Abstract
Background
Little is known about the association between juvenile idiopathic arthritis (JIA) and autoimmune thyroid disease (AITD) and therefore there are no indications for AITD screening in this population, which is possible using standard blood tests. The objective of this study is to determine the prevalence and predictors of symptomatic AITD in JIA patients from the international Pharmachild registry.
Methods
Occurrence of AITD was determined from adverse event forms and comorbidity reports. Associated factors and independent predictors for AITD were determined using univariable and multivariable logistic regression analyses.
Results
The prevalence of AITD after a median observation period of 5.5 years was 1.1% (96/8965 patients). Patients who developed AITD were more often female (83.3% vs. 68.0%), RF positive (10.0% vs. 4.3%) and ANA positive (55.7% vs. 41.5%) than patients who did not. AITD patients were furthermore older at JIA onset (median 7.8 years vs. 5.3 years) and had more often polyarthritis (40.6% vs. 30.4%) and a family history of AITD (27.5% vs. 4.8%) compared to non-AITD patients. A family history of AITD (OR = 6.8, 95% CI: 4.1 – 11.1), female sex (OR = 2.2, 95% CI: 1.3 – 4.3), ANA positivity (OR = 2.0, 95% CI: 1.3 – 3.2) and older age at JIA onset (OR = 1.1, 95% CI: 1.1 – 1.2) were independent predictors of AITD on multivariable analysis. Based on our data, 16 female ANA positive JIA patients with a family history of AITD would have to be screened during ±5.5 years using standard blood tests to detect one case of AITD.
Conclusions
This is the first study to report independent predictor variables for symptomatic AITD in JIA. Female ANA positive JIA patients with positive family history are at increased risk of developing AITD and thus might benefit from yearly serological screening.
Keywords: Juvenile idiopathic arthritis, Autoimmune thyroid disease, Hashimoto’s disease, Graves’ disease, Screening, Epidemiology, Registry
Background
Juvenile idiopathic arthritis (JIA) is a diagnosis of exclusion that includes all forms of chronic arthritis of unknown origin with onset below the age of 16 years [1]. It is the most common childhood rheumatic disease with an estimated global incidence of 1.6 – 23 cases per 100,000 children [2] and often persists into adulthood [3]. The International League of Associations for Rheumatology (ILAR) distinguishes seven JIA categories with different clinical and laboratory measures [4], although another classification system is under development [5].
There is some evidence that children with JIA suffer more often from autoimmune thyroid disease (AITD) than the general pediatric population [6–8]. AITD comprises Hashimoto’s thyroiditis which causes hypothyroidism and Graves’ disease which causes hyperthyroidism. If undiagnosed and thus left untreated, hyper- and hypothyroidism may lead to a variety of complaints, such as constipation or diarrhea, irritability, fatigue, hair loss but ultimately also growth retardation and depression [9].
Currently, little is known about the association between JIA and AITD and therefore there are no indications for AITD screening in this population, which is possible using standard blood tests. Previous studies reported a prevalence of (subclinical) AITD in JIA varying from 1 to 44% [7, 8, 10–16]. One study of 81 JIA patients reported a significant association between a family history of thyroid disease and AITD [17]. Nevertheless, studies that primarily focus on AITD in JIA are scarce and most include not only symptomatic but also subclinical AITD. Furthermore, no study has yet established independent predictor variables for AITD in JIA.
The purpose of this study is to determine the prevalence of symptomatic AITD in JIA and moreover to identify independent predictors for AITD using data from the international observational Pharmachild registry.
Methods
Pharmachild
Pharmachild was set up in 2011 with the primary aim of studying safety and effectiveness of drug therapies in JIA. Pharmachild collects demographic, clinical and laboratory data of JIA patients from 85 Paediatric Rheumatology International Trials Organisation (PRINTO) medical centers from 31 countries across the globe [18]. Inclusion criteria are JIA classified according to ILAR criteria while under treatment or previously treated with nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids, systemic corticosteroids, and/or conventional synthetic (cs-) or biological (b-) disease-modifying antirheumatic drugs (DMARD) as per physician decision. The registry consists of two cohorts. The first is a cohort of all included patients with retrospective information about drug exposure and adverse events (AEs) from disease onset until registration into Pharmachild. The second is a cohort of patients with additional prospective information about disease activity and patient-reported outcomes for hospital visits after registration into Pharmachild. More information about the Pharmachild registry is published elsewhere [19]. Data lock occurred on 18 December, 2019 and all patients at that time were included in the present study.
Outcome and determinants
The outcome of interest in this study was the ever occurrence of symptomatic AITD (Hashimoto’s thyroiditis, Graves’ disease and non-specified AITD). This outcome (yes/no) was evaluated for all patients from two sources: free-text fields for comorbidity reporting at registration into Pharmachild and AE forms. AEs in Pharmachild are reported using the Medical Dictionary of Regulatory Activities (MedDRA) coding system (version 22) with a three-level monitoring check for consistency by the treating physician, medical monitor (JS) and PRINTO certified MedDRA coders [20]. The following MedDRA preferred terms were considered as AITD: “hypothyroidism”, “autoimmune thyroiditis”, “thyroiditis”, “hyperthyroidism” and “Basedow’s disease”. Goiter and congenital thyroid disorder were not considered as AITD. Laboratory results were not considered for determining AITD, since these were likely to involve subclinical cases. All mentions of possible AITD cases were retrieved and reviewed by one researcher (LB) and event descriptions were subsequently independently evaluated by two other researchers (JS and JvS). In order to explore the coexistence of endocrinopathies, mentions of growth retardation/short stature, diabetes mellitus and celiac disease were retrieved from both free-text comorbidity reports and AE forms. For this assessment, the following mentions were included: “growth retardation”, “growth retarded”, “short stature”, “stature short”, “coeliac disease”, “celiac disease”, “type 1 diabetes mellitus”, “diabetes”, “type I diabetes mellitus” and “diabetes mellitus insulin-dependent”. In addition, the following patient characteristics were collected for all patients: sex, ethnicity, age at JIA onset, ILAR category, anti-nuclear antibodies (ANA) status, rheumatoid factor (RF) status, human leukocyte antigen (HLA)-B27 status, family history of autoimmune disease and AITD (in first, second and/or third-degree relatives), observation period (time from JIA onset until last Pharmachild visit), the number of active joints at JIA diagnosis (max. 12 months later) and drug history at last visit. Ethnicity was reported by the treating physician from a fixed set of categories. For ANA positivity, only one positive ANA test was required. A positive RF status was defined as two positive RF determinations at least 3 months apart. Drugs included were NSAIDs, intraarticular corticosteroids, systemic corticosteroids, cs- and b-DMARDs.
Statistical analysis
The retrospective and prospective Pharmachild cohorts were analyzed together. Patient characteristics were compared between patients with and without AITD using univariable logistic regression analyses. When the 95% confidence interval (CI) of the odds ratio (OR) did not contain 1, this was considered a statistically significant effect. All variables that differed statistically significant between AITD and non-AITD patients were considered for inclusion into a complete case multivariable logistic regression model in order to identify independent predictors of AITD. Predictors were selected using a stepwise backward procedure based on the Akaike’s Information Criterion (AIC). This measure is used to select a model that best predicts the observed data while adding a penalty for the number of variables in the model [21]. Because onset dates of AITD were not available for all cases, the observation period and drug history were not considered for inclusion into the multivariable model. The active joint count was also not considered since this measure was only available for (part of the) patients from the prospective Pharmachild cohort. Numerical variables were tested for a linear relationship with the logit outcome using the Box-Tidwell test. The performance of the multivariable model in distinguishing between AITD and non-AITD patients was evaluated by the area under the receiver operating characteristic curve (AUC). Based on the prevalence of AITD in patients at increased risk for developing AITD following our prediction model, we calculated a number needed to screen (NNS) (1 divided by the absolute risk reduction). IBM SPSS statistics (version 25.0.0.2) and R (version 4.0.3) were used for the statistical analyses.
Results
Patient characteristics
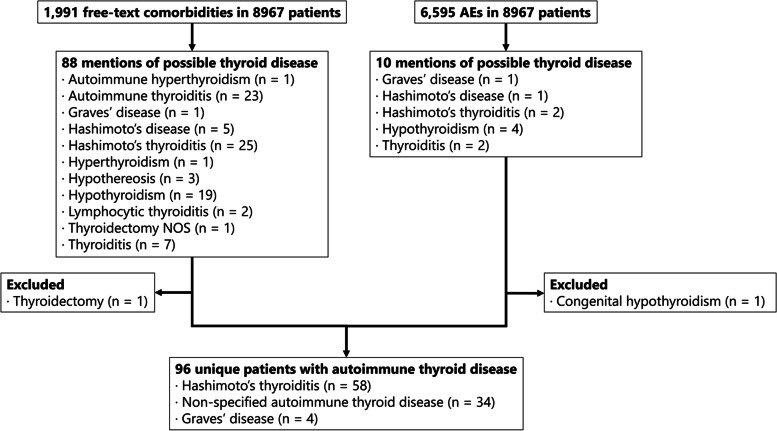
A total of 8965 patients were included from the Pharmachild registry for analysis with a total observation period of 57,053 years (median 5.5 years, IQR: 2.8–9.0). Within these patients, 96 cases of clinical AITD (1.1%) were identified (Fig. 1). Hashimoto’s thyroiditis occurred in 58/96 (60.4%) cases and Graves’ disease in 4/96 (4.2%) of cases. The remaining 34/96 (35.4%) of AITD cases were unspecified. Patients who developed AITD were more often female, RF positive and ANA positive than patients who did not develop AITD (Table 1). Furthermore, AITD patients were older at JIA onset and had more often polyarthritis and a family history of autoimmune disease and AITD compared to non-AITD patients. Celiac disease and diabetes mellitus were reported significantly more often in AITD patients compared to patients without AITD: 4.2% vs. 0.6% (P < 0.01) and 2.1% vs. 0.3% (P = 0.04), respectively. No significant difference was found for growth retardation/short stature: 0.0% for AITD patients and 0.3% for non-AITD patients (P = 1.0). AITD patients had less often systemic arthritis than non-AITD patients. The observation period, HLA-B27 status, active joint count at JIA diagnosis, ethnicity and drug history at last visit did not differ significantly between the two groups. A distribution of different AITD cases per ILAR category is provided in Table 2.
Fig. 1.
Flowchart of selected AITD cases. AE: adverse event, NOS: not otherwise specified
Table 1.
Characteristics of patients in Pharmachild with and without AITD
| Total (n = 8965) | No AITD (n = 8869) | AITD (n = 96) | |
|---|---|---|---|
| Observation period in years, median (IQR) | 5.5 (2.8 – 9.0) | 5.5 (2.8 – 9.0) | 4.9 (3.0 – 9.5) |
| Female sex, n (%) | 6107 (68.1%) | 6027 (68.0%) | 80 (83.3%) |
| Ethnicity, n (%) | |||
| European | 6940 (86.9%) | 6853 (86.9%) | 87 (90.6%) |
| Hispanic | 249 (3.1%) | 248 (3.1%) | 1 (1.0%) |
| Indian | 145 (1.8%) | 144 (1.8%) | 1 (1.0%) |
| Middle Eastern | 196 (2.5%) | 193 (2.4%) | 3 (3.1%) |
| Multiethnic | 110 (1.4%) | 109 (1.4%) | 1 (1.0%) |
| North African | 154 (1.9%) | 152 (1.9%) | 2 (2.1%) |
| Southeast Asian | 64 (0.8%) | 64 (0.8%) | 0 (0.0%) |
| Sub-Saharan African | 78 (1.0%) | 78 (1.0%) | 0 (0.0%) |
| Other |
48 (0.6%) n = 7984 |
47 (0.6%) n = 7888 |
1 (1.0%) n = 96 |
| Family history of autoimmune disease, n (%) |
2632 (30.4%) n = 8669 |
2584 (30.1%) n = 8578 |
48 (52.7%) n = 91 |
| Family history of AITD, n (%) |
441 (5.1%) n = 8669 |
416 (4.8%) n = 8578 |
25 (27.5%) n = 91 |
| Age at JIA onset in years, median (IQR) | 5.3 (2.4 – 9.9) | 5.3 (2.4 – 9.9) | 7.8 (3.1 – 12.7) |
| ILAR category, n (%) | |||
| Enthesitis-related arthritis | 969 (10.8%) | 961 (10.8%) | 8 (8.3%) |
| Oligoarthritis | 3370 (37.6%) | 3338 (37.6%) | 32 (33.3%) |
| Polyarthritis (RF-) | 2371 (26.4%) | 2341 (26.4%) | 30 (31.3%) |
| Polyarthritis (RF+) | 367 (4.1%) | 358 (4.0%) | 9 (9.4%) |
| Psoriatic arthritis | 298 (3.3%) | 292 (3.3%) | 6 (6.2%) |
| Systemic arthritis | 968 (10.8%) | 966 (10.9%) | 2 (2.1%) |
| Undifferentiated arthritis | 622 (6.9%) | 613 (6.9%) | 9 (9.4%) |
| Active joint count at JIA diagnosis, median (IQR) |
2.0 (0.0 – 5.0) n = 666 |
2.0 (0.0 – 5.0) n = 660 |
3.0 (1.3 – 4.0) n = 6 |
| ANA positive, n (%) |
3486 (41.7%) n = 8365 |
3437 (41.5%) n = 8277 |
49 (55.7%) n = 88 |
| RF positive, n (%) |
342 (4.3%) n = 7876 |
333 (4.3%) n = 7786 |
9 (10.0%) n = 90 |
| HLA-B27 positive, n (%) |
1122 (20.7%) n = 5414 |
1114 (20.8%) n = 5363 |
8 (15.7%) n = 51 |
| Drug history at last visit, n (%) | |||
| NSAIDs | 7375 (82.3%) | 7298 (82.3%) | 77 (80.2%) |
| Intraarticular corticosteroids | 4545 (50.7%) | 4496 (50.7%) | 49 (51.0%) |
| Systemic corticosteroids | 3582 (40.0%) | 3551 (40.0%) | 31 (32.3%) |
| cs-DMARDs | 7795 (86.9%) | 7712 (87.0%) | 83 (86.5%) |
| Methotrexate | 7524 (83.9%) | 7446 (84.0%) | 78 (81.2%) |
| b-DMARDs | 5946 (66.3%) | 5878 (66.3%) | 68 (70.8%) |
| Anti-TNF | 5248 (58.5%) | 5183 (58.4%) | 65 (67.7%) |
AITD autoimmune thyroid disease, ANA anti-nuclear antibodies, b biological, cs conventional synthetic, DMARDs disease-modifying antirheumatic drugs, HLA human leukocyte antigen, ILAR International League of Associations for Rheumatology, JIA juvenile idiopathic arthritis, NSAIDs nonsteroidal anti-inflammatory drugs, RF rheumatoid factor, TNF tumour necrosis factor
Table 2.
Distribution of different AITD cases per ILAR category
| ILAR category | Total AITD (n = 96) | Hashimoto’s disease (n = 58) | Graves’ disease (= 4) | Unspecified AITD (n = 34) |
|---|---|---|---|---|
| Enthesitis-related arthritis | 8 (8.3%) | 6 (10.3%) | 0 (0.0%) | 2 (5.9%) |
| Oligoarthritis | 32 (33.3%) | 17 (29.3%) | 3 (75.0%) | 12 (35.3%) |
| Polyarthritis (RF-) | 30 (31.3%) | 21 (36.2%) | 0 (0.0%) | 9 (26.5%) |
| Polyarthritis (RF+) | 9 (9.4%) | 4 (6.9%) | 0 (0.0%) | 5 (14.7%) |
| Psoriatic arthritis | 6 (6.2%) | 3 (5.2%) | 1 (25.0%) | 2 (5.9%) |
| Systemic arthritis | 2 (2.1%) | 1 (1.7%) | 0 (0.0%) | 1 (2.9%) |
| Undifferentiated arthritis | 9 (9.4%) | 6 (10.3%) | 0 (0.0%) | 3 (8.8%) |
AITD autoimmune thyroid disease, ILAR International League of Associations for Rheumatology, RF rheumatoid factor
Predictors for AITD
On multivariable analysis, a family history of AITD, female sex, ANA positivity and older age at JIA onset were independent predictors of AITD (Table 3). This model included 7345 patients and 82 AITD events due to 1620 patients with missing data. The model had good discriminatory power (AUC = 0.71, 95% CI: 0.65 – 0.78). Based on the data in Pharmachild, the number of female ANA positive JIA patients with a family history of AITD needed to screen to detect one case of AITD is 16. This number decreases with increasing age at JIA onset (Table 4).
Table 3.
Associated factors and independent predictors for AITD on univariable and multivariable analysis
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Family history of AITD | 7.43 | 4.56 – 11.74c | 6.84 | 4.07 – 11.14c |
| Female sex | 2.36 | 1.42 – 4.19c | 2.22 | 1.25 – 4.25c |
| ANA positive | 1.77 | 1.16 – 2.71c | 1.99 | 1.25 – 3.18c |
| Age at JIA onset in years | 1.10 | 1.05 – 1.15c | 1.12 | 1.07 – 1.18c |
| Observation period in yearsa | 1.02 | 0.98 – 1.07 | ||
| Ethnicity | ||||
| European | 1.00 | Reference | ||
| Sub-Saharan African | – | – | ||
| Hispanic | 0.32 | 0.02 – 1.44 | ||
| Indian | 0.55 | 0.03 – 2.48 | ||
| Middle Eastern | 1.22 | 0.30 – 3.30 | ||
| Multiethnic | 0.72 | 0.04 – 3.29 | ||
| North African | 1.04 | 0.17 – 3.32 | ||
| Southeast Asian | – | – | ||
| Other | 1.68 | 0.09 – 7.80 | ||
| Family history of autoimmune disease | 2.59 | 1.71 – 3.93c | ||
| ILAR category | ||||
| Oligoarthritis | 1.00 | Reference | ||
| Enthesitis-related arthritis | 0.87 | 0.37 – 1.80 | ||
| Polyarthritis (RF-) | 1.34 | 0.81 – 2.21 | ||
| Polyarthritis (RF+) | 2.62 | 1.17 – 5.31c | ||
| Psoriatic arthritis | 2.14 | 0.80 – 4.81 | ||
| Systemic arthritis | 0.22 | 0.03 – 0.71c | ||
| Undifferentiated arthritis | 1.53 | 0.68 – 3.09 | ||
| Active joint count at diagnosisb | 0.99 | 0.82 – 1.07 | ||
| RF positive | 2.49 | 1.15 – 4.73c | ||
| HLA-B27 positive | 0.71 | 0.31 – 1.43 | ||
| Drug history at last visita | ||||
| NSAIDs | 0.87 | 0.54 – 1.49 | ||
| Intraarticular corticosteroids | 1.01 | 0.68 – 1.52 | ||
| Systemic corticosteroids | 0.71 | 0.46 – 1.09 | ||
| cs-DMARDs | 0.96 | 0.55 – 1.81 | ||
| Methotrexate | 0.83 | 0.51 – 1.43 | ||
| b-DMARDs | 1.24 | 0.80 – 1.95 | ||
| Anti-TNF | 1.49 | 0.98 – 2.32 | ||
AITD autoimmune thyroid disease, ANA anti-nuclear antibodies, b biological, CI confidence interval, cs conventional synthetic, DMARD disease-modifying antirheumatic drug, HLA human leukocyte antigen, ILAR International League of Associations for Rheumatology, JIA juvenile idiopathic arthritis, NSAIDs nonsteroidal anti-inflammatory drugs, OR odds ratio, RF rheumatoid factor, TNF tumor necrosis factor
aNot considered for multivariable analysis due to missing AITD onset dates
bOnly available for patients from the prospective cohort and therefore not considered for multivariable analysis
cstatistically significant effect
Table 4.
Number of high-risk JIA patients needed to screen (NNS) to detect a case of AITD. The table summarizes the number of ANA positive girls with a family history of AITD who would have to be screened during a median observation period of 5.5 years to detect one case of AITD as a function of the age at JIA onset
| Age at JIA onset (years) | AITD prevalence | NNS |
|---|---|---|
| ≥0 | 14/196 (7.1%) | 16 |
| ≥4 | 10/85 (11.8%) | 9 |
| ≥8 | 5/34 (14.7%) | 7 |
| ≥12 | 4/18 (22.2%) | 5 |
AITD autoimmune thyroid disease, ANA anti-nuclear antibodies, JIA juvenile idiopathic arthritis, NNS number needed to screen
Discussion
The prevalence of AITD observed in the current study (1.1%) was lower compared to prevalence rates reported in the majority of previous studies about AITD in JIA (5.0 – 44.4%) [7, 8, 10, 11, 14–17]. These studies, however, also included cases of subclinical AITD based on active screening for serum levels of thyroid hormones (T3 and T4), thyroid-stimulating hormone (TSH) and anti-thyroid antibodies (TgA: thyroglobulin antibodies and/or TPOA: thyroid peroxidase antibodies). Two previous studies focused on clinical AITD in JIA and found similar prevalence rates as the current study (0.8 and 1.3%) [12, 13]. AITD has a varying prevalence in the general pediatric population (0.1 – 9.6%) according to the criteria used for diagnosis [22–26]. A population-based study from Scotland focused on clinical hypothyroidism in young people aged < 22 years and found a prevalence of 0.14% [27], which is over 4 times as low as the prevalence of clinical hypothyroidism in JIA found in the current study. In addition, several studies reported increased serum levels of anti-thyroid antibodies in children with JIA compared to healthy controls [7, 8, 15, 17].
Our study highlighted as independent predictors of AITD in JIA a family history of AITD, female sex, a positive ANA status and older age at JIA onset. In fact, previous studies about AITD in JIA also report a female predominance in the AITD group [7, 8, 10, 11, 17]. This can be explained by the predominance of girls in most JIA categories [28] and autoimmunity in general [29]. Previous studies have suggested that oligoarthritis might be associated with AITD in JIA [7, 8, 11, 15], but with the current study we conclude that this effect is likely explained by ANA positivity and female sex, which is highly frequent in oligoarthritis [28]. Similarly, the association between AITD and RF positivity and RF+ polyarthritis that we observed in univariable analyses is probably explained by older age and female sex [28].
This is the first study to report an (adjusted) association between AITD and a positive ANA status in JIA, likely due to a limited sample size in previous studies. An association between thyroid disorders and ANA positivity has previously been reported in adult RA [30] and a raised prevalence of ANA in AITD patients has also been previously reported, although the mechanism behind this phenomenon is not known [31–33]. More interestingly, we found a significant association between AITD in JIA patients and a family history of AITD, as described before in another study [17]. Previous studies on AITD patients have also reported high frequencies of familial AITD [34–36] or familial autoimmune disease in general [37]. The association between older age at JIA onset and AITD has not been previously reported. We hypothesize that this effect is caused by merely age rather than age at JIA onset, since older patients in general have an increased cumulative risk of developing any disease including AITD. In fact, it is known that the prevalence of pediatric AITD peaks during adolescence [25, 26, 38]. Systemic arthritis was observed considerably less in AITD patients than non-AITD patients in the current study, which might be explained by the fact that this JIA category resembles more an auto-inflammatory rather than an autoimmune disease and does not predominantly affect girls [39].
After a median observation period of 5.5 years, we observed a considerable increase in AITD prevalence for JIA patients at increased risk for developing AITD according to our analyses, providing rationale for yearly AITD screening in this high-risk group. Based on the Pharmachild data, only five ANA positive girls with a family history of AITD and an age at JIA onset of ≥12 years would have to be screened during 5.5 years to detect one case of clinical AITD. According to the coefficients in our prediction model, it is safe to conclude that a family history of AITD is the most important predictor of AITD in JIA, with an even larger OR for AITD than a 10-year increase in age at JIA onset. Hence, this would be the most important factor for clinicians to determine in JIA patients when estimating the risk of developing AITD. Screening for thyroid disease is based on abnormal levels of free thyroxine (T4) and thyroid stimulating hormone (TSH), which are standard blood tests. AITD is diagnosed when these abnormal levels are found in the presence of anti-thyroid antibodies. Interestingly, it has previously been mentioned that female sex, older age and a family history of autoimmune should raise suspicion for anti-thyroid antibodies screening in children with positive ANA of unknown cause [32].
This study has strengths and limitations. First of all, due to missing onset dates for AITD cases, no association between drug therapy, disease activity, disease duration, other endocrinopathies and AITD onset could be investigated. In the current study, we observed that AITD patients had more often received TNF inhibitors than non-AITD patients. However, although a decrease in thyroid dysfunction has been reported in autoimmune disease patients treated with TNF inhibitors [40–42], we cannot draw conclusions from our study since it is unknown whether TNF inhibitor therapy was received before or after AITD onset. Another limitation of our study is that baseline comorbidities and subsequent adverse events in Pharmachild are gathered using spontaneous reporting (i.e. these have to be reported by treating physicians), which might have led to an underestimation of the actual AITD prevalence. Furthermore, it is possible that the median observation period of 5.5 years was too short for patients with young age at JIA onset to develop AITD. The exact NNS reported in this study might therefore be applicable for a follow-up period of ±5.5 years after onset of JIA only, but higher or lower afterwards. Also, the results of our study might not be generalizable to all JIA patients, since the Pharmachild registry has a selection bias towards JIA patients with a more severe disease course requiring DMARD treatment, Nevertheless, this is the first study to report independent predictors of AITD in JIA. Contrary to most of the few previous studies on AITD in JIA, we report only symptomatic AITD cases and have included patients from multiple centers around the world.
Given the AUC of our prediction model, there is room for improvement in identifying other relevant predictive factors for AITD in JIA. Further research should therefore focus on incorporating drug therapy and disease duration. As suggested previously [10], the incidence of AITD increases with time from diagnosis of JIA and therefore disease duration might be a better predictor than age at JIA onset. Another relevant predictor might be iodine intake, since it is well-described that AITD is more common in iodine-replete areas around the world [22, 26, 43, 44].
Conclusions
To conclude, this is the first study to report independent predictors for AITD in JIA. These results provide evidence for the added value of yearly serological screening for AITD in ANA positive girls with positive family history, in order to guide a practical approach to the pediatric patient with JIA at risk of developing AITD.
Acknowledgements
The authors would like to acknowledge all PRINTO centers for contributing to the acquisition of data, PRINTO personnel for data quality control, and the European Reference Network for Immunodeficiency, Autoinflammatory, Autoimmune and Paediatric Rheumatic diseases (ERN-RITA). We also thank all patients and their parents/caregivers for their participation in Pharmachild.
Abbreviations
- AITD
Autoimmune thyroid disease
- ANA
Anti-nuclear antibodies
- b
Biological
- CI
Confidence interval
- cs
Conventional synthetic
- DMARDs
Disease-modifying antirheumatic drugs
- HLA
Human leukocyte antigen
- ILAR
International League of Associations for Rheumatology
- JIA
Juvenile idiopathic arthritis
- NNS
Number needed to screen
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- OR
Odds ratio
- RF
Rheumatoid factor
- TNF
Tumor necrosis factor
Authors’ contributions
GG, LG, JB, GV-C, VC, LH, SA, EG, NW, NR and JS collected data. JS, SdR, JvS and LB conceptualized and designed the study. LB, JS and JvS reviewed all AITD events. JvS performed the data-analysis and drafted the manuscript. All authors critically reviewed, revised and approved the manuscript.
Funding
This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology. Pharmachild has been supported by funding from the Italian public hospital IRCCS Istituto Giannina Gaslini and a grant from the European Union (grant 260353).
Availability of data and materials
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?id=19362).
Declarations
Ethics approval and consent to participate
Pharmachild and all participating centers obtained approval from their respective ethics committees and were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent/assent based on existing national regulations.
Consent for publication
Not applicable.
Competing interests
NR has received honoraria for consultancies or speaker bureaus from the following pharmaceutical companies in the past 3 years: 2 Bridge, Amgen, AstraZeneca, Aurinia, Bayer, Brystol Myers and Squibb, Celgene, inMed, Cambridge Healthcare Research, Domain Therapeutic, EMD Serono, Glaxo Smith Kline, Idorsia, Janssen, Eli Lilly, Novartis, Pfizer, Sobi, UCB. The IRCCS Istituto Giannina Gaslini (IGG), where NR works as full-time public employee has received contributions from the following industries in the last 3 years: Bristol Myers and Squibb, Eli-Lilly, F Hoffmann-La Roche, Novartis, Pfizer, Sobi. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment with third parties. All other authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martini A, Lovell DJ, Albani S, et al. Juvenile idiopathic arthritis. Nat Rev Dis Primers. 2022;8(1):5. Published 2022 Jan 27. 10.1038/s41572-021-00332-8. [DOI] [PubMed]
- 2.Palman J, Shoop-Worrall S, Hyrich K, McDonagh JE. Update on the epidemiology, risk factors and disease outcomes of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2018;32:206–222. doi: 10.1016/j.berh.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira RJ, Kishimoto ST, de Souza DP, Fernandes PT, Marini R, Appenzeller S. The importance of transition from pediatric to adult rheumatology care in juvenile idiopathic arthritis. Expert Rev Clin Immunol. 2021;17:155–161. doi: 10.1080/1744666X.2020.1865157. [DOI] [PubMed] [Google Scholar]
- 4.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 5.Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. 2019;46:190–197. doi: 10.3899/jrheum.180168. [DOI] [PubMed] [Google Scholar]
- 6.Del Giudice E, Swart JF, Wulffraat NM. Juvenile idiopathic arthritis. In: El Miedany Y, editor. Comorbidity Rheum dis. Springer International Publishing; 2017. pp. 265–288. [Google Scholar]
- 7.Robazzi TC, Adan LF, Pimentel K, Guimarães I, Magalhães Filho J, Toralles MB, et al. Autoimmune endocrine disorders and coeliac disease in children and adolescents with juvenile idiopathic arthritis and rheumatic fever. Clin Exp Rheumatol. 2013;31:0310–0317. [PubMed] [Google Scholar]
- 8.Stagi S, Giani T, Simonini G, Falcini F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Rheumatology (Oxford) 2005;44:517–520. doi: 10.1093/rheumatology/keh531. [DOI] [PubMed] [Google Scholar]
- 9.Swain M, Swain T, Mohanty BK. Autoimmune thyroid disorders—An update. Indian J Clin Biochem. 2005;20:9. doi: 10.1007/BF02893034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tronconi E, Miniaci A, Pession A. The autoimmune burden in juvenile idiopathic arthritis. Ital J Pediatr. 2017;43:1–6. doi: 10.1186/s13052-017-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpigiani M, Cerboni M, Bertini I, D’Annunzio G, Haupt R, Iester A, et al. Endocrine autoimmunity in young patients with juvenile chronic arthritis. Clin Exp Rheumatol. 2002;20:565–568. [PubMed] [Google Scholar]
- 12.Lovell DJ, Huang B, Chen C, Angeles-Han ST, Simon TA, Brunner HI. Prevalence of autoimmune diseases and other associated conditions in children and young adults with juvenile idiopathic arthritis. RMD Open. 2021;7(1):e001435. 10.1136/rmdopen-2020-001435. [DOI] [PMC free article] [PubMed]
- 13.Simon TA, Harikrishnan GP, Kawabata H, Singhal S, Brunner HI, Lovell DJ. Prevalence of co-existing autoimmune disease in juvenile idiopathic arthritis: a cross-sectional study. Pediatr Rheumatol. 2020;18:1–12. doi: 10.1186/s12969-020-00426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihailova D, Grigorova R, Vassileva B, Mladenova G, Ivanova N, Stephanov S, et al. Autoimmune thyroid disorders in juvenile chronic arthritis and systemic lupus erythematosus. Adv Exp Med Biol. 1999;455:55–60. doi: 10.1007/978-1-4615-4857-7_8. [DOI] [PubMed] [Google Scholar]
- 15.Harel L, Prais D, Uziel Y, et al. Increased prevalence of antithyroid antibodies and subclinical hypothyroidism in children with juvenile idiopathic arthritis. J Rheumatol. 2006;33(1):164–166. [PubMed] [Google Scholar]
- 16.Alhomaidah D, Alsagheir A, Al-Mayouf SM. Coexistence of endocrinopathies in children with rheumatic diseases. Int J Pediatr Adolesc Med. 2016;3:119–122. doi: 10.1016/j.ijpam.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ünsal E, Ören O, Salar K, Makay B, Abacı A, Özhan B, et al. The frequency of autoimmune thyroid disorders in juvenile idiopathic arthritis. Turk J Pediatr. 2008;50:462–465. [PubMed] [Google Scholar]
- 18.Ruperto N, Martini A. Networking in paediatrics: the example of the Paediatric rheumatology international trials organisation (PRINTO) Arch Dis Child. 2011;96:596–601. doi: 10.1136/adc.2010.188946. [DOI] [PubMed] [Google Scholar]
- 19.Swart J, Giancane G, Horneff G, Magnusson B, Hofer M, Alexeeva Е, et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther. 2018;20:285. doi: 10.1186/s13075-018-1780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giancane G, Swart JF, Castagnola E, Groll AH, Horneff G, Huppertz H-I, et al. Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild safety adjudication committee. Arthritis Res Ther. 2020;22:71. doi: 10.1186/s13075-020-02167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17:228–243. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasala P, Francis G. Autoimmune thyroid diseases in children. Expert Rev Endocrinol Metab. 2017;12:129–142. doi: 10.1080/17446651.2017.1300525. [DOI] [PubMed] [Google Scholar]
- 23.Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, et al. Thyroid nodules and Cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008;162:526–531. doi: 10.1001/archpedi.162.6.526. [DOI] [PubMed] [Google Scholar]
- 24.Aversa T, Lombardo F, Valenzise M, et al. Peculiarities of autoimmune thyroid diseases in children with Turner or Down syndrome: an overview. Ital J Pediatr. 2015;41:39. Published 2015 May 15. 10.1186/s13052-015-0146-2. [DOI] [PMC free article] [PubMed]
- 25.Admoni O, Rath S, Almagor T, Elias-Assad G, Tenenbaum-Rakover Y. Long-term follow-up and outcomes of autoimmune thyroiditis in childhood. Front Endocrinol (Lausanne) 2020;11:309. doi: 10.3389/fendo.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisafulli G, Gallizzi R, Aversa T, Salzano G, Valenzise M, Wasniewska M, et al. Thyroid function test evolution in children with Hashimoto’s thyroiditis is closely conditioned by the biochemical picture at diagnosis. Ital J Pediatr. 2018;44:1–6. doi: 10.1186/s13052-018-0461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter I, Greene S, MacDonald T, Morris A. Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child. 2000;83:207. doi: 10.1136/adc.83.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 29.Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10:2005. doi: 10.3201/eid1011.040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emamifar A, Hangaard J, Jensen Hansen IM. Thyroid disorders in patients with newly diagnosed rheumatoid arthritis is associated with poor initial treatment response evaluated by disease activity score in 28 joints-C-reactive protein (DAS28-CRP): An observational cohort study. Medicine (Baltimore). 2017;96(43):e8357. 10.1097/MD.0000000000008357. [DOI] [PMC free article] [PubMed]
- 31.Atzeni F, Doria A, Ghirardello A, Turiel M, Batticciotto A, Carrabba M, et al. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: prevalence and clinical value. Autoimmunity. 2008;41:111–115. doi: 10.1080/08916930701620100. [DOI] [PubMed] [Google Scholar]
- 32.Torok KS, Arkachaisri T. Autoimmune thyroiditis in antinuclear antibody positive children without rheumatologic disease. Pediatr Rheumatol. 2010;8:1–4. doi: 10.1186/1546-0096-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res. 2014;2014:150239. 10.1155/2014/150239. [DOI] [PMC free article] [PubMed]
- 34.Kust D, Matesa N. The impact of familial predisposition on the development of Hashimoto’s thyroiditis. Acta Clin Belg. 2018;75:104–108. doi: 10.1080/17843286.2018.1555115. [DOI] [PubMed] [Google Scholar]
- 35.Desai M, Karandikar S. Autoimmune thyroid disease in childhood: a study of children and their families. Indian Pediatr. 1999;36:659–668. [PubMed] [Google Scholar]
- 36.Dittmar M, Libich C, Brenzel T, Kahaly G. Increased familial clustering of autoimmune thyroid diseases. Horm Metab Res. 2011;43:200–204. doi: 10.1055/s-0031-1271619. [DOI] [PubMed] [Google Scholar]
- 37.Cárdenas-Roldán J, Rojas-Villarraga A, Anaya J-M. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med. 2013;11:1–22. doi: 10.1186/1741-7015-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RS. Autoimmune thyroiditis in childhood. J Clin Res Pediatr Endocrinol. 2013;5:45. doi: 10.4274/Jcrpe.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JJY, Schneider R. Systemic juvenile idiopathic arthritis. Pediatr Clin N Am. 2018;65:691–709. doi: 10.1016/j.pcl.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Paschou SA, Palioura E, Kothonas F, Myroforidis A, Loi V, Poulou A, et al. The effect of anti-TNF therapy on thyroid function in patients with inflammatory bowel disease. Endocr J. 2018;65:1121–1125. doi: 10.1507/endocrj.EJ18-0243. [DOI] [PubMed] [Google Scholar]
- 41.Raterman H, Jamnitski A, Lems W, Voskuyl A, Dijkmans B, Bos W, et al. Improvement of thyroid function in hypothyroid patients with rheumatoid arthritis after 6 months of adalimumab treatment: a pilot study. J Rheumatol. 2010;38:247–251. doi: 10.3899/jrheum.100488. [DOI] [PubMed] [Google Scholar]
- 42.Tarhan F, Orük G, Niflioğlu O, Ozer S. Thyroid involvement in ankylosing spondylitis and relationship of thyroid dysfunction with anti-TNF α treatment. Rheumatol Int. 2012;33:853–857. doi: 10.1007/s00296-012-2438-9. [DOI] [PubMed] [Google Scholar]
- 43.Skarpa V, Κousta E, Tertipi A, Anyfandakis K, Vakaki M, Dolianiti M, et al. Epidemiological characteristics of children with autoimmune thyroid disease. Hormones. 2011;10:207–214. doi: 10.14310/horm.2002.1310. [DOI] [PubMed] [Google Scholar]
- 44.Rose NR, Bonita R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1:97–103. doi: 10.1016/S1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?id=19362).