Abstract
Introduction
The complex and growing problem generated by the rapid expansion of unplanned urban areas includes high population density and increased infestation by vectors responsible for the transmission of several diseases. This requires interdisciplinary and intersectoral interventions due to the burden of diseases, especially those caused by arboviruses, which can present severe forms and generate significant pressure on health systems, especially in more vulnerable regions. The objective of this study was to analyze the spatial distribution of arboviruses transmitted by Aedes aegypti (dengue, Zika, and chikungunya) and assess their correlations with demographic, social, and environmental data from the state of Tocantins, Brazil.
Methods
This was an ecological time series study of the dengue, Zika, and chikungunya arboviruses in the state of Tocantins. Local Moran's indices were used to observe the spatial autocorrelation of cases and to delimit clusters of high and low risks, correlating them with socioenvironmental indicators, in addition to analyses to detect case clusters.
Results
The state reported a mean incidence of 591 annual cases of arbovirus infections per 100,000 inhabitants and a stationary trend with seasonal pattern. Female Pardo individuals aged 20–39 years, with an education level of below college education, were the most affected; Palmas and Araguaína, the two largest cities in the state in terms of economy and population, were the most affected.
Conclusion
A better understanding of the interaction between social characteristics, the environment, and ecology of wild animals and vectors is important for the development of mechanisms to predict outbreaks as well as to develop strategies to reduce and/or mitigate recurring arboviral epidemics and other diseases.
Keywords: Arboviruses, Epidemiology, Spatial analysis, One health
Highlights
-
•
DENV, ZIKV and CHIKV are endemic in the state of Tocantins, Brazil.
-
•
Stationary trend for arboviruses in the state of Tocantins, Brazil.
-
•
Socioeconomic aspects were related to cases of arboviruses.
-
•
Environmental factors were related to arboviruses in Tocantins, Brazil.
-
•
Presence of hot spots for arboviruses in the state of Tocantins, Brazil.
1. Introduction
Arboviruses represent a major challenge to public health in Brazil. This is due to its association with abrupt climate and environmental changes, deforestation, population migration, disorderly occupation of urban spaces, and precarious sanitation conditions, among others. There are currently 545 known species of arboviruses, of which 150 cause disease in humans [1,2].
Arboviruses are arthropod-borne viruses; transmission to humans occurs through the bite of hematophagous arthropods, the arbovirus vector. In the current epidemiological scenario, Brazil is facing a triple viral epidemic with a high number of dengue, Zika, and chikungunya cases, transmitted mainly by the Aedes aegypti (A. aegypti) mosquito. This is in addition to the transmission yellow fever, which, despite the eminently wild cases, remains endemic and enzootic in several tropical regions of the Americas, Asia, and Africa [[1], [2], [3]].
The state of Tocantins has a favorable environment for the proliferation of A. aegypti due to its urban and environmental characteristics. The hot and humid climate, vast network of rivers and streams, disorderly occupation of urban spaces, and low percentage of basic sanitation, along with the seasonal distribution of rainfall, significantly correlate with the high incidence of the arbovirus vector fauna [[4], [5],6].
Regions with low or few health services favor the worsening of this scenario, since non- or late diagnosis hinders the implementation of measures to combat and control these diseases [[7], [8], [9]].
Considering this perspective, the proposed One Health strategy acts as an integrated approach to understand and manage animal, social, and environmental determinants of complex problems such as arbovirus infections [10].
This implies the need to integrate knowledge in the fields of social, economic, biomedical, and ecological studies, among others, in addition to actions with the community and professionals working in the territory, to achieve greater effectiveness and coordination between stakeholders in the development of new surveillance, prevention, and mitigation approaches for multifactorial problems focused on One Health [11,12].
Given this scenario, the objective of this study was to analyze the trend of dengue, Zika, and chikungunya arbovirus infections and their relationship with socioeconomic and environmental determinants in the state of Tocantins, northern region of Brazil.
2. Materials and methods
This was an epidemiological and ecological study on the prevalence of confirmed cases of dengue, Zika, and chikungunya in the state of Tocantins. The inclusion criterion was a confirmed case of the disease, by place of residence, recorded between January 1, 2015 and December 31, 2019.
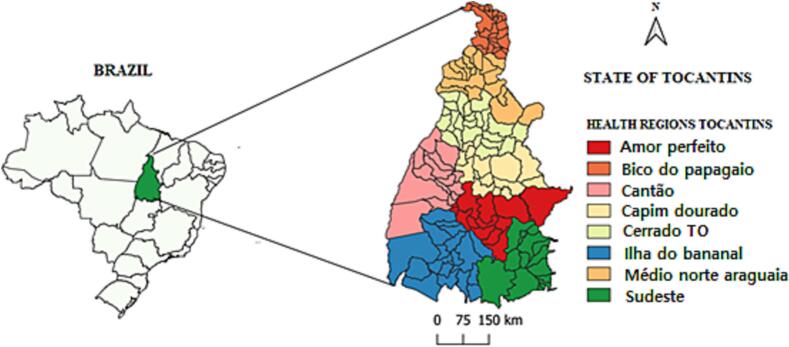
The study was conducted in the state of Tocantins in the northern region of Brazil, Legal Amazon, between the Parallels 5° e 13°, longitude 46° 00′ and 51° 00′ of Greenwich, and latitude 5° 00′ and 13° 00′ S. It has an estimated population of 1,515,126 and an area of 277,720.567 km2, totaling 139 municipalities with a demographic density of 4.98/km2. The state is subdivided into eight health microregions and two mesoregions [13].
The laboratory, epidemiological, and sociodemographic data analyzed in the research were exclusively those described in the patient databases provided by the GAL, the System of the Central Public Health Laboratory of Tocantins; there was no contact between the researcher and patient.
Data on building infestation index (BII) and percentage of primary health care coverage were collected from the State Secretariat of Epidemiological and Environmental Surveillance and from online epidemiological bulletins.
Climatological and rainfall data were collected from the National Meteorological Institute (INMET) database.
Socioeconomic information such as social vulnerability index (SVI), GINI coefficient, and GDP per capita were obtained from the Institute for Applied Economic Research (IPEA). Other socioeconomic indicators such as fertility rate, poverty rate, infant mortality rate, and illiteracy rate were collected from the Informatized Health System (DATASUS).
The annual population estimates used as denominators to calculate incidence and mortality coefficients for the disease, population density, and percentage of sanitary sewerage were obtained from the Brazilian Institute of Geography and Statistics (IBGE) and the National Sanitation Information System (SNIS). All dengue, Zika, and chikungunya cases with clinical/epidemiological and laboratory diagnosis in people residing in Tocantins in the period 2015–2019 were included in the study.
The study sample was composed of 100% of the population reported with dengue, Zika, and chikungunya with data duly registered in the database of the State Secretariat of Health of Tocantins.
Epidemiological data (sex, age group, and zone of residence) were represented by descriptive statistics using the Excel 2010 software (Microsoft Office) and statistically analyzed with the Minitab 19 software. The one-way ANOVA test was used to identify differences between the means of epidemiological and demographic variables. The Tukey's HSD post hoc test was performed to determine which mean differences were significant. Significant values were identified as those with p < 0.05.
The Prais-Winsten generalized linear analysis model was used for time series and trend analysis. This model is used to correct serial autocorrelation in time series. The confidence intervals of the estimates were calculated, and time series can be classified as increasing, decreasing, and stationary. Non-significant p-values (≥ 0.05) were interpreted as a stationary trend (accepting the null hypothesis that arboviruses did not change throughout the study period). Significant p-values (< 0.05) were interpreted as increasing (positive annual change) and decreasing (negative annual change) trends. The Prais-Winsten test was performed in Stata SE software 14.0 to test the statistical significance of the hypotheses at a 95% confidence level [14].
The statistical tools Variogram and bivariate local Moran (LISA) by queen-type contiguity neighborhood matrix generated with first order neighbors were used to analyze the spatial dependence of the incidence of arboviruses with environmental and social aspects, as these are spatialized variables that admit “negative” or “inverse” autocorrelation (I < 0), “randomness” (I = 0), and “positive” or “direct” (I > 0) hypotheses. This study considered a correlation as weak when the index value was close to 0 (−0.5–0.5) and strong when the value was close to −1 (< −0.5) and 1 (> 0.5), with spatial statistical significance determined by p < 0.05. Spatial autocorrelation measures the relationship between observations with spatial proximity, assuming that spatially close observations have similar values. The GeoDa 1.8 software was used to create a conditional “Map Matrix” that conjugates bivariate choropleth maps, combining the variables in pairs. The initial concept of the maps was based on the use of an analysis variable and one or two conditioning variables divided into groups, in this case division by quantile. Thus, each set of conditional maps was created using a socioeconomic and/or environmental indicator with the mean incidence rate of dengue, Zika, and chikungunya in the period.
This study was approved by the Research Ethics Committee (REC) of the School of Sciences of Tocantins (FACIT), TO, under approval no. 3,656,469.
3. Results
The state reported a mean incidence of 591 annual cases of the dengue, Zika, and chikungunya (CKG) arboviruses per 100,000 inhabitants, highlighting the years 2016 with 9902 cases (636 per 100,000/population) and 2019 with 13,954 cases (897 per 100,000/population), which was 300% more than that in the previous year (2018) with 3802 cases. Dengue was the most prevalent, representing 84.8% of the total cases. All four serotypes of the disease were identified in the period, with the most prevalent being DEN 2 (84%) and DEN 1 (13%), in relation to the total number of tests performed (SESAU/TO, 2020).
The analysis of the annual growth rate (AGR) and incidence trend of cases per health region in the state of Tocantins highlights that all regions showed a stationary trend, that is, the mean, variance, and auto correlation were constant over time. The state presented incidences above the number designated by WHO as high risk (> 300 cases per 100,000 inhabitants) in all years except 2018, highlighting the year 2019 with an incidence of approximately 900 cases (Table 1). (See Fig. 1.) (See Table 2.)
Table 1.
Incidence coefficient (per 100,000 inhabitants), annual growth rate and trend of dengue, Zika and chikungunya arboviruses by health region in the state of Tocantins (2015–2019).
| CI 95% |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | AGR% | IL | UL | P | Tendency | |
| DENGUE | ||||||||||
| Médio Norte Araguaia | 310.0755 | 374.3432 | 471.07619 | 144.7681 | 630.7518 | −4.28 | −93.47 | 84.89 | 0.85 | stationary |
| Bico do Papagaio | 95.80736 | 294.5719 | 165.39877 | 27.16925 | 150.1459 | −33.31 | −108.65 | 42.03 | 0.25 | stationary |
| Sudeste | 278.9132 | 596.367 | 312.38273 | 65.92493 | 904.6928 | −21.09 | −301.01 | 258.8 | 0.77 | stationary |
| Cerrado Tocantins Araguaia | 61.18589 | 94.56002 | 88.379624 | 32.13805 | 147.0934 | −1.24 | −27.38 | 24.9 | 0.85 | stationary |
| Ilha do Bananal | 217.6308 | 383.1605 | 87.377956 | 96.6042 | 640.4099 | 19.94 | −163.3 | 203.3 | 0.75 | stationary |
| Capim Dourado | 1183.629 | 588.2149 | 334.90386 | 335.1705 | 1441.473 | 16.92 | −518.3 | 552.1 | 0.92 | stationary |
| Cantão | 336.602 | 540.2539 | 74.544281 | 47.64686 | 725.4619 | −20.94 | −268.2 | 226.3 | 0.8 | stationary |
| Amor Perfeito | 163.3826 | 184.9275 | 57.453207 | 342.0261 | 1380.672 | 251.8 | −139.3 | 643.1 | 0.13 | stationary |
| Total | 443.2037 | 401.1785 | 241.47003 | 158.2462 | 789.4506 | 3.99 | −169.1 | 177.1 | 0.94 | stationary |
| ZIKA | ||||||||||
| Médio Norte Araguaia | 2.650218 | 108.9902 | 56.648402 | 3.312772 | 3.644049 | −14.65 | −55.82 | 26.51 | 0.34 | stationary |
| Bico do Papagaio | 5.243189 | 115.3501 | 28.599211 | 16.68287 | 53.38519 | −8.25 | −38.75 | −22.24 | 0.45 | stationary |
| Sudeste | 0 | 5.071148 | 5.0711482 | 2.028459 | 13.18499 | 1.28 | −1.4 | 3.97 | 0.17 | stationary |
| Cerrado Tocantins Araguaia | 11.12471 | 618.6574 | 72.310602 | 11.12471 | 6.180393 | −100.5 | −267.6 | 66.47 | 0.15 | stationary |
| Ilha do Bananal | 0.54272 | 46.67394 | 4.884482 | 0.54272 | 6.512643 | −6.63 | −19.32 | 6.05 | 0.19 | stationary |
| Capim Dourado | 2.133146 | 17.59845 | 0.7999296 | 0.79993 | 2.933075 | −271 | −7 | 1.56 | 0.13 | stationary |
| Cantão | 0 | 16.90695 | 1.5369955 | 2.305493 | 0.768498 | −2.32 | −6.48 | 1.83 | 0.17 | stationary |
| Amor Perfeito | 0 | 41.29449 | 31.419723 | 4.488532 | 4.488532 | −3.82 | −22.49 | 14.84 | 0.56 | stationary |
| Total | 2.924598 | 114.25 | 25.558439 | 4.895522 | 11.12619 | −16.12 | −48.46 | 16.2 | 0.21 | stationary |
| CHIKUNGUNYA | ||||||||||
| Médio Norte Araguaia | 0.331277 | 0 | 264.69049 | 26.83345 | 9.938316 | 5.36 | −107.9 | 118.6 | 0.89 | stationary |
| Bico do Papagaio | 0 | 1.429961 | 286.94541 | 29.55252 | 19.54279 | 7.37 | −113.3 | 128.06 | 0.85 | stationary |
| Sudeste | 0 | 3.042689 | 75.052993 | 14.19921 | 19.27036 | 5.02 | −23.27 | 33.33 | 0.61 | stationary |
| Cerrado Tocantins Araguaia | 0.618039 | 20.3953 | 708.89111 | 17.92314 | 12.97883 | 1.91 | −303.8 | 307.7 | 0.98 | stationary |
| Ilha do Bananal | 0 | 1.628161 | 122.11205 | 9.768964 | 11.93984 | 3.28 | −47.08 | 53.65 | 0.84 | stationary |
| Capim Dourado | 0 | 0 | 13.33216 | 4.532934 | 3.466362 | 1.24 | −3.85 | 6.34 | 0.49 | stationary |
| Cantão | 0 | 0.768498 | 51.489349 | 6.91648 | 15.36995 | 3.61 | −15.14 | 22.37 | 0.58 | stationary |
| Amor Perfeito | 0 | 0.897706 | 91.566049 | 16.15871 | 9.87477 | 3.85 | −34.04 | 41.74 | 0.76 | stationary |
| Total | 0.127156 | 2.797441 | 194.93078 | 15.7674 | 11.25334 | 3.78 | −78.59 | 86.16 | 0.89 | stationary |
CI: confidence index.
AGR: Annual growth rate.
IL: Inferior limit.
UL: Upper limit.
Fig. 1.
The state of Tocantins, Brazil and health regions.
Table 2.
Quantification and frequency of arbovirus cases in relation to the main epidemiological and demographic variables (2015–2019).
| Variable | Dengue | Zika | Chikungunya | Total | % | p-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| M | 17,308 | 1182 | 1330 | 19,820 | 44.8 | 0.68 |
| F | 19,897 | 2266 | 2207 | 24,370 | 55.1 | |
| Age groups (years) | ||||||
| < 5 | 1760 | 295 | 176 | 2231 | 5.0 | |
| 5–9 | 2293 | 227 | 191 | 2711 | 6.1 | |
| 10–19 | 7826 | 614 | 571 | 9011 | 20.4 | 0.005 |
| 20–39 | 15,255 | 1517 | 1271 | 18,043 | 40.8 | |
| 40–59 | 7693 | 654 | 915 | 9262 | 21.0 | |
| > 60 | 2380 | 137 | 413 | 2930 | 6.6 | |
| Education (years) | ||||||
| 0 | 332 | 18 | 57 | 407 | 0.9 | < 0.001 |
| < 9 | 9872 | 808 | 894 | 11,574 | 26.2 | |
| 9–12 | 11,261 | 1143 | 1156 | 13,560 | 30.7 | |
| > 12 | 4028 | 494 | 265 | 4787 | 10.8 | |
| Ignored | 11,718 | 985 | 1225 | 13,928 | 31.5 | |
| Race/color | ||||||
| White | 5647 | 652 | 496 | 6795 | 15.4 | |
| Black | 1760 | 79 | 197 | 2036 | 4.6 | |
| Pardo | 27,379 | 2474 | 2744 | 32,597 | 73.8 | < 0.0001 |
| Asian | 1046 | 68 | 78 | 1192 | 2.7 | |
| Indigenous | 127 | 17 | 22 | 166 | 0.4 | |
| Total cases | 37,211 | 3448 | 3537 | 44,196 | 100 | |
| Hospital admissions | 1979 | 119 | 177 | 2275 | 100 | – |
| Deaths | 20 | 1 | 3 | 24 | 100 | – |
The epidemiological profile showed that women had a higher incidence, mainly of Zika (66%). The highest percentage of cases occurred in Pardo individuals (73.4%) in the age group of 20–39 years (43.5%) with a maximum 9–12 years of education (29.2%).
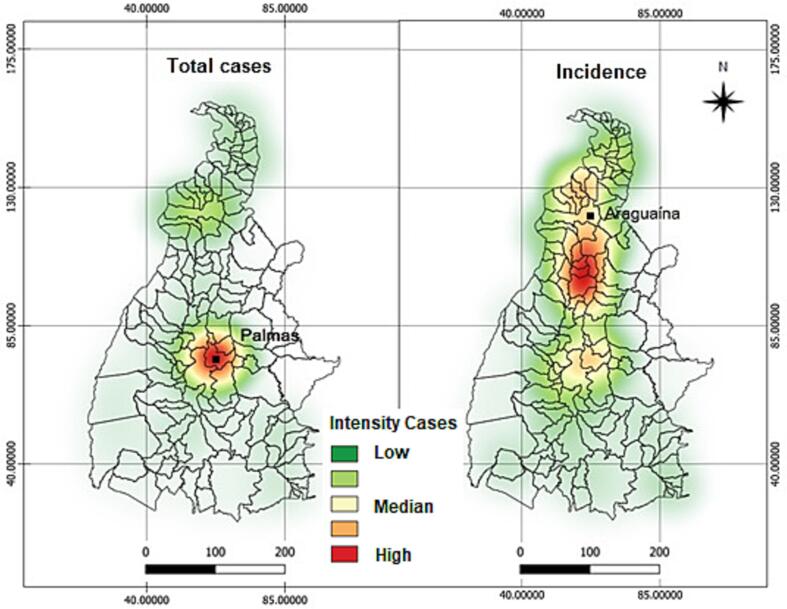
The analysis of absolute case clusters showed a strong spatial influence in the central and northern regions of the state, where the two largest cities in the state are located, the capital city of Palmas and Araguaína respectively. The analysis of incidence showed that the points of higher concentration presented more variability. According to the Kernel estimator, points of higher concentration intensity were located in the health microregions of Bico do Papagaio, in the northern region of the state, and Amor Perfeito and Capim Dourado regions in the center and southeast region, respectively. All these clusters showed a statistical significance >95% (Fig. 2).
Fig. 2.
Spatial distribution by the Kernel estimator of absolute cases and the mean incidence of arboviruses (2015–2019; TO, Brazil).
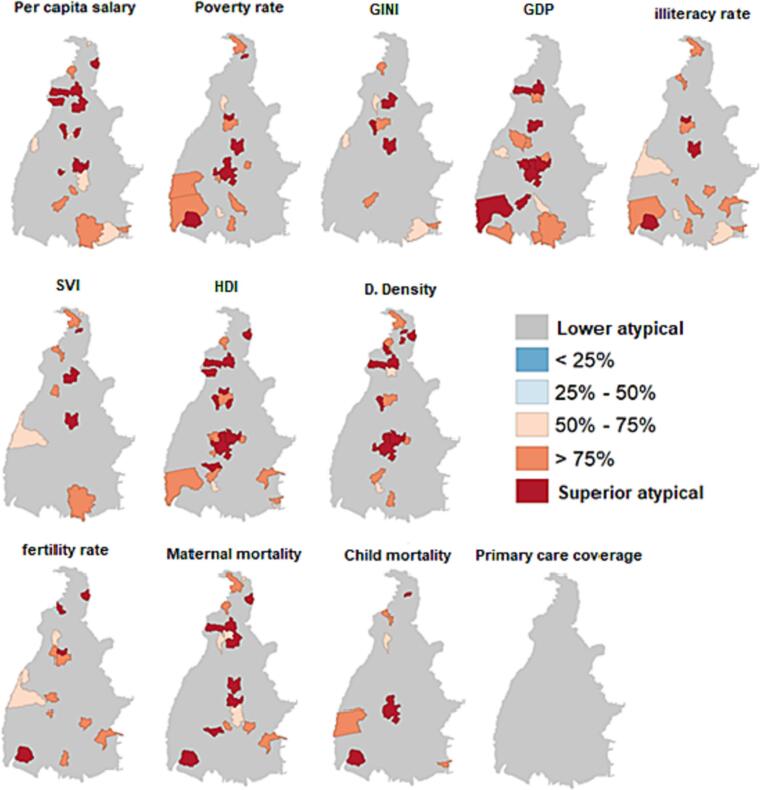
The analysis of the correlogram by the indicators that specifically represent the income distribution of the population studies, such as the case of the per capita salary, poverty rate, GINI coefficient, and GDP per capita, identified some important characteristics. Cities with high demographic density and good economic indicators, including low poverty rate, moderate per capita GDP, and low GINI index, such as Palmas, Araguaína, and Porto Nacional, presented high incidence of arboviruses. The other cities showed a strong influence of low economic indicators related to the distribution of the population's income on the incidence of arboviruses in those regions (Fig. 3).
Fig. 3.
Conditional maps of analyses of the level of correlation between socioeconomic factors and the incidence of arboviruses by city in the state of Tocantins (2015–2019).
This is demonstrated by social indicators, such as illiteracy rate, social vulnerability index, HDI, fecundity rate, and infant mortality, in which most cities presented a strong relationship between the occurrence of these diseases and a poor evaluation of these indicators, except for Palmas and Araguaína. All the cities presented an inverse correlation between the incidence of arboviruses and the coverage of primary health care, that is, the higher the level of coverage, the lower the incidence of the diseases in the city.
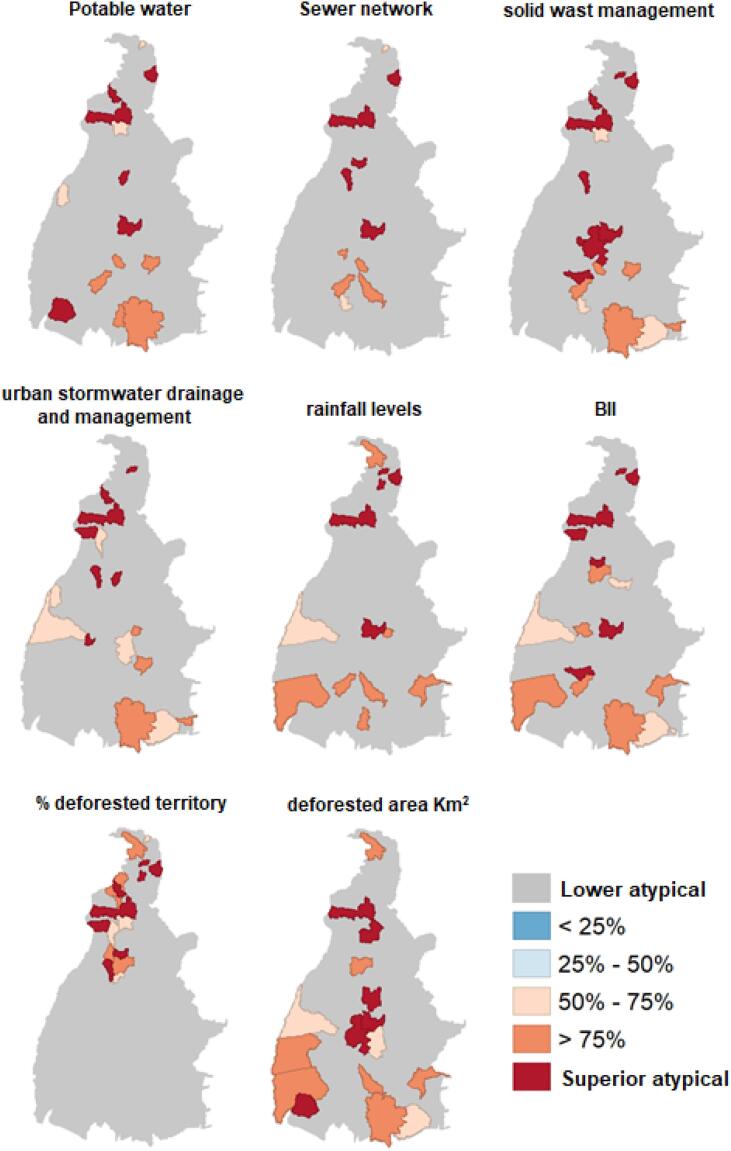
The analysis of environmental indicators in relation to the incidence of arboviruses and factors related to urban infrastructure such as basic sanitation, represented by the level of distribution of treated water, sewage collection and treatment network, solid waste management and collection, and drainage of urban rainwater, showed an inverse relationship in most cities with a high incidence of arboviruses and low sanitation levels, except in Palmas, Araguaína, and Tocantinópolis. These cities showed a strong relationship between arbovirus indices and rainfall levels in millimeters and BII (Fig. 4).
Fig. 4.
Conditional maps of analyses of the level of correlation between environmental factors and the incidence of arboviruses by city in the state of Tocantins (2015–2019).
The health regions of Bico do Papagaio and Médio Norte Araguaia, located in the northern part of the state, demonstrated a strong influence of the percentage (%) of territory deforestation in the cities on the incidence of arboviruses. The analysis by total deforested territory per square kilometers (km2) makes this relationship more heterogeneous in Tocantins, since the state has cities with considerable differences in territorial areas.
The region with the highest correlation between the deforested area in km2 and the incidence of arboviruses also demonstrated the highest cluster level by the Kernel estimator for the incidence of the diseases in the state of Tocantins (Fig. 2, Fig. 4).
The characteristics of the socioeconomic, environmental, and vector indicators of the study area compared with those of the states with the highest (Mato Grosso do Sul) and lowest (Rio Grande do Sul) incidence show that these indicators somehow influence the occurrence of arboviruses, as the states with more satisfactory indicators showed better dengue, Zika, and chikungunya incidence rates (Table 3).
Table 3.
Socioeconomic and environmental indicators for the states of Tocantins, Mato Grosso do Sul, and Rio Grande do Sul and for Brazil.
| Socioeconomic indicators | Tocantins | Brazil | Mato Grosso do Sul | Rio Grande do Sul | p-value |
|---|---|---|---|---|---|
| Salary per capita | 1.1 | 1.56 | 1.53 | 1.84 | 0.988 |
| Poverty rate1 (%) | 21.83 | 16.2 | 10.11 | 7.26 | |
| GINI index | 0.531 | 0.544 | 0.481 | 0.486 | |
| GDP per capita | 25,021 | 35,161 | 38,482 | 42,406 | |
| Illiteracy rate (%) | 9.7 | 6.6 | 5.1 | 2.4 | |
| SVI | 0.251 | 0.241 | 0.192 | 0.207 | |
| HDI | 0.69 | 0.77 | 0.76 | 0.78 | |
| Population density | 4.98 | 23.8 | 7.7 | 40.3 | |
| Fertility rate | 2.7 | 1.89 | 2.04 | 1.76 | |
| Maternal mortality rate2 | 83.8 | 59.1 | 62.9 | 39.1 | |
| Infant mortality rate3 | 15.3 | 11.5 | 13.6 | 9.3 | |
| Primary care coverage (%) | 95.4 | 74.6 | 73.04 | 73.3 | |
| Environmental indicators | |||||
| Treated water (%) | 76.6 | 97.1 | 85.8 | 86.6 | 0.440 |
| Sanitary sewerage (%) | 26.3 | 55 | 55.7 | 33.5 | |
| Solid waste management (%) | 83.2 | 90.5 | 87.9 | 92.5 | |
| Rainwater management and drainage | 10.6 | 21 | 7.4 | 21.5 | |
| Rainfall levels (mm) | 264 | 1460 | 1288 | 1229 | |
| Deforested territory (%) | 75.2 | 39 | 75.3 | 71.6 | |
| Deforested area (km2) | 30,663 | 1,160,634 | 60,446 | 56,018 | |
1 Poverty rate: income of up to ¼ of the minimum wage per capita.
2 Maternal mortality rate per 100,000 live births.
3 Number of deaths of residents under one year of age, divided by the total number of live births multiplied by one thousand.
4. Discussion
The state of Tocantins has historically presented favorable conditions for the dissemination of arboviruses, especially those transmitted by A. aegypti. Recurrent epidemics have been devastating to the state, such as those recorded in 2002, 2007, 2008 and 2012. This is a fact that corroborates the study by Böhm et al. [15] which shows that all Brazilian states showed a decreasing or stable trend between 2000 and 2010, except Tocantins and Alagoas, which showed an increasing trend for the disease. Despite having presented a stable tendency in the period studied, it is still not possible to consider a significant advance in the combat of arboviruses, since the indices are stationary at a high-risk coefficient, with a mean incidence close to 600 cases per 100,000 inhabitants [3].
In the period studied, the occurrence of these diseases presented heterogeneous characteristics for each sub-region and period, with dengue having the highest incidence and reaching the highest recorded incidence of the decade in 2019, with epidemic levels with predominance of serotypes DENV-2 and DENV-1, respectively, followed by Zika in 2016 and chikungunya in 2017.
These variations in the occurrence of epidemic outbreaks were directly related to the insertion of a new specific viral serotype in the epidemiological cycle, with no significant group immunity to that serotype, presenting an ideal scenario of vertebrate susceptibility to the virus. The detection of simultaneous infections between DENV and CHIKV in clinical samples analyzed in the state should be highlighted [[16], [17], [18], [19], [20]].
The phenomenon described as “feminization of poverty” and the prevalence of infectious diseases corroborates several studies on the profile of the occurrence of arboviruses, a fact well described in the studies by Freitas [21] and Coutinho [22]. These studies clearly present the profile of mothers of children with congenital Zika syndrome, a worrisome and aggravating incidence for the social pattern of women as the incapacitating sequels caused by this syndrome directly impact the reinsertion of these mothers in the labor market.
On the other hand, Johansen et al. [23] described that despite the fact that most reported cases are in women, they have greater health care and seek health services more often, suggesting the hypothesis of a possible greater underreporting in men, especially of acute diseases, aligned with the vector characteristic of having higher intra and peridomicile prevalence, an environment often frequented by women [24].
A well-defined characteristic regarding incidence clusters in the state occurs in cities along the highway that connects Belém to Brasília. This is another important risk factor in the region, since the main means of intercity travel in the state is by land, bringing to light the problem of traveler's disease since the state geographically acts as an epidemiological corridor between different ecosystems (Amazon and Cerrado), each with its biotic and abiotic pattern of disease distribution [25]. The same phenomenon was also reported in the studies by Almeida [26] and Sá [27], who reported that human mobility and cargo flow contributed to vector dispersion and to the occurrence of endemic and non-endemic diseases in the region.
As cities advance and deforestation increases, preexisting viral species, with an eminently wild circulation, start to circulate in the urban environment, and a new transmission chain may emerge. Other factors such as intensification of agriculture, cattle ranching, mining, and large hydroelectric developments increase deforestation, and are associated with people mobility and human density, thereby changing the ecological pattern of virus-vector-host interaction, providing a scenario completely favorable to the spread of diseases such as dengue, Zika, and chikungunya [28].
The complex phenomenon of the occurrence of infectious diseases, especially those with multicausal characteristics, comes to light when analyzing the socioeconomic characteristics of cities, with quite convergent findings in several of the studies analyzed. Souto-Marchand [29] and Cordeiro et al. [30] emphasized that regions with greater economic potential tended to have higher population density and high population migration, generating social problems such as disorderly growth with points of high social vulnerability and greater inequality. Such phenomena characterize the richest cities as those with the highest burden of infectious diseases, similarly to what occurred in Tocantins, where the cities with better economic indicators and higher population concentration showed high prevalence of the diseases analyzed [13,31,32].
Considering the continental size and the heterogeneity of Brazilian regions, and in several situations, socioenvironmental divergences in municipal boundaries, most studies demonstrated a strong correlation between socioeconomic indicators (GDP per capita, HDI, GINI, SVI, and poverty rate) and high levels of arboviruses. This situation is well demonstrated in large cities with high economic power, but with outbreaks concentrated in peripheral areas with low urban/health structure and high social vulnerability [29,33,34].
Greater effectiveness of public policies on the environment and health in integration with the community are necessary, since discoveries involving sanitary issues and the adaptability of A. aegypti regarding reproduction and of larva and pupa contamination have brought forth new hypotheses about the vector. A study by Chitolina et al. [35] suggests that A. aegypti can adapt to new places and lay eggs in polluted water such as raw sewage. Du et al. [36], on the other hand, identified that mosquito larvae and pupae could acquire ZIKV from contaminated aquatic systems, resulting in ZIKV infection in adult females.
In recent years, humans have been challenged with epidemics. In the last decade, several public health emergencies have shown that humans and health systems are poorly prepared for these events. The intriguing fact of this situation is that most of these episodes are known events, and their emergencies and re-emergences are the result of historical negligence from the co-responsible public and private agencies [37].
Despite advances in the dissemination of scientific and practical knowledge on successful One Health actions, the implemented proposals are still very limited, either by institutional and/or corporativist resistance or by the low inclusion of this interdisciplinary method in the professional training process [38,39].
In several different regions of the world, successful approaches have been adopted to fight against arboviruses with a One Health approach, such as the Early Warning and Response System for Dengue Outbreaks (EWARS) in Mexico, which processes epidemiological, meteorological, and entomological alarm indicators to predict dengue outbreaks and trigger early response activities [40]. In the Mediterranean region, the “EpiSouth” stands out as a project that creates a collaborative framework on epidemiological issues to improve communicable disease surveillance, communication, and training in the Mediterranean and Balkan countries [41,42].
In Brazil, the robust implementation of this strategy is still very discrete. In the scenario of public policies, isolated actions such as the inclusion of veterinarians as a part of primary health care (PHC) teams is already underway; however, with the immense challenges posed by socioenvironmental characteristics and the high burden of communicable zoonotic diseases, the inclusion of other areas of knowledge working in an integrated and targeted social, environmental, faunal, and epidemiological specificity regarding the region of operation, it would be possible to glimpse an effectiveness closer to the ideal of what is proposed in One Health [43].
Another bottleneck for an effective and proactive One Health action is the disarticulation between the potential tools for health, environmental, and animal surveillance, in addition to inequities in investment and supply of health services to meet needs related to the heterogeneous profile of Brazilian regions. It is possible to cite the extreme urgency in the expansion of high-tech laboratory and biomedical network in the Amazonian context, a region with a high need for constant environmental, animal, human, and vector surveillance [43,44].
Despite the challenges, successful experiences with One Health approaches have already been described in several regions, such as the elimination of canine visceral leishmaniasis in the city of Palotina, Paraná. In addition, the investment and preparation of professionals working with zoonosis, vector-borne diseases, and venomous animals to form a highly qualified and skilled team within the One Health approach has resulted in satisfactory results in Foz do Iguaçu, Paraná [45].
The advanced and efficient surveillance and control method by the stable introduction of Wolbachia bacteria (strain w Mel) in A. aegypti in Niterói, RJ, should be highlighted, which reduced dengue rates by 69%, proving to be an effective and sustainable method to control dengue and other diseases transmitted by A. aegypti, even in large and complex urban environments [46].
Tocantins presented a high prevalence for arboviruses (dengue, Zika, and chikungunya) in the period studied (2015–2019). Arboviruses showed a stationary trend with high-risk incidence levels, that is, above 300 cases per one-hundred thousand population, with emphasis on the cities of Palmas and Araguaína, the most populous and economically influential in the state.
5. Conclusions
The results showed a heterogeneous presentation of disease occurrences in the territory, and places with a high social vulnerability index and high population density should be monitored due to their association with an increased occurrence of arbovirus infection. Several cities showed a high correlation between the incidence of arboviruses and socioeconomic and environmental factors, especially Palmas and Araguaína.
Thus, an interdisciplinary approach, with a One Health focus aimed at integrating different fields of knowledge, will improve the understanding of the important social and environmental obstacles faced by control services, especially in the complex urban areas of Brazilian cities. The dialogue with other forms of knowledge will allow the definition of more viable strategies according to different political, social, environmental, and wildlife realities.
The use of secondary data is a limitation of this study, given that asymptomatic and oligosymptomatic patients choose not to visit health services, as well as individuals living in cities with lower health service provision, which can generate underreporting. Another challenge is the triple burden of the arboviruses, DENV, ZIKV, and CHIKV, which present similar signs and symptoms that can influence the differential diagnosis. This situation can lead to possible notification errors.
New studies are necessary to more accurately analyze the role of each indicator described in this study in the occurrence of arboviruses in specific regions. A better understanding of the interaction between social characteristics, the environment, and ecology of wild animals and vectors is important for the development of mechanisms to predict outbreaks and to develop strategies to reduce and/or mitigate recurring arboviral epidemics and other diseases.
Funding
This work was supported by National Institute of Science and Technology for Emerging and Reemerging Viruses - INCT‐VER/CNPq - 406360/2022‐7. National Council for Scientific and Technological Development (CNPq)/Brazil ‐ 308600/2022‐3.
Authors' contribution
Gomes H, De Jesus AG and Quaresma JAS contributed to the conception and design of the study, data collection, image processing, analysis and interpretation of results, writing and critical review of the manuscript's content. All authors have approved the final version of the manuscript and are responsible for all aspects of it, including ensuring its accuracy and integrity.
Author statement
I, Helierson Gomes, declare, for the purposes of submission to One Health journal, that the article “Identification of risk areas for arboviruses transmitted by Aedes aegypti in northern Brazil: A One Health analysis”, is original, unpublished and has not been submitted to another journal, as well as express consent regarding the Submission and Editorial Policy, Guidelines for Publication and Declaration of Copyright, which will apply in case of publication of the aforementioned work. I also declare, as the author of the manuscript, that I participated in the construction and formation of this study, and I assume public responsibility for its content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to PAPQ program (PROPESP / UFPA).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100499
Contributor Information
Helierson Gomes, Email: helierson@mail.uft.edu.br.
Juarez Antônio Simões Quaresma, Email: juarez@ufpa.br.
Appendix A. Supplementary data
Supplementary material: The state of Tocantins, Brazil and health regions
Data availability
Data will be made available on request.
References
- 1.Avelino-Silva V.L., Ramos J.F. Arboviroses e políticas públicas no Brasil/Arboviruses and public policies in Brazil. Rev. Ciências Saúde. 2017;7(3):1–2. [Google Scholar]
- 2.Sangkaew S., Ming D., Boonyasiri A., et al. Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Lancet Infect. Dis. 2021;21:1014–1026. doi: 10.1016/S1473-3099(20)30601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasil . 2018. Secretaria de vigilância em saúde. Monitoramento dos casos de Dengue, Febre de Chikungunya e Zika até a semana Epidemiológica 08/2018: Brasília – Df; p. 13. [Google Scholar]
- 4.Clancy I.L., Jones R.T., Power G.M., et al. Public health messages on arboviruses transmitted by Aedes aegypti in Brazil. BMC Public Health. 2021;21:1362. doi: 10.1186/s12889-021-11339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leite A.C.C.F. 2014. Biodiversidade e Ecologia de Mosquitos (Diptera: Culicidae), Vetores Potenciais de Doenças Humanas, em Áreas da Usina Hidrelétrica de São Salvador, Estado do Tocantins, Brasil. Dissertação (Mestrado) – Instituto Oswaldo Cruz, Pós-graduação em Biodiversidade e Saúde. [Google Scholar]
- 6.Júnior F.E.O.L., Macoris M.L.G., Santos R.F., et al. Estudo experimental sobre a ação de larvicidas em populações de Aedes aegypti do município de Itabuna, Bahia, em condições simuladas de campo. Epidemiol. Serviços Saúde [online]. 2019;28(1) doi: 10.5123/S1679-49742019000100004. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro V.S.T., Telles J.P., Tuon F.F. Arboviral diseases and Covid-19 in Brazil: Concerns regarding climatic, sanitation, and endemic scenario. J. Med. Virol. 2020;92:2390–2391. doi: 10.1002/jmv.26079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavany S.M., España G., Vazquez-Prokopec G.M., Scott T.W., et al. Pandemic-associated mobility restrictions could cause increases in dengue virus transmission. PLoS Negl. Trop. Dis. 2021;15(8) doi: 10.1371/journal.pntd.0009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicente C.R., Da Silva T.C.C., Pereira L.D.A., et al. Impact of concurrent epidemics of dengue, chikungunya, zika, and COVID-19. Rev. Soc. Bras. Med. Trop. 2021;v:54. doi: 10.1590/0037-8682-0837-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benelli G., Duggan M.F. Management of arthropod vector data – Social and ecological dynamics facing the One Health perspective. Acta Trop. 2018;182:80–91. doi: 10.1016/j.actatropica.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Fawzy M., Helmy Y.A. The One Health Approach is Necessary for the Control of Rift Valley Fever Infections in Egypt: A Comprehensive Review. Viruses. 2019;11:139. doi: 10.3390/v11020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavian C., Dulcey M., Munoz O., Salemi M., Vittor A.Y., Capua I. Islands as Hotspots for Emerging Mosquito-Borne Virus: A One-Health Perspective. Viruses. 2019;11(1) doi: 10.3390/v11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IBGE Instituto Brasileiro de Geografia e Estatística. 2022. http://www.cidades.ibge.gov.br/xtras/home.php Disponível em: acesso: 10/08/2022.
- 14.Antunes J.L.F., Cardoso M.R.A. Uso da análise de séries temporais em estudos epidemiológicos. Epidemiol. Serv. Saude. jul-set. 2015;24(3):565–576. [Google Scholar]
- 15.Böhm A.W., Costa C.S., Neves R.G., et al. Tendência da incidência de dengue no Brasil, 2002-2012. Epidemiol. Serviços Saúde [online]. 2016;25(4):725–733. doi: 10.5123/S1679-49742016000400006. [DOI] [PubMed] [Google Scholar]
- 16.Donalisio M.R., Freitas A.R.R., Von Zuben A.P.B. Arboviruses emerging in Brazil: challenges for clinic and implications for public health. Rev. Saude Publica. 2017;51:30. doi: 10.1590/S1518-8787.2017051006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza H.P., Oliveira W.T.G.H., Santos J.P.C., et al. Infectious and parasitic diseases in Brazil, 2010 to 2017: Considerations for surveillance. Rev. Panam. Salud Publica. 2020;44 doi: 10.26633/RPSP.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paploski I.A.D., Cardoso C.W., Kikuti M., Prates A.P.P.B., Tauro L.B., Silva M.M.O., et al. (2017) Unrecognized Emergence of Chikungunya Virus during a Zika Virus Outbreak in Salvador, Brazil. PLoS Negl. Trop. Dis. 2017;11(1) doi: 10.1371/journal.pntd.0005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinho R.S.S., Duro R.L.S., Santos G.L., Hunter J., et al. Detection of coinfection with Chikungunya virus and Dengue virus serotype 2 in serum samples of patients in State of Tocantins, Brazil. J. Infect. Public Health. 2020;13(5):724–729. doi: 10.1016/j.jiph.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 20.SVS Secretaria de Vigilância em Saúde. Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes (dengue, chikungunya e Zika), Semanas Epidemiológicas 01 a 52. 2020. https://antigo.saude.gov.br/images/pdf/2020/janeiro/20/Boletim-epidemiologico-Svs-02-1-.pdf Disponível em: Acesso em 20/09/2021.
- 21.PSS Freitas, Soares G.B., Mocelin H.J.S., LCX Lacerda, Prado T.N., CMM Sales, et al. Congenital Zika syndrome: sociodemographic profile of mothers Síndrome congénito por el virus del Zika: perfil sociodemográfico de las madres. Rev. Panam. Salud Publica. 2019;43 doi: 10.26633/RPSP.2019.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutinho R.Z., Montalvo A.V., Weitzman A., et al. Zika virus public health crisis and the perpetuation of gender inequality in Brazil. Reprod. Health. 2021;18:40. doi: 10.1186/s12978-021-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen I.C., Castro M.C.D., Alves L.C., Carmo R.L.D. Population mobility, demographic, and environmental characteristics of dengue fever epidemics in a major city in Southeastern Brazil, 2007-2015. Cadernos de Saúde Pública. 2021;37 doi: 10.1590/0102-311X00079620. [DOI] [PubMed] [Google Scholar]
- 24.Silva P.L.N., Marques A.C.R., Souza K.S., et al. Análise da incidência de dengue em pacientes notificados em Montes Claros entre 2017 e 2019. Res. Nurs. 2021;24:5642–5655. [Google Scholar]
- 25.Boulos M. Doenças dos Viajantes no Contexto do Mundo Globalizado. BEPA, Bol. epidemiol. paul. (Online), São Paulo. 2012;9(spe) [Google Scholar]
- 26.Almeida G.C. Instituto de biociências; 2017. Modelo matemático espaço-discreto para análise de propagação de dengue. Dissertação Mestrado Universidade Estadual Paulista Júlio de Mesquita Filho (UNESP) p. 88. [Google Scholar]
- 27.Sá E.L.R., Rodovalho C.M., Sousa N.P.R., et al. Evaluation of insecticide resistance in Aedes aegypti populations connected by roads and rivers: the case of Tocantins state in Brazil. Mem. Inst. Oswaldo Cruz [online]. 2019;114 doi: 10.1590/0074-02760180318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida L.S., Cota A.L.S., Rodrigues D.F. Sanitation, Arboviruses, and Environmental Determinants of Disease: impacts on urban health. Ciência & Saúde Coletiva [online]. 2020;25(10):3857–3868. doi: 10.1590/1413-812320202510.30712018. [DOI] [PubMed] [Google Scholar]
- 29.Souto-Marchand A.S. Tese Doutorado – Instituto Oswaldo Cruz – Pós graduação em Medicina tropical – Rio de Janeiro. 2017. Doenças infecciosas e suas correlações com indicadores socioeconômicos e demográficos: Estudo ecológico em diferentes estados brasileiros; p. 120. [Google Scholar]
- 30.Cordeiro D.C., Fonseca F.L.A., Arab C.L., et al. Factors associated with dengue cases in brazilian industrial area: an ecological study. J. Human Growth Develop. 2020;30(3):451–460. [Google Scholar]
- 31.Freitas L.P., Schmidt A.M., Cossich W., et al. Spatio-temporal modelling of the first Chikungunya epidemic in an intra-urban setting: The role of socioeconomic status, environment and temperature. PLoS Negl. Trop. Dis. 2021;15(6) doi: 10.1371/journal.pntd.0009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SINAN / SUS . 2021. Ministério da Saúde. Sistema de informação de agravos de notificação (SINAN / SUS) Acessado em 15 ago. 2021. [Google Scholar]
- 33.Almeida E.M. UFLA; Lavras: 2018. Métodos de processos pontuais para análise de interação entre árvores de espécies nativas da Amazônia / Elianara Martins de Almeida. 65 p.: il. Dissertação (mestrado acadêmico) – Universidade Federal de Lavras, 2018. [Google Scholar]
- 34.Santos S.D., Ribeiro M.C.S.A.R. Incidência de dengue e indicadores socioeconômicos e entomológicos em Santos, São Paulo, 2012 – 2016. Rev. Nurs. 2021;24:5229–5242. [Google Scholar]
- 35.Chitolina R.F., Anjos F.A., Lima T.S., et al. Raw sewage as breeding site to Aedes (Stegomyia) aegypti (Diptera, culicidae) Acta Trop. 2016;V164 doi: 10.1016/j.actatropica.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Du S., Liu Y., Liu J., et al. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat. Commun. 2019;10:1324. doi: 10.1038/s41467-019-09256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organização Mundial de Saúde (OMS) Especialistas debatem sobre o conceito de saúde única. 2020. https://www.afro.who.int/pt/news/especialistas-debatem-sobre-o-conceito-saude-unica-para-reforcar-integracao-das-vertentes Disponível. acesso 10/05/2021.
- 38.Cavalcante A.C.P., De Olinda R.A., Gomes A., et al. Spatial modelling of the infestation indices of Aedes aegypti: an innovative strategy for vector control actions in developing countries. Parasit. Vectors. 2020;13:197. doi: 10.1186/s13071-020-04070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider M., Munoz-Zanzi C., Min K., Aldighieri S. Oxford Research Encyclopedia of Global Public Health; 2019. “One Health” From Concept to Application in the Global World. [Google Scholar]
- 40.Benitez-Valladares D., Kroeger A., Tejeda G.S., et al. Validation of the Early Warning and Response System (EWARS) for dengue outbreaks: evidence from the national vector control program in Mexico. PLoS Negl. Trop. Dis. 2021;15(12) doi: 10.1371/journal.pntd.0009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dente M.G., Riccardo F., Nacca G., Ranghiasci A., Escadafal C., et al. Strengthening preparedness for arbovirus infections in mediterranean and black sea countries: a conceptual framework to assess integrated surveillance in the context of the one health strategy. Int. J. Environ. Res. Public Health. 2018;15:489. doi: 10.3390/ijerph15030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.EPISOUTH A Network for Communicable Disease Control in Southern Europe and Mediterranean Countries. 2022. http://www.episouthnetwork.org/content/episouth-project Disponível em. Acesso 28/01/2022.
- 43.Bastos V., Mota R., Guimarães M., Richard Y. Challenges of rabies surveillance in the Eastern Amazon: the need of a one health approach to predict rabies transborde. Frente. Saúde Pública. 2021;9:624574. doi: 10.3389/fpubh.2021.624574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espeschit I.D.F., Santana C.M.E., Moreira M.A.S. Public policies and one health in Brazil: the challenge of the disarticulation. Frente. Saúde Pública. 2021;9:644748. doi: 10.3389/fpubh.2021.644748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osaki S.C., Bregonde R.B., Dahm V., et al. Characterization of a municipality as free of canine visceral leishmaniasis in the context of One Health. Rev. Bras. Parasitol. Vet. 2021;30(2) doi: 10.1590/S1984-29612021038. [DOI] [PubMed] [Google Scholar]
- 46.Pinto S.B., Riback T.I.S., Sylvestre G., et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: a quasi-experimental study. PLoS Negl. Trop. Dis. 2021;15(7) doi: 10.1371/journal.pntd.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: The state of Tocantins, Brazil and health regions
Data Availability Statement
Data will be made available on request.