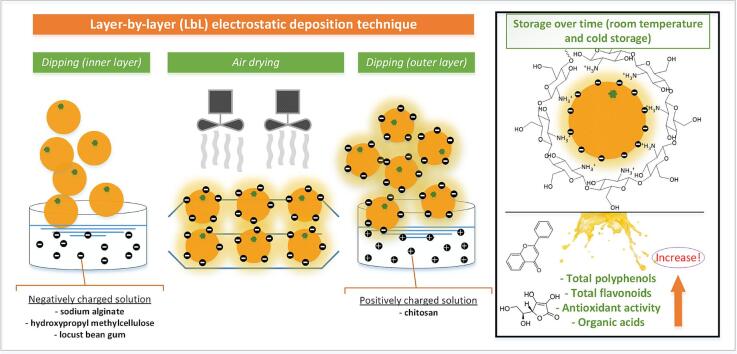
Graphical abstract
Abbreviations: Ctrl, control; C, chitosan single-coated mandarins; AC, alginate/chitosan-coated mandarins; HC, hydroxypropyl methylcellulose/chitosan-coated mandarins; GC, locust bean gum/chitosan-coated mandarins; LbL, Layer-by-Layer; CIE, International Commission on Illumination; HPLC, high-performance liquid chromatography; DAD, diode-array detector; ANOVA, analysis of variance; ABTS, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2,2-diphenyl-1-picrylhydrazyl; TSS, Total soluble solids; TA, Titratable acidity; TPC, Total polyphenolic content; TF, Total flavonoids; PCA, Principal component analysis; KMO, Kaiser-Meyer-Olkin measure of sampling adequacy; RT, Room temperature; CS, Cold storage
Keywords: Polyelectrolyte complex, Chitosan, Edible coatings, Mandarin fruit, Bioactive compounds, Organic acids
Highlights
-
•
Mandarin fruit quality was improved throughout storage using edible coatings.
-
•
Edible coatings influence mandarin fruit metabolism throughout storage.
-
•
Bioactive compounds in mandarin fruit with edible coatings were preserved.
-
•
Antioxidant activity can be retained in mandarin fruit coated with edible coatings.
-
•
Edible coatings preserve organic acids in mandarin fruits.
Abstract
The layer-by-layer application of biopolymeric coatings to mandarin fruits as a postharvest treatment to improve fruit coating efficacy has been reported. A single 1 % (w/v) chitosan application was evaluated, and polyelectrolyte complexes such as 1.5 % (w/v) alginate/chitosan, 1 % (w/v) hydroxypropyl methylcellulose/chitosan, and 0.2 % (w/v) locust bean gum/chitosan were applied to mandarin fruits. The quality of coated mandarin fruits was observed at temperatures: 20 ± 2 °C (up to 10 days) and 5 °C (up to 28 days). Changes in the fruit metabolism were observed by evaluating bioactive compounds (polyphenolic compounds and flavonoids), antioxidant activity, and organic acids during the preservation of mandarin fruits. All of the tested combinations of layer-by-layer coatings significantly impacted the quality of mandarin fruits throughout storage, both at room temperature and cold storage, respectively. The overall best performance was observed for a layer-by-layer hydroxypropyl methylcellulose/chitosan coating in terms of visual aspects, bioactive compounds, antioxidant activity, and organic acids content.
Introduction
Mandarins are among the most widely consumed citrus fruits, and due to their distinctive flavor and nutritional benefits, demand for them is rising on a global scale. Improper harvesting, handling, packaging, and shipping procedures significantly impact mandarin fruit quality loss. The losses can continually develop during the trading process, where quantitative losses of fresh fruits are easier to assess than qualitative losses (e.g. nutritional value, consumer acceptability, and edibility) (Kader, 2005). Food and Agriculture Organization (FAO) estimated a quantitative 48 % of fruit and vegetable losses on a global base in the postharvest chain (from harvest to consumption) (FAO, 2011). Mandarin fruits are non-climacteric, perishable, and cannot be kept for a long time during transportation and storage (Rokaya et al., 2016). Appropriate postharvest treatment can minimize fruit loss dramatically, increase fruit quality, and result in higher profitability.
The development of new technologies (like postharvest heat treatments or ultraviolet A irradiation) for prolonging shelf life with a lower cost of distribution is crucial to improving post-harvest handling (Queb-González et al., 2020, Yamaga and Nakamura, 2022). Maintaining the post-harvest life of fruit is a complex process. Fruits are prone to quality loss during harvesting, storage, and distribution until it reaches the consumers (Md Nor & Ding, 2020). A natural metabolic response leads to fruit senescence. The fruit can only retain its edible quality for a limited time. This process cannot be fully terminated and can only be managed by delaying the deterioration. The application of fruit coating before storage can minimize substantial privation and alleviate these deleterious effects (Md Nor & Ding, 2020). By decreasing moisture and solute migration, gas exchange, respiration, and reaction rates, as well as minimizing or suppressing physiological changes, edible coatings may help to prolong the shelf life of fruits. Another advantage of edible coatings application is the reduction of packaging waste due to their biodegradable nature (Dhall, 2013). Furthermore, appropriate coating components can even enhance the gloss and visual attractiveness of the fruits (Arnon et al., 2015).
Most biopolymeric coatings are formulated as liquid solutions to regulate moisture, solutes, and gaseous exchange between the internal and external atmosphere (Ncama et al., 2018). The semi-permeable characteristic of coating allows the minimal exchange of compounds but does not create an anaerobic condition that causes degradation of fruit quality (Bill et al., 2014). This type of surrounding can effectively slow down respiration rate, conserve stored energy, delay microbial growth, and therefore extend the shelf life of fruit (Salas-Méndez et al., 2019). Polysaccharide-based biopolymeric coatings are highly available, are not prone to evoke allergenic reactions, are mostly soluble in water, and frequently have good mechanical properties (Arnon-Rips & Poverenov, 2018). The main advantage of polysaccharide-based coatings is their defined chemical structure for each monomer unit. This can help in controlling the coating properties and predicting its behavior (Arnon-Rips & Poverenov, 2018).
Chitosan is one of the most popular polysaccharide coatings for various fruits due to its capability to delay water loss, slow down the browning process, and suppress fungal disease (Obianom et al., 2019). Due to its remarkable advantages, chitosan has positive feedback from many publications employing chitosan as a fruit coating. The frontiers of the chitosan-based preservation approach are rapidly evolving. Novel methods such as the layer-by-layer (LbL) assembly have been relatively uncommon in the chitosan-based fruit preservation field. These novel methods are still far from perfect, and improvements considering the mechanical strength of films, simplicity of the application process, and other factors are required to advance performance and move closer to practical application. As a sustainable material, chitosan represents one of the most important biopolymers used in postharvest technology (Arnon-Rips & Poverenov, 2018).
Due to their biodegradability, biocompatibility, nontoxicity, and physicochemical characteristics, sodium alginate, hydroxypropyl methylcellulose, and locust bean gum have shown considerable potential for application as fruit coatings (Belščak-Cvitanović et al., 2017). All of the mentioned polysaccharides have desirable properties but a single coating material can hardly satisfy variable requests such as balanced gas and water vapor permeability, adequate antibacterial activity, and extraordinarily excellent adhesion. As a result, there has been renewed interest in producing composite edible coatings that combine many benefits of their diverse components (Arnon-Rips & Poverenov, 2018). Combining two or more biopolymers can improve edible coatings' properties since it allows advantages from several materials. Electrostatic interactions are the most common chemical forces used to prepare layer-by-layer (LbL) structures. The LbL method involves alternating sequential deposition of polyelectrolytes, polycations, and polyanions on a charged surface, followed by a rinsing step after each deposition (Azinfar et al., 2021). Overall electroneutrality of the LbL structures is gained by a charge overcompensation mechanism based on intrinsic and extrinsic charge compensations and competitive ion pairing. Intrinsic charge compensation involves the pairing of certain polyelectrolytes with oppositely charged polyelectrolytes. In the end, no counter-ions are present within the bulk of the multilayer and all exchangeable charges are located at the surface. Extrinsic compensation is based on ion pairing with charged counter-ions. Upon LbL assembly, this extrinsic charge compensation is converted into intrinsic charge compensation, leading to the release of counter-ions and solvating water molecules, additionally providing an entropic driving force for the LbL assembly process (Mateos-Maroto et al., 2022).
The dipping technique is the oldest commercial coating technique but is still relevant due to its continuous development. The concept of the dipping technique is by immersing fruit in a coating solution to allow complete wetting on the fruit surface. Afterward, the coating solution is drained out to remove the excess coating from the surface. Finally, the fruit is dried to form a well-intact coating with the fruit surface. Dipping is a universal technique as it can be applied in a wide range of viscosity of coating solutions (Fathi et al., 2021). Commonly the dipping technique is applied to produce a single-layer coating. However, recent advancements, introduce the technique of dipping evolved to produce multilayer coating known as the layer-by-layer method (Md Nor & Ding, 2020).
In this research, the objective was to observe the effects of a single chitosan coating as well as LbL composite coatings (using chitosan as an outer layer and sodium alginate, hydroxypropyl methylcellulose, or locust bean gum as the inner layer) on mandarin fruits. Effects were studied both at room temperature and cold storage over specific periods. The postharvest quality of mandarin fruits was examined and a special focus was given to the impact of the coatings on bioactive compounds, antioxidant activity, and organic acids metabolism of mandarin fruit.
Materials and methods
Plant material
Mandarins 'Owari' (Citrus unshiu Marcovitch) were obtained from one of the commercial orchards (Mandarinko d.o.o.) located at Opuzen, Neretva valley, Croatia (Latitude: 43.0176, Longitude: 17.5623; 43°1′3″ North, 17°33′44″ East). The fruits were harvested (21st October 2021) at optimal maturity and immediately delivered to the laboratory for further processing. Harvest maturity parameters of the mandarin fruit ‘Owari’ was recorded for 30 randomly selected mandarin fruits. Fruit weight was 124.49 ± 34.69 g; firmness 18.81 ± 3.34 N; total soluble solids 11.76 ± 0.76 %; titratable acidity 1.04 ± 0.15 mg citric acid/100 g f.w.; total soluble solids to titratable acidity ratio 11.47 ± 1.38; color parameters as L* was 62.48 ± 8.86, a* 21.60 ± 3.65 and b* 59.36 ± 7.44.
Chemicals
High molecular weight chitosan (CAS Number: 9012-76-4, molecular weight: 310000–375000 Da; 800–2000 cP, 1 wt% in 1 % acetic acid (25 °C, Brookfield) (lit.)), low viscosity sodium alginate (CAS Number: 9005-38-3; Brookfield viscosity 4–12 cps (1 % in H2O at 25 °C)) and locust bean gum from Ceratonia siliqua seeds (CAS Number: 9000-40-2) were purchased from Sigma-Aldrich (USA). Hydroxypropyl methylcellulose (CAS Number: 9004-65-3) was obtained from Alfa Aesar (TermoFisher GmbH, USA). All of the analytical standards and chemicals used in the chemical analysis were purchased from Sigma-Aldrich (USA). All other chemicals were of analytical grade and used as received without further purification.
Preparation of biopolymeric coatings and dipping
Prior to the investigation, a literature survey (Arnon et al., 2014, Arnon et al., 2015, Li et al., 2021, Poverenov et al., 2014) and preliminary trials were performed to select adequate biopolymer concentrations for coatings (keeping in mind the simplicity of biopolymeric solution preparation). A volume of 10 L of 1 % (w/v) chitosan was prepared by dissolving it in a sterile 2 % (w/v) citric acid solution. The volume of 5 L of 1.5 % (w/v) sodium alginate, 1 % (w/v) hydroxypropyl methylcellulose, and 0.2 % (w/v) locust bean gum solutions were dissolved in sterile distilled water and left overnight under constant stirring at room temperature (20 ± 2 °C).
Solutions were then transferred in separate baths (intended for dipping). Mandarins were washed with cold tap water and air-dried. Per 180 (30 × 6 repetitions) mandarins were randomly selected for each treatment. Mandarins were submerged in a biopolymer solution of chitosan, sodium alginate, hydroxypropyl methylcellulose, or locust bean gum for 3 min, to gain the first (inner) coat. Mandarins were then left to dry in a room ventilated with laboratory fans to ensure good airflow (approx. after 4 h). Coated mandarins with an inner layer were submerged in the chitosan solution for 3 min (LbL complex was formed). The process of air drying was repeated after the second coat was applied. Single coated (chitosan), LbL coated and uncoated (control) mandarins were set in a room temperature setting (20 ± 2 °C, 60 ± 10 % relative humidity) (30 mandarins × 3 per treatment), and under refrigeration in a cold chamber (5 ± 1 °C) with fixed moisture content (90–95 %) (30 mandarins × 3 per treatment). A total of 900 mandarins were used in the experiment. Samples under room temperature (RT) were observed after 2, 4, 7, and 10 days while samples under cold storage at 5 °C and 90 % RH (CS) after 7, 14, 21, and 28 days. Samples were taken as per 6 mandarines for each repetition, a total of 18 mandarines for each relative day, and treatment (i.e. 90 mandarines per storage type for each sampling).
Fruit gloss, visual observation over time, and CIE (International Commission on Illumination) color variables
Fruit gloss was evaluated on a 0–10 scale in which 0 was equal to no gloss and 10 to very glossy. Per 10 mandarins in 6 repetitions were randomly selected for each treatment. Randomized blocks in 6 repetitions were prepared for evaluation. Twelve people, previously trained for this evaluation, subjectively graded treated mandarins (paired comparison blind test) (Arnon et al., 2015). 30 randomly selected (control and coated) mandarin fruits were set up at room temperature and photographed on the inspection dates.
The mandarin fruit color variables were measured according to the CIE Lab system, using a colorimeter (ColorTec PCM; ColorTec Associates Inc., USA) (Jatoi et al., 2017). Fruit peel color was measured with ColorTec-PCM Plus 30 mm Benchtop Colorimeter (ColorTec Associates, Inc. Clinton, New Jersey, USA) in CIE L*a*b* color systems. Measured color parameters were used to calculate the total color difference. Total color difference (ΔE) was calculated from CIE L*a*b* parameters measured after 2, 4,7, and 10 days (room temperature stored fruit) or 7, 14, 21, and 28 days (cold-stored fruit) compared to the relative harvest stage (day 0). Initially, color variables were recorded for each fruit (i.e. 180 (30 × 6 repetitions) mandarins per treatment). Further, color variables were recorded for all mandarin fruits (a total of 900) minus the take for sampling for each relative day, where these fruits were processed for chemical analysis (per 6 mandarines for each repetition, a total of 18 mandarines for each relative day). The total color difference was calculated by using the following equation [1] (Pathare et al., 2013):
| (1) |
Weight loss and fruit firmness
Fruit weight loss and firmness was measured before the treatment and after each sampling (day 2, 4, 7, 10 RT or 7, 14, 21, 28 CS) for every remaining mandarin thereafter (same as for color variable measurements). The fruit weight loss (%) was determined according to equation [2]:
| (2) |
where a is the initial weight at the start of the storage, and b is the weight on the inspection date or the final weight.
The highest force necessary to penetrate the entire mandarin fruit was used to measure firmness. A texture analyzer (FTA Fruit Texture Analyzer, GÜSS Manufacturing (Pty) Ltd., Cape Town, South Africa) with a cylindrical probe of 5 mm diameter was used, under the following conditions (Won & Min, 2018): a pre-test speed of 3.0 mm s−1, a test speed of 1.0 mm s−1, a post-test speed of 3.0 mm s−1, and a penetration distance of 3 mm.
Preparation of mandarin fruit juice
Six mandarines per 3 repetitions were used in juice preparation (a total of 3 juices per sample). Mandarin fruits were weighted, peeled, and blended using FOSS homogenizer 2094 (Hillerød, Denmark). Mass and volume for each sample after homogenization was recorded, respectively. The obtained puree was transferred in falcon tubes (50 mL) and centrifuged at 11,180 g and 4 °C, for 10 min. The supernatant was filtered using Whatman No.4 filter paper and was used for further analysis. The juice was diluted when necessary. Total soluble solids, titratable acidity, total polyphenolic content, total flavonoids, antioxidant activity, and organic acids were determined from juice samples.
Total soluble solids and titratable acidity
The total soluble solids (TSS) of mandarins were determined using a digital hand refractometer (PAL-1; Atago, Tokyo, Japan) and expressed in percentage (%). The titratable acidity (TA) was obtained by titrating 10 mL of mandarine juice containing phenolphthalein as an indicator with 0.1 mol/L NaOH using a digital burette and expressed as g/L citric acid. TSS and TA was measured before the treatment and after each sampling (day 2, 4, 7, 10 RT or 7, 14, 21, 28 CS) for every remaining mandarin thereafter.
Determination of total polyphenolic content, total flavonoids, and antioxidant activity
The modified Folin Ciocalteu’s method (Singleton et al., 1999) was used to determine total polyphenolic content (TPC). Briefly, mandarin juice (0.1 mL) was mixed with 7.9 mL of distilled water and 0.5 mL of Folin Ciocalteu’s reagent (diluted with distilled water in a 1:2 ratio). A volume of 1.5 mL of 20 % (w/v) sodium carbonate was added to the suspension which was vortexed and left for 2 h to react. Gallic acid was selected as a standard compound. Absorbance was measured at 765 nm, and the data are expressed as mg of gallic acid equivalents per g of freshweight mandarins (mg g−1).
Total flavonoid (TF) content was determined by the modified spectrophotometric method of Ivanova et al. (Ivanova et al., 2010). Briefly, 1 mL of mandarin juice was added to a 10 mL volumetric flask containing 4 mL of distilled water. Then, 0.3 mL of NaNO2 (0.5 g/L) solution was added. After 5 min, 0.3 mL of AlCl3 (1 g/L) solution was added and 6 min later, 2 mL of NaOH (1 mol/L) was added to the mixture. The final volume was made up to 10 mL with the addition of distilled water. The solution was mixed and the absorbance was measured at 360 nm. Quercetin was selected as a standard compound. Results are expressed as mg of quercetin equivalents per g of fresh weight mandarins (mg g−1).
The antioxidant potential of mandarin juice was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) reagents, according to the known procedures (Brand-Williams et al., 1995, Re et al., 1999), respectively. For the DPPH method, a volume of 3.9 mL methanolic DPPH solution was added to the test tube containing 100 μL of mandarin juice. The free radical-scavenging capacity of the sample was determined by measuring the absorbance decrease at 517 nm after 30 min of incubation against the blank sample. For the ABTS method, an amount of 40 μL of mandarin juice was added to 4 mL of the ABTS radical solution in a test tube, and the absorbance readings were taken after exactly 6 min against the appropriate reagent blank instead of the sample. A water-soluble vitamin E analog Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) was selected as a standard compound. Results are expressed as mmol Trolox equivalents per 100 g of fresh weight mandarins.
Measurements were performed using a UV–vis spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan).
Determination of organic acids
A modified HPLC (high-performance liquid chromatography) method was used to determine the content of oxalic, malic, ascorbic, and citric acids in mandarine juices (Nour et al., 2010). Mandarine juices were diluted at 1:50 for citric acid determination and 1:5 for other observed acids. The dilutions were filtered by LLG-RC 0.45 μm syringe filters (LLG Gmbh, Grevenbroich, Germany) before analysis of organic acids. Agilent 1260 Infinity II System (Agilent, Waldbronn, Germany), equipped with an autosampler, column thermostat, and DAD (diode-array detector) was used. The separation of organic acids was performed on COSMOSIL C18-PAQ (250 mm × 4.6 mm i.d., 5 μm) at 40 °C. The detection wavelengths were 254 nm for ascorbic acid and 210 nm for other acids. Injected sample volume was 20 μL. The mobile phase was 50 mM phosphate buffer with isocratic elution at a flow rate of 0.7 mL min−1 for the determination of oxalic, malic, ascorbic, and citric acids. Results are expressed as mg of acid per 100 g of fresh weight mandarins.
Statistical analysis
The obtained dataset was analyzed using IBM SPSS Statistics 22 and XLSTAT add-on for Microsoft Office 2016. The data are represented as means with standard deviations obtained from measurements of triplicates (additional triplicate for each replicate was performed, and a total of 9 values were obtained per sample, per relative day, and for each of the respective measurements). A Repeated measure ANOVA (analysis of variance) was used. The significance (p < 0.05) was established using the post hoc t-tests with Bonferroni adjustment. Pearson correlation analysis was also performed using the same statistical package. Relative change (Rc%) compared to the control samples (uncoated) was calculated in terms of eq. [3]:
| (3) |
where a is coated, and b uncoated (control) sample. Multivariate analysis (principal component analysis; PCA) was performed using the XLSTAT statistical software. PCA is based on the correlation matrix among the values of the studied parameters, and it indicates the contribution of each variable, independent of the range of its obtained values. Bartlett’s tests of sphericity and the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy were used before the PCA analysis. Bartlett's tests of sphericity revealed a significant difference in the variances (p < 0.0001). Kaiser-Meyer-Olkin measure gave a value of 0.84 indicating sampling quality adequate (above 0.8 = meritorious).
Results and discussion
Fruit gloss, visual observation over time, and CIE color variables
A layer-by-layer (LbL) method was used to prepare composite polysaccharide coatings for mandarin fruit quality preservation. The application of selected biopolymers and their respective concentrations were based on our preliminary data. Due to the feasibility of selected coatings preparation, and ease of application, this work represents real value to prospective mandarin producers. Herein we have investigated the influence of a single chitosan coating and LbL composite coatings with chitosan as an outer layer. Selected biopolymers that served as an inner coat; sodium-alginate, hydroxypropyl methylcellulose, and locust bean gum were found to easily adhere to the mandarin's surface. No negative sensory quality (i.e. stickiness, peeling off, curling, etc.) was observed for any of the treatments throughout the storage under room temperature and cold storage conditions. Chitosan addition (outer layer) with the initial (inner) coatings (sodium-alginate, hydroxypropyl methylcellulose, and locust bean gum) added enhanced gloss to the mandarin fruits (Fig. S1). This was also previously reported by Arnon et al. (2015), where chitosan-coated mandarin fruits had a higher gloss rate compared to the uncoated ones. Furthermore, they found that chitosan coating reduced the porosity of the layer. In general, chitosan coatings demonstrated superior gloss enhancement but with some drawbacks, like the accumulation of ethanol in the fruit juice. Arnon et al. (2015) also revealed that the addition of an inside coat (i.e., carboxymethyl cellulose) using the LbL method, prevents the accumulation of ethanol in the fruit juice with levels close to the uncoated mandarins. Herein we report that hydroxypropyl methylcellulose/chitosan (HC) coating and single chitosan (C) coat had the most pronounced effect on the fruit gloss, even though all of the investigated treatments were significantly higher evaluated compared to the uncoated mandarins (Fig. S1).
Another drawback to the addition of a single chitosan coating directly to the mandarin peel is that it affects normal gas exchange processes (Md Nor & Ding, 2020). The use of selected biopolymers as internal layers in this work is based on the electrostatic interactions between negatively charged sodium-alginate, hydroxypropyl methylcellulose, and locust bean gum aqueous solutions and positively charged ammonium groups of chitosan dissolved in citric acid. The application of internal layers limits the direct contact of the chitosan with the fruit peel. Arnon et al. (2015) investigated LbL biopolymer coatings' influence on the mandarin fruit quality in terms of firmness, ethanol concentration in juice, weight loss, internal atmosphere analysis, gloss, and sensory evaluations after 10 days of storage at 20 °C and relative humidity of 60–70 % (shelf-life conditions). The authors found a significant influence of carboxymethyl cellulose/chitosan coating on the fruit quality, remarking it as a considerable alternative to the conventional polyethylene-based waxes for mandarin fruits.
Furthermore, considering the visual aspects degradation of fruit was visible for control samples over room temperature storage time (2, 7, and 10 days) but not for treated samples. Also, it is worth noting that the fruit peel glossiness remained constant for coated mandarins, while uncoated showed serious signs of decay on the last day of RT storage (Fig. S2). Throughout the cold storage period (28 days), we observed no visible changes or degradation of fruit. It is important to consider that applied coatings should have either no negative or preferably a positive influence on the sensory aspects of the fruit. In general, it is essential to ensure that all ingredients used for the coating development have little to no impact on the sensory quality of coated fruit in terms of color, gloss, basic tastes (bitterness, sourness, and sweetness), aroma, and firmness since consumers’ first acceptation in rating the fruit quality is the appearance of the fruit. Even though, glossy, bright colors and appealing odor can sometimes make the fruit more attractive to the consumer (da Luz et al., 2018).
CIE color variables
The effect of coatings on peel color during storage at room temperature and cold storage is presented in Table S1-S3. The total color difference (ΔE) of mandarin fruits was higher at room temperature than in cold storage, respectively. Adekunte et al. (2010) classify color differences as very distinct (ΔE greater than 3), distinct (1.5 < ΔE < 3), and small differences (ΔE < 1.5). Thus, it is noticeable that the total color difference was very distinct at each storage period (2, 4, 7, 10, 14, 21, and 28 days) compared to the harvest date (day 0). Most citrus peels are high in pigments such as chlorophylls and carotenoids and their colors change during ripening and senescence. Chlorophyll pigments in citrus peel progressively vanish during maturity, but carotenoid pigments keep increasing. During ripening, sugars such as sucrose accumulate, enhancing the color break of citrus peel and consequently changing the peel color (Won & Min, 2018). Higher storage temperatures accelerate color change. Significantly, the highest values of total color difference were obtained for alginate/chitosan-treated mandarins (36.97 %) compared to control samples at the end of RT storage. The highest values were obtained on the last day of cold storage for HC-coated mandarins (99.17 %) compared to control samples.
Weight loss and fruit firmness
Regarding weight loss (Fig. S3), a significantly lower weight loss was observed for all postharvest treatments (coatings) after 10 days of storage at room temperature. Expectedly, no significant changes were observed during the cold storage (for 28 days), due to the relatively slow gas and water vapor exchange in a high-humidity environment (90 %). Obtained results are following the previously published research where LbL carboxymethyl cellulose/chitosan-coated mandarins with a higher concentration of second chitosan coat (1.5 % w/v) showed lower weight loss after 10 days of storage at 20 °C. In our case, we observed a significant influence of all treatments including the single chitosan coat, and this can partially be due to the use of high molecular weight chitosan in our work. Arnon et al. (2015), used medium molecular weight chitosan with a higher initial chitosan concentration (2 %). Weight loss caused by moisture escape leads to significant economic losses since food products are frequently sold by weight. Employing the use of an edible coating formulation which can reduce their water vapor permeability and inhibit weight loss is of vital importance (Arnon-Rips & Poverenov, 2018).
Firmness (Table S4) was evaluated for all tested samples, under both storage conditions and over time. No statistically significant (posthoc t-tests with Bonferroni adjustment, p < 0.05) was observed either for single chitosan coat or LbL coatings (data not shown). This is contrary to the results of Arnon et al. (2015) where significant influence was observed for LbL coatings. The differences between the studies are possible due to the use of different biopolymer combinations (carboxymethyl cellulose/chitosan) and the use of less rigorous Tukey’s HSD pairwise comparison to test the significance. Based on their report, single chitosan coating also showed no influence on the mandarin fruit firmness, which is following our data. The use of chitosan coat as an effective treatment for fungal decay was previously well-studied on various fruits (Candir et al., 2018), and is important to highlight that the chitosan layer has potent antimicrobial protection against bacteria, yeast, and molds, inhibiting thus fruit degradation, firmness loss, and formation of undesirable off-flavor volatiles. Regarding the fruit firmness, Poverenov et al. (2014) coated melons using the same LbL method, which resulted in a much firmer product than their uncoated or single-component-coated analogs. They also note a remarkable effect of LbL coatings in the improvement of gas exchange and water vapor permeability properties.
Total soluble solids and titratable acidity
Relative to the control samples highest relative change for titratable acidity (TA) can be observed after 10 days of storage at room temperature. A 51.02 % higher TA values were recorded for chitosan-coated fruits (1.48 mg CA/100 g f.w.) compared to the control samples (0.98 mg CA/100 g f.w.). Furthermore, after the 28 days of cold storage, an increase of 69.88 % TA content was recorded for chitosan-coated fruits (1.41 mg CA/100 g f.w.) compared to the control samples (0.83 mg CA/100 g f.w.). Event hough the relative change is high no significant influence of any investigated postharvest treatments for both storage types and relative times was observed for the mandarin fruits in terms of total soluble solids (TSS) (Table S4) and titratable acidity (TA) (Fig. S4) according to the post hoc t-test with Bonferroni adjustment (p < 0.05).
This is also following the existing literature where two types of mandarins, oranges, and grapefruits were investigated as potential citrus fruits with edible coatings (Arnon et al., 2014). LbL coatings and commercial wax coating had no significant effects on the total soluble solids and acidity levels of the mandarin fruit juice (Arnon-Rips & Poverenov, 2018). The same conclusion was recorded in the strawberry studies where both chitosan single-coat and LbL coatings had little to no effect on the total soluble solids and total acids after eight days of storage (Yan et al., 2019) at 0 °C.
Determination of total polyphenolic content, total flavonoids, and antioxidant activity
To the best of our knowledge and literature survey, there is no data on bioactive compound changes in the mandarin fruit treated with biopolymeric coatings. Relative to the control samples under both storage types, the room temperature, and cold storage, throughout the whole period of storage, we can observe a significant influence of both single chitosan coating and LbL coatings on total polyphenols (Table 1) and total flavonoids (Table 2) retention and synthesis. Remarkable results are following single chitosan and specifically, HC LbL coatings. An increase of 50.04 % in total polyphenols was found with chitosan-coated mandarins after 10 days of room temperature storage, relative to the control sample. A high relative increase (55.69 %) compared to the uncoated mandarins was observed during the cold storage (after 28 days) for HC-coated ones. It is important to note that the increase (C for RT; HC for CS) is also significantly higher than the other treatments respective to the storage conditions. Results of TPC are in correlation () with the results of total flavonoid (TF) content where again similar results can be observed. An increase of 45.83 % in TF for chitosan-coated after 10 days of RT and an increase of 56.43 % in TF for HC-coated mandarins after 28 days in CS were found. Previous research has shown that phytochemical biosynthesis is influenced by internal and external stress stimuli (Hanhineva, 2011). These phytochemicals accumulated when plants are exposed to harmful environmental conditions, like low temperatures and UV radiation (Severo et al., 2015). The single chitosan coating and LbL coatings reduced the variation of phytochemical metabolites, indicating that observed coatings can offer protection to fruits from exposure to environmental stress stimuli. According to other research, concerning the volatile bioactive compounds, chitosan and LbL coatings for strawberry fruits resulted in a delay of volatiles decomposition and the maintenance of characteristic volatiles after storage of strawberries for 8 days at 0 °C (Yan et al., 2019). Authors also report from the metabolomic perspective that chitosan coating may aggravate the exogenous environmental stress stimuli, as it promoted flavonoid biosynthesis. They indicate that chitosan coating was able to maintain better fruit quality during cold storage. Not only did the LbL coating decrease the resistance to environmental stress stimuli from the exogenous coating, but it also slowed down the primary and secondary metabolism as well as delayed the strawberry senescence during storage. According to other research, flavonoid content would first increase during postharvest storage, to deal with the sudden change in the environment, and then decrease with an enormous consumption of flavonoid compounds (Petriccione et al., 2015). Even though LbL coatings could delay the decline of most flavonoid derivatives in the present research, chitosan coating was able to promote the biosynthesis of flavonoids. This could be a response to the stress of chitosan acid solution being applied directly on the surface of the mandarin fruit, which caused the fruit to induce the biosynthesis of flavonoids in response to this environmental change (acidic environment), hence the relatively higher flavonoid values after RT storage. To alleviate possible future problems regarding this, the inner layer of the LbL coating may serve to moderate the stress of the chitosan acid solution, but this needs a more detailed approach (metabolomics aspect). When comparing single chitosan coating with AC or GC LbL coatings we can observe that the composition of the LbL polyelectrolyte complex does influence fruit metabolism. For e.g., total flavonoid change (at RT) over time was significantly different when comparing single chitosan and AC or GC whereas the HC combination proved favorable. This is why it is essential to investigate how different combinations of LbL influence the synthesis of specific compounds over storage time under specific conditions (in this case only at RT storage). As mentioned above, this might be explained by the fact that chitosan coating may stimulate and promote flavonoid biosynthesis. In the case of LbL complexes reported herein, chitosan is an outer coating, which might limit its ability to interact with the tissue surface and stimulate flavonoid synthesis. Even though, HC LbL coating showed a similar response as single chitosan coating. This is important to take into account for future studies where the type of polyelectrolyte complex (with chitosan, a polycationic compound) might play a crucial role to stimulate the specific compound synthesis of treated fruits.
Table 1.
Total polyphenolic content (TPC) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins during two storage conditions (room temperature (RT) and cold storage (CS)) over time.
| TPC / mg g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Room Temperature | |||||||||
| Treatment | Day 2 | Day 4 | Day 7 | Day 10 | Means | ||||
| Ctrl | 1.24 ± 0.14a | *Rc% | 1.11 ± 0.09a | *Rc% | 1.17 ± 0.15a | *Rc% | 1.15 ± 0.06a | *Rc% | 1.17a |
| C | 1.54 ± 0.09b | 24.27 | 1.63 ± 0.26b | 46.65 | 1.64 ± 0.02b | 40.56 | 1.72 ± 0.11b | 50.04 | 1.63b |
| AC | 1.48 ± 0.18bc | 19.40 | 1.27 ± 0.07c | 14.54 | 1.27 ± 0.04a | 8.79 | 1.43 ± 0.19c | 24.31 | 1.36ab |
| HC | 1.67 ± 0.12b | 34.19 | 1.57 ± 0.09b | 41.50 | 1.55 ± 0.02c | 32.65 | 1.47 ± 0.05c | 27.66 | 1.57b |
| GC | 1.38 ± 0.11ac | 11.36 | 1.24 ± 0.08c | 12.09 | 1.46 ± 0.12c | 25.46 | 1.49 ± 0.04c | 30.04 | 1.39ab |
| Cold Storage | |||||||||
| Treatment | Day 7 | Day 14 | Day 21 | Day 28 | Means | ||||
| Ctrl | 1.13 ± 0.06a | *Rc% | 1.12 ± 0.13a | *Rc% | 1.11 ± 0.12a | *Rc% | 1.23 ± 0.10a | *Rc% | 1.15a |
| C | 1.42 ± 0.17bc | 25.55 | 1.50 ± 0.06b | 33.56 | 1.53 ± 0.16b | 37.13 | 1.58 ± 0.03b | 28.83 | 1.51b |
| AC | 1.40 ± 0.10bc | 23.86 | 1.37 ± 0.05c | 22.32 | 1.60 ± 0.15bc | 43.38 | 1.73 ± 0.09c | 40.96 | 1.53ab |
| HC | 1.50 ± 0.07b | 32.74 | 1.77 ± 0.15d | 57.82 | 1.78 ± 0.07d | 59.83 | 1.91 ± 0.08d | 55.69 | 1.74b |
| GC | 1.28 ± 0.11c | 13.68 | 1.38 ± 0.08c | 22.76 | 1.67 ± 0.06c | 52.13 | 1.66 ± 0.08c | 35.15 | 1.50ab |
Values superscripted with the same letter within a column (and according to the relative storage type) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
Table 2.
Total flavonoids (TF) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins during two storage conditions (room temperature and cold storage) over time.
| TF / mg g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Room Temperature | |||||||||
| Treatment | Day 2 | Day 4 | Day 7 | Day 10 | Means | ||||
| Ctrl | 1.06 ± 0.09a | *Rc% | 0.97 ± 0.06a | *Rc% | 0.98 ± 0.10a | *Rc% | 0.92 ± 0.05a | *Rc% | 0.98a |
| C | 1.18 ± 0.06b | 11.94 | 1.27 ± 0.21b | 30.72 | 1.41 ± 0.05b | 44.62 | 1.34 ± 0.09b | 45.83 | 1.30b |
| AC | 1.29 ± 0.11b | 21.72 | 1.06 ± 0.09a | 8.82 | 1.04 ± 0.01a | 6.44 | 1.16 ± 0.19c | 25.94 | 1.14a |
| HC | 1.45 ± 0.09c | 37.31 | 1.22 ± 0.15b | 26.28 | 1.42 ± 0.04b | 45.42 | 1.22 ± 0.05b | 32.02 | 1.33b |
| GC | 1.10 ± 0.10ab | 4.22 | 0.95 ± 0.05a | −2.10 | 1.23 ± 0.13c | 26.19 | 1.12 ± 0.02c | 22.18 | 1.10a |
| Cold Storage | |||||||||
| Treatment | Day 7 | Day 14 | Day 21 | Day 28 | Means | ||||
| Ctrl | 0.91 ± 0.06a | *Rc% | 1.01 ± 0.05a | *Rc% | 1.12 ± 0.08a | *Rc% | 0.98 ± 0.07a | *Rc% | 1.01a |
| C | 1.23 ± 0.20b | 36.16 | 1.18 ± 0.04a | 16.90 | 1.20 ± 0.14ab | 6.84 | 1.19 ± 0.07b | 20.41 | 1.20b |
| AC | 1.07 ± 0.06c | 18.09 | 1.09 ± 0.09a | 7.87 | 1.29 ± 0.11c | 14.39 | 1.37 ± 0.08c | 38.98 | 1.21ab |
| HC | 1.24 ± 0.08b | 37.17 | 1.48 ± 0.13b | 47.05 | 1.51 ± 0.04d | 34.58 | 1.54 ± 0.08b | 56.43 | 1.44b |
| GC | 0.97 ± 0.12c | 6.63 | 1.09 ± 0.11a | 8.45 | 1.37 ± 0.09c | 21.70 | 1.33 ± 0.05c | 35.21 | 1.19ab |
Values superscripted with the same letter within a column (and according to the relative storage type) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
Furthermore, when comparing biopolymeric coatings with modified atmosphere packaging, Candir et al. (2018) reported that a single chitosan coating on the pomegranate fruit was the most effective treatment provided with higher total polyphenolic content and antioxidant capacity and a more intense aril color of the fruit. Another study reported the application of alginate-chitosan films on figs. Coated and uncoated figs were stored at 6 °C and 95 % of relative humidity for 15 days. Authors report that coated figs had promoted controlled synthesis of polyphenol compounds ensuring better retention and increase in some of these compounds. Furthermore, it was reported that during the storage period the antioxidant capacity and chlorogenic acid content of coated figs were preserved (Reyes-Avalos et al., 2019). Antioxidant capacity (Table 3 and Table 4) determined with two methods (ABTS and DPPH) for mandarin fruits is in correlation with results of TPC and TF (; ; ; ). The highest and significantly different from control and LbL coatings (except for HC coating) antioxidant capacity were observed for chitosan-coated mandarins after 10 days of RT storage (47.99 % and 43.34 % higher compared to the control samples, respectively). At the end of the CS storage, i.e., day 28, the highest values and significantly different antioxidant activity were measured for HC-coated mandarin samples (81.95 % and 99.66 % higher compared to the control samples, respectively). Throughout the whole period of storage (both conditions) we can observe significantly higher values when comparing them to the control samples. Similar data were reported on strawberry fruits after postharvest chitosan coating addition. The authors recorded that the changes in the total polyphenol, anthocyanin, and flavonoid contents and the antioxidant capacity (measured with the DPPH method) of chitosan-coated strawberry fruits were significantly delayed. Additionally, chitosan coating enhanced the activity of some antioxidant enzymes, preventing flesh browning and reducing membrane damage (Petriccione et al., 2015). Some studies suggest that postharvest application of external elicitors, such as chitosan, to a vegetative tissue, can trigger plant resistance. Chitosan activates the key enzyme in the phenol synthesis pathway, such as phenylalanine ammonia-lyase, which increases total polyphenols and influences the antioxidant capacity of fruits due to their high antioxidant potential (Romanazzi et al., 2017).
Table 3.
Antioxidant activity (DPPH method) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins during two storage conditions (room temperature and cold storage) over time.
| DPPH / mmol (100 g)−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Room Temperature | |||||||||
| Treatment | Day 2 | Day 4 | Day 7 | Day 10 | Means | ||||
| Ctrl | 0.33 ± 0.03a | *Rc% | 0.31 ± 0.06a | *Rc% | 0.28 ± 0.06a | *Rc% | 0.32 ± 0.02a | *Rc% | 0.31a |
| C | 0.40 ± 0.02b | 22.15 | 0.44 ± 0.08bc | 42.28 | 0.40 ± 0.02b | 41.97 | 0.48 ± 0.02b | 47.99 | 0.43b |
| AC | 0.43 ± 0.06bc | 31.25 | 0.35 ± 0.02ac | 12.26 | 0.32 ± 0.03c | 11.97 | 0.40 ± 0.04c | 25.08 | 0.38ab |
| HC | 0.48 ± 0.04c | 44.81 | 0.37 ± 0.04 cd | 18.24 | 0.40 ± 0.01b | 40.56 | 0.39 ± 0.01c | 21.77 | 0.41b |
| GC | 0.39 ± 0.05b | 19.60 | 0.33 ± 0.01ad | 6.40 | 0.35 ± 0.06c | 24.29 | 0.41 ± 0.02c | 26.09 | 0.37ab |
| Cold Storage | |||||||||
| Treatment | Day 7 | Day 14 | Day 21 | Day 28 | |||||
| Ctrl | 0.29 ± 0.03a | *Rc% | 0.35 ± 0.06a | *Rc% | 0.33 ± 0.02a | *Rc% | 0.33 ± 0.02a | *Rc% | 0.33a |
| C | 0.39 ± 0.06bc | 33.51 | 0.42 ± 0.03bc | 19.36 | 0.44 ± 0.02b | 32.11 | 0.45 ± 0.02b | 36.67 | 0.43b |
| AC | 0.38 ± 0.03bc | 30.83 | 0.39 ± 0.03ab | 12.36 | 0.44 ± 0.04b | 33.18 | 0.52 ± 0.05c | 57.89 | 0.43ab |
| HC | 0.40 ± 0.03b | 35.92 | 0.44 ± 0.04c | 26.58 | 0.44 ± 0.05b | 31.86 | 0.60 ± 0.03d | 81.95 | 0.47b |
| GC | 0.35 ± 0.03c | 19.19 | 0.36 ± 0.03a | 2.86 | 0.43 ± 0.02b | 29.45 | 0.54 ± 0.03c | 63.93 | 0.42ab |
Values superscripted with the same letter within a column (and according to the relative storage type) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
Table 4.
Antioxidant activity (ABTS method) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins during two storage conditions (room temperature and cold storage) over time.
| ABTS/ mmol (100 g)−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Room Temperature | |||||||||
| Treatment | Day 2 | Day 4 | Day 7 | Day 10 | Means | ||||
| Ctrl | 0.57 ± 0.09a | *Rc% | 0.61 ± 0.07a | *Rc% | 0.57 ± 0.09a | *Rc% | 0.62 ± 0.07a | *Rc% | 0.59a |
| C | 0.74 ± 0.05b | 29.19 | 0.90 ± 0.13b | 48.22 | 0.79 ± 0.09b | 38.97 | 0.89 ± 0.05b | 43.34 | 0.83b |
| AC | 0.70 ± 0.10b | 21.64 | 0.73 ± 0.03c | 21.01 | 0.64 ± 0.03c | 13.02 | 0.75 ± 0.10c | 20.28 | 0.71ab |
| HC | 0.81 ± 0.09c | 41.55 | 0.77 ± 0.04c | 26.47 | 0.93 ± 0.01d | 63.42 | 0.74 ± 0.02c | 18.68 | 0.81b |
| GC | 0.97 ± 0.52d | 68.20 | 0.62 ± 0.05a | 3.09 | 0.79 ± 0.08b | 39.36 | 0.82 ± 0.05d | 31.78 | 0.80b |
| Cold Storage | |||||||||
| Treatment | Day 7 | Day 14 | Day 21 | Day 28 | Means | ||||
| Ctrl | 0.57 ± 0.06a | *Rc% | 0.58 ± 0.10a | *Rc% | 0.58 ± 0.03a | *Rc% | 0.46 ± 0.05a | *Rc% | 0.55a |
| C | 0.72 ± 0.10b | 26.95 | 0.72 ± 0.05b | 25.82 | 0.71 ± 0.07b | 22.09 | 0.65 ± 0.09b | 42.05 | 0.70b |
| AC | 0.73 ± 0.05b | 28.69 | 0.68 ± 0.04b | 18.32 | 0.76 ± 0.10b | 30.16 | 0.77 ± 0.07c | 69.14 | 0.74bc |
| HC | 0.82 ± 0.07c | 44.78 | 0.81 ± 0.04c | 40.36 | 0.83 ± 0.14c | 42.39 | 0.91 ± 0.10d | 99.66 | 0.84c |
| GC | 0.71 ± 0.06b | 24.23 | 0.71 ± 0.04b | 24.04 | 0.85 ± 0.03c | 46.12 | 0.87 ± 0.11d | 91.36 | 0.79bc |
Values superscripted with the same letter within a column (and according to the relative storage type) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
LbL coatings have a great potential to extend shelf-life and maintain the postharvest quality of fruits. The application of biopolymeric coatings can have a positive influence by decreasing the primary metabolism resulting from environmental stress. Obtained results are also consistent with other research and supported by the ability of edible coatings to lower the respiration rate in strawberries, as the coatings offer a micro gas atmosphere modification to inhibit energy metabolism (Yan et al., 2019).
Determination of organic acids
Although there were no significant changes in total soluble solids (TSS) or titratable acidity (TA), there were significant changes in organic acid composition determined by HPLC. This is very important to highlight, because, to the best of our knowledge, the influence of edible coatings on the individual organic acid content of fruits during storage has never been studied. Furthermore, TA measures the total acid concentration in a sample, and it is important to consider that its determination in a highly acid fruit cannot be sufficiently accurate to study any subtle changes in acid composition throughout the storage period. Thus, the profile of organic acids (oxalic, malic, ascorbic, and citric) in mandarin fruits was determined during all postharvest treatments (coatings) at room temperature for 10 days (Table 5) and cold storage for 28 days (Table 6) using HPLC. Citric and malic acids were the most abundant in all treatments. At room temperature storage, citric, malic, and oxalic acid levels decreased first and then increased. During room temperature storage, chitosan-coated mandarins increased 36.47 % for malic and 45.60 % for citric acid relative to the control. The oxalic acid revealed the same trend for chitosan-coated mandarins, with 68.31 % higher content compared to control samples. On the last day of cold storage, LbL-coated mandarins showed 114.72 % (sodium alginate/chitosan) and 102.36 % (hydroxypropyl methylcellulose/chitosan) higher content of malic acid than control samples. Also, citric acid levels were 79.85 % (AC) and 83.26 % (HC) higher than in control samples. Citric and malic acids are the primary acids found in citrus fruits and their levels decrease during the ripening and storage period. There is a relationship between postharvest treatments and organic acid metabolism before cold storage. Zhou et al. (Zhou et al., 2019) demonstrated that UV-treated peaches stored at 1 °C were characterized by a down-regulation of aconitase and NADP-malic enzyme activities and gene expression levels, but higher levels of citrate synthase and NAD-malate dehydrogenase, resulting in reduced degradation of citric and malic acids. Pre-cold storage hot air treatment (40 °C, 48 h) in ponkan orange accelerated citric acid degradation, attributed to regulation by ATP citrate lyase (ACL) and γ-aminobutyric acid (GABA) pathways (Gao et al., 2018). A combination of LbL coatings and cold storage in this research increased the content of organic acids by more than 100 % concerning control samples. Ascorbic acid is the most abundant antioxidant present in plant cells and plays important role in several processes, including defense mechanisms, photosynthesis, cell division, growth regulation, and senescence (Alós et al., 2014). Ascorbic acid showed the same trend as other organic acids in this research. At room temperature storage, chitosan-coated mandarins had a significantly higher ascorbic acid content (59.43 %) than control fruits. These results are in comparison with Candir et al. (2018) who reported a single chitosan coating on the pomegranate fruit as an effective treatment that provided a more intense aril color of fruit with higher ascorbic acid content. At the end of cold storage (after 28 days) higher content of ascorbic acid was found in LbL-coated mandarins. The overall best results were for HC and GC treatments. HC treatment had the highest levels of ascorbic acid 103.46 % compared to control samples followed by GC (91.96 %) and AC treatments (67.01 %), respectively. Obtained results are comparable to Gol et al. (Gol et al., 2015) who used one layered coating in combination with 1 % hydroxypropyl methylcellulose and 1 % chitosan on strawberry fruit and stored at 11 °C maintaining the ascorbic acid at higher levels during the entire storage period. The combination of cold storage and LbL coatings lowered the activity of the enzymes and prevented the oxidation of ascorbic acid. All coated fruit (under RT and CS) had higher organic acid content than the control fruit in this research. Edible coatings provide a controlled environment with low oxygen stress, resulting in decreased enzyme activity and oxidation.
Table 5.
Organic acids (mg (100 g)−1) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins stored at room temperature over time.
| Room Temperature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Day 2 | Day 4 | Day 7 | Day 10 | Means | |||||
| Ctrl | Oxalic acid | 9.94 ± 0.84a | *Rc% | 9.22 ± 1.16a | *Rc% | 11.56 ± 1.95a | *Rc% | 10.35 ± 0.61a | *Rc% | 10.27a |
| C | 16.55 ± 1.47b | 66.60 | 15.05 ± 1.48b | 63.29 | 15.90 ± 1.27bc | 37.47 | 17.42 ± 0.93b | 68.31 | 16.23b | |
| AC | 13.83 ± 2.01c | 39.19 | 10.96 ± 0.64a | 18.86 | 10.92 ± 0.95a | −5.53 | 13.82 ± 1.55c | 33.53 | 12.38ac | |
| HC | 15.73 ± 1.53bc | 58.27 | 13.90 ± 1.71b | 50.75 | 14.04 ± 0.82c | 21.43 | 15.72 ± 0.49b | 51.88 | 14.85bc | |
| GC | 12.72 ± 1.69a | 28.06 | 12.05 ± 1.07b | 30.68 | 12.59 ± 1.44a | 8.90 | 14.50 ± 1.57c | 40.10 | 12.97c | |
| Ctrl | Malic acid | 75.07 ± 12.30a | *Rc% | 46.06 ± 7.87a | *Rc% | 46.64 ± 8.03ab | *Rc% | 65.31 ± 6.86a | *Rc% | 58.27a |
| C | 61.19 ± 10.32a | −18.49 | 64.19 ± 8.60a | 39.36 | 55.15 ± 1.79ab | 18.26 | 89.13 ± 8.69a | 36.47 | 67.42a | |
| AC | 55.74 ± 16.67a | −25.75 | 56.58 ± 6.78a | 22.84 | 51.10 ± 3.99ab | 9.57 | 70.82 ± 8.79a | 8.44 | 58.56a | |
| HC | 74.68 ± 2.38a | −0.52 | 62.15 ± 9.32a | 34.94 | 68.31 ± 10.43a | 46.47 | 71.41 ± 5.36a | 9.34 | 69.14a | |
| GC | 70.45 ± 8.04a | −6.16 | 51.04 ± 2.94a | 10.82 | 45.64 ± 2.60b | −2.14 | 76.50 ± 3.92a | 17.13 | 60.91a | |
| Ctrl | Ascorbic acid | 45.60 ± 4.53a | *Rc% | 36.57 ± 2.77a | *Rc% | 39.06 ± 8.51a | *Rc% | 39.46 ± 2.62a | *Rc% | 40.17a |
| C | 55.14 ± 3.91ab | 20.92 | 53.77 ± 7.30b | 47.05 | 56.60 ± 2.38b | 44.88 | 62.91 ± 2.62b | 59.43 | 57.11b | |
| AC | 55.55 ± 8.09ab | 21.83 | 43.93 ± 1.12b | 20.15 | 42.51 ± 3.45ac | 8.81 | 43.53 ± 9.13a | 10.31 | 46.38ab | |
| HC | 62.51 ± 4.09b | 37.10 | 53.18 ± 3.98b | 45.43 | 52.89 ± 1.46ab | 35.41 | 50.35 ± 1.54c | 27.60 | 54.73b | |
| GC | 54.58 ± 9.87ab | 19.71 | 44.35 ± 4.90ab | 21.28 | 52.31 ± 5.05ab | 33.90 | 50.99 ± 6.15c | 29.22 | 50.56ab | |
| Ctrl | Citric acid | 1216.25 ± 123.28a | *Rc% | 866.84 ± 100.93a | *Rc% | 1176.91 ± 249.83a | *Rc% | 1165.23 ± 28.12a | *Rc% | 1106.31a |
| C | 1395.65 ± 178.81ab | 14.75 | 1165.55 ± 264.08ab | 34.46 | 1529.01 ± 40.13a | 29.92 | 1696.57 ± 89.30bc | 45.60 | 1446.70ab | |
| AC | 1709.28 ± 376.73ab | 40.54 | 1078.63 ± 102.57ab | 24.43 | 1424.97 ± 172.18a | 21.08 | 1526.78 ± 287.59ac | 31.03 | 1434.92ab | |
| HC | 1657.04 ± 89.25b | 36.24 | 1217.27 ± 118.98b | 40.43 | 1520.75 ± 150.55a | 29.22 | 1513.81 ± 116.34bc | 29.92 | 1477.22b | |
| GC | 1485.07 ± 225.32ab | 22.10 | 827.92 ± 116.10a | −4.49 | 1420.51 ± 161.58a | 20.70 | 1324.41 ± 80.75ac | 13.66 | 1264.48ab | |
Values superscripted with the same letter within a column (and observed acid) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
Table 6.
Organic acids (mg (100 g)−1) determined in uncoated (control – Ctrl), single coated with chitosan (C), and LbL coated with sodium alginate/chitosan (AC), hydroxypropyl methylcellulose/chitosan (HC), and locust bean gum/chitosan (GC) mandarins stored in cold storage over time.
| Cold Storage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Day 7 | Day 14 | Day 21 | Day 28 | Means | |||||
| Ctrl | Oxalic acid | 11.31 ± 1.30a | *Rc% | 11.26 ± 1.24a | *Rc% | 10.31 ± 1.41a | *Rc% | 8.09 ± 0.60a | *Rc% | 10.24a |
| C | 14.75 ± 2.36a | 30.42 | 15.83 ± 0.91b | 40.58 | 16.85 ± 1.39b | 63.39 | 10.11 ± 0.89a | 25.00 | 14.39ab | |
| AC | 13.55 ± 2.65a | 19.81 | 14.11 ± 0.25b | 25.35 | 18.06 ± 2.09b | 75.15 | 11.31 ± 0.90bc | 39.72 | 14.26ab | |
| HC | 15.49 ± 1.07b | 36.96 | 17.25 ± 1.70b | 53.24 | 20.34 ± 1.69b | 97.20 | 13.74 ± 0.76c | 69.85 | 16.71b | |
| GC | 14.04 ± 0.83a | 24.14 | 13.29 ± 1.85a | 18.04 | 18.22 ± 1.09b | 76.63 | 11.39 ± 1.16bc | 40.78 | 14.24ab | |
| Ctrl | Malic acid | 66.89 ± 1.95a | *Rc% | 60.11 ± 3.98a | *Rc% | 34.89 ± 6.65a | *Rc% | 27.14 ± 3.62a | *Rc% | 47.26a |
| C | 80.45 ± 11.60a | 20.27 | 91.14 ± 5.60b | 51.61 | 55.41 ± 7.05b | 58.84 | 35.45 ± 3.44a | 30.60 | 65.61ab | |
| AC | 75.02 ± 11.02a | 12.15 | 82.34 ± 6.91b | 36.97 | 78.22 ± 8.06c | 124.22 | 58.28 ± 15.08b | 114.72 | 73.47b | |
| HC | 79.57 ± 5.13b | 18.96 | 75.16 ± 9.03a | 25.02 | 69.69 ± 12.12bc | 99.75 | 54.93 ± 2.51b | 102.36 | 69.84ab | |
| GC | 87.34 ± 12.28a | 30.57 | 81.87 ± 7.69b | 36.19 | 74.59 ± 6.39bc | 113.79 | 46.38 ± 3.29b | 70.87 | 72.55a | |
| Ctrl | Ascorbic acid | 38.03 ± 2.03a | *Rc% | 39.34 ± 6.50a | *Rc% | 33.97 ± 3.75a | *Rc% | 30.47 ± 1.85a | *Rc% | 35.45a |
| C | 49.90 ± 8.06ab | 31.21 | 53.55 ± 2.27b | 36.12 | 56.14 ± 3.43b | 65.25 | 48.18 ± 2.53b | 58.11 | 51.94b | |
| AC | 45.41 ± 11.24ab | 19.41 | 47.17 ± 3.93ab | 19.92 | 54.13 ± 7.72b | 59.33 | 50.89 ± 4.48c | 67.01 | 49.40b | |
| HC | 55.26 ± 4.64b | 45.31 | 52.81 ± 3.27b | 34.24 | 56.25 ± 6.51b | 65.56 | 62.00 ± 3.17d | 103.46 | 56.58b | |
| GC | 46.90 ± 5.52ab | 23.32 | 39.58 ± 3.96a | 0.61 | 56.55 ± 1.77b | 66.44 | 58.50 ± 4.88 cd | 91.96 | 50.38ab | |
| Ctrl | Citric acid | 1135.38 ± 178.48a | *Rc% | 1196.95 ± 136.88a | *Rc% | 1105.90 ± 193.46a | *Rc% | 987.70 ± 144.65a | *Rc% | 1106.48a |
| C | 1346.96 ± 63.15a | 18.64 | 1559.15 ± 85.46b | 30.26 | 1607.30 ± 132.07b | 45.34 | 1437.73 ± 83.67b | 45.56 | 1487.79b | |
| AC | 1295.34 ± 238.27a | 14.09 | 1447.64 ± 50.17a | 20.94 | 1719.38 ± 157.33b | 55.47 | 1776.41 ± 275.14bc | 79.85 | 1559.69b | |
| HC | 1466.61 ± 47.57a | 29.17 | 1712.28 ± 98.00b | 43.05 | 1551.73 ± 123.39b | 40.31 | 1810.07 ± 51.32c | 83.26 | 1635.17b | |
| GC | 1362.09 ± 47.09a | 19.97 | 1205.62 ± 117.06a | 0.72 | 1544.30 ± 60.74b | 39.64 | 1562.31 ± 112.71b | 58.18 | 1418.58a | |
Values superscripted with the same letter within a column (and observed acid) are not significantly different according to the post hoc t-test with Bonferroni adjustment (p < 0.05). *Rc% represents a relative change (%) relative to the control sample.
Principal component analysis
Principal component analysis (PCA) was used to indicate multivariate dependence between the selected variables. The weighting of the results was made for bioactive compounds (total polyphenols and total flavonoids), antioxidant activity (determined via two methods – ABTS and DPPH), and organic acids (oxalic, malic, ascorbic, and citric acids). For Bartlett’s Sphericity test, the risk for the rejection of the null hypothesis H0, while it was true, was < 0.01 %. Alpha was set to 0.05, and the p-value was < 0.0001. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy gave a value of 0.84 indicating high sampling quality adequacy (Table S5). High factor loading scores mean a tighter association with the same principal component. PCA revealed the two significant components which explain altogether 80.22 % of the total variance between the studied variables (Fig. S5). Factor 1 (F1) describes 67.24 % of the total variance and was tightly associated with the total polyphenolic content, total flavonoids, antioxidant activity (ABTS and DPPH), ascorbic and citric acids, whereas Factor 2 (F2) was associated with oxalic and malic acid as well as titratable acidity with 12.97 % of the total variance. Fig. S5 presents the distribution of the data for F1 and F2 and the correlation between the measured variables. From the PCA biplot, in the left two quadrants, a grouping of control samples (at all investigated storage times and types) can be observed in the left top and bottom quadrants. These samples measured the lowest values in bioactive compounds, antioxidant activity, and organic acids, over time. On the other hand, it can be observed that in the right two quadrants of the biplot, samples with the relative highest values are positioned, and these are values from mandarins coated with hydroxypropyl methylcellulose/chitosan LbL and single chitosan coating. Mandarins coated with locust bean gum/chitosan LbL showed a relatively lower influence on the juice bioactive compounds concentration, and antioxidant activity but still significantly higher (p < 0.05) respectively than the control samples.
Conclusions and prospects
All of the treatments revealed a significant influence on the mandarine fruit quality during storage under room temperature (up to 10 days) and cold storage (up to 28 days). Because of the sensitivity of mandarine fruits in terms of storage and bioactive compounds degradation we can conclude that the best performance was observed either with hydroxypropyl methylcellulose layer-by-layer coating or single chitosan coating. Fruit gloss was rated highest for hydroxypropyl methylcellulose/chitosan-coated mandarines, with remarkable influence on the preservation of bioactive compounds and organic acids in fruits. The application of edible coatings is simple and can be relatively easily applied during the postharvest period. Fruits can be dipped in the primary coat, while the second coat can be added through the next line of dipping. Selected combinations of biopolymers proved promising and future research should upgrade on this and focus on the inclusion of various bioactive compounds into the coatings. The results of this research are remarkable and can lead to the development of sustainable fruit preservation using simple environmentally friendly technology and economically very affordable green materials such as selected biopolymers.
Funding
The authors would like to acknowledge the financial support of the Croatian Science Foundation through a project entitled Mandarins from Neretva valley – Chemical characterization and Innovative postharvest TREAtments (CITREA) (UIP-2020-02-7496).
CRediT authorship contribution statement
Slaven Jurić: Conceptualization, Methodology, Writing – original draft. Marija Sigurnjak Bureš: Formal analysis, Investigation. Kristina Vlahoviček-Kahlina: Formal analysis, Investigation. Katarina Sopko Stracenski: Formal analysis. Goran Fruk: Methodology. Nenad Jalšenjak: Methodology. Luna Maslov Bandić: Conceptualization, Writing – original draft, Methodology.
Declaration of Competing Interest
The authors declare no financial interests/personal relationships which may be considered as potential competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100575.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Alós E., Rodrigo M.J., Zacarías L. Differential transcriptional regulation of l-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta. 2014;239(5):1113–1128. doi: 10.1007/s00425-014-2044-z. [DOI] [PubMed] [Google Scholar]
- Arnon-Rips H., Poverenov E. Vol. 75. Elsevier Ltd.; 2018. Improving food products’ quality and storability by using Layer by Layer edible coatings; pp. 81–92. (Trends in Food Science and Technology). [DOI] [Google Scholar]
- Arnon H., Granit R., Porat R., Poverenov E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chemistry. 2015;166:465–472. doi: 10.1016/j.foodchem.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Arnon H., Zaitsev Y., Porat R., Poverenov E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biology and Technology. 2014;87:21–26. doi: 10.1016/J.POSTHARVBIO.2013.08.007. [DOI] [Google Scholar]
- Azinfar A., Neuber S., Vancova M., Sterba J., Stranak V., Helm C.A. Self-patterning polyelectrolyte multilayer films: Influence of deposition steps and drying in a vacuum. Langmuir. 2021;37(35):10490–10498. doi: 10.1021/acs.langmuir.1c01409. [DOI] [PubMed] [Google Scholar]
- Belščak-Cvitanović A., Jurić S., Đorđević V., Barišić L., Komes D., Ježek D.…Nedović V. Chemometric evaluation of binary mixtures of alginate and polysaccharide biopolymers as carriers for microencapsulation of green tea polyphenols. International Journal of Food Properties. 2017;20(9):1971–1986. doi: 10.1080/10942912.2016.1225762. [DOI] [Google Scholar]
- Bill M., Sivakumar D., Korsten L., Thompson A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Protection. 2014;64:159–167. doi: 10.1016/J.CROPRO.2014.06.015. [DOI] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Candir E., Ozdemir A.E., Aksoy M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Scientia Horticulturae. 2018;235:235–243. doi: 10.1016/J.SCIENTA.2018.03.017. [DOI] [Google Scholar]
- da Luz L.N., Vettorazzi J.C.F., Santa-Catarina R., Barros F.R., Barros G.B.A., Pereira M.G., Cardoso D.L. Sensory acceptance and qualitative analysis of fruits in papaya hybrids. Anais Da Academia Brasileira de Ciencias. 2018;90(4):3693–3703. doi: 10.1590/0001-3765201820170111. [DOI] [PubMed] [Google Scholar]
- Dhall, R. K. (2013). Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. In Critical Reviews in Food Science and Nutrition (Vol. 53, Issue 5, pp. 435–450). https://doi.org/10.1080/10408398.2010.541568. [DOI] [PubMed]
- FAO. (2011). Global Food Losses and Food Waste – Extent, Causes and Prevention.
- Fathi M., Vinceković M., Jurić S., Viskić M., Režek Jambrak A., Donsì F. Food-grade colloidal systems for the delivery of essential oils. Food Reviews International. 2021;37(1):1–45. doi: 10.1080/87559129.2019.1687514. [DOI] [Google Scholar]
- Gao Y., Kan C., Chen M., Chen C., Chen Y., Fu Y.…Chen J. Effects of chitosan-based coatings enriched with cinnamaldehyde on mandarin fruit cv. Ponkan during room-temperature storage. Coatings. 2018;8(10) doi: 10.3390/COATINGS8100372. [DOI] [Google Scholar]
- Gol N.B., Chaudhari M.L., Rao T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. Journal of Food Science and Technology. 2015;52(1):78–91. doi: 10.1007/s13197-013-0988-9. [DOI] [Google Scholar]
- Hanhineva K. Recent advances in strawberry metabolomics. Genes, Genomes and Genomics. 2011;5:65–75. http://www.weizmann.ac.il/plants/aharoni/PDFs/b1.pdf [Google Scholar]
- Ivanova V., Stefova M., Chinnici F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. Journal of the Serbian Chemical Society. 2010;75(1):45–59. doi: 10.2298/JSC1001045I. [DOI] [Google Scholar]
- Jatoi M.A., Jurić S., Vidrih R., Vinceković M., Vuković M., Jemrić T. The effects of postharvest application of lecithin to improve storage potential and quality of fresh goji (Lycium barbarum L.) berries. Food Chemistry. 2017;230:241–249. doi: 10.1016/J.FOODCHEM.2017.03.039. [DOI] [PubMed] [Google Scholar]
- Kader, A. A. (2005). Increasing Food Availability by Reducing Postharvest Losses of Fresh Produce (Vol. 682).
- Li T., Liu R., Zhang C., Meng F., Wang L. Developing a green film from locust bean gum/carboxycellulose nanocrystal for fruit preservation. Future Foods. 2021;4 doi: 10.1016/J.FUFO.2021.100072. [DOI] [Google Scholar]
- Mateos-Maroto A., Fernández-Peña L., Abelenda-Núñez I., Ortega F., Rubio R.G., Guzmán E. Polyelectrolyte Multilayered Capsules as Biomedical Tools. Polymers. 2022;14(3) doi: 10.3390/polym14030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Nor, S., & Ding, P. (2020). Trends and advances in edible biopolymer coating for tropical fruit: A review. In Food Research International (Vol. 134, p. 109208). Elsevier. doi: 10.1016/j.foodres.2020.109208. [DOI] [PubMed]
- Ncama K., Magwaza L.S., Mditshwa A., Tesfay S.Z. Vol. 16. Elsevier; 2018. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review; pp. 157–167. (Food Packaging and Shelf Life). [DOI] [Google Scholar]
- Nour V., Trandafir I., Ionica M.E. HPLC organic acid analysis in different citrus juices under reversed phase conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38(1):44–48. www.notulaebotanicae.ro [Google Scholar]
- Obianom C., Romanazzi G., Sivakumar D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biology and Technology. 2019;147:214–221. doi: 10.1016/J.POSTHARVBIO.2018.10.004. [DOI] [Google Scholar]
- Pathare P.B., Opara U.L., Al-Said F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology. 2013;6(1):36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- Petriccione, M., Mastrobuoni, F., Pasquariello, M. S., Zampella, L., Nobis, E., Capriolo, G., Scortichini, M., & Barringer, S. (2015). Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. 4, 501–523. doi: 10.3390/foods4040501. [DOI] [PMC free article] [PubMed]
- Poverenov E., Danino S., Horev B., Granit R., Vinokur Y., Rodov V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate-chitosan combination. Food and Bioprocess Technology. 2014;7(5):1424–1432. doi: 10.1007/s11947-013-1134-4. [DOI] [Google Scholar]
- Queb-González D.B., Lopez-Malo A., Sosa-Morales M.E., Villa-Rojas R. Postharvest heat treatments to inhibit Penicillium digitatum growth and maintain quality of Mandarin (Citrus reticulata blanco) Heliyon. 2020;6(1):e03166. doi: 10.1016/j.heliyon.2020.e03166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reyes-Avalos M.C., Minjares-Fuentes R., Femenia A., Contreras-Esquivel J.C., Quintero-Ramos A., Esparza-Rivera J.R., Meza-Velázquez J.A. Application of an alginate-chitosan edible film on figs (Ficus carica): Effect on bioactive compounds and antioxidant capacity. Food and Bioprocess Technology. 2019;12(3):499–511. doi: 10.1007/s11947-018-2226-y. [DOI] [Google Scholar]
- Rokaya P.R., Baral D.R., Gautam D.M., Shrestha A.K., Paudyal K.P. Effect of pre-harvest application of gibberellic acid on fruit quality and shelf life of mandarin (<i>Citrus reticulata</i> Blanco) American Journal of Plant Sciences. 2016;07(07):1033–1039. doi: 10.4236/ajps.2016.77098. [DOI] [Google Scholar]
- Romanazzi G., Feliziani E., Baños S.B., Sivakumar D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Critical Reviews in Food Science and Nutrition. 2017;57(3):579–601. doi: 10.1080/10408398.2014.900474. [DOI] [PubMed] [Google Scholar]
- Salas-Méndez, E. de J., Vicente, A., Pinheiro, A. C., Ballesteros, L. F., Silva, P., Rodríguez-García, R., Hernández-Castillo, F. D., Díaz-Jiménez, M. de L. V., Flores-López, M. L., Villarreal-Quintanilla, J. Á., Peña-Ramos, F. M., Carrillo-Lomelí, D. A., & Jasso de Rodríguez, D. (2019). Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 150, 19–27. doi: 10.1016/j.postharvbio.2018.12.008.
- Severo J., de Oliveira I.R., Tiecher A., Chaves F.C., Rombaldi C.V. Postharvest UV-C treatment increases bioactive, ester volatile compounds and a putative allergenic protein in strawberry. LWT – Food Science and Technology. 2015;64(2):685–692. doi: 10.1016/J.LWT.2015.06.041. [DOI] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Won M.Y., Min S.C. Coating Satsuma mandarin using grapefruit seed extract–incorporated carnauba wax for its preservation. Food Science and Biotechnology. 2018;27(6):1649–1658. doi: 10.1007/s10068-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaga I., Nakamura S. Penicillium growth inhibition, fruit decay reduction, and polymethoxyflavones and scoparone induction in satsuma mandarin irradiated with ultraviolet-A light-emitting diodes. Scientia Horticulturae. 2022;303 doi: 10.1016/J.SCIENTA.2022.111197. [DOI] [Google Scholar]
- Yan J., Luo Z., Ban Z., Lu H., Li D., Yang D.…Li L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biology and Technology. 2019;147:29–38. doi: 10.1016/J.POSTHARVBIO.2018.09.002. [DOI] [Google Scholar]
- Zhou D., Chen S., Xu R., Tu S., Tu K. Interactions among chilling tolerance, sucrose degradation and organic acid metabolism in UV-C-irradiated peach fruit during postharvest cold storage. Acta Physiologiae Plantarum. 2019;41(6):1–16. doi: 10.1007/s11738-019-2871-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.