characteristics to Spiroplasma citri (13–15). Additionally, some assays had a high LOD (1,000 CFU/mL) or failed to detect Mycoplasma pulmonis and Mycoplasma salivarium.
TABLE 2.
Limit of detection studies carried out on Mollicutes type strains within this studya
LOD was defined as the terminal dilution in which all replicates were detected. Green denotes an LOD of ≤10 CFU/mL to replace culture-based methods according to the European and Japanese pharmacopeia. Red denotes organisms that failed to be detected at ≥1,000 CFU/mL.
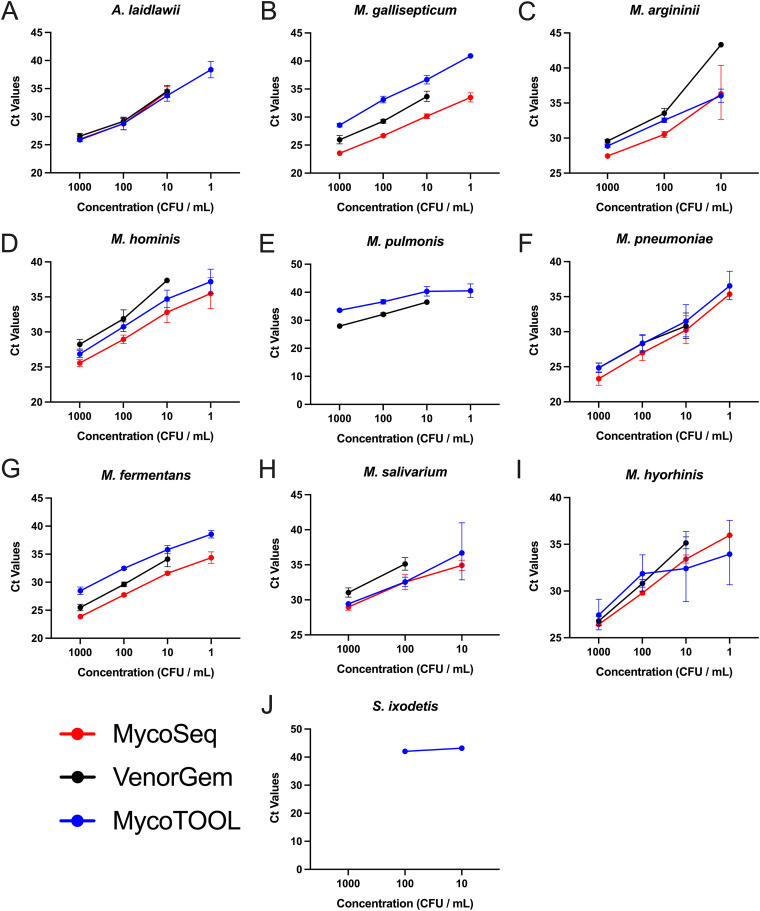
Given the differences between platforms for LOD, linearity was tracked to examine possible interassay differences for the three real-time PCR assays (Fig. 2). The MycoSEQ and MycoTOOL assays had comparable linear ranges, with most species detected across 3 to 4 log10 dilutions. On average, lower cycle threshold (CT) values were reported by the MycoSEQ assay. With the exception of the Biofire Mycoplasma assay, none of the remaining real-time PCR assays (i.e., the VenorGEM assay) were able to adequately detect Mollicutes below 10 CFU/mL, with no more than 67% being detected for any species across any real-time qPCR assay (Fig. 2; Table S1). S. ixodetis was detected only by the MycoTOOL but in only 16.7% of replicates at any one dilution.
FIG 2.
Linear range for limit of detection studies on Mollicutes spp. Linear range of MycoSEQ (red), VenorGEM (black), and MycoTOOL (blue) qPCR assay cycle threshold (CT) values comparative to bacterial cell concentration (CFU/mL) for the following species: A. laidlawii (A), M. gallisepticum (B), M. arginini (C), M. hominis (D), M. pulmonis (E), M. pneumoniae (F), M. fermentans (G), M. salivarium (H), M. hyorhinis (I), and S. ixodetis (J). Graphs represent geometric mean and standard deviation for each test condition for all examined species.
Similar assay performance was demonstrated for Mycoplasma hyorhinis, Mycoplasma pneumoniae, and Acholeplasma laidlawii at serial dilutions ranging from 1,000 to 10 CFU/mL in the presence of TILs. However, in some cases, only 50% of replicates were detected at 10 CFU/mL (Table 3).
TABLE 3.
Tumor infiltrative lymphocyte cell detection experiments carried out within this studya
Green indicates all replicates detected. Orange (*) indicates a test combination where only 50% of replicates were detected. Red indicated no detection across all replicates.
DISCUSSION
In this study, we conducted a proof-of-principle analysis to evaluate the performance of five commercially available molecular assays marketed for the detection of Acholeplasma, Mycoplasma and Spiroplasma spp. using a panel of 10 type strains. All but one assay was tested in sextuplicate across two different analysts to assess for LOD and repeatability; the Biofire Mycoplasma assay was completed only in triplicate runs per test condition due to assay cost. Overall, our results indicate the superior performance of the Biofire Mycoplasma assay, while the MycoSEQ and the MycoTOOL assays were comparable (Table 2; Table S1). Little assay interference was observed when testing was conducted in the presence of TILs (Table 3). European and Japanese pharmacopeia standards accept molecular assays if the LOD is ≤10 CFU/mL compared with culture and ≤100 CFU/mL compared with the indicator cell method (2, 3). Based on these standards alone, our preliminary data suggest that only the Biofire Mycoplasma assay was able to meet the equivalency standards to replace gold standard culture, although Mycoplasma orale and Mycoplasma synoviae could not be assessed. Increased sensitivity with the Biofire assay may be attributable to the nested PCR design. However, more extensive evaluation on a larger number of clinical isolates (acquired from animal, human, and/or cell lines), beyond banked type strains, will be required for further study before definitive conclusions can be drawn. The differences in LOD between assay and organism combinations are not necessarily a limitation, as the selection for quality-control (QC) strains for testing is dependent on the origin of the raw materials used in product manufacturing as well as the intended product recipient.
Surprisingly, S. ixodetis failed to be detected by all assays except for the ATCC universal Mycoplasma detection kit, despite genetic and growth similarities to S. citri (13–15). In this study, S. ixodetis was used in lieu of S. citri due to our inability to purchase the S. citri ATCC 27556 type strain. The overall impact for failing to detect S. ixodetis in this study is theoretically low for biological products, unless the final product incorporated raw materials associated with ticks (such as Ixodes pacificus) and other arthropods (14, 15). Nonetheless, S. ixodetis may be of increasing medical and industrial importance, so its inclusion in this study should not be overlooked entirely (16). S. citri, however, is recommended by the international pharmacopeias when there is exposure to insect or plant material during production. Therefore, depending on the circumstances of product manufacturing and the intended use, further investigation would be required to determine assay suitability.
Surprisingly, proficiency testing in the cGMP setting is not a regulatory requirement. Prior to 2022, Quality Control for Molecular Diagnostics (QCMD) offered an external quality assessment (EQA) for Mycoplasma molecular assays. Unfortunately, low participation rates (n = 13 to 15 laboratories annually) between 2018 and 2021 have led to the discontinuation of this program. Nevertheless, a review of participant summaries between 2018 and 2021 showed consistent failures to detect M. pulmonis, Mycoplasma fermentans, Mycoplasma gallisepticum, and at times Mycoplasma arginini. Participants reported the use of commercial platforms (including four evaluated in this study, namely, MycoSEQ, MycoTOOL, VenorGEM, and ATCC universal Mycoplasma detection kit), as well as a few in-house developed assays. While a breakdown of each assay’s performance is not disclosed, M. pulmonis and M. fermentans were consistently detected <80% of the time, while M. gallisepticum and M. arginini were consistently detected <85% of the time. Of the 12 samples included in each survey, 6 to 8 were classified as core challenges intended for positive Mycoplasma detection (as opposed to educational challenges). Performance for positive core challenge samples was poor, ranging between 17% and 63% for the 4 years studied. Most importantly, M. fermentans and M. gallisepticum (included in every QCMD challenge) are considered indicator organisms required by all international pharmacopeia standards. The testing of M. fermentans is required for vaccines and cell banks for human use, while M. gallisepticum testing is required for products where avian material has been used during production. Inadequate performance of certain platforms to detect M. fermentans and M. gallisepticum in the QCMD surveys highlights areas for concern. In our study, all but one assay was able to detect M. fermentans and M. gallisepticum at the LOD requirement of ≤10 CFU/mL to replace culture-based methods as per the European and Japanese pharmacopeias (Table 2).
All assays evaluated in this study provided generic qualitative results for the presence or absence of Mycoplasma. No commercial assays on the market have been designed to provide specific organism identification. This gap is attributed mostly to the fact that these assays (and many in-house assays) target numerous universal regions within the 16S rRNA that are specific for Mycoplasma, where sequence diversity is limited for the differentiation of Mollicutes (17). It is possible that species-specific Mycoplasma assays could be developed to supplement deficiencies observed with commercial platforms; however, the cost-benefit would need to be carefully balanced with regulatory requirements. Furthermore, as per the international pharmacopeias, the specificity and sensitivity of an assay should be target specific for strains directly related to the origin of materials and the intended product recipient.
LOD studies of A. laidlawii, M. hyorhinis, and M. pneumoniae performed in the presence and absence of TILs showed little interference with the detection of clinically relevant strains (Table 3). Testing in the presence of product, known as method suitability testing, is a regulatory requirement for any new product formulation to verify the absence of product interference for the detection of low-level (defined as <100 CFU) contaminants. Preliminary testing in the presence of TILs showed promise; however, further evaluation of other cell types and matrices is required at our institution due to the wide spectrum of investigational new drugs manufactured at the NIH for phase I and phase II clinical trials. Other institutions with a narrower product scope may find it easier to select and validate an appropriate Mycoplasma PCR assay to replace conventional USP <63> methods.
To date, there are limited data available in the peer-reviewed literature that describe the performance characteristics of various Mycoplasma assays by independent study groups, beyond work published by the manufacturers themselves. The Biofire Mycoplasma assay was presented recently by the manufacturer in a poster abstract format with results similar to ours (18). The MycoTOOL assay was studied by a Canadian group for rapid validation; however, only serial dilutions of M. hominis and M. arginini genomic DNA were studied (19). A subsequent study by the same group showed good performance (<10 CFU/mL detection) of M. hominis and M. arginini genomic DNA in the presence of chimeric antigen receptor T cells (20). The ATCC universal Mycoplasma (21–23) and the VenorGEM kits (24) have been used widely for the determination of cell line infection status, but both lacked performance data concerning assay cross-performance beyond the manufacturer’s claims. The data outcomes from our study show variations in LOD based on organism, thus highlighting the importance for rigorous end-user validation based on material origin and intended product recipient.
There are several limitations to our study. In the United States, USP <1223> requires that the alternative assay demonstrate equivalency and noninferiority to the compendial method (i.e., USP <63>). The number of replicates is at the discretion of the end-user to demonstrate statistical power; this discretion contrasts with Ph. Eur. 2.6.7 which requires at least 24 replicates per test condition. This study served as a proof-of-principle analysis to evaluate overall performance using six replicates per test conditions. Further testing will be required for application of any assay in the cGMP setting. Additionally, excessive replicate testing for the Biofire Mycoplasma assay was not possible in this study due to cost; however, the 2-min assay setup time, open access availability, and 1-h turnaround time offer significant advantages in cost savings in time and labor compared with other assays evaluated in this study. Further replicate testing focused on product-relevant and clinically relevant strains will be required if the Biofire Mycoplasma assay is chosen for implementation and product release testing. Finally, culture conditions used in this study followed procedures used in clinical reference laboratories, which differ slightly to the procedures prescribed in USP <63> (10). The impact of this amendment is minimal as culture recovery was successful and colony counts were verified for each dilution during each test condition. Frozen eluates from a single extraction of each organism-concentration combination were used to evaluate all assays except for the Biofire assay, so it is possible that DNA degradation may have contributed to the LOD variations observed between assays, although the effect is deemed minimal, as testing for each organism-concentration combination was completed within a 1-month time frame with minimal freeze-thaw cycles. In this study, we were unable to culture M. orale and M. synoviae using commercially available media. Both these organisms are considered compendial indicator organisms, and the failure to include them is a limitation for assay comparability in this study. The use of exogenous medium additions (such as NAD+ to enhance growth of M. synoviae in broth culture [25]) requires rigorous raw material testing and is generally avoided for cGMP applications.
In conclusion, we present a comprehensive comparative evaluation of five commercially available assays for the detection of Mollicutes spp. Our data show that not all assays were capable of meeting the ≤10 CFU/mL requirement to replace culture-based methods based on European and Japanese pharmacopeias. There is a critical need in the biopharmaceutical field to utilize rapid methods that offer sensitive detection of contaminants while conserving product volume. Caution must be applied to ensure that test methods are not overly sensitive, particularly in the absence of being able to confirm Mycoplasma viability through molecular testing alone and the questionable potential for clinical infectious risk which may jeopardize the release of a personalized, potentially lifesaving, costly product. Based on the strains studied here, our proof-of-principle analysis indicates promising broad-range sensitive detection using the Biofire Mycoplasma assay. The MycoSEQ and MycoTOOL assays also demonstrated good performance for selected strains. This study will help guide end-users for assay selection and further evaluation in the presence of product depending on the product type, the origin of raw materials used during manufacturing, and the intended product recipient.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Supplemental material is available online only.
Contributor Information
Anna F. Lau, Email: anna.lau@nih.gov.
Nathan A. Ledeboer, Medical College of Wisconsin
REFERENCES
- 1.United States Pharmacopeia. 2022. General chapter, <63> mycoplasma tests, USP41-NF36. United States Pharmacopeia, Rockville, MD. [Google Scholar]
- 2.European Pharmacopeia. 2008. 2.6.7. Mycoplasmas. European Directorate for the Quality of Medicines and Healthcare, Council of Europe, Strasbourg, France. [Google Scholar]
- 3.Pharmaceuticals and Medical Devices Agency. 2021. Japanese pharmacopoeia 18th edition. https://www.pmda.go.jp/english/rs-sb-std/standards-development/jp/0029.html.
- 4.Golchin A, Farahany TZ. 2019. Biological products: cellular therapy and FDA approved products. Stem Cell Rev Rep 15:166–175. 10.1007/s12015-018-9866-1. [DOI] [PubMed] [Google Scholar]
- 5.Nikfarjam L, Farzaneh P. 2012. Prevention and detection of Mycoplasma contamination in cell culture. Cell J 13:203–212. [PMC free article] [PubMed] [Google Scholar]
- 6.Olarerin-George AO, Hogenesch JB. 2015. Assessing the prevalence of mycoplasma contamination in cell culture via a survey of NCBI’s RNA-seq archive. Nucleic Acids Res 43:2535–2542. 10.1093/nar/gkv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drexler HG, Uphoff CC. 2002. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39:75–90. 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Pharmacopeia. 2022. General chapter, <1223> validation of alternative microbiological methods, USP41-NF36. United States Pharmacopeia, Rockville, MD. [Google Scholar]
- 9.Gebo JET, East AD, Lau AF. 2021. A side-by-side comparison of clinical versus current good manufacturing practices (cGMP) microbiology laboratory requirements for sterility testing of cellular and gene therapy products. Clin Microbiol Newsl 43:181–191. 10.1016/j.clinmicnews.2021.10.001. [DOI] [Google Scholar]
- 10.Waites KB, Bade DJ, Bebear C, Brown SD, Davidson MK, Duffy LB, Kenny G, Matlow A, Shortridge D, Talkington D, Totten PA, Watts JL, Zheng X. 2011. Methods for antimicrobial susceptibility testing for human mycoplasmas, approved guideline. CLSI, Wayne, PA. [PubMed] [Google Scholar]
- 11.Totten AH, Crawford CL, Dalecki AG, Xiao L, Wolschendorf F, Atkinson TP. 2019. Differential susceptibility of Mycoplasma and Ureaplasma species to compound-enhanced copper toxicity. Front Microbiol 10:1720. 10.3389/fmicb.2019.01720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. 2003. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 26:332–342. 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tully JG, Whitcomb RF, Clark HF, Williamson DL. 1977. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science 195:892–894. 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- 14.Tully JG, Rose DL, Yunker CE, Carle P, Bove JM, Williamson DL, Whitcomb RF. 1995. Spiroplasma ixodetis sp. nov., a new species from Ixodes pacificus ticks collected in Oregon. Int J Syst Bacteriol 45:23–28. 10.1099/00207713-45-1-23. [DOI] [PubMed] [Google Scholar]
- 15.Vera-Ponce Leon A, Dominguez-Mirazo M, Bustamante-Brito R, Higareda-Alvear V, Rosenblueth M, Martinez-Romero E. 2021. Functional genomics of a Spiroplasma associated with the carmine cochineals Dactylopius coccus and Dactylopius opuntiae. BMC Genomics 22:240. 10.1186/s12864-021-07540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matet A, Le Fleche-Mateos A, Doz F, Dureau P, Cassoux N. 2020. Ocular Spiroplasma ixodetis in Newborns, France. Emerg Infect Dis 26:340–344. 10.3201/eid2602.191097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega-Orellana O, Poveda JB, Rosales RS, Bradbury JM, Poveda CG, Mederos-Iriarte LE, Tavio MM, Ramirez AS. 2017. Comparison of different NAT assays for the detection of microorganisms belonging to the class Mollicutes. BMC Vet Res 13:195. 10.1186/s12917-017-1116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paris A, Barry W, Toxopeus C, Cassard S, Brown J, Kornowske L, Andjelic C, Kim M, Phillips C. 2020. Performance evaluation of a rapid, fully automated mycoplasma detection system for cell and gene therapy products. Cryotherapy 22:S167–S168. 10.1016/j.jcyt.2020.03.352. [DOI] [Google Scholar]
- 19.Chisholm J, Bhatt S, Chaboureau A, Viswanathan S. 2017. Strategy for an abbreviated in-house qualification of a commercially available Rapid Microbiology Method (RMM) for Canadian regulatory approval. Cytotherapy 19:1529–1536. 10.1016/j.jcyt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Dreolini L, Cullen M, Yung E, Laird L, Webb JR, Nelson BH, Hay KA, Balasundaram M, Kekre N, Holt RA. 2020. A rapid and sensitive nucleic acid amplification technique for Mycoplasma screening of cell therapy products. Mol Ther Methods Clin Dev 17:393–399. 10.1016/j.omtm.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell BJ, Horiuchi K, Velez JO, Goodman CH, Johnson BW. 2020. Mycoplasma detection in a historical arbovirus repository: commercial kit comparison and implications for improved repository management. J Virol Methods 276:113769. 10.1016/j.jviromet.2019.113769. [DOI] [PubMed] [Google Scholar]
- 22.Amanat F, White KM, Miorin L, Strohmeier S, McMahon M, Meade P, Liu WC, Albrecht RA, Simon V, Martinez-Sobrido L, Moran T, Garcia-Sastre A, Krammer F. 2020. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr Protoc Microbiol 58:e108. 10.1002/cpmc.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langdon CG, Gadek KE, Garcia MR, Evans MK, Reed KB, Bush M, Hanna JA, Drummond CJ, Maguire MC, Leavey PJ, Finkelstein D, Jin H, Schreiner PA, Rehg JE, Hatley ME. 2021. Synthetic essentiality between PTEN and core dependency factor PAX7 dictates rhabdomyosarcoma identity. Nat Commun 12:5520. 10.1038/s41467-021-25829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandrangi SL, Raju Bagadi SA, Sinha NK, Kumar M, Dada R, Lakhanpal M, Soni A, Malvia S, Simon S, Chintamani C, Mohil RS, Bhatnagar D, Saxena S. 2014. Establishment and characterization of two primary breast cancer cell lines from young Indian breast cancer patients: mutation analysis. Cancer Cell Int 14:14. 10.1186/1475-2867-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DaMassa AJ, Adler HE. 1975. Growth of Mycoplasma synoviae in a medium supplemented with nicotinamide instead of B-nicotinamide adenine dinucleotide. Avian Dis 19:544–555. 10.2307/1589080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.01498-22-s0001.xlsx, XLSX file, 0.01 MB (13.1KB, xlsx)