Abstract
Aims
Hematological markers that can be used for prognosis prediction for stage I lung adenocarcinoma (LUAD) are still lacking. Here, we examined the prognostic value of a combination of the red cell distribution width (RDW) and carcinoembryonic antigen (CEA), namely, the RDW-CEA score (RCS), in stage I LUAD.
Materials and methods
A retrospective study with 154 patients with stage I LUAD was conducted. Patients were divided into RCS 1 (decreased RDW and CEA), RCS 2 (decreased RDW and increased CEA, increased RDW and decreased CEA), and RCS 3 (increased RDW and CEA) subgroups based on the best optimal cutoff points of RDW and CEA for overall survival (OS). The differences in other clinicopathological parameters among RCS subgroups were calculated. Disease-free survival (DFS) and OS among these groups were determined by Kaplan–Meier analysis, and risk factors for outcome were calculated by a Cox proportional hazards model.
Results
Seventy, 65, and 19 patients were assigned to the RCS 1, 2, and 3 subgroups, respectively. Patients ≥ 60 years (P < 0.001), male sex (P = 0.004), T2 stage (P = 0.004), and IB stage (P = 0.006) were more significant in the RCS 2 or 3 subgroups. The RCS had a good area under the curve (AUC) for predicting DFS (AUC = 0.81, P < 0.001) and OS (AUC = 0.93, P < 0.001). The DFS (log-rank = 33.26, P < 0.001) and OS (log-rank = 42.05, P < 0.001) were significantly different among RCS subgroups, with RCS 3 patients displaying the worst survival compared to RCS 1 or 2 patients. RCS 3 was also an independent risk factor for both DFS and OS.
Conclusions
RCS is a useful prognostic indicator in stage I LUAD patients, and RCS 3 patients have poorer survival. However, randomized controlled trials are needed to validate our findings in the future.
Introduction
Lung cancer is still the leading cause of cancer-related death worldwide, with an estimated 1.8 million deaths in 2020 [1]. Fortunately, with the increasing popularity of low-dose computed tomographic screening, the mortality of the disease has decreased remarkably in recent years, and patients can be treated at early stages [2, 3]. However, recurrence is frustratingly unavoidable, and the 5-year overall survival (OS) rates are 68–92% for stage I cases according to the eighth edition of the Tumor-Node-Metastasis (TNM) classification [4]. Reliable prognostic markers that can be used for these early patients are still needed.
Previously, except for the risk factors identified in the National Comprehensive Cancer Network guidelines for non-small cell lung cancer (NSCLC) [5] and pathologically micropapillary and solid patterns [6], tumor spread through air spaces [7] is regarded as an additional risk factor for stage I cases, and patients with these features are thought to be candidates to receive and benefit from adjuvant therapies (ADTs). However, hematological markers, which can be easily obtained from routine blood tests that could also guide the treatment for these patients, are still lacking. Red cell distribution width (RDW), which can reflect the size heterogeneity of circulating erythrocytes, was also found to be associated with inflammation, malnutrition, and impaired kidney function [8]. The prognostic value of RDW has been registered in many cancers according to a meta-analysis [9]. In lung cancer, Wang et al. conducted a meta-analysis and indicated that a higher value of pretreatment RDW was significantly associated with worse OS and disease-free survival (DFS) [10]; however, single RDW was less efficient in predicting the outcome since the reported area under the curve (AUC) ranged from 0.565 [11] to 0.629 [12, 13]. Additionally, these studies conventionally included stage I–IV cases, which may have greatly biased the results [11–13]. To date, only two studies have explored the prognostic value of RDW in stage I lung cancer [14, 15]; however, without reporting the definite AUC, more studies are still needed.
Carcinoembryonic antigen (CEA) is a tumor maker in NSCLC [16], and its prognostic value is under extensive study [17]. However, the positive rate of CEA is still limited (ranging from 19 to 33.6% [18–20]) in stage I cases, and its prognostic value is under debate in such a scenario. For example, Kuo et al. conducted a study with 758 stage I patients (541 adenocarcinomas (AD), 83 squamous cell carcinomas (SCC), and 134 others) and found that CEA was an independent risk factor for recurrence [21]. However, Blankenburg et al. conducted a study with 240 stage I NSCLC patients (91 AD, 100 SCC, and 32 others) and found that preoperative CEA could not be used to predict the 3- or 5-year OS with a cutoff value of 6.7 ng/mL [22]; in line with this, Maeda et al. performed a study that enrolled 229 stage IA patients (195 AD, 34 others) and suggested that preoperative CEA was not an independent risk factor for poor prognosis [23]. Nonetheless, it was notable that CEA was much more efficient in AD than other pathological phenotypes [24, 25] in NSCLC, and the majority of previous studies mixed with other pathological phenotypes could attenuate the prognostic efficacy of CEA. Interestingly, some authors have tried to combine hematological markers, such as platelets, with CEA to further improve its prognostic efficacy in NSCLC [26], and other studies have indicated that a combination of RDW with tumor markers would be useful for the prognosis of cancer patients [27, 28]. Based on these findings, we speculated that a combination of RDW and CEA could also be meaningful in the prognosis of stage I lung adenocarcinoma (LUAD); however, related reports are rare.
Here, we aimed to explore the prognostic value of a newly established indicator, namely, the combined RDW and CEA score (RCS), in stage I LUAD.
Materials and methods
Patients
From December 2012 to April 2019, data from patients who underwent surgery for lung cancer at Hainan Hospital of Chinese PLA General Hospital were retrospectively collected. Those who met any one of the following criteria were excluded: (1) suspected distant lesions by preoperative examinations, (2) any preoperative adjuvant therapies, (3) in situ lesions, (4) lack of preoperative laboratory tests, (5) lack of any pathological TNM (pTNM) information, (6) pTNM > I according to the 8th edition of AJCC [4], (7) pathological phenotypes other than AD, and (8) follow-up problems. Other parameters, including age, sex, type of resection, and others, were also recorded as in our previous reports [29, 30]. The study was performed in line with the principles stated in the Declaration of Helsinki and was supervised by the ethics committee of Hainan Hospital of Chinese PLA General Hospital. Written informed consent was waived due to its retrospective nature.
Defining RCS and other inflammatory prognostic indicators
The data from routine blood tests were obtained within 1 week before curative surgery as previously described [29, 30]. CEA measurement was conducted in automated Cobas e601 immunoanalyzer (Roche Diagnostics GmbH, Germany) using an electrochemiluminescence immunoassay method with a reference at 0–5.0 ng/mL. Patients were divided into RDW-low or RDW-high and CEA-low or CEA-high subgroups based on the optimal cutoff points in the statistical results below. Subsequently, patients were divided into 3 subgroups, namely, RCS 1: both decreased RDW and CEA; RCS 2: decreased RDW with increased CEA or increased RDW with decreased CEA; and RCS 3: both increased RDW and CEA. Other inflammatory prognostic indicators, including the other systematic inflammatory prognostic indicators, including the neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), prognostic nutritional index (PNI), and advanced lung cancer inflammation index (ALI), were determined as described in previous studies [31–33].
Follow-up and definition of DFS and OS
Patients were routinely followed according to our previous studies [30]. DFS was defined as the date of surgery to the point of any recurrence, metastasis, or death from any cause, and OS was defined from the identical point to the point of any cause of death. The latest follow-up point ended in March 2022.
Statistical analysis
All analyses were conducted by SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). The optimal cutoff points of RDW and CEA for the outcome were tested by receiver operating characteristic curve (ROC) analysis. The differences in NLR, LMR, PNI, and ALI in RCS subgroups were analyzed by one-way ANOVA (with LSD for pairwise comparison) or nonparametric tests if a Gaussian distribution was not reached. Survival differences among RCS subgroups were measured by Kaplan–Meier analysis. Risk factors for survival were determined by a Cox proportional hazards model with the iterative forward LR method. P < 0.050 was considered statistically significant.
Results
Demographic characteristics of the cohort and the efficacy of RCS in prognosis
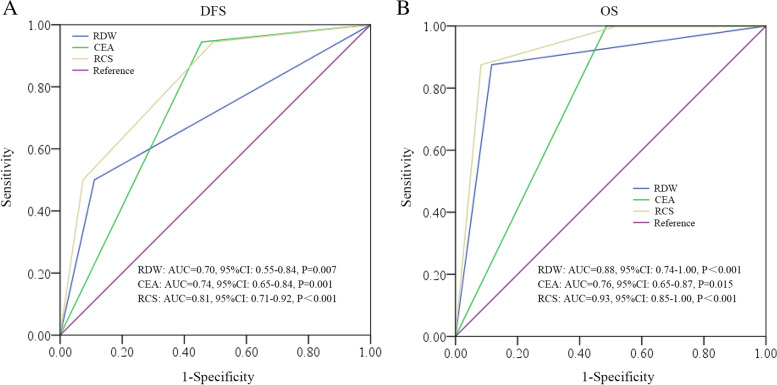
In total, 325 patients were included with 30 patients presenting distant lesions; in addition, 141 patients were excluded according to the exclusion criteria (Fig. 1). One hundred fifty-four patients were enrolled in the final cohort, with 124 IA and 30 IB cases. The ratio of female to male was 1:1 (n = 77 each), and the median follow-up was 49 months (m) (range: 9–118 m). During the follow-up, 16 patients experienced recurrence, and 8 patients died. By ROC analysis, the RCS was significant in predicting both DFS (AUC = 0.81, P < 0.001) and OS (AUC = 0.93, P < 0.001), and the AUC was larger than that of RDW or CEA alone (Fig. 2).
Fig. 1.
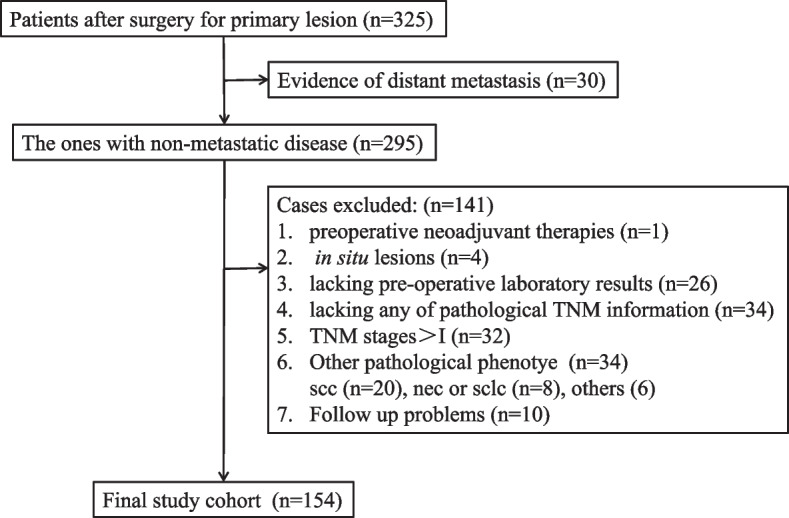
Flow chart of the study. TNM, Tumor-Node-Metastasis; scc, squamous carcinoma; nec, neuroendocrine carcinoma; sclc, small cell lung cancer
Fig. 2.
ROC analysis of RDW, CEA, and RCS in predicting DFS (A) and OS (B). RDW, red cell distribution width; CEA, carcinoembryonic antigen; RCS, red cell distribution width and carcinoembryonic antigen score
The differences in clinicopathological features among RCS subgroups
Taking OS as the end-point, the optimal cutoff points of RDW and CEA to the outcome were determined, and patients were divided into increased (≥ 13.45%) or decreased (< 13.45%) RDW and increased (≥ 1.20 ng/mL) or decreased (< 1.20 ng/mL) CEA subgroups. Based on these results, patients were divided into RCS 1 (n = 70, median age: 51 years, range: 29–72 years), RCS 2 (n = 65, median age: 60 years, range: 23–79 years), and RCS 3 (n = 19, median age: 65 years, range: 49–71 years) subgroups (Fig. 3). It was found that features such as ≥ 60 years (P < 0.001), male sex (P = 0.004), current or former tobacco use history (P = 0.001), T2 stage (P = 0.004), and IB stage (P = 0.006) were more significant in the RCS 2 or 3 subgroups (Table 1).
Fig. 3.
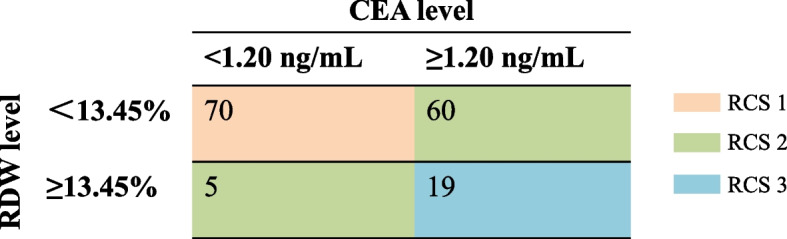
The assignment of patients into different RCS subgroups. RDW, red cell distribution width; CEA, carcinoembryonic antigen; RCS, red cell distribution width and carcinoembryonic antigen score
Table 1.
Differences of the clinicopathological features in RCS subgroups
| Features | Patient No | RCS | |||
|---|---|---|---|---|---|
| RCS 1 | RCS 2 | RCS 3 | P | ||
| Age (years) | < 0.001* | ||||
| < 60 | 90 | 52 | 33 | 5 | |
| ≥ 60 | 64 | 18 | 32 | 14 | |
| Gender | 0.004* | ||||
| Male | 77 | 25 | 39 | 13 | |
| Female | 77 | 45 | 26 | 6 | |
| Type of resection | 0.788 | ||||
| Lobectomy | 114 | 50 | 50 | 14 | |
| Segmentectomy | 40 | 20 | 15 | 5 | |
| Micropapillary or solid component | 0.830 | ||||
| Without or unknown | 120 | 56 | 50 | 14 | |
| With | 34 | 14 | 15 | 5 | |
| Tobacco use history | 0.001* | ||||
| Never | 119 | 63 | 45 | 11 | |
| Current + former | 35 | 7 | 20 | 8 | |
| Alcohol use history | 0.972 | ||||
| Never | 100 | 45 | 43 | 12 | |
| Current + former | 54 | 25 | 22 | 7 | |
| Hypertension | 0.060 | ||||
| Without | 119 | 60 | 45 | 14 | |
| With | 35 | 10 | 20 | 5 | |
| Combined T stages | 0.004* | ||||
| T1 | 123 | 63 | 49 | 11 | |
| T2 | 31 | 7 | 16 | 8 | |
| TNM stages | 0.006* | ||||
| IA | 124 | 63 | 50 | 11 | |
| IB | 30 | 7 | 15 | 8 | |
*With significant statistical difference
No Number, RCS Red cell distribution width and carcinoembryonic antigen score, TNM Tumor-Node-Metastasis
Correlation of RCS with other systematic inflammatory indicators
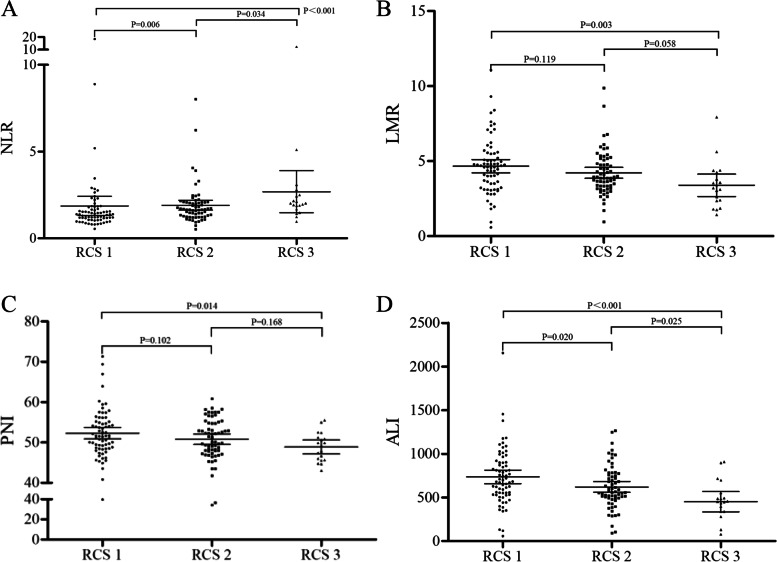
As shown in Fig. 4, significant differences were found for NLR, LMR, PNI, and ALI among the different RCS subgroups. In general, a significantly higher NLR was found between the RCS 3 and RCS 2 or 1 subgroups, whereas a significantly lower LMR, PNI, and ALI were also found between the RCS 3 and 1 subgroups and a significantly lower ALI was found between the RCS 3 and 2 subgroups.
Fig. 4.
The differences in NLR (A), LMR (B), PNI (C), and ALI (D) in the RCS subgroups. NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNI, prognostic nutritional index; ALI, advanced lung cancer inflammation index; RCS, red cell distribution width and carcinoembryonic antigen score
Survival differences among RCS subgroups
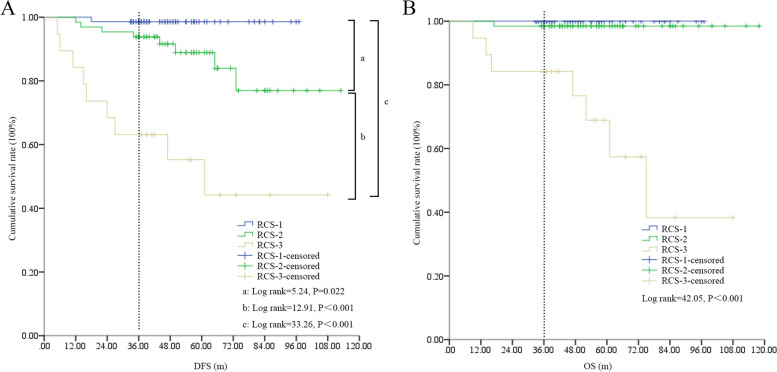
By Kaplan–Meier analysis, significant differences in DFS and OS were found among the different RCS subgroups (Fig. 5). Specifically, patients with different RCS could be well separated individually in DFS (the 3-year DFS rates in RCS 1, 2, and 3 were 98.57%, 93.84%, and 63.16%, respectively, P < 0.001); however, RCS 2 patients displayed an improved OS, which was similar to RCS 1 patients without a significant difference.
Fig. 5.
The survival differences among different RCS subgroups. A DFS differences in the study cohort. The dotted line indicates 3-year DFS in the subgroups. B OS differences in the study cohort. RCS, red cell distribution width and carcinoembryonic antigen score; DFS, disease-free survival; OS, overall survival
Univariate and multivariate analysis of risk factors for survival
By the Cox hazard model, with or without micropapillary or solid components, tobacco use history and RCS subgroups were identified as risk factors for both DFS and OS; in addition, with or without hypertension was a risk factor for DFS and TNM stage was a risk factor for OS (Table 2). When these factors were put into multivariate analysis, RCS was found to be an independent risk factor for both DFS and OS (Table 3).
Table 2.
Univariate tests for risk factors for DFS or OS
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| P | HR | 95%CI | P | HR | 95%CI | |
| Age (years) | ||||||
| < 60 | 1 | 1 | ||||
| ≥ 60 | 0.097 | 2.32 | 0.87–5.76 | 0.078 | 4.21 | 0.85–20.87 |
| Gender | ||||||
| Male | 1 | 1 | ||||
| Female | 0.187 | 0.52 | 0.19–1.38 | 0.215 | 0.36 | 0.07–1.80 |
| Type of resection | ||||||
| Lobectomy | 1 | 1 | ||||
| Segmentectomy | 0.635 | 1.36 | 0.39–4.77 | 0.650 | 1.64 | 0.19–13.88 |
| Micropapillary or solid component | ||||||
| Without or unknown | 1 | 1 | ||||
| With | 0.014* | 3.20 | 1.26–8.13 | 0.014* | 6.04 | 1.44–25.27 |
| Tobacco use history | ||||||
| Never | 1 | 1 | ||||
| Current + former | 0.001* | 5.10 | 2.01–12.96 | 0.010* | 6.59 | 1.57–27.68 |
| Alcohol use history | ||||||
| Never | 1 | 1 | ||||
| Current + former | 0.351 | 1.56 | 0.61–3.95 | 0.829 | 1.17 | 0.28–4.91 |
| Hypertension | ||||||
| Without | 1 | 1 | ||||
| With | 0.029* | 2.83 | 1.12–7.17 | 0.872 | 1.14 | 0.23–5.65 |
| Combined T stages | ||||||
| T1 | 1 | 1 | ||||
| T2 | 0.097 | 2.32 | 0.87–5.76 | 0.097 | 2.32 | 0.87–5.76 |
| TNM stages | ||||||
| IA | 1 | 1 | ||||
| IB | 0.094 | 2.32 | 0.87–6.21 | 0.027* | 4.84 | 1.20–19.58 |
| RCS subgroups | ||||||
| 1 + 2 | 1 | 1 | ||||
| 3 | < 0.001* | 8.64 | 3.42–21.79 | < 0.001* | 50.21 | 6.17–408.60 |
*With significant statistical difference
DFS Disease-free survival, OS Overall survival, HR Hazard ratio, CI Confidence interval, RCS Red cell distribution width and carcinoembryonic antigen score, TNM Tumor-Node-Metastasis
Table 3.
Multivariate tests for risk factors for DFS or OS
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| P | HR | 95%CI | P | HR | 95%CI | |
| Micropapillary or solid component | ||||||
| Without or unknown | 1 | 1 | ||||
| With | 0.047* | 2.69 | 1.01–7.16 | 0.035* | 4.74 | 1.12–20.11 |
| Tobacco use history | ||||||
| Never | 1 | |||||
| Current + former | 0.003* | 4.44 | 1.66–11.91 | |||
| RCS subgroups | ||||||
| 1 + 2 | 1 | 1 | ||||
| 3 | < 0.001* | 7.74 | 2.98–20.08 | < 0.001* | 44.69 | 5.47–365.29 |
*With significant statistical difference
DFS Disease-free survival, OS Overall survival, HR Hazard ratio, CI Confidence interval, RCS Red cell distribution width and carcinoembryonic antigen score
Discussion
In the present study, RCS was found to be a useful prognostic marker in stage I LUAD and could effectively separate the DFS among these patients. RCS 3 patients had worse outcomes than RCS 1 and RCS 2 patients, and it was an independent risk factor for survival. To the best of our knowledge, this is the first study concerning the utility of RCS in lung cancer.
The prognostic value of RDW and CEA has been registered in lung cancer previously. For RDW, Wang et al. conducted a meta-analysis that indicated that pretreatment RDW was significantly associated with poor DFS and OS [10]. However, it was notable that most of the studies enrolled imbalanced stages or mixed pathological phenotypes, and the prognostic value of RDW was likely to be more apparent in early-stage AD cases. For example, Liu et al. conducted a study with 750 stage I–III patients (stage I: n = 352, stage II: n = 134, stage III: n = 264) to explore the prognostic value of RDW, with more than half of the cases being AD (401/750) [34]. Matsui et al. also performed a study with 338 stage I–III patients (stage I: n = 289, stage II: n = 44, stage III: n = 5), and up to 70.38% of the cases were AD (259/338) [35]. To date, only two studies have reported the prognostic value of RDW (226 AD, 47 others [14]; 166 AD [15]) in stage I NSCLC. Moreover, the AUC of RDW alone in predicting survival has been relatively small [11–13]. These results indicated that further improvement of the prognostic efficacy is needed when explored in a specific stage or pathological phenotype. CEA was a useful tumor marker in lung cancer. Interestingly, the prognostic value of CEA in stage I cases has been extensively studied [36, 37]. However, the positive rate of CEA was higher in AD than other pathological phenotypes [24, 25], but similar to RDW, previous studies did not specifically explore its value in AD in stage I cases. In our study, we concurrently explored the prognostic value of RDW and CEA in stage I AD patients, and the results indicated that RCS displayed a larger AUC than RDW and CEA in predicting the outcome. Interestingly, a previous study also investigated the value of a combination of other hematological index (platelet, PLT) with CEA in NSCLC (stage I: n = 193, stage II + III: n = 83), and the results indicated that the survival was different when patients were divided into PLTnormal/CEAnormal, PLTnormal/CEAhigh, PLThigh/CEAnormal, and PLThigh/CEAhigh subgroups; however, it was also notable that such algorithm cannot separate the survival effectively in some subgroups [26]. In addition, although not conducted in NSCLC, other studies have indicated that a combination of RDW with tumor markers (CA125 in endometrial cancer [27]; CEA in colorectal cancer [28]) could have better efficacy in prognosis than used individually. Our study finds that RCS could be better in prognostic prediction and could separate the DFS effectively in stage I LUAD; additionally, RCS could be used to identify a cluster of patients with significant inferior OS.
Mechanistically, cancer-related inflammation is thought to be the seventh hallmark of cancer and plays a profound role in cancer initiation and development [38, 39]; in turn, cancer cells can also promote self-development by secreting inflammatory cytokines in an autocrine manner [40]. In lung cancer, some cytokines were significantly elevated in patients, such as interleukin-6 (IL-6) [41, 42], which has broad functions in regulating cancer cells. For example, IL-6 can promote cell proliferation [43], metastasis, and epithelial-mesenchymal transition (EMT) [44]; it can also contribute to treatment (cisplatin [45], EGF receptor (EGFR) tyrosine kinase inhibitors (TKIs) [46]) resistance. Many studies have indicated that abnormally elevated IL-6 predicts poor survival in lung cancer [41, 42, 47]. Interestingly, systematic evaluation of IL-6 can also result in a fluctuation of RDW, as an increase or decrease in RDW was closely correlated with high or low levels of IL-6, although not in the cancer background [48, 49]. In addition, the counts of circulating tumor cells (CTCs) were found to play an essential role in disease recurrence or metastasis in a postoperative setting [50, 51], and these cells were also found to be a major source of CEA in patients [52, 53]. Importantly, some CTCs share the characteristics of cancer stem cells (CSCs) [54, 55], which were identified as the ultimate source of cancer recurrence, metastasis, and treatment resistance [56, 57]. IL-6 can additionally amplify these cells, except for the aforementioned function in cancer cells [43–46]. Based on these findings, RCS 1 patients could mean a decreased concentration of IL-6 and low counts of CTCs or CSCs and also a low mutual promotion between them, which could have a superior outcome than the RCS 2 or 3 patients. Additionally, the prognostic value of other parameters including age [58], gender [58], micropapillary and solid patterns [6], type of resection [58, 59], and smoke and alcohol [60] have been under extensive study in stage I lung cancer, and hypertension was also found contribute to prognosis in lung cancer [61]. It was suggested that male patients smoke in particular after diagnosis correlated with poor survival in stage I cases [58, 60]. In our study, the proportion of male (25/70) and smoke (current + former) (7/70) in the RCS 1 group was significantly lower than in the RCS 2 and 3 subgroups, which may partly support its positive role in survival. Except these, although a high proportion of patients < 60 years was noted in the RCS 1 group in contrast to other subgroups; however, a great number of previous studies indicated that age was not a risk factor for survival in stage I lung cancer patients [62–64]. It was not definite to conclude that age plays a synergic role for good survival in the RCS 1 group at present. Interestingly, we also found a significant difference in other inflammatory prognostic indicators between RCS subgroups, as RCS 1 patients presented the highest NLR and lowest LMR, PNI, and ALI. Although it has not been extensively studied in stage I cases, the low levels of LMR [65], PNI [66], and ALI [67] have been found to be correlated with poor survival in patients after surgery, whereas the high level of NLR was found to be correlated with poor prognosis specifically in stage I cases [68]. These findings also support the notion that RCS 1 patients would have superior survival in our study.
Interestingly, RCS 2 included two subgroups of patients with a reversed increase or decrease in RDW or CEA and displayed a significantly poorer DFS but a similar OS compared with the RCS 1 subgroup. This could also be interpreted from the cancer-related inflammation and CTC perspectives. For DFS, the RCS 2 patients had relatively low levels of IL-6 with a high CTC count or vice versa. For the former, the high count of CTCs was a well-validated poor prognostic indicator in previous studies [50, 51]; for the latter, the CTCs could potentially quickly proliferate with the support of IL-6 [43], both of which could result in an early recurrence or metastasis in contrast to RCS 1 patients. For OS, the subsequent treatment after recurrence or metastasis should be taken into consideration. Notably, more than half of LUAD patients in China harbor EGFR mutations [69, 70], particularly females (66.67% in RCS 2 cases in our study) and never smokers (55.56% in RCS 2 cases in our study) [71, 72], and these patients are commonly treated with TKIs. Notably, it was found that treatment by TKIs such as gefitinib could lead to an obvious decrease in IL-6 in the patients [73]; in addition, such treatment could also shift a portion of patients with high counts of CTCs into low ones, which could have a significantly prolonged PFS [74], which is a surrogate for OS in NSCLC [75]. In addition, we noticed that RCS 3 patients displayed the worst DFS and OS in the cohort, even given that these patients have the same probability of harboring EGFR mutations and undergoing subsequent TKI treatment. We speculate that these patients may have a persistent expansion of CTCs, which could be promoted by a systematic-autocrine IL-6 loop [43] or a shift to the stemness feature of CTCs with IL-6 [76]. Clinically, adjuvant therapies (ADTs) (including chemotherapy, TKIs, or immunotherapy) were still not recommended for stage IA patients and were still controversial in stage IB cases. Based on our results, it was plausible that RCS 1 cases could be waived from ADTs safely due to the outstanding outcome, in addition, although RCS 2 patients were candidates for ADTs due to the relatively poor DFS; however, it was notable that the 3-year absolute benefits from such therapies (taken chemotherapy for example) was only 3.9% and the proportion of 3–4 grade adverse effects was up to 66% [77]; we thus believed “watch and wait” may also be an alternative option for these patients. RCS 3 patients presented the worst DFS and OS in our study, which indicated that these patients were candidates for ADTs and also receive more effective regimens, such as the additional agents anti-IL-6 (ALD518 [78] or siltuximab [79]), in addition to TKIs or TKIs plus chemotherapy based on our aforementioned speculation.
There are certain limitations to the present study. First, it was a retrospective study performed in a single hospital, and the sample size was relatively limited; in particular, only 19 patients were assigned to the RCS 3 group, which could bias the results. Second, the information of gene testing and the subsequent therapy regimens were not definite in our cohort, which therefore could not support our speculation. Some evidence for our speculation could be obtained if post hoc analysis could be conducted like the LACE study [77] where RCS could be analyzed as a stratification factor. Third, as pure solid type could have different CTCs in NSCLC [80], we could not analyze the pathological phenotype to the prognosis since 30 patients did not report definite pathological elements in resected samples. Fourth, taking into account the fact that RDW and CEA could be easily measured during subsequent treatment or follow-up, repeated assessments to validate its prognostic value in stage I LUAD are achievable. Nonetheless, more studies with a large sample size, in particular the randomized controlled trials, are the best way to validate our findings in the future.
Conclusion
Overall, our study indicated that RCS was a useful prognostic marker in stage I LUAD patients and RCS 3 patients had the worst survival. In addition, taking into account the fact that the poor OS in RCS 3 patients, we speculate that more effective ADTs should be an option for these patients; however, randomized controlled trials are needed in the future.
Acknowledgements
None.
Authors’ contributions
BY was responsible for the conception of the work. HX, JL and BY obtained the data. BY analyzed the data. HX, JL and BY wrote the manuscript. GZ and BY critically revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in line with the principles stated in the Declaration of Helsinki and was supervised by the ethics committee of Hainan Hospital of Chinese PLA General Hospital. Written informed consent was waived due to its retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hengliang Xu, Guangqiang Zhao, and Jixing Lin contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gierada DS, Pinsky PF. Survival following detection of stage I lung cancer by screening in the national lung screening trial. Chest. 2021;159(2):862–869. doi: 10.1016/j.chest.2020.08.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 6.Peng B, Li G, Guo Y. Prognostic significance of micropapillary and solid patterns in stage IA lung adenocarcinoma. Am J Transl Res. 2021;13(9):10562–10569. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D, Wang X, Zhang F, Han R, Ding Q, Xu X, et al. Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study. Ther Adv Med Oncol. 2020;12:1758835920978147. doi: 10.1177/1758835920978147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med. 2016;4(20):399. doi: 10.21037/atm.2016.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang PF, Song SY, Guo H, Wang TJ, Liu N, Yan CX. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: a meta-analysis of 49 studies. J Cancer. 2019;10(18):4305–4317. doi: 10.7150/jca.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou Y, Zhou K, Li J, Che G. Prognostic value of pre-treatment red blood cell distribution width in lung cancer: a meta-analysis. Biomarkers. 2020;25(3):241–247. doi: 10.1080/1354750X.2020.1731763. [DOI] [PubMed] [Google Scholar]
- 11.Kos M, Hocazade C, Kos FT, Uncu D, Karakas E, Dogan M, et al. Evaluation of the effects of red blood cell distribution width on survival in lung cancer patients. Contemp Oncol (Pozn) 2016;20(2):153–157. doi: 10.5114/wo.2016.60072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H, Tan X, Wang Y, Dai L, Liang G, Guo J, et al. Clinical Significance of red cell distribution width and circulating tumor cells with an epithelial-mesenchymal transition phenotype in lung adenocarcinoma. Cancer Manag Res. 2020;12:5105–5117. doi: 10.2147/CMAR.S251271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JL, Wu JN, Lv XD, Yang QC, Chen JR, Zhang DM. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS ONE. 2020;15(8):e0237947. doi: 10.1371/journal.pone.0237947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyokawa G, Shoji F, Yamazaki K, Shimokawa M, Takeo S. Significance of the red blood cell distribution width in resected pathologic stage I nonsmall cell lung cancer. Semin Thorac Cardiovasc Surg. 2020;32(4):1036–1045. doi: 10.1053/j.semtcvs.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Karube Y, Inoue T, Araki O, Maeda S, Matsumura Y, et al. Advanced lung cancer inflammation index predicts outcomes of patients with pathological stage IA lung adenocarcinoma following surgical resection. Ann Thorac Cardiovasc Surg. 2019;25(2):87–94. doi: 10.5761/atcs.oa.18-00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21–1 tumor markers in primary lung cancer. Lung Cancer. 2013;80(1):45–49. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Jiao Z, Cao S, Li J, Hu N, Gong Y, Wang L, et al. Clinical associations of preoperative and postoperative serum CEA and lung cancer outcome. Front Mol Biosci. 2021;8:686313. doi: 10.3389/fmolb.2021.686313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawabata N, Ohta M, Takeda S, Hirano H, Okumura Y, Asada H, et al. Serum carcinoembryonic antigen level in surgically resected clinical stage I patients with non-small cell lung cancer. Ann Thorac Surg. 2002;74(1):174–179. doi: 10.1016/S0003-4975(02)03662-7. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka K, Sumitomo S, Nakashima N, Nakajima D, Misaki N. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32(3):435–439. doi: 10.1016/j.ejcts.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Kawachi R, Nakazato Y, Takei H, Koshi-ishi Y, Goya T. Clinical significance of preoperative carcinoembryonic antigen level for clinical stage I non-small cell lung cancer: can preoperative carcinoembryonic antigen level predict pathological stage? Interact Cardiovasc Thorac Surg. 2009;9(2):199–202. doi: 10.1510/icvts.2009.206698. [DOI] [PubMed] [Google Scholar]
- 21.Kuo SW, Chen JS, Huang PM, Hsu HH, Lai HS, Lee JM. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148(4):1200–7.e3. doi: 10.1016/j.jtcvs.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Blankenburg F, Hatz R, Nagel D, Ankerst D, Reinmiedl J, Gruber C, et al. Preoperative CYFRA 21–1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer: external validation of a prognostic score. Tumour Biol. 2008;29(4):272–277. doi: 10.1159/000152945. [DOI] [PubMed] [Google Scholar]
- 23.Maeda R, Suda T, Hachimaru A, Tochii D, Tochii S, Takagi Y. Clinical significance of preoperative carcinoembryonic antigen level in patients with clinical stage IA non-small cell lung cancer. J Thorac Dis. 2017;9(1):176–186. doi: 10.21037/jtd.2017.01.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12(3):172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Szturmowicz M, Rudziński P, Kacprzak A, Langfort R, Bestry I, Broniarek-Samson B, et al. Prognostic value of serum C-reactive protein (CRP) and cytokeratin 19 fragments (Cyfra 21–1) but not carcinoembryonic antigen (CEA) in surgically treated patients with non-small cell lung cancer. Pneumonol Alergol Pol. 2014;82(5):422–429. doi: 10.5603/PiAP.2014.0055. [DOI] [PubMed] [Google Scholar]
- 26.Tomita M, Shimizu T, Ayabe T, Onitsuka T. Prognostic significance of the combined use of preoperative platelet count and serum carcinoembryonic antigen level in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2010;58(11):573–576. doi: 10.1007/s11748-010-0647-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhong W, Zhou C, Chen L, Wang Z, Lin H, Wu K, et al. The coefficient of variation of red blood cell distribution width combined with cancer antigen 125 predicts postoperative overall survival in endometrial cancer. Int J Gen Med. 2021;14:5903–5910. doi: 10.2147/IJGM.S323136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Xing C, Wei M, Wu H, Hu X, Li S, et al. Combining red blood cell distribution width (RDW-CV) and CEA predict poor prognosis for survival outcomes in colorectal cancer. J Cancer. 2019;10(5):1162–1170. doi: 10.7150/jca.29018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, You JH, Li F, Yan B. Postoperative fasting blood glucose predicts prognosis in stage I-III colorectal cancer patients undergoing resection. Gastroenterol Res Pract. 2020;2020:2482409. doi: 10.1155/2020/2482409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Liu Y, Qiu X, Yan B. Concurrent comparison of the prognostic values of tumor budding, tumor stroma ratio, tumor infiltrating pattern and lymphocyte-to-monocyte ratio in colorectal cancer patients. Technol Cancer Res Treat. 2021;20:15330338211045826. doi: 10.1177/15330338211045826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. 2018;484:272–277. doi: 10.1016/j.cca.2018.05.059. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Cao S, Geng J, Wang C, Meng Q, Yu Y. High prognostic nutritional index (PNI) as a positive prognostic indicator for non-small cell lung cancer patients with bone metastasis. Clin Respir J. 2021;15(2):225–231. doi: 10.1111/crj.13288. [DOI] [PubMed] [Google Scholar]
- 33.Ozyurek BA, Ozdemirel TS, Ozden SB, Erdoğan Y, Ozmen O, Kaplan B, et al. Does advanced lung inflammation index (ALI) have prognostic significance in metastatic non-small cell lung cancer? Clin Respir J. 2018;12(6):2013–2019. doi: 10.1111/crj.12768. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Zhang H, Qi Q, Zhang B, Yue D, Wang C. The preoperative platelet distribution width: a predictive factor of the prognosis in patients with non-small cell lung cancer. Thorac Cancer. 2020;11(4):918–927. doi: 10.1111/1759-7714.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui H, Taniguchi Y, Maru N, Utsumi T, Saito T, Hino H, et al. Prognostic effect of preoperative red cell distribution width on the survival of patients who have undergone surgery for non-small cell lung cancer. Mol Clin Oncol. 2021;14(5):108. doi: 10.3892/mco.2021.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanagiri T, Sugaya M, Takenaka M, Oka S, Baba T, Shigematsu Y, et al. Preoperative CYFRA 21–1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer. 2011;74(1):112–117. doi: 10.1016/j.lungcan.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Nasralla A, Lee J, Dang J, Turner S. Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non-small cell lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 2020;15(1):318. doi: 10.1186/s13019-020-01353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Butera G, Pacchiana R, Donadelli M. Autocrine mechanisms of cancer chemoresistance. Semin Cell Dev Biol. 2018;78:3–12. doi: 10.1016/j.semcdb.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Yan X, Han L, Zhao R, Fatima S, Zhao L, Gao F. Prognosis value of IL-6, IL-8, and IL-1β in serum of patients with lung cancer: a fresh look at interleukins as a biomarker. Heliyon. 2022;8(8):e09953. doi: 10.1016/j.heliyon.2022.e09953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, et al. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS ONE. 2017;12(7):e0181125. doi: 10.1371/journal.pone.0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng X, Lu G, Yao Y, Gu W. An autocrine IL-6/IGF-1R loop mediates EMT and promotes tumor growth in non-small cell lung cancer. Int J Biol Sci. 2019;15(9):1882–1891. doi: 10.7150/ijbs.31999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang GS, Liu L, Qin YW. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol Lett. 2017;13(6):4657–4660. doi: 10.3892/ol.2017.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan S, Tsai Y, Keng P, Chen Y, Lee SO, Chen Y. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget. 2015;6(29):27651–27660. doi: 10.18632/oncotarget.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F, Ha SJ, et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther. 2012;11(10):2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 47.Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132(9):1977–1985. doi: 10.1002/ijc.27892. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto K, Inai K, Takeuchi D, Shinohara T, Nakanishi T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J. 2015;79(5):1100–1106. doi: 10.1253/circj.CJ-14-1296. [DOI] [PubMed] [Google Scholar]
- 49.Fornal M, Wizner B, Cwynar M, Królczyk J, Kwater A, Korbut RA, et al. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin Hemorheol Microcirc. 2014;56(4):325–335. doi: 10.3233/CH-131745. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology. 2016;21(3):519–525. doi: 10.1111/resp.12696. [DOI] [PubMed] [Google Scholar]
- 51.Bayarri-Lara C, Ortega FG, CuetoLadrón de Guevara A. Puche JL, Ruiz Zafra J, de Miguel-Pérez D, et al. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS One. 2016;11(2):e0148659. doi: 10.1371/journal.pone.0148659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao H, Bai T, Takata K, Yokobori T, Ohnaga T, Hisada T, et al. High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells is associated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol Lett. 2018;15(3):3061–3067. doi: 10.3892/ol.2017.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita J, Matsuo A, Kurusu Y, Saishoji T, Hayashi N, Ogawa M. Preoperative evidence of circulating tumor cells by means of reverse transcriptase-polymerase chain reaction for carcinoembryonic antigen messenger RNA is an independent predictor of survival in non-small cell lung cancer: a prospective study. J Thorac Cardiovasc Surg. 2002;124(2):299–305. doi: 10.1067/mtc.2002.124370. [DOI] [PubMed] [Google Scholar]
- 54.Pantazaka E, Vardas V, Roumeliotou A, Kakavogiannis S, Kallergi G. Clinical relevance of mesenchymal- and stem-associated phenotypes in circulating tumor cells isolated from lung cancer patients. Cancers (Basel) 2021;13(9):2158. doi: 10.3390/cancers13092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obermayr E, Koppensteiner N, Heinzl N, Schuster E, Holzer B, Fabikan H, et al. Cancer stem cell-like circulating tumor cells are prognostic in non-small cell lung cancer. J Pers Med. 2021;11(11):1225. doi: 10.3390/jpm11111225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eramo A, Haas TL, De Maria R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene. 2010;29(33):4625–4635. doi: 10.1038/onc.2010.207. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Y, Wang L, Yin L, Yao Z, Tong R, Xue J, et al. Lung cancer stem cell markers as therapeutic targets: an update on signaling pathways and therapies. Front Oncol. 2022;12:873994. doi: 10.3389/fonc.2022.873994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer. 2010;68(3):398–402. doi: 10.1016/j.lungcan.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Chan EY, Amirkhosravi F, Nguyen DT, Chihara RK, Graviss EA, Kim MP. Lobectomy provides the best survival for stage I lung cancer patients despite advanced age. Ann Thorac Surg. 2022;114(5):1824–1832. doi: 10.1016/j.athoracsur.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 60.Christensen NL, Løkke A, Dalton SO, Christensen J, Rasmussen TR. Smoking, alcohol, and nutritional status in relation to one-year mortality in Danish stage I lung cancer patients. Lung Cancer. 2018;124:40–44. doi: 10.1016/j.lungcan.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Zeng X, Zeng D, Cheng J, Xu C, Sun C, Long H, Zhu B. Influence of hypertension on the survival of non-small cell lung cancer patients with type 2 diabetes mellitus. Med Sci Monit. 2020;26:e921676. doi: 10.12659/MSM.921676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palma DA, Tyldesley S, Sheehan F, Mohamed IG, Smith S, Wai E, Murray N, Senan S. Stage I non-small cell lung cancer (NSCLC) in patients aged 75 years and older: does age determine survival after radical treatment? J Thorac Oncol. 2010;5(6):818–824. doi: 10.1097/JTO.0b013e3181d6e052. [DOI] [PubMed] [Google Scholar]
- 63.Sigel K, Bonomi M, Packer S, Wisnivesky J. Effect of age on survival of clinical stage I non-small-cell lung cancer. Ann Surg Oncol. 2009;16(7):1912–1917. doi: 10.1245/s10434-009-0475-8. [DOI] [PubMed] [Google Scholar]
- 64.Fujita T, Koyanagi A, Kishimoto K. Older age is not a negative factor for video-assisted thoracoscopic lobectomy for pathological stage I non-small cell lung cancer: a single-center, retrospective, propensity score-matching study. Surg Today. 2023. 10.1007/s00595-022-02628-y. Epub ahead of print. [DOI] [PubMed]
- 65.Ramos R, Macía I, Navarro-Martin A, Déniz C, Rivas F, Ureña A, et al. Prognostic value of the preoperative lymphocyte-to-monocyte ratio for survival after lung cancer surgery. BMC Pulm Med. 2021;21(1):75. doi: 10.1186/s12890-021-01446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao J, Li J, Zhang XL, Wang G. Prognostic significance of the preoperative controlled nutritional status score in lung cancer patients undergoing surgical resection. Nutr Cancer. 2021;73(11–12):2211–2218. doi: 10.1080/01635581.2020.1850814. [DOI] [PubMed] [Google Scholar]
- 67.Tomita M, Ayabe T, Nakamura K. The advanced lung cancer inflammation index is an independent prognostic factor after surgical resection in patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2018;26(2):288–292. doi: 10.1093/icvts/ivx329. [DOI] [PubMed] [Google Scholar]
- 68.Liu W, Zhang T, Li L, Zou J, Xu C. Assessing the prognostic value of the neutrophil-to-lymphocyte ratio in stage I non-small-cell lung cancer with complete resection. Can Respir J. 2022;2022:6837872. doi: 10.1155/2022/6837872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology-mainland china subset analysis of the PIONEER study. PLoS ONE. 2015;10(11):e0143515. doi: 10.1371/journal.pone.0143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pi C, Xu CR, Zhang MF, Peng XX, Wei XW, Gao X, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: Analysis based on large-scale data from China. Thorac Cancer. 2018;9(7):814–819. doi: 10.1111/1759-7714.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Y, Wang WY, Zheng K, Jiang L, Zou Y, Su XY, et al. EGFR mutations in non-small cell lung cancer: an audit from West China Hospital. Expert Rev Mol Diagn. 2016;16(8):915–919. doi: 10.1080/14737159.2016.1199961. [DOI] [PubMed] [Google Scholar]
- 72.Cheng Y, Wang Y, Zhao J, Liu Y, Gao H, Ma K, et al. Real-world EGFR testing in patients with stage IIIB/IV non-small-cell lung cancer in North China: a multicenter, non-interventional study. Thorac Cancer. 2018;9(11):1461–1469. doi: 10.1111/1759-7714.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheng J, Fang W, Liu X, Xing S, Zhan J, Ma Y, et al. Impact of gefitinib in early stage treatment on circulating cytokines and lymphocytes for patients with advanced non-small cell lung cancer. Onco Targets Ther. 2017;10:1101–1110. doi: 10.2147/OTT.S112158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang B, Qin A, Zhang K, Ren H, Liu S, Liu X, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung cancer patients. Oncol Res. 2017;25(9):1601–1606. doi: 10.3727/096504017X14928634401178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiteni F, Westeel V, Bonnetain F. Surrogate endpoints for overall survival in lung cancer trials: a review. Expert Rev Anticancer Ther. 2017;17(5):447–454. doi: 10.1080/14737140.2017.1316196. [DOI] [PubMed] [Google Scholar]
- 76.Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim NH, et al. Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol. 2012;41(1):310–316. doi: 10.3892/ijo.2012.1447. [DOI] [PubMed] [Google Scholar]
- 77.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJD, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 78.Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011;11(12):1663–1668. doi: 10.1517/14712598.2011.627850. [DOI] [PubMed] [Google Scholar]
- 79.Song L, Smith MA, Doshi P, Sasser K, Fulp W, Altiok S, et al. Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J Thorac Oncol. 2014;9(7):974–982. doi: 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawabata N, Kawaguchi T, Watanabe T, Yohikawa D, Ouji-Sageshima N, Ito T. Pure solid pattern of non-small cell lung cancer and clustered circulating tumor cells. Cancers (Basel) 2022;14(18):4514. doi: 10.3390/cancers14184514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.