Abstract
Objectives
Neonatal sepsis, a major cause of death amongst infants in sub-Saharan Africa, is often gut derived. Gut colonisation by Enterobacteriaceae producing extended spectrum beta-lactamase (ESBL) or carbapenemase enzymes can lead to antimicrobial-resistant (AMR) or untreatable infections. We sought to explore the rates of colonisation by ESBL or carbapenemase producers in two neonatal units (NNUs) in West and East Africa.
Methods
Stool and rectal swab samples were taken at multiple timepoints from newborns admitted to the NNUs at the University College Hospital, Ibadan, Nigeria and the Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, western Kenya. Samples were tested for ESBL and carbapenemase genes using a previously validated qPCR assay. Kaplan–Meier survival analysis was used to examine colonisation rates at both sites.
Results
In total 119 stool and rectal swab samples were taken from 42 infants admitted to the two NNUs. Colonisation with ESBL (37 infants, 89%) was more common than with carbapenemase producers (26, 62.4%; P = 0.093). Median survival time before colonisation with ESBL organisms was 7 days and with carbapenemase producers 16 days (P = 0.035). The majority of ESBL genes detected belonged to the CTX-M-1 (36/38; 95%), and CTX-M-9 (2/36; 5%) groups, and the most prevalent carbapenemase was blaNDM (27/29, 93%).
Conclusions
Gut colonisation of neonates by AMR organisms was common and occurred rapidly in NNUs in Kenya and Nigeria. Active surveillance of colonisation will improve the understanding of AMR in these settings and guide infection control and antibiotic prescribing practice to improve clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-023-01216-0.
Keywords: Carbapenemase, ESBL, Neonatal sepsis, Molecular diagnostics, Surveillance
Introduction
Sepsis is a major cause of neonatal morbidity and mortality, with an estimated 1.7 million cases globally in 2010, and 203,000 sepsis-attributable deaths [1]. Neonatal sepsis has a higher incidence and mortality in sub-Saharan Africa (sSA) than in other regions [1, 2]. It is often gut derived, with compromised immunity and an impaired gut barrier allowing colonisation by opportunistic pathogens such as Enterobacteriaceae to progress to blood stream infection [3]. Preterm and low birth weight infants are at the greatest risk. Colonisation by antimicrobial resistant organisms (AROs) is particularly problematic and can lead to infections that are difficult or impossible to treat.
Infections with extended spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae or carbapenemase producing organisms (CPOs), which confer resistance to 3rd generation cephalosporins and carbapenems respectively, are associated with poor clinical outcomes due to the increased likelihood of treatment failure [4]. Globally, organisms that produce either ESBL or carbapenemase enzymes, particularly Klebsiella pneumoniae, are a common cause of outbreaks in neonatal units (NNU) [5–7], often due to horizontal transmission from a colonised infant admitted to the ward [8]. Mortality rates of up to 64% have been reported [9], driven by the lack of effective treatment options. Outbreaks of ESBL/CPO K. pneumoniae have been described frequently in African neonatal units [10] caused by contaminated intravenous fluids/antibiotics [11], or associated with gut colonisation [12]. Treatment options for neonatal infections with this resistance profile in sub-Saharan Africa (sSA) are severely restricted, due to a lack of alternative antibiotics and contraindications in this age group [10, 13].
Data on the prevalence of gut colonisation in NNUs in sSA is limited due to a lack of routine surveillance. Defining ARO epidemiology and colonisation rates in NNUs in sSA is critical for guiding antimicrobial stewardship and to assess the impact of interventions [14]. Typically, culture-based methods from rectal swabs or stool samples [15] are used for detecting colonisation. However, increasingly, molecular tools are used to screen samples directly for the presence of AMR genes, foregoing a culture step, providing faster results and increased throughput [16, 17].
We sought to explore the frequency and rates of gut colonisation by AROs in two NNU’s, one in West and one in East Africa, using highly multiplexed molecular diagnostics.
Methods
Study sites
The study was facilitated by the Neonatal Nutrition Network for sSA established in 2018 with the goal of building research capacity and establishing an environment for future trials of neonatal nutritional and other interventions [18]. Of the 7 secondary/tertiary NNUs in Nigeria and Kenya engaged in the network, the study sites were the NNUs at the University College Hospital, Ibadan, Nigeria and the Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH), Kisumu, western Kenya.
Over a 6-month period between August 2018 and May 2019, the Ibadan NNU admitted 488 babies aged less than 48 h. Amongst those with available data, 94/444 (21.2%) were very low birth weight (< 1500 g) and 117/482 (24.3%) were very or extremely preterm (gestational age < 28 weeks). The Kisumu NNU admitted 584 babies aged less than 48 h of which 100/579 (17.3%) were very low birth weight and 88/550 (16.0%) were very or extremely preterm.
Participants and data collection
Parents of neonates admitted to the NNUs were provided with information about the study and asked to provide informed consent. Clinical details of enrolled neonates were extracted from case records and entered into a case report form which was updated with patient outcomes upon discharge from the unit. Suspected sepsis was based on clinical assessment that advised starting or changing antibiotic treatment. Confirmation of sepsis by laboratory analysis was rarely available. Antibiotic usage data for each participant was also available from the Ibadan NNU.
Sampling
Samples of neonatal stool were taken by swabbing faecal material from diapers or, if no stool was present, a rectal swab. Swabs were immediately frozen at − 20 °C prior to DNA extraction. Sampling was scheduled to occur on a weekly basis for six weeks post-admission. However, in practice, samples were often taken at opportunistically and at different timepoints due to the high clinical workload for nursing staff, and some babies were sampled throughout their stay (up to 58 days).
DNA extraction and molecular detection of AMR markers
DNA was extracted from the stool samples and rectal swabs using a Qiagen Fast DNA stool mini kit (Qiagen, Germany), following the manufacturer’s instructions. DNA was shipped to the UK for molecular analysis. Antimicrobial resistance genes were detected using a previously validated in-house high resolution melt (HRM) analysis qPCR assay [19] that detects the five main carbapenemase genes (blaVIM, blaIMP−1, blaKPC, blaNDM, blaOXA−48) and ESBL genes (CTX-M groups 1 and 9). Briefly, the 12.5 µl reactions contained 6.25 µl 2 × Type-It HRM mix (Qiagen, Germany), 1 µl primer mix (final primer concentrations range from 100 to 500 nM), 2.75 µl molecular grade water and 2.5 µl DNA. Reactions were carried out using a Rotor-Gene Q (Qiagen, Germany), with end point detection carried out via HRM. Positive controls were DNA samples extracted from isolates carrying each AMR gene as detailed previously [19].
Statistical analysis
A patient was defined as CPO or ESBL colonised on the date that a carbapenemase or ESBL gene was detected by the HRM analysis qPCR assay. Patients were assumed not to be colonised until a positive sample was obtained. Rates of colonisation of both CPO and ESBL producers were described using Kaplan–Meier survival curves (GraphPad Prism), with patient’s data censored at the point of their last sample [15]. All samples, including those taken beyond six week post admission, were included in the analysis.
To calculate the proportion of the various AMR genes for both the ESBL and CPO genotypes in the cohort, all genes detected in an individual patients’ samples across all time points were included and collated. Genes that were isolated in multiple samples in a single patient were only counted once and when first identified, as these were assumed to belong to the same colonising organism. Associations between clinical variables and ARO colonisation were analysed with Chi-Squared and Fishers Exact Tests using GraphPad Prism Version 5 (GraphPad Software Inc, United States). A p-value < 0·05 was considered significant.
Results
Demographics and clinical characteristics
A total of 42 neonates were enrolled in the study, including 24 in Ibadan and 18 in Kisumu (Table 1). Overall, 24 (57.1%) were female, and 6 (14.3%) were extremely preterm (gestational age < 28 weeks). Suspected sepsis based on clinical assessment occurred in the majority of infants (30/42; 71.4%).
Table 1.
Characteristics of the study participants, colonisation and mortality
| Characteristic | Ibadan n (%) | Kisumu n (%) | Total n (%) |
|---|---|---|---|
| Participants | 24 (57.1) | 18 (42.9) | 42 (100.0) |
| Female | 14 (58.3) | 10 (55.6) | 24 (57.1) |
| Very low birthweight (< 1500 g) | 24 (100) | 4 (22.2) | 28 (66.7) |
| Extremely preterm (gestational age < 28 completed weeks) | 1 (4.2) | 5 (27.8) | 6 (14.3) |
| Caesarean section delivery | 13 (54.2) | 6 (33.3) | 19 (45.2) |
| Mother HIV positive | 0 (0.0) | 3 (16.7) | 3 (8.6) |
| 1 or more episodes of suspected sepsis | 12 (50) | 18 (100.0) | 30 (71.4) |
| 1 or more episodes of necrotising enterocolitis | 5 (20.8) | 1 (5.5) | 6 (14.3) |
| ESBL colonised1 | 22 (91.6) | 14 (77.8) | 36 (85.7) |
| CPO colonised1 | 20 (83.3) | 8 (44.4) | 28 (66.7) |
| Uncolonized by antimicrobial-resistant organism1 | 0 (0.0) | 2 (11.1) | 2 (4.8) |
| Mortality in neonatal unit | 5 (20.8) | 7 (38.9) | 12 (28.6) |
ESBL extended spectrum beta-lactamase, CPO carbapenemase producing organism
1At any time during admission
Sampling
A total of 58 samples were obtained from the 24 neonates in Ibadan between 1- and 56-days post admission. The average number of infants sampled per day between days 1–15, 16–30 and 31–45 were 1.9, 1.3 and 0.4, respectively. In Kisumu, 57 samples were obtained from the 18 neonates (Fig.S1), taken between 1- and 46-days post admission. The average number of infants sampled per day between days 1–15, 16–30 and 31–45 were 2.1, 1.5 and 0.5, respectively.
Antibiotic usage
Antibiotic usage data was only available for the neonates in Ibadan NNU; antibiotics were used for both prophylaxis in newborns with risk factors for infection and treatment of suspected sepsis. The antibiotics received by the cohort were amikacin (received by all neonates), ampicillin/sulbactam (95.2%), ceftazidime (42.9%), gentamycin (38.1%), levofloxacin (19.1%), metronidazole (14.3%), piperacillin/tazobactam (9.52%), clindamycin (9.52%), cefotaxime (4.8%) ciprofloxacin (4.8%), vancomycin (4.8%), and meropenem (4.8%).
Colonisation status
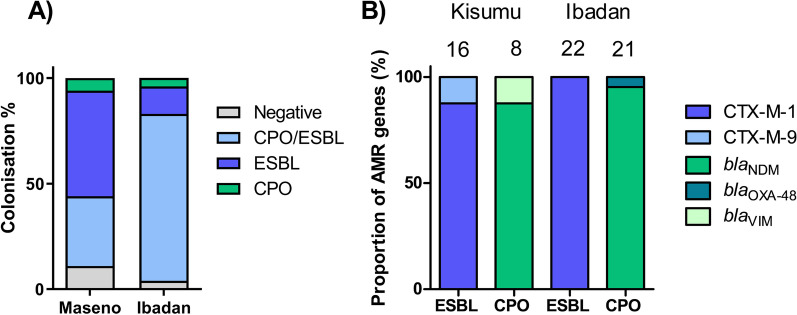
The overall colonisation rates among the study participants were 88.0% for ESBL and 64.3% for CPO organisms. A total of 50.0% (9/18), 5.5% (1/18), and 33.3% (6/18) of neonates in the Kisumu NNU were colonised by ESBL, CPO, or both ESBL/CPO, respectively at any point during their admission (Fig. 1A). Colonisation rates were higher in Ibadan, with 79.2% (19/24) of neonates colonised by both ESBL/CPO organisms during their admission, 12.5% (3/24) ESBL only, 4.2% (1/24) CPO only, and only 4.2% (1/24) uncolonised by either organism. Of the three infants who remained uncolonised across the two sites, all submitted a single sample on days one or two, with one being discharged from the ward and the remaining dying during the study. Colonisation according the demographic and clinical variables is shown in Additional file 1: Table S1.
Fig. 1.
A The percentage of participants at each site colonised by ESBL, CPO or both during the study period. B Number of participants at each site positive for various ESBL and carbapenemase genes identified during the study
Colonisation rates
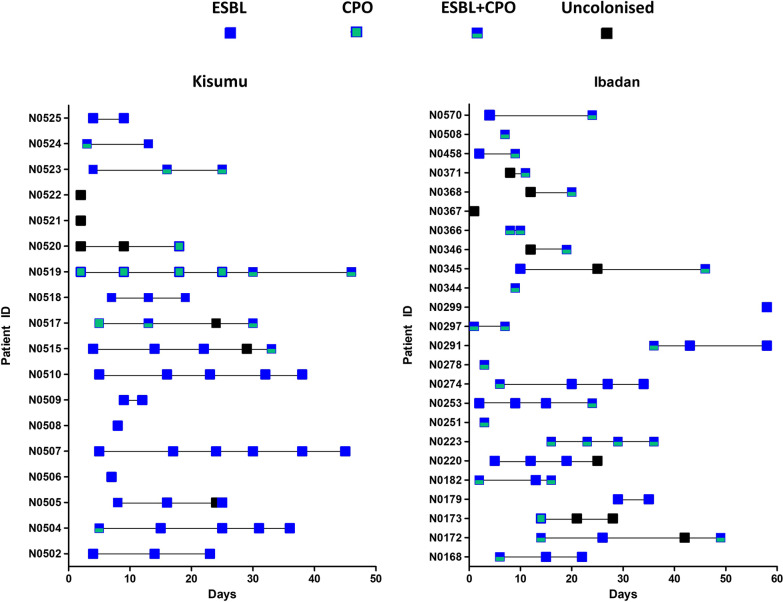
Longitudinal sampling revealed that colonisation occurred rapidly (Fig. 2) and had often occurred by the first sampling point (35/42; 83.3%). Median survival time to colonisation by ESBL organisms was 7 days with a maximum of 97% of colonisation by day 45 (Fig. 3). In contrast, the rate of CPO colonisation was significantly lower (P = 0.035, Mantel-Cox Test), with a median survival time before colonisation of day 16, but also with a maximum of 97% by day 45. Colonisation was also dynamic; in 13 (31%) cases either ESBL or CPO carriage was detected but then absent in a subsequent sample.
Fig. 2.
Longitudinal acquisition, carriage, and loss of ESBL and CPOs according to study site. Note: Each box represents a stool or rectal swab sample. Days are counted from the admission of each individual participant and do not run concurrently
Fig. 3.
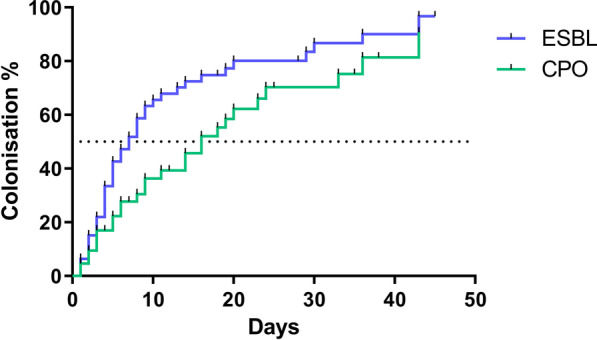
Kaplan–Meier survival analysis showing time to first colonisation by ESBL and CPOs. Notes: Dotted line indicates time to 50% of infants colonised. ESBL extended spectrum beta-lactamase, CPO carbapenemase producing organism
Molecular epidemiology of AMR genes
In Kisumu, the majority of ESBL genes detected belonged to the CTX-M-1 family (14/16, 87.5.%), and CTX-M-9 (2/16, 12.5%) (Fig. 1B.). The most prevalent carbapenemase was blaNDM (7/8, 87.5%). A single blaVIM gene was also detected (1/8, 12.5%). In Ibadan, CTX-M-1 was again the dominant ESBL family (22/22, 100%), and blaNDM the dominant carbapenemase (20/21, 95.2%). One sample was positive for blaOXA-48 (1/22, 4.8%). We did not detect any of the carbapenemases blaKPC or blaIMP genes in either site.
Discussion
Using a combination of longitudinal sampling and molecular testing for a panel of AMR genes we were able to demonstrate rapid rates of colonisation of neonates admitted to two neonatal units by ESBL and carbapenemase producing organisms. The prevalence of colonisation by ESBLs in infants admitted to both the Kisumu and Ibadan NNUs was higher than previously reported in sSA NNUs including Tanzania [12] and Kenya [20]. A systematic review of gut ESBL colonisation in sSA reported rates ranging from 25 to 74% [21], which are all below the levels found in our study. Colonisation of infants with ESBL producers was rapid, with mean time to colonisation of 8 days. This is comparable to studies in Asia [22] where the use of carbapenems and 3rd generation cephalosporins is widespread. In a minority of infants, colonisation was dynamic, with acquisition and loss of either ESBL or CPO bacteria occurring during the study. Whilst colonisation can last for months or even years [23], rapid acquisition and loss has been shown in travellers to areas with high ESBL prevalence [24].
Data on gut colonisation by CPOs in sSA is limited; however, this is assumed to be less common than ESBL colonisation, in part due to the lower usage of expensive carbapenem drugs in this setting [25]. A recent study from Nigeria, including patient isolates from the same hospital involved in our study, reported 69.1% of a cohort of hospital inpatients were colonised by CPOs, and highlighted the circulation of blaNDM and blaVIM carbapenemases [26]. A country specific meta-analysis found the prevalence of carbapenem resistance amongst Enterobacteriaceae to be 5% in Nigeria and 0.3% in Kenya [27]. The proportion of neonates colonised by CPOs in Ibadan exceeds the highest proportions reported in previous meta-analysis studies [28] carried out in non-NNU hospital and community settings in Africa and highlights that NNUs could be a major reservoir of CPOs.
The majority of CTX-M ESBL genes detected were in CTX-M group one, which includes blaCTX-M−15 and blaCTX-M−1 enzymes [29]. These are the most widespread ESBL genes globally and have previously been reported as the most prevalent genes in sSA [29]. The discovery of blaNDM as the most common carbapenemase gene present in both the Ibadan and Kisumu tertiary NNUs is in agreement with previous studies; A 2012–2014 survey found blaNDM and blaVIM genes to be the only reported carbapenemases in both Nigeria and Kenya [30]. A high prevalence of blaNDM−1 was found in the Ibadan NNU despite only a single dose of carbapenem antibiotics being given over the study period. However, 95.2% of neonates in Ibadan received a β-lactam antibiotic, and 46.7% a 3rd generation cephalosporin, and the use of these antibiotics could potentially select for bacteria producing any circulating enzyme capable of providing resistance, including carbapenemases. The frequent co-localisation of blaNDM−1 with other AMR genes on mobile genetic elements means that co-selection of blaNDM−1 can also occur during exposure to other antibiotic classes such as aminoglycosides [31].
The transmission of AROs from the hospital environment to the neonatal gut has been well documented, with health care workers [32], sinks and taps [33], surface environments [34] and maternal colonisation [32] all implicated as potential transmission sources. Molecular surveillance coupled with immediate patient isolation has been demonstrated to be an effective infection control measure in intensive care units [35], and the enhanced speed of molecular testing can be useful in ensuring prompt intervention to prevent hospital or unit wide outbreaks [15]. However, these interventions require the infrastructure capacity for isolation units. Successful control measures that focus on preventing colonisation include the use of disposable gloves and gowns for single patient use [36], improved hand hygiene, and intensive bio-cleaning of wards [37]. The assay we used in this study has since been validated in a dry-format, requiring only the addition of DNA and nuclease-free water to resuspend the dried reagents [38]. This eliminates the need for a cold chain for shipping and storage, making it more applicable to this setting. By directly testing faecal samples or swabs without a culture step, this method can be used to provide same day results. Although this approach does not identify the bacterial species, there are companion molecular assays that can be run alongside if this information is desired [39].
Our study had several limitations. The small sample size limited our ability to explore associations between demographic and clinical variables and colonisation. Nevertheless, the very high proportions of ESBL and/or CPO colonised infants provides important epidemiological information to inform infection control strategies. Whilst utilising molecular methods has several advantages, including the identification of specific AMR genes, this approach prevented the linkage of AMR genes to particular isolates, and therefore, we could not determine the species or sequence type of the AROs detected. Our pragmatic approach to sampling around clinical care also meant that sampling did not occur at regular intervals, and not all participants were sampled within their first week of admission to the NNU. This could affect the results, particularly the time-to-colonisation analysis.
Conclusion
This report highlights that gut colonisation of infants by AROs in NNUs in Kenya and Nigeria is common and occurs rapidly. The carbapenemase blaNDM−1 and group 1 blaCTX-M genes were the most prevalent resistance genes at both sites. The study demonstrates that active surveillance of colonisation can be used to improve the understanding of AMR in NNUs in sSA, and these data could guide infection control and prescribing practice to improve clinical outcomes.
Supplementary Information
Additional file 1: Table S1. Occurrence of selected variables in participants according to colonisation with ESBL and CPOs.
Additional file 2: Fig. S1. Sample collection rates in Kisumu and Ibadan NNUs. Days are counted from first day of life per participant.
Acknowledgements
We acknowledge the technical staff in the diagnostic microbiology laboratories at Kenya Medical Research Institute, Centre for Global Health Research, Kisumu, Kenya and the University College Hospital, Ibadan, Nigeria. We thank the parents and NNU staff who very kindly supported this study.
Author contributions
TE, OOT, HN, ERA and SA conceptualised the study. PA, WO, MO, GN, AA, and OOT managed the clinical studies, including collecting patient information and samples. TE, CTW, OOT, HN, ERA and SA contributed to the experimental design and data analysis. TE, CTW and TR carried out laboratory experiments. TE, CW, AICA and SA wrote the first draft of the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the MRC Confidence in Global Nutrition and Health Research Institutional “pump-priming” award 2018-2020 (MC_PC_MR/R019789/1).
Availability of data and materials
An anonymized database of the microbiological data related to study participants is available from the corresponding authors.
Declarations
Ethics approval and consent to participate
The study was approved by the Liverpool School of Tropical Medicine Research Ethics Committee (study number 18-021), the University of Ibadan/University College Hospital Ethics Committee (UI/EC/18/0446) and the JOOTRH Ethics Review Committee (ERC.IB/VOL.1/510).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Glob Health. 2018;3(1):e000347. doi: 10.1136/bmjgh-2017-000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):731–741. doi: 10.1016/S1473-3099(14)70804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haussner F, Chakraborty S, Halbgebauer R, Huber-Lang M. Challenge to the intestinal mucosa during sepsis. Front Immunol. 2019;10(891):85. doi: 10.3389/fimmu.2019.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin D, Zhang L, Wang A, He L, Cao Y, Hu F, et al. Clinical and molecular epidemiologic characteristics of carbapenem-resistant Klebsiella pneumoniae infection/colonization among neonates in China. J Hosp Infect. 2018;100(1):21–28. doi: 10.1016/j.jhin.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ghaith DM, Zafer MM, Said HM, Elanwary S, Elsaban S, Al-Agamy MH, et al. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur J Clin Microbiol Infect Dis. 2019;2:89. doi: 10.1007/s10096-019-03761-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Hu X, Chen F, Li H, Wang D, Li X, et al. Co-outbreak of multidrug resistance and a novel ST3006 Klebsiella pneumoniae in a neonatal intensive care unit: a retrospective study. Medicine. 2019;98(4):e14285. doi: 10.1097/MD.0000000000014285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton PJM, Murphy M, McCallion N, Brennan M, Cunney R, Drew RJ. Outbreaks of extended spectrum β-lactamase-producing Enterobacteriaceae in neonatal intensive care units: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):72. doi: 10.1136/archdischild-2015-308707. [DOI] [PubMed] [Google Scholar]
- 9.Stoesser N, Giess A, Batty EM, Sheppard AE, Walker AS, Wilson DJ, et al. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community- versus hospital-associated transmission in an endemic setting. Antimicrob Agents Chemother. 2014;58(12):7347–7357. doi: 10.1128/AAC.03900-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornick J, Musicha P, Peno C, Seager E, Iroh Tam P-Y, Bilima S, et al. Genomic investigation of a suspected Klebsiella pneumoniae outbreak in a neonatal care unit in sub-Saharan Africa. Microb Genom. 2021;7(11):59. doi: 10.1099/mgen.0.000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okomo U, Senghore M, Darboe S, Bojang E, Zaman SMA, Hossain MJ, et al. Investigation of sequential outbreaks of Burkholderia cepacia and multidrug-resistant extended spectrum β-lactamase producing Klebsiella species in a West African tertiary hospital neonatal unit: a retrospective genomic analysis. Lancet Microbe. 2020;1(3):e119–e129. doi: 10.1016/S2666-5247(20)30061-6. [DOI] [PubMed] [Google Scholar]
- 12.Marando R, Seni J, Mirambo MM, Falgenhauer L, Moremi N, Mushi MF, et al. Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital. Tanzania Int J Med Microbiol Suppl. 2018;308(7):803–811. doi: 10.1016/j.ijmm.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen SCH, Ezure Y, Rolley L, Spurling G, Lau CL, Riaz S, et al. Gram-negative neonatal sepsis in low- and lower-middle-income countries and WHO empirical antibiotic recommendations: a systematic review and meta-analysis. PLoS Med. 2021;18(9):e1003787. doi: 10.1371/journal.pmed.1003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants' and children's health? J Pediatr Gastroenterol Nutr. 2015;60(3):294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee R, Humphries R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):427–439. doi: 10.1080/21505594.2016.1185577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko YJ, Kim J, Kim H-N, Yoon S-Y, Lim CS, Lee CK. Diagnostic performance of the Xpert Carba-R assay for active surveillance of rectal carbapenemase-producing organisms in intensive care unit patients. Antimicrob Res Infect Control. 2019;8(1):127. doi: 10.1186/s13756-019-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CT, Edwards T, Adams ER, Feasey NA, Musicha P. ChloS-HRM, a novel assay to identify chloramphenicol-susceptible Escherichia coli and Klebsiella pneumoniae in Malawi. J Antimicrob Chemother. 2020;74(5):1212–1217. doi: 10.1093/jac/dky563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabwera HM, Wang D, Tongo OO, Andang’o PEA, Abdulkadir I, Ezeaka CV, et al. Burden of disease and risk factors for mortality amongst hospitalized newborns in Nigeria and Kenya. PLOS One. 2021;16(1):e0244109. doi: 10.1371/journal.pone.0244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards T, Williams C, Teethaisong Y, Sealey J, Sasaki S, Hobbs G, et al. A highly multiplexed melt-curve assay for detecting the most prevalent carbapenemase, ESBL and AmpC genes. Diagn Microbiol Infect Dis. 2020;8:115076. doi: 10.1016/j.diagmicrobio.2020.115076. [DOI] [PubMed] [Google Scholar]
- 20.Kagia N, Kosgei P, Ooko M, Wafula L, Mturi N, Anampiu K, et al. Carriage and acquisition of extended-spectrum β-Lactamase–producing Enterobacterales among neonates admitted to Hospital in Kilifi, Kenya. Clin Infect Dis. 2019;69(5):751–759. doi: 10.1093/cid/ciy976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis JM, Lester R, Garner P, Feasey NA. Gut mucosal colonisation with extended-spectrum beta-lactamase producing Enterobacteriaceae in sub-Saharan Africa: a systematic review and meta-analysis. Wellcome Open Res. 2019;4:160. doi: 10.12688/wellcomeopenres.15514.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts T, Limmathurotsakul D, Turner P, Day NPJ, Vandepitte WP, Cooper BS. Antimicrobial-resistant Gram-negative colonization in infants from a neonatal intensive care unit in Thailand. J Hosp Infect. 2019;103(2):151–155. doi: 10.1016/j.jhin.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, et al. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 24.Kantele A, Kuenzli E, Dunn S, Dance D, Newton P, Davong V, et al. Real-time sampling of travelers shows intestinal colonization by multidrug-resistant bacteria to be a dynamic process with multiple transient acquisitions. bioRxiv. 2019;8:827915. [Google Scholar]
- 25.Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–1052. doi: 10.1016/S1473-3099(17)30394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogbolu DO, Piddock LJV, Webber MA. Opening Pandora's box: high level resistance to antibiotics of last resort in Gram negative bacteria from Nigeria. J Glob Antimicrob Res. 2020;21:211–217. doi: 10.1016/j.jgar.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Mitgang EA, Hartley DM, Malchione MD, Koch M, Goodman JL. Review and mapping of carbapenem-resistant Enterobacteriaceae in Africa: using diverse data to inform surveillance gaps. Int J Antimicrob Agents. 2018;52(3):372–384. doi: 10.1016/j.ijantimicag.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Manenzhe RI, Zar HJ, Nicol MP, Kaba M. The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother. 2014;70(1):23–40. doi: 10.1093/jac/dku356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 30.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;60(2):1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnning A, Karami N, Tång Hallbäck E, Müller V, Nyberg L, Buongermino Pereira M, et al. The resistomes of six carbapenem-resistant pathogens-a critical genotype-phenotype analysis. Microb Genom. 2018;4(11):e000233. doi: 10.1099/mgen.0.000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tschudin-Sutter S, Frei R, Battegay M, Hoesli I, Widmer AF. Extended spectrum ß-lactamase-producing Escherichia coli in neonatal care unit. Emerg infect Dis. 2010;16(11):1758–1760. doi: 10.3201/eid1611.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H, et al. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Res Infect Control. 2017;6(1):24. doi: 10.1186/s13756-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bokulich NA, Mills DA, Underwood MA. Surface microbes in the neonatal intensive care unit: changes with routine cleaning and over time. J Clinical Microbiol. 2013;51(8):2617–2624. doi: 10.1128/JCM.00898-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou M, Kudinha T, Du B, Peng J, Ma X, Yang Y, et al. Active surveillance of carbapenemase-producing organisms (CPO) colonization with xpert carba-r assay plus positive patient isolation proves to be effective in CPO containment. Front Cell Infect Microbiol. 2019;9(162):89. doi: 10.3389/fcimb.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AD, Morgan DJ, Pineles L, Magder L, O’Hara LM, Johnson JK. Acquisition of antibiotic-resistant Gram-negative bacteria in the Benefits of Universal Glove and Gown (BUGG) Cluster Randomized Trial. Clin Infect Dis. 2020;8:69. doi: 10.1093/cid/ciaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferry A, Plaisant F, Ginevra C, Dumont Y, Grando J, Claris O, et al. Enterobacter cloacae colonisation and infection in a neonatal intensive care unit: retrospective investigation of preventive measures implemented after a multiclonal outbreak. BMC Infect Dis. 2020;20(1):682. doi: 10.1186/s12879-020-05406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cubas-Atienzar AI, Williams CT, Karkey A, Dongol S, Sulochana M, Rajendra S, et al. A novel air-dried multiplex high-resolution melt assay for the detection of extended-spectrum β-lactamase and carbapenemase genes. J Glob Antimicrob Res. 2021;27:123–131. doi: 10.1016/j.jgar.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards T, Sasaki S, Williams C, Hobbs G, Feasey NA, Evans K, et al. Speciation of common Gram-negative pathogens using a highly multiplexed high resolution melt curve assay. Sci Rep. 2018;8(1):1114. doi: 10.1038/s41598-017-18915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Occurrence of selected variables in participants according to colonisation with ESBL and CPOs.
Additional file 2: Fig. S1. Sample collection rates in Kisumu and Ibadan NNUs. Days are counted from first day of life per participant.
Data Availability Statement
An anonymized database of the microbiological data related to study participants is available from the corresponding authors.