Abstract
Imaging plays a central role in neuro-oncology including primary diagnosis, treatment planning, and surveillance of tumors. The emergence of quantitative imaging and radiomics provided an uprecedented opportunity to compile mineable databases that can be utilized in a variety of applications. In this review, we aim to summarize the current state of conventional and advanced imaging techniques, standardization efforts, fast protocols, contrast and sedation in pediatric neuro-oncologic imaging, radiomics-radiogenomics, multi-omics and molecular imaging approaches. We will also address the existing challenges and discuss future directions.
Introduction
Over the past decade, the field of neuro-oncologic imaging has experienced a tremendous shift from qualitative assessment of images by radiologists to the integration of imaging biomarkers and quantitative imaging approaches in clinical practice. This trend in imaging coincided with a paradigm shift in the diagnosis of central nervous system (CNS) neoplasms that started with the 2016 World Health Organization (WHO) classification of tumors of the CNS to incorporate both histologic features and genetic alterations. This trend continued with the 2021 WHO classification which added further molecular features. Quantitative imaging biomarkers and precision imaging now provide the opportunity to influence patient outcomes by generating tumor volumes, and growth curves and provide information about tumor microenvironment on the cellular level. Here we review the updated WHO classification of pediatric tumors of the CNS and how it relates to neuro-oncologic imaging, conventional and advanced imaging techniques, standardization efforts, fast protocols, contrast, and sedation in pediatric neuro-oncologic imaging, radiomics-radiogenomics, multi-omics, and molecular imaging approaches.
The 2021 WHO classification of tumors of the central nervous system
Here we focus on changes to glioma taxonomy in the revised 2021 WHO classification system, and summarize other key changes relevant to the practicing pediatric neuro-oncologist. In reviewing the arc taken by the WHO classification system over its various iterations, the new 5th edition (released in 2021) redoubles the influence of molecular biomarkers on the categorization of CNS tumors. Phenotypic and histologic features, which have traditionally been the basis for tumor categorization and grading, have an increasingly subordinate role in the world of emerging molecular information. Many histopathologic observations can seem arbitrary for classification purposes when compared with their newly uncovered genetic underpinnings. This paradigm shift toward molecular markers was felt to be significant enough to warrant an update to the 4th edition released by the WHO in 2016. We can now see the trend was in its infancy, with a particularly strong impetus from the contemporaneous advances in glioma and small round blue cell biology [1]. The most significant changes in the new system involve glioma and tumors of glioneuronal, neuronal, and embryonal origin [2]. For the first time, diffuse gliomas are divided into adult- and pediatric-type. Pediatric-type diffuse glioma is further split into a low- and high-grade categories. A fourth group of diffuse glioma is now recognized as circumscribed astrocytic gliomas. Circumscribed astrocytic gliomas include previously present entities such as pilocytic astrocytomas and subependymal giant cell astrocytomas the with addition of two new entities, high-grade astrocytomas with piloid features and astroblastoma, MN1-altered. In addition, fourteen glioma/glioneuronal tumors are newly recognized in the revised classification [2].
Pediatric-type diffuse low-grade gliomas now include the MYB or MYBL1-altered variety, angiocentric glioma, a newly recognized “oligodendroglioma-like” polymorphous low-grade neuroepithelial tumor of the young, (similar histologically to oligodendroglioma with MAPK pathway alterations and variable morphology,) and the MAPK pathway altered diffuse low-grade glioma [1]. In distinction, the categorization of pediatric-type diffuse high-grade gliomas relies to a greater extent upon molecular features, including diffuse midline glioma, H3K27-altered (previously referred to as “mutant”,) diffuse hemispheric glioma, H3G34/H3F3A-mutant, (similar to GBM histologically but with primitive embryonal features,) diffuse pediatric-type high-grade glioma harboring both H3- and IDH-wild type (several possible genotypes) and infant-type hemispheric glioma with alterations in ALK/ROS1/NTRK/MET, (similar to GBM) [1,2].
Another major departure from the 2016 WHO update is that ependymomas are now grouped by location: supratentorial, posterior fossa, and spinal cord with further molecular subgrouping in each location. Supratentorial ependymomas have two subgroups: 1) ZFTA fusion–positive ependymomas (formerly RELA-fusion) and can occur in both children and adults and are designated grade 2 or 3 neoplasms and 2) YAP1-fusion ependymomas which have a better prognosis than ZFTA ependymomas and are predominantly found in children younger than 3 years. Posterior fossa ependymomas now include two molecularly defined types: 1) group PFA which occur mainly in infancy and exhibit loss of H3K27me3 expression and EZHIP overexpression, and have worse outcome compared to 2) group PFB that are more common in older children and adults. There is a new type of spinal cord ependymoma with MYCN–amplification [1]. Finally, there have been several new additions to the embryonal tumors including cribriform neuroepithelial tumor, CNS tumor with BCOR internal tandem duplication and CNS neuroblastoma, FOXR2-activated in addition to the previously present atypical teratoid/ rhabdoid tumor (ATRT) and embryonal tumor with multilayered rosettes (ETMR) [1].
The update also incorporates a more exact nomenclature with respect to the various subgroups, with the terms “type” and “subtype” now preferred over the more ambiguous “entity” and “variant” used previously. Grading for CNS tumors in the 5th Edition has been adjusted to more closely resemble tumor grading in other body systems, now assigning a grade to tumor separately within each tumor type. For the first time, molecular features are explicitly included in the grading system. Roman numerals will no longer be used for grade since other classification systems use Arabic numerals. The grades again range from 1 (best prognosis) to 4 (worst prognosis), but some exceptions have been added to both incorporate genetic information, particularly those thought to most affect prognosis, and to avoid confusion with previous classifications. For example, there is no grade 1 available for IDH-mutant astrocytomas, only 2-4. IDH1 mutations in pediatric glioma are rare and likely represent the lower end of the age spectrum of adult-type IDH-mutant gliomas. They are usually present in the context of either 1p/19q codeletion or associated with TP53 and ATRX mutations [3]. Also inherent to the new grading system is an implicit nod to placing less importance on grade, as factors like available targeted therapies and tumor location are often more valuable to assess the prognosis. For example, the WNT-activated medulloblastoma subtype has a relatively good prognosis despite being grade 4 [4].
The new classification system strives to facilitate an integrated diagnosis which incorporates histology, grade and molecular data. Some examples of this approach in the new system can be seen with low-grade versus high-grade subcategories of pediatric diffuse glioma, CDKN2A/B homozygous deletion for astrocytoma and subtyping based on DNA-methylation profiling, TERT promoter mutations and EGFR amplification. Dropped now is the term “anaplastic,” previously common as a modifier for a given subtype. Thus, the previous “anaplastic astrocytoma” will now be referred to as “astrocytoma, IDH-mutant, CNS WHO grade 3.” [1].
Conventional and advanced imaging techniques
The conventional workhorse and gold standard for CNS tumor evaluation remains MRI with CT continuing to serve a vital role in the emergency setting particularly when looking for secondary effects of brain tumors. Conventional MR short and long echo sequences, T1 and T2- fluid-attenuated inversion recovery (FLAIR), are often enough to accurately characterize the tumor anatomy, establish the mass effect on adjacent structures and interrogate characteristics such as surrounding vasogenic edema, cystic versus solid components, cortical infiltration and intratumoral hemorrhage. Post-contrast spin echo and gradient T1 with and without fat suppression reliably measure the blood-brain barrier (BBB) disruption. The consensus recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group proposed strategies for the use of brain and spine MR to develop a balanced protocol that can be applicable to both 3 and 1.5 T scanners and could reach large-scale compliance and acceptance from the community to be used in the setting of multisite clinical trials and routine clinical care. Importantly, the RAPNO working group has defined standard brain and spine MR sequences [4], [5], [6], [7], [8].
Diffusion-weighted imaging (DWI) is a technique that measures the degree of movement of water molecules and probes their relation to the surrounding environment. The apparent diffusion coefficient (ADC) derived from diffusion-weighted imaging (DWI) quantifies the magnitude of water diffusion within a voxel, which varies based on the presence and organization of barriers to water diffusion. In brain tumors, ADC is a marker of cellularity [5] and has been shown to be inversely correlated with cellular proliferation [5]. ADC can be used to differentiate medulloblastoma, ependymoma, and pilocytic astrocytoma in posterior fossa [6] and may assist in differentiating molecular subgroups of pediatric low-grade [7] and to differentiate pediatric medulloblastoma histological variants and molecular groups [8] Higher baseline ADC values have been reported to be associated with a more favorable outcome in patients of diffuse midline glioma [9], [10], [11].
Advanced MR techniques are indispensable in modern oncologic imaging. Notable and widely utilized is MR perfusion, which has informed management by helping distinguish tumor progression from radiation effects and pseudo-progression [17]. Dynamic contrast-enhanced perfusion (DCE), dynamic susceptibility contrast (DSC) and arterial spin labeling perfusion use different approaches to detect increased plasma volume (Vp), relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF) as a biomarker for tumor neoangiogenesis, in contrary to tissues that demonstrate contrast enhancement secondary to treatment-related disruption in BBB. These perfusion techniques differ in their mechanism, with the first two employing a contrast agent and the latter uses magnetic blood labeling of intravascular protons to measure perfusion. Both DSC and DCE perfusion techniques require the use of a power injector to rapidly inject intravenous contrast. This somewhat limits the use of these techniques in pediatric gliomas compared to adults; however, a recent study demonstrated the feasibility of safe and high quality DSC imaging in children with brain tumors who received a standard dose of MRI contrast by an automated power injector [18]. Multiple studies have assessed the utility of DSC perfusion calculated relative cerebral blood volume (rCBV) in pediatric brain tumors. While these studies demonstrated a positive correlation between tumor grade and rCBV and high negative predictive value in in excluding high grade glioma in patients with low rCBV, they also showed limited specificity as high rCBV values can be seen in a subgroup of low-grade pediatric tumors such as pilocytic and pilomyxoid astrocytomas, ganglioglioma and choroid plexus papilloma [19,20]. DCE perfusion studies also demonstrated a significant positive correlation between DCE metrics (Ktrans, Kep, and Ve) and tumor grade in pediatric brain tumors [21,22].
Functional MRI (fMRI) commonly utilizes task-based paradigms to map the eloquent areas of the brain, most commonly motor and language function in patients where tumor involves the eloquent areas [23,24]. fMRI uses differences in magnetic properties of oxy- and deoxyhemoglobin to measure changes in blood flow within and in the vicinity of blood vessels while a subject performs a task [25]. When an area of the brain is activated due to a task, blood flow to that area increases, resulting in a higher concentration of oxyhemoglobin and a decrease in deoxygenated hemoglobin. This change in the concentration of hemoglobin causes a change in the magnetic properties of the blood, leading to a decrease in the dephasing of protons and an increase in the MR signal, a phenomenon known as the blood oxygen-level dependent (BOLD) effect. The BOLD effect allows fMRI to measure changes in brain activity and identify which areas of the brain are involved in a particular task [24]. Task-based presurgical BOLD fMRI is the most reliable and proven way to use fMRI in a clinical setting. Task-based fMRI requires that the patient follows commands during the scan which may not be possible for all patients, such as small children. In these cases, resting state fMRI (RS-fMRI) can be used as an alternative. RS-fMRI evaluates the patterns of BOLD signal while the patient is resting and can identify areas of the brain that have synchronized activity, known as resting-state networks [24].
Diffusion within white matter is anisotropic as water movement is restricted to a greater extent in the direction perpendicular to the axons than parallel to them. Diffusion tensor imaging (DTI) can detect such anisotropic diffusion by measuring diffusion in at least six directions to tease out the orientation of main white matter tracts that dictate the direction of the maximum diffusivity of the water molecules. The fractional anisotropy (FA) measure of this directed diffusion, with values ranging from 0 to 1. A value of 0 indicates isotropic (undirected) diffusion, which appears as a sphere, while a value of 1 represents totally directed diffusion, which appears as a straight line. The fractional anisotropy (FA) in biological tissues, including white matter axons, appears as an ellipsoid shape [24].
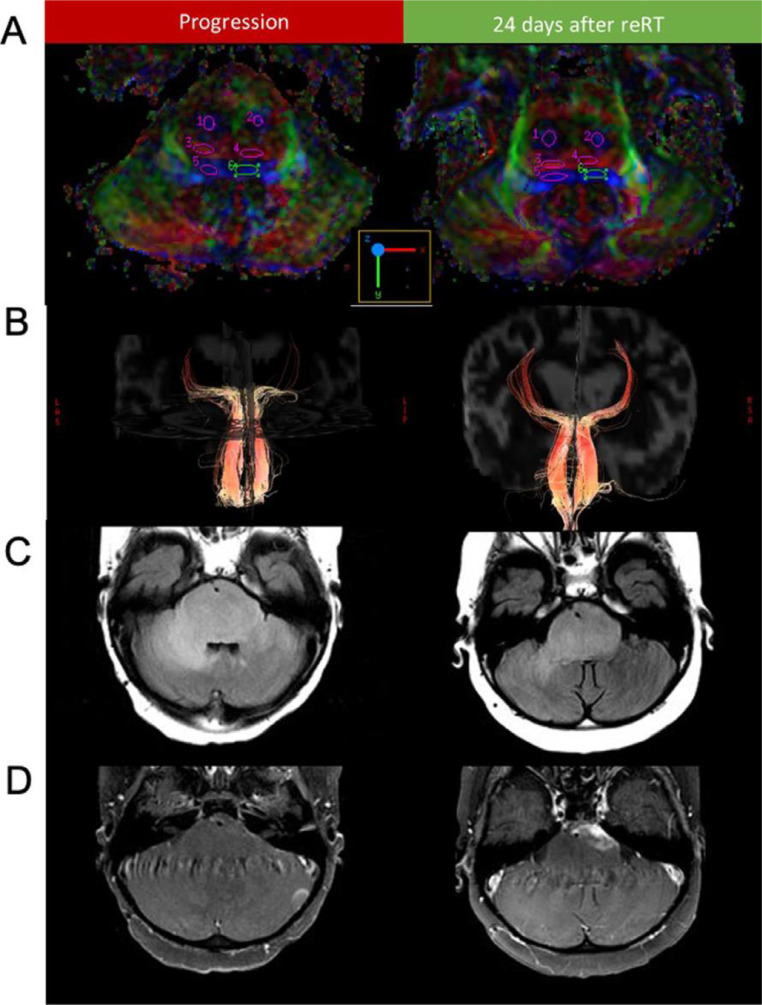
DTI tractography can be used to guide surgery by highlighting important pathways, such as the corticospinal tract, optic tract, superior longitudinal fasciculus, and arcuate fasciculus in relation to tumor [26]. DTI has also been used to differentiate different histologies in the posterior fossa [27,28], determination of tracts involvement in children with pontine tumors [29], monitoring white matter tracts before and after treatment [30], [31], [32] (Fig. 1) and prediction of motor outcomes [33].
Fig. 1.
(A) Diffusion tensor imaging color fractional anisotropy maps and region of interest delineation of the corticospinal (1,2), transverse pontine (3,4), and medial lemniscus (5,6) fibers before and after reirradiation are shown for patient 1. (B) Diffusion tensor imaging tractography of the medical lemniscus tracts before and after reirradiation. T2-FLAIR (C) and T1-post-contrast (D) sequences are shown before reirradiation and after reirradiation (This figure was published in Advances in Radiation Oncology, Vol 7, Bronk et al. Sequential Diffusion Tensor Imaging and Magnetic Resonance Spectroscopy in Patients Undergoing Reirradiation for Progressive Diffuse Intrinsic Pontine Glioma. Fig. 1, Copyright Elsevier (2022), reproduced with permission).
Updates on safety including contrast, fast protocols, and sedation
Pediatric imaging research can carry additional risks when compared to adults. Unlike adults who can consent and cooperate, children depend on parents or caregivers to consent on their behalf. Children also often require sedation for imaging, which carries additional potential risks [34]. For these reasons, in pediatric imaging research, one must consider the risks of the imaging technique itself (ionizing radiation, exposure to powerful magnetic fields and noise, and reactions to contrast agents) along with the added risk of sedation [35].
Contrast
Gadolinium-based contrast agents (GBCAs) have been in routine clinical use since 1988 when they received FDA approval. Intravenous GBCA administration during MRI increases diagnostic accuracy by delineating pathology due to increased vasculature and/or disruption of the BBB, which is critical for imaging in neuro-oncology. These contrast agents have become indispensable to clearly define tumor for diagnosis, treatment monitoring, and trial response assessment, as described in the proceeding section. GBCAs share a common structure of an organic ligand that tightly binds to (or chelates) and improves the stability, solubility, and safety of the central gadolinium ion. Historically, linear chelating agents were used, currently, macrocyclic agents are used which are much safer, likely due to stronger chelation of the gadolinium ion [36,37]. There remain risks associated with contrast agents. Namely, allergies, nephrogenic systemic fibrosis (NSF), and deposition of gadolinium.
Allergies
The frequency of adverse reactions to GBCAs ranges from 0.04 to 2.2% with most reactions being mild (up to 95%) and anaphylactic shock rare (0.004 to 0.01%) [37,38]. Allergic reactions are more frequent in patients with a previous reaction, females, macrocyclic structure agents, and with hepatic, abdominal, and thoracic examinations. The prevalence is lower in the pediatric population. The main risk factor for adverse reactions is a history of previous reactions to GBCAs [37].
NSF
Historically, administration of intravenous GBCAs was considered safe in patients with impaired renal function. In 2006, the FDA first reported an association between NSF and intravenous gadolinium administration [39]. NSF is a rare systemic disease characterized by fibrosis of the skin and other tissues throughout the body. It is generally seen in the middle-aged, but has also been reported in the elderly and in children [40].
The exact etiology of NSF remains unclear but most reported cases have been in patients with severe acute or chronic renal failure, with a glomerular filtration rate (GFR) < 30. Only two cases of NSF have been reported in patients with a GFR > 30 [41]. Recent data suggests that the risk of NSF is linked to the gadolinium binding strength of the chelates that form GBCAs, with most cases associated with weaker, linear chelates. Stable macrocyclic agents such as gadoterate acid (Dotarem®) and gadobutrol (Gadavist®) are thought to minimize the risk of NSF.
Gadolinium deposition
Current evidence suggests that all GBCAs are incompletely cleared from the body and that small amounts are retained in the brain, bones, and other tissues of healthy subjects with normal renal function and intact BBB [42,43]. These studies have shown that concentration depends on cumulative dose and the chemical stability of the agent. Deposition rates are higher in patients with impaired renal function and diseases with altered BBB integrity including some pediatric brain tumors [44] Gadolinium deposited in bone can be released during times of increased osteoblastic or osteoclastic activity [45]
The authors have not encountered any literature showing proven clinical long-term adverse effects from GBCAs in subjects with normal renal function. Although there is insufficient knowledge of long-term effects, gadolinium deposition raises a potentially greater concern in childhood due to brain development and rapid skeletal growth. Because long-term effects of GBCA deposition are not known, the authors suggest that they should be administered with caution. For some patients, pre-contrast images are reviewed in real-time to assess the need for GBCA. Use of macrocyclic agents over linear agents is preferred whenever possible, given stronger chelation. The radiologist and referring provider should evaluate whether administration of GBCA would add value, especially in neonates who have lower GFR and children who may receive multiple GBCA administrations. There is significant interest in alternatives to GBCAs and administration of chelating agents which would bind free gadolinium. For additional current best practices on contrast agent administration the reader is encouraged to refer to the American College of Radiology (ACR) Manual on Contrast Media [37]
Sedation
The risks of sedation during imaging can be direct or indirect. Direct risks relate to an adverse event occurring during or immediately after sedation. Their incidence depends on sedation technique, training of sedating health care providers, and patient population. Fortunately, at most institutions performing imaging research, sedation is administered at dedicated pediatric imaging centers by experienced subspecialty providers with trained support personnel/resources. Indirect risk remains theoretical, that of neurotoxicity from anesthetic agents [46], [47], [48], [49], [50]. Although there is no data showing neurotoxicity from anesthetic agents in children, data showing neurotoxicity in animal models suggests that judicious use of sedation is prudent [34].
The long scan times, narrow scanner bore, and noise of the MRI scanner make for a challenging environment for imaging children [34,51]. Multiple strategies can be used to increase the likelihood of a successful MRI examination without sedation. A child-friendly environment can reduce anxiety and increase cooperation [52,53].
Prior to scanning, exposure to photographs, models, or even mock scanners [54] with noise feed of the scanner can familiarize children and their caretakers with the MRI environment [34]. Despite these efforts, sedation is often required. While there is a low risk of acute complications with commonly used sedatives, long-term neurological and cognitive side effects of sedation remain uncertain.
In neonates, techniques for reducing sedation are centered around encouraging natural sleep during the exam. Noise reduction with ear covers or quieter MR pulse sequences have been shown to improve the success rate of unsedated scans [55]. “Feed and swaddle” approach is commonly employed; where the shortened scan is scheduled to occur after a feed, followed by induction of natural sleep and immobilization with swaddling [34]. This is not always possible for lengthier research protocols, and for these examinations it is critical to run the most important and/or quieter sequences first. In some cases, multiple imaging sessions might be required.
For older children, strategies center on minimizing anxiety and distracting the child during the MRI. This can include entertainment with items such as headphones or video goggles [51]. In certain research scenarios, particularly functional imaging, these techniques may not be possible as they would influence the data being acquired. In these cases, engaging with child life specialists and involving the patient's parents can improve the child's comfort with the exam and increase the likelihood of success. Child life specialists can be invaluable in working with both children and their families to reduce fears and prepare the child for the study. Their use in imaging has been shown to reduce exposure to the risks, cost, and inconvenience of general anesthesia without a decrease in diagnostic image quality [56].
MRI's level of noise due to gradient coils can wake a sleeping or sedated child, leading to aborted examinations or need for additional or deeper sedation. Some scanning parameters can be adjusted to reduce noise such as the use of deceased gradient slew rate and slow ramps for k-space readout [34]. This reduces noise beyond earplugs and insulation of the inner bore of the magnet. Newer “silent” MRI techniques can dramatically reduce acoustic noise while providing diagnostic image quality [34,57,58].
Fast protocols
Acquisition techniques that can help accelerate scan time include: radial k-space sampling [59] compressed sensing [60], use of 3D sequences, use of single shot fast spin echo [61,62]
More recently, artificial intelligence tools have been introduced which denoise and interpolate data during image reconstruction, to improve image quality, and further accelerate MRI scan times [63]. These techniques can be combined with k-space undersampling to enhance image quality. Several reports have shown non-inferiority with these techniques [63,64]. Due to advances in processing hardware, many of these tools can provide near real time results in near real time Rapid acquisition of images with MRI using a tailored protocol to answer a focused clinical question is standard of care to reduce scan duration, the need for sedation, and serve as an alternative to CT. As an example, echo-planar fast spin echo sequences, such as half-Fourier acquisition single-shot turbo spin echo, single-shot fast spin echo, and single-shot turbo spin echo, have been used for evaluation of children with ventriculoperitoneal shunts and are effective for evaluation of shunt position and size of the ventricular system; albeit limited for further evaluation of the brain [34]. In the research setting, where high-quality structural imaging is required, other image acceleration factors are preferable.
Synthetic MRI is one of the major advances in the field of MR physics and allows reconstruction of multiple MR tissue contrasts for qualitative and quantitative analysis from a single acquisition [65,66]. This method allows reduction of MR imaging time to minutes and has been applied to multiple disease processes including cancer [67], multiple sclerosis [68], and stroke [69] Both the STAGE and the MAGiC approaches are acquired over around 5 minutes and allow for reconstruction of standard tissue contrasts (T1, T2, FLAIR, SWI) [65]. Please refer to Tanenbaum et al for further detail [65]. Synthetic MRI will likely be critical in pediatric neuro-oncologic imaging as it not only allows for a dramatic reduction in scan time which will increase the time available for research sequences, but provides additional quantitative data. Although uncertainly about the edge case performance and potential failure modes of these sequences has limited their current clinical use, development of applications combining synthetic MRI with molecular or functional imaging may be of significant future value.
Beyond faster protocols, newer hybrid scanners such as PET/MRI offer significant benefits to clinical and research imaging in pediatrics. Combining MRI and PET imaging in a single scanner results in a decrease in the time required for imaging, decreases the number of sedation instances for the patient, and has the potential for improved image quality [70,71]. This latter point is particularly true for the PET imaging component of the scan where deep learning methods are being applied to improve attenuation correction and image reconstruction [72,73].
Radiomics, radiogenomics, and multi-omics approaches
Radiomics is high-throughput extraction of quantitative and computational features from medical images that allows for characterization of the underlying heterogeneity of the disease [74,75]. Radiomic methods were first introduced into the discipline of cancer imaging around a decade ago. Ever since, the literature has witnessed an exponential growth in the number of publications that include “radiomics” methodologies, to the extent that radiomics may now be considered a field of its own. Imaging genomics, or “radiogenomics”, refers to the application of radiomics for prediction of molecular characteristics of tumors [76], [77], [78]. Radiogenomics may overcome the limitations of histopathological assessments, such as molecular analysis of only a small portion of the tumor, sampling error from biopsy procedure, unavailability of a tissue sample for deep-seated or unresectable tumors, or lack of access to sequencing panels. This can be achieved by quantification of spatial heterogeneity of the tumors and providing non-invasive and in-vivo surrogate markers for molecular alterations based on pre-operative or pre-treatment MRI scans. In other words, radiogenomics may serve as a “virtual biopsy” tool to portray the molecular landscape of tumors based on readily available MRI scans and bridge the gap between imaging and genomics disciplines [76,77]. We refer the readers interested to learn about the existing radiomic and radiogenomic studies in pediatric brain tumors to a recent review paper [79].
While the field of radiomics in adult brain tumors has witnessed exponential growth over the past decade, such studies have been fairly limited in pediatric brain tumors [79], which mainly could be due to significantly lower incidence rate (on the order of one fifth) of brain tumors in children compared to their adult counterparts [80]. . As many pediatric brain tumor cohorts reported in the literature are small, some of the proposed methods may not be generalizable to unseen data from the same or different centers. The Children's Brain Tumor Network (CBTN) [81], Pediatric Brain Tumor Consortium (PBTC), and Pacific Neuro-Oncology Consortium (PNOC) have facilitated access to larger multi-institutional cohorts through their cloud-based platforms. Such consortiums are paving the path towards advancement of discoveries and available tools for diagnosis and treatment of pediatric brain tumors by increasing the statistical power for research studies. In the future, addition of data from multiple centers across the globe can further contribute to development of more generalizable predictive tools based on widely available MRI scans, built based on diverse datasets that are inclusive of patients from different countries with different economic and social backgrounds [79]. Such tools can allow their application in lower-income countries without access to sophisticated sequencing methods. Nonetheless, computational methods developed based on multi-institutional datasets face the challenge of harmonization when different MRI protocols have been implemented for image acquisition [79]. Standardization of image acquisition protocols and definitions in diagnosis and response assessment of pediatric brain tumors, as addressed in a few consensus recommendation studies in adult brain tumors can be helpful for reliable tumor response assessment [79,82]. The Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group has provided guidelines and recommendations [[12], [13],[14], [15], [16]], which if followed, can potentially help in reproducibility of extracted radiomic features among different scanners and centers, and further contribute to development of generalizable predictive models.
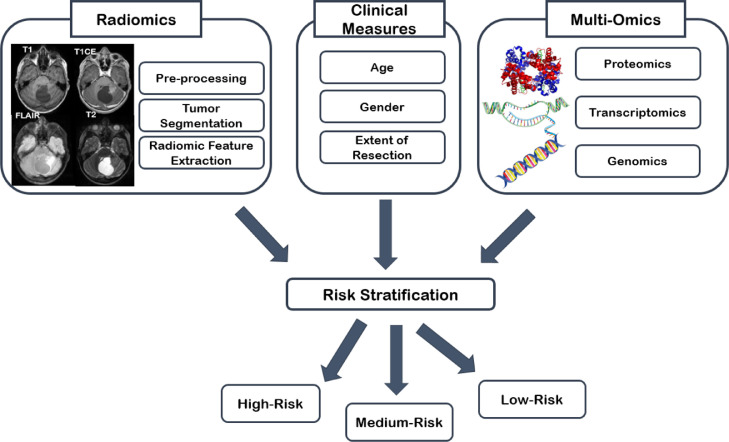
The overarching goal of radiomic and radiogenomic studies is to improve personalized decision-making through establishing predictive models for upfront prediction of therapeutic outcomes that can help in determining the risks and benefits of different treatment options [83]. Therefore, for comprehensive characterization of tumor landscape, integration of multi-scale information, from phenotype to genotype is critical [84]. The 2021 WHO classification of CNS tumors [1] introduced the integrated diagnosis, using histological and molecular features, for parsing the formerly broad categories of pediatric gliomas into more distinctive subtypes that correlate with clinical outcomes. Availability of “multi-omics” data including pathomics, proteomics, transcriptomics, metabolomics, and genomics can further revolutionize integrated diagnostic approaches [85] (Fig. 2). Combination of radiomics with multi-omics data provides an additional layer of information by demonstrating spatial heterogeneity of the tissue [84]. Guided localized biopsies based on pre-operative radiomic analysis may also facilitate the flow of information from one layer to another and bridge the gap between genotype and phenotype. Currently, the efforts at the CBTN consortium are focused on providing a platform for multi-omics data analysis, visualization, and interpretation of pediatric brain tumors [81]. In a near future, with the advent of targeted therapies, comprehensive multi-omics approaches can better tailor the course of treatment for individual patients and provide truly personalized patient care and therapies.
Fig. 2.
Integrated diagnostics based on a multi-omics approach in pediatric neuro-oncology.
Molecular imaging
Anatomic and physiologic MRI can provide crucial information in pediatric brain tumor patients such as tumor size, location, cystic versus solid components, contrast enhancement, cellularity, and vascularity. Molecular imaging techniques allow us to go even beyond assessing gross morphological and physiological characteristics and to gain insight into various aspects of tumor microenvironment.
Proton MR spectroscopy (MRS) has been used to assess different aspects of pediatric glioma metabolism for the past couple of decades [86], [87], [88]. Earlier studies were mainly focused on increased Cho/NAA ratio and lactate for tumor grading [89,90]. More recently, MRS was used to determine various metabolites to assess metabolic pathways, and metabolic subgrouping and to integrate metabolic and epigenetic reprogramming in pediatric gliomas [86], [87], [88]. In addition, MRS demonstrated excellent accuracy of 2-HG MRS to predict IDH mutant gliomas [91]. Despite these promising studies, proton MRS has limitations such as the inability to measure metabolic fluxes [92].
Hyperpolarized carbon-13 (HP-13C) MR imaging presents a promising relatively new technique that allows noninvasive investigation of in vivo tumor metabolism by using dynamic nuclear polarization to transiently enhance the signal for molecular probes that can assess different aspects of tumor metabolism. A recent feasibility study in participants with diffuse intrinsic pontine glioma demonstrated the safety of HP [1-13C]pyruvate injection and characteristic conversion to [1-13C]lactate and [13C]bicarbonate in the brain as biomarkers of glycolysis and oxidative phosphorylation respectively; however, the study was closed prematurely due to poor accrual [93]. Despite promises of HP [1-13C] MRI, the short lifetime of 13C magnetization requires very tight study logistics which can be challenging in pediatric glioma patients. Deuterium metabolic imaging (DMI) is a novel MRI molecular imaging technique that can be performed with oral intake or intravenous infusion of nonradioactive 2H-labeled substrates followed by deuterium magnetic resonance spectroscopic imaging and enables noninvasive metabolic imaging of gliomas [92]. DMI has been used [6,6′-2H2]glucose to monitor flux to lactate and glx (sum of glutamine and glutamate) and to visualize tumor burden in glioblastoma patients [94]. In addition, a recent animal study demonstrated that DMI can be used to non-invasively assess alternative lengthening of telomeres (ALT)-linked modulation of glycolytic flux and to monitor response to ALT-inhibitor therapy [94]. Chemical exchange saturation transfer (CEST) is a molecular MRI imaging technique that is based on applying an off-resonance that can detect very low concentrations of molecules by the presence of groups with exchangeable protons, such as hydroxyls, amides, and amines [95]. Many studies demonstrated that amide proton transfer (APT) imaging can predict tumor grading [96], IDH status [97], response to treatment [98]and differentiate progression from pseudoprogression [99]. Amine-weighted CEST imaging has also been used to monitor tumor PH [100] and for metabolic characterization of IDH mutant and wild-type gliomas [101] and finally to monitor tumor response to bevacizumab treatment [102]. Given the promising literature on adult gliomas, future studies on pediatric gliomas are needed to confirm their utility in this age group.
Positron emission tomography (PET) has also been utilized in pediatric brain tumors. While 18F-FDG PET has been used to answer various questions such as differentiation of neoplastic from non-neoplastic entities, tumor grading, biopsy planning, prognostication, and assessment of treatment response, amino acid radiotracers showed a better performance in these clinical settings [103,104]. In a study of 97 patients with suspected pediatric brain tumors, the addition of 18F-FET PET to MRI helped to discriminate tumors from non-tumor lesions with high accuracy [105]. A recent study of post-treatment pediatric high-grade glioma demonstrated that qualitative evaluation of 11C- methionine -PET has higher sensitivity, and accuracy for predicting tumor recurrence than does MRI and quantitative 11C-MET-PET can predict overall survival [106]. In another study, in pediatric HGG, 11C- methionine -PET was able to delineate non-enhancing tumor regions at high risk for recurrence [107]. Finally, most newly diagnosed diffuse intrinsic pontine gliomas were successfully visualized by 11C-methionine-PET and baseline PET uptake was able to delineate regions at increased risk of recurrence [108]. Despite these encouraging studies, amino acid PET imaging is still used only in a few academic centers in the US and future studies with widely available radiotracers are needed.
Future directions
The standard brain and spine MR sequences defined by the RAPNO working group can help to overcome the challenges with response assessment in clinical trials. Future prospective trials are needed to evaluate their feasibility and validate these criteria with patient outcomes. In addition, standardization of image acquisition and image harmonization can facilitate the utilization of multi-institutional databases which will be key to the advancement of radiomics and radiogenomics in pediatric neuro-oncology. Standardization of the reporting of radiomics- radiogenomics studies, similar to diagnostic performance studies will be another important step to increase the chance of reproducibility and validation of studies [109]. Finally, despite significant advances in experimental and molecular imaging approaches, participation in nontherapeutic research studies can be challenging as patients and families are usually overwhelmed by a large number of clinically relevant imaging, hospital visits, and therapeutic trials [93]. A necessary solution is the early integration of experimental imaging trials as a biomarker that can inform therapeutic research studies.
CRediT authorship contribution statement
Ali Nabavizadeh: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Matthew J Barkovich: Investigation, Methodology, Writing – original draft, Writing – review & editing. Ali Mian: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Van Ngo: Investigation, Methodology, Writing – original draft. Anahita Fathi Kazerooni: Investigation, Methodology, Writing – original draft, Writing – review & editing. Javier E Villanueva-Meyer: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborn A.G., Louis D.N., Poussaint T.Y., Linscott L.L., Salzman K.L. The 2021 World Health Organization classification of tumors of the central nervous system: what neuroradiologists need to know. AJNR Am. J. Neuroradiol. 2022;43(7):928–937. doi: 10.3174/ajnr.A7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryall S., Tabori U., Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020;8(1):30. doi: 10.1186/s40478-020-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen AR. Brain tumors in children. N. Engl. J. Med. 2022;386(20):1922–1931. doi: 10.1056/NEJMra2116344. [DOI] [PubMed] [Google Scholar]
- 5.Higano S., Yun X., Kumabe T., Watanabe M., Mugikura S., Umetsu A., et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–846. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 6.Dury R.J., Lourdusamy A., Macarthur D.C., Peet A.C., Auer D.P., Grundy R.G., et al. Meta-Analysis of Apparent Diffusion Coefficient in Pediatric Medulloblastoma, Ependymoma, and Pilocytic Astrocytoma. J. Magn. Reson. Imaging. 2022;56(1):147–157. doi: 10.1002/jmri.28007. [DOI] [PubMed] [Google Scholar]
- 7.Shrot S., Kerpel A., Belenky J., Lurye M., Hoffmann C., Yalon M. MR Imaging characteristics and ADC histogram metrics for differentiating molecular subgroups of pediatric low-grade gliomas. AJNR Am. J. Neuroradiol. 2022;43(9):1356–1362. doi: 10.3174/ajnr.A7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonçalves F.G., LO Tierradentro-Garcia., Kim JDU., Zandifar A., Ghosh A., Viaene A.N., et al. The role of apparent diffusion coefficient histogram metrics for differentiating pediatric medulloblastoma histological variants and molecular groups. Pediatr. Radiol. 2022;52(13):2595–2609. doi: 10.1007/s00247-022-05411-w. [DOI] [PubMed] [Google Scholar]
- 9.Poussaint T.Y., Kocak M., Vajapeyam S., Packer R.I., Robertson R.L., Geyer R., et al. MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC) Neuro-oncol. 2011;13(4):417–427. doi: 10.1093/neuonc/noq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lober R.M., Cho Y.J., Tang Y., Barnes P.D., Edwards M.S., Vogel H., et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J. Neurooncol. 2014;117(1):175–182. doi: 10.1007/s11060-014-1375-8. [DOI] [PubMed] [Google Scholar]
- 11.Aboian M.S., Tong E., Solomon D.A., Kline C., Gautam A., Vardapetyan A., et al. Diffusion characteristics of pediatric diffuse midline gliomas with histone H3-K27M mutation using apparent diffusion coefficient histogram analysis. AJNR Am. J. Neuroradiol. 2019;40(11):1804–1810. doi: 10.3174/ajnr.A6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooney T.M., Cohen K.J., Guimaraes C.V., Dhall G., Leach J., Massimino M., et al. Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e330–e3e6. doi: 10.1016/S1470-2045(20)30166-2. [DOI] [PubMed] [Google Scholar]
- 13.Erker C., Tamrazi B., Poussaint T.Y., Mueller S., Mata-Mbemba D., Franceschi E., et al. Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e317–ee29. doi: 10.1016/S1470-2045(20)30173-X. [DOI] [PubMed] [Google Scholar]
- 14.Fangusaro J., Witt O., Hernáiz Driever P., Bag A.K., de Blank P., Kadom N., et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e305–ee16. doi: 10.1016/S1470-2045(20)30064-4. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay H.B., Massimino M., Avula S., Stivaros S., Grundy R., Metrock K., et al. Response assessment in paediatric intracranial ependymoma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2022;23(8):e393–e401. doi: 10.1016/S1470-2045(22)00222-4. [DOI] [PubMed] [Google Scholar]
- 16.Peng J., Zhou H., Tang O., Chang K., Wang P., Zeng X., et al. Evaluation of RAPNO criteria in medulloblastoma and other leptomeningeal seeding tumors using MRI and clinical data. Neuro-oncol. 2020;22(10):1536–1544. doi: 10.1093/neuonc/noaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel P., Baradaran H., Delgado D., Askin G., Christos P., John Tsiouris A., et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro-oncol. 2017;19(1):118–127. doi: 10.1093/neuonc/now148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudino S., Martucci M., Botto A., Ruberto E., Leone E., Infante A., et al. Brain DSC MR perfusion in children: a clinical feasibility study using different technical standards of contrast administration. AJNR Am. J. Neuroradiol. 2019;40(2):359–365. doi: 10.3174/ajnr.A5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho C.Y., Cardinal J.S., Kamer A.P., Kralik S.F. Relative cerebral blood volume from dynamic susceptibility contrast perfusion in the grading of pediatric primary brain tumors. Neuroradiology. 2015;57(3):299–306. doi: 10.1007/s00234-014-1478-0. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim M., Ghazi T.U., Bapuraj J.R., Srinivasan A. Contrast pediatric brain perfusion: dynamic susceptibility contrast and dynamic contrast-enhanced MR imaging. Magn. Reson. Imaging Clin. N. Am. 2021;29(4):515–526. doi: 10.1016/j.mric.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Vajapeyam S., Stamoulis C., Ricci K., Kieran M., Poussaint T.Y. Automated processing of dynamic contrast-enhanced mri: correlation of advanced pharmacokinetic metrics with tumor grade in pediatric brain tumors. AJNR Am. J. Neuroradiol. 2017;38(1):170–175. doi: 10.3174/ajnr.A4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta P.K., Saini J., Sahoo P., Patir R., Ahlawat S., Beniwal M., et al. Role of dynamic contrast-enhanced perfusion magnetic resonance imaging in grading of pediatric brain tumors on 3T. Pediatr. Neurosurg. 2017;52(5):298–305. doi: 10.1159/000479283. [DOI] [PubMed] [Google Scholar]
- 23.Jones J.Y., Selvaraj B., Ho M.L. Pediatric functional neuroimaging: practical tips and pearls. AJR Am. J. Roentgenol. 2020;214(5):995–1007. doi: 10.2214/AJR.19.22178. [DOI] [PubMed] [Google Scholar]
- 24.Nikam R.M., Yue X., Kaur G., Kandula V., Khair A., Kecskemethy H.H., et al. Advanced neuroimaging approaches to pediatric brain tumors. Cancers. 2022;14(14) doi: 10.3390/cancers14143401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavhan G.B., Babyn P.S., Thomas B., Shroff M.M., Haacke E.M. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salama G.R., Heier L.A., Patel P., Ramakrishna R., Magge R., Tsiouris A.J. Diffusion weighted/tensor imaging, functional MRI and perfusion weighted imaging in glioblastoma-foundations and future. Front. Neurol. 2017;8:660. doi: 10.3389/fneur.2017.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duc N.M. The role of diffusion tensor imaging metrics in the discrimination between cerebellar medulloblastoma and brainstem glioma. Pediatr. Blood. Cancer. 2020;67(9):e28468. doi: 10.1002/pbc.28468. [DOI] [PubMed] [Google Scholar]
- 28.Minh Duc N. The performance of diffusion tensor imaging parameters for the distinction between medulloblastoma and pilocytic astrocytoma. Minerva Pediatr. 2021 doi: 10.23736/S2724-5276.21.05955-7. [DOI] [PubMed] [Google Scholar]
- 29.Helton K.J., Phillips N.S., Khan R.B., Boop F.A., Sanford R.A., Zou P., et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am. J. Neuroradiol. 2006;27(4):786–793. [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhu S.P., Ng S., Vajapeyam S., Kieran M.W., Pollack I.F., Geyer R., et al. DTI assessment of the brainstem white matter tracts in pediatric BSG before and after therapy: a report from the Pediatric Brain Tumor Consortium. Childs Nerv. Syst. 2011;27(1):11–18. doi: 10.1007/s00381-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khong P.L., Kwong D.L., Chan G.C., Sham J.S., Chan F.L., Ooi G.C. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. AJNR Am. J. Neuroradiol. 2003;24(4):734–740. [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu D., Kwong D.L., Chan G.C., Leung L.H., Khong P.L. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? Int. J. Radiat. Oncol. Biol. Phys. 2007;69(3):846–851. doi: 10.1016/j.ijrobp.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Lui Y.W., Law M., Chacko-Mathew J., Babb J.S., Tuvia K., Allen J.C., et al. Brainstem corticospinal tract diffusion tensor imaging in patients with primary posterior fossa neoplasms stratified by tumor type: a study of association with motor weakness and outcome. Neurosurgery. 2007;61(6):1199–1207. doi: 10.1227/01.neu.0000306098.38141.81. discussion 207-8. [DOI] [PubMed] [Google Scholar]
- 34.Barkovich M.J., Xu D., Desikan R.S., Williams C., Barkovich A.J. Pediatric neuro MRI: tricks to minimize sedation. Pediatr. Radiol. 2018;48(1):50–55. doi: 10.1007/s00247-017-3785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman T.R., Mustafa A., Rowe E. Pediatric CT radiation exposure: where we were, and where we are now. Pediatr. Radiol. 2019;49(4):469–478. doi: 10.1007/s00247-018-4281-y. [DOI] [PubMed] [Google Scholar]
- 36.Attari H., Cao Y., Elmholdt T.R., Zhao Y., Prince M.R. A systematic review of 639 patients with biopsy-confirmed nephrogenic systemic fibrosis. Radiology. 2019;292(2):376–386. doi: 10.1148/radiol.2019182916. [DOI] [PubMed] [Google Scholar]
- 37.ACR Committee on Drugs and Contrast Media . 2022. ACR Manual on Contrast Media. [DOI] [PubMed] [Google Scholar]
- 38.Prince M.R., Zhang H., Zou Z., Staron R.B., Brill P.W. Incidence of immediate gadolinium contrast media reactions. AJR Am. J. Roentgenol. 2011;196(2):W138–W143. doi: 10.2214/AJR.10.4885. [DOI] [PubMed] [Google Scholar]
- 39.Public Health Advisory Gadolinium-containing Contrast Agents for Magnetic Resonance Imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance 2006 [Available from: http://web.archive.org/web/20060613172647/http://www.fda.gov/cder/drug/advisory/gadolinium_agents.htm.
- 40.Kaewlai R., Abujudeh H. Nephrogenic systemic fibrosis. AJR Am. J. Roentgenol. 2012;199(1):W17–W23. doi: 10.2214/AJR.11.8144. [DOI] [PubMed] [Google Scholar]
- 41.Schieda N., Blaichman J.I., Costa A.F., Glikstein R., Hurrell C., James M., et al. Gadolinium-based contrast agents in kidney disease: a comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can. J. Kidney Health Dis. 2018;5 doi: 10.1177/2054358118778573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald R.J., Levine D., Weinreb J., Kanal E., Davenport M.S., Ellis J.H., et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology. 2018;289(2):517–534. doi: 10.1148/radiol.2018181151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumfield E., Swenson D.W., Iyer R.S., Stanescu A.L. Gadolinium-based contrast agents - review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients. Pediatr. Radiol. 2019;49(4):448–457. doi: 10.1007/s00247-018-4304-8. [DOI] [PubMed] [Google Scholar]
- 44.Layne K.A., Dargan P.I., Archer JRH., Wood D.M. Gadolinium deposition and the potential for toxicological sequelae - A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018;84(11):2522–2534. doi: 10.1111/bcp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lancelot E. Revisiting the pharmacokinetic profiles of gadolinium-based contrast agents: differences in long-term biodistribution and excretion. Invest. Radiol. 2016;51(11):691–700. doi: 10.1097/RLI.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 46.Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 47.Jevtovic-Todorovic V., Hartman R.E., Izumi Y., Benshoff N.D., Dikranian K., Zorumski C.F., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23(3):876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bercker S., Bert B., Bittigau P., Felderhoff-Müser U., Bührer C., Ikonomidou C., et al. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox. Res. 2009;16(2):140–147. doi: 10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 49.Bajic D., Commons K.G., Soriano S.G. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int. J. Dev. Neurosci. 2013;31(4):258–266. doi: 10.1016/j.ijdevneu.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olney J.W., Wozniak D.F., Jevtovic-Todorovic V., Farber N.B., Bittigau P., Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barkovich M.J., Li Y., Desikan R.S., Barkovich A.J., Xu D. Challenges in pediatric neuroimaging. Neuroimage. 2019;185:793–801. doi: 10.1016/j.neuroimage.2018.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGuirt D. Alternatives to sedation and general anesthesia in pediatric magnetic resonance imaging: a literature review. Radiol. Technol. 2016;88(1):18–26. [PubMed] [Google Scholar]
- 53.Dean D.C., 3rd, Dirks H., O'Muircheartaigh J., Walker L., Jerskey B.A., Lehman K., et al. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatr. Radiol. 2014;44(1):64–72. doi: 10.1007/s00247-013-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Bie H.M., Boersma M., Wattjes M.P., Adriaanse S., Vermeulen R.J., Oostrom K.J., et al. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur. J. Pediatr. 2010;169(9):1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Zwart J.A., van Gelderen P., Kellman P., Duyn J.H. Reduction of gradient acoustic noise in MRI using SENSE-EPI. Neuroimage. 2002;16(4):1151–1155. doi: 10.1006/nimg.2002.1119. [DOI] [PubMed] [Google Scholar]
- 56.Durand D.J., Young M., Nagy P., Tekes A., Huisman T.A. Mandatory child life consultation and its impact on pediatric MRI workflow in an academic medical center. J. Am. Coll. Radiol. 2015;12(6):594–598. doi: 10.1016/j.jacr.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo-Hagiyama C., Watanabe Y., Tanaka H., Takahashi H., Arisawa A., Yoshioka E., et al. Comparison of silent and conventional mr imaging for the evaluation of myelination in children. Magn. Reson. Med. Sci. 2017;16(3):209–216. doi: 10.2463/mrms.mp.2016-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alibek S., Vogel M., Sun W., Winkler D., Baker C.A., Burke M., et al. Acoustic noise reduction in MRI using Silent Scan: an initial experience. Diagn. Interv. Radiol. 2014;20(4):360–363. doi: 10.5152/dir.2014.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vertinsky A.T., Rubesova E., Krasnokutsky M.V., Bammer S., Rosenberg J., White A., et al. Performance of PROPELLER relative to standard FSE T2-weighted imaging in pediatric brain MRI. Pediatr. Radiol. 2009;39(10):1038–1047. doi: 10.1007/s00247-009-1292-8. [DOI] [PubMed] [Google Scholar]
- 60.Vasanawala S.S., Alley M.T., Hargreaves B.A., Barth R.A., Pauly J.M., Lustig M. Improved pediatric MR imaging with compressed sensing. Radiology. 2010;256(2):607–616. doi: 10.1148/radiol.10091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel D.M., Tubbs R.S., Pate G., Johnston J.M., Jr., Blount J.P. Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus. J. Neurosurg. Pediatr. 2014;13(4):440–447. doi: 10.3171/2014.1.PEDS13447. [DOI] [PubMed] [Google Scholar]
- 62.Forbes K.P., Pipe J.G., Karis J.P., Farthing V., Heiserman J.E. Brain imaging in the unsedated pediatric patient: comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. AJNR Am. J. Neuroradiol. 2003;24(5):794–798. [PMC free article] [PubMed] [Google Scholar]
- 63.Rudie J.D., Gleason T., Barkovich M.J., Wilson D.M., Shankaranarayanan A., Zhang T., et al. Clinical assessment of deep learning-based super-resolution for 3D volumetric brain MRI. Radiol. Artif. Intell. 2022;4(2) doi: 10.1148/ryai.210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bash S., Wang L., Airriess C., Zaharchuk G., Gong E., Shankaranarayanan A., et al. Deep learning enables 60% accelerated volumetric brain MRI while preserving quantitative performance: a prospective, multicenter, multireader trial. AJNR Am. J. Neuroradiol. 2021;42(12):2130–2137. doi: 10.3174/ajnr.A7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanenbaum L.N., Tsiouris A.J., Johnson A.N., Naidich T.P., DeLano M.C., Melhem E.R., et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am. J. Neuroradiol. 2017;38(6):1103–1110. doi: 10.3174/ajnr.A5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji S., Yang D., Lee J., Choi S.H., Kim H., Kang K.M. Synthetic MRI: technologies and applications in neuroradiology. J. Magn. Reson. Imaging. 2022;55(4):1013–1025. doi: 10.1002/jmri.27440. [DOI] [PubMed] [Google Scholar]
- 67.Blystad I., Warntjes JBM., Smedby Ö., Lundberg P., Larsson E.M., Tisell A. Quantitative MRI using relaxometry in malignant gliomas detects contrast enhancement in peritumoral oedema. Sci. Rep. 2020;10(1):17986. doi: 10.1038/s41598-020-75105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagiwara A., Hori M., Yokoyama K., Takemura M.Y., Andica C., Tabata T., et al. Synthetic MRI in the detection of multiple sclerosis plaques. AJNR Am. J. Neuroradiol. 2017;38(2):257–263. doi: 10.3174/ajnr.A5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.André J., Barrit S., Jissendi P. Synthetic MRI for stroke: a qualitative and quantitative pilot study. Sci. Rep. 2022;12(1):11552. doi: 10.1038/s41598-022-15204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gatidis S., la Fougere C., Schaefer J.F. Pediatric oncologic imaging: a key application of combined PET/MRI. Rofo. 2016;188(4):359–364. doi: 10.1055/s-0041-109513. [DOI] [PubMed] [Google Scholar]
- 71.Ehman E.C., Johnson G.B., Villanueva-Meyer J.E., Cha S., Leynes A.P., Larson PEZ., et al. PET/MRI: Where might it replace PET/CT? J. Magn. Reson. Imaging. 2017;46(5):1247–1262. doi: 10.1002/jmri.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladefoged C.N., Marner L., Hindsholm A., Law I., Hojgaard L., Andersen F.L. Deep learning based attenuation correction of PET/MRI in pediatric brain tumor patients: evaluation in a clinical setting. Front. Neurosci. 2018;12:1005. doi: 10.3389/fnins.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schramm G., Rigie D., Vahle T., Rezaei A., Van Laere K., Shepherd T., et al. Approximating anatomically-guided PET reconstruction in image space using a convolutional neural network. Neuroimage. 2021;224 doi: 10.1016/j.neuroimage.2020.117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gatenby R.A., Grove O., Gillies R.J. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269(1):8–15. doi: 10.1148/radiol.13122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fathi Kazerooni A., Bakas S., Saligheh Rad H., Davatzikos C. Imaging signatures of glioblastoma molecular characteristics: a radiogenomics review. J. Magn. Reson. Imaging. 2020;52(1):54–69. doi: 10.1002/jmri.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazurowski M.A. Radiogenomics: what it is and why it is important. J. Am. Coll. Radiol. 2015;12(8):862–866. doi: 10.1016/j.jacr.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Zinn P.O., Mahmood Z., Elbanan M.G., Colen R.R. Imaging Genomics in Gliomas. Cancer J. 2015;21(3):225–234. doi: 10.1097/PPO.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 79.Madhogarhia R., Haldar D., Bagheri S., Familiar A., Anderson H., Arif S., et al. Radiomics and radiogenomics in pediatric neuro-oncology: a review. Neurooncol. Adv. 2022;4(1):vdac083. doi: 10.1093/noajnl/vdac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ostrom Q.T., Gittleman H., Liao P., Vecchione-Koval T., Wolinsky Y., Kruchko C., et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-oncol. 2017;19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lilly J.V., Rokita J.L., Mason J.L., Patton T., Stefankiewiz S., Higgins D., et al. The children's brain tumor network (CBTN) - accelerating research in pediatric central nervous system tumors through collaboration and open science. Neoplasia. 2023;35 doi: 10.1016/j.neo.2022.100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaufmann T.J., Smits M., Boxerman J., Huang R., Barboriak D.P., Weller M., et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro-oncol. 2020;22(6):757–772. doi: 10.1093/neuonc/noaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanduleanu S., Woodruff H.C., de Jong EEC., van Timmeren J.E., Jochems A., Dubois L., et al. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. 2018;127(3):349–360. doi: 10.1016/j.radonc.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 84.Fathi Kazerooni A., Bagley S.J., Akbari H., Saxena S., Bagheri S., Guo J., et al. Applications of radiomics and radiogenomics in high-grade gliomas in the era of precision medicine. Cancers. 2021;13(23) doi: 10.3390/cancers13235921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subramanian I., Verma S., Kumar S., Jere A., Anamika K. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights. 2020;14 doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung C., Sweha S.R., Pratt D., Tamrazi B., Panwalkar P., Banda A., et al. Integrated metabolic and epigenomic reprograming by H3K27M mutations in diffuse intrinsic pontine gliomas. Cancer Cell. 2020;38(3):334–349. doi: 10.1016/j.ccell.2020.07.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panwalkar P., Tamrazi B., Dang D., Chung C., Sweha S., Natarajan S.K., et al. Targeting integrated epigenetic and metabolic pathways in lethal childhood PFA ependymomas. Sci. Transl. Med. 2021;13(614):eabc0497. doi: 10.1126/scitranslmed.abc0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamrazi B., Venneti S., Margol A., Hawes D., Cen S.Y., Nelson M., et al. Pediatric atypical teratoid/rhabdoid tumors of the brain: identification of metabolic subgroups Using In Vivo (1)H-MR spectroscopy. AJNR Am. J. Neuroradiol. 2019;40(5):872–877. doi: 10.3174/ajnr.A6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimizu H., Kumabe T., Tominaga T., Kayama T., Hara K., Ono Y., et al. Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1996;17(4):737–747. [PMC free article] [PubMed] [Google Scholar]
- 90.Sutton L.N., Wang Z., Gusnard D., Lange B., Perilongo G., Bogdan A.R., et al. Proton magnetic resonance spectroscopy of pediatric brain tumors. Neurosurgery. 1992;31(2):195–202. doi: 10.1227/00006123-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Suh C.H., Kim H.S., Jung S.C., Choi C.G., Kim S.J. 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro-oncol. 2018;20(12):1573–1583. doi: 10.1093/neuonc/noy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Feyter H.M., Behar K.L., Corbin Z.A., Fulbright R.K., Brown P.B., McIntyre S., et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018;4(8):eaat7314. doi: 10.1126/sciadv.aat7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Autry A.W., Park I., Kline C., Chen H.Y., Gordon J.W., Raber S., et al. Pilot study of hyperpolarized (13)C metabolic imaging in pediatric patients with diffuse intrinsic pontine glioma and other CNS cancers. AJNR Am. J. Neuroradiol. 2021;42(1):178–184. doi: 10.3174/ajnr.A6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taglang C., Batsios G., Mukherjee J., Tran M., Gillespie A.M., Hong D., et al. Deuterium magnetic resonance spectroscopy enables non-invasive metabolic imaging of tumor burden and response to therapy in low-grade gliomas. Neuro-oncol. 2022 doi: 10.1093/neuonc/noac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones K.M., Pollard A.C., Pagel M.D. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J. Magn. Reson. Imaging. 2018;47(1):11–27. doi: 10.1002/jmri.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Togao O., Yoshiura T., Keupp J., Hiwatashi A., Yamashita K., Kikuchi K., et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-oncol. 2014;16(3):441–448. doi: 10.1093/neuonc/not158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paech D., Windschuh J., Oberhollenzer J., Dreher C., Sahm F., Meissner J.E., et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro-oncol. 2018;20(12):1661–1671. doi: 10.1093/neuonc/noy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meissner J.E., Korzowski A., Regnery S., Goerke S., Breitling J., Floca R.O., et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J. Magn. Reson. Imaging. 2019;50(4):1268–1277. doi: 10.1002/jmri.26702. [DOI] [PubMed] [Google Scholar]
- 99.Ma B., Blakeley J.O., Hong X., Zhang H., Jiang S., Blair L., et al. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J. Magn. Reson. Imaging. 2016;44(2):456–462. doi: 10.1002/jmri.25159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harris R.J., Cloughesy T.F., Liau L.M., Nghiemphu P.L., Lai A., Pope W.B., et al. Simulation, phantom validation, and clinical evaluation of fast pH-weighted molecular imaging using amine chemical exchange saturation transfer echo planar imaging (CEST-EPI) in glioma at 3 T. NMR Biomed. 2016;29(11):1563–1576. doi: 10.1002/nbm.3611. [DOI] [PubMed] [Google Scholar]
- 101.Yao J., Chakhoyan A., Nathanson D.A., Yong W.H., Salamon N., Raymond C., et al. Metabolic characterization of human IDH mutant and wild type gliomas using simultaneous pH- and oxygen-sensitive molecular MRI. Neuro-oncol. 2019;21(9):1184–1196. doi: 10.1093/neuonc/noz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yao J., Tan CHP., Schlossman J., Chakhoyan A., Raymond C., Pope W.B., et al. pH-weighted amine chemical exchange saturation transfer echoplanar imaging (CEST-EPI) as a potential early biomarker for bevacizumab failure in recurrent glioblastoma. J. Neurooncol. 2019;142(3):587–595. doi: 10.1007/s11060-019-03132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cistaro A., Albano D., Alongi P., Laudicella R., Pizzuto D.A., Formica G., et al. The Role of PET in supratentorial and infratentorial pediatric brain tumors. Curr. Oncol. 2021;28(4):2481–2495. doi: 10.3390/curroncol28040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dunkl V., Cleff C., Stoffels G., Judov N., Sarikaya-Seiwert S., Law I., et al. The usefulness of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the clinical evaluation of brain tumors in children and adolescents. J. Nucl. Med. 2015;56(1):88–92. doi: 10.2967/jnumed.114.148734. [DOI] [PubMed] [Google Scholar]
- 105.Marner L., Lundemann M., Sehested A., Nysom K., Borgwardt L., Mathiasen R., et al. Diagnostic accuracy and clinical impact of [18F]FET PET in childhood CNS tumors. Neuro-oncol. 2021;23(12):2107–2116. doi: 10.1093/neuonc/noab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bag A.K., Wing M.N., Sabin N.D., Hwang S.N., Armstrong G.T., Han Y., et al. (11)C-Methionine PET for identification of pediatric high-grade glioma recurrence. J. Nucl. Med. 2022;63(5):664–671. doi: 10.2967/jnumed.120.261891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lucas J.T., Jr., Serrano N., Kim H., Li X., Snyder S.E., Hwang S., et al. (11)C-Methionine positron emission tomography delineates non-contrast enhancing tumor regions at high risk for recurrence in pediatric high-grade glioma. J. Neurooncol. 2017;132(1):163–170. doi: 10.1007/s11060-016-2354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tinkle C.L., Duncan E.C., Doubrovin M., Han Y., Li Y., Kim H., et al. Evaluation of (11)C-methionine PET and anatomic MRI associations in diffuse intrinsic pontine glioma. J. Nucl. Med. 2019;60(3):312–319. doi: 10.2967/jnumed.118.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pfaehler E., Zhovannik I., Wei L., Boellaard R., Dekker A., Monshouwer R., et al. A systematic review and quality of reporting checklist for repeatability and reproducibility of radiomic features. Phys. Imaging Radiat. Oncol. 2021;20:69–75. doi: 10.1016/j.phro.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]