Abstract
Background
The ability to proactively predict the epidemiological dynamics of infectious diseases such as coronavirus disease 2019 (COVID-19) would facilitate efficient public health responses and may help guide patient management. Viral loads of infected people correlate with infectiousness and, therefore, could be used to predict future case rates.
Aim
In this systematic review, we determine whether there is a correlation between severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) real-time reverse-transcription polymerase chain reaction (RT–PCR) cycle threshold (Ct) values (a proxy for viral load) and epidemiological trends in patients diagnosed with COVID-19, and whether Ct values are predictive of future cases.
Methods
A PubMed search was conducted on August 22 2022, based on a search strategy of studies reporting correlations between SARS-CoV-2 Ct values and epidemiological trends.
Results
Data from 16 studies were relevant for inclusion. RT–PCR Ct values were measured from national (n = 3), local (n = 7), single-unit (n = 5), or closed single-unit (n = 1) samples. All studies retrospectively examined the correlation between Ct values and epidemiological trends, and seven evaluated their prediction model prospectively. Five studies used the temporal reproduction number (Rt) as the measure of the population/epidemic growth rate. Eight studies reported a prediction time in the negative cross-correlation between Ct values and new daily cases, with seven reporting a prediction time of ~1–3 weeks, and one reporting 33 days.
Conclusion
Ct values are negatively correlated with epidemiological trends and may be useful in predicting subsequent peaks in variant waves of COVID-19 and other circulating pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00772-7.
Keywords: COVID-19, Ct value, Epidemiological trends, RT-PCR, SARS-CoV-2, Viral load
Key Summary Points
| SARS-CoV-2 real-time RT-PCR Ct values are associated with epidemiological trends. |
| Negative cross-correlation between Ct values and new daily cases were observed. |
| Ct values may be useful in predicting upcoming peaks in variant waves. |
| Predictive modeling using Ct values may enable assessment of epidemic trajectory. |
Introduction
In a world of increasing travel and migration, the numbers of known infections and deaths from the coronavirus disease 2019 (COVID-19) global pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), highlight the rapid spread of this pandemic and the importance of understanding infectious disease dynamics to be better prepared for a future pandemic. As of July 2022, there have been almost 548 million confirmed cases worldwide (229.6 million in Europe and 86.7 million cases in the USA), including more than 6.3 million deaths [1]. One important lesson from the COVID-19 pandemic is that correctly predicting epidemic waves and implementing timely appropriate preventative measures could potentially save millions of lives.
COVID-19 epidemic monitoring has focused on case counts, environmental levels from wastewater monitoring, test positivity rates, and reported deaths or hospitalizations. These parameters are used to estimate the growth rate of positive tests (the estimated effective reproduction number, Rt). These traditional estimates of the epidemic trajectory can result in a limited, biased, and delayed view of the epidemic. Viral loads of infected people are associated with infectivity and could be used to predict future case rates; a higher viral load in a patient suggests that more virus is being shed, and thus increases the chance of others being exposed to an infectious dose permissible for acquiring disease. High viral loads in a community can indicate increasing prevalence of disease. Viral load measures can improve epidemic predictions, especially in low surveillance settings where true case counts over time are not easily available [2].
Real-time reverse transcription polymerase chain reaction (RT–PCR) is regarded as the gold standard method for COVID-19 diagnosis. Although a qualitative result is usually obtained, certain instruments provide end users with access to cycle threshold (Ct) values. Real-time RT–PCR Ct values represent the number of amplification cycles required for the target gene to exceed a threshold level, with a low Ct value corresponding to a high pathogen load. Ct values are inversely related to viral load and can provide an indirect method for quantifying the copy number of sample viral ribonucleic acid (RNA) [3]. Several studies have demonstrated a link between lower Ct values and increased disease severity [4–6]. Ct values can support public health, infection control, and patient management decisions [7, 8].
Symptomatic presentation of COVID-19 has been significantly associated with lower Ct values, meaning higher viral load and prolonged virus shedding, which may play a role in determining the transmissibility and contagiousness of disease [3]. Therefore, a possible link between Ct values and epidemiology trends needs to be explored. Using population-based variations in Ct values could improve Rt predictions of a 7-day period or longer.
The main aim of this systematic review is to identify the presence or absence of a correlation between Ct values of patients diagnosed with COVID-19 and population dynamics of the disease, and determine whether temporal trends in SARS-CoV-2 Ct levels are predictive of future population epidemiology trends.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The review was undertaken according to the principles outlined in the Cochrane Handbook [9]. A comprehensive search of PubMed was conducted on August 22 2022, to identify studies reporting on the association between real-time RT–PCR Ct values of SARS-CoV-2 and epidemiological trends. The search strategy involved the terms “Ct value” or “viral load,” “SARS-CoV-2” and “epidemiological data,” along with relevant synonyms. The full search string is presented in the supplementary data (see Supplementary Table 1).
All studies conducted in humans diagnosed with COVID-19 and reporting on the presence or absence of an association between real-time RT–PCR Ct values, or viral load specifically determined via real-time RT–PCR Ct values, and epidemiological trends were included. Exclusion criteria included the following: prereview/preprint articles, animal/nonhuman and studies on wastewater, review articles, manuscripts not in English, and studies not reporting SARS-CoV-2 RT–PCR Ct values, population epidemiological trends of SARS-CoV-2, or correlations between the two.
Titles and abstracts were screened for relevance by two independent reviewers, while a third reviewer resolved conflicts. The full texts of relevant studies were assessed for inclusion with a focus on the association between SARS-CoV-2 RT-PCR Ct values or viral loads specifically determined via RT-PCR Ct values and epidemiological trends by two independent reviewers. The process for study selection was reported using the flow diagram of the Preferred-Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [10]. Key data from all included studies were captured using a data extraction form. All extracted data were verified by an independent reviewer. Outcomes were reported in a narrative format and meta-analyses were deemed inappropriate owing to wide variation in reported outcomes.
Given the novelty of RT–PCR Ct values as an epidemiological tool, there was no precedent in risk assessment for this study’s reference. To assess the methodological quality of the included studies, the risk of bias for these studies was assessed using an adaption of the modified Newcastle–Ottawa Scale [11, 12].
Results
Included Studies
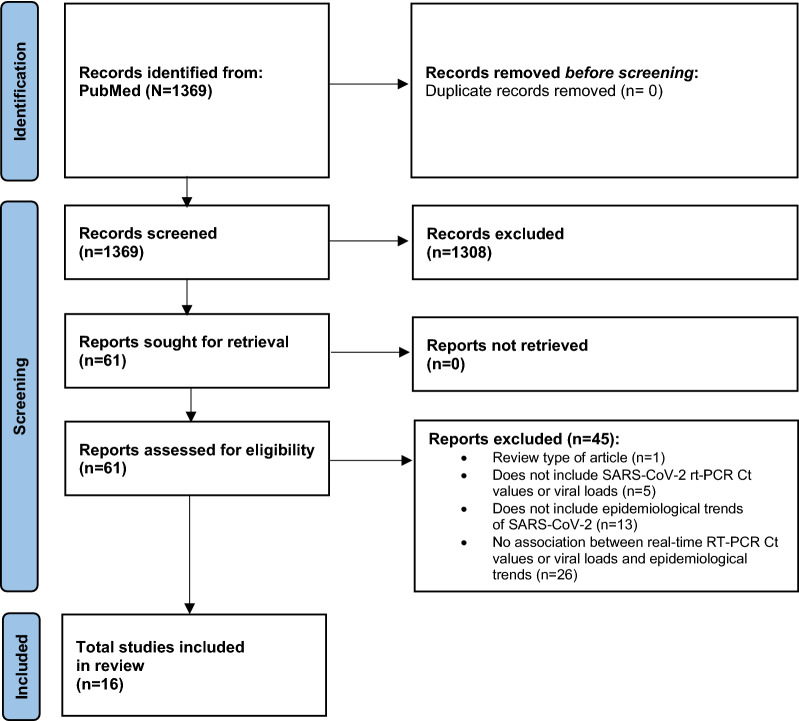
PubMed searches identified 1369 unique records for screening. The PRISMA flowchart of included studies is shown in Fig. 1. Studies that were excluded at the full-text stage are outlined (including the reason why they were excluded) in Supplementary Table 2. Data from the 16 studies that were relevant for inclusion, and their respective study characteristics, are outlined in Table 1. Study outcomes of the 16 included studies detailing correlation between SARS-CoV-2 real-time RT–PCR Ct values and epidemiological trends are summarized in Table 2, and as follows.
Fig. 1.
PRISMA flow diagram. Ct cycle threshold, RT–PCR real-time reverse transcription polymerase chain reaction, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2
Table 1.
Study characteristics of included studies
| Study | Year | Country | Study objective | Study design | Exposure samples (patient Ct testing) | Outcome population | Findings/outcome description |
|---|---|---|---|---|---|---|---|
| Abdulrahman et al. [3] | 2021 | Kingdom of Bahrain | To determine the relationship between Ct values and percentage of positive tests, and if there is an association with future COVID-19 cases to provide data for the development of a predictive epidemic model | Retrospective | National | National | A decrease in mean Ct values in 1 week was best correlated with an increase in cases 1 week forward from that point in time |
| Alizon et al. [18] | 2022 | France | To explore the possibility of using Ct values from SARS-CoV-2 screening tests to better understand the spread of an epidemic and to better understand the biology of the infection | Retrospective | National | National | The main factors affecting Ct values of SARS-CoV-2 RT–PCR in this multivariate linear model were the assay type; the laboratory; the level of positivity; the days post-symptom onset; the sample type; age (per 20 years older); whether target gene was N, ORF1, or S; or the date (per 71 days later) |
| Andriamandimby et al. [22] | 2022 | Madagascar | To estimate COVID-19 epidemic growth rates at the national level and in two major administrative regions of Madagascar, and evaluate the robustness of this Ct-based method in comparison with epidemic growth rates derived from more traditional case-count methods applied to the same regions and at the national level | Real-time and retrospective | Local/regional | National | Public reporting of Ct values could enable forecasting of impending incidence peaks in regions with limited case reporting |
| Avadhanula et al. [15] | 2021 | USA | To determine the potential of the superspreader by examining the viral load of SARS-CoV-2 in adults during the first and second wave of coronavirus disease 2019 pandemic at the local level | Cross-sectional observational cohort | Single unit/local | Single unit/local | The median Ct of the weekly viral load from nasopharyngeal samples of hospitalized patients on admission at an individual level may help to predict epidemic trend at the population level. During two epidemic waves the peak in viral load (determined by Ct) preceded the peak in positivity by 2–3 weeks |
| Calistri et al. [21] | 2021 | Italy | To analyze the trend of the Ct values in samples collected from March to mid-December 2020 | Real-time | Local | Local | There was a strong inverse correlation between Ct values and the trend in incident cases in the local population |
| El Zein et al. [16] | 2021 | USA | To evaluate the role of Ct values in samples as a prognostic marker in hospitalized patients | Retrospective cohort study | Single unit/local | Regional | A steady decrease in the percent of positive samples determined by Ct values was associated with the evolution of the pandemic from April to June 2020 |
| Hay et al. [2] | 2021 | USA | To develop a new method that uses information inherent in Ct values from RT-qPCR tests to robustly estimate the epidemic trajectory from multiple or even a single cross-section of positive samples | Real-time | Single unit/closed system | Single unit followed by regional | The local epidemic trajectory of SARS-CoV-2 was accurately estimated from Ct values of routine hospital admissions |
| Khalil et al. [26] | 2022 | Lebanon | To use data-driven modeling that utilizes Ct values and previous number of cases to forecast the trajectory of the spread of COVID-19 | Retrospective analysis | Single unit / local | National | A polynomial regression and support vector machine regression model using Ct values demonstrated potential for predicting COVID-19 incidences in institutions |
| Lin et al. [24] | 2022 | China | To analyze the viral load data on confirmed cases during two local epidemics in Hong Kong to explore the possibility of a correlation between temporal changes in the distribution of viral loads (measured by RT-qPCR Ct values) and estimates of Rt based on case counts | Real-time | Local | Local | A log linear regression was fitted to daily incidence-based Rt, on daily mean and skewness of Ct values at sampling during the third wave (considered the training period for this study) for real-time assessment of COVID-19 transmission in the community using Ct values. This trained model was applied to the daily Ct distributions in the fourth wave to estimate Rt in real-time in the fourth wave |
| Mishra et al. [25] | 2022 | India | To use Ct values as an early indicator for upcoming COVID-19 waves | Retrospective | Local/regional | National | Significantly lower Ct values were found in the second compared with the first pandemic wave. The second pandemic wave had a unprecedented rapid surge of cases |
| Penney et al. [17] | 2022 | USA | To examine the association between Ct values from patients in a tertiary care ER department with the weekly state hospitalizations to evaluate the utility of using Ct values to estimate epidemiological trends and anticipate the next phase of the pandemic | Retrospective | Single unit | Local | There was a significant inverse correlation between median weekly Ct values and weekly incident hospitalizations for SARS-CoV-2 infections in Massachusetts |
| Phillips et al. [13] | 2022 | USA | To determine if temporal trends in SARS-CoV-2 Ct values from clinical testing were predictive of future cases to aid hospital-level surge planning | Prospective | Single unit | Single unit | Temporal trends in SARS-CoV-2 Ct values were predictive of future admission case numbers of COVID-19 at a specific hospital |
| Stevens et al. [23] | 2022 | Australia | Analyzing SARS-CoV-2 real-time PCR test Ct values across a population to determine the usefulness in assisting public health efforts and adding refinement to epidemiological models | Observational | Local | Local | Ct values across a population demonstrated potential to predict community transmission, owing to the increased proportion of high Ct values as case numbers declined and the increased proportion of low Ct values as the case numbers increased in the community |
| Tso et al. [14] | 2021 | USA | Aimed to conduct an exploratory analysis of potential correlations between the population distribution of Ct values and COVID-19 dynamics, which were operationalized as percent positivity, Rt, and COVID-19 hospitalization count | Real-time | Local | Local | There was a negative correlation between median Ct and Rt, median Ct and hospitalization count (including a time delay), and median Ct and percent positivity. Cross-correlation plots of medial Ct values demonstrated a significant relationship with future hospitalization counts and not with percent positivity rates or Rt values |
| Walker et al. [19] | 2021 | UK | To investigate predictors of median Ct values (as proxy for viral load) using quartile regression | Real-time | National | National | Decreases in population level Ct values in July/early August 2020 preceded increases in positivity rates in England, whereas Ct rates in November 2020 to January 2021 did not precede positivity rates, but correlated with positivity rates in this infection wave |
| Yin et al. [20] | 2021 | Belgium | To assess the usefulness of SARS-CoV-2 RT–PCR Ct trends produced by the LHUB-ULB (a consolidated microbiology laboratory located in Brussels, Belgium) for monitoring the epidemic’s dynamics at local and national levels, and for improving forecasting models | Retrospective | Local/regional | National | A deterministic, continuous age-structured compartmental model (extended SEIR-type) found that Ct values negatively correlated with the estimated prevalence |
COVID-19 coronavirus disease 2019, Ct cycle threshold, ER emergency room, LHUB-ULB University Hospital Laboratory of Brussels, RT–PCR reverse transcription polymerase chain reaction, RT-qPCR reverse transcription quantitative polymerase chain reaction, NR not recorded, Rt transmission rate, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, SEIR susceptible-exposed-infectious-recovered
Table 2.
Summary of reported data relating to SARS-CoV-2 real-time RT–PCR Ct value correlation with epidemiological trends
| Study | Patient setting/exposure | Number of PCR + patients | PCR assay | RT–PCR gene target | Sample type | Timepoint of assessment after symptom onset (if symptomatic) | SARS-CoV-2 variant (if known) | Epidemiology/population outcome measure | Correlations between Ct value and community prevalence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct value | Prediction time | Correlation statistics | |||||||||
| Abdulrahman et al. [3] | Hospital and public |
63,879 Ct cutoff for positivity ≤ 40 for E gene |
TaqPath, 1-Step RT-qPCR Master Mix, CG and the SuperScript III Platinum One-Step qRT–PCR Kit, Thermo Fisher Scientific, Waltham, MA, USA, LightMix1 Modular SARS-CoV (COVID-19) (TIB MOLBIOL, Berlin, Germany) The RT–PCR was conducted on the Applied Biosystems 7500 Fast Dx RealTime PCR Instrument, LightCycler 480 Instrument II, Roche Molecular Systems, Inc, Pleasanton, CA, USA |
E, N, RdRp | Nasopharyngeal | NR (35.25% were symptomatic) | NR |
The mean weekly proportion of positive tests (taking into consideration incubation periods) New daily cases |
NR | 1 week |
A pilot study for the development of a predictive model Pearson’s correlation between Ct values with new daily cases −0.06 (95% CI: −0.06, −0.05, P < 0.001) A decrease in the standardized Z-score of mean Ct values in 1 week was best correlated with an increase in the mean weekly proportion of positive cases 1 week later |
| Alizon et al. [18] | Public |
793,479 tests Only tests with a Ct value were included, meaning that negative results were less represented in the final database as negative samples do not usually have any reported Ct value |
PerkinElmer, Alinity, Abbott Laboratories, Abbott Park, IL, USA, Allplex, Seegene, Argene, BioMerieux, BGI, CNR Paris, Cobas 6800, Roche, Cobas 8800, Roche, Daan Gene, Appolon Biotek, Rhone, France, Genefinder, Technique Charite, Thermo Fisher Scientific, Waltham, MA, USA |
E, N, N and O, targeted together, ORF1, S | Nasopharyngeal, lower respiratory tract, feces, saliva |
Known for 9%: 0–4 days (reference), 4–7 days, 8–14 days, > 14 days |
NR | The Rt of the epidemic (measured via national hospital admission data and the EpiEstim method) | NR | ~ 1 week |
The Rt (based on hospitalization and screening data) on the date of sampling was not significantly associated with Ct values Using an ARIMA predictive model to estimate whether the Ct data improves short-term predictions of the disease epidemiology, 6–7 days appeared to be the most significant time lag for cross-correlation between Ct and Rt (tested between 0 and 20 days) The mean absolute percentage error in predicting Rt using the ARIMA model improved when including Ct quartiles and Ct skewness |
| Andriamandimby et al. [22] | Public |
5310 Ct cutoffs for positive results from different PCR assays: Charity Berlin: ≤ 38; Hong Kong: ≤ 40; Da An: ≤ 40; LightMix SarbeCoV/SarbeCoV TibMolBiol ≤ 38; TaqPath ≤ 37 for 2 of 3 targets; GeneXpert: ≤ 40) To control for extensive variation in qPCR test and target a TaqPath N-corrected Ct value was calculated with a Ct cutoff of ≤ 37 |
Seven WHO recommended kits and corresponding protocols: Charity Berlin kit, Hong Kong University kit, Da An gene kit, Da An Gene Co. Ltd. Sun Yatsen University, Guangzhou, China, LightMix SarbeCoV E-gene plus EAV control, TIB MolBiolBiolmo Berlin, Germany SarbeCoV TibMolBiol, TIB Biolmol, Berlin, Germany TaqPath COVID-19 Combo kit, Life Technologies Ltd, Paisley, UK GeneXpert, Cepheid, Sunnyvale CA, USA |
E, N, Orf1a/b or S gene | Nasopharyngeal and oropharyngeal | NR | NR | Real-time estimates of COVID-19 prevalence taken from publicly reported incidence data and retrospective results from the National Influenza Centre across three administrative regions | NR | NR |
A decline in population-level Ct was associated with the epidemic peak observed both regionally and nationally Used a SEIR model and a flexible GP model Both Ct-derived epidemic growth models predicted the epidemic trajectory with increasing growth rates in the months preceding epidemic sub-peaks |
|
Avadhanula et al. [15] |
Hospital | 828 unique patients | Centers for Disease Control and Prevention 2019-novel coronavirus (2 019-ncoV) real time RT–PCR diagnostic panel | N1, N2 | Mid-turbinate swab (98%), nasopharyngeal (2%) | NR (asymptomatic: 68% wave 1, 61% wave 2) | NR | COVID-19 prevalence in individuals who worked within the medical center or used the healthcare services of the medical center | Wave 1 peak viral load median Ct 21.3. Wave 2 peak viral load median Ct 21.7 | 2–3 weeks |
No predictive modeling used in this study During the first wave the peak in the weekly viral load preceded the highest positivity rate of 15% by 3 weeks. Similarly, during the second wave the weekly viral load peaked 2 weeks before the highest positivity rate of 20% in the medical center |
| Calistri et al. [21] | Public | 12,880 | Adopted molecular test [TaqMan 2019-nCoV (qPCR) assay kit v2, Thermo Fisher Scientific, Waltham, MA, USA] | N | Nasopharyngeal/oropharyngeal | NR | B.1.177 | Incidence of COVID-19 cases locally |
Wave 1 (March–April) median Ct: 31 Inter-epidemic period (May–September) median Ct: 32 Wave 2 (October–December) median Ct: 28 |
NR |
No predictive modeling used in this study A strong inverse correlation (Pearson correlation coefficient = −0.978) was observed between the median Ct value in the three test periods and incidence cases of COVID-19 |
| El Zein et al. [16] | Hospital | 461 | GeneXpert, Cepheid, Sunnyvale CA, USA | N | Nasopharyngeal | NR | NR | Qualitative trends in regional case counts |
Start of April 2020 Ct values predominantly (46%), Ct 25–37. By start of June 2020 Ct values predominantly (67%), Ct ≥ 37 |
NR |
No predictive modeling used in this study Case counts in the region declined during the study period (not quantified). An increasing trend of high Ct values corresponded with a decrease in all-cause in-hospital mortality rates, which were a proxy for severity of the pandemic |
| Hay et al. [2] | Hospital and care home | NR |
Massachusetts long-term care facilities data processed in an FDA emergency use authorized lab-developed assay Data from Brigham and Women's Hospital, Boston, MA, USA was processed on a Hologic Panther Fusion SARS-CoV-2 assay |
N1, N2, and ORF1ab | Nasopharyngeal | NR | NR | Daily confirmed case counts for Massachusetts based on information from state and local health agencies | NR | 7-day |
The population level distribution of Ct values varied with Rt. The median and skewness of a single cross-sectional set of random Ct samples within a 1- to 2-month epidemic seed time could be used to estimate Rt Multiple cross-section models including the more complex GP prior for the underlying daily probabilities of infection allow for a more flexible approach to estimating the epidemic trajectory. The GP model provided growth-rate estimates that followed those estimated using observed case counts for the whole state |
| Khalil et al. [26] | Hospital | NR | NR | NR | NR | NR | NR | National daily COVID-19 confirmed case counts were obtained from the Lebanese Ministry of Public Health and Worldometers website | NR | 7-day |
There was a temporal delay between the observed Ct values and the incidence rate with a trough in mean Ct values (on October 8 2020) followed by an increase in case numbers 3 weeks later (on October 29 2020) There was an inverse correlation between mean Ct values and number of cases (P < 0.001), (Spearman correlation) Six data-driven models that utilized Ct values and number of cases from a previous wave (training dataset) were used to predict the epidemic trajectory. This was evaluated using MSE The sequence-to-sequence model MSE = 0.025 The polynomial regression (OLS) and SVR MSE = 0.1596, and MSE = 0.16754, respectively |
| Lin et al. [24] | Public | 8268 |
LightMix Modular SARS-CoV-2 (COVID-19) E-gene, TIB Molbiol/Roche, Berlin, Germany |
E gene | NR | Specific time points NR; however, adjusting for delays from illness onset to sampling did not alter the association between population Ct distribution and incidence-based Rt | NR | Public health surveillance of local Rt during two local epidemics | NR | Real-time estimates | The Spearman’s correlation coefficient of incidence-based Rt with decreasing average Ct values was ρ = −0.79, P < 0:001 for the first wave (a training wave) and ρ = −0.52, P < 0:001 for the next wave) |
| Mishra et al. [25] | Hospital and public | 13,816 | NR | N, ORF, RdRp | NR | NR | Delta variant (B.1.617) confirmed for second wave included in this study | Positive samples reported during the first and second wave | > 25, 25–30, and > 30 were categorized as high, moderate, and low viral load respectively | NR |
No predictive modeling used in this study The study did not include correlation statistics but rather examined the Ct values over time of the two waves and found that the proportion of Ct values < 25 was significantly higher before the peak of the second wave of infections, which was associated with a more rapid surge of cases nationally |
| Penney et al. [17] | Hospital | 342 | Abbott M2000 SARS-CoV-2 assay, Abbott Park, IL, USA | N, RdRp | Nasopharyngeal | NR | NR | Epidemiological trends and anticipating the next phase of the pandemic using median weekly incident hospitalizations due to SARS-CoV-2 infection in Massachusetts, obtained from the Massachusetts Department of Public Health | NR | NR |
No predictive modeling used in this study A strong inverse correlation (Pearson's correlation r = −0.76 (P < 0.05)) was observed between the median Ct value and median weekly incident hospitalizations due to SARS-CoV-2 infection in Massachusetts, obtained from the Massachusetts Department of Public Health |
| Phillips et al. [13] | Hospital |
2114 Lowest Ct value associated > 0 for E and N2 region for Xpert Xpress SARSCoV-2 assay OR a single Ct value that corresponds to the lowest Ct value > 0 for the Xpert Xpress SARS-CoV-2/Flu/RSV assay |
Xpert Xpress SARS-CoV-2 Assay, Cepheid, Sunnyvale CA, USA Xpert Xpress SARS-CoV-2/Flu/RSV assay, Cepheid, Sunnyvale CA, USA Both run on Infinity and XVI systems |
E, N2 | Nasopharyngeal or anterior nasal | NR | NR | Future changes in institutional COVID-19 cases | NR | 2 weeks |
When compared temporally, the average weekly Ct values for all tests performed negatively correlated with the number of cases occurring 2 weeks after specimen collection (Pearson’s correlation r = –0.75, P < 0.0001) Predictive modeling using Ct values from the epidemic surge October 2020–March 2021 correctly predicted whether cases would increase or decrease 65% of the time for a subsequent surge (May–July 2021) |
| Stevens et al. [23] | Hospital and public | ~ 26,388 |
Five testing platforms used: BD Max; Liat, Roche Diagnostics; Cobas, Roche Diagnostics; GeneSig, Primerdesign; Seegene, Korea |
E, M, N, N1, N2, ORF1a ORF1a and N gene RdRp and N gene |
NR | NR |
Omicron strain (B.1.1.529) and Omicron BA.2 sub-variant |
Community prevalence | < 30 | NR |
No predictive modeling used in this study There was an increased proportion of Ct > 30 as case numbers declined and the opposite was true as numbers increased again in the community |
| Tso et al. [14] | Public |
36,306 Ct ≤ 37 in at least two genes |
TaqPath COVID-19 Combi Kit, Thermo Fisher Scientific, Waltham, MA, USA | N, ORF1ab, S, | Mostly nasopharyngeal, also some anterior nares, saliva or unknown sample type | NR | NR | The community disease dynamics included the percent positivity, Rt and the COVID-19 hospitalization count in the El Paso area | NR | 33 days |
No predictive modeling used in this study A negative correlation between median Ct and Rt (P < 0.001), negative correlation between median Ct and hospitalization count (P < 0.001) (with time delay of 33 days), and negative correlation between median Ct and changes in percent positivity (P < 0.001) Visual trends suggested time delays in median Ct values and outbreak measures, but a statistically significant delay in predicting community disease dynamics was detected only with COVID-19 hospitalization counts (P < 0.001), not with Rt or change in percent positivity |
| Walker et al. [19] | Public | 21,831 |
TaqPath RT–PCR COVID-19 Kit, Thermo Fisher Scientific, Waltham, MA, USA Analyzed using UgenTec Fast Finder 3.300.5 (TaqMan 2019-nCoV Assay Kit V2 UK NHS ABI 7500 v2.1) |
N, ORF1ab, S, | Nose and throat swabs | NR | Alpha / B.1.1.7 | National positivity rate | NR | NR |
No predictive modeling used in this study Decreases in population level Ct values in July/early August 2020 preceded increases in positivity rates in this country. However, later declines in Ct in early December coincided with, rather than preceded, increases in positivity possibly due to the expansion of a new variant |
| Yin et al. [20] | Public |
7906 Ct > 22.3 were considered as “weak positive” (viral load < 103 RNA copies/mL) |
Abbot m2000 RealTime SARS-CoV-2 assay, Abbott Laboratories, Abbott Park, IL, USA | N, RdRp | Nasopharyngeal | NR | NR | National prevalence as extracted from the “total number of tests by date” and the “confirmed cases by date, province, age and sex” public dataset available on the Sciensano website | NR | 17 days |
Each epidemic wave was preceded a number of days earlier by a drastic decrease in Ct values A 17-day time lag in the 14-day median Ct value negatively correlated with the 14-day mean daily positive tests The 14-day mean daily positive tests in a central laboratory in Brussels were strongly correlated with the 14-day mean confirmed cases in the Brussels-Capital and in the whole country with coinciding start, peak, and end of each wave of the epidemic A deterministic, continuous age-structure compartmental model (extended SEIR-type) found that Ct values negatively correlated to the estimated prevalence in Belgium |
ARIMA autoregressive integrated moving average, CI confidence interval, COVID-19 coronavirus disease 2019, Ct cycle threshold, FDA US Food and Drug Administration, GP Gaussian process, MSE mean square error, NR not recorded, OLS ordinary least squares, (q)PCR quantitative polymerase chain reaction, RNA ribonucleic acid, Rt reproduction number, RT–PCR reverse transcription polymerase chain reaction, SARS-CoV-2 severe acute respiratory syndrome coronavirus-2, SEIR susceptible-exposed-infectious-recovered, SVR support vector machine regression
The studies were conducted in the USA [2, 13–17], Europe [18–21], Africa [22], Australia [23], China [24], India [25], the Kingdom of Bahrain [3], and Lebanon [26]. RT–PCR Ct values were measured from public samples only (national or local), or from hospital and public samples in ten studies [3, 14, 18–25], and from hospital only or hospital and care home samples only in six studies [2, 13, 15–17, 26]. Eleven studies included nasopharyngeal swabs [2, 3, 13–18, 20–22], one study used nose and throat swabs [19], and four studies did not record the sample type [23–26]. At least 30 different polymerase chain reaction (PCR) assays were included in the studies, but not all were described in sufficient detail to give a precise count, with two studies not recording the PCR assay used [25, 26]. The real-time RT–PCR targets included the E gene, M gene, N gene, S gene, RdRp, and ORF1ab. Ten studies included more than one gene target. The time point of assessment after symptom onset was not recorded for most studies. The Rt number was used as the measure of the population/epidemic trend levels in five studies [2, 14, 18, 22, 24]. Seven of the studies used predictive models based on the inverse association between Ct values and epidemiological trends to prospectively predict the epidemiological trajectory [2, 13, 18, 20, 22, 24, 26].
Risk of Bias of Included Studies
A modified Newcastle–Ottawa Scale was used to assess risk of bias of the included studies. All the studies were classed as being of low quality (Supplementary Table 3). The reasons for low quality included a lack of comparability of the RT–PCR Ct values of the exposure samples between studies, a lack of comparability of the temporal cohorts at the population level within each study, and that there is no way of quantifying respondents and nonrespondents. In addition, the novelty of this review adds to the challenge of the determination of the risk of bias of included studies (Supplementary Table 3).
Correlation between Ct Value and Community Prevalence of SARS-CoV-2
All the included studies examined the association between Ct values and epidemiological trends (Table 2). Many retrospectively examined the cross-correlation (lag lead) between Ct values and epidemiological trends. Almost half the studies reported a time lag in the negative cross-correlation between Ct values and new daily cases, with seven studies reporting time lags between 1 and 3 weeks [2, 3, 13, 15, 18, 20, 26], and one study reporting a time lag of 33 days [14] (Table 2). Five studies looked at the correlation between Ct values and the stages of different epidemic waves of SARS-CoV-2 [15–17, 21, 23]. These studies found that as case numbers increased towards the peak of an epidemic wave there was an increase in viral loads as determined by low Ct values; towards the end of epidemic waves, there was also a higher proportion of patients with high Ct values, and therefore a decrease in viral load (Table 2).
Predictive Models of Future Epidemic Trends
To assess whether Ct values themselves predicted future SARS-CoV-2 prevalence, eight studies used the data from the retrospective analysis of the correlation between Ct values and community prevalence to develop models predicting future epidemic trends [2, 3, 13, 18, 20, 22, 24, 26], although one of these did not test their predictive model prospectively [3].
In one US study (Hay et al.), models that use information inherent in Ct values were developed to estimate the epidemic trajectory from a cross-section of positive samples (Table 1) [2]. The authors used three mathematical models to describe daily SARS-CoV-2 transmission: (1) The deterministic susceptible-exposed-infectious-recovered (SEIR) model; (2) the Exponential Growth Model that assumes new infections arise under a constant exponential growth rate; and (3) the Gaussian Process (GP) Model that describes the epidemic trajectory as a vector of daily infection probabilities, where the GP prior ensures that daily infection probabilities are correlated in time [2]. The model outcomes using Ct values were comparable to a modification of the SEIR model, the SEEIRR model (that included additional compartments for PCR negative exposed individuals and PCR positive individuals who are recovered), which used prevalence data to determine baseline estimates in a closed population. More complex epidemic trajectories were estimated using cross-section models including the GP model using Ct values of hospital-based surveillance at a local hospital compared with daily confirmed case counts for the state (Table 2) [2]. The estimated epidemic trajectory using hospital Ct values correlated with community-level viral load changes obtained from wastewater monitoring [2].
Khalil et al. evaluated six data-driven models that utilized Ct and previous number of cases to predict the epidemic trajectory using mean square error (MSE). The ordinary least squares (OLS) polynomial regression and support vector machine regression (SVR) had the best performance during independent validation (MSE = 0.1596 and MSE = 0.16754, respectively). The OLS and SVR models also accurately predicted the COVID-19 incidence in an external institution (Table 2) [26].
Abdulrahman et al. carried out a pilot study for the development of a predictive model based on the linear regression analysis on the association between Ct values and new daily cases. There was a significant negative correlation between Ct values with new daily cases (Pearson’s correlation r = −0.06 (95% CI: −0.06, −0.05, P < 0.001). The average weekly Ct values negatively correlated with daily cases occurring 1 week after specimen collection (Table 2) [3].
The predictive model in Phillips et al. was created using linear regression analysis on data from all Ct values within a closed institute setting between October 2020 and March 2021. Average weekly Ct values negatively correlated with the number of cases occurring 2 weeks after specimen collection (Pearson’s correlation r = −0.75, P < 0.0001). Ct values for symptomatic patients correlated with tests performed the week of collection, whereas asymptomatic patient Ct values correlated best with tests performed 1 month later [13]. The data from this model was validated when Ct values collected May–July 2021 were compared with future cases, correctly predicting increases or decreases 65% of the time for this surge (Table 2). The predictive model performed better when cases increased dramatically (greater than 30% increase), with the model capturing 82% of these increases in cases at the individual hospital level (Table 2) [13].
Alizon et al. used Rt of the epidemic as a measure of prevalence for the predictive model. A time series analysis using autoregressive integrated moving average (ARIMA) predictive models was used to estimate whether Ct data improves short-term predictions of disease epidemiology. A time lag of 6–7 days appeared to be the most significant time lag for cross-correlation between Ct and Rt (tested between 0 and 20 days) (Table 2). Rt was calculated using hospital admissions data for COVID-19 in addition to RT–PCR screening data. The error in predicting Rt improved when Ct quartiles and Ct skewness were known. The prediction error of Rt using Ct values was lower than that using the ratio of positive tests [18].
Andriamandimby et al. compared the results of cross-sectional Ct distributions with Rt estimates derived from more traditional case count methods to estimate the epidemic growth rate (Table 1) [22]. A decline in population-level Ct was associated with the epidemic peak both regionally and nationally. A population-level SEIR model framework, and the flexible GP model developed by Hay et al. [2], correctly estimated epidemic growth rates from Ct distributions locally in the months preceding epidemic sub-peaks (Table 2). The epidemic growth estimates were largely congruent with those using Rt estimates from daily reported incidence. The authors noted that cross-sectional Ct distributions would have predicted the possibility of an epidemic resurgence in Madagascar that had been missed during declining surveillance [22].
Lin et al. fitted a log linear regression to the daily Rt, on daily mean and skewness of Ct values at sampling during a training period wave to determine real-time assessment of transmission in the community using Ct values (Table 2). The results from the training model were used to successfully predict real-time estimations of Rt in a subsequent wave [24]. The temporal Ct distribution correlated with the incidence-based Rt over both epidemic waves. Higher values of incidence-based Rt were associated with decreasing average Ct values (Spearman’s correlation coefficient, ρ = −0.79, P < 0.001, and ρ = −0.52, P < 0.001, for the initial and subsequent wave respectively).
Yin et al. found that each epidemic wave was preceded by a drastic decrease in Ct values several days earlier. There was a 17-day time lag in the median Ct value negative correlation with the mean daily positive tests. An extended SEIR-type model found that the Ct values by all ages and age classes was negatively correlated with the estimated daily prevalence in Belgium (Table 2) [20].
Discussion
To the best of our knowledge, this is the first review to systematically assess and consolidate available evidence on associations between SARS-CoV-2 Ct values and epidemiological trends. Previous systematic reviews have demonstrated Ct value correlations with disease severity and transmissibility [12, 27, 28]. Our review differs by highlighting the potential relevance for determining trends in COVID-19 epidemiology, such as predicting peaks in variant waves, which in turn may be useful for investigations into other circulating respiratory pathogens.
All 16 studies included in this review reported an inverse correlation between SARS-CoV-2 Ct values and epidemiological trends at a single unit, locally, or nationally. Eight of the studies observed the inverse correlation of Ct values and incidence rates over two waves of the epidemic [15, 19–21, 23–26]. Seven of the studies reported a prediction time in the negative cross-correlation between Ct values and new daily cases of around 1–3 weeks [2, 3, 13, 15, 18, 20, 26]. Mathematical models using Ct values predicted epidemic trajectories of a few weeks to longer term incidence curves [2, 3, 13, 18, 20, 22, 24, 26]. Many of these epidemic models accounted for complex populations, where interventions may be implemented and relaxed over time and new variants may arise.
Existing surveillance systems using case-counting methods and test positivity rates to estimate the epidemic trajectories can suffer from reliability issues. Predictive modeling using Ct values might provide a more reliable estimate, or useful adjunct to assessing the epidemic trajectory. These models could be extended to predict the epidemic trajectory of other contagious viral diseases diagnosed by RT–PCR after at least one wave of disease. Better estimates of epidemic trajectories can also allow for better epidemic planning and the implementation of more targeted epidemiological measures.
Similar to using patient viral loads as a proxy to population-level exposure to SARS-CoV-2, the association between exposure to high viral loads in the environment and epidemiological trends has also been investigated with wastewater viral loads. Several studies have examined how wastewater surveillance can complement clinical surveillance to infer COVID-19 prevalence. A systematic review by Shah et al. found an association between wastewater viral load and prevalence in the community in 53 studies [7]. In addition, wastewater sample positivity preceded confirmed cases in the community up to 63 days, with 13 out of 87 studies reporting wastewater sample positivity before the detection of cases in the community [7].
The possible impact of the later identified variants of SARS-CoV-2 may be similar to the earlier variants. Stevens et al. [23] analyzed the SARS-CoV-2 real-time PCR test Ct values in a population in Australia from the end of November 2021 to the end of March 2022 including ~26,388 PCR positive samples. This period overlapped with the emergence of the Omicron variant and there was indeed an inverse relationship between the Ct value and the number of cases of the Omicron variant in this included paper as in the Alpha and Delta variants from the other included studies.
This review has several limitations due to heterogeneity among the included studies. The number of PCR positive samples included in the studies varied widely: not recorded in three studies [2, 23, 26], < 500 in two studies [16, 17] and 500 to ~800,000 in the remaining studies. There were several Ct value-related limitations such as a broad range of RT–PCR assays and PCR target genes. As SARS-CoV-2 viral load varies over time from initial infection [29–35], the absence of recording time since symptom onset in many studies was a limitation. Sample type can also affect Ct values [32] and less than half the included studies used nasopharyngeal samples with others using either a mix of sample types or not recording it. In addition, actual exposure was not measured, most studies looked at local populations for exposure (Ct values) and larger populations for outcome. However, one study validated a predictive model of SARS-CoV-2 prevalence trends from Ct values of samples from a closed system/single unit and then developed predictive models from Ct values of samples in a single unit to predict the regional epidemic trajectories [2]. Another study looked at temporal trends in Ct values in a single unit and how they correlated with future case numbers in that single unit [13].
The limitations of the included studies notably strengthen the findings of this review. A correlation between Ct values and future number of cases is demonstrable despite the heterogeneity in sample types, PCR assays, and variants. The studies revealed sufficient evidence that the number of COVID-19 positive cases in the community rise around 2 weeks after a drop in the Ct values; thus, predicting a wave 1 month in advance may even be possible.
However, there are several considerations for future work. Establishing an aggregated Ct cutoff value that predicts new waves with greater accuracy would be useful. As different RT–PCR methods report different Ct values, standard curves should be used to accurately quantify the expected viral copy number [36]. This would translate into better comparability between cohorts and more accurate correlation and prediction times. In addition, as sample type is known to affect Ct values [32], it would be useful to determine the impact of sample type on the correlation and prediction time of epidemic spread for future studies. The effect of the length of time from symptom onset to sample collection and testing should also be determined to examine whether there is an optimal time from symptom onset for correlation with disease epidemiology. The minimum number of cases needed to correctly assess the crossing of the Ct cutoff should also be established, and predictions should be validated across different pathogens and their variants. Public health decision-makers could then use the aggregated Ct cutoff value to estimate the prediction time to an upcoming wave and implement appropriate epidemic planning more efficiently in low-surveillance settings [2].
Conclusions
Ct values of current positive COVID-19 cases can be used to predict community infection rates at least 2 weeks in advance, irrespective of the prevalent variant or sample type and RT–PCR method used for diagnosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study received funding from QIAGEN Manchester Ltd. The funder had the following involvement with the study: article processing fees and provision of medical writing support.
Medical Writing and/or Editorial Assistance
Medical writing support, under the guidance of the authors, was provided by Costantinos Bezos, of 3 Stories High (3SH), UK, and was funded by QIAGEN Manchester Ltd, in accordance with Good Publication Practice (GPP) guidelines (Ann Intern Med 2022;175: 1298–1304). Amanda Weber and Costantinos Bezos of 3SH aided with literature screening.
Author Contributions
Ester Sala: Conceptualization, Writing—review & editing. Isheeta S Shah: Conceptualization, Writing—review & editing. Davide Manissero: Conceptualization, Writing—review & editing. Marti Juanola: Conceptualization, Writing—review & editing. Anne-Marie Quirke: Writing—original draft, Writing—review & editing, Methodology, Data Curation, Formal Analysis. Sonia N Rao: Conceptualization, Writing—review & editing. All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Ester Sala is an employee of STAT-Dx Life, a QIAGEN company, Isheeta S Shah is an employee of QIAGEN, Davide Manissero is an employee of QIAGEN, Marti Juanola is an employee of STAT-Dx Life, a QIAGEN company, Anne-Marie Quirke is an employee of 3SH, which received funding from QIAGEN to conduct the study, Sonia N Rao is an employee of Roche
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 06 Jul 2022.
- 2.Hay JA, Kennedy-Shaffer L, Kanjilal S, et al. Estimating epidemiologic dynamics from cross-sectional viral load distributions. Science. 2021 doi: 10.1126/science.abh0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdulrahman A, Mallah SI, Alawadhi A, Perna S, Janahi EM, AlQahtani MM. Association between RT-PCR Ct values and COVID-19 new daily cases: a multicenter cross-sectional study. Infez Med. 2021;29(3):416–426. doi: 10.53854/liim-2903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8(9):e70. doi: 10.1016/s2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabaan AA, Tirupathi R, Sule AA, et al. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics (Basel). 2021;11(6). 10.3390/diagnostics11061091. [DOI] [PMC free article] [PubMed]
- 7.Shah VP, Farah WH, Hill JC, et al. Association between SARS-CoV-2 cycle threshold values and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Open Forum Infect Dis 2021;8(9): 453. 10.1093/ofid/ofab453. [DOI] [PMC free article] [PubMed]
- 8.Tom MR, Mina MJ. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020;71(16):2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JT, Chandler J, Cumpston J, Lu M, Page T, Welch MJ (eds). Cochrane handbook for systematic reviews of interventions,. https://training.cochrane.org/handbook/current. Accessed July 2022.
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 2022.
- 12.Bonacorsi S, Visseaux B, Bouzid D, et al. Systematic review on the correlation of quantitative PCR cycle threshold values of gastrointestinal pathogens with patient clinical presentation and outcomes. Front Med (Lausanne) 2021;8:711809. doi: 10.3389/fmed.2021.711809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips MC, Quintero D, Wald-Dickler N, Holtom P, Butler-Wu SM. SARS-CoV-2 cycle threshold (Ct) values predict future COVID-19 cases. J Clin Virol. 2022;150–151:105153. doi: 10.1016/j.jcv.2022.105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tso CF, Garikipati A, Green-Saxena A, Mao Q, Das R. Correlation of Population SARS-CoV-2 cycle threshold values to local disease dynamics: exploratory observational study. JMIR Public Health Surveill. 2021;7(6):e28265. doi: 10.2196/28265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avadhanula V, Nicholson EG, Ferlic-Stark L, et al. Viral load of severe acute respiratory syndrome coronavirus 2 in adults during the first and second wave of coronavirus disease 2019 pandemic in Houston, Texas: the potential of the superspreader. J Infect Dis. 2021;223(9):1528–1537. doi: 10.1093/infdis/jiab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Zein S, Chehab O, Kanj A, et al. SARS-CoV-2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One. 2021;16(9):e0255981. doi: 10.1371/journal.pone.0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penney JA, Jung AW, Koethe BC, Doron SI. Utility of the cycle threshold in anticipating the next phase of the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022;43(6):800–801. doi: 10.1017/ice.2022.41. [DOI] [PubMed] [Google Scholar]
- 18.Alizon S, Selinger C, Sofonea MT, et al. Epidemiological and clinical insights from SARS-CoV-2 RT-PCR crossing threshold values, France, January to November 2020. Euro Surveill. 2022. 10.2807/1560-7917.ES.2022.27.6.2100406. [DOI] [PMC free article] [PubMed]
- 19.Walker AS, Pritchard E, House T, et al. Ct threshold values, a proxy for viral load in community SARS-CoV-2 cases, demonstrate wide variation across populations and over time. Elife. 2021 doi: 10.7554/eLife.64683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin N, Dellicour S, Daubie V, et al. Leveraging of SARS-CoV-2 PCR cycle thresholds values to forecast COVID-19 trends. Front Med (Lausanne). 2021;8:743988. doi: 10.3389/fmed.2021.743988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calistri P, Danzetta ML, Amato L, et al. Epidemiological significance of SARS-CoV-2 RNA dynamic in naso-pharyngeal swabs. Microorganisms. 2021;9(6):1264. doi: 10.3390/microorganisms9061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andriamandimby SF, Brook CE, Razanajatovo N, et al. Cross-sectional cycle threshold values reflect epidemic dynamics of COVID-19 in Madagascar. Epidemics. 2022;38:100533. doi: 10.1016/j.epidem.2021.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens R, Pratama R, Naing Z, Condylios A. Analysis of SARS-CoV-2 real-time PCR test CT values across a population may afford useful information to assist public health efforts and add refinement to epidemiological models. Pathology. 2022;54(6):800–802. doi: 10.1016/j.pathol.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Yang B, Cobey S, et al. Incorporating temporal distribution of population-level viral load enables real-time estimation of COVID-19 transmission. Nat Commun. 2022;13(1):1155. doi: 10.1038/s41467-022-28812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra B, Ranjan J, Purushotham P, et al. High proportion of low cycle threshold value as an early indicator of COVID-19 surge. J Med Virol. 2022;94(1):240–245. doi: 10.1002/jmv.27307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil A, Al Handawi K, Mohsen Z, et al. Weekly nowcasting of new COVID-19 cases using past viral load measurements. Viruses. 2022;14(7):1414. doi: 10.3390/v14071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouzid D, Vila J, Hansen G, et al. Systematic review on the association between respiratory virus real-time PCR cycle threshold values and clinical presentation or outcomes. J Antimicrob Chemother. 2021;76(Suppl 3):iii33–iii49. doi: 10.1093/jac/dkab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 30.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 31.Huang JT, Ran RX, Lv ZH, et al. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(16):2158–2166. doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Chen S, Yang Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201(11):1435–1438. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/s1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71(15):793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han MS, Byun JH, Cho Y, Rim JH. RT-PCR for SARS-CoV-2: quantitative versus qualitative. Lancet Infect Dis. 2021;21(2):165. doi: 10.1016/s1473-3099(20)30424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.