Abstract
Objective:
The first annual Women’s Vascular Summit highlighted sex and gender-related knowledge gaps in vascular disease diagnosis and treatment. This suggests an opportunity for further research to improve care and outcomes in people who identify as women, specifically. The purpose of this study was to use a large national dataset to identify all operations performed for abdominal aortic aneurysm (AAA), carotid artery stenosis (CAS), and peripheral arterial disease (PAD) in the US, and to provide data on sex-related disparities in treatment.
Methods:
All hospitalizations of adult patients (≥18 years old) diagnosed with AAA, CAS, or PAD who underwent vascular surgery from 2000–2016 were identified in the Healthcare Cost and Utilization Project National Inpatient Sample. Sex-stratified US Census data and sex-specific population disease prevalence estimates from the National Institute of Health and Agency for Healthcare Research and Quality were used to calculate the number of US adults with AAA, CAS, and PAD. Sex-stratified rates of surgery and incidence rate ratios (IRR) were estimated using Poisson regression. Among those undergoing surgery, multivariable logistic regression was used to assess differences in endovascular versus open approach.
Results:
Over 16 years, there were 1,021,684 hospitalizations for vascular surgery: 13% AAA (21% female, 79% male), 40% CAS (42% female, 58% male), and 47% PAD (42% female, 58% male). Females were older than males at time of surgery (median age 71.3 vs 69.7) and less likely to have private insurance (18% vs 23%); minimal differences were seen across race/ethnicity, comorbidities, and hospital characteristics. After accounting for disease prevalence, females were still 25% less likely to undergo surgery for AAA and 30% less likely to undergo surgery for PAD, compared to males with the same disease. These results were consistent over time. After adjustment, females, compared to males, were less likely to receive an endovascular procedure compared to open for AAA or CAS, and more likely to receive one for PAD.
Conclusions:
From 2000–2016 in the US, females were less likely to undergo intervention for AAA and PAD than males. This is particularly significant for PAD, as the prevalence is the same for both sexes, indicating that females are likely undertreated for PAD. Additionally, females were less likely to have endovascular surgery for AAA and more likely to have endovascular surgery for PAD than males. These findings suggest that improvement in AAA and PAD identification and management in females may improve outcomes.
Table of Contents Summary
Females are significantly undertreated for AAA and PAD in this review of over 1 million hospitalizations from the National Inpatient Sample. Aggressive efforts to diagnose and treat arterial diseases in females are warranted.
INTRODUCTION
The first annual Women’s Vascular Summit highlighted knowledge gaps in how vascular disease presents in people who identify as women and how interventions and outcomes may be impacted by sex and gender. This summit is an annual meeting for medical professionals who treat vascular disease in women and is designed to present and discuss what we do and do not know about how vascular disease presents differently in females, and how intervention is impacted by sex and gender. Across a broad spectrum of diseases, including aortic aneurysms and dissections, cerebrovascular disease, and peripheral vascular disease, there were recurring themes that females tend to present with atypical symptoms, present later, and have worse outcomes. This suggests an opportunity for further research to improve care and outcomes for females with vascular disease. A broader understanding of current vascular practice is required in order to explore and address sex and gender-related disparities in the identification and management of vascular disease.
Generally speaking, females are underrepresented in vascular literature, particularly in landmark clinical trials that guide everyday vascular practice. Abdominal aortic aneurysm (AAA) is 3–4 times less prevalent in females than males,1 but is still a potentially life threatening pathology for all who have it. Females, however, comprised less than 8% of the patients in the DREAM trial and were not studied in the MASS and OVER trials.2–4 There is a slight male preponderance for carotid artery stenosis (CAS), but we would expect 40% instead of 30–35% of the patients in ACAS, NASCET, and CREST to be females.5–7 Many are surprised to discover the prevalence of peripheral arterial disease (PAD) is equal for males and females, yet only 40% of study participants in BASIL were females.8 Although females are underrepresented in clinical trials, it is unclear if this is the result of trial recruitment or consistent under-treatment of arterial disease in females.
The aim of this study was to use a large national dataset to identify all operations performed for AAA, CAS, and PAD in the United States, and to provide data on sex-related disparities in the intervention rate and type of intervention performed (open versus endovascular). Based on the timing and atypical nature of how females may present with vascular disease, we hypothesized that females are undertreated for AAA, CAS, and PAD. We also hypothesized that, due to smaller vessel diameter, females undergo a higher proportion of open procedures versus endovascular procedures when compared to males.9,10
METHODS
Data source and study population
We identified all hospitalizations of adult patients (≥18 years old) undergoing vascular surgery between January 1, 2000 and December 31, 2016 in the Healthcare Cost and Utilization Project National Inpatient Sample (NIS). The NIS is the largest publicly available all-payer inpatient database in the United States and includes roughly 7 million hospitalizations (35 million weighted hospitalizations) from community hospitals in the US.11 Participating states cover >97% of the US population and >96% of all community-hospital discharges, and there is no evidence to suggest there is any selection bias in the dataset. Discharge weights to obtain national estimates were calculated by HCUP and account for changes in sampling over time and the stratified sampling approach. Sampling strata include Census division, location (urban vs. rural), teaching status, hospital control, and bed size. Diagnoses and procedures of interest were captured using International Classification of Disease, 9th revision, Clinical Modification (ICD-9-CM) and International Classification of Disease, 10th revision, Clinical Modification (ICD-10-CM) codes. ICD-9-CM codes were identified using clinical review. ICD-10-CM codes were identified by first using forward and backwards mapping to the Generalized Equivalence Mappings (GEMs) published by CMS and then clinical review.
Hospitalizations of patients diagnosed with an AAA, CAS, or PAD and underwent vascular surgery for their condition were included (Supplemental Table I). Hospitalizations missing patient sex(n=262, unweighted) were excluded.
Statistical Analysis
Descriptive statistics were used to compare patient and hospital characteristics among females and males for each surgery type (AAA, CAS, PAD). When datasets are large (i.e. thousands of observations), studies become over-powered to conduct bivariable analyses. This means, that any difference, no matter how small, will be statistically significant between groups without reflecting clinically meaningful differences. In accordance with the STROBE statement for reporting outcomes from observational studies, we do not include p-value comparisons between the two groups in our description.12
Trends in the yearly rate of vascular surgery between females and males were also assessed. The estimated number of surgeries each year, stratified by type, were obtained from HCUP NIS. The number of males and females ‘at risk’ for surgery (i.e. the number of males and females in the US with AAA, CAS, and PAD) were estimated using two data sources. First, the number of adults living in the United States each year was obtained from US Census data. Then, sex-stratified counts for 2000 and 2010 were directly obtained from US Census summary files.13,14 For the years 2001–2009 and 2011–2016, sex-stratified population estimates were predicted using the 2000 and 2010 available data with linear regression.
Next, sex-specific population estimates of AAA, CAS, and PAD burden were obtained from the National Institute of Health (NIH) and Agency for Healthcare Research and Quality (AHRQ) (Supplemental Table II).1,15,16 We assumed the prevalence of disease was consistent across the study period. The yearly population data was then multiplied by the prevalence of each disease to estimate the number of males and females at risk for vascular surgery each year. Yearly rates, per 100,000 adults with disease, and trends in vascular surgery rates were then estimated using Poisson regression. Time was treated as a continuous, linear variable.
Differences in the use of endovascular surgery and elective admissions between females and males were also assessed. Multivariable logistic regression models were used to compare the odds of each among those undergoing vascular surgery for AAA, CAS, and PAD, respectively. Models were each adjusted for year of surgery, age (treated as a restricted quadratic spline), race/ethnicity, primary insurance type, median ZIP code level income, comorbidities (diabetes, hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease [COPD], renal insufficiency), hospital bed size, hospital location and teaching status, and hospital region. Models assessing odds of endovascular surgery were also adjusted for admission status.
All analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC). The complex sampling design and weighting were appropriately accounted for in all analyses;17 cluster variables included year and hospital ID. Weighted results are reported unless otherwise noted. The Institutional Review Board of the University of North Carolina determined the study met criteria for exemption because only deidentified data were used.
RESULTS
From 2000 to 2016, there were 1,021,684 hospitalizations (4,893,960 weighted hospitalizations included) for adult patients (≥18 years old) undergoing vascular surgery. Overall, 13% underwent surgery for AAA (21% female, 79% male), 40% underwent surgery for CAS (42% female, 58% male), and 47% underwent surgery for PAD (42% female, 58% male). Females were older than males at time of surgery (median age 71.3 vs 69.7), less likely to have private insurance (18% vs 23%), and less likely to have coronary artery disease (CAD, 38% vs 47%) as a comorbidity. Minimal differences were seen across race/ethnicity, other comorbidities, and hospital characteristics (Table I).
Table 1.
Patient and hospital characteristics among adult patients undergoing vascular surgery between 2000 and 2016.
| AAA |
CAS |
PAD |
||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| 140,492 | 524,248 | 817,782 | 1,131,796 | 981,441 | 1,343,431 | |
| (21%) | (79%) | (42%) | (58%) | (42%) | (58%) | |
|
| ||||||
| Age, median (IQR) | 75 (68–80) | 72 (66–77) | 72 (65–78) | 71 (64–77) | 70 (61–78) | 67 (59–75) |
| Race/ethnicity, n (%) | ||||||
| Non-Hispanic White | 99,706 (86) | 387,116 (89) | 586,867 (88) | 825,536 (89) | 584,962 (72) | 851,261 (76) |
| Non-Hispanic Black | 8,597 (7) | 162,209 (4) | 33,338 (5) | 28,372 (3) | 138,728 (17) | 144,865 (13) |
| Hispanic | 3,658 (3) | 14,226 (3) | 25,732 (4) | 39,076 (4) | 61,008 (7) | 85,335 (8) |
| Asian or Pacific Islander | 1,552 (1) | 6,389 (1) | 5,223 (1) | 10,264 (1) | 8,735 (1) | 11,815 (1) |
| Native American | 401 (<1) | 1,756 (<1) | 2,892 (<1) | 3,701 (<1) | 5,200 (1) | 5,957 (1) |
| Other | 2,262 (2) | 8,790 (2) | 12,044 (2) | 18,295 (2) | 17,414 (2) | 26,374 (2) |
| Missing a | 24,316 | 86,762 | 151,686 | 206,552 | 165,393 | 217,825 |
| Primary insurance, n (%) | ||||||
| Private | 17460 (12) | 104647 (20) | 155,664 (19) | 256,277 (23) | 165,449 (17) | 320,739 (24) |
| Medicare/Medicaid | 120240 (86) | 404447 (77) | 639,471 (78) | 842,714 (75) | 784,858 (80) | 961,605 (72) |
| Other/self-pay | 2535 (2) | 13812 (3) | 20,516 (3) | 29,800 (3) | 27,867 (3) | 553,446 (4) |
| ZIP code median income b , n (%) | ||||||
| Low | 30870 (22) | 103447 (20) | 180,730 (22) | 231,434 (21) | 275,421 (29) | 349,408 (27) |
| Medium | 37588 (27) | 137217 (27) | 230,917 (29) | 307,479 (28) | 255,883 (27) | 352,621 (27) |
| High | 335149 (25) | 134618 (26) | 203,592 (25) | 285,771 (26) | 226,126 (23) | 318,770 (24) |
| Highest | 34299 (25) | 138416 (27) | 188,093 (23) | 284,711 (26) | 306,726 (21) | 293,963 (22) |
| Comorbidities, n (%) | ||||||
| Diabetes | 19,856 (14) | 86,150 (16) | 247,353 (30) | 343,122 (30) | 428,592 (44) | 599,660 (45) |
| Hypertension | 86,502 (62) | 311,083 (59) | 599,004 (73) | 775,761 (69) | 569,000 (58) | 728,775 (54) |
| Coronary artery disease | 43,438 (31) | 223,421 (43) | 294,795 (36) | 545,446 (48) | 391,428 (40) | 639,133 (48) |
| Congestive heart failure | 15,677 (11) | 4,956 (10) | 64,700 (8) | 82,625 (7) | 159,312 (16) | 200,946 (15) |
| COPD | 49,132 (35) | 151,311 (29) | 146,548 (18) | 190,167 (19) | 206,410 (21) | 296,266 (22) |
| Renal insufficiency | 13,140 (9) | 48,671 (9) | 42,459 (5) | 73,304 (6) | 157,405 (16) | 231,694 (17) |
| Elective admission, n (%) | 101,669 (73) | 396,897 (77) | 656,923 (81) | 907,698 (81) | 570,380 (59) | 803,549 (61) |
| Hospital size c , n (%) | ||||||
| Small | 11,699 (8) | 42,227 (8) | 78,861 (10) | 106,410 (9) | 97,009 (10) | 134,355 (10) |
| Medium | 29,599 (21) | 109,855 (21) | 190,321 (23) | 260,760 (23) | 230,591 (24) | 311,661 (23) |
| Large | 98,900 (71) | 370,702 (71) | 546,527 (67) | 762,025 (67) | 651,389 (67) | 893,916 (67) |
| Hospital type, n (%) | ||||||
| Urban, teaching | 84,875 (61) | 310,310 (59) | 383,459 (47) | 551,171 (49) | 526650 (54) | 731785 (55) |
| Urban, non-teaching | 47,813 (34) | 184,329 (35) | 362,356 (44) | 488,142 (43) | 387696 (40) | 522238 (39) |
| Rural, non-teaching | 7,511 (5) | 28,145 (5) | 69,895 (9) | 89,881 (8) | 64643 (7) | 85909 (6) |
| Hospital region, n (%) | ||||||
| Northeast | 31,558 (22) | 102,442 (20) | 137,610 (17) | 194,926 (17) | 182,705 (19) | 258,459 (19) |
| Midwest | 36,211 (26) | 129,206 (25) | 204,265 (25) | 282,269 (25) | 244,795 (25) | 328,990 (24) |
| South | 52,175 (37) | 205,304 (39) | 353,426 (43) | 479,419 (42) | 40,356 (41) | 547,678 (41) |
| West | 20,548 (14) | 87,296 (17) | 122,480 (15) | 175,182 (15) | 15,885 (15) | 208,305 (16) |
Abbreviations: AAA, abdominal aortic aneurysm; CAS, carotid artery stenosis; PAD, peripheral artery disease; IQR, interquartile range; COPD, chronic obstructive pulmonary disease
Several states do not report patient race to HCUP (n=177,637 unweighted, 17%)
Between 2000 and 2002 median household income for each patient’s ZIP code was characterized by the following categories: $1–$24,999, $25,000–$34,999 (medium), $35,000–$44,999 (high), and $45,000 and above (highest); from 2003 onward, income was characterized into quartiles
Hospital size categories are based on the number of hospital beds; cut points were chosen for each region and location (rural, non-teaching, urban non-teaching, and urban teaching) combination so that approximately 1/3 of hospitals would appear in each size category
After accounting for disease prevalence, females were still 25% less likely to undergo surgery for AAA and 30% less likely to undergo surgery for PAD, compared to males with the same disease (IRR 0.76, 95% CI 0.75, 0.76 and IRR 0.69, 95% CI 0.69, 0.69, respectively; Table II). Interestingly, minimal difference in treatment rate was noted between males and females with CAS (IRR 0.96, 95% CI 0.95, 0.96). These results were consistent over time (Figure 1). Overall, 36% of females and 37% of males underwent endovascular procedures, compared to open. After adjustment, females, compared to males, were less likely to receive an endovascular procedure compared to open for AAA or CAS (OR 0.55, 95% CI 0.53, 0.57 and OR 0.94, 95% CI 0.91, 0.94), and more likely to receive one for PAD (OR 1.28, 95% CI 1.26, 1.30), Table III. These results were also consistent over time (Figure 2).
Table 2.
Estimated Sex-stratified vascular surgery rates for adults with AAA, CAS, and PAD in the United States, 2000–2016.
| Number of Surgeries | Estimated Disease Prevalencea | Surgery rate per 1000 with disease | IRR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Female | Male | Female | Male | Female | Male | ||
|
| |||||||
| AAA | 140,492 | 524,248 | 20,095,539 | 56,746,591 | 7.0 | 9.2 | 0.76 (0.75, 0.76) |
| CAS | 817,782 | 1,131,796 | 54,257,954 | 71,879,015 | 15.1 | 15.7 | 0.96 (0.95, 0.96) |
| PAD | 981,441 | 1,343,431 | 86,410,816 | 81,336,781 | 11.4 | 16.5 | 0.69 (0.69, 0.69) |
Abbreviations: IRR, incidence rate ratios; CI, confidence intervals; AAA, abdominal aortic aneurysm; CAS, carotid artery stenosis; PAD, peripheral arterial disease
Sex-stratified US population counts were obtained using 2000 and 2010 Census data and modeled using linear regression to get yearly estimates which accounted for population increases; sex-stratified disease prevalence was obtained from the National Institute of Health and Agency for Healthcare Research and Quality (AAA: male 3.0%, female: 1.0%; CAS: male 3.8%, female 2.7%; PAD: male 4.3%, female 4.3%); population estimates and disease prevalence were multiplied together to obtain yearly estimates
Figure 1.
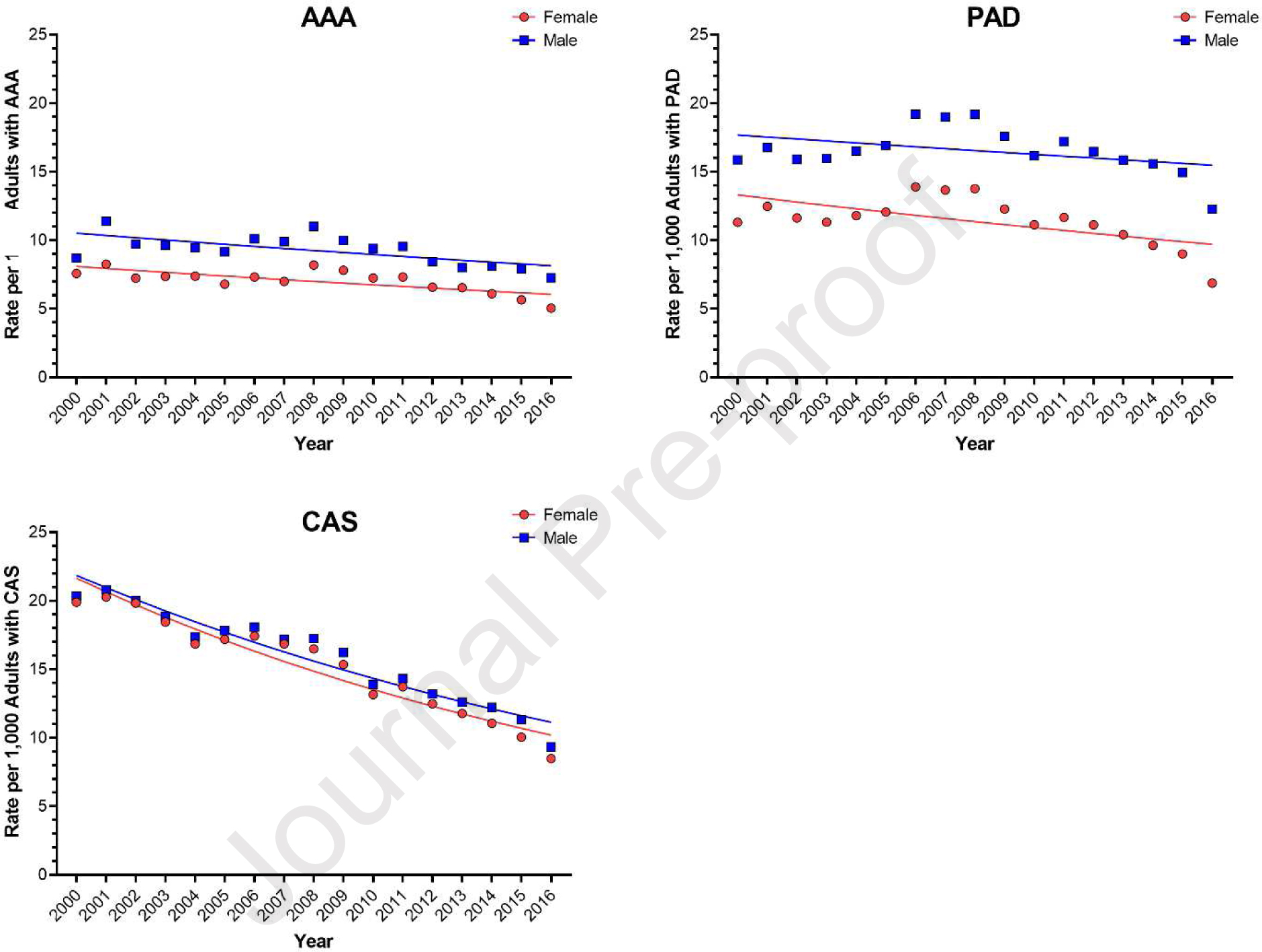
Estimated rates of vascular surgery, among adults with abdominal aortic aneurysm (AAA), peripheral arterial disease (PAD), and carotid artery stenosis (CAS), between 2000 and 2016, stratified by patient sex.
Table 3.
Adjusted odds of females undergoing an endovascular procedure, versus an open procedure, compared to males.
| Female | Male | ||
|---|---|---|---|
| N (%) | N (%) | OR (95% CI)a | |
|
| |||
| Procedure | |||
| AAA | 73,199 (52) | 331,056 (63) | 0.55 (0.53, 0.57) |
| CAS | 68,203 (8) | 106,398 (9) | 0.94 (0.91, 0.96) |
| PAD | 544,861 (56) | 664,137 (49) | 1.28 (1.26, 1.30) |
| Overall | 681,217 (36) | 1,088,358 (37) | 0.93 (0.91, 0.94) |
Abbreviations: AAA, abdominal aortic aneurysm; CAS, carotid artery stenosis; PAD, peripheral artery disease; OR, odds ratio; CI, confidence interval
Adjusted for year of surgery, patient age, race/ethnicity, primary insurance, ZIP code median household income, comorbidities, elective admission, hospital type, hospital bed size, and hospital region
Figure 2.
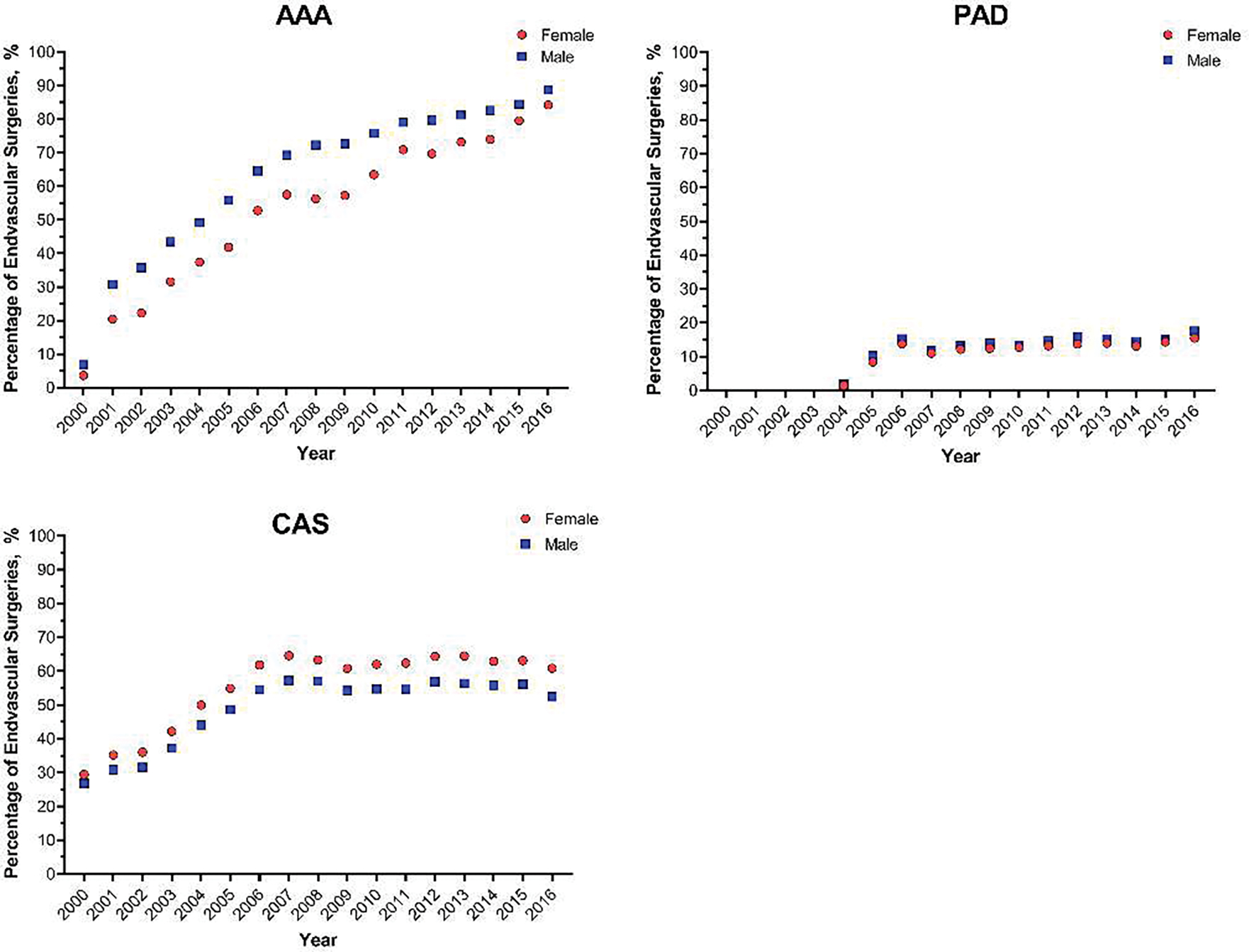
Estimated rates of endovascular operations, among adults with abdominal aortic aneurysm (AAA), peripheral arterial disease (PAD), and carotid artery stenosis (CAS), between 2000 and 2016, stratified by patient sex.
DISCUSSION
This study evaluated a large national dataset to explore sex-related disparities in the management of AAA, CAS, and PAD in the United States. Specifically, we sought to identify sex-related disparities in the number of procedures performed for each disease process, after accounting for known sex-related differences in the prevalence of disease. In addition, we examined whether there were sex-related disparities in type of intervention performed (open versus endovascular). We found that females were significantly less likely to undergo intervention for PAD and AAA than males, even when accounting for sex-specific prevalence estimates. We also found that femaleswere less likely to undergo endovascular intervention for AAA and CAS, and more likely to undergo endovascular intervention for PAD.
Healthcare disparities, in general, is not a new topic of interest. Over the past 15 years, multiple studies have been conducted to evaluate race/ethnicity, income, and sex/gender-related disparities within a variety of specialties. A study on catheter use and dialysis access in the US revealed that females and minorities spent more time on a central venous catheter, when compared to their male counterparts, despite efforts to transition patients to permanent access more quickly.18 Another study demonstrated significant disparities in the presentation, management, and outcomes between males and females with acute myocardial infarctions.19 One article even assessed the biological and environmental factors contributing to racial, ethnic, and sex disparities in vascular surgery.20 While biology and anatomy related to sex differences may account for some of the differences in care, it is quite possible that an additional explanation is that disparities are related to gender as well. Exposing potential disparities is the first step in improving care, and our aim was to highlight sex-disparities in the treatment of three major disease entities in vascular surgery: AAA, CAS, and PAD.
AAA
The overall prevalence of AAA in the population is approximately 5%, but it is estimated that males are up to six times more likely to develop an AAA than females.1,21,22 Due to the assumption that females are disproportionately unaffected by aneurysmal disease, they have been underrepresented in randomized control trials.23 Although males are more likely to develop an AAA, multivariable analyses have shown that, once developed, the growth rate of aortic aneurysms is twice as fast in females compared to males.1,9,24 Females with AAA tend to present later, have a greater number of comorbidities, and are more likely to rupture at smaller aneurysm size with a higher rate of fatality (14% vs 5%).25–28 There are also a handful of studies showing that menopausal females have a higher risk of developing an AAA than younger females, suggesting that estrogen has a premenopausal protective effect.29–32 Current SVS guidelines recommend ultrasound screening for AAA for all males ≥ 65 and for females ≥ 65 with a history of smoking or family history of AAA. These guidelines also suggest that females with an AAA measuring 5.0–5.4 cm may benefit from early repair.32 Even though some efforts have been made to address specific guidelines for females with AAA, further investigation into sex-specific AAA screening recommendations, size threshold for AAA repair in females, and the impact of sex hormones on aneurysmal development will be important in improving the diagnosis and treatment of females with AAA.
PAD
In regards to PAD, our results showed that females were 30% less likely to undergo surgery than males, which is especially surprising considering the estimated prevalence of PAD is the same for males and females (4.3%).17 Females with PAD tend to have atypical symptoms, present later, and are more likely to have chronic limb threatening ischemia at presentation compared to males.34,35 Discrepancies in the description of lower-extremity pain between males and females, even when PAD is known, have been known to influence the diagnosis and treatment plan for females, regardless of objective data.36 Evidence describing the efficacy of lower extremity revascularization for PAD is largely derived from predominantly male study populations.37 Considering PAD studies have shown that female sex is associated with more advanced disease, incisional complications, early graft failure, and increased risk for 30-day morbidity and mortality, female-focused studies are severely lacking.38–44 Future areas of research for females with PAD should focus on biological, clinical, anatomic, and procedural characteristics. Interestingly, in this cohort, females were more likely to undergo endovascular revascularization for infrainguinal PAD than males, so this will be particularly important to evaluate. In the meantime, PAD in females should be approached with a high index of suspicion, liberal use of screening, and aggressive risk factor modification.
CAS
Intervention rates for CAS were the most comparable between males and females (IRR = 0.96). Although our results indicate that females are receiving an appropriate number of procedures for CAS in comparison to males based on prevalence values, females have been consistently underrepresented in CAS clinical trials.5–7 Studies have shown that 1 in 5 females in the US will have a stroke in their lifetime and, compared to males, females tend to have more severe strokes that result in worse return to baseline.45–47 This indicates that females could still benefit from an increase in sex-specific or sex comparison CAS studies.
Endovascular vs Open Procedures.
There are known anatomical differences in stature and blood vessel size between males and females, with females typically having smaller vessel diameter.9,10 As hypothesized, we found that females were less likely to receive an endovascular repair for AAA and CAS. Previous studies have shown that females with AAA have a higher 30-day mortality than males for both open and endovascular procedures, females had a higher rate of periprocedural stroke during carotid artery stenting than with carotid endarterectomy (5.5% vs 2.2%), while there was no difference identified in males, and regardless of the type of operation for PAD, females had increased mortality compared to males.10,41,48 However, we were surprised to find females were more likely to receive an endovascular repair for PAD. This may be an artifact of the NIS database, which only includes inpatient procedures. Females are more likely to be admitted to the hospital after peripheral vascular interventions compared to males and may be skewing the dataset.49,50 Further sex-specific randomized control trials are necessary to improve the efficacy of treatment for AAA, CAS, and PAD in females.
Limitations
This study is not without limitations. We used sex-specific prevalence estimates of AAA, CAS, and PAD from the NIH and AHRQ, but prevalence estimates for these diseases vary depending on the literature, with sex-specific estimates being especially variable. This variation could be attributed to females frequently presenting with atypical symptoms for AAA, CAS, and PAD, making diagnosis of these diseases difficult. These difficulties may have led to an under-diagnosis of these vascular diseases and could have skewed prevalence estimates from the true values. Therefore, we caution that the overall sex-specific prevalence estimates of AAA, CAS, and PAD used throughout this paper are speculative. A large epidemiologic sex-specific natural history study is necessary to determine the true burden of AAA, CAS, and PAD in females.
While the procedure counts are more accurate, they may be overestimated for both males and females since we are unable to assess repeat procedures in this de-identified dataset. We expect that this impact is minimal since the overall rates of surgery are low (7–16.5 operations per 1000 with disease) and repeat operations are not that common, particularly in carotid and aortic disease. Additionally, the procedure rates for all PAD, AAA, and CAS procedures for both males and females declined steadily over the 16 year study period (Figure 1). Our findings of declining operative rates in AAA and CAS are consistent with other studies. Our study reports a decline in operations for PAD that require an overnight hospital stay. Since there has been a dramatic rise in outpatient endovascular PAD procedures, which are not captured in this dataset, it appears as if treatment for PAD is declining when that is not the case. This, accompanied by the switch from ICD-9-CM to ICD-10-CM in 2015, may have impacted our findings.
Additionally, there are inherent limitations to using a large dataset like the NIS, which only captures a nationally representative sample of inpatient hospitalizations and not outpatient treatment. Specific variables related to unique patient factors, including indications for treatment, and operative technical details are not available, and we must rely on diagnosis and procedure codes. Due to the limited number of covariates that can be controlled for, there could be other reasons that explain the differences in treatment rates making the true difference smaller than we report; however, disease characteristics (e.g., aneurysm growth rate or burdens of atherosclerosis) could actually make the treatment disparity based on disease severity even greater than reported. Furthermore, the switch to ICD-10-CM in October 2015 may have impacted our findings. We attempted to mitigate some of the discrepancies due to the switch to ICD-10-CM by using forward and backward mapping with GEMs, but discrepancies in the coding may still exist. Finally, the sampling strategy for the NIS was altered in 2012, from a 20% stratified random sample of hospitals to a 20% stratified random sample of hospitalizations,51 although the updated weighting schemes used to account for this change was utilized in our study. This change in sampling resulted in a slight decrease in total hospitalizations in 2012.
Future Directions
Our results showed that females were significantly less likely to undergo intervention for PAD and AAA than males, even after accounting for sex-specific prevalence estimates. We also found that females are less likely to undergo endovascular intervention for AAA and CAS, and more likely to undergo endovascular intervention for PAD. Underrepresentation of females in clinical trials makes treatment decisions and expected outcomes difficult to generalize to this population. Further randomized trials specifically addressing endovascular and open treatment strategies in females are needed to improve technical decision making, treatment efficacy, and evaluation of long-term outcomes. Further investigation into sex-specific screening recommendations, comorbidity management, and objective thresholds for offering treatment is needed in order to address the under treatment of AAA and PAD in females. As we begin to understand more about sex-related concepts, addressing the intersectionality between sexand race will be important as well.
CONCLUSION
This study identified significant sex-related disparities in the treatment of AAA, PAD and CAS in the United States. Based on our analysis of the NIS from 2000–2016, females were undertreated for AAA and PAD.
Supplementary Material
Type of Research:
Retrospective review of the National Inpatient Sample Database
Key Findings:
In over 1 million hospitalizations from 2000–2016, even after accounting for differences in disease prevalence, females were 25% less likely to undergo surgery for AAA and 30% less likely to undergo surgery for PAD, compared to males with the same disease. No difference in treatment rates were noted for carotid artery stenosis.
Take home Message:
There are significant sex-related disparities in the treatment of AAA and PAD in the United States. Accounting for disease prevalence, females were undertreated for AAA and PAD.
Footnotes
Presentation: This work was presented as a poster at the 2020 Vascular Annual Meeting of the Society for Vascular Surgery, virtual meeting, June 2020.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lo RC, Schermerhorn ML. Abdominal aortic aneurysms in women. J Vasc Surg. 2016;63(3):839–844. doi: 10.1016/j.jvs.2015.10.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven ELG, Cuypers PWM, et al. Long-Term Outcome of Open or Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2010;362(20):1881–1889. doi: 10.1056/NEJMoa0909499 [DOI] [PubMed] [Google Scholar]
- 3.Ashton HA, Buxton MJ, Day NE, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet Lond Engl. 2002;360(9345):1531–1539. doi: 10.1016/s0140-6736(02)11522-4 [DOI] [PubMed] [Google Scholar]
- 4.Weinkauf C, George E, Zhou W. Open versus endovascular aneurysm repair trial review. Surgery. 2017;162(5):974–978. doi: 10.1016/j.surg.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Goldstein LB Carotid Endarterectomy for Asymptomatic Carotid Stenosis. Stroke. 2004;35(10):2425–2427. doi: 10.1161/01.STR.0000141706.50170.a7 [DOI] [PubMed] [Google Scholar]
- 6.Ferguson Gary G, Michael Eliasziw, Barr Hugh WK, et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30(9):1751–1758. doi: 10.1161/01.STR.30.9.1751 [DOI] [PubMed] [Google Scholar]
- 7.Howard VJ, Lutsep HL, Mackey A, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol. 2011;10(6):530–537. doi: 10.1016/S1474-4422(11)70080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson RA, Meecham LA, Hewitt CA, Bradbury AW. Comparison of Immediate and Long-term Outcomes in Men and Women Undergoing Revascularisation for Chronic Limb Threatening Ischaemia in the Bypass vs. Angioplasty in Severe Ischaemia of the Leg (BASIL-1) Trial. Eur J Vasc Endovasc Surg. 2019;58(2):224–228. doi: 10.1016/j.ejvs.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 9.Katie Cheung, Munir Boodhwani, Leung Chan Kwan, Luc Beauchesne, Alexander Dick, Thais Coutinho. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J Am Heart Assoc. 6(2):e003792. doi: 10.1161/JAHA.116.003792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rango P, Brown MM, Didier L, Howard VJ, Moore WS, Paciaroni M, et al. Management of carotid stenosis in women. Neurology. 2013;80(24):2258–2268. doi: 10.1212/WNL.0b013e318296e952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality R, MD. In. [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2000 Census Summary File 1 [SF1]. https://www.census.gov/data/datasets/2000/dec/summary-file-1.html
- 14.2010 Census Summary File 1 [SF1]. https://www.census.gov/data/datasets/2010/dec/summary-file-1.html
- 15.Mathiesen EB, Joakimsen O, Bønaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromsø Study. Cerebrovasc Dis Basel Switz. 2001;12(1):44–51. doi: 10.1159/000047680 [DOI] [PubMed] [Google Scholar]
- 16.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0 [DOI] [PubMed] [Google Scholar]
- 17.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA. 2017;318(20):2011–2018. doi: 10.1001/jama.2017.17653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arya S, Melanson TA, George EL, Rothenberg KA, Kurella Tamura M, Patzer RE, et al. Racial and Sex Disparities in Catheter Use and Dialysis Access in the United States Medicare Population. J Am Soc Nephrol JASN. 2020;31(3):625–636. doi: 10.1681/ASN.2019030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liakos M, Parikh PB. Gender Disparities in Presentation, Management, and Outcomes of Acute Myocardial Infarction. Curr Cardiol Rep. 2018;20(8):64. doi: 10.1007/s11886-018-1006-7 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen LL, Henry AJ. Disparities in Vascular Surgery: Is It Biology or Environment? J Vasc Surg. Published online April 2010. doi: 10.1016/j.jvs.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villard C, Hultgren R. Abdominal aortic aneurysm: Sex differences. Maturitas. 2018;109:63–69. doi: 10.1016/j.maturitas.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 22.Boese AC, Chang L, Yin K-J, Chen YE, Lee J-P, Hamblin MH. Sex differences in abdominal aortic aneurysms. Am J Physiol-Heart Circ Physiol. 2018;314(6):H1137–H1152. doi: 10.1152/ajpheart.00519.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoel AW, Kayssi A, Brahmanandam S, Belkin M, Conte MS, Nguyen LL. Underrepresentation of women and ethnic minorities in vascular surgery randomized controlled trials. J Vasc Surg. 2009;50(2):349–354. doi: 10.1016/j.jvs.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mofidi R, Goldie VJ, Kelman J, Dawson ARW, Murie JA, Chalmers RTA. Influence of sex on expansion rate of abdominal aortic aneurysms. BJS Br J Surg. 2007;94(3):310–314. doi: 10.1002/bjs.5573 [DOI] [PubMed] [Google Scholar]
- 25.Nienaber Christoph A, Rossella Fattori, Mehta Rajendra H, et al. Gender-Related Differences in Acute Aortic Dissection. Circulation. 2004;109(24):3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C [DOI] [PubMed] [Google Scholar]
- 26.Liang NL, Genovese EA, Al-Khoury GE, Hager ES, Makaroun MS, Singh MJ. Effects of Gender Differences on Short-term Outcomes in Patients with Type B Aortic Dissection. Ann Vasc Surg. 2017;38:78–83. doi: 10.1016/j.avsg.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(1):17–28. doi: 10.1016/S0003-4975(01)03236-2 [DOI] [PubMed] [Google Scholar]
- 28.Skibba AA, Evans JR, Hopkins SP, Yoon HR, Katras T, Kalbfleisch JH, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62(6):1429–1436. doi: 10.1016/j.jvs.2015.07.079 [DOI] [PubMed] [Google Scholar]
- 29.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, et al. Gender Differences in Experimental Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 2004;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84 [DOI] [PubMed] [Google Scholar]
- 30.Dehaini H, Fardoun M, Abou-Saleh H, El-Yazbi A, Eid AA, Eid AH. Estrogen in vascular smooth muscle cells: A friend or a foe? Vascul Pharmacol. 2018;111:15–21. doi: 10.1016/j.vph.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 31.Wu X-F, Zhang J, Paskauskas S, Xin S-J, Duan Z-Q. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg. 2009;197(1):49–54. doi: 10.1016/j.amjsurg.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 32.Villard C, Eriksson P, Kronqvist M, Lengquist M, Jorns C, Hartman J, et al. Differential expression of sex hormone receptors in abdominal aortic aneurysms | Elsevier Enhanced Reader. doi: 10.1016/j.maturitas.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 33.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: Executive summary. J Vasc Surg. 2009;50(4):880–896. doi: 10.1016/j.jvs.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 34.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic Peripheral Arterial Disease Is Independently Associated With Impaired Lower Extremity Functioning. Circulation. 2000;101(9):1007–1012. doi: 10.1161/01.CIR.101.9.1007 [DOI] [PubMed] [Google Scholar]
- 35.Schramm K, Rochon P. Gender Differences in Peripheral Vascular Disease. - Abstract - Europe PMC. Accessed April 29, 2020. https://europepmc.org/article/PMC/5886764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigvant B, Lundin F, Nilsson B, Bergqvist D, Wahlberg E. Differences in presentation of symptoms between women and men with intermittent claudication. BMC Cardiovasc Disord. 2011;11(1):39. doi: 10.1186/1471-2261-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, et al. A Call to Action: Women and Peripheral Artery Disease. Circulation. 2012;125(11):1449–1472. doi: 10.1161/CIR.0b013e31824c39ba [DOI] [PubMed] [Google Scholar]
- 38.Harthun NL, Cheanvechai V, Graham LM, Freischlag JA, Gahtan V. Arterial occlusive disease of the lower extremities: do women differ from men in occurrence of risk factors and response to invasive treatment? J Thorac Cardiovasc Surg. 2004;127(2):318–321. doi: 10.1016/j.jtcvs.2003.10.020 [DOI] [PubMed] [Google Scholar]
- 39.Moitra VK, Flynn BC, Mazzeffi M, Bodian C, Bronheim D, Ellis JE. Indication for Surgery, the Revised Cardiac Risk Index, and 1-Year Mortality. Ann Vasc Surg. 2011;25(7):902–908. doi: 10.1016/j.avsg.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 40.Flu HC, Tamsma JT, Lindeman JHN, Hamming JF, Lardenoye JHP. A Systematic Review of Implementation of Established Recommended Secondary Prevention Measures in Patients with PAOD. Eur J Vasc Endovasc Surg. 2010;39(1):70–86. doi: 10.1016/j.ejvs.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 41.Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51(4):878–885.e1. doi: 10.1016/j.jvs.2009.10.102 [DOI] [PubMed] [Google Scholar]
- 42.Lancaster RT, Conrad MF, Patel VI, Cambria RP, LaMuraglia GM. Predictors of Early Graft Failure After Infrainguinal Bypass Surgery: A Risk-adjusted Analysis from the NSQIP. Eur J Vasc Endovasc Surg. 2012;43(5):549–555. doi: 10.1016/j.ejvs.2012.01.026 [DOI] [PubMed] [Google Scholar]
- 43.Soma G, Greenblatt DY, Nelson MT, Rajamanickam V, Havlena J, Fernandes-Taylor S, et al. Early graft failure after infrainguinal arterial bypass. Surgery. 2014;155(2):300–310. doi: 10.1016/j.surg.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 44.Brahmbhatt R, Brewster LP, Shafii S, et al. Gender and frailty predict poor outcomes in infrainguinal vascular surgery. J Surg Res. 2016;201(1):156–165. doi: 10.1016/j.jss.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 45.Persky RW, Turtzo LC, McCullough LD. Stroke in Women: Disparities and Outcomes. Curr Cardiol Rep. 2010;12(1):6–13. doi: 10.1007/s11886-009-0080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu C, An Z, Zhao W, Wang W, Gao C, Liu S, et al. Sex Differences in Stroke Subtypes, Severity, Risk Factors, and Outcomes among Elderly Patients with Acute Ischemic Stroke. Front Aging Neurosci. 2015;7. doi: 10.3389/fnagi.2015.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jim J, Dillavou ED, Upchurch GR, Osborne NH, Kenwood CT, Siami FS, et al. Gender-specific 30-day outcomes after carotid endarterectomy and carotid artery stenting in the Society for Vascular Surgery Vascular Registry. J Vasc Surg. 2014;59(3):742–748. doi: 10.1016/j.jvs.2013.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT. Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet Lond Engl. 2017;389(10088):2482–2491. doi: 10.1016/S0140-6736(17)30639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bechter-Hugl B, Falkensammer J, Gorny O, Greiner A, Chemelli A, Fraedrich G. The influence of gender on patency rates after iliac artery stenting. Journal of Vascular Surgery. 2014;59(6):1588–1596. doi: 10.1016/j.jvs.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 50.Ortiz D, Jahangir A, Singh M, Allaqaband S, Bajwa T, Mewissen M. Access Site Complications Following Peripheral Vascular Interventions: Incidence, Predictors and Outcomes. Circ Cardiovasc Interv. 2014;7(6):821–828. doi: 10.1161/CIRCINTERVENTIONS.114.001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide inpatient sample (NIS) redesign final report. U.S. Agengy for Healthcare Research and Quality. Published online 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


