Abstract
Neurological disorders are the leading cause of disability and the second leading cause of death worldwide. In the past 30 years, the absolute numbers of deaths and people with disabilities owing to neurological diseases have risen substantially, particularly in low-income and middle-income countries, and further increases are expected globally as a result of population growth and ageing. This rise in absolute numbers of people affected suggests that advances in prevention and management of major neurological disorders are not sufficiently effective to counter global demographic changes. Urgent measures to reduce this burden are therefore needed. Because resources for health care and research are already overstretched, priorities need to be set to guide policy makers, governments, and funding organisations to develop and implement action plans for prevention, health care, and research to tackle the growing challenge of neurological disorders.
Introduction
The burden of deaths and disability caused by neurological disorders is increasingly being recognised as a global publich health challenge, and this burden is set to rise during the next few decades. In the past 30 years, the absolute numbers of deaths have increased by 39% and disability-adjusted life-years (DALYs; the sum of years of life lost and years lived with disability) have increased by 15%, despite declines in communicable neurological disorders. The greatest burden is in low-income and middle-income countries (LMICs).1 The large and increasing burden of neurological disorders is driven by global population growth and ageing; understanding the burden of neurological disease has, in turn, been complicated by these fast-paced changes in demographic characteristics, as well as changes in risk factors (eg, overweight, obesity) in both high-income counries (HICs) and LMICs. Policy makers and funding bodies therefore need regular and reliable up-to-date estimates of the burden of neurological disorders and of determinants of changes in the burden, across different countries and populations. Such estimates would enable cost-effective health-care planning and resource allocation, and assessments of the impact of responsive policy interventions. Furthermore, knowledge of the effects of sociodemographic changes (eg, at a national level) on neurological disorders and overall population health is needed to inform policy and set priorities for research and health-care service development.
Epidemiological studies of neurological disorders have traditionally been restricted to particular disorders (eg, stroke, dementia) or confined by geographical area (eg, district, city, region), database settings (eg, official reports, mortality or hospitalisation statistics), or demographics (eg, specific ethnicities or age groups). Differences in study designs, methodology, diagnostic criteria, and completeness of case ascertainment complicate comparisons between studies. The Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study2 aims to address these challenges in the most systematic, comprehensive, and consistent way. Metrics of the burden of neurological disorders are available at the global, regional income (eg, LMICs or HICs), and national (and in some instances subnational) levels, categorised by age, sex, and with time, across 195 countries. The study uses all available published and unpublished data, including hospital data and insurance claims, that meet the study inclusion criteria1 from 1990 to the present, and various techniques are applied to produce accurate and reliable estimates of the burden of neurological disorders.
In this Policy View, we highlight the scale of the challenge of neurological disorders, with an overview of their burden using GBD Study findings,1,3–11 and we discuss implications and priorities for policy making and funding for prevention, health care, and research. The specific aims of this Policy View are listed in panel 1. Explanations of the GBD Study concept and key GBD metrics are given in panel 2.
Panel 1: Aims of this Policy View.
To provide an understanding of the burden of neurological disorders, and evidence for risk factors, primarily focusing on Global Burden of Diseases, Injuries, and Risk Factors (GBD) 2016 Study estimates1,3–11
To discuss implications of GBD estimates of the burden of neurological disorders for prevention, disease management and services, and research, including implications for low-income and middle-income settings
To provide recommendations and set priorities for policy makers and funding organisations to develop strategies to reduce the burden of neurological disorders, with examples of successful translation of policy into practice
Panel 2: Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study concept and key metrics for neurological disorders.
Concept
Although accurate data on incidence, prevalence, mortality, disability, and risk factors for neurological disorders are essential for evidence-based health-care planning and resource allocation, data are scarce for most countries, especially up-to-date data and data collected during one or more decades covering not only a single neurological disorder (eg, stroke or Parkinson’s disease) but a whole range of disorders. The GBD Study1–11 aims to fill this gap in data and to provide the best possible estimates on incidence, prevalence, mortality, disability, and risk factors for neurological disorders for 195 countries, from 1990 to the present, using all available epidemiological data (both published and unpublished), official mortality data, and other statistics and databases. For countries with no relevant epidemiological data, estimates are made from neighbouring regions and any available local health and sociodemographic statistics. The GBD estimates are available for global, regional, and national levels, thus providing an opportunity to examine the most important contributors to health loss to facilitate decision making at different levels of policy making.
Key metrics and definitions
Mortality
Mortality is the occurrence of death caused by a neurological disorder, following the tradition of the International Classification of Diseases to assign a single underlying cause for each death.
Incidence
Incidence is the occurrence of a new neurological disorder; GBD estimates of the incidence of neurological disorders include only first-ever-in-a-lifetime events.
Prevalence
Prevalence is the proportion of people who have a neurological disorder and are alive at the time of measurement. It is a function of past incidence and the average survival time of people with the disease or of the event. In the GBD Study, prevalence is expressed per 100000 people.
Disability-adjusted life-years (DALYs)
DALYs refer to years of healthy life lost due to premature death and disability, calculated as the sum of years of life lost (YLLs) and years lived with disability (YLDs) due to a neurological disorder. One DALY equals one lost year of healthy life.
Years of life lost
YLLs are years lost due to premature mortality, calculated by subtracting the age at death from the longest possible life expectancy for a person at that age. This aspirational life expectancy is derived from the lowest observed mortality rates age-by-age in any population.
Years lived with disability
YLDs are years lived in less than ideal health, including conditions such as migraine, which may last for only a few days, or epilepsy, which can last a lifetime. It is measured by taking the prevalence of the condition multiplied by the disability weight (the severity of health loss associated with a health state that is developed through surveys of the general public) for that condition.
Risk factors and attributable burden of neurological disorders
Risk factors are potentially modifiable causes of neurological disorders. In the GBD Study, attributable burden is the share of DALYs that can be estimated to occur because of exposure to a particular risk factor or risk factor cluster in comparison with a theoretical minimum level of exposure.
GBD regions
GBD regions are groups of countries that are geographically close and epidemiologically similar. The high-income North America GBD region, for example, contains the USA and Canada, and the south Asia GBD region contains Bangladesh, Bhutan, India, Nepal, and Pakistan. The GBD regions are themselves grouped into GBD super-regions, which have similar cause-of-death patterns. The Latin America and Caribbean super-region, for example, contains the Caribbean, central Latin America, tropical Latin America, and Andean Latin America regions.
Sociodemographic index (SDI)
SDI is a summary measure that identifies where countries or geographical areas sit on the spectrum of development. Expressed on a scale of 0 to 1, SDI is a composite of income per capita, average educational attainment in the population older than 15 years, and total fertility rate (the average number of children a woman would deliver over her lifetime) of all areas in the GBD Study. For example, a low SDI will be assigned to a country with lower income per capita, fewer average years of schooling, and higher total fertility rate than other countries.
Burden of neurological disorders
The neurological disorders included in the GBD estimates, how to interpret the estimates, and data for 20161 are in the appendix (pp 6–7), and findings can also be viewed interactively through an online data visualisation tool on the GBD Compare Viz Hub website. The GBD estimates of deaths, DALYs, incidence, and prevalence per 100000 people by neurological disorder are presented as absolute numbers and age-standardised rates for 2016 (age-standardised to the world population for comparison between populations and regions and over time), with percentage changes from 1990 to 2016.2 Each GBD estimate is presented with a 95% uncertainty interval (UI), which is a range of values that is likely to include the correct estimate of health loss for a given cause. In the absence of data, neurological burden is extrapolated from countries with similar causation to provide informed estimates.12
Depending on the geographical level of the estimates, GBD findings can have global, regional, or national implications for policy development and research funding. For example, the findings could have implications for policies related to social development goals (global, national), population-wide prevention measures (national), workforce development (national), and health-care service planning (national and subnational; eg, absolute numbers could be used to estimate the number of hospital beds and community services required to care for patients with neurological disorders, overall and for each neurological disorder).
Despite decreases in age-standardised rates of incidence, prevalence, mortality, and DALYs across all neurological disorders analysed from 1990 to 2016,1 the absolute numbers of people with incident, prevalent, and fatal cases of non-communicable neurological disorders and their associated DALYs have significantly increased (appendix, pp 2–6). The bulk of the burden (78·5% deaths and 77·3% DALYs) is in LMICs. Globally, in 2016, neurological disorders were the leading cause of DALYs (276 million; 95% UI 247–308 million) and the second leading cause of deaths (9·0 million; 95% UI 8·8–9·4 million).
The decline in age-standardised DALYs and mortality rates between 1990 and 2016 is probably due to advances in prevention and management (particularly of communicable neurological disorders and stroke) during that period of time. However, the comparatively low rates of decline in LMICs compared with those in HICs1 highlights the inequality between regions in the accessibility, intensity, comprehensiveness, and quality of preventive interventions, which needs to be addressed within LMICs.
The increase in the absolute numbers of people—including incident and prevalent cases—who died or remained disabled from non-communicable neurological disorders suggests that the advances in prevention and management of these disorders have not been sufficiently effective to counterbalance ongoing population growth and ageing. Improved case finding and reporting over the past 30 years might be a contributing factor, but global demographic changes are likely to be primarily responsible for the large increase in numbers of people affected by non-communicable neurological disorders.
The principal metric of GBD studies, DALYs, helps decision makers compare the impact of different diseases and injuries, not only in terms of early deaths, but also in terms of suffering for different groups of people.13 Overall, the major contributors to DALYs were stroke (42·2%), migraine (16·3%), Alzheimer’s disease and other dementias (10·4%), and meningitis (7·9%; figure 1). For combined neurological disorders, the 2016 age-standardised DALY rates were significantly higher in men than in women (male-to-female ratio 1·12; 95% UI 1·05–1·20), whereas headaches, Alzheimer’s disease and other dementias, and multiple sclerosis were more common and caused more burden in women than in men (male-to-female ratio ranging from 0·54 [95% UI 0·53–0·56] for migraine to 0·90 [0·88–0·92] for Alzheimer’s disease and other dementias). Globally, the three leading neurological causes of deaths were stroke (67·4%), Alzheimer’s disease and other dementias (20·3%), and meningitis (3·7%; figure 1).
Figure 1: Proportional contribution of various neurological disorders to the overall burden of neurological disorders.
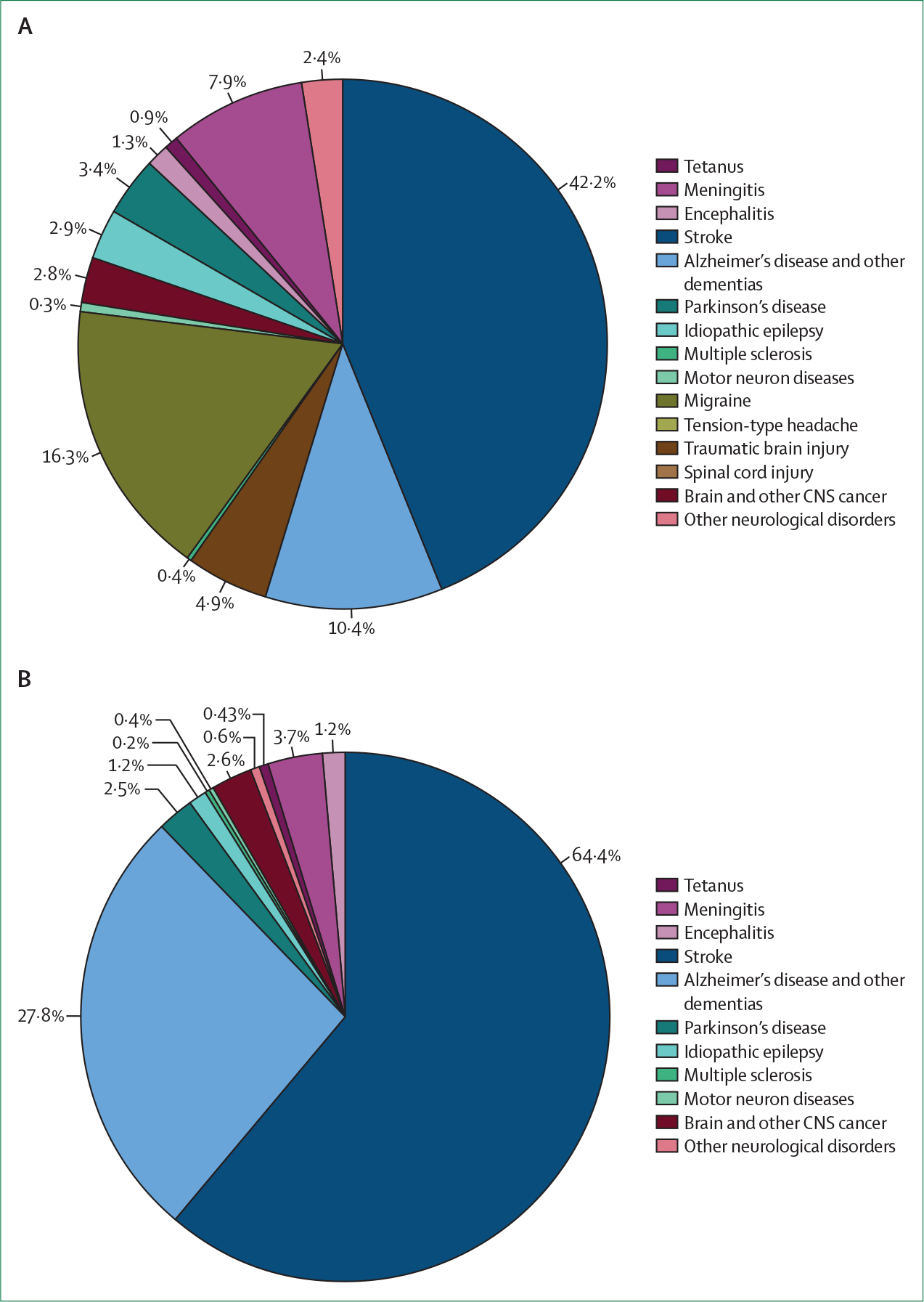
Proportions (%) of disability-adjusted life-years (A) and deaths (B). Based on data from Feigin and colleagues.1
There were also significant differences in the age patterns of neurological disorders (figure 2). For example, although for children younger than 5 years communicable neurological disorders (particularly meningitis) were the main causes of neurological DALYs, migraine and tension-type headache were large contributors in young and middle-aged adults. Stroke burden was greatest between age 60 and 84 years, and the burden due to Alzheimer’s disease and other dementias was greatest from age 85 years to older than 95 years. For stroke, epilepsy, and meningitis, age-standardised DALY rates were higher in regions with lower socioeconomic development, measured with the sociodemographic index (SDI). For brain and other CNS cancers, Parkinson’s disease, multiple sclerosis, and motor neuron diseases, DALY rates increased with increasing SDI, whereas for Alzheimer’s disease and other dementias and for migraine and tension-type headache no associations with SDI were found.
Figure 2: Global DALYs by age for various neurological disorders in 2016.
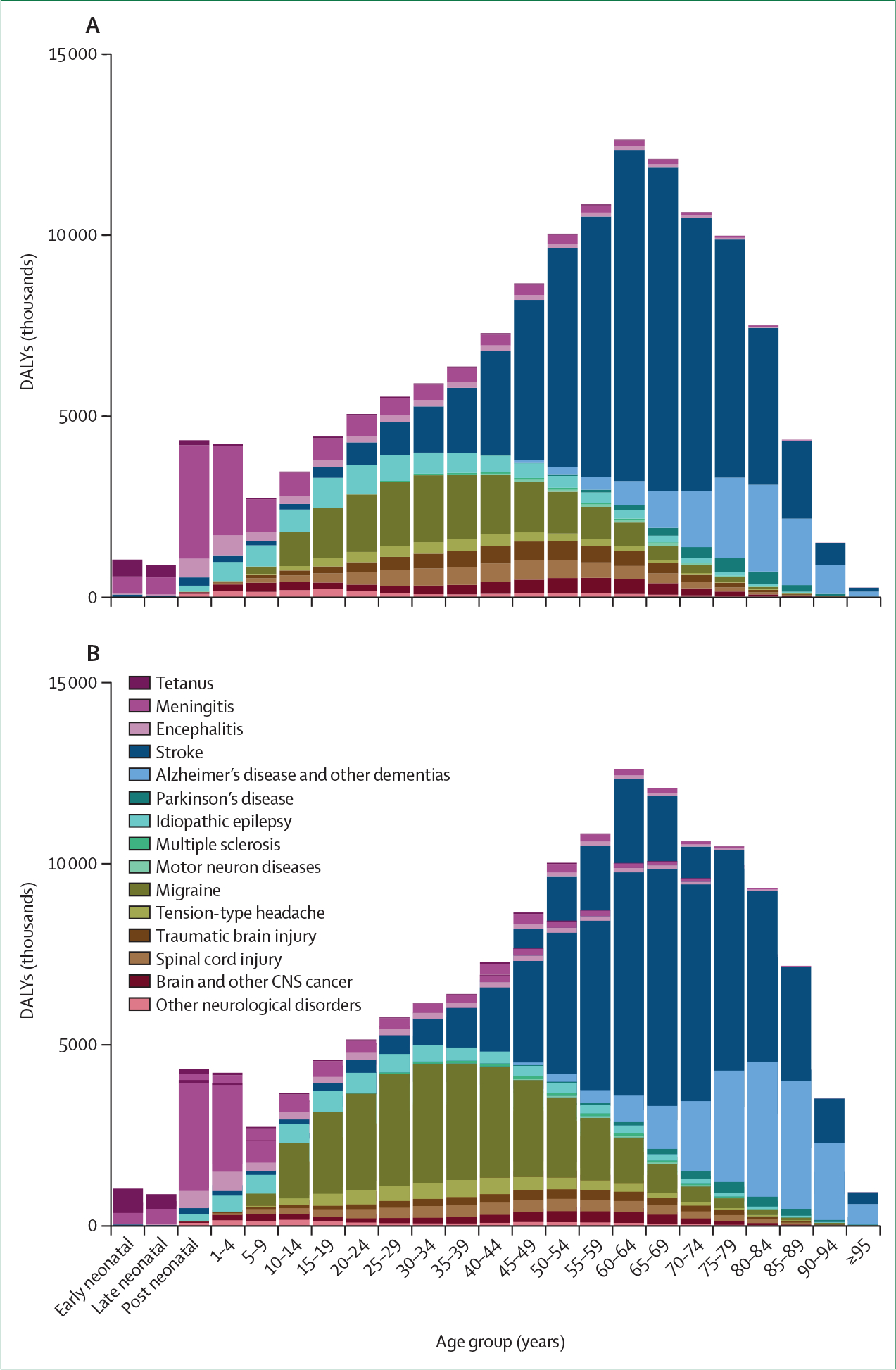
Proportions for men (A) and women (B). Early neonatal is 0–7 days; late neonatal is 7–28 days; post-neonatal is 28 days to 1 year. Reproduced from Feigin and colleagues,1 by permission of Elsevier. DALY=disability-adjusted life-year.
Understanding risk factors and improving prevention
In the GBD 2016 Study,1 risk factors were analysed in terms of DALYs due to individual neurological disorders or all neurological disorders combined, attributable to 84 individual behavioural, environmental and occupational, and metabolic risk factors, as well as their clusters (eg, the behavioural cluster included a combined effect of smoking, poor diet, physical inactivity, and high body-mass index).14 These estimates showed a proportion of DALYs that could be avoided if the population concerned had a theoretical minimum level of exposure to a risk. The criteria for including risks in the GBD Study were the availability of sufficient evidence to establish a causal relationship between a risk factor and one or more disease or injury outcomes; sufficient evidence to support the generalisability of an effect size beyond the populations included in epidemiological studies; sufficient data and methods to enable the estimation of exposure by country; and the probable importance of a risk factor to disease burden or policy considerations.
Our estimates showed that the vast majority of stroke burden can be attributed to risk factors measured in the GBD Study (88·8% of stroke DALYs; 95% UI 86·5–90·9).4 Metabolic risks, including high systolic blood pressure, high body-mass index, high fasting plasma glucose, high total cholesterol, and low glomerular filtration rate, accounted for 72·1% (95% UI 66·4–77·3) of stroke DALYs. Behavioural factors, including smoking, poor diet, and low physical activity, accounted for 66·3% (95% UI 59·3–73·1) of attributable DALYs, and environmental risks, such as air pollution and lead exposure, were responsible for a further 28·1% (25·3–30·9). For neurological disorders with established risk factors, such as stroke, efforts must be made to broaden implementation of proven, effective primary and secondary prevention strategies. The GBD data at national levels—covering 195 countries—on stroke risk factors and lifetime risk of stroke5 can be used by health-care policy makers and service providers to inform country-specific primary stroke prevention interventions and educational campaigns (including stroke awareness) and targeted research funding.
Our finding that an exceedingly high proportion of stroke-related DALYs is potentially avoidable, together with the continuously increasing stroke burden across nearly all countries, suggests that the primary stroke prevention strategies that are currently used are either not sufficiently effective or not implemented widely enough, or both.15,16 Because high systolic blood pressure is the single most important risk factor for stroke globally, a mixed service provision should be implemented worldwide at all levels (global, regional, and national). Such mixed service provision should ensure the availability and proper use of antihypertensives, combined with some population-based strategies (eg, WHO best buys)17, including interventions towards salt reduction, tobacco control, reduction in the harmful use of alcohol, healthy diet, and increased physical activity.18
There is strong evidence that tobacco,19–21 salt,22,23 and alcohol24–28 taxations are effective strategies to improve health and generate considerable revenue for governments. As advocated by WHO,29 revenue from such taxations should be used by governments to fund the development of neurological services (including workforce development), the development and implementation of preventive and disease-modifying interventions, and the improvement of access to and reduction of disparities in service provision for neurological disorders and other major health conditions. National engagement in and discussions about the use of tax revenue to support research initiatives and the development and implementation of preventive and disease-modifying interventions would improve the public acceptability of such initiatives.16 These strategies for preventing stroke and other non-communicable diseases should be complemented by measures to control other behavioural, metabolic, and environ mental risks. For example, air pollution is modifiable through regulating exposure to indoor and outdoor air pollution and through funding the transition towards clean stove technology to reduce indoor air pollution.30
With the exception of stroke, and to a lesser extent Alzheimer’s disease and other dementias, the 84 risk factors analysed in the GBD Study explained little to none of the burden of neurological disorders.3 The absence of associations between established, modifiable risk factors and most neurological disorders necessitates further research to identify new risk factors and effective preventive strategies for these disorders. In the absence of optimal strategies to reduce the risk of these neurological disorders, governments and policy makers need to take measures to improve early diagnosis and optimise treatment and rehabilitation to reduce their burden. The sex and age differences in the burden of neurological disorders should be used to define corresponding priorities in prevention. For example, the finding that the greatest burden of Alzheimer’s disease and other dementias, headaches, and multiple sclerosis is in women1 suggests that women should be given priority for early identification and prevention measures to mitigate the risk and impact of these neurological disorders and prevent further increases in their burden. The relatively stable lifetime risk of stroke in people aged 20–75 years5 and the increased stroke burden in people aged 60–84 years4 indicate that the intensity of primary stroke prevention interventions should continue throughout adult life.
Implications for disease management and neurological services
The GBD 2016 Study data3–11 suggest that advances in the management of neurological disorders are not keeping up with the increasing burden of these diseases. From a public health perspective, this is worrisome because the affected people require adequate care in hospital or community settings, or both, but the health-care resources are already overstretched, even in HICs.35 If the changes in demographic characteristics continue, steep increases in deaths and disability from neurological disorders will be inevitable unless the accessibility and affordability of health care is improved and more effective therapeutic, rehabilitative, and restorative interventions are introduced, in addition to enhanced preventive measures.
Neurological examination, diagnostic tests, and hospital care and outpatient services are essential for the adequate diagnosis and management of neurological disorders. Different neurological disorders require different diagnostic tests and health-care services. For neurological service planning, we suggest a framework for minimal, essential, and advanced levels of global neurological services (panel 3). Our framework is based on the Global Stroke Services Guidelines developed by the World Stroke Organization (WSO),36 and encompasses access to health-care personnel, laboratory screening tests and neuroimaging, medicines and other treatments, and preventive and rehabilitation services. The goal of the framework is to achieve the most advanced level of neurological service that is realistically and reasonably attainable, given local resources and practice. Our list of essential neurological medicines includes the 2015 WHO Model List of 15 neurological medicines,37 which are classified into disease-specific categories, and 11 other medicines used to treat neurological disorders, which are classified by pharmacological effect.37 As proposed by the WSO36 and Rimmer and colleagues,37 the 2015 WHO Model List should be expanded to include aspirin and recombinant tissue plasminogen activator for ischaemic stroke (recently added to the WHO Model List), labetalol, nicardipine, dopamine agonists for Parkinson’s disease, lamotrigine for epilepsy, sumatriptan for migraine, cholinesterase inhibitors for dementia, glatiramer acetate for multiple sclerosis, carbamazepine for neuropathic pain, propranolol and primidone for essential tremor, and azathioprine for myasthenia gravis.
Panel 3: Proposed framework for levels of access to global neurological services*.
Minimal neurological services
Variable access to health-care workers, including nurses or lay workers
Variable access to neurologists
Limited access to the most basic preventive and rehabilitation services
Access to care provided in local communities without coordination across defined geographical regions
Essential neurological services
Access to nurses
Access to neurologists and physicians
Access to basic laboratory screening tests, including blood, urine, and CSF tests, X-rays, electrocardiogram, telemetry, and Holter monitoring
Access to basic neuroimaging tests, including CT, EEG, electromyography, myelography, and ultrasound imaging
Access to essential treatments, including but not limited to the 2015 WHO Model List and additional neurological medicines from an expanded WHO Model List36,37
Variable access to hospital and outpatient rehabilitation, treatment, and preventive services
Advanced neurological services*†
Access to neurologists with disease-specific expertise
Access to advanced laboratory tests, including genetic testing, monitoring of therapeutic drugs, amniocentesis, chorionic villus sampling, uterine ultrasound, fluoroscopy, biopsy, and long-term heart rate monitoring (eg, reveal device)
Access to neuroimaging tests, including CT, MRI, discography, angiography, evoked potentials, and transcranial doppler ultrasound
Access to advanced treatments, including thrombectomy, novel oral anticoagulants and other proven effective medications for various neurological disorders, interventional radiology, and neurosurgery
Access to specialist rehabilitation therapy, including cognitive therapy treatment
Access to community rehabilitation programmes
Access to fully coordinated and multidisciplinary neurological care across geographically discrete regions
*These guidelines, based on guidelines for stroke developed by the World Stroke Organization, are intended as a starting point for developing a framework for levels of access to neurological services to support health service planning. The goal is to achieve the most advanced level of neurological services that is realistically and reasonably attainable, given local resources and practice, which can potentially vary within and between regions (eg, urban vs rural). Adapted from Lindsay and colleagues,36 by permission of John Wiley & Sons.
†Advanced neurological services should also include the essential services listed.
We believe that equitable—meaning reliable and affordable—access to neurological services is crucial to reducing the diagnostic and management gaps in high-burden neurological disorders globally. In parallel with the goal of the WHO Model List of Essential Medicines, there must be concerted efforts towards affordable prices at the different SDI strata if there is to be equitable access.
Policy implications for LMICs
The GBD finding that neurological disorders are the leading cause of DALYs among all disease groups globally1 is particularly relevant in LMICs, because disability is an important cause of poverty in LMICs, which in turn exacerbates the burden of neurological diseases, such as stroke, epilepsy, and meningitis. To break this cycle, more investment is needed to improve prevention and increase accessibility to services, with improved specialised management of neurological disorders in LMICs.
As shown in the WHO Atlas: Country Resources for Neurological Disorders,35 there is a disproportionate gap between the large and increasing global burden of neurological disorders and the resources available to meet this challenge, especially in LMICs. According to the Atlas, only 24% of countries globally have stand-alone neurological health policies, and this proportion is much lower for LMICs; furthermore, only 12% of countries have a separate budget for neurological disorders. Although globally 58% of countries provide financial support for people with neurological disorders, only 24% of LMICs provide such support. Access to essential medications for neurological disorders is low in primary care settings across WHO regions, particularly in Africa and southeast Asia. HICs have a median of 7·1 members of the total neurological workforce (total number of neurologists, neurosurgeons, and child neurologists) per 100 000 population, compared with 0·1 members per 100 000 population in low-income countries.35 With the large and increasing burden of neurological disorders worldwide, a substantial shortage in neurological work forces is now observed even in HICs.38,39
Given the huge burden of neurological disorders compared with other conditions,1 these disorders deserve a high priority and an increased budget to address the research and service gaps in epidemiological surveillance, prevention, acute care, and rehabilitation in LMICs. To drive the necessary change, it is crucial to revitalise and expand the health workforce. This change requires planning and funding for medical education and other strategies to increase the number and quality of personnel who can deliver the necessary services. Strategies could include promotion of self-efficacy (creating an environment that encourages best workplace performance), task-sharing (delegating roles and responsibilities, and professional collaboration), and task-shifting (moving tasks, where appropriate, to other health workers who have had shorter training and have fewer qualifications). Furthermore, the curriculum at all levels of medical educational systems needs to be enriched with content promoting basic understanding of the common neurological disorders, their predominant risk factors, and the lifestyle measures that prevent them.
Improved preventive services for infectious neurological diseases that reach entire populations should also be high on the policy agenda. Although the overall burden from tetanus, meningitis, and encephalitis has decreased in almost all countries, it remains substantial in many LMICs, particularly in sub-Saharan Africa.1,7 Insufficient vaccination coverage, especially in rural and remote areas in LMICs, is one reason that meningitis and encephalitis are ongoing problems. Insufficient access to vaccinations might be exacerbated by poverty, conflict, and also climate change.40 Many patients with encephalitis die without a diagnosis, in some cases owing to insufficient diagnostic capabilities for virus detection. Changes in disease-causing pathogens due to HIV/AIDS infection or exposure to novel viruses in immune-naive people, as a result of human encroachment on wildlife environments, might also contribute to the ongoing diagnostic challenge of infectious neurological disorders. Improved preventive strategies could be achieved and sustained through primary health-care systems that are reworked to integrate orthodox (ie, western medicine) and non-orthodox (eg, faith healing, traditional and alternative medicine) systems to screen for and control common risk factors. Geographical and virtual communities can also be sensitised and engaged through social media and mobile health technologies.
Health financing solutions in LMICs are urgently needed. Financing strategies should include universal health insurance systems and non-governmental sources of funding from implementation and development partners at national and international levels. It is also crucial to involve, engage, and empower all stakeholders, including patients, the general public, providers, and policy makers,41 and to promote collaboration between multiple sectors of society (eg, government, public health, and research and education) under a One Health initiative.42 According to the WHO definition,42 One Health is an approach to designing and implementing programmes, policies, legislation, and research, in which multiple sectors communicate and work together to achieve better public health outcomes. Such an approach is required to achieve and sustain health targets within the context of all UN Sustainable Development Goals.
From GBD findings to research priorities
Reliable morbidity and mortality data on neurological disorders and their risk factors are the backbone of evidence-based health-care planning, priority setting (including acute hospital care, community care, rehabilitation, and prevention), and resource allocation (including research funding). However, methodologically sound epidemiological studies are scarce for most neurological disorders, which limits the ability to compare and replicate findings. The GBD Study of neurological disorders goes some way towards addressing these limitations, but the accuracy of the estimates is directly related to the availability of reliable epidemiological data from the countries and regions concerned; 3–11 42% of countries did not report data on neurological disorders in the past 2 years.35 Therefore, more high-quality epidemiological studies of the burden of various neurological disorders are needed for many countries, especially LMICs, and these studies should be considered a high priority for public health research funding.
Unlike the effect of risk factors on DALYs due to stroke, the absence of association between the risk factors analysed in the GBD 2016 Study and the burden of other neurological disorders should prompt further research into risk factors for neurological disorders in different countries and populations (eg, by ethnic group, age, and sex). Much of the progress in neurological health care is due to basic research, but many areas—such as neurodegeneration or inflammatory mechanisms—are currently underfunded, given the large disease burden and the rapidly increasing incidence of neurological disorders.43
To address the burden in LMICs, a range of additional studies is needed, including social science studies about disease concepts,44 research into how best to deliver treatment and care to patients with non-communicable neurological diseases (eg, through hospital, outpatient settings, self-management), and studies of treatment acceptability, interventions, and procedures in different ethnic populations. Studies on the cost-effectiveness of interventions and the best methods for policy transfer are also essential.
Appropriate funding at national and international levels is therefore vital to study and monitor frequency, outcomes, and predictors of neurological disorders, as well as prevention, health-care and treatment strategies, including translational research.
Progress in translation to policy and practice
There are several examples of successful implementation of evidence-based strategies for the prevention and management of neurological disorders, and this progress should be built on to counter the effects of the growing burden of these diseases. Globally, the most impressive achievements have been made in communicable neurological disorders (eg, tetanus, meningitis, encephalitis), for which there have been substantial reductions not only in the rates of mortality and DALYs, but also in absolute numbers.1 These achievements are largely owing to wider community and country-wide vaccination programmes for tetanus and meningitis, although, as already noted, substantial gaps in vaccine coverage need to be addressed in many LMICs.45
For non-communicable neurological disorders, the most notable achievements have been observed for stroke. Wide implementation of evidence-based stroke management strategies (eg, stroke units, thrombolysis, national smoking cessation campaign) in the USA for just 20 years has shifted stroke from the second to the fourth leading cause of death.45,47 An initiative to translate evidence into practice was set up in 2018 by the first Ministerial Stroke Meeting, conducted concurrently with the 21st Ibero–American Stroke Organization Congress in Gramado, Brazil. There, the Gramado declaration was adopted, pledging to implement specific measures to improve primary stroke prevention and acute and community care and rehabilitation (eg, stroke riskometer app for primary stroke prevention, increased number of acute and community care and rehabilitation services, training of health professionals) across all Latin American countries.32
Other interventions that have been explored on a national level include integrated care models for Parkinson’s disease, such as ParkinsonNet in the Netherlands, USA, and Norway.48,49 For headache, multidisciplinary care models have been developed and already partly tested in Germany and other countries, and have been shown to be cost-effective.50,51 Additionally, the Value of Treatment project of the European Brain Council has examined the value of interventions for prevention, screening, diagnosis, treatment, and rehabilitation for a range of neurological and mental health disorders. This initiative aims to identify areas that need to be prioritised for policy recommendations to optimise research and improve services at a European level.52 Not all interventions are appropriate for all countries, however, and more research into existing interventions and their effectiveness is needed to develop customised national or subnational solutions.
Accumulating evidence demonstrates the feasibility and preliminary effectiveness of telemedicine and mobile technologies for remote delivery of health care and education in neurology.53,54 Some examples include promoting a healthy lifestyle,16,55–57 preventing stroke,32,58–60 and managing Parkinson’s disease,60 multiple sclerosis,61 migraine,62 dementia,63 and epilepsy,65,66 among others. Supported by WHO,67 validated mobile technologies are evolving quickly and are already showing unprecedented uptake among the public and health professionals. Such technologies have the potential to transform neurological health care and prevention.16
Conclusions and recommendations
Neurological disorders are collectively the leading cause of disability and the second highest cause of death, globally, and the magnitude of their burden is likely to rise owing to population growth and ageing, particularly in LMICs. Governments and public policy makers need to take urgent action to mitigate the risks and impact of neurological disorders. Given the limited resources and competing health issues all policy makers are facing around the globe, coordinated advocacy efforts are required at the individual, institutional, local, and national government levels, with support from relevant non-governmental organisations and patient organisations. Such efforts should encourage prioritisation and promote the funding and implementation of strategies to reduce the burden of neurological disorders. Funding bodies and policy makers should make use of the GBD global, regional, and national estimates of the burden of neurological disorders, risk factors, and their trends1,3–11 to define health-care and research priorities, and to advance prevention, management, and service and workforce development. Panel 4 gives a summary of priorities and recommendations for policy making on the basis of the GBD findings. The GBD findings should place prevention of neurological disorders as the main priority for public-health authorities, neurology services as a main priority for health-care systems, and epidemiological monitoring (observational and experimental) studies, the assessment of preventive and disease-modifying interventions, and translational studies as top priorities for research funding agencies.68 Moreover, addressing social determinants of the burden of neurological disorders, including targeting SDI as a highly predictive factor,69 could be effective in reducing the burden, and should be carried out at international or governmental levels.
Panel 4: Overview and summary of priorities and recommendations for policy making.
Burden of neurological disorders
In 2016, neurological disorders were the leading cause of disability and the second leading cause of death3
Almost one in three people worldwide has a neurological disorder at some point in their lifetime; the most common neurological causes of disability are stroke, migraine, and Alzheimer’s disease and other dementias3
In the past 30 years, the number of deaths has increased by 39% and disability-adjusted life-years lost by 15%3
Most of the burden (nearly 80%) is borne by low-income and middle-income countries (LMICs), whose total neurological workforce is 70 times less than that of high-income countries3
Priorities and recommendations
Funding bodies and policy makers should make use of the GBD global, regional, and national estimates of the burden of neurological disorders, risk factors, and their trends1,3–11 to define health-care and research priorities, and to advance prevention, management, and service and workforce development
Coordinated advocacy efforts at the individual, institutional, local, and national government levels are required to encourage prioritisation, funding, and implementation of strategies to reduce the burden of neurological disorders
Governments should consider developing and implementing strategies to counter established risk factors for neurological disorders (eg, taxation strategies to tackle unhealthy behaviours, such as tobacco use, salt intake, sugar consumption, and alcohol use); the revenue from these taxations could be used to fund advances in neurological care, workforce development, development and implementation of disease-modifying interventions, and the provision of culturally appropriate and population-specific preventive strategies
To be most cost-effective, preventive strategies for neurological disorders must be integrated with prevention strategies for other non-communicable diseases and health conditions, including ischaemic heart disease, chronic kidney disease, cancer, and diabetes
Governments and policy makers need to take measures to improve early diagnosis, treatment, and rehabilitation to reduce the burden of neurological disorders in the absence of optimal strategies to reduce the risk of many of these disorders
Urgent efforts are needed for adequate neurological service development, on the basis of our proposed framework for access to minimal, essential, and advanced services, with the aim of achieving the most advanced level of services possible, given local resources and practice (panel 3)
To achieve global impact in reducing the burden of neurological diseases, LMICs, which bear the greatest burden, require targeted interventions to rapidly improve the accessibility and delivery of primary, population-wide and individual, motivational prevention strategies and services for acute care and rehabilitation of neurological disorders
An increase in the size and quality of the health workforce is needed in LMICs to improve access to services
Collaboration between health, sociopolitical, and economic sectors31 at the local, national, regional, and international levels is key to achieving good neurological health for global populations
Targeted investments on the international and national levels are required to study and monitor frequency, outcomes, and predictors of neurological disorders, and their preventive and disease-modifying interventions (including translational research); funding for research focused on epidemiological surveillance, prevention, and treatment and care tailored to local circumstances in LMICs is also vital
Similar to initiatives for stroke32,33 and the Global Action Against Dementia,34 concerted, collaborative, and comprehensive global initiatives should be developed for other major neurological disorders (including traumatic brain injury) at WHO and national levels; to be effective and sustainable, these actions need to be supported by sufficient funding, with clear goals and outcomes
More data on the burden of neurological disorders in all countries and populations, especially in LMICs, are sorely needed to inform policy and guide improvements in health-care services. Additionally, improvements in the quality of data collection via health information systems in LMICs are crucial for understanding the magnitude of the problem and for developing and providing solutions that are calibrated to local settings. Widely accessible prevention strategies and expanded and enhanced workforces for better health-care delivery in LMICs are essential to reduce the global burden of neurological disorders. Innovations that have been successfully developed in high-income settings will have to be adjusted to local circumstances to improve the quality of neurology services for the high number of people living with neurological diseases in LMICs.
Following the Global Action Against Dementia initiative34 and initiatives for stroke (eg, the World Stroke Organization initiative to “Cut stroke in half”,33 the first Ministerial Stroke Meeting of Latin American countries,32 and the Action Plan for Stroke in Europe)70, comprehensive and collaborative efforts should be developed at international and national levels on a similar scale for other major neurological disorders, with sufficient funding and clear goals and outcomes. Telemedicine and mobile health technologies are showing promise for providing health care and education remotely for a range of neurological disorders.32,53,54,58–66 Further development of these technologies and upscaling of widely accessible and proven effective mobile technologies should be one of the priorities for funding bodies and policy makers.32,60 Precision medicine71,72 and artificial intelligence73 are emerging technologies that could contribute to a reduction in the burden of neurological disease through improved personalised detection, prevention, and treatment of these clinically and biologically complex disorders.
Regular updates and evaluation of the burden of neurological disorders at various levels through future GBD estimates and other epidemiological studies will be essential for monitoring progress and understanding responses to interventions, and for further driving the appropriate allocation of resources, building on successful policies, and developing new policies. Commitment to action is now needed at individual, organisational, governmental, and international levels to tackle the already immense and fast-increasing global burden of neurological disorders and other non-communicable diseases.
Supplementary Material
Search strategy and selection criteria.
We searched PubMed and the internet (using Google and other search engines) for articles published in English during the period Jan 1, 1990, to Sept 30, 2019, using the terms “neurological disorders”, “stroke”, “brain and other nervous system cancers”, “traumatic brain injury”, “spinal cord injury”, “Alzheimer’s disease”, “dementias”, “Parkinson’s disease”, “multiple sclerosis”, “motor neuron disease”, “epilepsy”, “migraine”, “tension-type headache”, “tetanus”, “meningitis”, and “encephalitis”, combined with either “Global Burden of Disease”, “global”, “burden”, “cost”, “taxation”, “policy”, “funding”, “priority”, “prioritisation”, or “action plan”. We concentrated on Global Burden of Disease estimates and nationally representative and international reports. Additionally, we manually searched the reference lists of relevant publications and consulted with experts in the area of neurological disorders and other relevant stakeholders.
Acknowledgments
Funding for our travelling and meetings was provided by the Bill & Melinda Gates Foundation.
Footnotes
Declaration of interests
VLF reports that he is a CEO of the New Zealand Stroke Education (charitable) Trust, outside the submitted work. GD has served as a consultant for Boston Scientific, Cavion, and Functional Neuromodulation; has received lecture fees from Boston Scientific and royalties from Thieme publishers; and is a government employee and receives funding for his research through his institution from the German Research Council, the German Ministry of Education and Research, and Medtronic, outside the submitted work. WMC reports travel fees from Sanofi Genzyme, Biogen, and Merck, and travel and personal fees from Roche and Novartis, outside the submitted work. All other authors declare no competing interests.
See Online for appendix
For more on GBD Compare Viz Hub see https://vizhub.healthdata.org/gbd-compare/
Contributor Information
Valery L Feigin, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Sciences, Auckland University of Technology, Auckland, New Zealand.
Theo Vos, Institute for Health Metrics Evaluation, University of Washington, Seattle, WA, USA.
Emma Nichols, Institute for Health Metrics Evaluation, University of Washington, Seattle, WA, USA.
Mayowa O Owolabi, Center for Genomic and Precision Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria.
William M Carroll, Sir Charles Gairdner Hospital, Perth, WA, Australia.
Martin Dichgans, Institute for Stroke and Dementia Research, University Hospital, and Munich Cluster of Systems Neurology, Ludwig-Maximilians University, Munich, Germany.
Günther Deuschl, Department of Neurology, University Centre Schleswig-Holstein, Christian-Albrechts University, Kiel, Germany.
Priya Parmar, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Sciences, Auckland University of Technology, Auckland, New Zealand.
Michael Brainin, Department of Neurosciences and Preventive Medicine, Danube University Krems, Krems, Austria.
Christopher Murray, Institute for Health Metrics Evaluation, University of Washington, Seattle, WA, USA.
References
- 1.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Vos T. Global burden of neurological disorders: from Global burden of disease estimates to actions. Neuroepidemiology 2019; 52: 1–2. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zunt JR, Kassebaum NJ, Blake N, et al. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 1061–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logroscino G, Piccininni M, Marin B, et al. Global, regional, and national burden of motor neuron diseases 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 1083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SL, Theadom A, Ellenbogen RG, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Health Metrics and Evaluation. Protocol for the Global Burden of Disease, injuries, and risk factors Study (GBD), version 3.0. Feb 26, 2018. http://www.healthdata.org/sites/default/files/files/Projects/GBD/GBD_Protocol.pdf (accessed April 3, 2019). [Google Scholar]
- 13.Institute for Health Metrics and Evaluation. Rethinking development and health: findings from the Global Burden of Disease Study. Seattle: Institute for Health Metrics and Evaluation, 2016. [Google Scholar]
- 14.Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feigin VL. Primary stroke prevention needs overhaul. Int J Stroke 2017; 12: 5–6. [DOI] [PubMed] [Google Scholar]
- 16.Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA. Prevention of stroke: a strategic global imperative. Nat Rev Neurol 2016; 12: 501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen LN, Pullar J, Wickramasinghe KK, et al. Evaluation of research on interventions aligned to WHO “Best Buys” for NCDs in low-income and lower-middle-income countries: a systematic review from 1990 to 2015. BMJ Glob Health 2018; 3: e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization, World Economic Forum and the Harvard School of Public Health. From burden to. “best buys”: reducing the economic impact of non-communicable diseases in low- and middle-income countries. Geneva: World Economic Forum, 2011. http://www.who.int/nmh/publications/best_buys_summary.pdf (accessed Sept 11, 2018). [Google Scholar]
- 19.Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med 2001; 20 (suppl): 16–66. [DOI] [PubMed] [Google Scholar]
- 20.Goodchild M, Perucic AM, Nargis N. Modelling the impact of raising tobacco taxes on public health and finance. Bull World Health Organ 2016; 94: 250–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. Geneva: WHO, 2015. [Google Scholar]
- 22.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet 2007; 370: 2044–53. [DOI] [PubMed] [Google Scholar]
- 23.Wilson N. Salt tax could reduce population’s salt intake. BMJ 2004; 329: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier PS, Holmes J, Angus C, Ally AK, Meng Y, Brennan A. Estimated effects of different alcohol taxation and price policies on health inequalities: a mathematical modelling study. PLoS Med 2016; 13: e1001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delcher C, Maldonado-Molina MM, Wagenaar AC. Effects of alcohol taxes on alcohol-related disease mortality in New York State from 1969 to 2006. Addict Behav 2012; 37: 783–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: a systematic review. Am J Public Health 2010; 100: 2270–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaloupka FJ, Grossman M, Saffer H. The effects of price on alcohol consumption and alcohol-related problems. Alcohol Res Health 2002; 26: 22–34. [PMC free article] [PubMed] [Google Scholar]
- 28.Elder RW, Lawrence B, Ferguson A, et al. The effectiveness of tax policy interventions for reducing excessive alcohol consumption and related harms. Am J Prev Med 2010; 38: 217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Strategic investment of tobacco tax revenue. Geneva: WHO, 2018. https://www.tobaccofreekids.org/ssets/global/pdfs/en/strategic_investment_tobacco_tax_revenue.pdf (accessed Feb 26, 2019). [Google Scholar]
- 30.World Health Organization. Clean air for health: Geneva action agenda: first WHO global conference on air pollution and health—summary report. Nov 1, 2018. https://www.who.int/phe/news/clean-air-for-health/en/ (accessed Feb 22, 2019). [Google Scholar]
- 31.World Health Organization. Social determinants of health: intersectoral action. https://www.who.int/social_determinants/thecommission/countrywork/within/isa/en/ (accessed Oct 1,2019).
- 32.Ouriques Martins SC, Sacks C, Hacke W, et al. Priorities to reduce the burden of stroke in Latin American countries. Lancet Neurol 2019; 18: 674–83. [DOI] [PubMed] [Google Scholar]
- 33.Brainin M, Feigin V, Martins S, et al. Cut stroke in half: polypill for primary prevention in stroke. Int J Stroke 2018; 13: 633–47. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. First WHO ministerial conference on global action against dementia, 16–17 March 2015, Geneva, Switzerland: meeting report. Geneva: WHO, 2015. https://apps.who.int/iris/bitstream/handle/10665/179537/9789241509114_eng.pdf (accessed Feb 22, 2019). [Google Scholar]
- 35.World Health Organization. Atlas: country resources for neurological disorders, 2nd edn. Geneva: WHO, 2017. [Google Scholar]
- 36.Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke 2014; 9 (suppl A100): 4–13. [DOI] [PubMed] [Google Scholar]
- 37.Rimmer K, Shah H, Thakur K. Expanding medicines for neurologic disorders on the WHO Model List. Neurology 2017; 88: e87–91. [DOI] [PubMed] [Google Scholar]
- 38.Burton A. How do we fix the shortage of neurologists? Lancet Neurol 2018; 17: 502–03. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Neurology. Neurology workforce data. https://www.aan.com/siteassets/home-page/policy-and-guidelines/policy/public-policy-resources/2014-neurology-workforce-data.pdf (accessed Oct 1, 2019).
- 40.Guo B, Naish S, Hu W, Tong S. The potential impact of climate change and ultraviolet radiation on vaccine-preventable infectious diseases and immunization service delivery system. Expert Rev Vaccines 2015; 14: 561–577. [DOI] [PubMed] [Google Scholar]
- 41.Owolabi M, Miranda JJ, Yaria J, Ovbiagele B. Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Glob Health 2016; 1: e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. One Health. September 2017. https://www.who.int/features/qa/one-health/en/ (accessed Aug 16, 2019). [Google Scholar]
- 43.Fereshtehnejad SM, Vosoughi K, Heydarpour P, et al. Burden of neurodegenerative diseases in the eastern Mediterranean region, 1990–2016: findings from the Global Burden of Disease Study 2016. Eur J Neurol 2019; 26: 1252–65. [DOI] [PubMed] [Google Scholar]
- 44.Kleinman A. Four social theories for global health. Lancet 2010; 375: 1518–19. [DOI] [PubMed] [Google Scholar]
- 45.Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ 2008; 86: 140–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towfighi A, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke 2010; 41: 499–503. [DOI] [PubMed] [Google Scholar]
- 47.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011; 42: 2351–55. [DOI] [PubMed] [Google Scholar]
- 48.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 2018; 8: S3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darweesh SKL, Raphael KG, Brundin P, et al. Parkinson matters. J Parkinsons Dis 2018; 8: 495–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallasch T-M, Kropp P Multidisciplinary integrated headache care: a prospective 12-month follow-up observational study. J Headache Pain 2012; 13: 521–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen RH, Rasmussen A. Multidisciplinary team approach to headache care. Clin Pract 2012; 9: 181–88. [Google Scholar]
- 52.European Brain Council. Early intervention: bridging the early diagnosis and treatment gap: white paper towards optimizing research and care for brain disorders. https://www.braincouncil.eu/wp-content/uploads/2017/06/EBC_white_policy_paper_DEF26072017_Low.pdf (accessed Oct 17, 2019).
- 53.Janssen F, Awadallah M, Alhalabi A, et al. Telemedicine in general neurology: use of audiovisual consultation for on call back-up service in an acute care hospital. J Neurol 2018; 265: 880–84. [DOI] [PubMed] [Google Scholar]
- 54.Dorsey ER, Glidden AM, Holloway MR, Birbeck GL, Schwamm LH. Teleneurology and mobile technologies: the future of neurological care. Nat Rev Neurol 2018; 14: 285–97. [DOI] [PubMed] [Google Scholar]
- 55.Spring B, Schneider K, McFadden HG, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med 2012; 172: 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bond DS, Thomas JG, Raynor HA, et al. B-MOBILE—a smartphone-based intervention to reduce sedentary time in overweight/obese individuals: a within-subjects experimental trial. PLoS One 2014; 9: e100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glynn LG, Hayes PS, Casey M, et al. Effectiveness of a smartphone application to promote physical activity in primary care: the SMART MOVE randomised controlled trial. Br J Gen Pract 2014; 64: e384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnamurthi R, Hale L, Barker-Colllo SAT, et al. Mobile technology for primary stroke prevention: a proof-of-concept pilot randomised controlled trial—a brief report. Stroke 2018; 50: 196–98. [DOI] [PubMed] [Google Scholar]
- 59.Feigin VL, Norrving B, Mensah GA. Primary prevention of cardiovascular disease through population-wide motivational strategies: insights from using smartphones in stroke prevention. BMJ Global Health 2017; 2: e000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wardlaw JM, Bath PM. Stroke research in 2018: extended time windows, refined benefit, and lifestyle prevention targets. Lancet Neurol 2019; 18: 2–3. [DOI] [PubMed] [Google Scholar]
- 61.Linares-Del Rey M, Vela-Desojo L, Cano-de la Cuerda R. Mobile phone applications in Parkinson’s disease: a systematic review. Neurologia 2019; 34: 38–54. [DOI] [PubMed] [Google Scholar]
- 62.Zayas-Garcia S, Cano-de-la-Cuerda R. Mobile applications related to multiple sclerosis: a systematic review. Rev Neurol 2018; 67: 473–83. [PubMed] [Google Scholar]
- 63.Stubberud A, Linde M. Digital technology and mobile health in behavioral migraine therapy: a narrative review. Curr Pain Headache Rep 2018; 22: 66. [DOI] [PubMed] [Google Scholar]
- 64.Klimova B, Bouckova Z, Toman J. Mobile phone apps as support tools for people with dementia. In: Park J, Loia V, Choo KK, Yi G (eds). Advanced multimedia and ubiquitous engineering: lecture notes in electrical engineering, Singapore. Springer, 2019. 518: 7–12. [Google Scholar]
- 65.Ryvlin P, Beniczky S. Seizure detection and mobile health devices in epilepsy: update and future developments. Epilepsia 2018; 59 (suppl 1): 7–8. [DOI] [PubMed] [Google Scholar]
- 66.Afra P, Bruggers CS, Sweney M, et al. Mobile software as a medical device (SaMD) for the treatment of epilepsy: Development of digital therapeutics comprising behavioral and music-based interventions for neurological disorders. Front Hum Neurosci 2018; 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. mHealth: new horizons for health through mobile technologies. Geneva: WHO, 2011. https://www.who.int/ehealth/mhealth_summit.pdf (accessed Nov 8, 2019). [Google Scholar]
- 68.The Lancet Neurology. The data to put neurology on top of the public-health agenda. Lancet Neurol 2019; 18: 1. [DOI] [PubMed] [Google Scholar]
- 69.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Socio-Demographic Index (SDI) 1950–2017. Seattle: Institute for Health Metrics and Evaluation, 2018. [Google Scholar]
- 70.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J 2018; 3: 309–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gibbs RM, Lipnick S, Bateman JW, et al. Toward precision medicine for neurological and neuropsychiatric disorders. Cell Stem Cell 2018; 23: 21–24. [DOI] [PubMed] [Google Scholar]
- 72.Tan L, Jiang T, Tan L, Yu JT. Toward precision medicine in neurological diseases. Ann Transl Med 2016; 4: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ridler C Artificial intelligence accelerates detection of neurological illness. Nat Rev Neurol 2018; 14: 572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


