Abstract
Background
Obesity is accompanied by low-grade inflammation and leucocytosis and increases the risk of venous thromboembolism. Associations with platelet count, however, are unclear, because several studies have reported positive associations only in women. Associations with body shape are also unclear, because waist and hip circumferences reflect overall body size, as well as body shape, and are correlated strongly positively with body mass index (BMI).
Methods
We evaluated body shape with the allometric body shape index (ABSI) and hip index (HI), which reflect waist and hip size among individuals with the same weight and height and are uncorrelated with BMI. We examined the associations of BMI, ABSI, and HI with platelet count, mean platelet volume (MPV), and platelet distribution width (PDW) in multivariable linear regression models for 125,435 UK Biobank women and 114,760 men. We compared men with women, post-menopausal with pre-menopausal women, and older (≥ 52 years) with younger (< 52 years) men.
Results
BMI was associated positively with platelet count in women, more strongly in pre-menopausal than in post-menopausal, and weakly positively in younger men but strongly inversely in older men. Associations of BMI with platelet count were shifted towards the inverse direction for daily alcohol consumption and current smoking, resulting in weaker positive associations in women and stronger inverse associations in men, compared to alcohol ≤ 3 times/month and never smoking. BMI was associated inversely with MPV and PDW in pre-menopausal women but positively in post-menopausal women and in men. ABSI was associated positively with platelet count, similarly in women and men, while HI was associated weakly inversely only in women. ABSI was associated inversely and HI positively with MPV but not with PDW and only in women. Platelet count was correlated inversely with platelet size and positively with leucocyte counts, most strongly with neutrophils.
Conclusions
Competing factors determine the associations of BMI with platelet count. Factors with sexually dimorphic action (likely thrombopoietin, inflammatory cytokines, or cortisol), contribute to a positive association, more prominently in women than in men, while age-dependent factors (likely related to liver damage and fibrosis), contribute to an inverse association, more prominently in men than in women.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-023-00494-y.
Keywords: Obesity, Body shape, Waist size, Hip size, ABSI, Platelets
Highlights
We examined a large middle-aged population-based cohort, excluding participants with prevalent cancer, endocrine and metabolic conditions, and severe illnesses.
Body mass index (BMI) was associated positively with platelet count in women (strongest in pre-menopausal women) and inversely in men (strongest in older men), but inversely with platelet size in pre-menopausal women and positively in post-menopausal women and men.
Alcohol consumption and smoking shifted the association of BMI with platelet count towards the null for women and towards a stronger inverse association for men, but did not affect the associations with waist or hip size.
Waist size was associated positively with platelet count in women and men, with only a weak inverse association for hip size.
Waist size was associated inversely and hip size positively with platelet size, mainly in women.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-023-00494-y.
Background
Obesity is associated with chronic low-grade inflammation and a higher risk of venous thromboembolism [1, 2] and contributes to the development of cardiovascular diseases and some cancers [3, 4]. Although platelets have long been considered mainly participants in haemostasis and thrombosis, it is clear now that they also act as inflammatory effector cells and modulate leucocyte-mediated sterile inflammatory processes, such as atherosclerosis [5]. Platelets carry angiogenic growth factors which facilitate cancer development and, when activated by cancer cells, regulate immune cell migration towards the tumour cite and contribute to cancer metastasis and progression [5, 6]. Correspondingly, high platelet count, even when remaining within the clinical reference range, has been associated with cancer development [7, 8]. Further to platelet count, large platelet size, as reflected in large mean platelet volume (MPV), and variable platelet size, as reflected in large platelet size distribution width (PDW), are indicative of platelet activation and thrombosis [9, 10], and have also been associated with diabetes mellitus, diabetes-related complications, and some cancers [11].
We have previously shown that in UK Biobank, inflammatory biomarkers represented by C-reactive protein and leucocyte subtypes, including lymphocytes, monocytes, and neutrophils, were higher for higher body mass index (BMI), both in women and men [12]. Associations of platelet count with obesity, however, are unclear. Studies investigating platelets in relation to obesity are small scale and limited. Some have not examined separately women and men [13, 14] and those that have separated them have reported higher platelet count with obesity only in women but not in men [15, 16]. Studies on platelet size are also small scale and limited, reporting an inverse correlation of MPV with platelet count, but larger platelet size in obesity [17].
Unclear is also the association of body shape with platelets, not least because waist and hip circumferences, traditionally used to evaluate body shape, are dependent on overall body size and are correlated strongly positively with BMI [18]. The allometric body shape index (ABSI) and hip index (HI), on the other hand, reflect waist and hip size among individuals with the same weight and height and are uncorrelated with BMI [19, 20]. We have previously shown that in UK Biobank, mainly ABSI but not HI were associated with inflammatory biomarkers, represented by C-reactive protein (CRP), neutrophils and monocytes and only lymphocytes resembled glycemia-related biomarkers, with an additional inverse association with hip size [12]. Clarifying the associations of body shape with platelets matters, because the cardiometabolic complications of obesity depend on fat distribution, with visceral fat being associated with higher risk and gluteofemoral fat with lower risk [21].
In this study, we have used UK Biobank data to examine the association of body size and body shape, evaluated with allometric anthropometric indices, with platelet count and size. To clarify sex differences, we have examined separately and have compared men with women. To clarify the potential influence of endogenous sex steroids, we have compared post-menopausal with pre-menopausal women, which differ dramatically with respect to oestrogen production, and older with younger men. We have also examined heterogeneity of the associations with body size according to alcohol consumption and smoking, to account for differences in these lifestyle characteristics between women and men, and heterogeneity in the associations with body shape according to body size.
Methods
Study population
UK Biobank includes half a million individuals from the general population of England, Scotland, and Wales, living within 40 km of an assessment centre and aged 40–70 years at recruitment (between 2006 and 2010) [22]. As in our previous study examining associations of body size and body shape with metabolic and inflammatory biomarkers [12], we restricted the data set to participants with self-reported white ancestry and excluded participants with missing or extreme anthropometric measurements, genetically determined sex not matching the self-reported sex, and pregnant women. To minimise the influence of underlying medical conditions, which could influence body size and shape and haematological and biomarker measurements, we similarly excluded participants with prevalent cancer at recruitment (defined as in [23]), incident cancer or death within 2 years after recruitment, self-reported diabetes mellitus, or endocrine non-cancer illness, or chronic respiratory disease, or inflammatory bowel disease, or liver disease, or kidney or heart failure at recruitment, or receiving lipid lowering drugs, or exogenous steroids, or anti-hypertensive drugs at recruitment. For this study, we additionally excluded participants with cardiovascular and haematological conditions, or receiving anticoagulants, or missing all platelet measurements. The total number of excluded participants was 262,218 (52.2%). For further details on exclusions see Additional file 1: Table S1.
Anthropometric indices
Trained UK Biobank technicians measured waist circumference at the natural indent or the umbilicus and hip circumference at the widest point [24]. We calculated ABSI for women and men and HI for women with coefficients from the National Health and Nutrition Examination Survey (NHANES) [19, 20], multiplying ABSI by 1000 to obtain numbers in the magnitude of waist circumference. For HI in men, we used coefficients previously derived from UK Biobank data [18], because HI, calculated with the original coefficients from NHANES, was correlated inversely with BMI in UK Biobank men [23]. (WC—waist circumference; HC—hip circumference)
To standardise the anthropometric indices on a continuous scale, we calculated sex-specific z-scores (value minus mean, divided by standard deviation, SD). Similarly to our previous study [18], we dichotomised ABSI (cutoffs ≥ 73 for women, ≥ 80 for men) and HI (cutoffs ≥ 64 for women, ≥ 49 for men) and defined body shape phenotypes with an ABSI-by-HI cross-classification as “pear”—small-ABSI–large-HI (reference), “slim”—small-ABSI–small-HI, “wide”—large-ABSI–large-HI, “apple”—large-ABSI–small-HI. For categorisation of BMI, we used World Health Organisation criteria and defined normal weight (BMI ≥ 18.5 to < 25 kg/m2, reference), overweight (BMI ≥ 25 to < 30 kg/m2), and obese (BMI ≥ 30 to < 45 kg/m2). To examine heterogeneity of the associations with body shape according to body size, we defined a combined cross-classification BMI-by-ABSI-by-HI (“pear” normal weight reference).
Haematological and biomarker measurements
UK Biobank participants provided blood samples throughout the day (8 am to 9 pm), irrespective of fasting status. Samples for haematological measurements were collected in EDTA (ethylenediaminetetraacetic acid) vacutainers and were analysed within 24 h of blood draw on Beckman Coulter LH750 automated analysers [25]. The analysers measured directly platelet count (109/L), but derived platelet size, represented by MPV (fL) and PDW (%), from scatter plots and histograms of platelet size. In very few participants, haematological measurements were recorded as zero and we replaced these with half of the lowest non-zero value. We have previously described the measurements for metabolic and inflammatory biomarkers, liver function test, sex hormone binding globulin (SHBG), and sex steroids [12, 26]. In this study, we examined only the correlation of blood biomarkers with platelet count and size. As our previous study on sex steroids included fewer exclusions [26], we imputed testosterone in women using, as previously, quantile regression imputation of truncated left-censored data (QRILC) [27], but estimated the parameters of the distribution based on the restricted data set of the current study. In addition, as previously [26], we calculated free testosterone with law-of-mass-action equations [28].
To mitigate the influence of right-skewness of the distributions, we log-transformed all haematological and biomarker measurements. To provide a standardised scale for comparability, we calculated sex-specific z-scores.
Statistical analysis
We examined separately women overall and men overall and the following subgroups: pre-menopausal and post-menopausal women, and younger (< 52 years at recruitment) and older (≥ 52 years) men. Menopausal status was self-reported, considering women with bilateral oophorectomy as post-menopausal and women with hysterectomy as undetermined menopausal status [12]. The age cutoff in men was selected to provide mean ages of the subgroups in men comparable to the mean ages of the subgroups by menopausal status in women.
We calculated SD differences (95% confidence intervals) for each platelet parameter with multivariable linear regression models using three combinations of the anthropometric indices as exposure variables. To examine associations with body size and body shape independent of each other, we used an additive model including BMI, ABSI, and HI on a continuous scale (interpreting estimates as SD difference in platelet parameters per one SD increment of the anthropometric index). To examine associations with body shape phenotypes, we used an additive model including body shape phenotypes (ABSI-by-HI cross-classification, “pear” reference, “slim”, “wide”, “apple”) and BMI categories (normal weight reference, overweight, obese) (interpreting estimates as SD difference in platelet parameters compared to the reference category). To examine heterogeneity of the associations with body shape according to body size, we used an interaction model including BMI-by-ABSI-by-HI cross-classification. As in our previous study [12], we adjusted all models for height (continuous), age at recruitment (continuous), weight change within the last year preceding recruitment (weight loss, stable weight, weight gain), smoking status (never, former occasional, former regular, current), alcohol consumption (≤ 3 times/month, ≤ 4 times/week, daily), physical activity (active, moderately active, inactive), Townsend deprivation index (sex-specific tertiles), region of the assessment centre, time of blood collection (8:00 to < 12:00, 12:00 to < 16:00, 16:00 to ≤ 20:15), fasting time (0–2 h, 3–4 h, ≥ 5 h), use of nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol use, and for women also menopausal status (pre-menopausal, post-menopausal, undetermined, only for women overall), HRT use (never or former, for women overall and post-menopausal), oral contraceptives use (never or former), and age at the last live birth (no live births, < 30 years, ≥ 30 years). We replaced the limited number of missing values for covariates (< 2%, see Additional file 1: Table S2) with the median sex-specific value or category.
We used Wald tests to evaluate the statistical significance of individual terms. Separately for women and men and within subgroups by menopausal status and age, we used likelihood ratio tests to evaluate the significance of the associations with body shape phenotypes overall, comparing a model including BMI categories and covariates with a model additionally including ABSI-by-HI cross-classification, as in our previous study [12]. To evaluate heterogeneity of the associations with body shape phenotypes according to BMI, we compared with a likelihood ratio test the additive model including ABSI-by-HI cross-classification, BMI categories, and covariates, with the interaction model including BMI-by-ABSI-by-HI cross-classification and covariates. To evaluate heterogeneity of the associations with anthropometric indices according to alcohol consumption or smoking status, we compared with a likelihood ratio test models with and without interaction terms of BMI, ABSI, or HI with alcohol consumption or smoking status categories (a separate model for each combination of an anthropometric index and a lifestyle variable).
To evaluate differences in the associations of body size with platelet parameters according to sex, we used an interaction term of BMI with sex (men vs. women) from a model including women and men, adjusted for ABSI, HI, and all covariates (except female-specific), and additionally including an interaction term of age with sex, to account for potential differences by menopausal status in women. To evaluate differences in the associations of body shape with platelet parameters according to sex, we used interaction terms of ABSI and HI with sex, both in the same model, including women and men and adjusted for BMI and all co-variates (except female-specific) and also including an interaction term of age with sex. To evaluate differences according to menopausal status in women, we similarly used interaction terms of anthropometric indices with menopausal status (post-menopausal vs. pre-menopausal). To evaluate differences according to age in men, we used interaction terms with age on a continuous scale. To evaluate differences in body shape phenotypes between women and men, we used likelihood ratio tests comparing fully adjusted models (including an interaction of age with sex) with and without interaction terms of ABSI-by-HI or BMI-by-ABSI-by-HI with sex. Tests for statistical significance were two-sided. We considered p < 0.05 as suggestive, p < 0.001 as evidence for association (equivalent to Bonferroni correction for 50 comparisons), and p < 1 × 10–6 as strong evidence for association (equivalent to Bonferroni correction for 50,000 comparisons).
To examine the associations of platelet parameters with liver function test, metabolic, inflammatory, and sex steroid biomarkers, we calculated partial Pearson correlation coefficients with adjustment for BMI, ABSI, and HI (continuous scale, z-scores), and covariates (except for region of the assessment centre), in a subset of participants with available all biomarker measurements.
In sensitivity analyses, we examined fully adjusted additive models, including BMI categories and tertiles of ABSI and HI, to explore potential non-linearity. To explore the influence of recent weight change, we restricted the data set to participants with stable weight within the year preceding recruitment. To explore the influence of medication use, we restricting the data set to participants not receiving NSAIDs or paracetamol. To explore the influence of covariates, we examined unadjusted models.
We used R version 4.1.3 for all analyses [29].
Results
Cohort characteristics
The study included 125,435 women and 114,760 men. Women overall had lower BMI and ABSI compared to men and lower PDW but higher platelet count and MPV (Table 1). Post-menopausal women had higher BMI and ABSI and a substantially larger proportion of “wide” phenotype compared to pre-menopausal women, but lower platelet counts and MPV. Older men also had higher ABSI and a substantially larger proportion of “wide” phenotype compared to younger men, as well as lower platelet counts and MPV, but had similar BMI (Table 1). Men were more likely to be current smokers, or to consume alcohol daily, as we have previously described [12], but in both sexes, there was no major overlap between daily alcohol consumption and current smoking (Additional file 1: Table S2). Post-menopausal women and older men were more likely to consume alcohol daily compared to pre-menopausal women and younger men, correspondingly, but were less likely to be current smokers (Additional file 1: Table S2), as we have previously described for a larger part of the UK Biobank data set [26].
Table 1.
Anthropometric characteristics and platelet parameters of study participants
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Overall | Pre-MP | Post-MP | Overall | < 52 years | ≥ 52 years | |
| Cohort: n (% sex) | 125,435 (52.2) | 37,917 (30.2) | 75,491 (60.2) | 114,760 (47.8) | 44,593 (38.9) | 70,167 (61.1) |
| Age (years)a,*** | 55.5 (7.9) | 46.6 (3.9) | 60.1 (5.3) | 55.0 (8.1) | 46.3 (3.3) | 60.4 (4.8) |
| Anthropometric characteristicsa,*** | ||||||
| BMI (kg/m2) | 26.2 (4.4) | 25.9 (4.6) | 26.2 (4.3) | 27.1 (3.7) | 27.3 (3.9) | 27.0 (3.6) |
| Weight (kg) | 69.7 (12.3) | 70.0 (12.9) | 69.2 (11.8) | 84.5 (12.9) | 86.0 (13.5) | 83.6 (12.5) |
| Height (cm) | 163.1 (6.2) | 164.4 (6.2) | 162.4 (6.1) | 176.5 (6.7) | 177.6 (6.7) | 175.8 (6.6) |
| WC (cm) | 82.4 (11.1) | 81.0 (11.1) | 82.8 (10.9) | 94.8 (10.1) | 94.1 (10.3) | 95.1 (10.0) |
| HC (cm) | 102.1 (9.1) | 101.7 (9.3) | 102.0 (8.9) | 102.6 (6.7) | 103.0 (6.8) | 102.4 (6.6) |
| ABSI | 73.3 (4.8) | 72.4 (4.6) | 73.8 (4.9) | 79.2 (4.0) | 78.2 (3.9) | 79.9 (4.0) |
| HI | 64.3 (2.4) | 64.1 (2.4) | 64.4 (2.4) | 49.1 (1.6) | 49.0 (1.6) | 49.2 (1.6) |
| BMI categoryb,*** | ||||||
| 18.5 to < 25 kg/m2 | 57,521 (45.9) | 19,175 (50.6) | 33,634 (44.6) | 34,326 (29.9) | 13,116 (29.4) | 21,210 (30.2) |
| 25 to < 30 kg/m2 | 45,845 (36.5) | 12,353 (32.6) | 28,941 (38.3) | 58,546 (51.0) | 22,322 (50.1) | 36,224 (51.6) |
| 30 to < 45 kg/m2 | 22,069 (17.6) | 6389 (16.8) | 12,916 (17.1) | 21,888 (19.1) | 9155 (20.5) | 12,733 (18.1) |
| Body shape phenotypesb,*** | ||||||
| Pear | 33,369 (26.6) | 11,402 (30.1) | 18,775 (24.9) | 30,990 (27.0) | 14,170 (31.8) | 16,820 (24.0) |
| Slim | 28,836 (23.0) | 10,566 (27.9) | 15,501 (20.5) | 35,393 (30.8) | 16,339 (36.6) | 19,054 (27.2) |
| Wide | 36,548 (29.1) | 8711 (23.0) | 24,404 (32.3) | 30,391 (26.5) | 8613 (19.3) | 21,778 (31.0) |
| Apple | 26,682 (21.3) | 7238 (19.1) | 16,811 (22.3) | 17,986 (15.7) | 5471 (12.3) | 12,515 (17.8) |
| Platelet parametersc,*** | ||||||
| Platelet count (109/L) | 259 (166 to 403) | 262 (166 to 413) | 257 (166 to 398) | 234 (150 to 365) | 238 (154 to 367) | 231 (148 to 363) |
| MPV (fL) | 9.31 (7.47 to 11.59) | 9.41 (7.55 to 11.72) | 9.26 (7.44 to 11.53) | 9.20 (7.41 to 11.42) | 9.24 (7.45 to 11.44) | 9.18 (7.38 to 11.41) |
| PDW (%) | 16.41 (15.47 to 17.41) | 16.39 (15.46 to 17.38) | 16.42 (15.48 to 17.41) | 16.54 (15.57 to 17.58) | 16.52 (15.55 to 17.55) | 16.56 (15.58 to 17.59) |
ABSI a body shape index (cutoffs ≥ 73 for women, ≥ 80 for men); Apple large-ABSI–small-HI; BMI body mass index; HC hip circumference; HI hip index (cutoffs ≥ 64 for women, ≥ 49 for men); MPV mean platelet volume; PDW platelet distribution width (coefficient of variation of platelet size); Pear small-ABSI–large-HI; Post-MP post-menopausal women; Pre-MP pre-menopausal women; Slim small-ABSI–small-HI; WC waist circumference; Wide large-ABSI–large-HI
aMean (standard deviation)
bCount (percentage from total per column)
cGeometric mean (reference range)
***Pairwise comparisons for men vs. women overall, Post-MP vs. Pre-MP women, and men ≥ 52 years vs. < 52 years were performed with t test for continuous variables (log-transformed platelet parameters) or χ2-test for categorical variables. All comparisons were significant at p < 1 × 10–6
Associations of body size with platelets
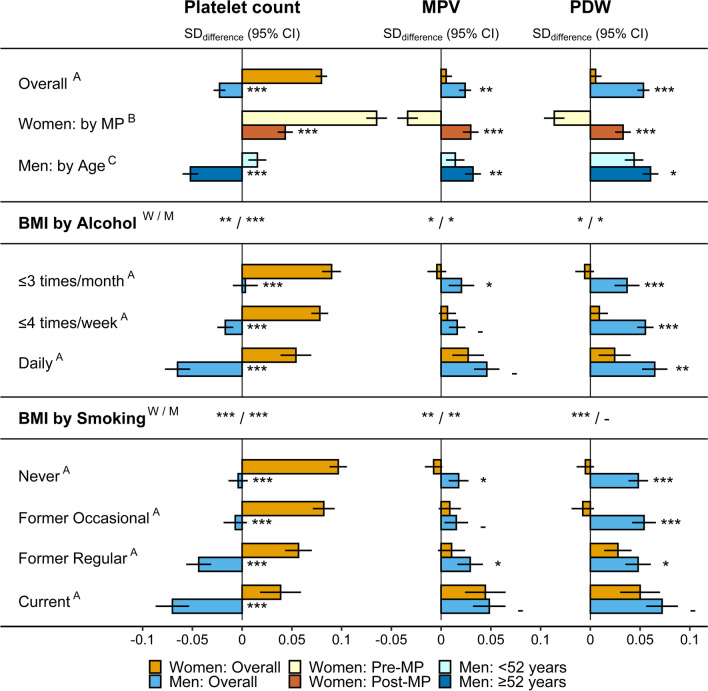
BMI was associated positively with platelet count only in women, with a stronger association in pre-menopausal compared to post-menopausal women, but was associated inversely in men overall, with a weak positive association in younger men and a strong inverse association in older men (Fig. 1). The association of BMI with platelet count was positive for all subgroups according to alcohol consumption and smoking status in women, but was strongest for low alcohol consumption (≤ 3 times/month) and for never smokers and was weakest for daily alcohol consumption and current smoking (Fig. 1). In men, there was no evidence for association of BMI with platelet count for low alcohol consumption and in never smokers, but a strong inverse association for daily alcohol consumption and in current smokers (Fig. 1).
Fig. 1.
Associations of BMI with platelet parameters. BMI body mass index; CI confidence interval; MPV mean platelet volume; PDW platelet distribution width; Post-MP post-menopausal women; Pre-MP pre-menopausal women; SD standard deviation. SDdifferences (95% CI) in platelet parameters per one SD increment of BMI from multivariable linear regression models including each platelet parameter as an outcome variable (sex-specific z-scores, following log-transformation) and BMI, ABSI, and HI as exposure variables (sex-specific z-scores), with adjustment for height, age, weight change (last year), smoking status (except for subgroups by smoking status), alcohol consumption (except for subgroups by alcohol consumption), physical activity, Townsend deprivation index, region of the assessment centre, time of blood collection, fasting time, use of nonsteroidal anti-inflammatory drugs, paracetamol use, menopausal status (women overall), hormonal replacement therapy use (women overall and Post-MP), oral contraceptives use and age at the last live birth (all women). Numerical values are shown in Additional file 1: Table S3. A p value for the interaction term of BMI with sex, from a model including women (reference) and men (separately for subgroups by alcohol consumption and smoking status), with adjustment for ABSI, HI, covariates (except female-specific), and including an interaction term of age with sex, to account for potential differences by menopausal status in women. B p value for the interaction term of BMI with menopausal status, from a model including Pre-MP (reference) and Post-MP women, with adjustment for ABSI, HI, and covariates. C p value for the interaction term of BMI with age (continuous), from a model including all men, with adjustment for ABSI, HI, and covariates (except female-specific). W/M p value (women/men) derived from likelihood ratio tests comparing models with and without an interaction term of BMI with either alcohol consumption or smoking status, separately for women and men, with adjustment for ABSI, HI, and covariates. p values - p ≥ 0.05; *p < 0.05; **p < 0.001; ***p < 1 × 10–6
For platelet size, BMI was associated positively with MPV and more strongly with PDW in men, especially in older men. No evidence for association in women overall, however, was accounted for by an inverse association in pre-menopausal and a positive association in post-menopausal women, similarly for MPV and PDW (Fig. 1). In subgroups according to alcohol consumption and smoking status, BMI showed the strongest positive associations with MPV in women and men and with PDW in women for daily alcohol consumption and in current smokers, but there was little evidence for heterogeneity of the association of BMI with PDW in men (Fig. 1).
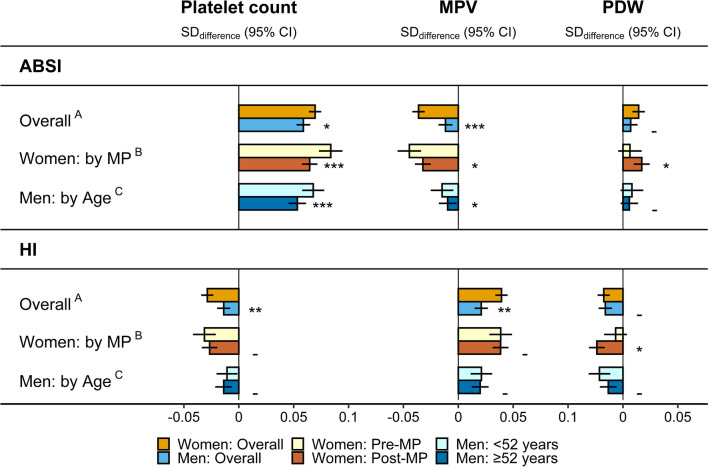
Association of body shape with platelets
ABSI was associated positively with platelet count, with little difference between women and men but somewhat stronger in pre-menopausal women and younger men compared to post-menopausal women and older men, correspondingly (Fig. 2). HI was associated more weakly inversely with platelet count, mainly in women. Opposite to platelet count, ABSI was associated inversely and HI positively with MPV, mainly in women, while associations with PDW were directionally consistent with associations with platelet count (positive for ABSI and inverse for HI), but were very weak (Fig. 2). Neither alcohol consumption nor smoking status influenced materially the associations of ABSI and HI with platelet parameters (Additional file 1: Fig. S1).
Fig. 2.
Associations of ABSI and HI with platelet parameters. ABSI a body shape index; CI confidence interval; HI hip index; MPV mean platelet volume; PDW platelet distribution width; Post-MP post-menopausal women; Pre-MP pre-menopausal women; SD standard deviation. SDdifferences (95% CI) in platelet parameters per one SD increment of ABSI or HI from multivariable linear regression models including each platelet parameter as an outcome variable (sex-specific z-scores, following log-transformation) and body mass index (BMI), ABSI, and HI as exposure variables (sex-specific z-scores), with adjustment for height, age, weight change (last year), smoking status, alcohol consumption, physical activity, Townsend deprivation index, region of the assessment centre, time of blood collection, fasting time, use of nonsteroidal anti-inflammatory drugs, paracetamol use, menopausal status (women overall), hormonal replacement therapy use (women overall and Post-MP), oral contraceptives use and age at the last live birth (all women). Numerical values are shown in Additional file 1: Table S4. A p values for the interaction terms of ABSI and HI with sex, from a model including women (reference) and men, with adjustment for BMI, covariates (except female-specific), and including an interaction term of age with sex, to account for potential differences by menopausal status in women. B p values for the interaction terms of ABSI and HI with menopausal status, from a model including Pre-MP (reference) and Post-MP women, with adjustment for BMI and covariates. C p values for the interaction terms of ABSI and HI with age (continuous), from a model including all men, with adjustment for BMI and covariates (except female-specific). p values - p ≥ 0.05; *p < 0.05; **p < 0.001; *** p < 1 × 10–6
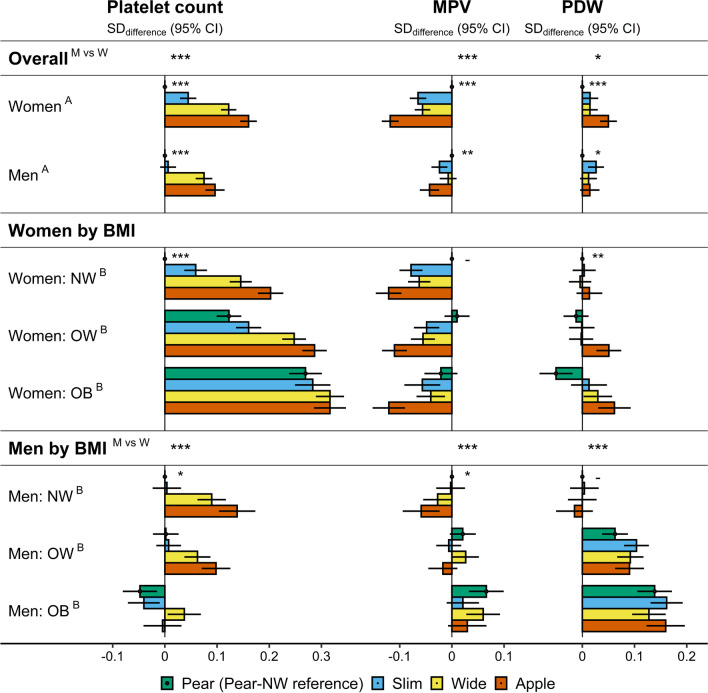
Corresponding to the predominant positive association with ABSI, the highest platelet counts were for “apple” and “wide” phenotypes in women and men (using “pear” phenotype as reference), but “slim” phenotype showed slightly higher platelet counts only in women (Fig. 3). On the contrary, MPV was lowest for “apple” and highest for “pear” phenotype, with both “wide” and “slim” phenotypes showing similar intermediate levels, mainly in women. In addition, in women, PDW was higher only for “apple” phenotype (Fig. 3).
Fig. 3.
Associations of body shape phenotypes with platelet parameters. ABSI a body shape index (cutoffs ≥ 73 for women; ≥ 80 for men); Apple large-ABSI–small-HI; BMI body mass index; CI confidence interval; HI hip index (cutoffs ≥ 64 for women; ≥ 49 for men); MPV mean platelet volume; NW normal weight (BMI ≥ 18.5 to BMI < 25 kg/m2); OB obese (BMI ≥ 30 to BMI < 45 kg/m2); OW overweight (BMI ≥ 25 to BMI < 30 kg/m2); PDW platelet distribution width; Pear small-ABSI–large-HI; SD standard deviation; Slim small-ABSI–small-HI; Wide large-ABSI–large-HI. SDdifferences (95% CI) in platelet parameters compared to the reference category from multivariable linear regression models including each platelet parameter as an outcome variable (sex-specific z-scores, following log-transformation) and as exposures, ABSI-by-HI (“pear” reference) and BMI categories (A), or BMI-by-ABSI-by-HI (“pear” NW reference) (B), with adjustment for height, age, weight change (last year), smoking status, alcohol consumption, physical activity, Townsend deprivation index, region of the assessment centre, time of blood collection, fasting time, use of nonsteroidal anti-inflammatory drugs, paracetamol use, and for women, menopausal status, hormonal replacement therapy use, oral contraceptives use, and age at the last live birth. Numerical values are shown in Additional file 1: Table S5. A/B p values for body shape overall (next to “pear”) or for heterogeneity of the associations with body shape according to BMI category (next to “pear-NW”) from likelihood ratio tests, separately for women and men, comparing fully adjusted models including BMI categories with and without ABSI-by-HI (A), or the fully adjusted additive model, including ABSI-by-HI and BMI categories, with the interaction model, including BMI-by-ABSI-by-HI (B). M vs W p values for men vs. women from likelihood ratio tests comparing models including women and men with and without an interaction term of ABSI-by-HI with sex (row Overall) or of BMI-by-ABSI-by-HI with sex (row Men), adjusted for covariates (except female-specific), and including an interaction term of age with sex. p values - p ≥ 0.05; *p < 0.05; **p < 0.001; ***p < 1*10–6
Within BMI categories, the associations of body shape phenotypes with platelet count were stronger for normal weight and overweight BMI in both women and men, but were considerably weaker for obese BMI (Fig. 3, see also Additional file 1: Table S5 for comparisons between “apple” and “pear” phenotype within each BMI category). The association pattern with MPV, however, was maintained for all BMI categories in women, while the difference in PDW between “apple” and “pear” phenotype was largest in obese women (Fig. 3).
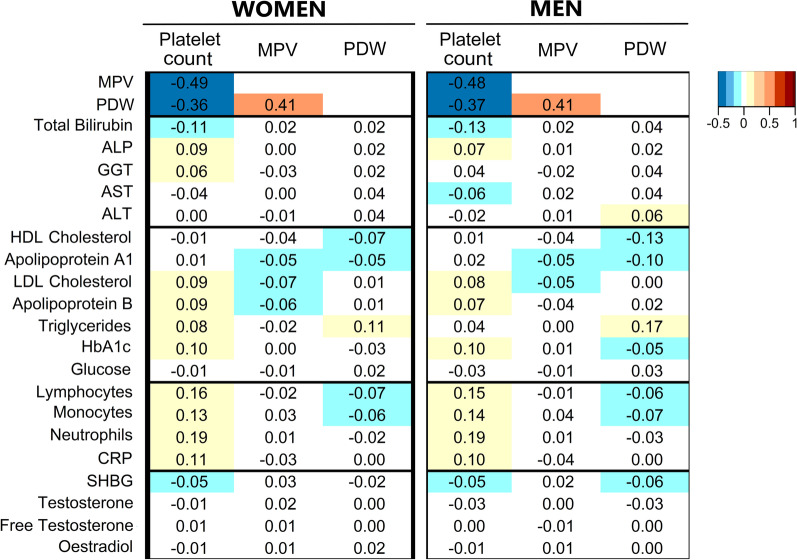
Correlation of platelet count and size with metabolic and inflammatory biomarkers
Independent of body size, body shape, and covariates, platelet count was correlated substantially inversely with both MPV and PDW, which were correlated positively with each other (Fig. 4). Platelet count was correlated weakly positively with all leucocyte subtypes but most strongly with neutrophils and, to some extent, with C-reactive protein and glycated haemoglobin, but weakly inversely with total bilirubin. MPV showed no material associations with biomarkers or leucocyte counts and PDW was only correlated weakly positively with triglycerides and weakly inversely with high-density lipoprotein cholesterol and apolipoprotein A1, mainly in men. Neither platelet count nor platelet size showed any material correlations with testosterone, oestradiol, or SHBG.
Fig. 4.
Correlation of platelet parameters with liver function tests, metabolic, inflammatory, and sex steroid biomarkers. ALP alkaline phosphatase; ALT alanine aminotransferase; AST aspartate aminotransferase; CRP C-reactive protein; GGT gamma-glutamyltransferase; HbA1c haemoglobin A1c (glycated haemoglobin); HDL high-density lipoprotein; LDL low-density lipoprotein; MPV mean platelet volume; PDW platelet distribution width; SHBG sex hormone binding globulin. Values represent partial Pearson correlation coefficients with adjustment for body mass index, a body shape index, hip index, height, age, weight change (last year), smoking status, alcohol consumption, physical activity, Townsend deprivation index (continuous), time of blood collection (continuous), fasting time (continuous), use of nonsteroidal anti-inflammatory drugs, paracetamol use, and for women, menopausal status, hormone replacement therapy use, oral contraceptives use, and age at the last live birth. Correlation coefficients were calculated in a subset of participants with available all biomarker measurements (94,285 (75.2%) women; 87,702 (76.4%) men)
Sensitivity analyses
Examining BMI categories and tertiles of ABSI and HI, there were no indications for U-shaped associations or for major deviations from linearity. Suggestive of a plateau (similar associations for overweight and obese compared to normal weight) were only the positive associations of BMI with platelet count in post-menopausal women and in younger men, while MPV was lower only for obese and not for overweight pre-menopausal women and was higher only for obese young men (Additional file 1: Fig. S2). Restricting the data set to participants with stable weight (Additional file 1: Fig. S3) or without medication use (Additional file 1: Fig. S4) made no material difference to association estimates. Omitting all covariates, however, resulted in slightly stronger positive associations of BMI with platelet count in women and in younger men but a weaker inverse association in older men (Additional file 1: Fig. S5). The unadjusted association estimates of ABSI with platelet count were also somewhat weaker in women and in older men.
Discussion
In the middle-aged UK Biobank cohort, excluding participants with prevalent cancer, endocrine and metabolic conditions, and severe illnesses, we report directional sex-specific differences in the associations of obesity indices with platelet count and platelet size.
Sex differences in platelet count, higher in women compared to men, are well established [30–32] and suggest an involvement of sex steroids in platelet production. This is supported further by the sex differences in the associations of BMI with platelet count that we have shown, positive in women but inverse in men. Although platelet count was lower in post-menopausal compared to pre-menopausal women and in older compared to younger men, in accordance with smaller scale studies [30, 31], sex differences in platelet count and its associations with BMI were retained for all ages. However, post-menopausal women not using HRT, which have lower circulating oestradiol than men [26], maintained higher platelet count than men and a positive association with BMI and, notably, platelet count was not correlated with circulating sex steroids. Our results, therefore, suggest, that the response of platelet production to BMI-related factors is set differently in women and men, most likely during intrauterine development or during puberty, but does not depend further on the levels of circulating sex steroids in middle adulthood. Nevertheless, the association between BMI and platelet count was shifted towards the inverse direction for both post-menopausal women and older men, suggesting that an age-related factor alters the influence of BMI on platelet count similarly in women and men.
Our study, therefore, is the first to show that competing BMI-related factors influence platelet count, one group contributing to a positive association and subject to sex differences, and another contributing to an inverse association and related to age. Our findings would, therefore, explain the discrepancies in published studies for the association of obesity with platelet count. The direction of the association would be determined by the balance between the opposed BMI-related factors, which would depend on the proportion of women and men in the study (for joint association estimates) and on the age of study participants. Thus, studies examining separately women and men with mean age around 40 years, have reported a positive association of BMI with platelet count in women but no evidence for association in men [15, 16]. However, a study including younger participants, 80% of whom were men but with an average age around 30 years, has reported higher platelet count for obese compared to normal weight individuals [14]. This is compatible with the weak positive association of BMI with platelet count that we have shown for younger men.
The positive association of BMI with platelet count matches the positive associations of BMI with leucocyte subtype counts [12]. This is compatible with their coordinated function in inflammation, especially for neutrophils, as platelet–neutrophil interactions are required for neutrophil activation and recruitment to inflamed tissues [33, 34]. Therefore, inflammatory cytokines could be responsible for the positive association of BMI with platelet count. Correspondingly, animal studies have shown that obesity, induced by high fat diet, results in increased production of inflammatory cytokines, bone marrow hyperplasia, and leucocytosis [35–37]. It is less clear, however, whether these changes are coordinated with thrombocytosis, as some factors such as cytokine receptor-like factor 3 (CRLF3) regulate platelet formation in the hematopoietic bone marrow compartment without affecting leucocyte lineages [38]. Another factor contributing to platelet production is thrombopoietin, which is higher in obesity, at least in women [39]. Cortisol could also be contributing to a positive association with BMI, as glucocorticoids promote the responsiveness to thrombopoietin receptor agonists [40] and contribute to granulocytosis by delaying apoptosis and increasing the release of polymorphonuclear cells from the bone marrow and from the endothelial surface [41]. Patients with Cushing’s syndrome have higher platelet count compared to healthy controls and an overall state of hypercoagulability [42], as well as higher neutrophil count, which decrease following treatment [43].
The inverse association of BMI with platelet count is most likely related to liver damage and fibrosis resulting from liver fat infiltration, which in more extreme cases leads to non-alcoholic fatty liver disease (NAFLD) [44] and would further be aggravated by liver damage related to older age, high alcohol consumption, and smoking. Low platelet count is, indeed, a hallmark of fibrosis associated with cirrhosis and gradually decreases for several years prior to the overt clinical presentation of the disease [45]. Correspondingly, platelet count is included in most non-invasive liver fibrosis indices [46]. Smoking can also contribute to the development of liver fibrosis [47], in addition to high alcohol consumption [48], thus explaining why both shifted the association of BMI with platelet count towards the inverse direction. Female sex and oestrogens, however, show an apparent protective effect against NAFLD-related fibrosis [49], which could explain why women are more likely than men to balance the opposing BMI-related factors in the positive direction and why the positive association with BMI was strongest in pre-menopausal women.
Platelet size and platelet count were correlated inversely, in accordance with small scale studies [17], and showed associations with BMI in opposite directions. This is in accordance with opposite changes, a reduction of platelet count and an increase in MPV, after bariatric surgery [50, 51] and in association with liver fibrosis [45, 52]. The association of BMI with MPV and PDW, however, was inverse only for pre-menopausal women, but was positive for post-menopausal women, as well as for men. This could not have been identified in small scale studies combining both sexes and all ages, which have reported higher MPV and PDW for obesity [13, 17, 53]. Given that large and variable platelet size is indicative of platelet activation and thrombosis [9, 10], our findings of higher MPV and PDW in obese post-menopausal women and men are compatible with higher risk of thrombosis and cardiovascular diseases in these groups [54].
Another possibility for a trade-off between platelet size and count is suggested by the mechanism of platelet formation. Megakaryocytes in bone marrow, derived from haematopoietic stem cells, form platelets by extending long cytoskeletal processes and releasing circular-preplatelets in the circulation. Preplatelets evolve into barbell-shaped proplatelet intermediates, which undergo fission and form two smaller size mature platelets [55]. Megakaryocyte maturation and formation of proplatelets depend heavily on actin and microtubule cytoskeletons and mutations in some of the cytoskeletal proteins are associated with both large platelet size and reduced platelet number [56]. RNA processing is another mechanism controlling platelet formation. Deletion of the RNA-binding protein serine–arginine-rich splicing factor 3 (SRSF3) in mice results in arrest of megakaryocyte maturation, with sequestration of mRNAs in the megakaryocyte nucleus and production of a smaller number of dysfunction platelets with abnormally large size [57]. CRLF3 also plays a role in the maturation of proplatelets, with CRLF3 deficiency contributing to thrombocytopenia due to inefficient thrombopoiesis, with preplatelets, which are larger than the mature platelets, circulating for longer and being destroyed in the spleen before managing to mature into platelets [38].
While the associations of BMI with platelet count and size showed clear sex and age differences and a similar association pattern for MPV and PDW, the association of body shape with platelet count showed small sex differences and minimal age differences. Body shape was also associated only with MPV, in opposite directions to the associations with platelet count, but mainly in women and there was little evidence for associations of body shape with PDW. The predominant positive association of ABSI with platelet count in women and men, with similarly higher levels in both “apple” and “wide” compared to “pear” phenotype, resembled the association patterns of body shape with monocyte and neutrophil counts and C-reactive protein [12], but unlike them, the associations of ABSI with platelet count was weaker for obese BMI. Cortisol is among the main factors determining body shape [58] that could contribute to a positive association with ABSI, as well as with BMI, similarly for platelet and neutrophil counts. It is unclear, however, what factors could explain the associations of body shape with platelet size.
A major strength of our study is the very large sample size compared to previous reports, which enabled us to examine in more detail subgroups by menopausal status in women and by age in men. We have also applied rigorous exclusion criteria, which has minimised the possibility for reverse causality. We were able to adjust for major lifestyle and reproductive factors, which clearly influenced the association estimates, and we have thus minimised confounding. Our study was not affected by bias from self-reporting, as anthropometric measurements were obtained by trained personnel according to standardised procedures. Measurement errors were also minimised by the standardised approach to blood sample collection and storage, the use of a single type of automatic blood count analyser for all samples, and the use of systematic quality control for all biomarker measurements.
Our study, admittedly, has several limitations. Most importantly, we did not have information on haemocoagulation and platelet activity and could not evaluate to what extent differences in platelet count and size contributed to different thrombotic states. We were unable to examine early or late adulthood, because UK Biobank is a middle-aged cohort. We could not examine underweight, or severe obesity, or ethnic variations either, due to limited numbers in these categories. A misclassification of medication use or self-reported disease status at recruitment is also possible, which may have affected the exclusions. Our study was also cross-sectional and we could not assess temporality. Last, UK Biobank participants have a healthier lifestyle and are not representative of the overall UK population [59].
Conclusions
Competing BMI-related factors determine the associations of BMI with platelet count. Factors with sexually dimorphic actions contribute to a positive association, more prominent in women compared to men, and age-dependent factors, aggravated by smoking and alcohol consumption, contribute to an inverse association. Inflammatory cytokines, thrombopoietin, and cortisol may contribute to the positive association with BMI, while the inverse association would likely be explained by liver damage and fibrosis. Associations of body shape with platelet count, predominantly positive with waist size and with minor sex differences, resemble the association patterns of body shape with inflammatory biomarkers (Creactive protein, neutrophil and monocyte counts). Platelet size is correlated inversely with platelet count, both for MPV and PDW, and shows associations with BMI in opposite direction to platelet count but only MPV is associated with body shape and only in women.
Perspectives and significance
Our study shows that, in addition to the constitutively higher platelet count and size in women compared to men, there are directional differences in the associations of obesity indices with platelet parameters. In women, especially in pre-menopausal women, appear more prominent obesity-related factors stimulating platelet synthesis, with more complete maturation of platelet particles and smaller platelet size. In men, especially in older men, as well as in smokers and in daily alcohol consumers, apparently gain more prominence restrained platelet maturation and platelet destruction, facilitated by obesity-related liver damage. It is important, therefore, when evaluating platelet status in clinical practice to consider how sex, age, menopause, lifestyle factors, and obesity are balanced for each individual and, in research, to consider how these factors are balanced for a given data set. It would further be important to evaluate in future studies the impact of obesity on the diagnostic value of platelet-based indices, such as the platelet-to-leucocyte ratio or the non-invasive indices of liver fibrosis. Sex differences in platelet–neutrophil co-operations and in platelet contributions to obesity-related complications, such as atherosclerosis and cancer development, should also be examined.
Supplementary Information
Additional file 1: Table S1 Flow chart of UK Biobank participants in the study. Table S2 Lifestyle and reproductive characteristics of study participants. Table S3 Associations of BMI with platelet parameters. Table S4 Associations of ABSI and HI with platelet parameters. Table S5 Associations of body shape phenotypes with platelet parameters. Figure S1 Associations of ABSI and HI with platelet parameters (subgroups by alcohol consumption and smoking status). Figure S2 Associations of anthropometric index categories with platelets. Figure S3 Associations of anthropometric indices with platelets in participants with stable weight. Figure S4 Associations of anthropometric indices with platelets in participants without NSAID or paracetamol use. Figure S5 Unadjusted associations of anthropometric indices with platelets.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application number 41952 (https://www.ukbiobank.ac.uk/about-biobank-uk/).
Abbreviations
- ABSI
A body shape index
- BMI
Body mass index
- CI
Confidence interval
- CRLF3
Cytokine receptor-like factor 3
- HC
Hip circumference
- HI
Hip index
- HR
Hazard ratio
- MPV
Mean platelet volume
- NAFLD
Non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- NSAID
Nonsteroidal anti-inflammatory drugs
- PDW
Platelet distribution width
- SD
Standard deviation
- WC
Waist circumference
Author contributions
SC, ER and KKT conceived and designed the study. KKT and EE provided statistical advice. SC led the research and performed the statistical analysis. SC had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SC drafted the paper with contributions from ER, KKT and EE. All authors: SC, KKT, EE and ER were involved in the interpretation of the results and the critical revisions of the paper. All authors have read and approved the manuscript.
Funding
This work was supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC), which provided infrastructure support for the Department of Epidemiology and Biostatistics at Imperial College London (UK). KKT was supported by Cancer Research UK (Grant PPRCPJT\100005). The funders had no role in the design and conduct of the study, the collection, analysis, and interpretation of the data, or the preparation, review, and approval of the manuscript, or in the decision to submit the manuscript for publication.
Availability of data and materials
The data supporting the findings of the study are available to bona fide researchers upon approval of an application to the UK Biobank (https://www.ukbiobank.ac.uk/researchers/) and a material transfer agreement.
Declarations
Ethics approval and consent to participate
This research was conducted according to the principles expressed in the Declaration of Helsinki. The UK Biobank cohort has been approved by the North West Multicenter Research Ethics Committee, UK (Ref.: 16/NW/0274). Written informed consent has been obtained from all study participants. The current study was approved by the UK Biobank access management board. Participants who had withdrawn consent by the time of the analysis were excluded from the analysis data set.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: a PRISMA-compliant systemic review and meta-analysis. Medicine. 2016;95(32):e4495. doi: 10.1097/md.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi: 10.1172/jci92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–1500. doi: 10.1161/circresaha.120.316101. [DOI] [PubMed] [Google Scholar]
- 4.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, et al. Innate immune receptors in platelets and platelet–leukocyte interactions. J Leukoc Biol. 2020;108(4):1157–1182. doi: 10.1002/jlb.4mr0620-701r. [DOI] [PubMed] [Google Scholar]
- 6.Palacios-Acedo AL, Mège D, Crescence L, Dignat-George F, Dubois C, Panicot-Dubois L. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol. 2019;10:1805. doi: 10.3389/fimmu.2019.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannakeas V, Kotsopoulos J, Cheung MC, Rosella L, Brooks JD, Lipscombe L, et al. Analysis of platelet count and new cancer diagnosis over a 10-year period. JAMA Netw Open. 2022;5(1):e2141633. doi: 10.1001/jamanetworkopen.2021.41633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mounce LT, Hamilton W, Bailey SE. Cancer incidence following a high-normal platelet count: cohort study using electronic healthcare records from English primary care. Br J Gen Pract. 2020;70(698):e622–e628. doi: 10.3399/bjgp20X710957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzi B, Gialluisi A, Gianfagna F, Orlandi S, De Curtis A, Magnacca S, et al. Platelet distribution width is associated with p-selectin dependent platelet function: results from the Moli-family cohort study. Cells. 2021;10(10):2737. doi: 10.3390/cells10102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogorzelska K, Krętowska A, Krawczuk-Rybak M, Sawicka-Żukowska M. Characteristics of platelet indices and their prognostic significance in selected medical condition—a systematic review. Adv Med Sci. 2020;65(2):310–315. doi: 10.1016/j.advms.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Christakoudi S, Riboli E, Evangelou E, Tsilidis KK. Associations of body shape index (ABSI) and hip index with liver, metabolic, and inflammatory biomarkers in the UK Biobank cohort. Sci Rep. 2022;12(1):8812. doi: 10.1038/s41598-022-12284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuncuoğlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300–1306. [PubMed] [Google Scholar]
- 14.Kornblith LZ, Howard B, Kunitake R, Redick B, Nelson M, Cohen MJ, et al. Obesity and clotting: body mass index independently contributes to hypercoagulability after injury. J Trauma Acute Care Surg. 2015;78(1):30–38. doi: 10.1097/ta.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, et al. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008;2008:834153. doi: 10.1155/2008/834153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charles LE, Fekedulegn D, McCall T, Burchfiel CM, Andrew ME, Violanti JM. Obesity, white blood cell counts, and platelet counts among police officers. Obesity (Silver Spring) 2007;15(11):2846–2854. doi: 10.1038/oby.2007.338. [DOI] [PubMed] [Google Scholar]
- 17.Nkambule BB, Mxinwa V, Nyambuya TM, Dludla PV. The mean platelet volume and atherosclerotic cardiovascular-risk factors in adults with obesity: a systematic review and meta-analysis of observational studies. BMC Nutr. 2022;8(1):47. doi: 10.1186/s40795-022-00541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. Association of body-shape phenotypes with imaging measures of body composition in the UK Biobank cohort: relevance to colon cancer risk. BMC Cancer. 2021;21(1):1106. doi: 10.1186/s12885-021-08820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krakauer NY, Krakauer JC. An anthropometric risk index based on combining height, weight, waist, and hip measurements. J Obes. 2016;2016:8094275. doi: 10.1155/2016/8094275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 22.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakoudi S, Tsilidis KK, Evangelou E, Riboli E. A body shape index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. Cancer Med. 2021;10(16):5614–5628. doi: 10.1002/cam4.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Biobank Coordinating Centre; UK Biobank: Protocol for a large-scale prospective epidemiological resource. Protocol No: UKBB-PROT-09-06 (Main Phase); 21 March 2007 (AMENDMENT ONE FINAL). https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf. Accessed 05 Dec 2022.
- 25.UK Biobank Haematology Data Companion Document; 24 October 2017. https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/haematology.pdf. Accessed 05 Dec 2022.
- 26.Christakoudi S, Riboli E, Evangelou E, Tsilidis KK. Associations of body shape phenotypes with sex steroids and their binding proteins in the UK Biobank cohort. Sci Rep. 2022;12(1):10774. doi: 10.1038/s41598-022-14439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep. 2018;8(1):663. doi: 10.1038/s41598-017-19120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org. Accessed 05 Dec 2022.
- 30.Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, et al. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects' data. PLoS ONE. 2013;8(1):e54289. doi: 10.1371/journal.pone.0054289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006;16(2):123–130. doi: 10.1016/j.annepidem.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 32.Ittermann T, Feig MA, Petersmann A, Radke D, Greinacher A, Völzke H, et al. Mean platelet volume is more important than age for defining reference intervals of platelet counts. PLoS ONE. 2019;14(3):e0213658. doi: 10.1371/journal.pone.0213658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornerup KN, Salmon GP, Pitchford SC, Liu WL, Page CP. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol (1985). 2010;109(3):758–767. doi: 10.1152/japplphysiol.01086.2009. [DOI] [PubMed] [Google Scholar]
- 34.Page C, Pitchford S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int Immunopharmacol. 2013;17(4):1176–1184. doi: 10.1016/j.intimp.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36(2):379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 36.Pini M, Rhodes DH, Fantuzzi G. Hematological and acute-phase responses to diet-induced obesity in IL-6 KO mice. Cytokine. 2011;56(3):708–716. doi: 10.1016/j.cyto.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do Carmo LS, Rogero MM, Paredes-Gamero EJ, Nogueira-Pedro A, Xavier JG, Cortez M, et al. A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp Biol Med (Maywood). 2013;238(4):375–384. doi: 10.1177/1535370213477976. [DOI] [PubMed] [Google Scholar]
- 38.Bennett C, Lawrence M, Guerrero JA, Stritt S, Waller AK, Yan Y, et al. CRLF3 plays a key role in the final stage of platelet genesis and is a potential therapeutic target for thrombocythemia. Blood. 2022;139(14):2227–2239. doi: 10.1182/blood.2021013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maury E, Brichard SM, Pataky Z, Carpentier A, Golay A, Bobbioni-Harsch E. Effect of obesity on growth-related oncogene factor-alpha, thrombopoietin, and tissue inhibitor metalloproteinase-1 serum levels. Obesity (Silver Spring) 2010;18(8):1503–1509. doi: 10.1038/oby.2009.464. [DOI] [PubMed] [Google Scholar]
- 40.Poston JN, Gernsheimer TB. Glucocorticoids promote response to thrombopoietin-receptor agonists in refractory ITP: a case series. Int J Hematol. 2019;110(2):255–259. doi: 10.1007/s12185-019-02638-6. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa M, Terashima T, D'Yachkova Y, Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98(21):2307–2313. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 42.Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, et al. Blood coagulation and fibrinolysis in patients with Cushing's syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest. 2009;32(2):169–174. doi: 10.1007/bf03345709. [DOI] [PubMed] [Google Scholar]
- 43.Masri-Iraqi H, Robenshtok E, Tzvetov G, Manistersky Y, Shimon I. Elevated white blood cell counts in Cushing's disease: association with hypercortisolism. Pituitary. 2014;17(5):436–440. doi: 10.1007/s11102-013-0522-0. [DOI] [PubMed] [Google Scholar]
- 44.Engin A. Non-alcoholic fatty liver disease. Adv Exp Med Biol. 2017;960:443–467. doi: 10.1007/978-3-319-48382-5_19. [DOI] [PubMed] [Google Scholar]
- 45.Gotlieb N, Schwartz N, Zelber-Sagi S, Chodick G, Shalev V, Shibolet O. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis. World J Gastroenterol. 2020;26(38):5849–5862. doi: 10.3748/wjg.v26.i38.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Feng Y, Ma X, Wang G, Wu H, Xie X, et al. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS ONE. 2017;12(8):e0182969. doi: 10.1371/journal.pone.0182969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munsterman ID, Smits MM, Andriessen R, van Nieuwkerk CMJ, Bloemena E, Mulder CJJ, et al. Smoking is associated with severity of liver fibrosis but not with histological severity in nonalcoholic fatty liver disease. Results from a cross-sectional study. Scand J Gastroenterol. 2017;52(8):881–885. doi: 10.1080/00365521.2017.1315169. [DOI] [PubMed] [Google Scholar]
- 48.Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutluturk F, Ozsoy Z. Effect of sleeve gastrectomy on platelet counts and mean platelet volumes. Obes Surg. 2018;28(10):3159–3164. doi: 10.1007/s11695-018-3287-8. [DOI] [PubMed] [Google Scholar]
- 51.Johansson HE, Wåhlén A, Aldenbäck E, Haenni A. Platelet counts and liver enzymes after gastric bypass surgery. Obes Surg. 2018;28(6):1526–1531. doi: 10.1007/s11695-017-3035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kosekli MA. Mean platelet volume and platelet to lymphocyte count ratio are associated with hepatitis B-related liver fibrosis. Eur J Gastroenterol Hepatol. 2022;34(3):324–327. doi: 10.1097/meg.0000000000002219. [DOI] [PubMed] [Google Scholar]
- 53.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59(8):981–982. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 54.Armeni E, Lambrinoudaki I. Menopause, androgens, and cardiovascular ageing: a narrative review. Ther Adv Endocrinol Metab. 2022;13:1–23. doi: 10.1177/20420188221129946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemble S, Dalby A, Lowe GC, Nicolson PLR, Watson SP, Senis Y, et al. Analysis of preplatelets and their barbell platelet derivatives by imaging flow cytometry. Blood Adv. 2022;6(9):2932–2946. doi: 10.1182/bloodadvances.2021006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulter NS, Thomas SG. Cytoskeletal regulation of platelet formation: coordination of F-actin and microtubules. Int J Biochem Cell Biol. 2015;66:69–74. doi: 10.1016/j.biocel.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Heazlewood SY, Ahmad T, Mohenska M, Guo BB, Gangatirkar P, Josefsson EC, et al. The RNA-binding protein SRSF3 has an essential role in megakaryocyte maturation and platelet production. Blood. 2022;139(9):1359–1373. doi: 10.1182/blood.2021013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasquali R. The hypothalamic–pituitary–adrenal axis and sex hormones in chronic stress and obesity: pathophysiological and clinical aspects. Ann N Y Acad Sci. 2012;1264:20–35. doi: 10.1111/j.1749-6632.2012.06569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Flow chart of UK Biobank participants in the study. Table S2 Lifestyle and reproductive characteristics of study participants. Table S3 Associations of BMI with platelet parameters. Table S4 Associations of ABSI and HI with platelet parameters. Table S5 Associations of body shape phenotypes with platelet parameters. Figure S1 Associations of ABSI and HI with platelet parameters (subgroups by alcohol consumption and smoking status). Figure S2 Associations of anthropometric index categories with platelets. Figure S3 Associations of anthropometric indices with platelets in participants with stable weight. Figure S4 Associations of anthropometric indices with platelets in participants without NSAID or paracetamol use. Figure S5 Unadjusted associations of anthropometric indices with platelets.
Data Availability Statement
The data supporting the findings of the study are available to bona fide researchers upon approval of an application to the UK Biobank (https://www.ukbiobank.ac.uk/researchers/) and a material transfer agreement.