Abstract
Over the years, betel quid chewing and tobacco use have attracted considerable interest as they are implicated as the most likely causative risk factors of oral and esophageal cancers. Although areca nut use and betel quid chewing may lead to apoptosis, chronic exposure to areca nut and slaked lime may promote pre-malignant and malignant transformation of oral cells. The putative mutagenic and carcinogenic mechanisms may involve endogenous nitrosation of areca and tobacco alkaloids as well as the presence of direct alkylating agents in betel quid and smokeless tobacco. Metabolic activation of carcinogenic N-nitrosamines by phase-I enzymes is required not only to elicit the genotoxicity via the reactive intermediates but also to potentiate the mutagenicity with the sporadic alkylations of nucleotide bases, resulting in the formation of diverse DNA adducts. Persistent DNA adducts provides the impetus for genetic and epigenetic lesions. The genetic and epigenetic factors cumulatively influence the development and progression of disorders such as cancer. Accumulation of numerous genetic and epigenetic aberrations due to long-term betel quid (with or without tobacco) chewing and tobacco use culminates into the development of head and neck cancers. We review recent evidence that supports putative mechanisms for mutagenicity and carcinogenicity of betel quid chewing along with tobacco (smoking and smokeless) use. The detailed molecular mechanisms of the extent of accumulation and patterns of genetic alterations, indicative of the prior exposure to carcinogens and alkylating agents because of BQ chewing and tobacco use, have not yet been elucidated.
Keywords: Areca nut, Tobacco, Alkaloids, N-nitrosamines, DNA adducts, Epigenetic modifications
Graphical Abstract
Highlights
-
•
There are more than 600 million betel quid (BQ) chewers worldwide.
-
•
BQ is often used with tobacco, either chewed or smoked.
-
•
BQ use is one of the strongest risk factors for head and neck cancers.
-
•
Potent mutagenic and carcinogenic substances in BQ are summarized.
-
•
Both the genetic and epigenetic instability induced by BQ are firstly summarized.
1. Introduction
As a result of rapid globalization, changing socio-economic status and attitude, lifestyle habits such as tobacco, areca nut or betel quid (BQ) use with or without tobacco has spread far and wide across the world among the adolescent and adult populations [1], [2], [3]. It is postulated that culturally accepted BQ use is the potential initiation stimuli for the tobacco use later [4]. Tobacco use and areca nut use confers higher risk for oral, esophageal, and stomach cancers [1], [3], [5]. The frequency and duration of BQ chewing, in a dose-dependent manner, is implicated for the development of oral leukoplakia, erythroplakia, and the concomitant oral cancer [6]. In fact, oral cancer incidence associated with BQ chewing and smoking was much higher in Taiwan than in South Asia by virtue of the number of BQs consumed per day as well as region-specific variations in BQ preparations [7]. In addition, alkaline BQ chewing without tobacco is implicated in the very high oral squamous cell carcinoma (OSCC) incidence in the New Guinean population which is also indicative of the carcinogenic potential of BQ and areca nut consumption [6], [8].
Alkaloids present in the lifestyle products such as areca nut, tobacco are susceptible to endogenous nitrosation in presence of other precursors under physiological conditions such as nitrite or nitric oxide which leads to the formation of nitrosamines [9]. The ensuing enzyme-induced metabolic activation of nitrosamines potentiates the carcinogenic and genotoxic attributes of nitrosamines. The metabolites of nitrosamines covalently bind with the DNA bases and form DNA adducts including methylated DNA bases [10], [11]. In addition, few DNA adducts are known to inhibit DNA repairing [9]. The accumulation of sporadic DNA adducts are responsible for aberrant mutations (G→A transitions and G→T transversions for nitrosamines of tobacco) which play an important role in the deregulation of cellular signalling pathways along with chromosomal instability and related carcinogenesis [9], [10]. The regulation of gene expression via epigenetic modifications, including DNA methylation and microRNAs, can control all pathways in the cellular network. Aberrant DNA methylations and dysfunctional histone modifications are the manifestations of exposure to various lifestyle risk factors and they can be considered as one of the important early biomarkers to predict the susceptibility of individuals to head and neck cancer [12], [13], [14]. We review recent genetic and epigenetic instabilities that strongly support putative mechanisms for the mutagenicity and carcinogenicity of BQ chewing along with tobacco (smoking and smokeless) use.
2. Areca nut and betel quid
Areca nut (AN), the endosperm of the Areca palm (Areca catechu) fruit, is considered an important carcinogenic ingredient of different BQ preparations by virtue of its astringent polyphenols and potent alkaloids contents [15], [16], [17], [18]. Due to a multitude of ingredients employed for BQ preparations, and diverse combinations of BQ usage patterns render the assessment of relative risks of BQ chewing in different regions rather difficult [5], [7], [18], [19]. Although alkaloids constitute only as minor components in AN, they are important psychoactive and pro-carcinogenic chemical species. The major alkaloid arecoline can stimulate collagen synthesis with the activation of pro-collagen genes [20], [21]. BQ Chewing releases tannins and catechins into the oral cavity [15]. Tannins precipitate mucins in saliva that may lead to mucins depleted oral mucosa [16]. In addition, micro-nutrient elements including Cu2+ are released into the saliva during mastication and Cu2+ species can potentiate the cytotoxicity of AN extract by virtue of its’ synergetic interaction with organic components of areca nut [20]. The ensuing exposure to Cu2+ species and alkaloids in AN extract entails epithelial atrophy and chronic inflammation followed by progressive kertinization [22]. Concomitantly, chronic inflammation, a characteristic feature of OSF, activates the immune system to release cytokines. Many heavy elements are indeed co-carcinogens and they act synergistically in association with other carcinogenic species, thereby cause DNA damage (Fig. 1). Interestingly, under alkaline conditions (vide infra), Cu2+ species forms coordination complexes with the areca nut alkaloids and induce reactive oxygen species (ROS) generation [22]. Upregulation of transglutaminase 2, a Ca2+-dependent protein, catalyses protein-protein cross linkages leading to the BQ-chewing associated oral submucous fibrosis (OSF) by virtue of the intracellular ROS induced by arecoline [23]. Precancerous conditions such as OSF and oral lichen planus also causes hyposecretion of mucins. These conditions facilitate the penetration of small molecules into exposed oral mucosal cells and hence promote the development of oral cancer [16]. Oral mucosa cells, probably exposed in oral cavity of BQ-chewers, can metabolize the areca-specific alkaloids including arecoline, a possible carcinogen to humans [24], [25], [26]. Therefore, oral pre-malignant disorders such as leukoplakia, erythroplakia, OSF are the early manifestations of oral cancer [6].
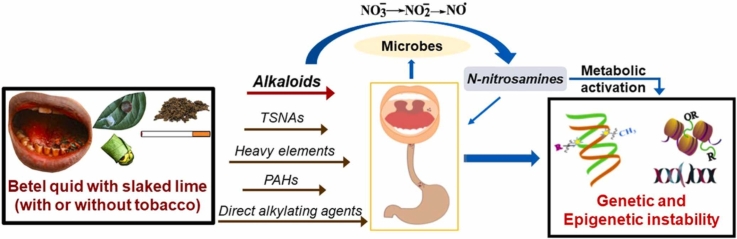
Fig. 1.
Overview of carcinogenic and genotoxic attributes of BQ chewing. Addictive lifestyle habits such as AN and BQ chewing (with or without tobacco) concurrently with smoking causes long-term oral exposure to alkaloids, nitrate, carcinogens (heavy elements, PAHs, N-nitrosamines) and alkylating agents. Oral microbial flora converts nitrate to nitrite. Nitrite in oral cavity is subsequently transformed to nitrosating species in mouth and stomach (stomach tissue in inflammation also releases inducible nitric oxide, a potential nitrosating species). The ensuing encounter between nitrosating species and alkaloids in oral cavity and stomach favorably enhances the endogenous formation of carcinogenic N-nitrosamines. In addition to the metabolic activation of N-nitrosamines, exposure to carcinogens and direct alkylating agents in situ induces sporadic genetic and epigenetic aberrations.
Furthermore, addition of slaked lime as an alkaline agent to BQ serves two purposes: Firstly, it moderates the astringency arising from tannins of areca nut, and secondly, it facilitates the availability of ‘un-ionized’ alkaloids leading to the rapid absorption of arecoline in the oral mucosa [6], [25]. However, at high concentration levels, tannins and polyphenols also elicit pro-oxidant effect under alkaline conditions (pH ≥ 9.5) due to the addition of slaked lime and in presence of redox-active transition metals such as Fe2+, Cu2+ [27]. BQ containing AN and slaked lime (Ca(OH)2) with betel inflorescence generated significant levels of ROS than BQ containing AN and slaked lime with betel piper leaves. The generation of ROS is more likely due to the presence of polyphenols which elicit superoxide radicals by autoxidation in presence of Fe2+, and Cu2+ species [6]. When calf thymus DNA was incubated, under alkaline conditions, with an aqueous extract of AN in vitro, it also instigated the 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo; so-called 8-OHdG) species formation. The BQ chewing induced oxidative stresses further corroborated by the observation of higher frequency of micronucleated oral epithelial cells of Indian BQ chewers with tobacco and slaked lime than the controls with no habits [6]. Under alkaline conditions (vide supra), the oral mucosal cells efficiently hydrolyse arecoline to yield arecaidine, while guvacoline is hydrolysed to yield guvacine. In addition, trace levels of N-methylnipecotic acid (NMPA), a nitrosylated metabolite of arecaidine is also detected in the BQ chewer’s saliva [25]. Arecoline is further metabolized to arecoline-N-oxide, and subsequently glutathionylated, as a part of detoxification pathway, to the corresponding mercapturic acid [28].
In vitro studies have shown that various AN products, viz., unripe tender areca nut (used in Taiwan as a component of BQ), ripe and mature areca nut (consumed in Northeast Indian States of India), pan masala (a mixture of pre-packed finely chopped areca nut pieces, slaked lime, and condiments without tobacco), and gutkha (a mixture of pre-packed finely chopped areca nut, slaked lime, and condiments with tobacco flakes) [19] exhibit DNA alkylation potency in a dose-dependent manner. DNA alkylation potency of the aqueous extract of different areca nut products has shown the following order: gutkha > unripe tender areca nut > ripe and mature areca nut > pan masala [29]. This data commensurate with the arecoline contents in AN products, viz., unripe areca nut>ripe mature areca nut>processed areca nut [4]. More importantly, highly polar chemical constituents of the aqueous extract of AN, which can be easily absorbed trans-orally, may be responsible for the DNA alkylation. As noted above, it is most likely that alkaloids and other polar compounds of tobacco and AN may augment the DNA alkylation potency of gutkha, in an additive manner rather than synergistic manner [28]. In addition, oral pre-malignant disorders (OPMDs), the manifestations of BQ as well as AN chewing, are also common in gutkha users [20].
Moreover, ROS inflicted DNA damage [30], probably, contributes to the development of carcinogenesis [31]. Importantly, with the downregulation of cell cycle inhibitors, P21 and P27 via rapamycin complex-1 (mTOR) pathway and the upregulation of transcriptional repressor Snail, BQ-chewing induced increase in the intracellular ROS production results in the development of OSCC. Arecoline also plays an anti-apoptotic role with the inhibition of AMPK through ROS production [32]. The bio-activation of carcinogens by the enzyme-mediated phase-I metabolic processes play a major role in chemical carcinogenesis. Arecoline-N-oxide, an oxidative metabolite of arecoline exerted DNA damage and cytotoxicity, possibly, through the Cu2+ ion-mediated Fenton type reaction induced ROS production [27], [32]. Arecoline-N-oxide exposure also stimulates an elevated collagen expression and deposition in vivo [33]. It should be noted that significant levels of arecoline and arecoline-N-oxide (ARNO) were detected in OSCC tumor tissues [31]. ARNO elicits strong cytotoxicity effects, higher intracellular ROS levels, and depleted antioxidants and antioxidant enzyme levels, whereas a metabolite of arecoline and ARNO, viz., arecoline-N-oxide mercapturic acid ameliorates oral carcinogenesis [31].
3. Nitrate, nitrite, and endogenous nitrosation of AN alkaloids
After ingestion, nitrate is actively absorbed in the upper gastroinstestinal tract. In addition, modest amounts of nitrate can be endogenously formed as an oxidized end-product of the nitric oxide at the small intestine, by gut microflora [34]. Approximately 25 % of exogenous and endogenous nitrate reaches salivary glands through systemic circulation. Besides, lifestyle habits, viz., smokeless tobacco use, AN chewing, may supplement smaller amounts of nitrate in oral cavity [35]. At the oral cavity, oral microbiome reduces 10–90 % of nitrate to nitrite depending upon the oral hygiene (Fig. 1) [34]. The elevated endogenous nitrosation process in subjects with poor oral hygiene is more likely due to the bacterial enzyme-mediated reduction of nitrate to nitrite and the concomitant formation of N-nitrosamines in oral cavity [36]. At stomach, where mild alkaline saliva meets acidic gastric juice, nitrite is further reduced to nitric oxide (NO•) (Fig. 1) [34], [37]. In presence of nitrite-rich saliva, the acidic stomach also provides an ideal platform for the endogenous nitrosation process as it is an important site for S-nitrosation, N-nitrosation and O-nitrosation reactions [37]. Therefore, IARC designated that ingested nitrate or nitrite that result in endogenous nitrosation is probably carcinogenic to humans (Group 2 A) [38]. However, Nitrite reduction to nitric oxide in the stomach is strongly pH dependent and can be suppressed by high salt intake, proton pump inhibitor drugs as well as Helicobacter pylori infection that increase the gastric pH and also attenuates the ascorbic acid levels in gastric juice [39]. Moreover, consumption of fruits and vegetables enriched with antioxidants can inhibit the endogenous nitrosation, whereas ingestion of processed red meat rich in nitrite and high animal fat entails the elevation of nitrosation [38]. Tobacco products use, BQ use with or without tobacco, and high salt intake may act as the gastric mucosal irritants or damage the gastric mucosa and may also modulate the gastric acid secretion. It should be noted that high salt intake may accentuate carcinogenesis induced by other carcinogens [39], [40].
At neutral pH, entero-salivary nitrite is rather unreactive, albeit it becomes activated when pH < 4 [41]. Due to the presence of gastric juice refluxate at lower-esophageal segment (Barrett’s segment) during the acid reflux episode, ‘activated’ nitrite can be transformed into potent nitrosative species such as NO+, N2O3, as well as NOSCN (arising from salivary SCN−). In addition, with the ingestion of high-fat meal, lipid molecules can function as a “solvent” as they can dissolve and bring NO• (generated from nitrite and inflammation induced iNOS) and O2 to close proximity, favourably enhance the formation of N2O3 [42]. These potent nitrosating species generate the endogenous N-nitrosamines when they encounter tertiary amine and secondary amine-containing alkaloids at Barrett’s segment in tobacco and BQ users (Fig. 1). Thus, gastric acid is an important etiological factor that causes the erosion and ulceration of squamous mucosa which results in reflux oesophagitis [34], [43]. With the erosion induced damage, due to gastric refluxate onslaught, luminal nitrosative chemistry occurs at Barrett’s esophageal segment, predisposing to adenocarcinoma [42], [43].
More akin to nitrate, essentially derived from the diet, thiocynate (SCN−) is released in saliva, ranging from 0.1 to 2 mM, as a host defence against pathogenic microbes. However, in smoker’s saliva, the quantity of thiocyanate is significantly higher than a non-tobacco user by virtue of the exposure to hydrogen cyanide in tobacco smoke and concomitantly the depletion of glutathione is also observed [6]. The AN alkaloid-derived N-nitrosamines due to the exposure to AN alkaloids via BQ-chewing are 3-(N-nitrosomethylamino)-propionitrile (MNPN), 3-(N-nitrosomethylamino) propionaldehyde (MNPA), N-nitrosoguvacoline (NGCO) and N-nitrosoguvacine (NGCI) [6], [17], [44], [45]. Despite the knowledge on the formation of areca nut nitrosamines (ASNAs), a comprehensive understanding of the human phase-I enzymes that metabolize the ASNAs till remains to elucidated [31], [32], [45]. The current wisdom is that areca nut N-nitrosamines are metabolically activated via α-hydroxylation pathways mainly by the ubiquitous cyt-P450 systems [28], [45], [46]. Most N-nitrosamines in biological milieu are metabolically activated to exert their genotoxicity via the formation of reactive electrophilic intermediates. These transient electrophilic alkyl-azo-hydroxide species are potent alkylating agents that can induce G-C to A-T transitions (Fig. 2) [37]. Detoxification pathways involve phase-II enzyme(s)-mediated glucuronidation and flavin-containing monoxygenases catalysed N-oxidation of nitrosamines for TSNAs and ASAs [28], [46], [47].
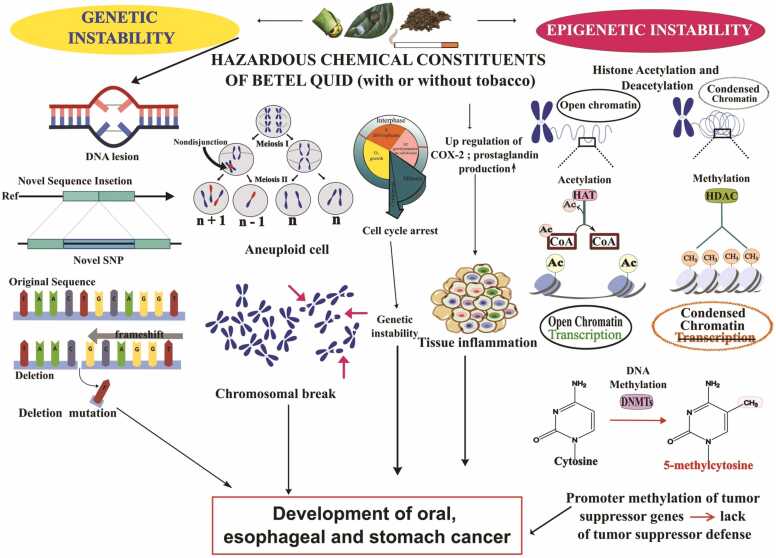
Fig. 2.
Induction of genetic and epigenetic instability due to betel quid chewing. Genetic instability includes formation of DNA adducts leading to deleterious DNA damage and promotion of chromosomal breakage. Besides, DNA mutations such as formation of novel SNP (Single Nucleotide Polymorphism), insertion-deletion mutation as well as the generation of aneuploid cells is also evident. Alterations in cell cycle pathways due to mutations and epigenetic modifications of certain cell cycle regulator genes can be observed. Epigenetic instability includes histone acetylation, methylation and promoter methylation of key regulatory genes involved in tumor suppressor defense, i.e., normal cell cycle regulation. Promotor DNA hypermethylations of tumor suppressor genes leads to the upregulation of COX-2 via NFκβ pathway. Concomitant prostaglandins production reflects the inflammation and mucosal damage, which may eventually promote the development of oral, esophageal or gastric cancer.
4. Tobacco-specific nitrosamines
Nicotine, nornicotine, anatabine, anabasine are the major alkaloids of tobacco and precursors of potent carcinogenic tobacco-specific nitrosamines (TSNAs). Tobacco carcinogenicity is relatively well established and manifestations of tobacco carcinogenicity are also extensively studied [27], [47], [48], [49]. Overwhelmingly diverse types of smokeless tobacco products (STPs) with a wide range of carcinogenic properties are consumed worldwide. These STPs contain a wide range of compounds including alkaloids, nitrate, nitrite, TSNAs, and toxic contaminants such as pesticide residues, aflatoxins, and other mycotoxins [35], [50], [51]. Few of these chemical species can be classified by the IARC, based on their carcinogenic potential as Group 1 (aflatoxins, NNN, NNK, Cd2+), Group 2 A (nitrite, Pb2+), Group 2B (acetaldehyde, crotonaldehyde), and Group 3 (N′-nitrosoanatabine, N′-nitroso-anabasine) carcinogenic species as they are important determinants of oral, esophageal and stomach cancers [51].
TSNAs are biosynthesized during the curing and fermentation as a part of processing the tobacco matter. Microbial population at the surface of tobacco leaves effected the reduction of nitrate to nitrite. Concomitantly, TSNAs, viz., N′-nitrosonornicotine [NNN], N′-nitrosoanatabine (NAT), N′-nitroso-anabasine (NAB), 4-(methylnitrosamino)− 1-(3-pyridyl)− 1-butanone (NNK), 4-(methylnitrosamino)− 4-(3-pyridyl)-butanal (NNAL), 4-(methylnitrosamino)− 4-(3-pyridyl)− 1-butanol (iso-NNAL), and 4-(methylnitrosamino)− 4-(3-pyridyl)butyric acid (iso-NNAC) are formed during the curing, aging and the processing of tobacco leaves [52]. Interestingly, nitrosamines are also formed due to the interaction of transition metal ion, Cu2+ with alkaloids in tobacco [53]. Importantly, TSNAs are volatilized and also formed de novo (in moderate levels) during the combustion of tobacco matter in smoking tobacco products [54]. Metabolism of some TSNAs potentiates the carcinogenicity of TSNAs. Among the TSNAs, NNN, NNK, NNAL are strong carcinogenic species (IARC group 1 carcinogen), while NAT and NAB are weakly carcinogenic, whereas iso-NNAL, iso-NNAC are negatively associated with carcinogenicity [49]. In animal model studies, it is established that NNN as an oral and esophageal carcinogen, and NNK as a lung carcinogen. NNAL is known to elicit lung, liver and pancreatic tumors [49]. Importantly, NNN is the preponderant N-nitrosamine species along with nitrate ion in STPs available in South Asia [35], [48], [55] albeit NNN is most likely an oral and esophageal carcinogen in humans [10]. NNN absorption due to STPs use may be correlated with high esophageal cancer incidence in India (malignancy location is preponderantly at the mid and distal esophagus, and 79 % dominated by squamous cell carcinoma) with the male to female ratio of 1.67 [56], [57].
Carcinogens can be metabolically transformed, in a diverse manner, either as metabolically activated metabolites or detoxified and eliminated [10], [52]. Metabolically activated products can exert carcinogenesis as they act, in multiple ways, through the formation of DNA and protein adducts or through the generation of ROS [32], [52]. Secondary amine or tertiary amine containing alkaloids undergo nitrosation reactions and the N-atoms of these alkaloids are attacked by the electrophilic nitroso compounds and the formation of N-N bonds on alkaloids gives rise to N-nitrosamines [58]. Chronic use of alkaloids rich lifestyle products such as tobacco or AN provides a facile environment for the endogenous formation of N-Nitrosamines in the metabolic redox milieu (Fig. 1) as well as exposure to exogenously formed N-nitrosamines [54]. Concomitantly, the metabolic activation of N-nitrosamines potentiates their respective carcinogenic activities [49]. Metabolic activation of N-nitrosamines generates electrophiles which can effectively interact with DNA and proteins of important cellular pathways to form DNA and proteins alkylation adducts to initiate the carcinogenesis process [11].
Metabolic activations of tobacco-specific N-nitrosamines such as NNN and NNK lead to the formation of unstable electrophilic diazohydroxide intermediates, concomitantly transformed to short-lived alkyl-diazonium ion intermediate species [47]. These unstable electrophilic species attack nucleophilic sites of DNA, RNA, and protein systems to form the corresponding adducts (Fig. 2). The activated form of N-nitrosamines, by SN1-nucleophilic substitution, induce various DNA adducts [59], and DNA-RNA crosslinks [60]. The formation of DNA adducts provides the impetus for somatic mutations [47], [49]. The accumulation of genetic and epigenetic abnormalities constitutes carcinogenesis.
An increase in the frequency of adenocarcinoma of distal esophagus and gastric cardia is generally observed by virtue of high dietary intake of nitrate along with processed meat products, whereas squamous cell cancer is prevalent in population with nutritional deficiencies including reduced water soluble vitamin intake [34], [38]. Chronic H. pylori colonization and the concomitant exposure to H. pylori toxins lead to atropic gastritis [39]. Moreover, chronic H. pylori infections could contribute to the development of distal gastric cancers due to attenuated gastric acid and ascorbic acid secretions. Furthermore, H. pylori infections also modulate the promotor methylation [39]. In fact, the exposure to H. pylori toxins is characterized by gastric oxidative stress, reactive aldehyde formation, cellular RNA and DNA damage, DNA promoter genes hypermethylation, persistent mucosal inflammation, and achlorhydria [40]. H. pylori infection is the one of the important causal factors of distal gastric adenocarcinoma [61]. In Northeast India (NEI), esophageal and stomach cancers depicts more pronounced male preponderance [61], [62]. Moreover, induction of neutrophil myeloperoxidase and macrophage Ca2+/calmodulin dependent nitric oxide synthase (NOS) enzymes and the associated ROS pathways involving hydrogen peroxide is also observed. Interestingly, carcinogenic nitrosamines are formed in gastric mucosa when inducible nitric oxide synthase activity is increased along with attenuated gastric mucosal ascorbic acid levels. This synergistic epithelial oxidative stress, deregulated promotor methylations and the attenuated antioxidative capacity portend the promotion of carcinogenesis [39], [40].
5. Betel quid and its associated genetic instability
Areca nut (AN) is an essential constituent of BQ and is declared as a group I carcinogen by the International Agency for Research on Cancer (IARC) [18], [48]. However, the molecular and cellular mechanisms regarding the carcinogenicity of AN are not fully understood. Genomic instability is one of the hallmarks of cancer that is associated with disease progression [63]. Arecoline is not genotoxic albeit cytotoxic in vitro and tumorigenic in vivo [64]. However, AN alkaloids may be important environmental risk factors for OSCC [65]. Abnormalities in the genetic and epigenetic factors induced by long-term BQ chewing cumulatively influence the development of head and neck cancers [44], [66]. In addition, due to the presence of hazardous substances (arecoline, aflatoxin B1) in fermented AN, changes in numerous biological pathways, including aberrant gene expressions associated with xenobiotic metabolism, chromosomal structural integrity, and DNA repair mechanisms, have been reported. Toxicity of arecoline was linked to novel point mutations of AR, BRCA1, IL8, and TP53 genes. Aflatoxin B1 has been linked to the BARD1, BRCA2, CCND2, IGF1R, MSH6, and RASSF1 genes with novel deletion as well as the APC, BRMS1, CDK2AP1, CDKN2B, GAS1, IGF1R, and RB1 genes with novel insertion [67].
In OSCC patients, BQ-derived carcinogens may encourage DNA damage that facilitates genomic instability. BQ-related OSCC is linked to somatic aberrations such INDELs (insertion/deletion mutations), SV-related breakpoints, and certain mutational signatures (signatures 1, 7, and 13). In cases of oral mucosal epithelial malignancies, BQ users have a higher number of INDELs than non-BQ users. Besides, compared to non-users, in BQ-users a greater number of chromosomal aberrations are also observed. The greater prevalence of C>T substitutions in tongue carcinomas is linked to habitual BQ chewing [68]. Aqueous extract of raw mature areca nut (RAN) stimulates stomach cancer [69]. By virtue of persistent RAN consumption with slaked lime, larger amounts of precocious anaphase and aneuploidy cells (Fig. 2) were detected in the bone marrow cells. Overexpression of p53, Bax, Securin, and p65 are observed in the stomach and esophageal cells (Table 1) [69].
Table 1.
BQ and its associated genetic instability.
| Model | Dose/Exposure | Biological Sample |
Method | Observation | Ref |
|---|---|---|---|---|---|
| N = 25 (patients of oral cancer) | Habitual chewers | Tissue samples collected from endoscopic biopsy | 1. Next Generation sequencing 2. Comparative Toxogenomic database tool (for the analysis of association between toxic compounds present in BQ and oral cancer) |
|
[67] |
| N = 196 male patients with OSCC (N = 95 habitual BQ chewers, N = 101 non-BQ users) | chewing one or more BQ-related products daily for at least 1 year |
Fresh-frozen tumors and matched whole blood | 1. Exome sequencing 2. Detection of Single Nucleotide Variation and Insertion/ Deletion 3. Analysis of mutational signatures |
|
[68] |
| 3 groups of (Swiss albino mice) mice (N = 25 in each) and another 3 groups of mice (N = 15 in each) for tumor induction studies | RAN-extract ad libitum in drinking water with or without lime (1 mg/day for first 60 days, subsequently2 mg/day till 120 days) | Bone marrow cells, esophageal cells and stomach cells | 1. Real-time RT-PCR 2. Immunoblotting |
|
[69] |
| N = 10,657 participants (all male patients age ≥ 18 years) [ranged from 18 to 96 years with an average age of 55.2 years (±18.6 years)] |
Habitual chewers | Oral inspection of participants | 1. Real-time RT-PCR 2. Bivariate analysis 3. Logistic Regression model |
|
[70] |
| N = 100 BQ-chewers N = 100 non-BQ chewers N = 100 oral cancer patients |
Habitual chewers | 1. Buccal cells 2. Peripheral blood samples |
1. Real-time RT-PCR 2. Data analysis of STR markers |
|
[71] |
| Cell line - Gingival Keratinocyte (Human GK) | Areca Nut Extract (200–800 µg/ml) (24 h exposure) | Human GK (Gingival Keratinocyte) | 1. Real-time RT-PCR 2. Immunoblotting |
|
[73] |
| Cell line-Normal Oral Human Keratinocyte (NHOK) | Ripe Areca Nut Extract (ANE) | Normal Oral Human Keratinocyte (NHOK) | 1 Real-time RT-PCR 2. EMSA 3. Western blot analysis 4. 5-bromo-20-deoxyuridine (BrdU) incorporation assay 6. Senescence-associated b-galactosidase (SA-b-Gal) assays 7. Combined SA-b-Gal and BrdUlabeling assay 8. Flow Cytometry |
|
[74] |
| N = 35 Swiss albino mice N = 32 humans |
RAN extract ad libitum in the drinking water with lime (pH 9.8) Initially 1 mg extract/day for 60 days then the dose was increased 1–2 mg/day till 120 days |
Mouse bone marrow cells and peripheral blood from human donors | 1. Immunobotting 2. Histopathological evaluation 3. Western blotting |
|
[75] |
| Cell line (HEp-2 and KB) | Arecoline, a major alkaloid of areca nut (0.3 mM arecoline) | HEp-2 (Human larynx epithelioma cancer) and KB (Human epithelial carcinoma) cell line | 1. Real-time RT-PCR 2. Western blotting 3. Flow cytometry 4. In vitro microtubule assembly assay |
|
[76] |
| Young male and female Swiss albino mice | aqueous extract of AN(AEBN) in drinking water (2 mg.ml−1) for up to 24weeks for chronic exposure and |
Whole homogenates of liver, spleen cells, enlarged lymph nodes, pus-filled sacs and solid tumors |
1. Slot blot and Western immunoprobing 2. Imaging and densitometric analysis 3. Molecular modeling of predicted protein sequences 4. DNA sequencing |
|
[77] |
| N = 116 HNSCC | Habitual chewers | Squamous cell carcinoma and peripheral blood | 1. Multiplex PCR 2. Capillary array electrophoresis |
|
[78] |
Individuals who have the habits of smoking, alcohol consumption and BQ chewing were at higher risk of developing oral cancer (odds ratio: 46.87, 95 % confidence interval: 31.84–69.00) [70]. In oral cancer patients, two forms of DNA instability (allelic alterations including the expansion and contraction) are also observed (Table 1) [71]. Arecoline potentiates collagen synthesis with the activation of TGF-β pathway that causes OPMDs [20]. Under oxygen depleted environments in vitro, HIF‐1α enhances epithelial–mesenchymal transition (EMT) and promotes fibrogenesis with the overexpression of extracellular matrix‐modifying factors and lysyl oxidase genes leading to the formation of OSF that may be attributed to BQ or areca nut chewing [72]. In addition, through its stimulatory action on prostaglandins, COX-2 production, and related tissue inflammation, ripe areca nut extract (ANE) promotes OSF and oral cancer in Human GK (Gingival Keratinocyte) (Fig. 2) [73]. ANE induces G1 cell arrest by upregulation of p16 and p21 expression in NHOK (Normal Oral Human Keratinocyte). It is also clear that late-passaged NHOK exhibits elevated IL-6 and COX-2 mRNA expressions [74].
When treated with RAN and slaked lime, mouse bone marrow cells (BMC) as well as human PBL of heavy RAN users exhibited high levels of premature anaphase and aneuploidy cells. Securin and p53 proteins were found in high concentrations, indicative of chromosome instability and related carcinogenesis. Besides, cancerous tumor also appeared in mouse stomach tissue by the stimulatory action of RAN and slaked lime [75]. Arecoline stabilizes the mitotic spindle assembly, causing erratic organization of the mitotic spindle. Additionally, it causes chromosome misalignment and the deregulation of spindle assembly checkpoint genes (SAC), which induces prometaphase arrest in HEp-2 cell line [76]. Choudhury et al., observed the repression of BRCA1, BRCA2, and p53 that signify the lack of tumour suppressor defence. Consequently, the likelihood of developing cancer was significantly increased by the presence of mutation in exon 11 of BRCA1 in Swiss albino mice [77]. Microsatellite changes were evident in head and neck squamous cell carcinoma (HNSCC) patients with BQ chewing habit (Table 1) [78].
6. Genetic instability due to smokeless tobacco products use
By virtue of the hydrophilic and direct acting DNA alkylating agents present in tobacco matter, long-term STPs use portends adverse oral health effects [29], [79]. In India, the world’s second-largest tobacco consumer, where predominantly STPs are used extensively by > 200 million people and STPs act as a putative causative factor of various cancers [80], [81]. Some states of NEI have the highest prevalence of STPs use such as Mizoram has the highest prevalence of STPs use (47.8 ± 1.2) followed by Manipur among females (46.1 ± 0.7) (by National Family Health Survey-4, NFHS-4) [82]. Most popular STPs include khaini, gutkha, zarda, paan or BQ with tobacco, loose tobacco leaf or sadapata, tuibur or hidakphu [81]. STPs can exasperate the risk of various cancers, viz., oral cancers, pancreatic cancer, oesophageal cancer, gastric cancer [83]. STPs contain diverse carcinogens, including alkaloids (nicotine, nornicotine, anabasine etc.), carbonyl compounds (acrolein, crotonaldehyde), nitrosamines (NNN, NNK, NAB, NAT), polycyclic aromatic hydrocarbons (PAHs) and heavy elements (As, Pb, Cd, Cu, Hg, U, Po etc.). Chronic STPs use is also an important etiological factor for the development of many tobacco related disorders including cancer [81], [84], [85], [86].
Nicotine acts through Cyclooxygenase 2 dependent pathway [87]. It binds β adrenergic receptors and promotes cell survival and cell growth as well [88]. Nicotine undergoes peroxidation and forms myosmine that releases activated methyl-species and could methylate DNA and protein [89]. Nornicotine binds to nicotine acetylcholine receptor (AchR) activates signaling pathways that result in increased cell proliferation and cell survival. Rapid Akt activation and suppression of apoptosis is also reported. Nicotine and nornicotine act through NF-κβ and MAPK signalling pathways in case of gastrointestinal carcinogenesis. Arecoline and guvacoline also stimulate prostaglandin production and COX2 expression.
Metabolic activation of NNK and NNN by α-hydroxylation generates DNA methylating and pyridyl-oxobutylating intermediates that can cause deleterious mutations by forming DNA adducts in oncogene and tumour suppressor genes [11]. Novel SNPs in ATM, BRCA1, CDKN1A, EGFR, IL8, and TP53 genes of NEI population is linked to the TSNAs toxicity [67]. In oral and esophageal carcinoma, FAK/Src complex phosphorylation promotes MEK1/2 and ERK1/2 (MAP kinase family) phosphorylation [90]. This signal transduction cascade leads to enhanced MMP-2/MMP-9 protease secretion and the downregulation of TIMP which degrade extracellular matrix and cause cellular membrane disruption. Thus, overexpression of MMPs is directly correlated with cancer cell invasion and metastasis [44], [90], [91].
Nicotine also diminishes epithelial β-catenin and E-cadherin levels and concomitantly increases the mesenchymal proteins fibronectin and vimentin levels through the activation of PI3/AkT pathway. Thus, nicotine elicits EMT leading to the disruption of cell–cell adhesion. This modulation of different cellular cascades by nicotine more akin to arecoline renders the cell to acquire migratory and motility properties which may promote metastasis [72], [89], [92]. With the upregulation of MMPs, COX-2, VGFR, urokinase-type plasmogen activator (uPA), nicotine also promotes pro-angiogenic activity. Besides, a mimick of nicotine, nornicotine can be nitrosylated to form NNN, a human carcinogen, in saliva [89]. As a surrogate of nicotine, NNK binds to α7-nicotinic acetylcholine receptor and elicits the influx of Ca2+ as well as the release of serotonin which leads to the activation of protein kinase cascade (PKC/Raf-1/MEK/ERK1/2). As noted above, NNK stimulates the simultaneous phosphorylation of major oncoproteins, Bcl-2 and c-myc. Consequently, the direct interaction between Bcl-2 and c-myc deregulates multiple functions of c-myc protein such as the inhibition of apoptosis while promoting the development and proliferation of neoplasms [93]. Mutant copies of onco-proteins EGFR, K-ras, c-myc, and cyclin D1, have been implicated in the development of OSCC [94]. Miscoding of DNA due to DNA adduct formation in p53 leading to GC→AT transition mutations are also observed. Overexpression of aberrant p53 mutant proteins arising from these mutations are often detected in tumor tissues [95]. Specific p53 codon polymorphisms were implicated for the susceptibility to OSCC due to tobacco, BQ, alcohol use and HPV infection [96]. In fact, chronic HPV infection is causally linked to the development and progression of oro-laryngeal malignancy [44], [94]. Besides, these abnormal p53 proteins may promote cell migration, invasion and the concomitant metastasis with the induction of oncogenes and repression of tumor suppressor genes [44], [94], [95], [97].
7. Betel quid and its associated epigenetic instability
In South and Southeast Asia, areca nut use is a significant etiological factor for oral cancer, which is also the fourth most prevalent malignancy in men in Taiwan [98]. BQ chewers who also smoke, and consume alcohol, have a 123-times more oral cancer risk than non-users [99], [100]. The gene inactivation of methyl transferase, MGMT, by promotor hypermethylation, possibly plays a key role in the tumorigenesis, especially when exposed to direct DNA alkylating agents through chronic lifestyle habits, causing chromosomal aberrations (Fig. 1) [52], [101]. Penetration of small molecules into exposed oral mucosal cells due to AN chewing alters the epigenome during the development of cancer [44], [66]. DNA methylation prevents transcription factors from binding to DNA either directly by "masking the DNA" or inadvertently by attracting methyl-CpG binding proteins (MBPs), which have repressive chromatin-remodeling properties [66]. The majority of histone modifications, including acetylation, methylation, phosphorylation, ubiquitination, and SUMOylation, takes place in their unstructured, alkaline N-terminal tails. The regulation of chromatin structure by these post-transcriptional modifications has an impact on biological processes like gene expression, DNA repair, and chromosomal condensation (Fig. 2) [66]. It has been demonstrated that heavy metal ion cadmium, present at trace levels in lifestyle products (vide supra) [102], [103], can induce global DNA hypermethylations with the upregulation of DNMT genes (Fig. 1) [12], [79]. The development of OSCC is most likely the manifestation of numerous accumulated genetic and epigenetic alterations.
Carcinogens induce epigenetic alterations in oral mucosal cells and the long-term stimulation of those cells due to lifestyle habits such as AN or BQ chewing and tobacco use can cause oncogenes and tumour suppressors including p53, BRCA2, and XRCC4 to express abnormally [104], [105], [106], [107], [108]. Oral cancer and esophageal cancer are the two cancers that are most frequently linked to consuming areca nut. The comparison of aberrant mutations (C→A) as well as the methylation changes of TFAP2E in esophageal squamous cell carcinoma (ESCC) and the nearby healthy esophageal mucosal cells of Taiwanese ESCC patients may possibly be indicative of esophageal cancer risks [109]. Animal studies have shown that 3-(methylnitrosamino)-propionitrile (MNPN), a carcinogen detected in areca nut chewers' saliva, induces DNA methylation in the liver, oesophagus, and nasal mucosa [110]. Since H3K9 methylation is essential for preserving the stability of heterochromatin structures and suppressing euchromatic gene expression, a reduced amount of H3K9 methylation after arecoline induction impairs chromosome stability in leukemia K-562 cells (Table 2) [111].
Table 2.
BQ and its associated epigenetic instability.
| Model | Dose/Exposure | Biological Sample |
Method | Observation | Ref |
|---|---|---|---|---|---|
| Cell line (Human K-562 cells) | Cells were incubated with different concentrations of ARC (Arecoline) (0, 200, 400, 800, or 1600 μg/ml) for 24 h. |
Human leukemia K-562 cells | 1.Cytotoxicity Assay 2.Real-time RT-PCR 3.Western blotting |
|
[111] |
|
N = 74 patients (39 males, 35 females) N = 24 BQ chewers with OSCC, N = 25 BQ non-chewers with OSCC, N = 25 nonchewing healthy control subjects. |
Habitual chewers | OSCC and normal oral mucosa tissue samples |
1. Quantitative methylation-specific PCR 2. Real-time quantitative reverse-transcription PCR 3.Western blotting |
|
[14] |
| N = 48 resected primary oral cancers and nearby non- cancer tissue samples | Habitual chewers | OSCC | 1. Methylation specific PCR 2. Sequencing analysis |
|
[112] |
| N = 93 patients ESCC |
Raw AN (RAN) chewing (N = 34 are only RBN-chewing habit) | ESCC | 1. PCR 2. Methylation specific-PCR |
|
[114] |
| N = 64 patients (oral pre-cancerous) | Habitual BQ chewers | Tissue samples | 1.Methylation specific-PCR 2.Immunohisto-chemical staining |
|
[115] |
| N = 6 groups of mice (Swiss albino mice) | RAN (Raw Areca Nut) For 60 days, drinking water was treated with 1 mg of RAN extract with lime daily, ad libitum, and then the dosage was raised by 1 mg every 60 days following that. | Stomach tissue | 1. Histopathological evaluation 2. Immunoblotting 3.Immunohisto-chemistry 4. ChIP-qPCR assays |
|
[116] |
Previous studies have shown that BQ chewing alters the expression of various genes involved in histone methylation (MII, Setdb1, and Suv39h2), acetylation (Atf2), and demethylation (JMJD6) [110], [111]. Retinoic acid receptor β (RARB) gene promotor hypermethylation and the concomitant repression of BARB plays an important role in the BQ chewing related OSCC progression [100]. BQ chewing also results in DNA hypermethylation of sirtuin 1 (SIRT1), which may be a putative biomarker for malignant transformation. Individuals with oral cancer and precancerous lesions have DNA hypermethylation of tumour suppressor genes, and higher levels of SIRT1 methylation were found in OSCC patients who chewed BQ more frequently than those that did not (p 0.05), which contributed to oral carcinogenesis [14]. Aberrant methylation on the promoter of the P15, P16, P53 and the VHL genes are observed in habitual chewers [112]. Importantly, epigenetic changes including differential alternative splicing of mRNA precursors and insertion/deletion polymorphisms are the major aberrant mechanisms of P53, possibly due to BQ chewing [95], [113].
In previous studies, CDKN2A had considerably higher promoter hypermethylation in the patients with ESCC who have chewed RAN (raw areca nut) only (p = 0.01) [114]. Normal epithelium did not have any hypermethylation, whereas pre-cancerous lesions exhibited p14, p15, and p16 hypermethylation. Since, the hypermethylation was clearly visible even in p53-negative lesions, it is clear that p14, p15, and p16 hypermethylation takes place whether or not the lesions carry p53 mutations [115]. Overexpression of lysine-N-methyltransferase 2A, lysine-acetyltransferase, EP-300, and PCAF enzymes were observed after RAN treatment, which resulted in an increase in the trimethylation of H3 lysine 4 and the acetylation of H3 lysine 9 and 18 both globally and in the promoter region of the securin in Swiss albino mice (Table 2) [116]. Furthermore, RAN with lime treatment induces global hyperphosphorylation of Rb (retinoblastoma) and histone H3 trimethylation modifications relax the chromatin. These epigenetic modifications deregulate Rb-E2F1 pathway and causes the up-regulation of proto-ongenes, E2F1 and securin that may mediate oral carcinogenesis [117]. Promotor DNA hypermethylation of BEX1 and LDOC1 tumor suppressor genes and the consequent hyperactivation of NF-κβ signalling pathway is linked to the oncogenic effects of combined BQ and tobacco use that leads to increasing OSCC occurrence [118].
8. Conclusion
In summary, accumulating research evidence suggests that BQ and AN chewing with or without tobacco can induce OPMDs. Persistent AN and BQ chewing with OPMD conditions portend the oral or oro-pharyngeal cancer development. In presence of nitrosating agents such as nitrosonium ion (NO+) and nitrogen sesquioxide (N2O3), under the prevailing physiological conditions at stomach, the major alkaloids in areca nut and tobacco exhibit high propensity for the carcinogenic nitrosamines formation. Ingested as well as endogenously formed N-nitrosamines are, most likely, activated by metabolic phase-I metabolic enzymes to exert their mutagenic and carcinogenic effects. In addition, tobacco matter (smokeless tobacco) chewed along with BQ and to a lesser extent tobacco smoke also contains hydrophilic direct alkylating agents that can be easily absorbed by saliva that may elicit potent adverse health effects leading to the development of oral and esophageal cancers. The deleterious interaction of metabolically activated N-nitrosamines and direct alkylating agents of areca nut and tobacco with DNA entails genetic aberrations viz., aberrant DNA mutations, deregulated hyper- or hypomethylations of DNA and dysfunctional histone modifications. The genetic and epigenetic factors cumulatively influence the development of neoplasms. Accumulation of sporadic genetic and epigenetic lesions in the cellular signalling and regulatory pathways due to long-term BQ (with or without tobacco) chewing and tobacco use culminates into the development of head and neck cancers.
Our knowledge on the carcinogenesis of BQ and AN use has considerably evolved over past two decades, yet much remains unknown. Comprehensive data on the wide range of ingredients employed for BQ preparations, and diverse combinations of BQ usage patterns is warranted in order to understand and elucidate the genetic and epigenetic abnormalities associated with oral and oro-pharyngeal neoplasia. Although the endogenous formation and the corresponding metabolic activation of TSNAs is relatively well-known phenomenon, however, the molecular mechanisms of the endogenous formation of ASNAs, metabolic activation of ASNAs, and the concomitant DNA adducts formation propensity remains to be elucidated.
Lately, non-targeted analysis of DNA adducts (DNA adductomics [119]) and nucleic acid modifications (nucleic acid adductomics [120]) are emerging as powerful and versatile tools for comprehensively detecting both expected and unexpected DNA adducts and nucleic acid modifications using high resolution mass spectrometry. Ultimately, these adductomics approaches will provide us with a more comprehensive and in-depth understanding of the carcinogenic mechanisms underlying BQ and AN use.
Funding
R.B. Muthukumaran: Project Grant, BT/PR24211/NER/95/715/2017 - DBT, New Delhi, India; Advanced State Biotech Hub Grant to Mizoram University BT/04/NE/2009 - DBT, New Delhi, India; P. Bhattacharjee: Project Grant, BT/PR24211/NER/95/715/2017 - DBT, New Delhi, India; N.S. Kumar: Advanced State Biotech Hub Grant to Mizoram University BT/04/NE/2009 - DBT, New Delhi, India; M.R. Chao: Grant number, MOST 109-2314-B-040-018-MY3, the Ministry of Science and Technology, Taiwan; C.W. Hu: Grant number, MOST 111-2628-B-040-005, the Ministry of Science and Technology, Taiwan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
R.B. Muthukumaran was the recipient of a DBT Overseas Associateship (# BT/20/NE/2011) from DBT, Government of India, New Delhi; P. Bhowmick is thankful to the DBT, New Delhi, for the financial assistance in the form of Research fellowship; L. Zote is thankful to the Ministry of Tribal Affairs, Government of India, New Delhi, for the financial assistance in the form of research fellowship; Malsawmtluagi is thankful to the University Grants Commission (UGC), New Delhi, for the financial assistance in the form of Institute research fellowship.
Handling Editor: Prof. L.H. Lash
Contributor Information
Chiung-Wen Hu, Email: windyhu@csmu.edu.tw, cwhu0823@gmail.com.
Mu-Rong Chao, Email: chaomurong@gmail.com, mrchao@csmu.edu.tw.
Data availability
No data was used for the research described in the article.
References
- 1.Secretan B., Straif K., Baan R., Grosse Y., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens--part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 2.Sinha D.N., Suliankatchi R.A., Gupta P.C., Thamarangsi T., Agarwal N., Parascandola M., Mehrotra R. Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: systematic review and meta-analysis. Tob. Control. 2018;27:35–42. doi: 10.1136/tobaccocontrol-2016-053302. [DOI] [PubMed] [Google Scholar]
- 3.Cirillo N., Duong P.H., Er W.T., Do C.T.N., De Silva M.E.H., Dong Y., Cheong S.C., Sari E.F., McCullough M.J., Zhang P., et al. Are there betel quid mixtures less harmful than others? A scoping review of the association between different betel quid ingredients and the risk of oral submucous fibrosis. Biomolecules. 2022;12:664. doi: 10.3390/biom12050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A.K., Tulsyan S., Thakur N., Sharma V., Sinha D.N., Mehrotra R. Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul. Toxicol. Pharmacol. 2020;110 doi: 10.1016/j.yrtph.2019.104548. [DOI] [PubMed] [Google Scholar]
- 5.Sharma D.C. Betel quid and areca nut are carcinogenic without tobacco. Lancet Oncol. 2003;4:587. doi: 10.1016/s1470-2045(03)01229-4. [DOI] [PubMed] [Google Scholar]
- 6.Nair U., Bartsch H., Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 7.Gupta B., Johnson N.W. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PloS One. 2014;9 doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Regional Office for the Western Pacific Review of Areca (Betel) Nut and Tobacco Use in the Pacific: a Technical Report, WHO Regional Office for the Western Pacific, 2012;
- 9.Hoffmann D., Brunnemann K.D., Prokopczyk B., Djordjevic M.V. Tobacco-specific N-nitrosamines and areca-derived n-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- 10.Ma B., Stepanov I., Hecht S.S. Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics. 2019;7 doi: 10.3390/toxics7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Hecht S.S. Metabolic activation and DNA interactions of carcinogenic N-nitrosamines to which humans are commonly exposed. Int. J. Mol. Sci. 2022;23:4559. doi: 10.3390/ijms23094559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Lei Y., Wang C. Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium. Toxicol. Sci. 2012;125:412–417. doi: 10.1093/toxsci/kfr320. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran K., Singal R. DNA methylation and field cancerization. Epigenomics. 2012;4:243–245. doi: 10.2217/epi.12.12. [DOI] [PubMed] [Google Scholar]
- 14.Islam S., Uehara O., Matsuoka H., Kuramitsu Y., Adhikari B.R., Hiraki D., Toraya S., Jayawardena A., Saito I., Muthumala M., et al. DNA hypermethylation of sirtuin 1 (SIRT1) caused by betel quid chewing—a possible predictive biomarker for malignant transformation. Clin. Epigenetics. 2020;12:12. doi: 10.1186/s13148-019-0806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke A.A., Li X., Custer L.J., Lai J.F. Chemical markers for short- and long-term areca nut exposure. Subst. Use Misuse. 2020;55:1395–1402. doi: 10.1080/10826084.2019.1630442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menicagli R., Marotta O., Nunzia M., Casotti M.T. Betel chewing: a new analysis, in vitro and in vivo, of the risk factors in oral cancer. Gulf J. Oncol. 2019;1:13–21. [PubMed] [Google Scholar]
- 17.Myers A.L. Metabolism of the areca alkaloids – toxic and psychoactive constituents of the areca (betel) nut. Drug Metab. Rev. 2022;0:1–18. doi: 10.1080/03602532.2022.2075010. [DOI] [PubMed] [Google Scholar]
- 18.Jain V., Garg A., Parascandola M., Chaturvedi P., Khariwala S.S., Stepanov I. Analysis of alkaloids in areca nut-containing products by liquid chromatography-tandem mass-spectrometry. J. Agric. Food Chem. 2017;65:1977–1983. doi: 10.1021/acs.jafc.6b05140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta P.C., Ray C.S., Sinha D.N., Singh P.K. Smokeless tobacco: a major public health problem in the SEA region: a review. Indian J. Public Health. 2011;55:199–209. doi: 10.4103/0019-557X.89948. [DOI] [PubMed] [Google Scholar]
- 20.Tilakaratne W.M., Klinikowski M.F., Saku T., Peters T.J., Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral. Oncol. 2006;42:561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Muthukrishnan A., Warnakulasuriya S. Oral health consequences of smokeless tobacco use. Indian J. Med. Res. 2018;148:35–40. doi: 10.4103/ijmr.IJMR_1793_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan I., Pant I., Narra S., Radhesh R., Ranganathan K., Rao S.G., Kondaiah P. Epithelial atrophy in oral submucous fibrosis is mediated by copper (II) and arecoline of areca nut. J. Cell. Mol. Med. 2015;19:2397–2412. doi: 10.1111/jcmm.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.-S., Chen Y.-J., Tsai C.-H., Huang F.-M., Chang Y.-C. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin. Oral. Investig. 2016;20:1029–1034. doi: 10.1007/s00784-015-1579-0. [DOI] [PubMed] [Google Scholar]
- 24.Wild, C.P.; Weiderpass, E.; Stewart, B.W. , World Cancer Report: Cancer Research for Cancer Prevention, ISBN 978-92-832-0447-3.
- 25.Chang Y.-J., Muthukumaran R.B., Chen J.-L., Chang H.-Y., Hung Y.-C., Hu C.-W., Chao M.-R. Simultaneous determination of areca nut- and tobacco-specific alkaloids in saliva by LC-MS/MS: distribution and transformation of alkaloids in oral cavity. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128116. [DOI] [PubMed] [Google Scholar]
- 26.Marques M.M., Beland F.A., Lachenmeier D.W., Phillips D.H., Chung F.-L., Dorman D.C., Elmore S.E., Hammond S.K., Krstev S., Linhart I., et al. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol. 2021;22:19–20. doi: 10.1016/S1470-2045(20)30727-0. [DOI] [PubMed] [Google Scholar]
- 27.Nair J., Ohshima H., Nair U.J., Bartsch H. Endogenous formation of nitrosamines and oxidative DNA-damaging agents in tobacco users. Crit. Rev. Toxicol. 1996;26:149–161. doi: 10.3109/10408449609017928. [DOI] [PubMed] [Google Scholar]
- 28.Giri S., Idle J.R., Chen C., Zabriskie T.M., Krausz K.W., Gonzalez F.J. A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem. Res. Toxicol. 2006;19:818–827. doi: 10.1021/tx0600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C.-W., Chao M.-R. Direct-acting DNA alkylating agents present in aqueous extracts of areca nut and its products. Chem. Res. Toxicol. 2012;25:2386–2392. doi: 10.1021/tx300252r. [DOI] [PubMed] [Google Scholar]
- 30.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 31.Nithiyanantham S., Arumugam S., Hsu H.-T., Chung C.-M., Lee C.-P., Tsai M.-H., Yeh K.-T., Luo S.-Y., Ko Y.-C. Arecoline N-oxide initiates oral carcinogenesis and arecoline N-oxide mercapturic acid attenuates the cancer risk. Life Sci. 2021;271 doi: 10.1016/j.lfs.2021.119156. [DOI] [PubMed] [Google Scholar]
- 32.Wang T.-S., Lin C.-P., Chen Y.-P., Chao M.-R., Li C.-C., Liu K.-L., CYP450-Mediated Mitochondrial R.O.S. Production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018;33:1029–1038. doi: 10.1002/tox.22588. [DOI] [PubMed] [Google Scholar]
- 33.Kuo T.-M., Luo S.-Y., Chiang S.-L., Yeh K.-T., Hsu H.-T., Wu C.-T., Lu C.-Y., Tsai M.-H., Chang J.-G., Ko Y.-C. Fibrotic effects of arecoline N-oxide in oral potentially malignant disorders. J. Agric. Food Chem. 2015;63:5787–5794. doi: 10.1021/acs.jafc.5b01351. [DOI] [PubMed] [Google Scholar]
- 34.McColl K.E.L. When saliva meets acid: chemical warfare at the oesophagogastric junction. Gut. 2005;54:1–3. doi: 10.1136/gut.2004.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepanov I., Hecht S.S., Ramakrishnan S., Gupta P.C. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Int. J. Cancer. 2005;116:16–19. doi: 10.1002/ijc.20966. [DOI] [PubMed] [Google Scholar]
- 36.Knezevich A., Muzic J., Hatsukami D.K., Hecht S.S., Stepanov I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N′-nitrosonornicotine in humans. Nicotine Tob. Res. 2013;15:591–595. doi: 10.1093/ntr/nts172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi J. Effect of diet and gut environment on the gastrointestinal formation of N-nitroso compounds: a review. Nitric Oxide Biol. Chem. 2018;73:66–73. doi: 10.1016/j.niox.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Sinha R., Zhao N., Goedert J.J., Byrd D.A., Wan Y., Hua X., Hullings A.G., Knight R., Breda S., van; Mathijs K., et al. Effects of processed meat and drinking water nitrate on oral and fecal microbial populations in a controlled feeding study. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooke C.L., Torres J., Solnick J.V. Biomarkers of Helicobacter pylori-associated gastric cancer. Gut Microbes. 2013;4:532–540. doi: 10.4161/gmic.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toh J.W.T., Wilson R.B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iijima K., Henry E., Moriya A., Wirz A., Kelman A.W., McColl K.E.L. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122:1248–1257. doi: 10.1053/gast.2002.32963. [DOI] [PubMed] [Google Scholar]
- 42.Combet E., Preston T., McColl K.E.L. Development of an in vitro system combining aqueous and lipid phases as a tool to understand gastric nitrosation. Rapid Commun. Mass Spectrom. 2010;24:529–534. doi: 10.1002/rcm.4388. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H., Iijima K., Scobie G., Fyfe V., McColl K.E.L. Nitrate and nitrosative chemistry within barrett’s oesophagus during acid reflux. Gut. 2005;54:1527–1535. doi: 10.1136/gut.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farah C.S., Shearston K., Nguyen A.P., Kujan O. In: Premalignant Conditions of the Oral Cavity. Brennan P.A., Aldridge T., Dwivedi R.C., editors. Head and Neck Cancer Clinics, Springer; Singapore: 2019. Oral carcinogenesis and malignant transformation; pp. 27–66. ISBN 9789811329319. [Google Scholar]
- 45.Das A., Giri S. A review on role of arecoline and its metabolites in the molecular pathogenesis of oral lesions with an insight into current status of its metabolomics. Prague Med. Rep. 2020;121:209–235. doi: 10.14712/23362936.2020.19. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira N.G., Ramos D.L., Dinis-Oliveira R.J. Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: an updated review. Arch. Toxicol. 2021;95:375–393. doi: 10.1007/s00204-020-02926-9. [DOI] [PubMed] [Google Scholar]
- 47.Hecht S.S., Stepanov I., Carmella S.G. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 2016;49:106–114. doi: 10.1021/acs.accounts.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanfill S., Croucher R., Gupta P., Lisko J., Lawler T., Kuklenyik P., Dahiya M., Duncan B., Kimbrell J., Peuchen E., et al. Chemical characterization of smokeless tobacco products from South Asia: nicotine, unprotonated nicotine, tobacco-specific n-nitrosamines, and flavor compounds. Food Chem. Toxicol. 2018:118. doi: 10.1016/j.fct.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Hecht S.S. in: Smokeless Tobacco Products. Elsevier; 2020. Metabolism and DNA adduct formation of carcinogenic tobacco-specific nitrosamines found in smokeless tobacco products; pp. 151–166. ISBN 978-0-12-818158-4. [Google Scholar]
- 50.Pappas R.S., Stanfill S.B., Watson C.H., Ashley D.L. Analysis of toxic metals in commercial moist snuff and Alaskan Iqmik. J. Anal. Toxicol. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 51.Kaur J., Sharma A., Kumar A., Bhartiya D., Sinha D.N., Kumari S., Gupta R., Mehrotra R., Singh H. SLTChemDB: a database of chemical compounds present in smokeless tobacco products. Sci. Rep. 2019;9:7142. doi: 10.1038/s41598-019-43559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hecht S.S., Hatsukami D.K. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat. Rev. Cancer. 2022;22:143–155. doi: 10.1038/s41568-021-00423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haque S.A., Canete S.J.P. Facilitated tobacco-specific nitrosamine formation from nicotine in the presence of Cu2+ ions. Ind. Crops Prod. 2018;122:493–497. doi: 10.1016/j.indcrop.2018.06.022. [DOI] [Google Scholar]
- 54.Cogliano V., Straif K., Baan R., Grosse Y., Secretan B., El Ghissassi F. WHO international agency for research on cancer smokeless tobacco and tobacco-related nitrosamines. Lancet Oncol. 2004;5:708. doi: 10.1016/s1470-2045(04)01633-x. [DOI] [PubMed] [Google Scholar]
- 55.Stepanov I., Hatsukami D.K. in: Smokeless Tobacco Products. Elsevier; 2020. Chemical characterization of smokeless tobacco products and relevant exposures in users; pp. 121–150. ISBN 978-0-12-818158-4. [Google Scholar]
- 56.Krishnamurthy A., Behuria S.S. Demographic trends in carcinoma esophagus from india along with a brief comparative review of the global trends. South Asian J. Cancer. 2020;9:163–167. doi: 10.1055/s-0041-1723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choksi D., Kolhe K.M., Ingle M., Rathi C., Khairnar H., Chauhan S.G., Chaudhary V., Shukla A., Pandey V. Esophageal carcinoma: an epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J. Fam. Med. Prim. Care. 2020;9:1695–1699. doi: 10.4103/jfmpc.jfmpc_1111_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Rodríguez R., McManus J.A., Murphy N.S., Ott M.A., Burns M.J. Pathways for N-nitroso compound formation: secondary amines and beyond. Org. Process Res. Dev. 2020;24:1558–1585. doi: 10.1021/acs.oprd.0c00323. [DOI] [Google Scholar]
- 59.Li Y., Ma B., Cao Q., Balbo S., Zhao L., Upadhyaya P., Hecht S.S. Mass spectrometric quantitation of pyridyloxobutyl DNA phosphate adducts in rats chronically treated with N′-nitrosonornicotine. Chem. Res. Toxicol. 2019;32:773–783. doi: 10.1021/acs.chemrestox.9b00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dator R.P., Murray K.J., Luedtke M.W., Jacobs F.C., Kassie F., Nguyen H.D., Villalta P.W., Balbo S. Identification of formaldehyde-induced DNA–RNA cross-links in the A/J mouse lung tumorigenesis model. Chem. Res. Toxicol. 2022;35:2025–2036. doi: 10.1021/acs.chemrestox.2c00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghatak S., Chakraborty P., Sarathbabu S., Pautu J.L., Zohmingthanga J., Lalchhandama C., Kumar N.S. Influence of TP53 gene somatic mutations in Helicobacter pylori infected gastric tumor. Meta Gene. 2018;17:108–114. doi: 10.1016/j.mgene.2018.05.008. [DOI] [Google Scholar]
- 62.Ghatak S., Yadav R.P., Lalrohlui F., Chakraborty P., Ghosh S., Ghosh S., Das M., Pautu J.L., Zohmingthanga J., Senthil Kumar N. Xenobiotic pathway gene polymorphisms associated with gastric cancer in high risk mizo-mongoloid population, Northeast India. Helicobacter. 2016;21:523–535. doi: 10.1111/hel.12308. [DOI] [PubMed] [Google Scholar]
- 63.Yao Y., Dai W. Genomic instability and cancer. J. Carcinog. Mutagen. 2014:5. doi: 10.4172/2157-2518.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W.-C., Lee P.-L., Chou I.-C., Chang W.-J., Lin S.-C., Chang K.-W. Molecular and cellular cues of diet-associated oral carcinogenesis—with an emphasis on areca-nut-induced oral cancer development. J. Oral. Pathol. Med. 2015;44:167–177. doi: 10.1111/jop.12171. [DOI] [PubMed] [Google Scholar]
- 65.Lin K.-H., Lin C.-Y., Liu C.-C., Chou M.-Y., Lin J.-K. Arecoline N-oxide: its mutagenicity and possible role as ultimate carcinogen in areca oral carcinogenesis. J. Agric. Food Chem. 2011;59:3420–3428. doi: 10.1021/jf104831n. [DOI] [PubMed] [Google Scholar]
- 66.Wang T.-H., Hsia S.-M., Shih Y.-H., Shieh T.-M. Association of smoking, alcohol use, and betel quid chewing with epigenetic aberrations in cancers. Int. J. Mol. Sci. 2017;18:1210. doi: 10.3390/ijms18061210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yadav D.S., Chattopadhyay I., Verma A., Devi T.R., Singh L.C., Sharma J.D., Kataki A.C., Saxena S., Kapur S. A pilot study evaluating genetic alterations that drive tobacco- and betel quid-associated oral cancer in Northeast India. Tumour Biol. 2014;35:9317–9330. doi: 10.1007/s13277-014-2222-4. [DOI] [PubMed] [Google Scholar]
- 68.Su S.-C., Chang L.-C., Lin C.-W., Chen M.-K., Yu C.-P., Chung W.-H., Yang S.-F. Mutational signatures and mutagenic impacts associated with betel quid chewing in oral squamous cell carcinoma. Hum. Genet. 2019;138:1379–1389. doi: 10.1007/s00439-019-02083-9. [DOI] [PubMed] [Google Scholar]
- 69.Kurkalang S., Banerjee A., Ghoshal N., Dkhar H., Chatterjee A. Induction of chromosome instability and stomach cancer by altering the expression pattern of mitotic checkpoint genes in mice exposed to areca-nut. BMC Cancer. 2013;13:315. doi: 10.1186/1471-2407-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin W.-J., Jiang R.-S., Wu S.-H., Chen F.-J., Liu S.-A. Smoking, alcohol, and betel quid and oral cancer: a prospective cohort study. J. Oncol. 2011;2011 doi: 10.1155/2011/525976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pai C.-Y., Hsieh L.-L., Tsai C.-W., Chiou F.-S., Yang C.-H., Hsu B.-D. Allelic alterations at the STR markers in the buccal tissue cells of oral cancer patients and the oral epithelial cells of healthy betel quid-chewers: an evaluation of forensic applicability. Forensic Sci. Int. 2002;129:158–167. doi: 10.1016/s0379-0738(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 72.Ray J.G., Chatterjee R., Chaudhuri K. Oral submucous fibrosis: a global challenge. rising incidence, risk factors, management, and research priorities. Periodontol 2000. 2019;80:200–212. doi: 10.1111/prd.12277. [DOI] [PubMed] [Google Scholar]
- 73.Jeng J.H., Ho Y.S., Chan C.P., Wang Y.J., Hahn L.J., Lei D., Hsu C.C., Chang M.C. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 MRNA and protein expression of human oral keratinocytes. Carcinogenesis. 2000;21:1365–1370. doi: 10.1093/carcin/21.7.1365. [DOI] [PubMed] [Google Scholar]
- 74.Lu S.-Y., Chang K.-W., Liu C.-J., Tseng Y.-H., Lu H.-H., Lee S.-Y., Lin S.-C. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis. 2006;27:1273–1284. doi: 10.1093/carcin/bgi357. [DOI] [PubMed] [Google Scholar]
- 75.Kurkalang S., Banerjee A., Dkhar H., Nongrum H.B., Ganguly B., Islam M., Rangad G.M., Chatterjee A. Precocious anaphase and expression of securin and P53 genes as candidate biomarkers for the early detection in areca nut-induced carcinogenesis. Mutagenesis. 2015;30:381–389. doi: 10.1093/mutage/geu083. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y.-C., Tsai Y.-S., Huang J.-L., Lee K.-W., Kuo C.-C., Wang C.-S., Huang A.-M., Chang J.-Y., Jong Y.-J., Lin C.-S. Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint: implication for carcinogenesis. Oral. Oncol. 2010;46:255–262. doi: 10.1016/j.oraloncology.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Choudhury Y., Sharan R.N. Altered BRCA1 and BRCA2 responses and mutation of BRCA1 gene in mice exposed chronically and transgenerationally to aqueous extract of betel nut (AEBN) Environ. Toxicol. Pharmacol. 2011;31:57–69. doi: 10.1016/j.etap.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Lin J.-C., Wang C.-C., Jiang R.-S., Wang W.-Y., Liu S.-A. Microsatellite alteration in head and neck squamous cell carcinoma patients from a betel quid-prevalent region. Sci. Rep. 2016;6:22614. doi: 10.1038/srep22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu C.-W., Cooke M.S., Chang Y.-J., Chao M.-R. Direct-acting DNA ethylating agents associated with tobacco use primarily originate from the tobacco itself, not combustion. J. Hazard. Mater. 2018;358:397–404. doi: 10.1016/j.jhazmat.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Gupta P.C., Ray C.S. Smokeless tobacco and health in India and South Asia. Respirology. 2003;8:419–431. doi: 10.1046/j.1440-1843.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 81.Madewell Z.J., Kolaja C.A. Smokeless tobacco warnings in Indian mass media: intention and attempts to quit. Indian J. Med. Paediatr. Oncol. 2019;40:413–419. doi: 10.4103/ijmpo.ijmpo_135_19. [DOI] [Google Scholar]
- 82.Jitenkumar singh K., Haobijam N., Nair S., Sharmila Devi A., Roshan Singh S., Hijam M., Tawfeeq Alee N., Sharma S., Deepani V., Singh L., et al. Smokeless tobacco use among women in Northeastern States, India: a study of spatial clustering and its determinants using national family health survey-4 data. Clin. Epidemiol. Glob. Health. 2021;12 doi: 10.1016/j.cegh.2021.100840. [DOI] [Google Scholar]
- 83.Lyons K., Le L.C., Pham Y.T.-H., Borron C., Park J.Y., Tran C.T.D., Tran T.V., Tran H.T.-T., Vu K.T., Do C.D., et al. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur. J. Cancer Prev. 2019;28:397–412. doi: 10.1097/CEJ.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 84.Madathil S., Kumar N.S., Zodinpuii D., Muthukumaran R.B., Lalmuanpuii R., Nicolau B. Tuibur: tobacco in a bottle-commercial production of tobacco smoke-saturated aqueous concentrate. Addict. Abingdon Engl. 2018:113. doi: 10.1111/add.14117. [DOI] [PubMed] [Google Scholar]
- 85.Lalmuanpuii R. Mizoram University; 2016. Risk Factors of Tumorigenesis in Tuibur Consumers (Thesis) [Google Scholar]
- 86.Warnakulasuriya S., Straif K. Carcinogenicity of smokeless tobacco: evidence from studies in humans & experimental animals. Indian J. Med. Res. 2018;148:681–686. doi: 10.4103/ijmr.IJMR_149_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin V.Y., Cho C.-H. Nicotine and gastric cancer. Alcohol Fayettev. N. 2005;35:259–264. doi: 10.1016/j.alcohol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Singh S., Pillai S., Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J. Oncol. 2011;2011 doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanner T., Grimsrud T.K. Nicotine: carcinogenicity and effects on response to cancer treatment - a review. Front. Oncol. 2015;5:196. doi: 10.3389/fonc.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang C.-Y., Wu C.-Z., Chen P.-N., Chang Y.-C., Chuang C.-Y., Lai C.-T., Yang S.-F., Tsai L.-L. Antimetastatic potentials of salvianolic acid A on Oral squamous cell carcinoma by targeting MMP-2 and the c-Raf/MEK/ERK pathway. Environ. Toxicol. 2018;33:545–554. doi: 10.1002/tox.22542. [DOI] [PubMed] [Google Scholar]
- 91.Pramanik K.K., Mishra R. ERK-mediated upregulation of matrix metalloproteinase-2 promotes the invasiveness in human oral squamous cell carcinoma (OSCC) Exp. Cell Res. 2022;411 doi: 10.1016/j.yexcr.2021.112984. [DOI] [PubMed] [Google Scholar]
- 92.Dasgupta P., Rizwani W., Pillai S., Kinkade R., Kovacs M., Rastogi S., Banerjee S., Carless M., Kim E., Coppola D., et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin Z., Gao F., Flagg T., Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through Phosphorylation in Regulating Cell Survival and Proliferation. J. Biol. Chem. 2004;279:40209–40219. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 94.Mastronikolis N., Ragos V., Kyrodimos E., Chrysovergis A., Papanikolaou V., Mastronikolis S., Stamatelopoulos A., Tsiambas E. Mechanisms of C-Myc oncogenic activity in head and neck squamous cell carcinoma. J. BUON. 2019;24:2242–2244. [PubMed] [Google Scholar]
- 95.Ragos V., S Mastronikolis N., Tsiambas E., Baliou E., N Mastronikolis S., Tsoukalas N., E Patsouri E., P Fotiades P. P53 mutations in oral cavity carcinoma. J. BUON. 2018;23:1569–1572. [PubMed] [Google Scholar]
- 96.Ihsan R., Devi T.R., Yadav D.S., Mishra A.K., Sharma J., Zomawia E., Verma Y., Phukan R., Mahanta J., Kataki A.C., et al. Investigation on the role of P53 Codon 72 polymorphism and interactions with tobacco, betel quid, and alcohol in susceptibility to cancers in a high-risk population from North East India. DNA Cell Biol. 2011;30:163–171. doi: 10.1089/dna.2010.1119. [DOI] [PubMed] [Google Scholar]
- 97.Singh V., Singh L.C., Singh A.P., Sharma J., Borthakur B.B., Debnath A., Rai A.K., Phukan R.K., Mahanta J., Kataki A.C., et al. Status of epigenetic chromatin modification enzymes and esophageal squamous cell carcinoma Risk in Northeast Indian population. Am. J. Cancer Res. 2015;5:979–999. [PMC free article] [PubMed] [Google Scholar]
- 98.Yeh K.-T., Chang J.-G., Lin T.-H., Wang Y.-F., Chang J.-Y., Shih M.-C., Lin C.-C. Correlation between protein expression and epigenetic and mutation changes of Wnt pathway-related genes in oral cancer. Int. J. Oncol. 2003;23:1001–1007. [PubMed] [Google Scholar]
- 99.Ko Y.C., Huang Y.L., Lee C.H., Chen M.J., Lin L.M., Tsai C.C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral. Pathol. Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 100.Lai Z.-L., Tsou Y.-A., Fan S.-R., Tsai M.-H., Chen H.-L., Chang N.-W., Cheng J.-C., Chen C.-M. Methylation-associated gene silencing of RARB in areca carcinogens induced mouse oral squamous cell carcinoma. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/378358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur. J. Cancer. 2000;36:2294–2300. doi: 10.1016/s0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 102.Zote L., Lalrammawia K., Buragohain A., Lalrinhlupuii, Kakki B., Lalmuanpuii R., Pachuau Z., Vanlalhruaia J., Muthukumaran R.B., Kumar N.S., et al. Macro-, micro-, and trace element distributions in areca nut, husk, and soil of Northeast India. Environ. Monit. Assess. 2021;193:65. doi: 10.1007/s10661-021-08859-9. [DOI] [PubMed] [Google Scholar]
- 103.Lalrammawia K., Buragohain A., Kakki B., Zote L., Marak N.K., Lalrinhlupuii Malsawmtluangi, Lalmuanpuii R., Kumar N.S., Jahau L., et al. Determination of multi elements in tobacco plant of Northeast India by neutron activation analysis and atomic absorption spectrometry. Biol. Trace Elem. Res. 2022;200:4534–4549. doi: 10.1007/s12011-021-03040-2. [DOI] [PubMed] [Google Scholar]
- 104.Wong Y.K., Liu T.Y., Chang K.W., Lin S.C., Chao T.W., Li P.L., Chang C.S. P53 alterations in betel quid- and tobacco-associated oral squamous cell carcinomas from Taiwan. J. Oral. Pathol. Med. 1998;27:243–248. doi: 10.1111/j.1600-0714.1998.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 105.Xu J., Gimenez-Conti I.B., Cunningham J.E., Collet A.M., Luna M.A., Lanfranchi H.E., Spitz M.R., Conti C.J. Alterations of P53, cyclin D1, Rb, and H-Ras in human oral carcinomas related to tobacco use. Cancer. 1998;83:204–212. doi: 10.1002/(sici)1097-0142(19980715)83:2<204::aid-cncr2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 106.Chiu C.-F., Tsai M.-H., Tseng H.-C., Wang C.-L., Wang C.H., Wu C.-N., Lin C.-C., Bau D.-T. A novel single nucleotide polymorphism in XRCC4 gene is associated with oral cancer susceptibility in Taiwanese patients. Oral. Oncol. 2008;44:898–902. doi: 10.1016/j.oraloncology.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Tseng H.-C., Tsai M.-H., Chiu C.-F., Wang C.-H., Chang N.-W., Huang C.-Y., Tsai C.-W., Liang S.-Y., Wang C.-L., Bau D.-T. Association of XRCC4 codon 247 polymorphism with oral cancer susceptibility in Taiwan. Anticancer Res. 2008;28:1687–1691. [PubMed] [Google Scholar]
- 108.Yang C.-H., Lin Y.-D., Yen C.-Y., Chuang L.-Y., Chang H.-W. A systematic gene-gene and gene-environment interaction analysis of DNA repair genes XRCC1, XRCC2, XRCC3, XRCC4, and oral cancer risk. Omics J. Integr. Biol. 2015;19:238–247. doi: 10.1089/omi.2014.0121. [DOI] [PubMed] [Google Scholar]
- 109.Yamashita S., Kishino T., Takahashi T., Shimazu T., Charvat H., Kakugawa Y., Nakajima T., Lee Y.-C., Iida N., Maeda M., et al. Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. Proc. Natl. Acad. Sci. USA. 2018;115:1328–1333. doi: 10.1073/pnas.1717340115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jeng J.H., Chang M.C., Hahn L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral. Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 111.Lin P.-C., Chang W.-H., Chen Y.-H., Lee C.-C., Lin Y.-H., Chang J.-G. Cytotoxic effects produced by arecoline correlated to epigenetic regulation in human K-562 cells. J. Toxicol. Environ. Health A. 2011;74:737–745. doi: 10.1080/15287394.2011.539123. [DOI] [PubMed] [Google Scholar]
- 112.Yeh K.-T., Chang J.-G., Lin T.-H., Wang Y.-F., Tien N., Chang J.-Y., Chen J.-C., Shih M.-C. Epigenetic changes of tumor suppressor genes, P15, P16, VHL and P53 in oral cancer. Oncol. Rep. 2003;10:659–663. [PubMed] [Google Scholar]
- 113.Chen S.-H., Hsiao S.-Y., Chang K.-Y., Chang J.-Y. New insights into oral squamous cell carcinoma: from clinical aspects to molecular tumorigenesis. Int. J. Mol. Sci. 2021;22:2252. doi: 10.3390/ijms22052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rai A.K., Freddy A.J., Banerjee A., Kurkalang S., Rangad G.M., Islam M., Nongrum H.B., Dkhar H., Chatterjee A. Distinct involvement of 9p21-24 and 13q14.1-14.3 chromosomal regions in raw betel-nut induced esophageal cancers in the state of Meghalaya, India. Asian Pac. J. Cancer Prev. 2012;13:2629–2633. doi: 10.7314/apjcp.2012.13.6.2629. [DOI] [PubMed] [Google Scholar]
- 115.Takeshima M., Saitoh M., Kusano K., Nagayasu H., Kurashige Y., Malsantha M., Arakawa T., Takuma T., Chiba I., Kaku T., et al. High frequency of hypermethylation of P14, P15 and P16 in oral pre-cancerous lesions associated with betel-quid chewing in Sri Lanka. J. Oral. Pathol. Med. 2008;37:475–479. doi: 10.1111/j.1600-0714.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 116.Boruah N., Singh C.S., Swargiary P., Dkhar H., Chatterjee A. Securin overexpression correlates with the activated Rb/E2F1 pathway and histone H3 epigenetic modifications in raw areca nut-induced carcinogenesis in mice. Cancer Cell Int. 2022;22:30. doi: 10.1186/s12935-022-02442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swargiary P., Boruah N., Singh C.S., Chatterjee A. Genome-wide analysis of dnasei hypersensitivity unveils open chromatin associated with histone H3 modifications after areca nut with lime exposure. Mutagenesis. 2022;37:182–190. doi: 10.1093/mutage/geac015. [DOI] [PubMed] [Google Scholar]
- 118.Lee C.-H., Wong T.-S., Chan J.Y.-W., Lu S.-C., Lin P., Cheng A.-J., Chen Y.-J., Chang J.S.-M., Hsiao S.-H., Leu Y.-W., et al. Epigenetic regulation of the X-linked tumour suppressors BEX1 and LDOC1 in oral squamous cell carcinoma. J. Pathol. 2013;230:298–309. doi: 10.1002/path.4173. [DOI] [PubMed] [Google Scholar]
- 119.Peterson L.A., Balbo S., Fujioka N., Hatsukami D.K., Hecht S.S., Murphy S.E., Stepanov I., Tretyakova N.Y., Turesky R.J., Villalta P.W. Applying tobacco, environmental, and dietary-related biomarkers to understand cancer etiology and evaluate prevention strategies. Cancer Epidemiol. Biomark. Prev. 2020;29:1904–1919. doi: 10.1158/1055-9965.EPI-19-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cooke M.S., Chang Y.-J., Chen Y.-R., Hu C.-W., Chao M.-R. Nucleic acid adductomics - the next generation of adductomics towards assessing environmental health risks. Sci. Total Environ. 2023;856 doi: 10.1016/j.scitotenv.2022.159192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.